Abstract
In this study we describe the expression pattern of the Leuconostoc paramesenteroides citMCDEFGRP operon in response to the addition of citrate to the growth medium. An 8.8-kb polycistronic transcript, which includes the citMCDEFGRP genes, was identified; its synthesis was dramatically induced upon addition of citrate to the growth medium. We also found that expression of the cit operon is subjected to posttranscriptional regulation, since processing sites included in four complex secondary structures (I, II, III, and IV) were identified by Northern blot analysis and mapped by primer extension. Upstream of the citMCDEFGRP operon a divergent open reading frame, whose expression was also increased by citrate, was identified by DNA sequencing and designated citI. The start and end sites of transcription of the cit operon and citI gene were mapped. The start sites are separated by a stretch of 188 bp with a very high A+T content of 77% and are preceded by transcriptional promoters. The end sites of the transcripts are located next to the 3′ end of two secondary structures characteristic of ρ-independent transcriptional terminators. The effect of the citI gene on expression of the cit operon was studied in Escherichia coli. The presence of the citI gene in cis and in trans resulted in increased activity of the cit promoter. These data provide the first evidence that citrate fermentation in Leuconostoc is regulated at the transcriptional level by a transcriptional activator rather than by a repressor.
Citrate metabolism is carried out by only a few strains of lactic acid bacteria. This metabolic ability is invariable linked to endogenous plasmids that contain the gene encoding the transporter responsible for citrate uptake from the medium. Citrate transporters (CitPs) have been found in strains belonging to the genera Lactococcus and Leuconostoc, bacteria in which the mechanism of citrate fermentation has been studied in detail (1, 14–16). The first step in the breakdown of citrate inside the cell involves its conversion to acetate and oxalacetate by citrate lyase, a three-subunit enzyme (2). In the next step, oxalacetate is decarboxylated by oxalacetate decarboxylase, yielding pyruvate and carbon dioxide (for a review, see reference 9). The pathway generates a proton motive force (PMF) by a secondary mechanism (10). Electrogenic exchange of divalent citrate and monovalent lactate, catalyzed by CitP, efficiently generates a membrane potential, inside negative (17). Moreover a pH gradient (inside alkaline) is formed by the consumption of scalar protons in the decarboxylation of oxalacetate (10). Together, the membrane potential and pH gradient constitute the PMF, which seems to contribute significantly to the growth advantage observed during cometabolism of citrate and glucose in both Lactococcus and Leuconostoc (14, 17).
Like all the known citrate lyases, the Leuconostoc enzyme forms a functional complex of three proteins: a γ subunit (acyl carrier protein [ACP]), a β subunit (citryl-S-ACP lyase), and an α subunit (citrate:acetyl-ACP transferase) (2). This enzymatic complex is active only if the thioester residue of the prostethic group linked to the γ subunit is acetylated. This activation is catalyzed by an acetate:SH-citrate lyase ligase which converts HS-ACP in the presence of ATP and acetate to acetyl-S-ACP (2).
The genes encoding CitP (22) and the subunits of citrate lyase (2) have been independently cloned and sequenced from genomic DNA of two different strains of Leuconostoc mesenteroides. Moreover, it has been shown that the citCDEFG genes coding for the L. mesenteroides citrate lyase together with a putative malic enzyme gene constitute an operon, which is induced by citrate at the transcriptional level (3). However, little is known about the molecular mechanism(s) involved in regulation of the synthesis of the CitP permease. Marty-Teysset et al. (16) reported that in L. mesenteroides the activity of the transporter was increased when citrate was added to the growth medium. In agreement with these experiments, we recently found that the utilization of citrate by L. paramesenteroides was stimulated when cells were grown in a medium containing citrate (15). These observations suggest that the mechanism of regulation of Leuconostoc CitP is different from the one demonstrated for the 99% identical CitP from Lactococcus. In the latter organism, the presence of citrate in the growth medium does not influence the expression of citP (13); instead, expression is transcriptionally induced at acidic pHs (8).
To investigate the regulation of Leuconostoc CitP synthesis, we recently cloned the citP gene from L. paramesenteroides (15). This gene is carried by a 22-kb plasmid. It is included in an operon together with five genes coding for the citrate lyase multienzymatic complex (citCDEFG) (15) and two open reading frames (ORFs), named citM and citR, coding for a putative malic enzyme and a polypeptide with homology to a Lactococcus lactis cit regulator (12).
In the work presented here, we analyzed the expression pattern of the L. paramesenteroides citMCDEFGRP operon and showed unequivocally that its transcription is induced by citrate independently of the pH of the growth medium. We also present evidence that a regulatory protein, named CitI, encoded by an ORF found in the upstream region of citMC DEFGRP is a transcriptional activator of the cit operon. The proposed mechanism of citMCDEFGRP transcriptional activation provides an explanation for the induction of citrate fermentation in Leuconostoc when citrate is added to the growth medium.
MATERIALS AND METHODS
Bacterial strains, plasmids, and growth media.
The bacterial strains and plasmids used in this study are listed in Table 1. L. paramesenteroides J1 was grown at 30°C without shaking in modified MRS medium supplemented with 2% glucose (MRSG) as described previously (15). Escherichia coli was routinely grown in Luria-Bertani medium (19) and transformed as previously described. Ampicillin and kanamycin were added at a final concentrations of 100 and 30 μg/ml, respectively.
TABLE 1.
Bacterial strains and plasmids used in this study
| Designation | Relevant characteristics | Reference or source |
|---|---|---|
| Bacterial strains | ||
| L. paramesenteroides J1 | Lac+ Cit+ | 15 |
| E. coli DH5-α | supE44 ΔlacU169 (φ80 lacZM15) hsdR17 recA1 endA1 gyrA96 thi-1 relA1 | Laboratory stock |
| Plasmids | ||
| pCITJ1 | 21-kb Cit+ plasmid from L. paramesenteroides J1 | 15 |
| pMM13 | 5.8-kb fragment from pCITJ1 including citI, citCDE, and the 3′ end of citF cloned into pBluescript SK(+) | 15 |
| pMM8 | 2.4-kb fragment from pCITJ1 including the 3′ end of citR and citP cloned in pBluescript SK(+) | 15 |
| pJM116 | Promoter probe plasmid derivative of pBR322 containing a promoterless lacZ gene | 5 |
| pJMM1 | pJM116 derivative containing lacZ under control of the citMCDEFGRP promoter and citI | This work |
| pJMM12 | Deletion of a 0.75-kb fragment from citI contained in pJMM1 | This work |
| pSU39 | Low-copy-number cloning vector derivative of pACYC184 | 4 |
| pSUI | pSU39 derivative containing citI under control of the lacUV5 promoter | This work |
RNA analysis.
After growth overnight in MRSG medium, L. paramesenteroides J1 was sedimented by centrifugation and resuspended in saline solution. Appropriate aliquots of the cultures were used to inoculate MRSG fresh medium to give an initial A660 of approximately 0.05. For Northern analysis, the cultures were grown to an A660 of 0.2, then supplemented with 1% sodium citrate, and further incubated at 30°C. At the times indicated in the figure legends, aliquots were withdrawn and used for analysis of mRNA. For primer extension experiments, endonuclease S1 mapping, and dot blot analysis, MRSG or MRSG supplemented with 1% sodium citrate was inoculated with the overnight cultures as indicated above. Cultures were grown until they reached an A660 of 0.2 and then used for analysis of RNA. To adapt Leuconostoc cultures to acidic pHs, stock cultures previously grown at pH 7.0 and kept frozen at −70°C were used to inoculate MRSG medium adjusted at pH 5.0 and grown overnight. The overnight cultures were sedimented and resuspended in saline solution, and appropriate aliquots were used to inoculate fresh medium at the pH required to give an A660 of approximately 0.05 as indicated. These conditions of growth and dilution allowed the latent period of the cultures to be reduced and the contributions of the mRNA present in the overnight cultures to be minimized.
RNA manipulations.
For primer extension and endonuclease S1 mapping, RNA from L. paramesenteroides was isolated as previously described for L. lactis (12) except that cell lysis was performed by the addition of lysozyme at 30 μg/ml. For Northern blot hybridization, RNA was isolated with a Ribolyser and Recovery kit from Hybaid as specified by the supplier. The RNAs were checked for the integrity and yield of the rRNAs in all samples. The patterns of rRNAs were similar in all preparations. The total RNA concentrations were determined and quantified by UV spectrophotometry and by Gel Doc 1000 (Bio-Rad). Primer extension analysis was performed as previously described (12). The primers used for detection of the start sites of citMCDEFGRP and citI mRNAs were 5′-TGGGATTTGTGCACCTT-3′ and 5′-TCTTCGGCAATTTTAGC-3′, respectively, complementary to nucleotides (nt) +32 to +16 of citM and +35 to +19 of citI. The primers used for detection of the 5′-end of mRNA processed species were 5′-GTGTGCCGGCGACTGCA-3′, 5′-CCGGCCTTGACCATCGC-3′, 5′-CGTCATGCCATCGCGGA-3′, and 5′-GGCATGTGACCAACCTG-3′, complementary to nt +34 to +18 of citD, +272 to +256 of citE, +54 to +38 of citF, and +728 to +712 of citR, respectively. One picomole of either primer was annealed to 15 μg of total RNA. Primer extension reactions were performed by incubation of the annealing mixture with 20 U of avian myeloblastosis virus reverse transcriptase (Promega) at 42°C for 30 min. Endonuclease S1 mapping was performed as previously described (12). The probe used for determination of the 3′ end of citMCDEFGRP or citI was a 720-nt StyI-EcoRI or a 535-nt BanI-PstI DNA fragment from pMM8 or pMM13, respectively. Probes were 32P labeled at their unique 3′ recessive ends by fill-in with E. coli Klenow fragment and used to detect the citMCDEFGRP or citI transcripts. Size determination of the reaction products of primer extension and endonuclease S1 mapping were carried out in 8% polyacrylamide gels containing 7 M urea. Bands labeled with 32P were detected by autoradiography on Kodak X-Omat S films and were directly quantified with a PhosphorImager system (Molecular Dynamics). For Northern blot analysis, samples containing 6 μg of total RNA were fractionated in a 1% agarose gel. Transfer of nucleic acids to nitrocellulose membranes and Northern blot hybridization with 0.2 pmol of the appropriate probes were performed as previously described (12). The single-stranded probes used were synthesized as follows. The BglII-PstI fragment from pMM10 and the PstI insert of pMM8 were purified and 32P labeled in one strand with T7 DNA polymerase. Primers 5′-CTTTACTTGCTTGCTCG-3′ and 5′-AGCAAGCAATGCGTGCG-3′, complementary to nt +517 to 500 of citC and +1014 to 998 of citP, were used to give probes I and II (Fig. 1A). Bands labeled with 32P were detected and quantified as indicated above.
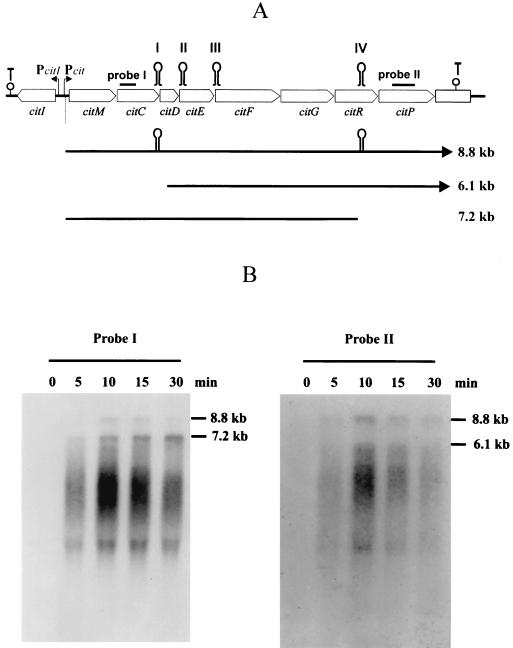
FIG. 1.
Organization of the citrate fermentation genes in L. paramesenteroides J1 (A) and Northern blot analysis of the citMCDEEFGRP operon (B). (A) The 11-kb DNA cluster encompassing nine genes involved in citrate utilization is shown in at the top. Pcit, promoter of the citMCDEEFGRP operon, PcitI, promoter of the citI gene. The secondary structures downstream of citP and citI represent ρ-independent transcriptional terminators. The stem-loop structures named I, II, III, and IV include the processing sites of citMCDEEFGRP mRNA mapped in Fig. 2. Probe I includes a 0.5-kb fragment of citC. Probe II includes a 0.5-kb fragment of citP. The major RNA species observed in the Northern blot shown in panel B are indicated. (B) Northern blot analysis was carried out as described in Materials and Methods. Strain J1 was grown to an A660 of 0.2 in MRSG medium. At this time cells, were supplemented with 1% citrate; total RNA was isolated at different times and probed with probe I or probe II.
DNA analysis and manipulation.
Plasmid DNA preparations for cloning and sequencing experiments as well as transformations of E. coli were performed as described elsewhere (21). Treatment of DNA with restriction enzymes and T4 DNA ligase was performed as recommended by the suppliers. DNA sequence of both strands of the citI gene was determined from plasmid pMM13 with automated DNA sequencing instrumentation (ABI PRISM; Perkin-Elmer) at the Centro de Investigaciones Biológicas.
Construction of plasmids pJMM1, pJMM12, and pSUI.
To construct a transcriptional fusion of the promoter of the citMCDEFGRP operon to the E. coli lacZ gene, a 2.2-kb EcoRI-EcoRI fragment from pMM13 including the promoter region and the citI gene under the control of its own promoter was purified and cloned into the unique EcoRI site of plasmid pJM116 to give pJMM1. To construct plasmid pJMM12, pJMM1 was digested with HindIII and the 0.8-kb fragment including the citMCDEFGRP and citI promoters was purified and ligated to HindIII-linearized pJM116. To construct plasmid pSUI, the citI gene was amplified by PCR by using pMM13 as the template and primers RegU (5′-GTGCAGAATTCGTCATCGACGGTGGATAC-3′) and RegM (5′-AAAAAAACTGCAGAATTTCCAGTTTAAATCTCG-3′). The underlined sequences indicate sites EcoRI and PstI, respectively. The PCR product was purified, digested with EcoRI and PstI, and ligated to EcoRI- and PstI-digested pSU39. The constructs were established in E. coli DH5-α by transformation.
β-Galactosidase assay.
E. coli cells were grown overnight in Luria-Bertani medium with aeration at 37°C, then diluted 1:100 in 10 ml of fresh medium, and grown to an A600 of 0.3. The final absorbance was measured after 10 min in ice bath to stop the cell growth, and aliquots of 1 ml were harvested. Pellets were frozen at −20°C until they were used. To induce citI expression from pSUI, E. coli cells were grown to an A600 of 0.2 and then induced by the addition of 1 mM isopropyl-β-d-thiogalactopyranoside (IPTG). At the times indicated in Table 2, samples were withdrawn and processed as described above. β-Galactosidase activity was measured by the method of Miller (19). Specific activities were expressed in units per absorbance of the cultures at 600 nm.
TABLE 2.
β-Galactosidase activities of E. coli DH5-α cells containing the indicated plasmidsa
| Plasmid(s) | Time of inductionb (min) | β-Gal activity (Miller units/A600) |
|---|---|---|
| pJMM1 | NI | 27,097 ± 1,046 |
| pJMM12 | NI | 8,613 ± 279 |
| pJM116 | NI | 1,188 ± 40 |
| pJM116 + pSUI | NI | 1,152 ± 56 |
| pJM116 + pSU39 | NI | 1,900 ± 212 |
| pJMM12 + pSUI | NI | 26,633 ± 473 |
| 20 | 30,443 ± 3,480 | |
| 40 | 37,110 ± 3,860 | |
| 60 | 50,806 ± 3,947 | |
| pJMM12 + pSU39 | NI | 7,343 ± 217 |
| 20 | 9,291 ± 440 | |
| 40 | 11,177 ± 826 | |
| 60 | 11,931 ± 1,181 |
Each value is the average of the values from at least three experiments.
Induction with IPTG was performed as described in Materials and Methods. NI, noninduced.
Nucleotide sequence accession number.
The nucleotide sequence of L. paramesenteroides J1 that contains the citI gene has been deposited at the EMBL database under accession no. AJ132782.
RESULTS
Transcription of the citMCDEFGRP operon is induced by citrate.
The clustering of the citMCDEFGRP genes (Fig. 1A) and their functional relationship suggest that these eight genes form a single transcriptional unit (15). To verify the operon structure and to test whether its transcription is regulated by citrate, Northern blot analysis was performed. Total cellular RNA was isolated from cultures of the L. mesenteroides J1 grown in medium lacking citrate and then supplemented with citrate for various times. The RNA was hybridized with two 32P-labeled probes (Fig. 1B). Probe I includes a 0.5-kb fragment covering the 5′ end of citC, while probe II covers a 0.57-kb internal fragment of citP (Fig. 1A). The larger RNA species detected with probe I also hybridized with probe II (Fig. 1B). This mRNA species could correspond to a 8.8-kb transcript starting upstream of citM and ending downstream of citP. Quantification of total citMCDEFGRP RNA by dot blotting allowed the detection of basal levels of transcription before the addition of citrate (time zero) and revealed a 13-fold increase of transcription after 10 min of induction (data not shown). These experiments demonstrated that the citMCDEF GRP operon is induced by citrate at the transcriptional level. Probe I also revealed another RNA specie of 7.2 kb (Fig. 1B), which was predominant 10 min after addition of citrate, while probe II detected a second band of 6.1 kb (Fig. 1B). The differential pattern obtained with both probes suggested that the 8.8-kb transcript is subjected to specific processing at several locations. Furthermore, analysis of this mRNA with the Fold program (23) using the University of Wisconsin Genetics Computer Group software package (7) predicted the existence of four complex secondary structures named I, II, III, and IV (Fig. 1A and 2) with predicted free energies of −31, −29, −31, and −42 kcal/mol, respectively. A cleavage at structures I and IV (Fig. 1A) could account for the existence of RNA species of 6.1 and 7.2 kb, respectively (Fig. 1B). To test that these structures as well as structures II and III were specific cleavage sites for endoribonucleases, a primer extension analysis was performed with four different primers located proximal to the 3′ end of these putative cleavage sites (for details, see Materials and Methods). The results obtained (Fig. 2) revealed that indeed processing at these secondary structures occurred. The two cleavages at structure I should disrupt citC and as a consequence impair its translation. Moreover, they could enhance expression of citD, since its predicted ribosomal binding site (RBS) is located at the base of the structure and it will be exposed to the ribosomes in the processed species. The three cleavages detected at structure II are located within citE, and they should abolish synthesis of CitE. Cleavages at structure III could determine expression of citF, since its RBS is buried in the structure. Finally, the three cleavages at structure IV should result in disruption of citR translation. Therefore, expression of the citMCDEFGRP operon seems to be subjected to posttranscriptional regulation, and the specific cleavages may determine that the cell synthesizes the different proteins, required for citrate utilization, in suitable proportions.
FIG. 2.
Analysis of processing of citMCDEFGRP mRNA in L. paramesenteroides by primer extension. Detection of the 5′ ends of processed species as well as the proposed putative structures (I, II, III, and IV) of the regions involved in the processing of citMCDEFGRP are depicted. The primer extension reactions were carried out as described in Materials and Methods. The specific cleavage sites (marked by arrows) at each structure were determined by comparing the extended fragments with a sequence reaction carried out with the same primer used in the extension reaction.
Identification of the citI gene and determination of the transcriptional signals of the citMCDEFGRP operon.
Northern analysis revealed that addition of citrate to Leuconostoc cultures induced the transcription of the citMCDEFGRP operon (Fig. 1B). In an attempt to identify a possible regulator responsible for this transcriptional induction, the DNA sequence of the region located upstream of the citMCDEFGRP operon was determined. An ORF of 1,095 nt located upstream and oriented inversely to the cit operon was detected and designated citI (Fig. 1A and 3C). A potential RBS (5′-AAGGA-3′) is located 9 nt upstream of the first ATG of citI (Fig. 3C). Thus, the gene should encode a protein of 322 amino acids with a predicted Mr of 36,488. Data bank searches revealed significant homology of this peptide with 11 characterized and putative transcriptional regulators belonging to the SorC family and included in the ProDom domain PD006970 (5). Among them, ClyR (accession no. O86289), a putative regulator of the maecitCDEFG from L. mesenteroides, showed the highest identity (54% in a 309-amino-acid overlap), while the other members of the family showed homology ranging from 26 to 20% (data not shown). These homologies strongly suggested that CitI could be a regulator involved in transcriptional induction of the cit operon by citrate.
FIG. 3.
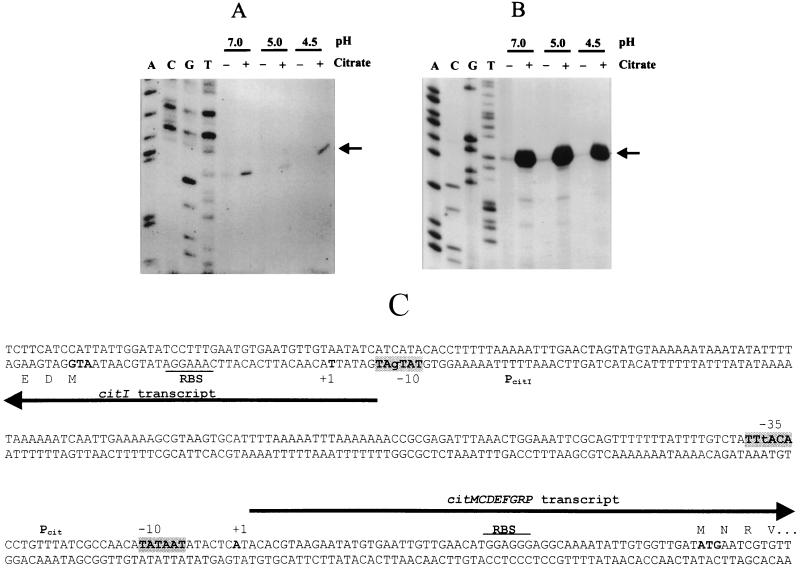
Primer extension analysis of the transcription start sites of citI (A) and citMCDEFGRP (B). The autoradiographs show primer extension experiments performed with RNA extracted from strain J1 grown with or without citrate in media buffered at pH 4.5, 5.0, and 7.0. Lanes A, C, G, and T show sequencing reactions performed with the same primers used in the extension reactions. Arrows indicate the 5′-extended fragments of citI (A) and citMCDEFGRP (B), respectively. (C) Nucleotide sequence of the citM-citI intergenic region containing the bidirectional promoter region of the citMCDEFGRP and citI genes. The −10 and −35 regions are indicated in grey boxes. The transcription initiation sites (+1) and the ATGs of citM and citI are in boldface. The putative RBSs are underlined. Arrows indicate the direction of transcription.
The region of DNA between the start sites of citI and citM is 188 nt in length and has an unusually high A+T content of 77% (Fig. 3C). Moreover, analysis of this stretch of DNA with the Bend program (Dnastar) predicts that this region has an intrinsic bending (data not shown), suggesting that transcription of these genes could be modulated by alteration of the curvature mediated by a regulatory protein. The overall results indicated that CitI could be a regulator involved in transcriptional induction of the cit operon by citrate. Therefore, to determine the steady-state levels of transcription of the citI gene and the cit operon, and to establish whether synthesis of the transcripts was driven from promoters localized in the intergenic region, we determined the start sites of transcription of citI (Fig. 3A) and citM (Fig. 3B) by primer extension. Taking into account that citP expression as well as citrate fermentation in L. lactis is induced at acidic external pHs (8), we extracted RNA from cultures of strain J1 grown in medium buffered at pH 7.0, 5.0, or 4.5 and containing or lacking citrate (Fig. 3A and B). Both transcripts start with an adenosine residue located 31 and 58 nt upstream of the start codons of citI and citM, respectively (Fig. 3C). In both cases and at all pHs tested, the detected extended products were more abundant in RNA preparations from cultures grown in the presence of citrate (Fig. 3A and B). Quantification of the extended products and of total RNA blotted to specific probes indicated that the levels of citI and of citM mRNAs were about 2.5 ± 0.75- and 30.0 ± 9-fold higher in cultures grown in the presence of citrate (data not shown). These experiments also showed that external pH did not affect expression of the citI gene or the cit operon. Therefore, we conclude that citrate is a transcriptional inductor of both the L. paramesenteroides cit operon and the citI gene and that these genes are not subjected to the pH regulation observed for the citQRP operon from L. lactis (8).
Preceding the start site of citM mRNA, we detected a canonical −10 hexamer TATAAT and a −35 hexamer TTtACA which shares five residues with the consensus sequence of ς70 promoters (Fig. 3C). Preceding the start site of citI mRNA, a −10 hexamer TATgAT, containing five residues identical to the consensus, was observed. However, no obvious −35 sequence was found at the appropriate distance (Fig. 3C). This lack of −35 hexamer is not unusual for promoters requiring an activator for binding of the RNA polymerase.
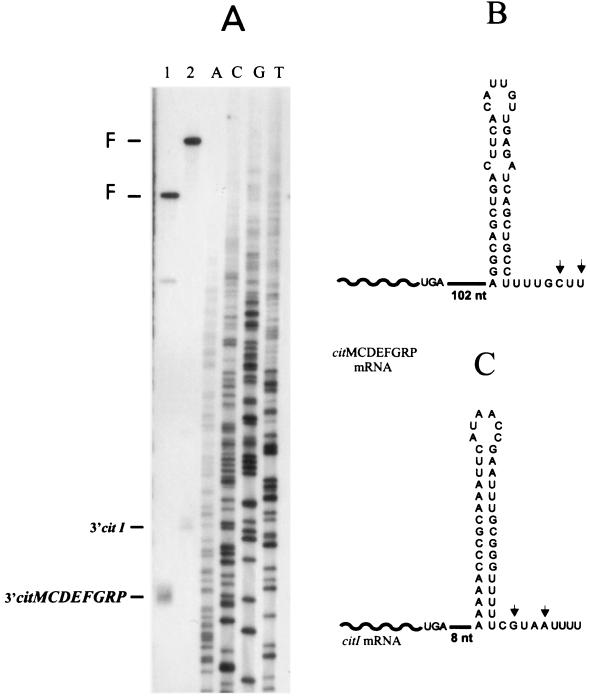
Analysis of the DNA sequence localized downstream of citI and citP showed the presence of putative ρ-independent transcriptional terminators (Fig. 1A). To assess whether these terminators were functional, the 3′ end of the cit transcripts was determined by endonuclease S1 mapping of total RNA extracted from citrate-induced cells (Fig. 4A). Both transcripts ended at two nucleotides (C and U for citMCDEFGRP mRNA or G and A for citI mRNA) located next to the 3′ end of the terminators (Fig. 4B and C). Thus, the location of the 5′ and 3′ ends of the cit transcripts confirmed the nature of the citMC DEFGRP operon and revealed that citI is included in a monocistronic mRNA.
FIG. 4.
Endonuclease S1 analysis of the transcription termination sites of citMCDEFGRP and citI. (A) Autoradiograph showing reactions performed with RNA extracted from strain J1 grown in medium supplemented with citrate. Lane 1, 3′ citI-protected fragment; lane 2, 3′ citMCDEFGRP-protected fragment. F, full-length restriction fragments. Lanes A, C, G, and T show unrelated sequencing reactions. (B and C) Predicted ρ-independent terminators of citMCDEFGRP and citI, respectively. Arrows indicate termination sites of the transcripts.
CitI is a transcriptional activator of the cit operon.
Since no useful techniques are available to analyze regulation of gene expression in Leuconostoc, we attempted to examine the role of the citI gene product in determining citMCDEFGRP promoter activity in an E. coli host. To this end, we constructed plasmids pJMM1 and pJMM12, based on the ColE1 replicon (Fig. 5). Plasmid pJMM1 contains the citI gene under the control of its own promoter and the promoter of the cit operon fused to the E. coli lacZ gene, while plasmid pJMM12 is a pJMM1 derivative in which most of the citI gene has been deleted. These constructs were used to transform E. coli DH5-α, and β-galactosidase activities of the resultant strains were measured. Cells of strain DH5-α harboring pJMM1 showed about 3.5-fold-higher β-galactosidase activity than pJMM12-containing cells (Table 2), suggesting that the presence of the citI gene increases the activity of the cit promoter. To determine whether the enhanced activity of the cit promoter in plasmid pJMM1 is due to cis-acting sequences contained in citI or if the observed effect is caused by the citI gene product, we constructed plasmid pSUI. This plasmid contains the Leuconostoc citI gene under the control of the E. coli lacZ promoter and bears the P15a replicon, which is compatible with ColE1 derivatives (Fig. 5). To test the induction of the cit-lacZ transcriptional fusion upon expression of CitI in trans, we assayed the β-galactosidase activities of strain DH5-α/pJMM12 transformed either with the parental vector pSU39 or with plasmid pSUI (Table 2). The levels of activity in DH5-α carrying plasmids pJMM12 and pSUI were more than threefold higher, upon treatment with IPTG, than those detected in DH5-α harboring plasmids pJMM12 and pSU39 (Table 2). Thus, induction of cit promoter (Pcit)-lacZ expression was increased upon overexpression of citI supplemented in trans. Since E. coli is unable to transport citrate due to lack of a functional transport system, these experiments were performed with cells growing in a medium devoid of citrate. Therefore, our results strongly suggest that CitI functions as a transcriptional activator of the citMCDEFGRP operon in the absence of citrate transport and utilization.
FIG. 5.
Schematic representation of plasmids used to study the role of the citI gene product in expression of the citMCDEFGRP operon. The construction of plasmids pJMM1, pJMM12, and pSU1 is detailed in Materials and Methods. Pcit, promoter of the citQMCDEEFGRP operon, PcitI, promoter of the citI gene; Plac, lacUV5 promoter.
DISCUSSION
The proteins specifically required for the first two steps of citrate fermentation (the citrate transporter and the citrate lyase) and the CitI regulator are encoded by a plasmid-borne 11-kb cluster harboring nine genes which are organized into two divergently transcribed units (Fig. 1A). The physical arrangement of the eight citMCDEFGRP genes suggested that they constitute an operon (15). This assumption was confirmed in this work by detection of the 8.8-kb cit transcript and by determination of its start and termination sites. In addition to the full-length transcript, we detected distinct smaller mRNA species. We demonstrated that they are formed by specific processing at complex structures, which seems to be target for endonucleolytic cleavage (Fig. 2). Northern blot analysis indicated that the cit transcript is predominantly processed at structures I and IV and that the more abundant RNA species are those including either the citMCDEFG or citDEFGRP cluster. These two RNAs should be suitable for synthesis of citrate lyase, and they could support the translation of either the citrate lyase ligase (CitC) or the citrate permease (CitP). Thus, the cell through processing might be able to regulate the synthesis of the different proteins in suitable proportions. The early degradation of the citC mRNA could make sense physiologically. It has been reported that Klebsiella pneumoniae cells require more copies of citrate lyase than the ligase necessary for activation (18). If citC is subjected to rapid degradation, as suggested by the Northern experiment, processing of the primary mRNA transcript would provide an appropriate mechanism to reduce the level of citC. Thus, RNA processing could be a mechanism ensuring that the catabolic enzyme citrate lyase is synthesized in excess to the citrate lyase ligase. Similarly, this mode of posttranscriptional regulation could be necessary to decrease and to uncouple the expression of citP from the citDEFG genes. If this is the case, this fate of citP could be correlated with the lack of linkage, and as a consequence presumably different levels of expression, of citrate permease and citrate lyase genes in L. mesenteroides (2) and L. lactis (12).
How could the transcription of the Leuconostoc citMCDEF GRP be regulated by CitI and by citrate? In this report, we have shown that transcription of the cit operon is induced about 30-fold when Leuconostoc cells are grown in citrate. Moreover, we have provided evidence that expression of cit operon is effected by transcriptional activation mediated by the citI gene product. The involvement of citI in the induction of the citMCDEFGRP promoter is evident from the increase in β-galactosidase activity of E. coli DH5-α expressing a Pcit-lacZ transcriptional fusion in the presence of the citI gene either in cis or in trans. This result indicates that the promoter region of the cit operon is a target for the CitI transcriptional activator.
E. coli is unable to transport citrate under aerobic conditions (20), and we were able to detect CitI-mediated activation of the cit promoter in E. coli cultures grown in medium lacking citrate and in the absence of CitP. Moreover, the induction of transcription of citI from Plac resulted in an enhanced expression of the Pcit-lacZ transcriptional fusion (Table 2). These facts suggested that citrate is not directly required for the activation of Pcit. In Leuconostoc, citI transcription is increased about threefold when cells are grown in a citrate-containing media. Thus, citrate could be necessary to stimulate the transcription of citI by binding either to its gene product, CitI, or to a not yet identified citrate sensor. If this is the case, a small increase in citI transcription could account for the large increase of the citMCDEFGRP mRNA detected in Leuconostoc (Fig. 3A and B). Why is the regulation of the cit operons different in Leuconostoc and Lactococcus? We have determined that transcription of the Leuconostoc cit operon is not increased by growing cells at acidic pHs. These experiments directly demonstrate that in addition to the difference in gene organization of the cit operons in Lactococcus and Leuconostoc, the mechanisms controlling their expression are different. While expression of the Lactococcus citQRP operon is transcriptionally regulated by external pH (8), transcription of the Leuconostoc citMCDEFGRP operon is regulated by citrate. The difference in regulation of expression is likely to reflect different physiological functions of citrate metabolism in the two bacteria. In the heterofermentative bacterium Leuconostoc, citrate degradation is induced by citrate in cultures growing exponentially. This results in a cometabolism of citrate and glucose leading to a growth advantage relative to growth of glucose alone (9, 17). This growth stimulation is attributed to a metabolic shift in the heterofermentative pathway for glucose breakdown yielding additional ATP (9, 17). On the other hand, induction of the citrate metabolic pathway under acidic conditions by the homofermentative bacterium L. lactis is used in the late exponential growth phase for alkalinization of the growth medium (8). In addition, the increased metabolism of citrate seems to make L. lactis cells more resistant to the inhibitory effect of the glucose fermentation product, lactate, that accumulates under these conditions (14).
ACKNOWLEDGMENTS
This work was partially supported by exchange grants of Consejo Superior de Investigaciones Científicas (CSIC) from Spain and Consejo Nacional de Investigaciones Científicas y Técnicas (CONICET) from Argentina. The work at IBR (Argentina) was supported by CONICET, Fundación Antorchas, and Agencia de Promoción Científica y Tecnológica (FONCYT). M. Martin is a fellow from CONICET. C. Magni and D. de Mendoza are Career Investigators of the same institution. The work at the Centro de Investigaciones Científicas (Spain) was under the auspices of CSIC and was supported by Comisión Interministerial de Ciencia y Tecnología (CICYT) grant BIO97-0347 and CICYT-European Union grant 2FD97-1025.
REFERENCES
- 1.Bandell M, Lhotte M E, Marty-Teyssset C, Veyrat A, Prevóst H, Dartois V, Diviès C, Konings W N, Lolkema J S. Mechanism of the citrate transporters in carbohydrate and citrate cometabolism in Lactococcus and Leuconostoc species. Appl Environ Microbiol. 1998;64:1594–1600. doi: 10.1128/aem.64.5.1594-1600.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bekal S, Van Beeumen J, Samyn B, Garmyn D, Heinini S, Diviès C, Prevóst H. Purification of Leuconostoc mesenteroides citrate lyase and cloning and characterization of the citCDEFG gene cluster. J Bacteriol. 1998;180:647–654. doi: 10.1128/jb.180.3.647-654.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bekal-Si Ali S, Diviès C, Prevost H. Genetic organization of the citCDEF locus and identification of mae and clyR genes from Leuconostoc mesenteroides. J Bacteriol. 1999;181:4411–4416. doi: 10.1128/jb.181.14.4411-4416.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Borja B, Jubete Y, Martínez E, de la Cruz F. Construction and properties of a family of pACYC184-derived cloning vectors compatible with pBR322 and its derivatives. Gene. 1991;102:75–78. doi: 10.1016/0378-1119(91)90541-i. [DOI] [PubMed] [Google Scholar]
- 5.Corpet F, Gouzy J, Kahn D. The ProDom database of protein domain families. Nucleic Acids Res. 1998;26:323–326. doi: 10.1093/nar/26.1.323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Dartois V, Djavakhishvili T, Hoch J A. Identification of a membrane protein involved in activation of the KinB pathway to sporulation in Bacillus subtilis. J Bacteriol. 1996;178:1178–1186. doi: 10.1128/jb.178.4.1178-1186.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Devereux J, Haeberli P, Smithies O. A comprehensive set of sequence analysis programs for the VAX. Nucleic Acids Res. 1984;11:387–395. doi: 10.1093/nar/12.1part1.387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.García-Quintáns N, Magni C, de Mendoza D, López P. The citrate transport system of Lactococcus lactis subsp. lactis biovar diacetylactis is induced by acid stress. Appl Environ Microbiol. 1998;64:850–857. doi: 10.1128/aem.64.3.850-857.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hugenholtz J. Citrate metabolism in lactic acid bacteria. FEMS Microbiol Rev. 1993;12:165–178. [Google Scholar]
- 10.Konings W N, Lolkema J S, Poolman B. The generation of metabolic energy by solute transport. Arch Microbiol. 1995;164:235–242. [Google Scholar]
- 11.Lolkema J S, Poolman B, Konings W N. Role of scalar protons in metabolic energy generation in lactic acid bacteria. J Bioenerg Biomembr. 1995;27:467–473. doi: 10.1007/BF02110009. [DOI] [PubMed] [Google Scholar]
- 12.López de Felipe F, Magni C, de Mendoza D, López P. Citrate utilization gene cluster of the Lactococcus lactis biovar. diacetylactis: organization and regulation of expression. Mol Gen Genet. 1995;246:590–599. doi: 10.1007/BF00298965. [DOI] [PubMed] [Google Scholar]
- 13.Magni C, López de Felipe F, Sesma F, López P, de Mendoza D. Citrate transport in Lactococcus lactis subsp. lactis biovar diacetylactis. Expression of the citrate permease P. FEMS Microbiol Lett. 1994;118:75–82. [Google Scholar]
- 14.Magni C, de Mendoza D, Konings W N, Lolkema J S. Mechanism of citrate metabolism in Lactococcus lactis: resistance against lactate toxicity at low pH. J Bacteriol. 1999;181:1451–1457. doi: 10.1128/jb.181.5.1451-1457.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Martin M, Corrales M A, de Mendoza D, López P, Magni C. Cloning and molecular characterization of the citrate utilization citMCDEFGRP cluster of Leuconostoc paramesenteroides. FEMS Microbiol Lett. 1999;174:231–238. doi: 10.1111/j.1574-6968.1999.tb13573.x. [DOI] [PubMed] [Google Scholar]
- 16.Marty-Teysset C, Lolkema J S, Schmitt P, Divies C, Konings W N. Membrane potential-generating transport of citrate and malate catalyzed by CitP of Leuconostoc mesenteroides. J Biol Chem. 1995;270:25370–25376. doi: 10.1074/jbc.270.43.25370. [DOI] [PubMed] [Google Scholar]
- 17.Marty-Teysset C, Posthuma C, Lolkema J S, Schmitt P, Divies C, Konings W N. Proton motive force generation by citrolactic fermentation in Leuconostoc mesenteroides. J Bacteriol. 1996;178:2178–2185. doi: 10.1128/jb.178.8.2178-2185.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Meyer M, Dimroth P, Bott M. In vitro binding of the response regulator CitB and its carboxy-terminal domain to A+T-rich DNA target sequences in the control region of the divergent citC and citS operons of Klebsiella pneumoniae. J Mol Biol. 1997;269:719–731. doi: 10.1006/jmbi.1997.1076. [DOI] [PubMed] [Google Scholar]
- 19.Miller J H. Experiments in molecular genetics. Cold Spring Harbor, N.Y: Cold Spring Harbor Laboratory; 1972. [Google Scholar]
- 20.Pos K M, Dimroth P, Bott M. The Escherichia coli citrate carrier CitT: a member of a novel eubacterial transporter family related to the 2-oxoglutarate/malate translocator from spinach chloroplasts. J Bacteriol. 1998;180:4160–4165. doi: 10.1128/jb.180.16.4160-4165.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sambrook J, Fritsch E F, Maniatis T. Molecular cloning: a laboratory manual. Cold Spring Harbor, N.Y: Cold Spring Harbor Laboratory; 1989. [Google Scholar]
- 22.Vaughan E E, David S, Harrington A, Daly C, Fitzgerald G F, de Vos W M. Characterization of plasmid-encoded citrate permease (citP) genes from Leuconostoc species reveals high sequence conservation with the Lactococcus lactis citP gene. Appl Environ Microbiol. 1995;61:3172–3176. doi: 10.1128/aem.61.8.3172-3176.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zuker M, Stiegler P. Optimal computer folding of large RNA sequences using thermodynamics and auxiliar information. Nucleic Acids Res. 1981;9:133–148. doi: 10.1093/nar/9.1.133. [DOI] [PMC free article] [PubMed] [Google Scholar]