Significance
Calcific aortic valve disease (CAVD) is a common aging-related cardiovascular disease with no pharmacological therapy presently. CAVD pathobiology has chronic inflammation and progressive calcification in aortic valve leaflets. We found that aortic valve interstitial cells (AVICs) from CAVD valves have lower levels of the anti-inflammatory protein IL-38. Recombinant IL-38 reduces spontaneous calcium deposition in AVICs, as well as calcium deposition in AVICs from non-CAVD valves when subjected to pro-inflammatory stimulation. Mechanistically, IL-38 inhibits the NLRP3 inflammasome and suppresses the inflammatory and osteogenic activities in AVICs. Aged IL-38–deficient mice display aortic valve calcification when fed a high-fat diet. Thus, IL-38 suppresses inflammation to alleviate calcification in the aortic valve and may have therapeutic potential for prevention of CAVD progression.
Keywords: IL-38, inflammation, calcification, aortic valve, NLRP3 inflammasome
Abstract
Calcific aortic valve disease (CAVD) is common in people over the age of 65. Progressive valvular calcification is a characteristic of CAVD and due to chronic inflammation in aortic valve interstitial cells (AVICs) resulting in CAVD progression. IL-38 is a naturally occurring anti-inflammatory cytokine; here, we report lower levels of endogenous IL-38 in AVICs isolated from patients’ CAVD valves compared to AVICs from non-CAVD valves. Recombinant IL-38 suppressed spontaneous inflammatory activity and calcium deposition in cultured AVICs. In mice, knockdown of IL-38 enhanced the production of inflammatory mediators in murine AVICs exposed to the proinflammatory stimulant matrilin-2. We also observed that in cultured AVICs matrilin-2 stimulation activated the NOD-, LRR-, and pyrin domain-containing protein 3 (NLRP3) inflammasome with procaspase-1 cleavage into active caspase-1. The addition of IL-38 to matrilin-2–treated AVICs suppressed caspase-1 activation and reduced the expression of intercellular adhesion molecule-1, vascular cell adhesion molecule-1, runt-related transcription factor 2, and alkaline phosphatase. Aged IL-38–deficient mice fed a high-fat diet exhibited aortic valve lesions compared to aged wild-type mice fed the same diet. The interleukin-1 receptor 9 (IL-1R9) is the putative receptor mediating the anti-inflammatory properties of IL-38; we observed that IL-1R9–deficient mice exhibited spontaneous aortic valve thickening and greater calcium deposition in AVICs compared to wild-type mice. These data demonstrate that IL-38 suppresses spontaneous and stimulated osteogenic activity in aortic valve via inhibition of the NLRP3 inflammasome and caspase-1. The findings of this study suggest that IL-38 has therapeutic potential for prevention of CAVD progression.
Calcific aortic valve disease (CAVD) is an aging-related cardiovascular disease and is becoming a significant healthcare issue worldwide, with an annual incidence of 12.6 million cases (1). In the United States alone, CAVD accounts for 15,000 deaths each year (2). Older males are at increased risk for CAVD (3). However, there are currently no pharmacological interventions for CAVD progression, and aortic valve replacement is the only available treatment when CAVD develops to severe aortic stenosis (4). Valve replacement and surgical complications result in significant health care costs (5). Effective pharmacological interventions are desperately needed for prevention of CAVD progression.
Chronic inflammation plays a major role in the development and progression of aortic valve lesions by promoting valvular fibrosis and calcification (6). In this regard, both human and mouse studies support the notion that inflammation leads to collagen and calcium deposition in aortic valves (7, 8). Calcification, however, is the main cause of valvular dysfunction and resultant heart failure (9). It is known that higher levels of calcification in aortic valve indicates worse morbidity and mortality in patients with CAVD. While macrophage and T lymphocyte accumulation have been observed in calcified aortic valves (10, 11), aortic valve interstitial cells (AVICs) are the most prevalent cells in the aortic valve, can become proinflammatory, and play a critical role in chronic valvular inflammation. In CAVD, proinflammatory activation of AVICs contribute to valvular extracellular matrix (ECM) remodeling leads to tissue fibrosis (12, 13) and calcium deposition (14). Understanding of the role of AVICs in the inflammatory mechanism of aortic valve calcification may lead to the development of therapeutic approaches for suppression of CAVD progression to severe aortic stenosis.
Matrilin-2 is one of the ECM proteins (15). It is present in various tissues, including heart, lung, kidney, esophagus, and bone (16). Matrilin-2 forms a filamentous network and serves as an adapter to connect other ECM proteins (16). Over-expression of matrilin-2 has been found in inflammatory conditions, such as neuroinflammation (17). In the aortic valve, matrilin-2 can function as a damage-associated molecular pattern (DAMP) when being in a soluble form due to valvular stress or injury (18). Soluble matrilin-2 has been found to elevate AVIC inflammosteogenic (inflammatory and osteogenic) activities via activation of Toll-like receptor 2 and Toll-like receptor 4 (14, 17, 19). Soluble matrilin-2 can also promote the interaction of monocytes with AVICs, leading to the enhancement of inflammatory activity in AVICs (20). Thus, soluble matrilin-2 plays a mechanistic role in the valvular inflammosteogenic activities that promotes CAVD progression.
IL-38 is a member of the IL-1 family of cytokines (21). It is expressed in many different tissues, including the heart, skin, tonsils, kidney, and brain (22) and has potent anti-inflammatory properties (21). In a study of overweight subjects, reduced plasma levels of IL-38 are associated with metabolic syndrome and increased risk of cardiovascular diseases (23). Low plasma IL-38 levels have also been correlated to chronic inflammatory diseases such as psoriasis (24, 25). In addition, recombinant IL-38 limits skin inflammation and nephritis in a mouse model of systemic lupus erythematosus (26). The anti-inflammatory mechanism of IL-38 involves interleukin-1 receptor 9 (IL-1R9, gene name IL1RAPL1), which is located on the X-chromosome (27). Currently, it is unknown whether IL-38 modulates the inflammatory activity in aortic valve.
In the current study, we tested the hypothesis that IL-38 suppresses AVIC inflammosteogenic activity to alleviate aortic valve calcification and determined first, whether human AVICs express IL-38 and whether the expression is altered in AVICs from aortic valves affected by CAVD; second, whether endogenous IL-38 down-regulates AVIC inflammosteogenic activity; third, whether recombinant IL-38 reverses the proinflammosteogenic phenotype of AVICs from CAVD valves and suppresses the inflammosteogenic activity evoked by a proinflammatory stimulant in AVICs from non-CAVD valves; and fourth, whether IL-38 deficiency exaggerates aortic valve calcification in vivo.
Results
IL-38 Suppresses Spontaneous Inflammosteogenic Activities in AVICs from CAVD Valves.
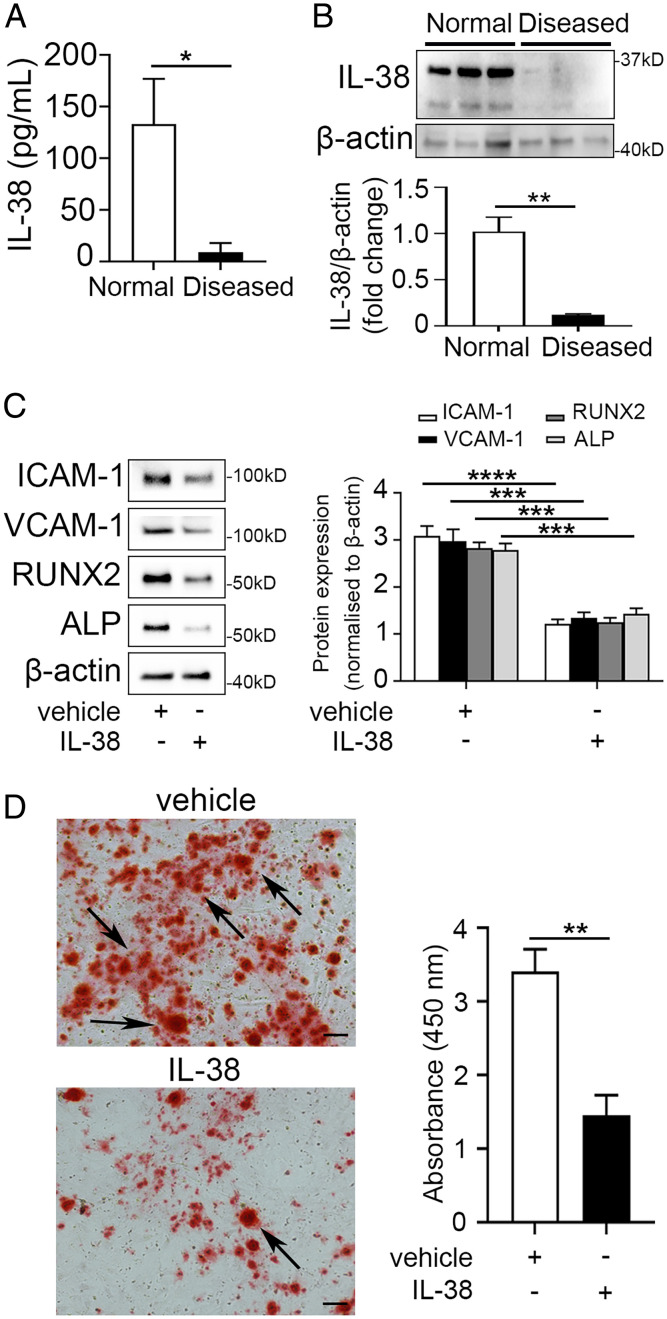
As shown in Fig. 1 A and B, IL-38 proteins were detected in AVICs and significantly lower levels of IL-38 were observed in AVICs isolated from CAVD aortic valve leaflets when compared to AVICs isolated from non-CAVD aortic valve leaflets. As AVICs from CAVD valves have greater spontaneous inflammatory and osteogenic activities (28), we determined the effect of recombinant IL-38 on the inflammosteogenic activity of AVICs from CAVD valves in the absence of any stimulation. The data presented in SI Appendix, Fig. S1 and Fig. 1C show that recombinant IL-38 down-regulated the expression of intercellular adhesion molecule 1 (ICAM-1, 2.5-fold, P < 0.0001), vascular cell adhesion molecule 1 (VCAM-1, 2.2-fold, P < 0.001), runt-related transcription factor 2 (RUNX2, 2.2-fold, P < 0.001), and alkaline phosphatase (ALP, 1.9-fold, P < 0.001). Moreover, recombinant IL-38 reduced spontaneous calcium deposition in AVICs from CAVD valves by 2.3-fold (P < 0.01) (Fig. 1D). Thus, human AVICs express IL-38. The spontaneous inflammosteogenesis in AVICs from CAVD valves is associated with IL-38 insufficiency, and recombinant IL-38 suppresses both inflammatory and osteogenic activities in AVICs from CAVD valves.
Fig. 1.
IL-38 suppresses the spontaneous inflammatory and osteogenic activities in human AVICs from CAVD valves. (A and B) IL-38 protein levels in lysates of human AVICs from CAVD and non-CAVD valves were analyzed by ELISA (A) and immunoblotting (B). Human AVICs from CAVD valves have much lower levels of IL-38. Values are mean ± SEM, n = 3 donors. *P < 0.05 and **P < 0.01 versus human AVICs from non-CAVD valves. (C) Human AVICs from CAVD valves were treated with recombinant IL-38 (10 ng/mL) or vehicle (normal saline) for 72 h. Representative immunoblots (Left) and densitometric data (Right) show that recombinant IL-38 reduced cellular levels of inflammatory (ICAM-1 and VCAM-1) and osteogenic (RUNX2 and ALP) mediators. Values are mean ± SEM, n = 4 donors. ***P < 0.001 and ****P < 0.0001 versus vehicle. (D) Representative images of Alizarin Red S staining (Left) and spectrophotometric data (Right) show that treatment for 14 d with recombinant IL-38 reduced calcium deposition in human AVICs from CAVD valves. Values are mean ± SEM, n = 4 donors. **P < 0.01 versus vehicle. (Scale bar, 100 µm).
IL-38 Reduces the Production of Inflammatory and Osteogenic Mediators in AVICs from Non-CAVD Valves Exposed to Soluble Matrilin-2.
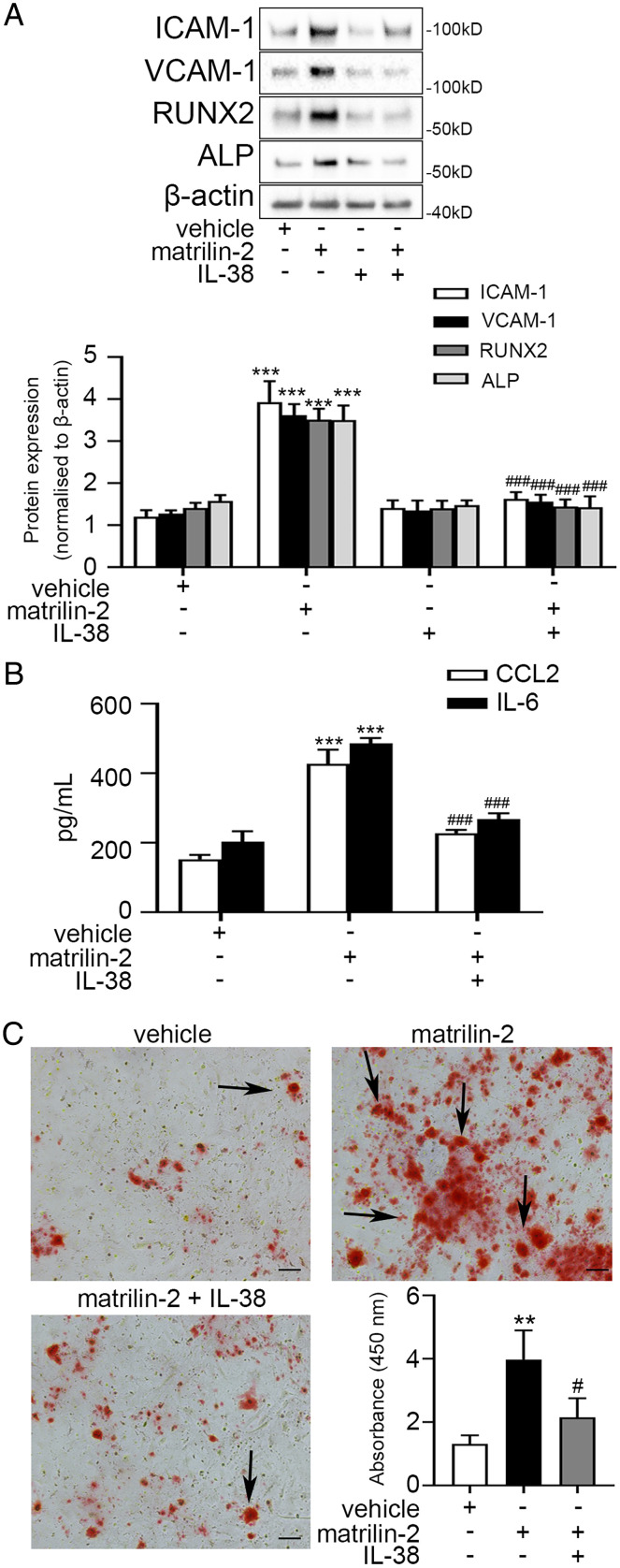
Soluble matrilin-2 is a potent inducer of inflammatory and osteogenic activities in AVICs (14, 19). We tested the hypothesis that recombinant IL-38 is capable of suppressing the inflammatory and osteogenic activities induced by ECM protein in AVICs from non-CAVD valves. We treated AVICs from non-CAVD valves with recombinant IL-38 followed by matrilin-2 stimulation for 24, 48, and 72 h. Recombinant IL-38 markedly reduced the levels of inflammatory (2.8- to 3.3-fold, P < 0.001) and osteogenic (2.1- to 2.5-fold, P < 0.001) mediators in AVICs from non-CAVD valves at these time points of stimulation (Fig. 2 A and B and SI Appendix, Fig. S2). In addition, recombinant IL-38 markedly reduced calcium deposition in AVICs from non-CAVD valves exposed to matrilin-2 by 1.8-fold (P < 0.01) (Fig. 2C).
Fig. 2.
IL-38 suppresses the inflammatory and osteogenic responses to proinflammatory stimulation in human AVICs from non-CAVD valves. (A) Human AVICs from non-CAVD valves were pretreated with recombinant IL-38 (10 ng/mL) for 2 h followed by matrilin-2 (2 µg/mL) treatment for 72 h. Representative immunoblots (Upper) and densitometric data (Lower) show that treatment with recombinant IL-38 reduced the inflammatory and osteogenic responses to matrilin-2. (B) ELISA data show that recombinant IL-38 suppressed the production of IL-6 and CCL2 in human AVICs from non-CAVD valves exposed to matrilin-2. (C) Representative images of Alizarin Red S staining and spectrophotometric data show that treatment for 14 d with recombinant IL-38 reduced calcium deposition in human AVICs from non-CAVD valves exposed matrilin-2. Values are mean ± SEM, n = 4 donors. **P < 0.01 and ***P < 0.001 versus vehicle; #P < 0.05 and ###P < 0.001 versus matrilin-2 alone. (Scale bar, 100 µm).
IL-38 Deficiency Enhances AVIC Inflammosteogenic Activity and Exacerbates Aortic Valve Calcification.
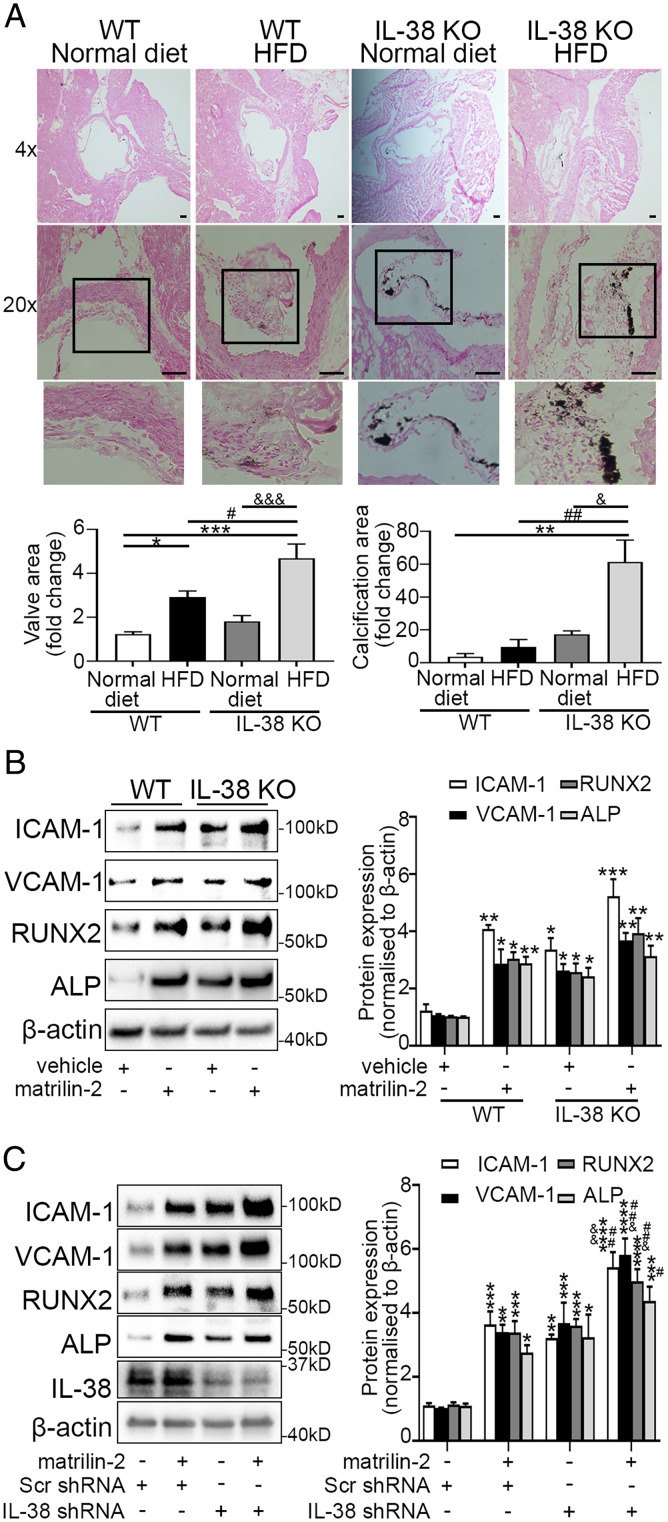
To evaluate the role of IL-38 in modulation of aortic valve pathobiology, we fed a high-fat diet (HFD) to old (16-mo-old) wild-type (WT) and IL-38–deficient mice for 4 mo. Von Kossa staining revealed thicker aortic valve leaflets and more calcification nodules in IL-38–deficient mice compared to WT mice following HFD treatment (Fig. 3A). To determine the impact of IL-38 deficiency on AVIC response to proinflammosteogenic stimulation, we treated AVICs isolated from WT and IL-38–deficient mice with soluble matrilin-2. AVICs from IL-38–deficient mice displayed higher spontaneous inflammosteogenic activities (1.4- to 2-fold, P < 0.05) in comparison to AVICs from WT mice. In addition, AVICs from IL-38–deficient mice had elevated inflammatory and osteogenic responses to matrilin-2 stimulation (Fig. 3B). However, the difference from those in AVICs from WT mice was not significant. In addition, shRNA-mediated knockdown of IL-38 reduced its levels in AVICs from non-CAVD valves (SI Appendix, Fig. S3) and markedly enhanced the production of inflammatory and osteogenic mediators in response to matrilin-2 stimulation (Fig. 3C). Together, these data demonstrate that IL-38 deficiency enhances AVIC response to endogenous proinflammosteogenic stimulant and exacerbates aortic valve osteogenesis in vivo.
Fig. 3.
IL-38 deficiency exacerbates aortic valve lesions in mice. (A) Old mice (16 mo old) were fed a standard diet or HFD for 4 mo. Representative Von Kossa staining images show that IL-38 KO-HFD mice displayed greater aortic valve leaflet thickening and calcification compared to WT-HFD mice. Values are mean ± SEM, n = 3 mice per group. *P < 0.05 and **P < 0.01 versus WT-standard diet; #P < 0.05 and ##P < 0.01 versus WT-HFD; &P < 0.05 and &&&P < 0.001 versus IL-38 KO-standard diet. (Scale bar, 100 µm). (B) Mouse AVICs from WT or IL-38 KO mice were treated with vehicle or matrilin-2 (2 µg/mL) for 72 h. Representative immunoblots (Left) and densitometric data (Right) show that AVICs from IL-38 KO mice exhibited higher spontaneous inflammatory and osteogenic activities in comparison to AVICs from WT mice. In addition, AVICs from IL-38 KO mice had moderately greater responses to matrilin-2 stimulation, but the difference between AVICs from IL-38 KO mice and AVICs from WT mice did not reach statistical significance. Values are mean ± SEM, n = 4 mice per group. *P < 0.05, **P < 0.01 and ***P < 0.001 versus WT vehicle. (C) Human AVICs from non-CAVD valves were pretreated with either scrambled shRNA or IL-38 shRNA prior to matrilin-2 (2 µg/mL) treatment for 72 h. Representative immunoblots (Left) and densitometric data (Right) show that knockdown of IL-38 markedly increased the inflammatory and osteogenic responses to matrilin-2 stimulation. Values are mean ± SEM, n = 4 donors. *P < 0.05, **P < 0.01, ***P < 0.001 and ****P < 0.0001 versus scrambled shRNA alone; #P < 0.05 and ##P < 0.01 versus scrambled shRNA + matrilin-2; &P < 0.05 and &&P < 0.01 versus IL-38 shRNA alone.
IL-1R9 Deficiency Also Exacerbates Aortic Valve Calcification.
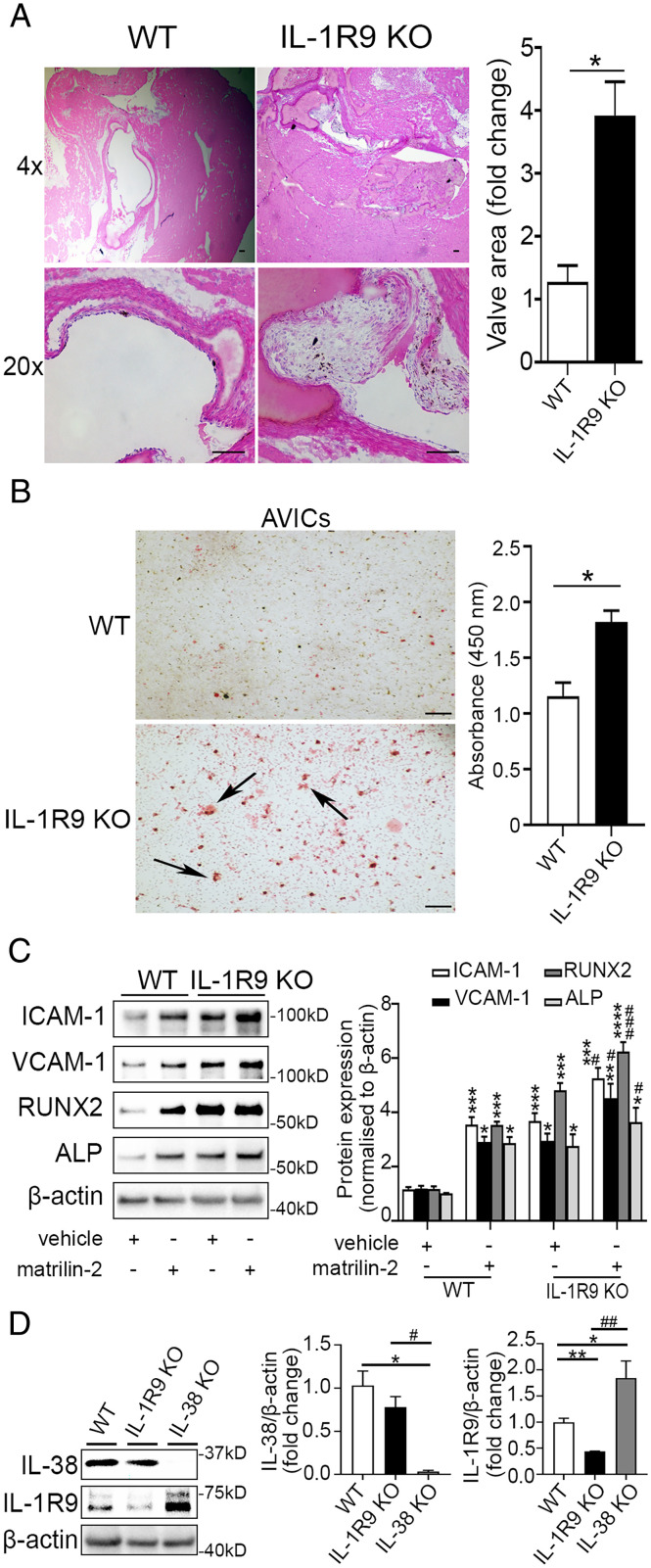
IL-1R9 mediates the anti-inflammatory function of IL-38 (27). We evaluated the effect of IL-1R9 deficiency on aortic valve lesions. Hematoxylin and (H&E) staining revealed that IL-1R9 deficiency resulted in aortic valve thickening in mice fed a normal diet (Fig. 4A). In addition, AVICs from IL-1R9–deficient mice displayed greater calcium deposition compared to AVICs from WT mice (Fig. 4B). In evaluation of the impact of IL-1R9 deficiency on AVIC inflammosteogenic response, we observed markedly higher levels of ICAM-1, VCAM-1, RUNX-2, and ALP in AVICs isolated from mice deficient in IL-1R9 with stimulation (3.6- to 5.3-fold, P < 0.01) or without stimulation (2.7- to 4.1-fold, P < 0.05) (Fig. 4C). IL-38 had a minimal effect on matrilin-2-induced inflammatory and osteogenic responses in AVICs lacking IL-1R9 (SI Appendix, Fig. S4). As shown in Fig. 4D, IL-1R9 protein levels are slightly increased (2.3-fold, P < 0.05) in AVICs from IL-38–deficient mice compared to WT mice, whereas IL-38 levels in AVICs of IL-1R9–deficient mice were comparable to those in AVICs of WT mice. These data support the concept that deficiency of IL-1R9 attenuates the effect of IL-38 on inflammosteogenic activity in aortic valves and AVICs.
Fig. 4.
Effects of IL-1R9 deficiency on aortic valve. (A) Representative images of hematoxylin and eosin staining of aortic valves show that valve leaflet thickening was occurred in IL-1R9 KO mice (12 wk old) in the absence of any treatment. Values are mean ± SEM, n = 3 mice per group. *P < 0.05 versus WT. (Scale bar, 100 µm). (B) Representative images of Alizarin Red S staining (Left) and spectrophotometric data (Right) show that mouse AVICs from IL-1R9 KO mice displayed greater calcium deposition compared to mouse AVICs from WT mice. Values are mean ± SEM, n = 3 mice per group. *P < 0.05 versus WT. Scale bar, 100 µm. (C) Mouse AVICs from WT and IL-1R9 KO mice were treated with vehicle or matrilin-2 (2 µg/mL) for 72 h. Representative immunoblots (Left) and densitometric data (Right) show that mouse AVICs from IL-1R9 KO mice had greater inflammatory and osteogenic responses to matrilin-2 stimulation. Values are mean ± SEM, n = 4 mice per group. *P < 0.05, **P < 0.01, ***P < 0.001 and ****P < 0.0001 versus WT vehicle; #P < 0.05 and ###P < 0.001 versus WT + matrilin-2. (D) Representative immunoblots (Left) and densitometric data (Right) show that IL-1R9 protein levels were slightly increased in mouse AVICs from IL-38 KO mice than those from WT mice, and IL-38 protein levels in mouse AVICs from IL-1R9 KO mice were similar to those from WT mice. Values are mean ± SEM, n = 3 mice per group. *P < 0.05 and **P < 0.01 versus WT; #P < 0.05 and ##P < 0.01 for IL-38 KO versus IL-1R9 KO.
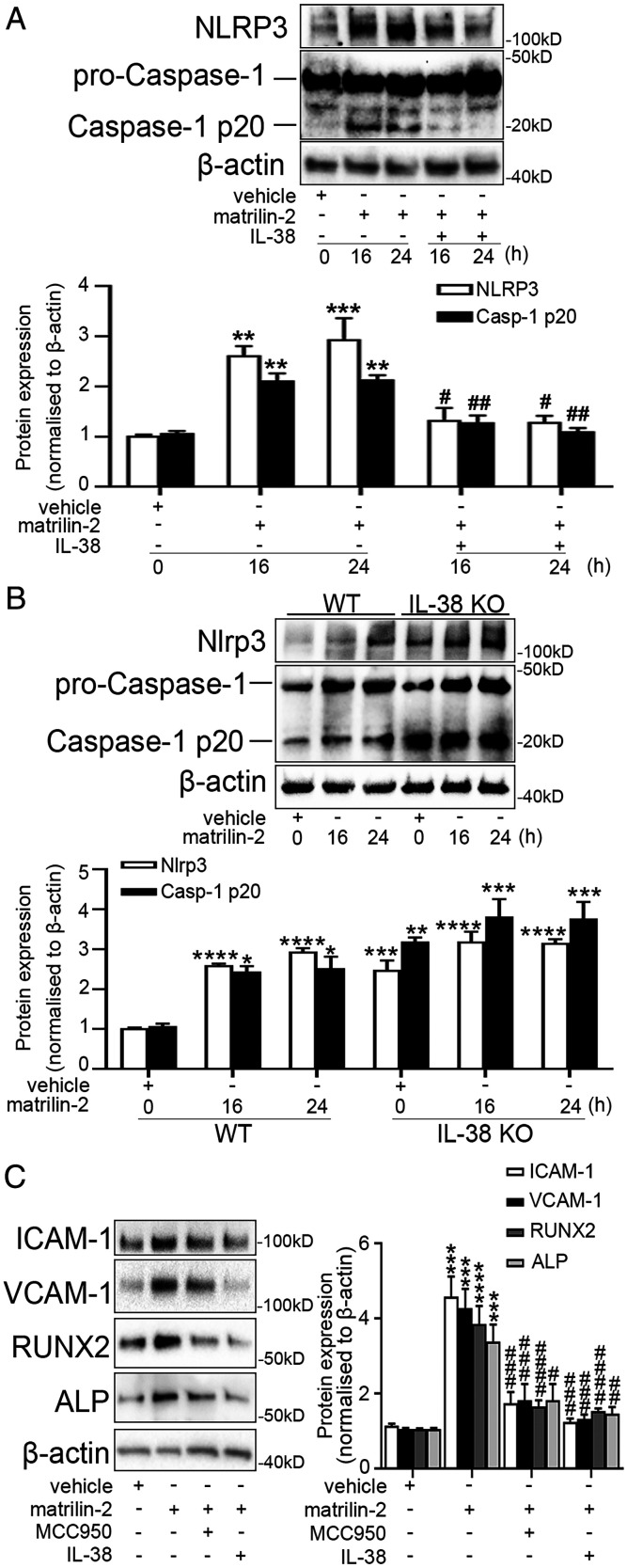
IL-38 Suppresses the Activation of NLRP3 Inflammasome and Caspase-1.
To investigate the mechanism by which IL-38 inhibits inflammation and osteogenesis, we examined the activation of NOD-, LRR-, and pyrin domain-containing protein 3 (NLRP3) inflammasome in AVICs from non-CAVD valves following stimulation with matrilin-2. As shown in SI Appendix, Fig. S5, matrilin-2 induced activation of NLRP3 inflammasome and caspase-1. Treatment with recombinant IL-38 suppressed NLRP3 inflammasome and caspase-1 activation (Fig. 5A) and reduced the secretion of IL-18 (SI Appendix, Fig. S6) induced by matrilin-2 in AVICs from non-CAVD valves. In addition, AVICs from IL-38–deficient mice displayed higher Nlrp3 inflammasome protein levels and enhanced caspase-1 activation in comparison to AVICs from WT mice, and the difference is greater in cells not exposed to matrilin-2 (Fig. 5B). As shown in Fig. 5C, both recombinant IL-38 and MCC950, a specific small molecule inhibitor of NLRP3, markedly suppressed the inflammatory and osteogenic responses to matrilin-2 in AVICs from non-CAVD valves. Therefore, IL-38 inhibits the NLRP3 inflammasome in AVICs to suppress inflammosteogenic activity.
Fig. 5.
IL-38 inhibits NLRP3 activation. (A) Human AVICs from non-CAVD valves were pretreated with recombinant IL-38 (10 ng/mL) for 2 h prior to matrilin-2 (2 µg/mL) treatment for 16 and 24 h. Representative immunoblots (Upper) and densitometric data (Lower) show that recombinant IL-38 suppressed NLRP3 inflammasome and caspase-1 p20 induced by matrilin-2. Values are mean ± SEM, n = 4 donors. **P < 0.01 and ***P < 0.001 versus vehicle; #P < 0.05 and ##P < 0.01 matrilin-2 alone. (B) Mouse AVICs from WT and IL-38 KO mice were treated with vehicle or matrilin-2 (2 µg/mL) for 16 and 24 h. Representative immunoblots (Upper) and densitometric data (Lower) show higher levels of Nlrp3 inflammasome and caspase-1 p-20 activation in mouse AVICs from IL-38 KO mice compared to mouse AVICs from WT mice, especially in the absence of matrilin-2 stimulation. Values are mean ± SEM, n = 3 per group. *P < 0.05, **P < 0.01, ***P < 0.001 and ****P < 0.0001 versus WT vehicle. (C) Human AVICs from non-CAVD valves were pretreated with recombinant IL-38 (10 ng/mL) or MCC950 (1 µM) for 2 h and then exposed to matrilin-2 (2 µg/mL) for 72 h. Representative immunoblots (Left) and densitometric data (Right) show that both NLRP3 specific inhibitor and IL-38 suppressed matrilin-2-induced inflammatory and osteogenic responses to matrilin-2. Values are mean ± SEM, n = 4 donors. ***P < 0.001 and ****P < 0.0001 versus vehicle; #P < 0.05, ##P < 0.01, ###P < 0.001 and ####P < 0.0001 versus matrilin-2 alone.
Discussion
Given the high incidence of CAVD and unavailability of pharmacological intervention for prevention of CAVD progression, we aimed to explore potential of IL-38 as a therapeutic agent. This study demonstrates that AVICs from CAVD valves have lower IL-38 levels in comparison to AVICs from non-CAVD valves. Treatment of AVICs from CAVD valves with recombinant IL-38 reduces their spontaneous osteogenic activity. Furthermore, both endogenous IL-38 and recombinant IL-38 are capable of suppressing the inflammosteogenic activation induced by soluble matrilin-2 in AVICs from non-CAVD valves. The anti-inflammosteogenic effects of IL-38 in AVICs are lost in the absence of IL-1R9, and AVICs from mice deficient in IL-1R9 exhibit greater spontaneous inflammosteogenic activity. Mechanistically, IL-38 inhibits the NLRP3 inflammasome and caspase-1 activation to suppress the inflammosteogenic activity in AVICs. More importantly, mice deficient in IL-38 develop aortic valve calcification after being on an HFD for a prolonged period. Overall, these novel findings suggest that IL-38 suppresses AVIC inflammatory and osteogenic activities, and that recombinant IL-38 has therapeutic potential for prevention of CAVD progression.
Chronic, low-grade inflammation plays an important role in the development and progression of several diseases, including CAVD (29). Studies have reported that soluble ECM proteins, including biglycan and matrilin-2, are capable of elevating the osteogenic activity in human AVICs (14, 30). Our recent studies found that soluble matrilin-2 enhances the interaction of monocytes with AVICs (20) and that activated monocytes secrete tumor necrosis factor (TNF)-α and Regulated upon activation, normal T cell expressed and secreted (RANTES) to up-regulate the osteogenic activity in human AVICs (31). IL-38 is a newly discovered anti-inflammatory cytokine (24). It acts similarly to IL-37, another inhibitory IL-1 family member (32) that is capable of preventing aortic valve lesions in laboratory animals (28). Serum levels of IL-38 are negatively correlated with C-reaction protein, and recombinant IL-38 inhibits IL-1β and IL-6 production by peripheral blood mononuclear cells of hyperlipidemia patients (23, 33). Our data demonstrate relative deficiency of IL-38 in AVICs from CAVD valves. This finding is in line with the observation that blood IL-38 levels are lower in overweight subjects at greater risk for cardiovascular diseases (23). ICAM-1, VCAM-1, CCL2 (C-C motif chemokine ligand 2), and IL-6 are inflammatory mediators essential for leukocyte infiltration (20, 34). ALP is a biomarker of early osteoblastic transition in AVICs and RUNX2 is an osteoblast biomarker (35). Both ALP and RUNX2 are over-expressed in human aortic valves affected by CAVD (36, 37). We found that recombinant IL-38 suppresses the spontaneous production of these inflammatory and osteogenic biomarkers in AVICs from CAVD valves. Further, we found that recombinant IL-38 also suppresses the inflammatory and osteogenic responses to soluble matrilin-2 in AVICs from non-CAVD valves. Since soluble matrilin-2 appears to contribute to the mechanisms of CAVD pathobiology by elevating AVIC inflammatory and osteogenic activities (14, 19), this observation suggests that recombinant IL-38 also suppresses AVIC inflammosteogenic activation evoked by an endogenous stimulant. Together, the results show that IL-38 insufficiency contributes to the elevated spontaneous inflammosteogenic activity in AVICs in CAVD valves, and recombinant IL-38 is capable of suppressing the spontaneous activity in AVICs of CAVD valves and stimulated activity in AVICs of non-CAVD valves.
Western diet is a risk factor for the development of CAVD (38). In this study, we used HFD to mimic the Western diet. We observed that IL-38–deficient mice fed an HFD have thicker aortic valve leaflets and more calcification nodules in comparison to WT mice fed the HFD diet. It is plausible that IL-38 deficiency elevates AVIC inflammosteogenic activity to promote valvular calcification. In this regard, lower levels of IL-38 in AVICs would reduce their anti-inflammatory capacity and thereby exacerbate inflammation-induced valvular calcification. Indeed, AVICs from IL-38–deficient mice exhibit greater spontaneous inflammosteogenic activity, a similar phenotypic change to that observed in human AVICs from CAVD valves. Moreover, knockdown of IL-38 in AVICs from non-CAVD valves markedly enhances cellular inflammatory and osteogenic responses to soluble matrilin-2. Together, these findings demonstrate that IL-38 deficiency elevates the pathobiological activity of AVICs and contributes to the promotion of aortic valve thickening and calcification. It is noteworthy that, unlike human AVICs with IL-38 knockdown, AVICs from IL-38–deficient mice only have moderately greater inflammatory and osteogenic responses to matrilin-2 stimulation in comparison to matrilin-2–stimulated AVICs from WT mice. It is possible that mouse AVICs and human AVICs respond to matrilin-2 somewhat differently in the condition of IL-38 deficiency. Alternatively, matrilin-2 in the dose used in this study induced responses close to maximal in mouse AVICs, and the exacerbating effect of IL-38 deficiency could not be revealed. Future studies will address this issue.
The NLRP3 inflammasome is required for caspase-1 activation that results in the processing of the inactive IL-1β and IL-18 precursors into active cytokines (39). Studies have reported that DAMPs can activate the NLRP3 inflammasome and thereby contributes to the mechanism of inflammatory diseases such as atherosclerosis (40–43). Recent studies have reported that IL-38 inhibits NLRP3 inflammasome activation induced by lipopolysaccharide (LPS) in macrophages and IL-1β production in chondrocytes (44, 45). In addition, IL-38– deficient mice subjected to dextran sulfate sodium colitis exhibit increased colonic Nlrp3 mRNA and protein expression, as well as caspase-1 processing in comparison to WT mice (46). In this study, we observed that matrilin-2 up-regulates NLRP3 inflammasome levels and caspase-1 cleavage in AVICs. Recombinant IL-38 attenuates the activation of the NLRP3 inflammasome and caspase-1, resulting in reduced inflammosteogenic activity in AVIC, whereas a deficiency of IL-38 has an opposite effect. The inhibitory effects of IL-38 on NLRP3 inflammasome activation and caspase-1 are comparable to the inhibitory effects of recombinant human IL-37 on NLRP3 inflammasome and caspase-1 (47). Taken together, the overlapping anti-inflammatory properties of recombinant IL-38 and recombinant IL-37 support the view that in the IL-1 family, there is functional duplication. Inhibition of the NLRP3 inflammasome with MCC950 also suppresses the expression of inflammatory and osteogenic responses to soluble matrilin-2 in AVICs from non-CAVD valves. Thus, the NLRP3 inflammasome plays a crucial role in AVIC pathobiology, and IL-38 inhibits this proinflammatory signaling mechanism to suppress AVIC inflammosteogenic activity associated with CAVD progression. The recent suspension of MCC950 in a phase II clinical trial due to hepatoxicity (48) further underscores IL-38 as a new potential therapeutic agent for the prevention of CAVD progression. Interestingly, we observed that recombinant IL-38 has a greater effect in suppressing AVIC inflammatory and osteogenic responses compared to NLRP3 inflammasome inhibitor. This observation indicates that IL-38 not only inhibits the NLRP3 inflammasome, but also modulates other signaling pathways, such as the Protein kinase R (PKR)/Nuclear factor kappa B (NF-ĸB) signaling pathway, utilized by soluble matrilin-2 in the induction of the inflammatory response in human AVICs (19).
IL-1R9 is a putative receptor of IL-38 and was identified initially in the brain (49). Mutations of IL-1R9 in men can cause X-linked mental retardation (50). Thus far, no relationship between IL-1R9 and aortic valve has been identified. Unexpectedly, we observed spontaneous aortic valve leaflet thickening in young IL-1R9–deficient mice fed a normal diet. IL-1R9 deficiency has a similar effect as IL-38 deficiency on the spontaneous inflammosteogenic activity in AVICs. However, IL-38 levels were comparable in AVICs from IL-1R9–deficient and WT mice. Interestingly, both unstimulated and stimulated AVICs from IL-1R9–deficient mice have markedly increased inflammosteogenic activity. It appears that the inability of IL-38 to suppress inflammatory activity due to IL-1R9 deficiency exaggerates proinflammatory up-regulation of AVIC inflammosteogenic activities. Together, these indicate that IL-38 requires IL-1R9 to exert its anti-inflammatory action in AVICs. Conversely, the levels of IL-1R9 are increased in AVICs from IL-38–deficient mice. This is expected when there is no ligand (IL-38). Overexpression of IL-1R9 in mouse neurons and HEK293 cells selectively activates the c-Jun N-terminal kinase (JNK) signaling (51, 52), a pathway that plays a role in AVIC pathobiology associated with CAVD progression (53, 54). It is likely that elevated expression of IL-1R9 promotes JNK activation to contribute to enhanced inflammosteogenesis in AVICs. Further investigation is required to test this hypothesis. Together, our data indicate that the IL-38 interacts with IL-1R9 to modulate aortic valve inflammosteogenic activity. As the IL-1 family often copies itself, the relationship between IL-38 and IL-1R9 may be similar to IL-37 as an anti-inflammatory ligand and IL-1R8, where IL-1R8 acts as the suppressive coreceptor for IL-37 (55). In further analogy, IL-1R8–deficient mice developed exacerbated autoimmune lung disease and lupus nephritis when compared to WT mice, which is similar with our findings on spontaneous aortic valve lesions in young IL-1R9–deficient mice (56).
One of the limitations of this study is the small sample size and associated variability. While specimens were collected from six donors, we did some experiments with cells from some donors as the study evolved over time since many assays need a large quantity of cells. Nevertheless, we repeated each experiment using samples from four distinct donors to make sure that the data are reproducible.
Our findings highlight the role of IL-38 in suppressing the inflammosteogenic activity in AVICs. The anti-inflammosteogenic properties of IL-38 depend on the inhibition of the NLRP3 inflammasome. Together, our data suggest that IL-38 is a potential therapeutic agent for the prevention of CAVD progression.
Materials and Methods
Chemicals and Reagents.
Recombinant human matrilin-2 (endotoxin-free, 3044-MN-050) and recombinant human IL-38 (amino acid 3-152) were obtained from R&D Systems. Tween-20 (P7949) and collagenase (C5138) were purchased from Sigma-Aldrich. Medium 199 (11150067) was purchased from Thermo Fisher Scientific. Antibodies against ICAM-1 (SC8439) and caspase-1 (SC56036) were obtained from Santa Cruz Biotechnology. Antibodies against VCAM-1 (EIE8X) and RUNX2 (DIH7) were obtained from Cell Signaling Technology. Antibodies against ALP (AB108337) and IL-38 (AB180898) were purchased from Abcam. NLRP3 antibody was purchased from Novus Biologicals (NBP2-12446).
Animals and Treatment.
All experimental protocols were approved by the Institutional Animal Care and Use Committee of the University of Colorado Denver. This study complied with the guidelines for the Care and Use of Laboratory Animals (National Research Council 1996).
Il38-deficient mice (GenBank accession number: NM_153077.2; Ensembl: ENSMUSG00000046845) were generated using CRISPR/Cas9 technology and Il1rapl1 deficient (IL-1R9-/y) mice (GenBank accession number: NM_001160403.1; Ensembl: ENSMUSG00000052372) were generated by deleting Exon 3 using CRISPR/Cas9 technology (Cyagen Biosciences). SI Appendix, Table S1 and S2 contains gRNA sequences and nucleotides to generate and confirm the IL-38 and IL-1R9 deficiency.
Cas9 mRNA and gRNA were generated by in vitro transcription and injected into fertilized C57BL/6 eggs. Founders were genotyped by PCR using TaKaRa TaqTM Hot Start Version (Takara) and the PCR product was purified using the MiniBEST Universal Genomic DNA Extraction Kit Ver.5.0, 9765 (Takara). The IL-38–deficient mice and IL-1R9–deficient mice were confirmed by DNA sequencing analysis. Following transport to our animal facility and further breeding, the presence of homozygote IL-38–deficient mice or WT alleles was confirmed by 3 d of age via use of Transnetyx testing of ear clippings.
IL-38–deficient male mice (16 to 18 mo) and C57BL/6 male mice (nonlittermate WT, 16 to 18 mo) were given a regular diet or an HFD (diet containing: 24% fat, 41% carbohydrate, and 24% protein; 45% of kilocalories from fat) and euthanized after being on the diet for 4 mo. The aortic valve morphology and calcification were examined by microscopy and evaluated in a blinded fashion.
Isolation and Culture of Human and Mouse AVICs.
Non-CAVD valves were collected from normal morphology of valves from explanted hearts of heart-transplant recipients with cardiomyopathy and CAVD valves were collected from aortic stenosis patients who underwent aortic valve replacement at the University of Colorado Hospital (SI Appendix, Table S3). This study was approved by the Institutional Review Board of University of Colorado and conducted in accordance with the Declaration of Helsinki 1964 and revision of 2013. Written informed consent was obtained from all donors.
Human aortic valve leaflets were washed in phosphate-buffered saline (PBS, 10-010-072, Thermo Fisher Scientific), cut into 1- to 2-mm pieces and treated with collagenase type I solution (1.0 mg/mL, Sigma-Aldrich) for 30 min at 37 °C to eliminate endothelial cells. Tissue digestion was performed with a fresh collagenase solution at 37 °C for 4 to 6 h. Cells suspensions were then centrifuged for 10 min at 1,000 rpm, and cell pellets were resuspended and cultured in medium 199, containing 10% fetal bovine serum (FBS) and 1% penicillin-streptomycin, in a 37 °C humidified incubator with 5% CO2.
The culture media were replaced every 3 d throughout the growth and experimental periods. AVICs of passage 3 to 6 at 80 to 90% confluence were used for the experiments. All experiments in this study were repeated using AVICs isolated from valves from 10 different donors.
AVICs of aortic valves from WT, IL-38–deficient and IL-1R9–deficient mice were isolated and cultured using the previously described method (28). Briefly, aortic valve tissues were washed in 1× PBS and treated with collagenase type I solution (1.0 mg/mL) to eliminate endothelial cells. Small pieces of aortic valve tissues were plated onto 1% gelatin-coated dishes and cultured with medium 199 containing 10% FBS and 1% penicillin-streptomycin for AVIC outgrowth.
Cells were treated with vehicle (saline), recombinant human IL-38 (10 ng/mL) or MCC950 (1 μM, 5479, Tocris Bioscience) (57, 58) prior to an exposure to rh-matrilin-2 (2.0 μg/mL) for 72 h (19). Cell lysates were used for the assessment of ICAM-1, VCAM-1, RUNX2, and ALP proteins. To evaluate the roles of NLRP3 and caspase-1 in mediating AVIC inflammosteogenic activity and determine the effect of IL-38 on NLRP3 and caspase-1, AVICs were pretreated with IL-38 (10 ng/mL) or MCC950 (1 μM) and then subjected to matrilin-2 stimulation for 16 and 24 h. Cell lysates were used for assessment of NLRP3 and caspase-1 activation.
shRNA IL-38.
AVICs of 80% confluence were transduced with lentiviral vectors carrying IL-38 shRNA, and cells were selected with 1 μg/mL puromycin (A1113803, Thermo Fisher Scientific). The lentiviral shRNA targeting human IL-38 (TRCN0000058441) and the scramble shRNA (pLKO.1-puro) were from the Functional Genomics Core Facility at the University of Colorado.
Immunoblotting.
The expression of ICAM-1, VCAM-1, RUNX2, ALP, NLRP3, and caspase-1 was detected by immunoblotting. AVICs were lysed in a 2× Laemmli sample buffer (161-0737, Biorad). Samples were electrophoresed on 4 to 20% sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) gels and transferred to nitrocellulose membranes. The membranes were incubated with 5% skim milk at room temperature for 1 h, followed by incubation with primary antibodies (1:200 to 1:500 volume dilution) at 4 °C overnight. Horseradish peroxidase-conjugated secondary antibodies (1:10,000 dilution) were applied, and enhanced chemiluminescence reagents were used for band visualization. β-actin was used as a protein loading control. ImageLab software (Bio-Rad) was used to quantify band.
Enzyme-Linked Immunosorbent Assay.
The levels of IL-38 (DY9110), CCL2 (DY279) and IL-6 (DY206) were measured by enzyme-linked immunodsorbent assay (ELISA, R & D Systems) following the manufacturer's instructions. An automatic microplate reader (Biotek) was used to measure samples and standards in triplicate at a wavelength of 450 nm.
Aortic Valve Histology.
Aortic valve tissues were embedded and frozen in optimal cutting temperature compound. Then, 5-μm thick cryosections were prepared and fixed in 4% paraformaldehyde for 15 min. H&E staining was performed to examine the morphology of aortic valve leaflets. To analyze calcium deposits in aortic valve tissues, Von Kossa staining was performed by the Histology Core of University of Colorado Denver.
Alizarin Red S Staining.
Alizarin Red S staining (A5533, Sigma-Aldrich) was used to detect calcium deposition in AVIC culture. Cells were incubated with procalcification media (medium 199 supplemented with 10 nmol/L of dexamethasone 10 mmol/L of β-glycerophosphate, 8 mmol/L of CaCl2 and 4.0 μg/mL of cholecalciferol) for 14 d. Cells were washed with PBS, fixed with 4% paraformaldehyde, and incubated with 0.2% Alizarin Red solution (pH 4.0 to 4.2) for 30 min. Excessive dye was removed by washing with distilled water. For quantitation, the stain was washed off with 10% acetic acid at 75 °C and measured using a spectrophotometer at 450 nm wavelength.
Statistical Analysis.
Significance of difference was evaluated with an unpaired, two-tailed Student t test or one-way ANOVA with Tukey multiple comparisons test by the GraphPad Prism 9 (GraphPad Software). Nonparametric Mann-Whitney U test was performed to confirm the difference of two group comparison. For multiple group comparisons, Tukey test was performed to confirm the differences. Statistical significance was defined as P ≤ 0.05. Data are presented as mean ± SEM.
Supplementary Material
Acknowledgments
This study was supported in part by National Institutes of Heart, Lung and Blood Grants HL106582 and HL121776. The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH. D.M.d.G. is supported by the Interleukin Foundation Fellowship. C.A.D. is supported by National Institute of Allergy and Infectious Disease Grant AI-15614. The authors thank Vassili Kalabokis, PhD, and Biotechne for providing recombinant human IL-38.
Footnotes
Reviewers: F.B., INSERM Delegation Regionale Grand Ouest; G.F., University of Cincinnati; C.L., East Tennessee State University; and M.N., Monash University, Melbourne, Australia.
The authors declare no competing interest.
This article contains supporting information online at https://www.pnas.org/lookup/suppl/doi:10.1073/pnas.2202577119/-/DCSupplemental.
Data, Materials, and Software Availability
All study data are included in the article and/or supporting information.
References
- 1.Yadgir S., et al. ; Global Burden of Disease Study 2017 Nonrheumatic Valve Disease Collaborators, Global, regional, and national burden of calcific aortic valve and degenerative mitral valve diseases, 1990–2017. Circulation 141, 1670–1680 (2020). [DOI] [PubMed] [Google Scholar]
- 2.Mozaffarian D., et al. , Executive summary: Heart disease and stroke statistics—2015 update: A report from the American Heart Association. Circulation 131, 434–441 (2015). [DOI] [PubMed] [Google Scholar]
- 3.Summerhill V. I., Moschetta D., Orekhov A. N., Poggio P., Myasoedova V. A., Sex-specific features of calcific aortic valve disease. Int. J. Mol. Sci. 21, 5620 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Head S. J., Çelik M., Kappetein A. P., Mechanical versus bioprosthetic aortic valve replacement. Eur. Heart J. 38, 2183–2191 (2017). [DOI] [PubMed] [Google Scholar]
- 5.Alsara O., Alsarah A., Laird-Fick H., Advanced age and the clinical outcomes of transcatheter aortic valve implantation. J. Geriatr. Cardiol. 11, 163–170 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lerman D. A., Prasad S., Alotti N., Calcific aortic valve disease: Molecular mechanisms and therapeutic approaches. Eur. Cardiol. 10, 108–112 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Coté N., et al. , Inflammation is associated with the remodeling of calcific aortic valve disease. Inflammation 36, 573–581 (2013). [DOI] [PubMed] [Google Scholar]
- 8.Sider K. L., Blaser M. C., Simmons C. A., Animal models of calcific aortic valve disease. Int. J. Inflamm. 2011, 364310 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Minners J., et al. , Sudden cardiac death in asymptomatic patients with aortic stenosis. Heart 106, 1646–1650 (2020). [DOI] [PubMed] [Google Scholar]
- 10.Kaden J. J., et al. , Inflammatory regulation of extracellular matrix remodeling in calcific aortic valve stenosis. Cardiovasc. Pathol. 14, 80–87 (2005). [DOI] [PubMed] [Google Scholar]
- 11.Hewing B., et al. , Severe aortic valve stenosis in adults is associated with increased levels of circulating intermediate monocytes. J. Cardiovasc. Transl. Res. 10, 27–34 (2017). [DOI] [PubMed] [Google Scholar]
- 12.Leopold J. A., Cellular mechanisms of aortic valve calcification. Circ. Cardiovasc. Interv. 5, 605–614 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Liu A. C., Joag V. R., Gotlieb A. I., The emerging role of valve interstitial cell phenotypes in regulating heart valve pathobiology. Am. J. Pathol. 171, 1407–1418 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Li F., et al. , ADAMTS5 deficiency in calcified aortic valves is associated with elevated pro-osteogenic activity in valvular interstitial cells. Arterioscler. Thromb. Vasc. Biol. 37, 1339–1351 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Deák F., Piecha D., Bachrati C., Paulsson M., Kiss I., Primary structure and expression of matrilin-2, the closest relative of cartilage matrix protein within the von Willebrand factor type A-like module superfamily. J. Biol. Chem. 272, 9268–9274 (1997). [DOI] [PubMed] [Google Scholar]
- 16.Zhang S., et al. , Matrilin-2 is a widely distributed extracellular matrix protein and a potential biomarker in the early stage of osteoarthritis in articular cartilage. BioMed Res. Int. 2014, 986127 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Jonas A., et al. , Axonally derived matrilin-2 induces proinflammatory responses that exacerbate autoimmune neuroinflammation. J. Clin. Invest. 124, 5042–5056 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Frevert C. W., Felgenhauer J., Wygrecka M., Nastase M. V., Schaefer L., Danger-associated molecular patterns derived from the extracellular matrix provide temporal control of innate immunity. J. Histochem. Cytochem. 66, 213–227 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.The E., et al. , Mechanistic Roles of matrilin-2 and Klotho in modulating the inflammatory activity of human aortic valve cells. Cells 9, 385 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Luo Z., et al. , Monocytes augment inflammatory responses in human aortic valve interstitial cells via β2-integrin/ICAM-1-mediated signaling. Inflamm. Res. 71, 681–694 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Xu W. D., Huang A. F., Role of interleukin-38 in chronic inflammatory diseases: A comprehensive review. Front. Immunol. 9, 1462 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lin H., et al. , Cloning and characterization of IL-1HY2, a novel interleukin-1 family member. J. Biol. Chem. 276, 20597–20602 (2001). [DOI] [PubMed] [Google Scholar]
- 23.de Graaf D. M., et al. , Reduced concentrations of the B cell cytokine interleukin 38 are associated with cardiovascular disease risk in overweight subjects. Eur. J. Immunol. 51, 662–671 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Garraud T., Harel M., Boutet M.-A., Le Goff B., Blanchard F., The enigmatic role of IL-38 in inflammatory diseases. Cytokine Growth Factor Rev. 39, 26–35 (2018). [DOI] [PubMed] [Google Scholar]
- 25.Mercurio L., et al. , IL-38 has an anti-inflammatory action in psoriasis and its expression correlates with disease severity and therapeutic response to anti-IL-17A treatment. Cell Death Dis. 9, 1104 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Xu W. D., et al. , IL-38: A novel cytokine in systemic lupus erythematosus pathogenesis. J. Cell. Mol. Med. 24, 12379–12389 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mora J., et al. , Interleukin-38 is released from apoptotic cells to limit inflammatory macrophage responses. J. Mol. Cell Biol. 8, 426–438 (2016). [DOI] [PubMed] [Google Scholar]
- 28.Zeng Q., et al. , Interleukin-37 suppresses the osteogenic responses of human aortic valve interstitial cells in vitro and alleviates valve lesions in mice. Proc. Natl. Acad. Sci. U.S.A. 114, 1631–1636 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.García-Rodríguez C., et al. , Toll-like receptors, inflammation, and calcific aortic valve disease. Front. Physiol. 9, 201 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Song R., et al. , Biglycan induces the expression of osteogenic factors in human aortic valve interstitial cells via Toll-like receptor-2. Arterioscler. Thromb. Vasc. Biol. 32, 2711–2720 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Zhang P., et al. , Pro-inflammatory mediators released by activated monocytes promote aortic valve fibrocalcific activity. Mol. Med. 28, 5 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Nold M. F., et al. , IL-37 is a fundamental inhibitor of innate immunity. Nat. Immunol. 11, 1014–1022 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Yang N., et al. , Elevated interleukin-38 level associates with clinical response to atorvastatin in patients with hyperlipidemia. Cell. Physiol. Biochem. 49, 653–661 (2018). [DOI] [PubMed] [Google Scholar]
- 34.Schnoor M., Alcaide P., Voisin M.-B., van Buul J. D., Crossing the vascular wall: Common and unique mechanisms exploited by different leukocyte subsets during extravasation. Mediators Inflamm. 2015, 946509 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Song R., Zhai Y., Ao L., Fullerton D. A., Meng X., MicroRNA-204 deficiency in human aortic valves elevates valvular osteogenic activity. Int. J. Mol. Sci. 21, 76 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Song R., et al. , BMP-2 and TGF-β1 mediate biglycan-induced pro-osteogenic reprogramming in aortic valve interstitial cells. J. Mol. Med. (Berl.) 93, 403–412 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Yang X., et al. , Bone morphogenic protein 2 induces Runx2 and osteopontin expression in human aortic valve interstitial cells: Role of Smad1 and extracellular signal-regulated kinase 1/2. J. Thorac. Cardiovasc. Surg. 138, 1008–1015 (2009). [DOI] [PubMed] [Google Scholar]
- 38.Shen M., Tastet L., Bergler-Klein J., Pibarot P., Clavel M.-A., Blood, tissue and imaging biomarkers in calcific aortic valve stenosis: Past, present and future. Curr. Opin. Cardiol. 33, 125–133 (2018). [DOI] [PubMed] [Google Scholar]
- 39.Kelley N., Jeltema D., Duan Y., He Y., The NLRP3 inflammasome: An overview of mechanisms of activation and regulation. Int. J. Mol. Sci. 20, 3328 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Wang Z., et al. , NLRP3 inflammasome and inflammatory diseases. Oxid. Med. Cell. Longev. 2020, 4063562 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Swanson K. V., Deng M., Ting J. P., The NLRP3 inflammasome: Molecular activation and regulation to therapeutics. Nat. Rev. Immunol. 19, 477–489 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Chen S., et al. , Sex-specific effects of the Nlrp3 inflammasome on atherogenesis in LDL receptor-deficient mice. JACC Basic Transl. Sci. 5, 582–598 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Lasithiotaki I., et al. , NLRP3 inflammasome expression in idiopathic pulmonary fibrosis and rheumatoid lung. Eur. Respir. J. 47, 910–918 (2016). [DOI] [PubMed] [Google Scholar]
- 44.Luo P., Zhao T., He H., IL-38-mediated NLRP3/caspase-1 inhibition is a disease-modifying treatment for TMJ inflammation. Ann. N. Y. Acad. Sci. 1508, 92–104 (2022). [DOI] [PubMed] [Google Scholar]
- 45.Ge Y., Chen J., Hu Y., Chen X., Huang M., IL-38 alleviates inflammation in sepsis in mice by inhibiting macrophage apoptosis and activation of the NLRP3 inflammasome. Mediators Inflamm. 2021, 6370911 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.de Graaf D. M., et al. , IL-38 gene deletion worsens murine colitis. Front. Immunol. 13, 840719 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Moretti S., et al. , IL-37 inhibits inflammasome activation and disease severity in murine aspergillosis. PLoS Pathog. 10, e1004462 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Mangan M. S. J., et al. , Targeting the NLRP3 inflammasome in inflammatory diseases. Nat. Rev. Drug Discov. 17, 588–606 (2018). [DOI] [PubMed] [Google Scholar]
- 49.Booker C. S., Grattan D. R., IL1R9 is evolutionarily related to IL18BP and may function as an IL-18 receptor. J. Immunol. 198, 270–278 (2017). [DOI] [PubMed] [Google Scholar]
- 50.Hayashi T., Yoshida T., Ra M., Taguchi R., Mishina M., IL1RAPL1 associated with mental retardation and autism regulates the formation and stabilization of glutamatergic synapses of cortical neurons through RhoA signaling pathway. PLoS One 8, e66254 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Khan J. A., Brint E. K., O’Neill L. A., Tong L., Crystal structure of the Toll/interleukin-1 receptor domain of human IL-1RAPL. J. Biol. Chem. 279, 31664–31670 (2004). [DOI] [PubMed] [Google Scholar]
- 52.Pavlowsky A., et al. , Neuronal JNK pathway activation by IL-1 is mediated through IL1RAPL1, a protein required for development of cognitive functions. Commun. Integr. Biol. 3, 245–247 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Liu F., et al. , Metformin ameliorates TGF-β1-induced osteoblastic differentiation of human aortic valve interstitial cells by inhibiting β-catenin signaling. Biochem. Biophys. Res. Commun. 500, 710–716 (2018). [DOI] [PubMed] [Google Scholar]
- 54.Yang L., et al. , MicroRNA-34c inhibits osteogenic differentiation and valvular interstitial cell calcification via STC1-mediated JNK pathway in calcific aortic valve disease. Front. Physiol. 11, 829 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 55.Nold-Petry C. A., et al. , IL-37 requires the receptors IL-18Rα and IL-1R8 (SIGIRR) to carry out its multifaceted anti-inflammatory program upon innate signal transduction. Nat. Immunol. 16, 354–365 (2015). [DOI] [PubMed] [Google Scholar]
- 56.Lech M., et al. , Tir8/Sigirr prevents murine lupus by suppressing the immunostimulatory effects of lupus autoantigens. J. Exp. Med. 205, 1879–1888 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Heiser D., et al. , Evaluation of protein kinase D auto-phosphorylation as biomarker for NLRP3 inflammasome activation. PLoS One 16, e0248668 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Huang Y., et al. , Dexmedetomidine attenuates myocardial ischemia-reperfusion injury in vitro by inhibiting NLRP3 Inflammasome activation. BMC Anesthesiol. 21, 1–12 (2021). Correction in: BMC Anesthesiol. 21, 141 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All study data are included in the article and/or supporting information.