Abstract
The superiority of early interventions for children with autism spectrum disorders (ASDs) compared to treatment as usual (TAU) has recently been questioned. This study was aimed to investigate the efficacy of early interventions in improving the cognitive ability, language, and adaptive behavior of pre-school children with ASDs through a systematic review of randomized controlled trials (RCTs). In total, 33 RCTs were included in the meta-analysis using the random effects model. The total sample consisted of 2581 children (age range: 12–132 months). Early interventions led to positive outcomes for cognitive ability (g = 0.32; 95% CI: 0.05, 0.58; p = 0.02), daily living skills (g = 0.35; 95% CI: 0.08, 0.63; p = 0.01), and motor skills (g = 0.39; 95% CI: 0.16, 0.62; p = 0.001), while no positive outcomes were found for the remaining variables. However, when studies without the blinding of outcome assessment were excluded, positive outcomes of early interventions only remained for daily living skills (g = 0.28; 95% CI: 0.04, 0.52; p = 0.02) and motor skills (g = 0.40; 95% CI: 0.11, 0.69; p = 0.007). Although early intervention might not have positive impacts on children with ASDs for several outcomes compared to controls, these results should be interpreted with caution considering the great variability in participant and intervention characteristics.
Keywords: autism spectrum disorders, early interventions, cognitive ability, language, meta-analysis
1. Introduction
The increasing prevalence rates in autism diagnoses during recent years (1 in every 150 children in 2000, 1 in every 68 children in 2012, and now 1 in every 44 children) [1] has enhanced the establishment of a variety of interventions for young children with ASDs [2,3,4,5,6]. Such approaches are classified according to their manual and targeted outcomes as behavioral interventions, developmental interventions, naturalistic developmental behavioral interventions (NCBI), TEACCH, sensory-based interventions, animal-assisted interventions and technology-based interventions [2]. Sensory-based interventions are motivated by the theory that children with ASDs may fail to respond to sensory inputs such as sound, touch, body movement, sight, taste, and smell. Within this concept, sensory integration therapy aims to help children with ASDs use their senses together to enhance their engagement and participation in a range of daily living activities. For example, sensory stimuli, such as a hug machine with deep pressure, can provide a calming effect and reduce unwanted movements in children with ASDs during travelling [7].
Despite the existence of various treatment programs, there is insufficient evidence for the superiority of a treatment model in improving core areas of deficits of children with ASDs, such as cognitive ability, language, communication, socialization and adaptive behavior. Recently, the American Psychological Association (APA) published a meta-analysis about the efficacy of early interventions. The authors concluded that, when no study quality criteria were considered, positive outcomes were found for behavioral, developmental and NCB interventions. However, when the analysis was limited to RCTs at a low risk of detection bias, there was no evidence of positive outcomes for young children with ASDs [2]. Partially consistent with the results published by APA is the meta-analysis conducted by Yi et al. (2019) [8]. The authors compared the effectiveness of applied behavior analysis (ABA), early start Denver model (ESDM), Picture Exchange Communication Systems (PECS), and discrete trial training (DTT) investigated via randomized controlled trials (RCTs). They found positive outcomes of ABA-based interventions in socialization, communication, and expressive language, but not in receptive language, adaptive behavior and cognitive ability. Rogers et al. (2021) [9], conducted a meta-analysis of non-randomized studies about the efficacy of ABA. They did not find any significant outcomes for cognitive ability or adaptive behavior, but children in the experimental group outperformed children in the control group in the adaptive behavior scale over a 2-year follow-up. Positive outcomes for children in cognitive competence, language-communication, social competence, and adaptive behavior were also reported in a substantial number of meta-analyses [10,11,12,13,14,15,16,17,18]. However, the aforementioned findings were not ubiquitous [19,20,21].
The majority of previous meta-analyses pointed out that the considered studies had considerable methodological limitations [1,18,22,23]. Moreover, if we carefully examine the studies testing the effectiveness of early interventions, we notice that regardless of their theoretical frameworks or “brand-name”, they presented extensive differences in terms of treatment intensity and duration. Some studies included more comprehensive interventions, focusing on core functional areas such as cognitive ability, language, or adaptive behavior, while others included targeted interventions addressing more restricted areas, such as joint attention and imitation. However, children with autism who present higher scores of joint attention, imitation, and object play in infancy are more likely to have stronger communication and intellectual skills in the subsequent years [24].
The aim of the current meta-analysis was to draw a valid conclusion about the efficacy of early intervention programs for pre-school children with ASDs compared to children that did not receive any of the abovementioned early intervention treatments in improving their cognitive ability, language skills, communication, socialization and adaptive behavior. Furthermore, this is the first meta-analysis to synthesize all available information from the included studies through mathematical formulas that enabled the combined testing of multiple measurements of the dependent variables across studies (e.g., single variables measured by different scales). Considering the impact that the improvement in proximal variables can have on distal variables [24], we included studies focusing on both comprehensive and targeted areas of functionality. We also aimed to extend the findings of Sandback et al., 2020 [2] and examine whether intervention duration and intensity can predict the performance of participants post treatment. To fulfil this purpose, we decided to only include RCTs, because this design can provide more reliable results regarding the causal effect of an intervention [25].
2. Materials and Methods
2.1. Search Strategy
We searched PsycInfo, ERIC and MEDLINE PubMed, and Google Scholar on 11 May 2022 without applying any time limit for peer-reviewed studies published in English language by entering the following keywords: (autism) OR (autistic) OR (developmental disorder) OR (autism spectrum disorder) OR (Asperger)) AND ((preschool age children) OR (young children) OR (toddlers) OR (pupils)) AND ((intervention) OR (comprehensive intervention) OR (parent training) OR (parent implemented) OR (comprehensive approach) OR (developmental approach) OR (behavioral approach) OR (therapy) OR (EIBI) OR (ABA)) AND ((cognition) OR (cognitive ability) OR (language) OR (adaptive behavio*)). A pilot search was first conducted in October 2021. The literature search was performed by two independent authors. Disagreements were solved after discussion and the re-evaluation of the relevance of each study until a consensus was reached.
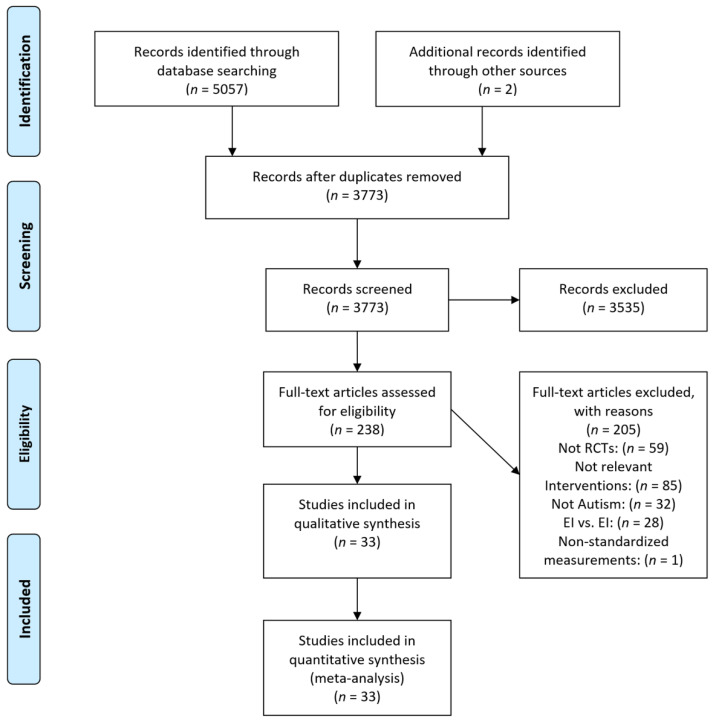
Query logic was adapted to each search database to optimize retrieval. Following the recommendations by [26], the study selection process was conducted and presented using the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (http://prisma-statement.org/, accessed on 26 May 2022) as a guide (see Figure 1). The PRISMA study selection process entails four phases: identification, screening, eligibility and final synthesis.
Figure 1.
Flow diagram of study selection.
2.2. Study Selection
We included studies that: (i) were randomized controlled trials (RCTs); (ii) focused on participants who were infants at risk of autism and preschool-aged children with a diagnosis of autistic spectrum disorders (ASDs), autistic disorder (AD), pervasive developmental disorders-not-otherwise-specified (PDD-NOS), and/or pervasive developmental disorders (PDDs) (studies including exclusively infants who were all under 18 months of age were not included, since we aimed at measuring a clear manifestation of the autistic or developmental symptoms); (iii) considered interventions that included psychosocial parent- or/and professional-implemented specialized interventions aiming at reducing ASD-related impairments (studies examining pharmacological treatments and alternative interventions such as music therapy or equine therapy were not included, and since we tested the overall effectiveness of early interventions, we did not include studies examining the effectiveness of different versions of the same approach, varying in intervention providers, setting, or dosage, for example, or studies comparing different kinds of early interventions); (iv) measured outcomes that included at least one of the following domains—cognitive ability, expressive language, receptive language, communication, socialization, adaptive behavior composite, daily living skills and motor skills—measured by standardized scales (outcomes had to be presented by means and standard deviations for both groups); and (v) were published in the English language and were peer-reviewed articles.
2.3. Data Extraction and Coding
We created three different spreadsheets in Excel (version 2206, Microsoft Excel, Microsoft Corporation, Redmont, WA, USA). The first spreadsheet contained descriptive information of each study including study name, date, study sample size, participant’s age, gender, and intervention-related information such as intervention type, duration, intensity, intervention providers, and intervention setting (Table 1). Experimental group interventions included any intervention. The intervention duration and intensity were coded as moderators of the result and were thus analyzed as independent variables. The second spreadsheet contained the means and standard deviations, as well as the scales of measurement of the examined variables for each outcome, and the third spreadsheet was identical to second for the combined outcomes (Table A1 and Table A2).
Table 1.
Characteristics of the included studies.
| Study | Participants n (% Males), Mean Age (Age Range) |
Experiment Group Intervention | Duration and Intensity of Intervention | Comparison Condition |
Duration | Intervention Providers | Setting |
|---|---|---|---|---|---|---|---|
| Aldred et al. (2004) [27] | n = 28 (89.29%, males); mean age: 49.5 (24–71) | Social Communication Intervention | 6 months with monthly sessions followed by another 6 months of 2-monthly consolidation sessions plus 30 min daily parent–child interaction | TAU | 12 months | Professionals and parents | Individual |
| Brian et al. (2017) [28] | n = 62 (75.8%, males); mean age: 25.26 (16–30) | Social ABCs | 12 weeks of 1.5 h home visits with tapering intensity (week 1: 3 visits; week 2: 2 visits; weeks 3–8: 1 visit/week; weeks 10 and 12: 1 “booster” visit/week; weeks 9 and 11: check-in phone call) |
TAU: 3 months: up to 1 h/week of “other” direct therapy | 3 months | Parents | Individual |
| Carter et al. (2011) [29] | n = 62 (82.26% males); mean age: 20.25 (15–25) | Hanen’s More Than Words (HMTW) | 8 group parent sessions of 2.5 h and three in-home individualized parent–child sessions of 1 h | No treatment | 3.5 months | Parents | Individual and group |
| Dawson et al. (2010) [30] | n = 48 (77.1% males); mean age: 23.5 (18–30) | The Early Start Denver Model (ESDM) | 15.2 h therapist-delivered and 16.3 h/week parent-delivered therapy, 5 days/week | TAU: 9.1 h of individual therapy and an average of 9.3 h/week of group interventions for 2 years | 2 years | Parents | Individual |
| Divan et al. (2019) [31] | n = 40 (87.5% males); mean age: 64 (27–105) | Parent mediated intervention for Autism Spectrum Disorder Plus (PASS Plus) | 12 fortnightly home-based session between the parent and the lay health worker after a 10 min period of play between the parent and the child |
TAU | 6 months | Parents | Individual |
| Drew et al. (2002) [32] | n = 24 (79.17% males); mean age: 22.5 (-) | Parent training intervention with a focus on the development of joint attention skills and joint action routines | 3 h parent sessions every 6 weeks | TAU: 3 months: 32.9 h/week | 12 months | Parents | Individual |
| Estes et al. (2015), follow-up of Dawson et al. (2010) [33] | n = 39 (77% males); mean age: 2.9 | The Early Start Denver Model (ESDM) | 15.2 h therapist-delivered and 16.3 h/week parent-delivered therapy, 5 days/week | TAU: 9.1 h of individual therapy and an average of 9.3 h/week of group interventions for 2 years | 2 years | Parents | Individual |
| Gengoux et al. (2019) [34] | n = 43 (88.4% males); mean age: 48.4 (24–60) | PRT-P | Weeks 1 to 12: weekly 60 min parent training sessions and 10 h per week of clinician delivered in-home treatment for children. Weeks 12 to 24: monthly 60 min parent training sessions and 5 h per week of in-home treatment for children | Waitlist and stable community treatments | 24 weeks | Professionals and parents | Individual |
| Green et al. (2010) [35] | n = 152 (90.79% males); mean age: 45 (24–60) | Parent-mediated communication-focused treatment in children with autism (PACT) |
2 h clinic sessions every 2 weeks for 6 months followed by monthly booster sessions for 6 months, plus 30 min of daily home practice | TAU | 13 months | Parents |
Individual |
| Green et al. (2022) [36] | n = 248 (79.4% males); mean age: 63 (24–132) | Paediatric Autism Communication Therapy-Generalised (PACT-G) | 12 intervention sessions over 6 months at home plus 12 sessions over 6 months, again with 50% remote delivery | TAU | 6 months | Professionals and parents | Individual |
| Hampton et al. (2020) [37] | n = 73 (79% males); mean age: 43 (36–60) | Caregiver training, Discrete Trial Teaching, and JASP + EMT + SGD | 36 sessions in the clinic and at home, 45–60 min per session | TAU | 4 months | Professionals and parents | Individual |
| Hardan et al. (2015) [38] | n = 47 (75% males); mean age: 49.2 (24–84) | Pivotal Response Treatment (PRT) | 1 session of 90 min/week | Psychoeducation: 12 weeks: 1 session of 60 min/week | 12 weeks | Parents | Individual |
| Kaale et al. (2014) [39] | n = 61 (78.7% males); mean age: 48.8 (24–60) | Social communication treatment | 2 daily 20 min sessions, including 5 min of table-top training and 15 min of floor play | TAU | 8 weeks | Teachers | Group |
| Kasari et al. (2015) [40] | n = 86 (81.4% males); mean age: 31.5 | Joint Attention, Symbolic Play, Engagement and Regulation (JASPER) | 2 sessions of 30 min per week | Psychoeducation: 1 h per week | 10 weeks | Professionals and parents | Individual |
| Landa et al. (2011) [41] | n = 48 (88.3% males); mean age: 28.7 (21–33) | Interpersonal Synchrony | 10 h per week in classroom, student-to-teacher ratio, schedule, home-based parent training (1.5 h per month), parent education (38 h), plus supplementary curriculum targeting socially engaged imitation, joint attention, and affect sharing | Non-Interpersonal Synchrony: 10 h per week in classroom, student-to-teacher ratio, schedule, home-based parent training (1.5 h per month), parent education (38 h) | 6 months | Teachers, Parents | Group, Individual |
| Oosterling et al. (2010) [42] | n = 65 (77.61% males); mean age: 34.32 (<12–42) | Joint attention and language skills stimulation | 2 h sessions with parents, 3 h home visits every 6 weeks during the first year. In the second year, 3-month intervals between home visits | TAU | 2 years | Parents | Individual |
| Parsons et al. (2019) [43] | n = 59 (81.4% males); mean age: 62.6 (24–72) | TOBY app targeting visual motor, imitation, language and social parameters | At least 20 min on the TOBY app daily for 3 months using an iPad | TAU | 3 months | - | Individual |
| Rahman et al. (2016) [44] | n = 65 (81.5% males); mean age: 64.5 24–108) | PASS (plus TAU) | 1 h sessions every 2 weeks for 6 months | TAU | 6 months | Professionals and parents | Individual |
| Reitzel et al. (2013) [45] | n = 11 (-); mean age: 58.5 (38–82) | Functional Behaviour Skills Training program (FBST) | 30 min parents-only training sessions, a simultaneous children’s activity session, and a 90 min combined children’s and parents’ training session | TAU | 4 months | Professionals and parents | Individual |
| Rickards et al. (2007) [46] | n = 59 (79.7% males); mean age: 43.87 (36–60) | Home-based Program (in addition to a center-based program) |
1 and 1½ h during school terms over a 12-month period plus 5 h spread over two weekly sessions during school terms | Center-based program: 5 h spread over two sessions weekly during school terms | 12 months | Professionals | Individual |
| Roberts et al. (2011) [47] | n = 56 (90.5% males); mean age: 42.6 (26.3–60.3) | Building Blocks home-based | Visit for 2 h once a fortnight over a 40-week period (20 sessions maximum) | Waitlist | 1 year | Parents | Individual |
| Rogers et al. (2012) [48] | n = 98 (77.55% males); mean age: 20.98 (12–24) | Brief Early Start Denver Model (P-ESDM) Parent-based Intervention | 1 session of 1 h/week | TAU | 12 weeks | Parents | Individual |
| Rogers et al. (2019) [49] | n = 118 (78% males); mean age: 21.02 (14–24) | Early Start Denver Model (ESDM) | 3 months of weekly parent coaching followed by 24 months of 15 h per week (on average) 1:1 treatment weekly on average in homes or daycare settings from supervised therapy assistants while parents received 4 h of coaching monthly from a certified ESDM therapist | TAU | 27 months | Professionals and parents | Individual |
| Scahill et al. (2016) [50] | n = 180 (87.7% males); mean age: 4.75 (36–83) | Parent training (PT) | Eleven 60-to-90-minute core sessions, up to 2 optional sessions, and a home visit over 16 weeks, as well as a home visit and 2 telephone booster sessions between weeks 16 and 24 | Structured parent education program (PEP): twelve 60-to-90-minute individually administered sessions and 1 home visit over 24 weeks | 24 weeks | Parents | Individual |
| Schertz et al. (2013) [51] | n = 23 (-); mean age: 26.11 (<30) | Joint Attention Mediated Learning (JAML) | 15 home visits included 10 min parent–child interaction plus 30 min daily parent–child interaction | TAU | 7 months | Parents | Individual |
| Siller et al. (2013) [52] | n = 70 (91% males); mean age: 57.1 (32–82) | Focused Playtime Intervention (FPI) | 1 session per week for 12 weeks, 90 min per session | PAC | 12 weeks | Parents | Individual |
| Solomon et al. (2014) [53] | n = 128 (78.91% males); mean age: 50.19 (32–71) | PLAY Project Home Consultation program (PLAY) | 3 h home visits/month | TAU: 2 h/week | 1 year | Parents | Individual |
| Strain and Bovey (2011) [54] | n = 294; mean age: 50.33 | LEAP intervention (Learning Experiences and Alternative Program for Preschoolers and Their Parents) | 2.75–3 h per day, 5 days per week | Intervention manuals and related written materials to preschool staff: 2.75–3 h per day, 5 days per week | 2 years | Professionals and parents | Group |
| Tonge et al. (2014) [55] | n = 70 (82.86% males); mean age: 46.56 (23–70) | Education and behavior management skills for Pre-schoolers with Autism (PEBM) | Ten 90-minute small group (4–5 families) sessions alternated with ten 60-minute individual family sessions over a 20-week period. | TAU | 20 weeks | Parents | Individual and group |
| Turner-Brown et al. (2019) [56] | n = 49 (85.7% males); mean age: 29.6 (17–35) | Family Implemented TEACCH for Toddlers (FITT) | Twenty 90-minute in-home sessions where the FITT coach works directly with the family and toddler and 4 parent group sessions. | TAU | 6 months | Parents and professionals | Individual and group |
| Valeri et al. (2020) [57] | n = 34 (79% males); mean age: 48.3 (24–132) | Cooperative parent-mediated therapy (CPMT) plus low-intensity psychosocial intervention (LPI) | 15 sessions of 60 min. 12 core sessions, 1 per week, were delivered in the first 3 months, followed by 3 monthly booster sessions plus 4 h LIP per week | Low-intensity psychosocial intervention (LPI) | 6 months | Parents and professionals | Individual |
| Vernon et al. (2019) [58] | n = 23 (87% males); mean age: 35.13 (18–56) | Pivotal Response Intervention for Social Motivation (PRISM) |
10 h a week of intervention: 8 h of one-on-one clinician-implemented treatment and 2 h of parent education in the intervention strategies with the child present | TAU | 6 months | Parents and professionals | Individual |
| Welterlin et al. (2012) [59] | n = 20 (90% males); mean age: 30.5 (24–39) | Home TEACCHing Program for Toddlers with Autism | 1.5 h per week for 12 sessions plus 30 min of parents’ psychoeducation | Waitlist | 12 weeks | Parents | Individual |
2.4. Risk of Bias
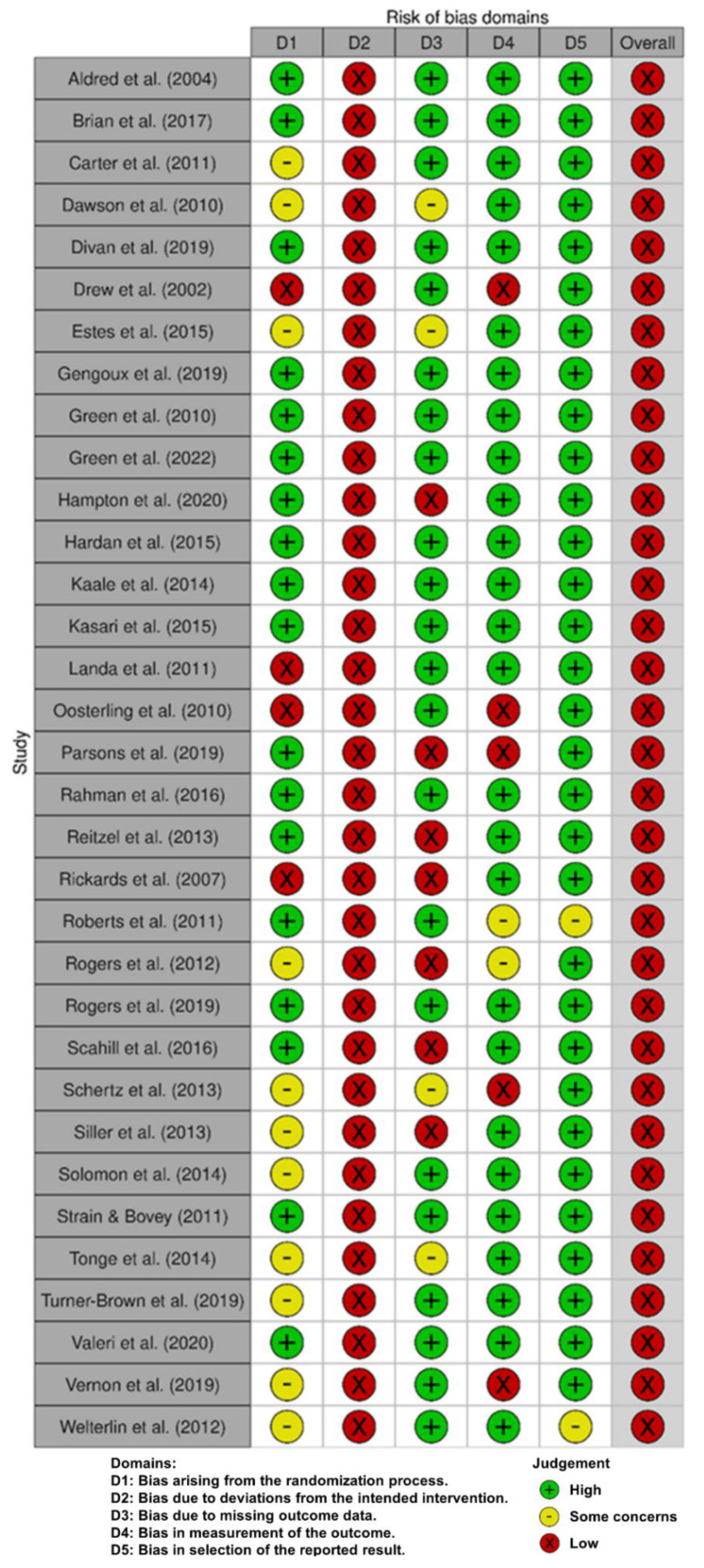
The quality of each study was assessed with the Cochrane risk of bias tool RoB 2 [60] by two independent examiners. This tool includes six items that cover the following bias domains: (i) bias arising from the randomization process, (ii) bias due to deviation from intended interventions, (iii) bias due to missing outcome data, (iv) bias in the measurement of the outcome, (v) bias in the selection of the reported results, and (vi) overall bias. This tool has three grading levels: (i) low, (ii) unclear, and (iii) high risk of bias. The worst grading in individual items define the overall risk of bias for each single study.
2.5. Data Analysis
Meta-analysis was performed using the Review Manager (RevMan, version 5.4.1, The Nordic Cochrane Centre, Copenhagen, Denmark). RevMan is the Cochrane Collaboration’s software for preparing and maintaining Cochrane reviews. Because there was meaningful variability across studies regarding participant and intervention characteristics, we analyzed the results using the random effects model of meta-analysis. In our study, we used variable instruments for assessing the same variable (e.g., expressive language was measured with Mullen Scales of Early Learning (MSEL), MacArthur Communicative Development Inventories (MCDI), or other standardized scales). We converted all the measurements to standardized mean differences and variances so that they could be comparable to each other. The standardized mean difference is the difference in mean outcome between groups divided by the standard deviation of outcome across participants [25]. Subsequently, based on the standardized mean differences and variances, we calculated Hedges’ g with RevMan software. Hedges’ g and Cohen’s d were interpreted in the same way according to the rule of thumb that Cohen suggested, where an effect size of 0.20 is small, an effect size of 0.50 is moderate, and an effect size of 0.80 is large [61].
After computing the effect sizes and their statistical significance, we conducted a heterogeneity test in order to establish whether our data were consistent. The heterogeneity was assessed using tau2, a metric that we used to define the variance of the true effects sizes and to determine the weight assigned to each included study analyzed with the random effects model [62]. Additionally, we calculated the I2 statistic, which describes the magnitude of heterogeneity across studies that is attributable to the true differences of the results rather than chance or sampling error [63]. Heterogeneity can be interpreted as low when I2 =0–40%, as moderate when I2 = 30–60%, as substantial when I2 = 50–90% and as considerable when I2 = 75–100% [62].
In the current review, many of the included studies contained outcomes that were measured by more than one scale (MSEL and MCDI for expressive language). We could not analyze the different outcomes as they were independent because this could lead to incorrect estimates of the variance for the summary effect [62]. Since we analyzed the standardized mean differences for each outcome, we calculated an effect size for all the multiple outcomes per variable for each study. In this case, we calculated the mean effect sizes and the variances for all the multiple outcomes and then the corresponding standard errors (SEs). To compute the combined variance, we applied the formulas suggested by Borenstein et al., (2021) [62]. In this way, we could include all the relevant and available information across studies and at the same time address the problem of non-independence, since all the measurements per study came from the same sample. The formulas were as follows.
-
(1)
Computed variance in case we had two outcomes per study.
-
(2)
Computed variance in case we had more than two outcomes per study.
3. Results
3.1. Search Results
A PRISMA flowchart summarizing the article selection process is presented in Figure 1. After the initial database search, 5057 studies, plus two studies identified in Google Scholar, were retrieved. After excluding duplicates and studies that did not meet our inclusion criteria, 33 studies were included in the analysis.
3.2. Study Characteristics
A full description of the included studies [27,28,29,30,31,32,33,34,35,36,37,38,39,40,41,42,43,44,45,46,47,48,49,50,51,52,53,54,55,56,57,58,59] is depicted in Table 1. The total number of children was 2581. All children had a diagnosis of either ASDs or PDD. The participant age at the beginning of the study ranged from 12 to 132 months.
Out of the 33 studies included, 12 studies were categorized as long-term interventions, 9 were categorized as medium-term interventions, and 12 were categorized as short-term interventions. Additionally, 10 studies implemented high-intensity interventions and 23 studies implemented low-intensity interventions. Twenty-two studies compared the interventions of our interest with TAU, three studies compared early interventions with no treatment or a WL, and eight studies included an altered or low intensity intervention compared to the intervention of the experimental group. The duration of the provided interventions ranged from 12 weeks to 2 years, and their intensity ranged from 3 h of parent sessions every 6 weeks to 15.2 h of therapist-delivered and 16.3 h per week of parent-delivered therapy 5 days per week for 2 years. Intervention providers were both professionals and parents in 13 studies, parents only in 17 studies, and professionals only in three studies. In 27 studies, the intervention setting was individual therapy; in two studies, the setting was both group and individual therapy; and in four studies, the setting was group therapy. All the studies included in meta-analysis reported results obtained from standardized tests.
3.3. Risk of Bias Assessment
All studies were assessed for risk of bias by two independent authors (Figure 2). Disagreements were solved through the re-evaluation of the original papers and discussion until a consensus was reached. In general, 11 studies were assessed as having some concerns due to a lack of specific information and three studies were assessed as having a high risk of bias in randomization process criterium. All studies were assessed as having a high risk of bias in deviation from intended intervention criterium because the participants and personnel were not blinded to intervention status. Four studies were assessed as having some concerns, and seven studies were assessed as having a high risk of bias due to missing outcome data criterium. Two studies were assessed as having some concerns, and five studies were assessed as having a high risk of bias in the measurement of the outcome criterium. Finally, two studies were assessed as having some concerns regarding bias in the selection of the reported results.
Figure 2.
Risk of bias assessment [27,28,29,30,31,32,33,34,35,36,37,38,39,40,41,42,43,44,45,46,47,48,49,50,51,52,53,54,55,56,57,58,59].
After the completion of the risk of bias assessment, studies that were assessed as having a high risk of bias in the measurement of the outcome criterium were excluded from the analysis.
3.4. Meta-Analysis
Appendix A (Table A1) contains the pre- and post-measurements for every variable across studies, and (Table A2) contains tables with the effect sizes of the combined outcomes after the statistical formulas were applied.
3.4.1. Sensitivity Analysis
In order to calculate the variance for the combined outcomes, we had to provide their correlation coefficients. Since we did not know the correlation between our combined outcomes, we assumed it was r = 0.5. Subsequently, we performed a sensitivity analysis for r = 0.25 and r = 0.75, and the results confirmed our assumption, since we did not observe any differences in the results.
3.4.2. Cognitive Ability Results
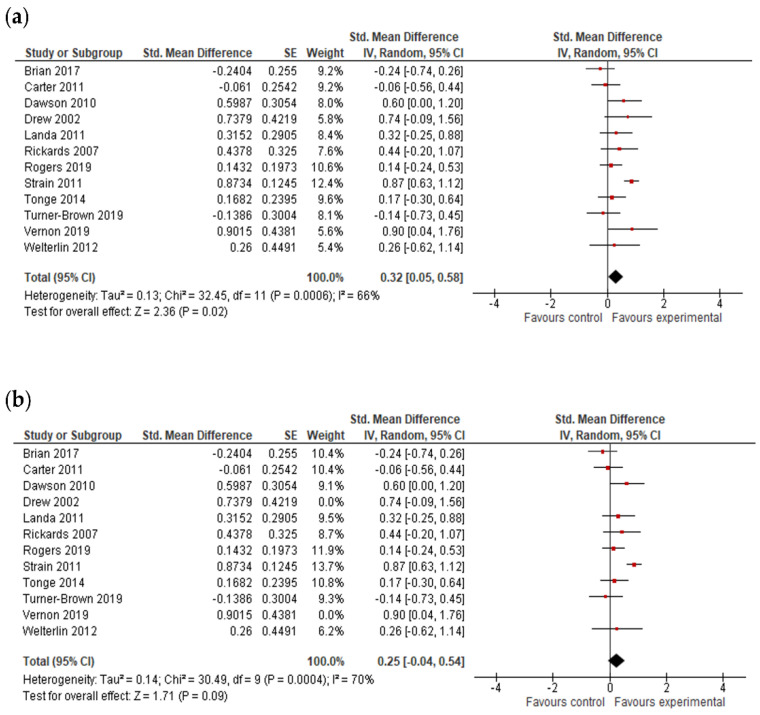
The overall effect size of cognitive ability was based on data from 12 studies (Figure 3). The overall result of the meta-analysis indicated that early intervention programs are efficacious in improving the cognitive ability of pre-school children with ASDs (g = 0.32; 95% CI: 0.05, 0.58; p = 0.02) based on the pre-treatment and post-treatment assessments.
Figure 3.
Forest plots for cognitive ability results. (a) Overall effect [28,29,30,32,41,46,49,54,55,56,58,59]. (b) Results after exclusion of studies with no blinding of outcome assessment [28,29,30,41,46,49,54,55,56,58,59].
A subgroup analysis was performed to test whether intervention intensity and intervention duration modified the effect of early intervention in comparison to control conditions (analysis not presented). However, the number of trials and participants contributing data to the intervention duration subgroups (5 trials and 543 participants for long-term interventions, 3 trials and 120 participants for medium-term interventions, and 4 trials and 214 participants for short-term interventions) and the intervention intensity subgroups (7 trials and 614 participants for high-intensity interventions and 5 trials and 263 participants for low-intensity interventions) was unequal, meaning that the analysis was unlikely to produce useful findings [64].
After the exclusion of studies with bias in measuring of the outcomes, there were no positive outcomes of early intervention for cognitive ability (g = 0.25; 95% CI: −0.04, 0.54; p = 0.09).
3.4.3. Language Results
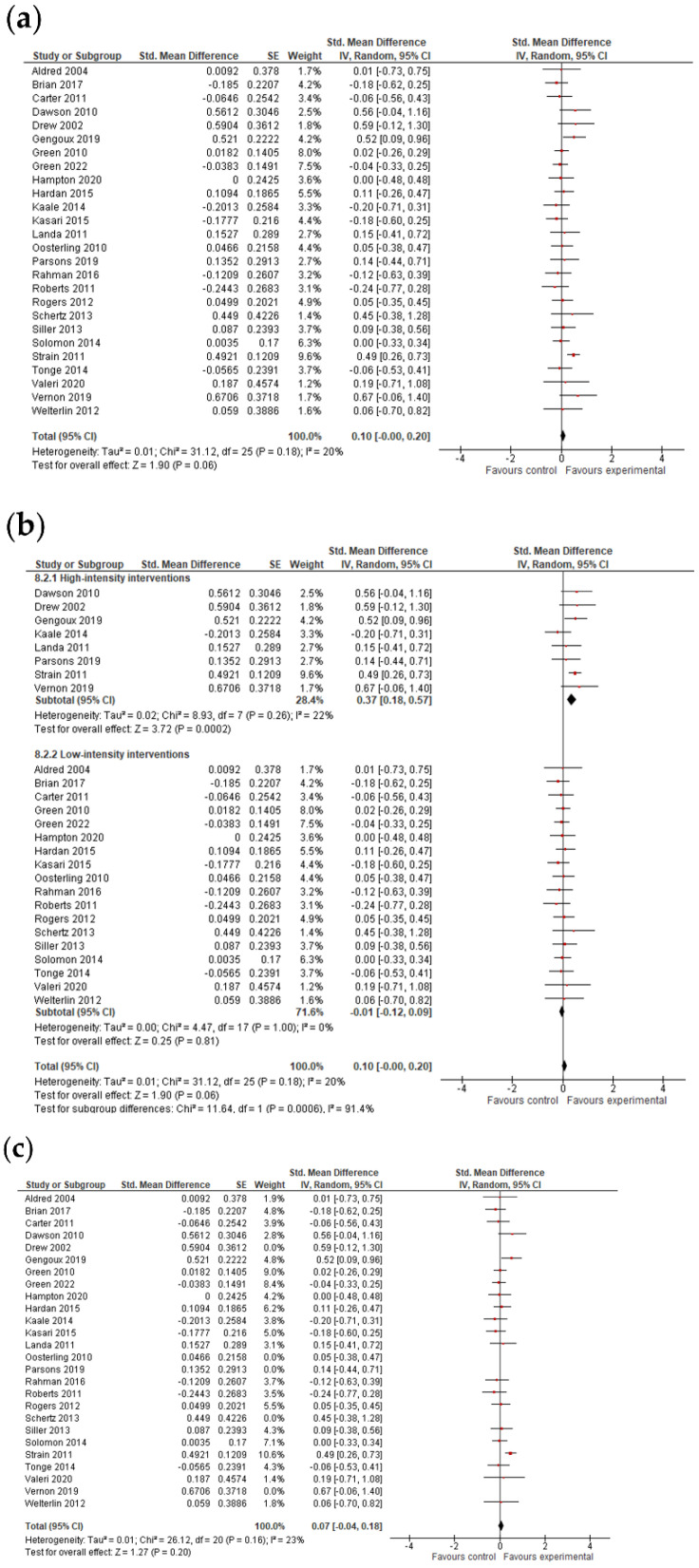
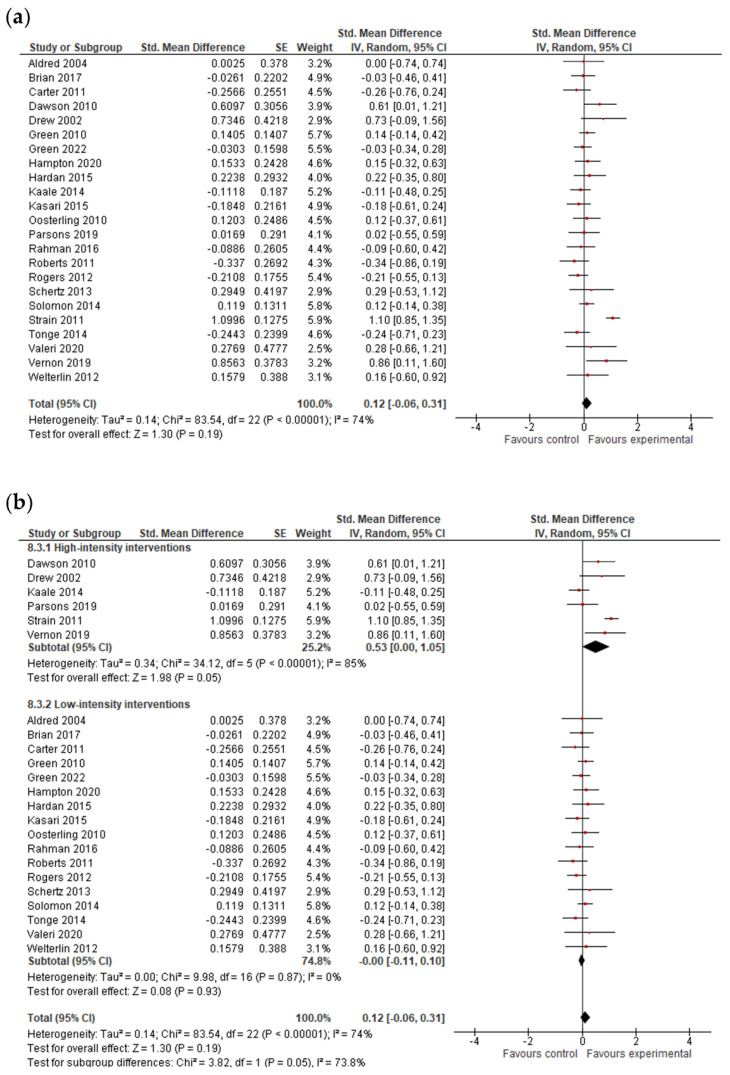
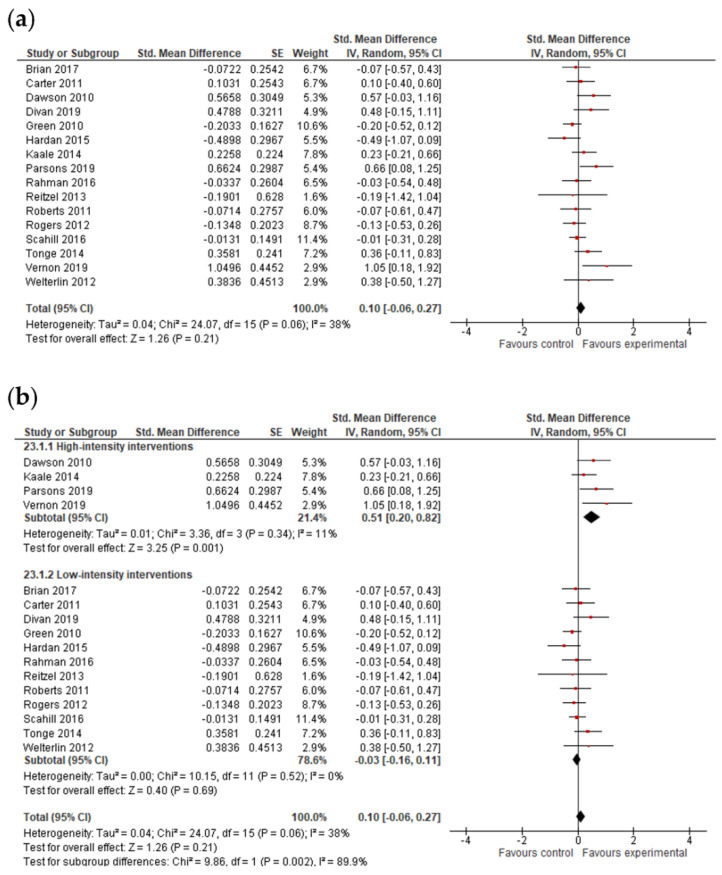
The analysis was based on 26 studies for expressive language and on 23 studies for receptive language (Figure 4 and Figure 5). After combining the results of studies with multiple outcomes, analysis showed that early interventions were marginally insignificant for expressive language (g = 0.10; 95% CI: −0.00, 0.20; p = 0.06) and not efficacious in improving the receptive language skills of pre-school children with ASDs (g = 0.12; 95% CI: −0.06, 0.31; p = 0.19). The I2-statistic showed that the heterogeneity among studies was insignificant for expressive language (Tau2 = 0.01; I2 =20%, p = 0.18) and substantial and significant for receptive language (Tau2 = 0.14; I2 =74%, p ≤ 0.0001).
Figure 4.
Forest plots for expressive language results. (a) Overall effect [27,28,29,30,32,34,35,36,37,38,39,40,41,42,43,44,47,48,51,52,53,54,55,57,58,59]. (b) Subgroup analysis: intensity of intervention. (c) Results after exclusion of studies with no blinding of outcome assessment [27,28,29,30,34,35,36,37,38,39,40,41,44,47,48,52,53,54,55,57,59].
Figure 5.
Forest plots for receptive language results. (a) Overall effect [27,28,29,30,32,35,36,37,38,39,40,42,43,44,47,48,51,53,54,55,57,58,59]. (b) Subgroup analysis: intensity of intervention.
The test for subgroup differences indicated that there was no statistically significant subgroup effect (p = 0.16 for expressive language and p = 0.09 for receptive language; analysis not presented), suggesting that intervention duration does not modify the effect of early intervention in comparison to control conditions. However, the number of trials and participants that contributed data to the subgroups was unequal, meaning that the analysis may not have been able to detect subgroup differences. Additionally, although the subgroup analysis performed to test whether intervention intensity modifies the effect of early intervention in comparison to control conditions (analysis not presented) indicated that there was a statistically significant subgroup effect, the number of trials and participants that contributed data to the intervention intensity subgroups (8 trials and 613 participants for high-intensity interventions and 18 trials and 1278 participants for low-intensity interventions for expressive language; 6 trials and 522 participants for high-intensity interventions and 17 trials and 1208 participants for low-intensity interventions for receptive language) was unequal, meaning that the analysis was also unlikely to produce useful findings [64].
However, when studies with bias in the measurement of the outcome were excluded from the analysis, the results remained insignificant for expressive language (g = 0.07; 95% CI: −0.04, 0.18; p = 0.20).
3.4.4. Adaptive Behavior Composite, Communication, Socialization, Daily Living Skills and Motor Skills Results
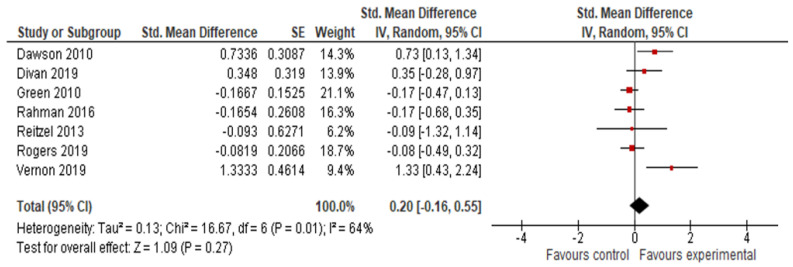
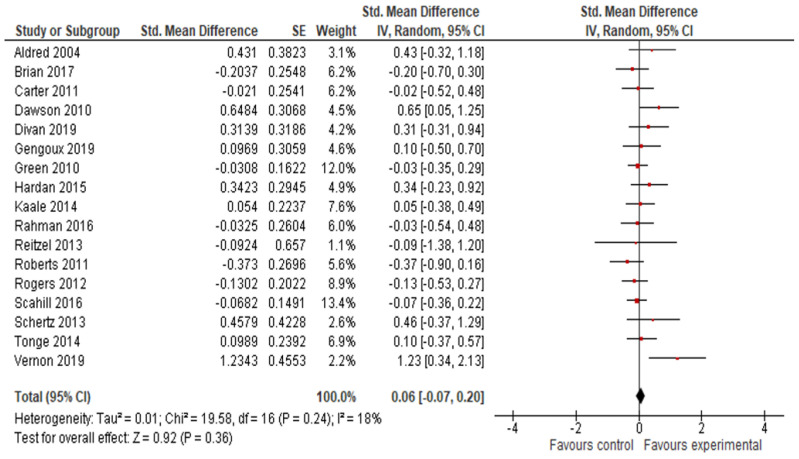
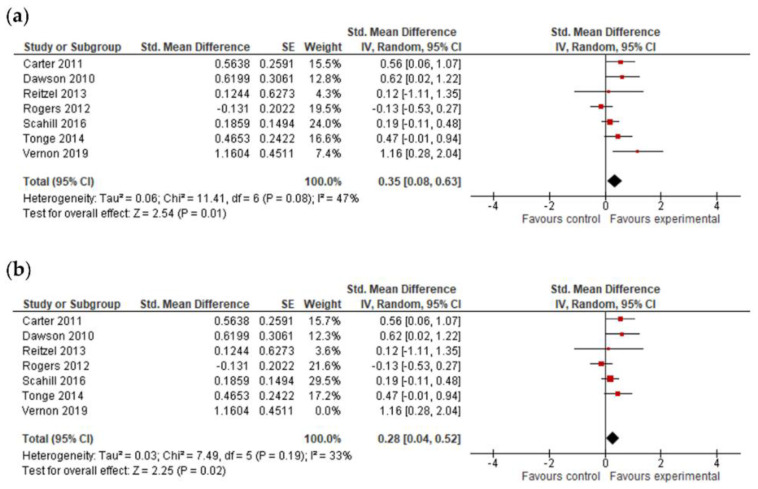
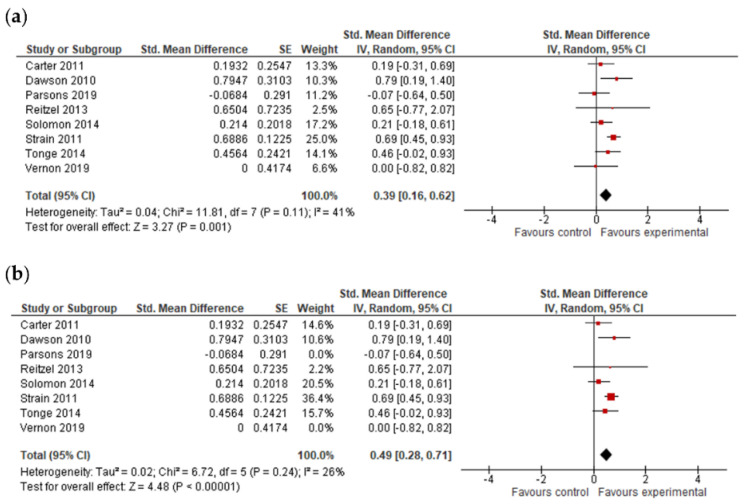
The final effect size for the adaptive behavior composite result was based on the results from seven studies (Figure 6) and showed that early intervention was not effective for the adaptive behavior composite (g = 0.20; 95% CI: −0.16, 0.55; p = 0.27). Analysis also indicated that early interventions were not statistically significant, either for improving communication (17 studies, g = 0.06; 95% CI: −0.07, 0.12; p = 0.36, Figure 7) and socialization (16 studies, g = 0.10; 95% CI: −0.06, 0.27; p = 0.21, Figure 8); on the other hand, early interventions were statistically significant for daily living (seven studies, g = 0.35; 95% CI: 0.08, 0.63; p = 0.01, Figure 9) and motor skills (sight studies, g = 0.39; 95% CI: 0.16, 0.62; p = 0.001, Figure 10).
Figure 6.
Forest plot for adaptive behavior composite. Overall effect [30,31,35,44,45,49,58].
Figure 7.
Forest plot for communication results. Overall effect [27,28,29,30,31,34,35,38,39,44,45,47,48,50,51,55,58].
Figure 8.
Forest plots for socialization results. (a) Overall effect [28,29,30,31,35,38,39,43,44,45,47,48,50,55,58,59]. (b) Subgroup analysis: intensity of intervention.
Figure 9.
Forest plots for daily living skills. (a) Overall effect [29,30,45,48,50,55,58]. (b) Results after exclusion of studies with no blinding of outcome assessment [29,30,45,48,50,55].
Figure 10.
Forest plots for motor skills. (a) Overall effect [29,30,43,45,53,54,55,58]. (b) Results after exclusion of studies with no blinding of outcome assessment [29,30,45,53,54,55].
The I2-statistics showed that the heterogeneity among studies was moderate for socialization (Tau2 = 0.07; I2 = 55%, p = 0.004) and statistically insignificant for all the other variables. Non-significant heterogeneity tests for the remaining outcomes possibly occurred due to a low power, since the number of studies included in these analyses was small [25].
The test for subgroup differences indicated that there was no statistically significant subgroup effect for the adaptive behavior composite (p = 0.70), communication (p = 0.54), socialization (p = 0.51), daily living skills (p = 0.56), and motor skills (p = 0.27), suggesting that intervention duration does not modify the effect of early interventions in comparison to control conditions. However, the number of trials and participants that contributed data to the subgroups was unequal, meaning that the analysis may not have been able to detect subgroup differences. Similarly, although the subgroup analysis performed to test whether intervention intensity modified the effect of early intervention in comparison to control conditions (analysis not presented) indicated that there was a statistically significant subgroup effect for socialization, the number of trials and participants that contributed data to the intervention intensity subgroups (4 trials and 191 participants for high-intensity interventions and 12 trials and 754 participants for low-intensity interventions) was unequal, meaning that the analysis was also unlikely to produce useful findings [64].
After the exclusion of studies with a high risk of bias, results remained positive for daily living skills (g = 0.28; 95% CI: 0.04, 0.52; p = 0.02) and motor skills (g = 0.49; 95% CI: 0.28, 0.79; p ≤ 0.00001).
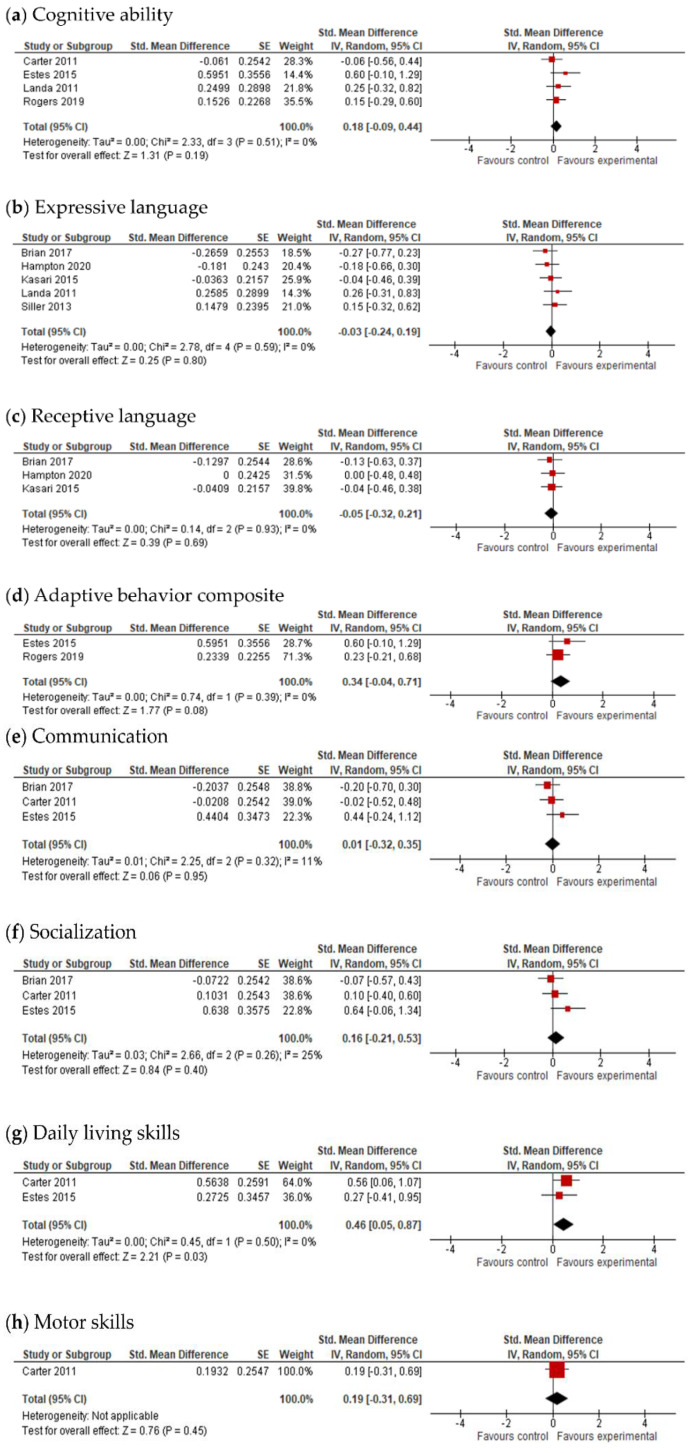
3.4.5. Follow-Up Data
Since early intervention may initiate a cascade of developmental events that is not yet apparent at post-treatment [65], follow-up data recorded sometime after the intervention period is over could provide evidence of the efficacy of early interventions [2]. For this reason, we conducted a separate analysis of follow-up data despite the limited number of studies that reported follow-up data. These results must be interpreted with caution. A detailed depiction of follow-up data is shown in Figure 11. Overall, the analysis of follow-up data did not provide evidence of the sustainability of positive outcomes. A positive result was found for daily living skills (g = 0.46, p = 0.03), and a marginally insignificant result was found for the adaptive behavior composite (g = 0.34, p = −0.04). However, due to the instability of the estimated pooled effects, we recommend a descriptive use of Figure 11 regarding the results of the included studies.
Figure 11.
Forest plots for follow-up data. (a) Cognitive ability [29,33,41,49]. (b) Expressive language [28,37,40,41,52]. (c) Receptive language [28,37,40]. (d) Adaptive behavior composite [33,49]. (e) Communication [28,29,33]. (f) Socialization [28,29,33]. (g) Daily living skills [29,33]. (h) Motor skills [29].
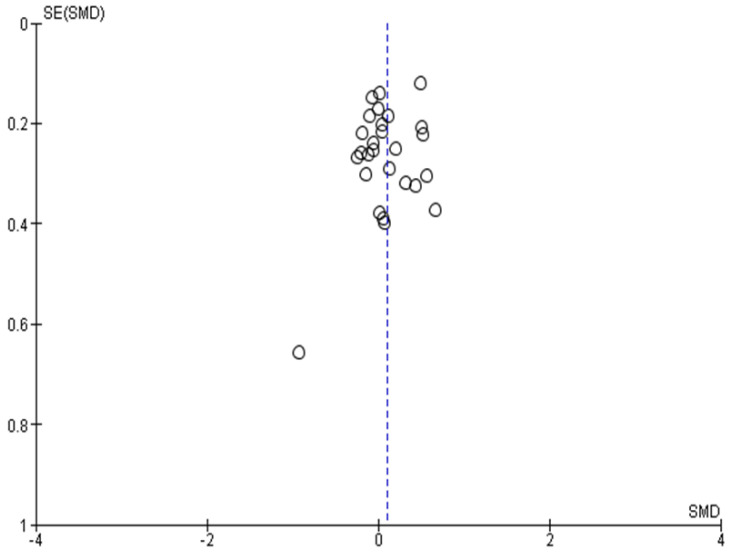
3.5. Publication Bias
A possible way to assess publication bias is via a funnel plot [66]. Regarding publication bias, the results of smaller studies were spread widely, due to lower precision, and asymmetrically around the average estimate compared to the results of larger studies. This asymmetry is suggestive of missing studies. In the absence of publication bias, individual study results are more evenly distributed around a pooled estimate. However, caution should be exercised when interpreting funnel plots, especially when the number of included studies is smaller than 10 (ref. Cochrane handbook) [29,67]. In our case, the funnel plot (Figure 12) indicates no publication bias.
Figure 12.
Funnel plot for publication bias.
4. Discussion
The purpose of this meta-analysis was to assess the efficacy of early interventions in pre-school children with ASDs and investigate how the intervention intensity and duration could mediate the final outcome. Considering the overall effect, when all the included studies were analyzed, early interventions showed significant effects on the cognitive ability, daily living skills, and motor skills of children with autism, while there were no additional benefits for expressive language, receptive language, communication, socialization, and adaptive behavior compared to whatever other interventions were provided. It should be noted here that the results for expressive language were marginally insignificant and worth further investigation. Moreover, when studies with detection bias were excluded from the analyses, positive early intervention effects were detected solely for daily living skills and motor skills. The subgroup analyses on the intervention intensity and duration did not reveal any significant effects. This could be attributed to the fact that the number of studies and participants included in the subgroups were unequal, limiting the power to identify any actual effects of these moderators on the tested outcomes.
Apart from daily living skills and motor skills, our main results are consistent with the meta-analysis of Sandback et al., (2020) [2], where no positive effect of early intervention was detected when the analysis was based only on RCTs with no detection bias. Similarly, Rogers et al., (2021) [8], in their meta-analysis of non-randomized studies about the efficacy of ABA, did not find any significant effects on cognitive ability or adaptive behavior. Additionally, the results of our study are in partial agreement with those of Tachibana et al., (2017) [21], who also only included RCTs and excluded studies with a high risk of bias or studies that did not meet other decisive quality criteria from the final analysis. That study indicated that early intervention did not appear to be efficacious for expressive language, receptive language, and adaptive behavior. Howlin et al., (2009) [68] also failed to find a significant effect of EIBI for expressive language. Nevil et al., (2016) [20] also based their analysis on RCTs and reported that a parent-mediated intervention only resulted in minor improvements in socialization and cognition of children with ASDs. They also reported insignificant improvements in communication language, which incorporated the expressive and receptive language variables. In addition, a recent meta-analysis conducted by Reichow et al., (2018) [18] that examined the efficacy of EIBI concluded that there is low-quality evidence that EIBI can improve IQ, language and adaptive behavior. The authors also underlined the fact that most of the data were derived from studies with many methodological limitations that could have affected the outcomes. Additionally, Speckley et al., (2009) [19] examined the efficacy of applied behavioral intervention and concluded that it did not lead to significant improvements in cognitive ability, language and adaptive behavior compared to TAU. Furthermore, the results of our study are in partial agreement with those of the meta-analysis conducted by Peters-Scheffer et al., (2011) [69], who indicated that EIBI only showed statistically significant effects for IQ, not for expressive and receptive language or for communication, socialization and daily living skills. The described inconsistencies with previous studies could be attributed to the different inclusion criteria applied in each case, which resulted in considerably different study samples. They could also be partly or entirely attributed to biases within the included studies or to the quality of the collected data. In our study, only five of the included studies were assessed as having a low risk of bias in all criteria.
Various studies on the efficacy of early interventions have also demonstrated positive effects in specific areas. A series of meta-analyses that tested high-intensity interventions showed positive outcomes. Yu et al. (2020) [8] concluded that ABA-based interventions can have a positive effect on socialization, communication, and expressive language but not on receptive language, adaptive behavior, daily living skills, IQ, verbal IQ, nonverbal IQ, and cognition. Fuller et al., (2020) [70] conducted a meta-analysis regarding the efficacy of ESDM for children with ASDs and found positive outcomes for cognition and language but not for adaptive behavior. Finally, Warren et al., (2011) [71], Makrygianni et al., (2018) [12], and Reichow et al., (2011) [13] concluded that early intervention is efficient in improving the IQ, language, and adaptive behavior of children with ASDs.
Based on the current evidence, it is not yet clear whether early intervention is effective. There seems to be a trend where older meta-analyses more often reported positive outcomes while more recent ones that included studies of higher quality did not show such strong overall effects, only improvements on isolated variables if on any variable at all. Especially when only RCTs were included, the claimed positive effects could not be established. Considering the extended heterogeneity presented by children with ASDs and the different types of interventions tested, we cannot conclude at present that early intervention is not an effective intervention. There could be many explanations for the absence of effects. We suggest that the main priority in relevant research should be to identify which treatment works best and for whom [72,73]. It is not yet clear which characteristics of a child and family may affect the success of an intervention. Early interventions target children even at infancy and until more than ten years of age. In our review, the age range of the participants was found to be 24–132 months. Although there have been a limited number studies examining the influence of age of therapy initiation on outcomes, research has shown that the sooner an intervention takes place, the higher the impact on the altered brain circuity of children with ASDs, resulting in positive outcomes [72,73]. In addition, the predictive value of cognitive ability at pre-treatment on participants’ performance is not yet clear [21,72]. Of course, different researchers have used different inclusion criteria to attempt to account for the limitations and controversies that the previous ASD research presents. On the other hand, these controversies may highlight the need for more targeted interventions in response to age of treatment onset and cognitive ability at baseline [74,75]. Improvements in daily living skills have been previously associated with increased age, higher developmental quotient and lower symptom severity, while children with lower IQs and more severe symptoms have shown slower daily living skills gains. Caregivers and treatment providers may need to adjust their interventions according each child’s developmental and autism level [76,77,78]. On the other hand, motor skills are an essential component for social communication and social engagement, since they influence how someone responds to social stimuli. Gestures and facial expressions rely on motor function and are crucial for the communication and social engagement of children with ASDs. Motor skill deficits are common for people with ASDs and influence how people with ASDs interact and perceive communication with other people and their environment. For this reason, in recent years, research has been focused on investigating the mechanisms of motor skills and the impact that motor skills interventions can have on ASD-related symptoms [79,80,81].
Lately, more and more studies have highlighted the need for parent involvement and parent training. When parents are actively involved, either as main intervention providers or as co-therapists, intervention is associated with more positive outcomes for children with ASDs. Current evidence suggests that effective skills need to be intensively practiced in everyday life so that an intervention can be effective. Therefore, interventions in which families are trained to work daily on the skills that children with ASDs need to acquire may be the most promising. In order to be applicable and to maximize the effects, such interventions should be individualized, considering the needs of the child, family life, and their interactions [82,83].
Although our subgroup analysis failed to demonstrate any mediating effect of the duration and intensity of interventions on outcomes, it is strongly recommended to compare the early interventions based on their intensity and duration and not just on their manuals. Given the fact that the majority of the control groups in the current meta-analysis were assigned to TAU, it should be noted that, particularly in the case of very low and of high-intensity experimental interventions, the TAU group should be receiving at least as much intervention as the experimental groups so that the comparisons are meaningful. This was not always the case. Additionally, some studies, such as those involving very short-term interventions (e.g., 12 weeks), were not designed to examine whether major changes occur in cognition and language as a result of treatment but rather whether intervention can differentially alter or accelerate development in specific skills in ASDs. However, by including these studies, we wanted to see whether this acceleration could indirectly influence more comprehensive skills such as cognition, language and adaptive behavior as distal variables [2]. Additionally, early intervention may initiate a cascade of developmental events that lead to an altered brain circuity, resulting in better developmental outcomes [65]. For this reason, we performed a meta-analysis of follow-up data. According to our analysis, there is no evidence that experimental groups perform better than control groups after the course of the intervention. However, follow-up data were not reported consistently, so this analysis can be considered unstable due to the limited number of available studies. Another possible explanation for the absence of effects is that standardized measures are not always sensitive to the kinds of change activated by treatment for children with ASDs since they measure molar aspects of behavior and fail to detect the acquisition of specific skills [2].
The current study has certain limitations. One of the main limitations was the high variability of the included studies in the participants’ age and the targeted outcomes, as reported above. The provided interventions also varied regarding their duration, intensity, and structural elements. The combination of outcomes in statistical analysis could have diluted or masked the isolated gains that might have occurred due to the intervention. In children with a complex, multi-system disorder such as an ASDs, even those isolated gains are noteworthy. Furthermore, the current meta-analysis included data from pilot studies, such as those of Divan et al., (2019) [36], Drew et al., (2002) [37], Vernon et al., (2019) [63], and Reitzel et al., (2013) [50], that did not have a large number of participants. Additionally, many participants across all studies received other kind of therapies apart from the examined interventions during the study periods, and this could have exerted some influence on the results.
On the other hand, apart from its limitations, the current meta-analysis has many strengths. The first and most important is that this meta-analysis reports outcomes based only on RCTs, so many study quality parameters, such as the adequate randomization procedure, the existence of a control condition, the comparability of the groups at baseline, and the use of standardized measurements were fulfilled across all studies. Additionally, following the quality assessment of the included studies, those assessed as having a high risk of bias in the measurement of the outcome were excluded from the analysis, so it can be assumed that the outcomes ere accurate and valid. Moreover, using the formula suggested by Borenstein et al., (2021) [29], it was possible to combine all outcomes for the same variable across studies using a valid method to calculate the variances of the multiple outcomes so that each study was appropriately weighted in the final analysis. In this way, we included all the available information derived from different tests and scales for each single variable.
5. Conclusions
In conclusion, the current meta-analysis has demonstrated that although early intervention generally might not lead to positive outcomes for cognitive ability, language, communication and socialization for children with ASDs, these results should be interpreted with caution considering the great variability in participant and intervention characteristics. Perhaps it is not that the intervention itself is ineffective but that researchers should consider (a) the need for participants to attain sufficient intervention dosage, since previous literature has yielded positive outcomes for high-intensity interventions; (b) the need to design or use more sensitive measures than standardized measures; and (c) the need to examine longer-term effects through follow-up studies, since early intervention programs initiate a circuit of developmental events and children who received early intervention may continue to progress well years after the initial intervention. On the other hand, we identified significant positive effects of early intervention on daily living and motor skills, which have implications for everyday life and social communication. We recommend that future research should focus on creating more specific intervention groups using participants with comparable cognitive ability at baseline and a smaller age range in order to explore whether specific subgroups of children with ASDs respond better to early interventions than others. Additionally, we underline the need for future studies that meet the quality research standards in order to draw more valid and accurate conclusions.
Acknowledgments
We are very grateful to Stefanie Schmidt, University of Bern, for their precious help and guidance during the implementation of the study and to Christine Gerhardt at the University of Bern for her invaluable statistical insights.
Appendix A
Table A1.
Follow up data used in the meta-analysis.
| Study | Outcome | Measure | Experimental Group | Control Group | ||||
|---|---|---|---|---|---|---|---|---|
| Mean | SD | N | Mean | SD | N | |||
| Aldred et al. (2004) [27] | Expressive | MCDI words post | 199.4 | 25.606 | 14 | 33.1 | 683 | 14 |
| Receptive | MCDI words post | 222.7 | 40.431 | 14 | 146.8 | 11,426 | 14 | |
| Communication | VABS post | 36.9 | 21.2 | 14 | 28.7 | 16.6 | 14 | |
| Brian et al. (2017) [28] | Expressive | MSEL follow up | 28.53 | 12.13 | 30 | 32 | 13.86 | 32 |
| Receptive | MSEL follow up | 29.07 | 13.17 | 30 | 30.87 | 14.51 | 32 | |
| Communication | VABS follow up | 75.63 | 13.18 | 30 | 78.34 | 13.42 | 32 | |
| Socialization | VABS follow up | 75.83 | 8.27 | 30 | 76.44 | 8.62 | 32 | |
| Cognitive Ability | MSEL | 65.2 | 16.23 | 30 | 69.81 | 21.57 | 32 | |
| Expressive | PLS-4 | 75.4 | 13.78 | 30 | 76.97 | 15.02 | 32 | |
| Receptive | PLS-4 | 70.33 | 17.7 | 30 | 68.91 | 19.21 | 32 | |
| Carter et al. (2011) [29] | Communication | VABS follow up | 76.14 | 13.85 | 32 | 76.43 | 14.05 | 30 |
| Socialization | VABS follow up | 71.42 | 7.07 | 32 | 70.7 | 6.89 | 30 | |
| Daily living skills | VABS follow up | 77.84 | 7.07 | 32 | 72.95 | 10.11 | 30 | |
| Motor skills | VABS follow up | 83.16 | 7.36 | 32 | 81.55 | 9.26 | 30 | |
| Cognitive Ability | MSEL follow up | 63.88 | 18.41 | 32 | 64.88 | 13.94 | 30 | |
| Receptive | MSEL | 15.52 | 6.93 | 32 | 17.48 | 8.33 | 30 | |
| Expressive | MSEL | 16.2 | 7.23 | 32 | 16.68 | 7.88 | 30 | |
| Dawson et al. (2010) [30] | Cognitive Ability | MSEL | 78.6 | 24.2 | 24 | 66.3 | 15.3 | 21 |
| Expressive | MSEL | 36.6 | 13.6 | 24 | 30 | 9.2 | 21 | |
| Receptive | MSEL | 40 | 16.3 | 24 | 31.5 | 10.6 | 21 | |
| Adaptive behaviour composite | VABS | 68.7 | 15.9 | 24 | 59.1 | 8.8 | 21 | |
| Communication | VABS | 82.1 | 21.8 | 24 | 69.4 | 15.8 | 21 | |
| Socialization | VABS | 69.2 | 11.6 | 24 | 63.1 | 9.3 | 21 | |
| Daily living skills | VABS | 64.7 | 12.4 | 24 | 58 | 8.1 | 21 | |
| Motor skills | VABS | 77.4 | 19.8 | 24 | 64.1 | 12.3 | 21 | |
| Divan et al. (2019) [31] | Adaptive behaviour composite | VABS | 65.00 | 12.69 | 19 | 60.53 | 12.98 | 21 |
| Communication | VABS | 60.93 | 17.88 | 19 | 55.90 | 14.15 | 21 | |
| Socialization | VABS | 68.47 | 10.26 | 19 | 63.25 | 11.45 | 21 | |
| Drew et al. (2002) [32] | Cognitive Ability | NVIQ | 77.9 | 14.8 | 12 | 66.1 | 17.1 | 12 |
| Expressive | MCDI words | 96.6 | 118.8 | 12 | 44 | 50.2 | 12 | |
| Expressive | MCDI gestures | 38.6 | 12.5 | 12 | 29.1 | 18.4 | 12 | |
| Receptive | MCDI words | 176.1 | 121.9 | 12 | 100.3 | 80.2 | 12 | |
| Estes et al. (2015) [33] | Cognitive Ability | MSEL follow up | 90.52 | 26.36 | 24 | 79.83 | 23.64 | 21 |
| Adaptive behaviour composite | VABS follow up | 81.41 | 17.27 | 17 | 72.06 | 13.86 | 16 | |
| Communication | VABS follow up | 88.35 | 19.76 | 17 | 79.71 | 18.53 | 17 | |
| Socialization | VABS follow up | 79.24 | 16.03 | 17 | 69.44 | 13.81 | 16 | |
| Daily living skills | VABS follow up | 83.06 | 21.56 | 17 | 77.71 | 16.4 | 17 | |
| Gengoux et al. (2019) [34] | Expressive | CDI 396 | 194.9 | 133.7 | 23 | 84.4 | 93.5 | 20 |
| Expressive | CDI 680 | 256.6 | 200.1 | 23 | 112.9 | 148.1 | 20 | |
| Expressive | VABS | 7.6 | 2.4 | 23 | 6.2 | 1.6 | 20 | |
| Expressive | PLS-5 | 58.7 | 10.2 | 23 | 56.9 | 10.5 | 20 | |
| Expressive | MSEL | 21 | 8.7 | 23 | 17.3 | 6.9 | 20 | |
| Communication | VABS | 63.8 | 14.8 | 23 | 62.5 | 11.6 | 20 | |
| Green et al. (2010) [35] | Expressive | PLS | 20 | 11.2 | 77 | 20 | 11.3 | 75 |
| Expressive | MCDI | 171.9 | 150.7 | 77 | 163.8 | 144.3 | 75 | |
| Receptive | PLS | 21.5 | 13 | 77 | 20.3 | 12.8 | 75 | |
| Receptive | MCDI | 233.7 | 129.6 | 77 | 209 | 131.3 | 75 | |
| Adaptive behaviour composite | VABS | 60.3 | 15.2 | 77 | 62.8 | 14.8 | 75 | |
| Communication | VABS | 6.6 | 3.3 | 77 | 6.7 | 3.2 | 75 | |
| Socialization | VABS | 9.2 | 3 | 77 | 9.8 | 2.9 | 75 | |
| Green et al. (2020) [36] | Expressive | PLS | 57.5 | 14.9 | 92 | 59.3 | 20 | 102 |
| Expressive | MCDI | 42.5 | 18.5 | 49 | 42 | 21.7 | 52 | |
| Receptive | PLS | 57.7 | 13.7 | 92 | 59.5 | 18.9 | 102 | |
| Receptive | MCDI | 43.2 | 20 | 39 | 42.2 | 21.9 | 42 | |
| Hampton et al. (2020) [37] | Expressive | PLS post | 25 | 5 | 34 | 25 | 5 | 34 |
| Expressive | PLS follow-up | 26 | 6 | 34 | 27 | 5 | 34 | |
| Receptive | PLS post | 26 | 7 | 34 | 25 | 6 | 34 | |
| Receptive | PLS follow-up | 28 | 8 | 34 | 28 | 7 | 34 | |
| Hardan et al. (2015) [38] | Expressive | CDI | 172.2 | 123.6 | 25 | 215 | 118.3 | 22 |
| Expressive | CDI | 289.1 | 181.9 | 25 | 239.9 | 187.1 | 22 | |
| Expressive | PLS | 63.9 | 11.6 | 25 | 63 | 13.4 | 22 | |
| Expressive | VABS | 41.7 | 14.7 | 25 | 34 | 18.9 | 22 | |
| Receptive | VABS | 21.5 | 14.7 | 25 | 18.9 | 6.5 | 22 | |
| Communication | VABS | 78.9 | 18.9 | 25 | 72.8 | 16.5 | 22 | |
| Socialization | SRS | 74.9 | 12.4 | 25 | 80.6 | 10.7 | 22 | |
| Kaale et al. (2014) [39] | Expressive | RDSL | 27.9 | 29.48 | 34 | 34.1 | 32.41 | 27 |
| Receptive | RDSL | 32.8 | 29.1 | 34 | 39.4 | 28.5 | 27 | |
| Communication | SCQ parents | 4.1 | 3.28 | 34 | 4.52 | 3.42 | 27 | |
| Communication | SCQ teachers | 4.88 | 3.28 | 34 | 4.12 | 3.22 | 27 | |
| Socialization | SCQ parents | 5.28 | 6.23 | 34 | 4.13 | 5.7 | 27 | |
| Socialization | SCQ teachers | 6.3 | 4.94 | 34 | 5.19 | 3.22 | 27 | |
| Kasari et al. (2015) [40] | Expressive | RDLS post | 18.42 | 8.03 | 43 | 19.83 | 7.84 | 43 |
| Expressive | RDLS follow up | 24.26 | 9.34 | 43 | 24.59 | 8.82 | 43 | |
| Receptive | RDLS post | 20.87 | 11.85 | 43 | 23.17 | 13.02 | 43 | |
| Receptive | RDLS follow up | 32.74 | 15.24 | 43 | 33.38 | 16 | 43 | |
| Landa et al. (2011) [41] | Non-verbal IQ | MSEL post | 36.75 | 14.54 | 24 | 32.24 | 14.07 | 24 |
| Non-verbal IQ | MSEL follow up | 34.44 | 16.67 | 24 | 30.28 | 16.62 | 24 | |
| Expressive | MSEL post | 34.08 | 14.59 | 24 | 31.92 | 13.67 | 24 | |
| Expressive | MSEL follow up | 34.52 | 12.33 | 24 | 31.36 | 12.12 | 24 | |
| Oosterling et al. (2010) [42] | Expressive | MCDI words | 182.3 | 201 | 34 | 157.8 | 206.96 | 31 |
| Expressive | MCDI gestures | 35.8 | 23.9 | 34 | 36.4 | 22.16 | 29 | |
| Receptive | MCDI words | 239.9 | 197.5 | 34 | 216.7 | 187.5 | 31 | |
| Parsons et al. (2019) [43] | Expressive | MSEL | 70.9 | 27.97 | 21 | 67.5 | 22.75 | 27 |
| Receptive | MSEL | 72.1 | 32.84 | 21 | 71.6 | 26.98 | 27 | |
| Fine motor | CSBS | 68.8 | 21.74 | 21 | 70.2 | 19.42 | 27 | |
| Socialization | MSEL | 36.2 | 7.07 | 21 | 31 | 8.4 | 27 | |
| Rahman et al. (2016) [44] | Expressive | MCDI | 6.69 | 2.51 | 29 | 6.97 | 2.11 | 30 |
| Receptive | MCDI | 9.24 | 2.65 | 29 | 9.47 | 2.54 | 30 | |
| Adaptive behaviour composite | VABS | 61.76 | 12.51 | 29 | 63.67 | 10.54 | 30 | |
| Communication | VABS | 60.28 | 13.84 | 29 | 60.73 | 13.84 | 30 | |
| Socialization | VABS | 61.41 | 9.02 | 29 | 61.73 | 8.07 | 30 | |
| Reitzel et al. (2013) [45] | Motor skills | VABS | 72.3 | 7.1 | 6 | 67.7 | 7 | 3 |
| Communication | VABS | 58 | 9.4 | 7 | 67 | 10.4 | 4 | |
| Socialization | VABS | 63.9 | 5.1 | 7 | 64.8 | 3.9 | 4 | |
| Daily living skills | VABS | 66.9 | 13.7 | 7 | 65.3 | 11 | 4 | |
| Adaptive behaviour composite | VABS | 62.7 | 6.8 | 7 | 63.3 | 5.7 | 4 | |
| Rickards et al. (2007) [46] | Cognitive Ability | Bayley/WPPSI-R | 57.2 | 21.9 | 18 | 48.6 | 17.5 | 21 |
| Roberts et al. (2011) [47] | Expressive | RDLS | 8.8 | 8.9 | 28 | 11.1 | 9.9 | 28 |
| Receptive | RDLS | 17.5 | 6.3 | 28 | 22 | 17.8 | 28 | |
| Communication | VABS | 68.4 | 15.6 | 28 | 74.2 | 15.5 | 28 | |
| Socialization | VABS | 66.4 | 7.7 | 28 | 73.1 | 10.8 | 28 | |
| Rogers et al. (2012) [48] | Expressive | MCDI | 42.27 | 61.99 | 49 | 38.87 | 73.71 | 49 |
| Receptive | MCDI | 106.51 | 96.81 | 49 | 125.72 | 106.39 | 49 | |
| Receptive | MCDI phrases | 12.73 | 9.11 | 49 | 14.77 | 8.14 | 49 | |
| Communication | VABS | 72.55 | 12.06 | 49 | 74.29 | 14.55 | 49 | |
| Socialization | VABS | 77.32 | 9.19 | 49 | 78.67 | 10.78 | 49 | |
| Daily living skills | VABS | 82.25 | 13.82 | 49 | 84.04 | 13.5 | 49 | |
| Rogers et al. (2019) [49] | Cognitive Ability | MSEL post | 72 | 18.55 | 51 | 69.4 | 17.77 | 52 |
| Cognitive Ability | MSEL follow up | 83.09 | 26.12 | 44 | 79.14 | 25.58 | 35 | |
| Adaptive behaviour composite | VABS post | 15.39 | 5.22 | 49 | 15.86 | 6.25 | 45 | |
| Adaptive behaviour composite | VABS follow up | 39.76 | 12.07 | 44 | 36.69 | 14.32 | 36 | |
| Scahill et al. (2016) [50] | Communication | VABS | 84.75 | 15.68 | 89 | 85.78 | 14.5 | 91 |
| Socialization | VABS | 76.46 | 13.51 | 89 | 76.63 | 12.28 | 91 | |
| Daily living skills | VABS | 82.39 | 14.61 | 89 | 79.6 | 15.39 | 91 | |
| Schertz et al. (2013) [51] | Expressive | MSEL | 33.27 | 15.79 | 11 | 27.17 | 11.21 | 12 |
| Receptive | MSEL | 28.27 | 11.35 | 11 | 25.33 | 8.52 | 12 | |
| Communication | VABS | 75.9 | 13.51 | 11 | 68.08 | 19.77 | 12 | |
| Siller et al. (2013) [52] | Expressive | MSEL post | 4.02 | 1.34 | 36 | 3.9 | 1.42 | 34 |
| Expressive | MSEL follow up | 4.38 | 1.42 | 36 | 4.17 | 1.42 | 34 | |
| Solomon et al. (2014) [53] | Expressive | MSEL | 52.82 | 28.1 | 52 | 48.33 | 29.08 | 47 |
| Expressive | MCDI gestures | 66.41 | 58.91 | 64 | 81.59 | 85.58 | 64 | |
| Expressive | MCDI sentences | 598.25 | 129.12 | 16 | 590.55 | 123.7 | 22 | |
| Receptive | MSEL | 59.1 | 31.76 | 52 | 53.84 | 29.97 | 47 | |
| Receptive | MCDI words | 285.2 | 123.98 | 64 | 276.2 | 128.51 | 64 | |
| Receptive | MCDI | 23.09 | 6.54 | 64 | 22.27 | 7.32 | 64 | |
| Motor skills | MSEL | 59.94 | 26.36 | 52 | 54.33 | 26.03 | 47 | |
| Strain & Bovey (2011) [54] | Cognitive Ability | MSEL | 68.5 | 7.5 | 177 | 61.4 | 9 | 117 |
| Expressive | MSEL | 38.7 | 6.4 | 177 | 35.9 | 4.4 | 117 | |
| Receptive | MSEL | 49.3 | 7.9 | 177 | 40.7 | 7.7 | 117 | |
| Fine motor skills | MSEL | 43.3 | 5.2 | 177 | 39.8 | 4.9 | 117 | |
| Tonge et al. (2014) [55] | Communication | VABS | 71.71 | 19.83 | 35 | 69.53 | 24.05 | 35 |
| Socialization | VABS | 73.31 | 16.59 | 35 | 67.35 | 16.7 | 35 | |
| Daily living skills | VABS | 68.26 | 16.46 | 35 | 60.09 | 18.59 | 35 | |
| Motor skills | VABS | 76.82 | 20.29 | 35 | 68.25 | 17.13 | 35 | |
| Cognitive Ability | PEP-R | 72.18 | 24.77 | 35 | 67.72 | 28.14 | 35 | |
| Expressive | RDLS | 17.17 | 17.07 | 35 | 18.24 | 20.65 | 35 | |
| Receptive | RDLS | 14.06 | 19.67 | 35 | 19.18 | 22.17 | 35 | |
| Turner-Brown et al. (2019) [56] | Cognitive Ability | MSEL | 67.10 | 23.39 | 32 | 70.33 | 23.16 | 17 |
| Valeri et al. (2020) [57] | Expressive | MCDI | 59.7 | 104.2 | 12 | 43.3 | 52.1 | 8 |
| Receptive | MCDI | 103.3 | 104.2 | 12 | 79.7 | 25.9 | 7 | |
| Vernon et al. (2019) [58] | Cognitive Ability | MSEL | 90.67 | 27.28 | 12 | 68.36 | 21.62 | 11 |
| Expressive | MSEL | 37.83 | 12.31 | 12 | 31.27 | 11.88 | 11 | |
| Receptive | MSEL | 47.17 | 14.79 | 12 | 31.91 | 14.83 | 11 | |
| Expressive | PLS | 87.50 | 15.75 | 12 | 74.27 | 17.39 | 11 | |
| Receptive | PLS | 90.17 | 27.78 | 12 | 73.27 | 20.99 | 11 | |
| Adaptive behaviour composite | VABS | 87.91 | 12.99 | 12 | 73.27 | 8.22 | 11 | |
| Communication | VABS | 88.45 | 16.23 | 12 | 70.91 | 11.59 | 11 | |
| Socialization | VABS | 85.18 | 10.56 | 12 | 74.27 | 10.21 | 11 | |
| Daily living skills | VABS | 92.45 | 10.08 | 12 | 80.45 | 10.62 | 11 | |
| Motor skills | VABS | 92.91 | 18.32 | 12 | 92.91 | 18.33 | 11 | |
| Welterlin et al. (2012) [59] | Cognitive Ability | MSEL | 63.7 | 17.4 | 10 | 58.1 | 25 | 10 |
| Expressive | MSEL | 58 | 21.9 | 10 | 62.4 | 32.6 | 10 | |
| Expressive | SIB | 16.2 | 7.1 | 10 | 14.2 | 7.1 | 10 | |
| Receptive | MSEL | 60.9 | 26.1 | 10 | 58.1 | 30.8 | 10 | |
| Receptive | SIB | 12 | 4.7 | 10 | 10.9 | 4.8 | 10 | |
| Socialization | SIB | 18.4 | 7.3 | 10 | 16 | 5 | 10 | |
VABS indicates Vineland Adaptive Behaviour Scale. MSEL indicates Mullen Scales of Early Learning. PLS-4 indicates Preschool Language scale. SCQ indicates Social Communication Questionnaire. RDLS indicates Reynell Developmental Language Scales. SRS indicates Social Responsiveness scale. WPPSI-R indicates Wechsler Preschool and Primary Scale of Intelligence-Revised. PEP-R indicates psycho-Educational Profile-Revised. SIB indicates Scales of Independent Behaviour scale.
Table A2.
List of combined variables for which there were multiple measurements per study.
| Study | Outcome | Scale | Hedges’ g | SE | Hedges’ g Combined | Variance Combined | Correlation | SE Combined |
|---|---|---|---|---|---|---|---|---|
| Brian et al. (2017) [28] | Expressive | MSEL | −0.2625 | 0.2553 | −0.1850 | 0.0487 | 0.5 | 0.2207 |
| Expressive | PLS-4 | −0.1074 | 0.2543 | |||||
| Receptive | MSEL | −0.1281 | 0.2544 | −0.0261 | 0.0485 | 0.5 | 0.2202 | |
| Receptive | PLS-4 | 0.0758 | 0.2542 | |||||
| Drew et al. (2002) [32] | Expressive | MCDI words | 0.5768 | 0.1736 | 0.5904 | 0.13046 | 0.5 | 0.3612 |
| Expressive | MCDI gestures | 0.604 | 0.1743 | |||||
| Gengoux et al. (2019) [34] | Expressive | CDI 396 | 0.9460 | 0.3223 | 0.5209 | 0.0493 | 0.5 | 0.2222 |
| Expressive | CDI 680 | 0.8077 | 0.3179 | |||||
| Expressive | VABS | 0.6769 | 0.3143 | |||||
| Expressive | PLS-5 | 0.1741 | 0.3063 | |||||
| Expressive | MSEL | 0.4673 | 0.3099 | |||||
| Green et al. (2010) [35] | Expressive | PLS | 0.0000 | 0.1622 | 0.0182 | 0.0197 | 0.5 | 0.1405 |
| Expressive | MCDI | 0.0546 | 0.1623 | |||||
| Receptive | PLS | 0.0925 | 0.1623 | −0.0303 | 0.0255 | 0.5 | 0.1598 | |
| Receptive | MCDI | 0.1884 | 0.1626 | |||||
| Green et al. (2022) [36] | Expressive | PLS | 0.0247 | 0.1991 | −0.0383 | 0.0222 | 0.5 | 0.1491 |
| Expressive | MCDI | 0.0476 | 0.2224 | |||||
| Receptive | PLS | −0.1013 | 0.1438 | −0.0303 | 0.0255 | 0.5 | 0.1598 | |
| Receptive | MCDI | −0.1082 | 0.1439 | |||||
| Hardan et al. (2015) [38] | Expressive | CDI | −0.3480 | 0.2946 | 0.1094 | 0.0348 | 0.5 | 0.1865 |
| Expressive | CDI | 0.2629 | 0.2936 | |||||
| Expressive | PLS | 0.0711 | 0.2924 | |||||
| Expressive | VABS | 0.4518 | 0.2961 | |||||
| Kaale et al. (2014) [39] | Communication | SCQ parents | −0.1257 | 0.2580 | 0.0540 | 0.0501 | 0.5 | 0.2237 |
| Communication | SCQ teachers | 0.2336 | 0.2586 | |||||
| Socialization | SCQ parents | 0.1916 | 0.2584 | 0.2258 | 0.0502 | 0.5 | 0.2230 | |
| Socialization | SCQ teachers | 0.2601 | 0.2588 | |||||
| Oosterling et al. (2010) [42] | Expressive | MCDI words | 0.1202 | 0.2486 | 0.0466 | 0.0471 | 0.5 | 0.2171 |
| Expressive | MCDI gestures | −0.0260 | 0.2528 | |||||
| Rogers et al. (2012) [48] | Receptive | MCDI | −0.1874 | 0.2025 | −0.2108 | 0.0308 | 0.5 | 0.1755 |
| Receptive | MCDI phrases | −0.2343 | 0.2027 | |||||
| Solomon et al. (2014) [53] | Receptive | MSEL | 0.1701 | 0.2016 | 0.1190 | 0.0172 | 0.5 | 0.1311 |
| Receptive | MCDI | 0.1181 | 0.1769 | |||||
| Receptive | MCDI words | 0.0713 | 0.1768 | |||||
| Expressive | MSEL | 0.1559 | 0.2016 | 0.0035 | 0.0289 | 0.5 | 0.17 | |
| Expressive | MCDI gestures | −0.2054 | 0.1772 | |||||
| Expressive | MCDI sentences | 0.0598 | 0.3286 | |||||
| Vernon et al. (2019) [58] | Expressive | MSEL | 0.5418 | 0.4250 | 0.6706 | 0.1383 | 0.5 | 0.3718 |
| Expressive | PLS-5 | 0.7993 | 0.4337 | |||||
| Receptive | MSEL | 1.0305 | 0.4442 | 0.8563 | 0.1431 | 0.5 | 0.3783 | |
| Receptive | PLS-5 | 0.6820 | 0.4294 | |||||
| Welterlin et al. (2012) [59] | Expressive | MSEL | −0.1517 | 0.4479 | 0.0590 | 0.1510 | 0.5 | 0.3886 |
| Expressive | SIB | 0.2698 | 0.4494 | |||||
| Receptive | MSEL | 0.0939 | 0.4475 | 0.1579 | 0.1506 | 0.5 | 0.3881 | |
| Receptive | SIB | 0.2218 | 0.4487 |
VABS indicates Vineland Adaptive Behaviour Scale. MSEL indicates Mullen Scales of Early Learning. PLS-4 indicates Preschool Language scale. SCQ indicates Social Communication Questionnaire. SIB indicates Scales of Independent Behaviour scale.
Author Contributions
Conceptualization, S.D. and H.Z.; methodology, S.D. and H.Z.; software, S.D.; data collection and analysis, S.D. and N.P.; data curation, S.D.; writing—original draft preparation, S.D.; writing—review and editing, N.P. and H.Z., visualization, S.D.; supervision, H.Z. All authors have read and agreed to the published version of the manuscript.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
All data are available in the main text or the extended data. The search protocols and datasets generated and/or analyzed during the current study are available from the corresponding author on reasonable request.
Conflicts of Interest
The authors declare no conflict of interest.
Funding Statement
This research received no external funding.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Maenner M.J., Graves S.J., Peacock G., Honein M.A., Boyle C.A., Dietz P.M. Comparison of 2 Case Definitions for Ascertaining the Prevalence of Autism Spectrum Disorder Among 8-Year-Old Children. Am. J. Epidemiol. 2021;190:2198–2207. doi: 10.1093/aje/kwab106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Sandbank M., Bottema-Beutel K., Crowley S., Cassidy M., Dunham K., Feldman J.I., Crank J., Albarran S.A., Raj S., Mahbub P., et al. Project AIM: Autism intervention meta-analysis for studies of young children. Psychol. Bull. 2020;146:1–29. doi: 10.1037/bul0000215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Dawson G. Early behavioral intervention, brain plasticity, and the prevention of autism spectrum disorder. Dev. Psychopathol. 2008;20:775–803. doi: 10.1017/S0954579408000370. [DOI] [PubMed] [Google Scholar]
- 4.Landa R.J. Efficacy of early interventions for infants and young children with, and at risk for, autism spectrum disorders. Int. Rev. Psychiatry. 2018;30:25–39. doi: 10.1080/09540261.2018.1432574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Schreibman L., Dawson G., Stahmer A.C., Landa R., Rogers S.J., McGee G.G., Kasari C., Ingersoll B., Kaiser A.P., Bruinsma Y., et al. Naturalistic developmental behavioral interventions: Empirically validated treatments for autism spectrum disorder. J. Autism Dev. Disord. 2015;45:2411–2428. doi: 10.1007/s10803-015-2407-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Boyd B.A., Hume K., McBee M.T., Alessandri M., Gutierrez A., Johnson L., Sperry L., Odom S.L. Comparative efficacy of LEAP, TEACCH and non-model-specific special education programs for preschoolers with autism spectrum disorders. J. Autism Dev. Disord. 2014;44:366–380. doi: 10.1007/s10803-013-1877-9. [DOI] [PubMed] [Google Scholar]
- 7.Afif I.Y., Manik A.R., Munthe K., Maula M.I., Ammarullah M.I., Jamari J., Winarni T.I. Physiological effect of deep pressure in reducing anxiety of children with ASD during traveling: A public transportation setting. Bioengineering. 2022;9:157. doi: 10.3390/bioengineering9040157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Yu Q., Li E., Li L., Liang W. Efficacy of interventions based on applied behavior analysis for autism spectrum disorder: A meta-analysis. Psychiatry Investig. 2010;17:432. doi: 10.30773/pi.2019.0229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Rodgers M., Simmonds M., Marshall D., Hodgson R., Stewart L.A., Rai D., Wright K., Ben-Itzchak E., Eikeseth S., Eldevik S., et al. Intensive behavioural interventions based on applied behaviour analysis for young children with autism: An international collaborative individual participant data meta-analysis. Autism. 2021;25:1137–1153. doi: 10.1177/1362361320985680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Liu Q., Hsieh W.Y., Chen G. A systematic review and meta-analysis of parent-mediated intervention for children and adolescents with autism spectrum disorder in mainland China, Hong Kong, and Taiwan. Autism. 2020;24:1960–1979. doi: 10.1177/1362361320943380. [DOI] [PubMed] [Google Scholar]
- 11.Parsons D., Cordier R., Vaz S., Lee H.C. Parent-mediated intervention training delivered remotely for children with autism spectrum disorder living outside of urban areas: Systematic review. J. Med. Internet Res. 2017;19:e6651. doi: 10.2196/jmir.6651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Makrygianni M.K., Gena A., Katoudi S., Galanis P. The effectiveness of applied behavior analytic interventions for children with Autism Spectrum Disorder: A meta-analytic study. Res. Autism Spectr. Disord. 2018;51:18–31. doi: 10.1016/j.rasd.2018.03.006. [DOI] [Google Scholar]
- 13.Reichow B. Evidence-Based Practices and Treatments for Children with Autism. Springer; Boston, MA, USA: 2011. Development, procedures, and application of the evaluative method for determining evidence-based practices in autism; pp. 25–39. [Google Scholar]
- 14.Reichow B., Wolery M. Comprehensive synthesis of early intensive behavioral interventions for young children with autism based on the UCLA young autism project model. J. Autism Dev. Disord. 2009;39:23–41. doi: 10.1007/s10803-008-0596-0. [DOI] [PubMed] [Google Scholar]
- 15.Eldevik S., Hastings R.P., Hughes J.C., Jahr E., Eikeseth S., Cross S. Meta-analysis of early intensive behavioral intervention for children with autism. J. Clin. Child Adolesc. Psychol. 2009;38:439–450. doi: 10.1080/15374410902851739. [DOI] [PubMed] [Google Scholar]
- 16.Makrygianni M.K., Reed P. A meta-analytic review of the effectiveness of behavioural early intervention programs for children with autistic spectrum disorders. Res. Autism Spectr. Disord. 2010;4:577–593. doi: 10.1016/j.rasd.2010.01.014. [DOI] [Google Scholar]
- 17.Virués-Ortega J. Applied behavior analytic intervention for autism in early childhood: Meta-analysis, meta-regression and dose–response meta-analysis of multiple outcomes. Clin. Psychol. Rev. 2010;30:387–399. doi: 10.1016/j.cpr.2010.01.008. [DOI] [PubMed] [Google Scholar]
- 18.Reichow B., Barton E.E., Boyd B.A., Hume K. Early intensive behavioral intervention (EIBI) for young children with autism spectrum disorders (ASD) Cochrane Database Syst. Rev. 2012;9:CD009260. doi: 10.1002/14651858.CD009260.pub2. [DOI] [PubMed] [Google Scholar]
- 19.Spreckley M., Boyd R. Efficacy of applied behavioral intervention in preschool children with autism for improving cognitive, language, and adaptive behavior: A systematic review and meta-analysis. J. Pediatrics. 2009;154:338–344. doi: 10.1016/j.jpeds.2008.09.012. [DOI] [PubMed] [Google Scholar]
- 20.Nevill R.E., Lecavalier L., Stratis E.A. Meta-analysis of parent-mediated interventions for young children with autism spectrum disorder. Autism. 2018;22:84–98. doi: 10.1177/1362361316677838. [DOI] [PubMed] [Google Scholar]
- 21.Tachibana Y., Miyazaki C., Ota E., Mori R., Hwang Y., Kobayashi E., Terasaka A., Tang J., Kamio Y. A systematic review and meta-analysis of comprehensive interventions for pre-school children with autism spectrum disorder (ASD) PLoS ONE. 2017;12:e0186502. doi: 10.1371/journal.pone.0186502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.French L., Kennedy E.M. Annual Research Review: Early intervention for infants and young children with, or at-risk of, autism spectrum disorder: A systematic review. J. Child Psychol. Psychiatry. 2018;59:444–456. doi: 10.1111/jcpp.12828. [DOI] [PubMed] [Google Scholar]
- 23.Maw S.S., Haga C. Effectiveness of cognitive, developmental, and behavioural interventions for Autism Spectrum Disorder in preschool-aged children: A systematic review and meta-analysis. Heliyon. 2018;4:e00763. doi: 10.1016/j.heliyon.2018.e00763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Poon K.K., Watson L.R., Baranek G.T., Poe M.D. To what extent do joint attention, imitation, and object play behaviors in infancy predict later communication and intellectual functioning in ASD? J. Autism Dev. Disord. 2012;42:1064–1074. doi: 10.1007/s10803-011-1349-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Higgins J.P., Green S., editors. Cochrane Handbook for Systematic Reviews of Interventions. The Cochrane Collaboration; London, UK: 2011. Version 5.1. 0 [Updated March 2011] [Google Scholar]
- 26.Moher D., Shamseer L., Clarke M., Ghersi D., Liberati A., Petticrew M., Shekelle P., Stewart L.A. Preferred reporting items for systematic review and meta-analysis protocols (PRISMA-P) 2015 statement. Syst. Rev. 2015;4:1–9. doi: 10.1186/2046-4053-4-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Aldred C., Green J., Adams C. A new social communication intervention for children with autism: Pilot randomised controlled treatment study suggesting effectiveness. J. Child Psychol. Psychiatry. 2004;45:1420–1430. doi: 10.1111/j.1469-7610.2004.00338.x. [DOI] [PubMed] [Google Scholar]
- 28.Brian J.A., Smith I.M., Zwaigenbaum L., Bryson S.E. Cross-site randomized control trial of the Social ABCs caregiver-mediated intervention for toddlers with autism spectrum disorder. Autism Res. 2017;10:1700–1711. doi: 10.1002/aur.1818. [DOI] [PubMed] [Google Scholar]
- 29.Carter A.S., Messinger D.S., Stone W.L., Celimli S., Nahmias A.S., Yoder P. A randomized controlled trial of Hanen’s ‘More Than Words’ in toddlers with early autism symptoms. J. Child Psychol. Psychiatry. 2011;52:741–752. doi: 10.1111/j.1469-7610.2011.02395.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Dawson G., Rogers S., Munson J., Smith M., Winter J., Greenson J., Donaldson A., Varley J. Randomized, controlled trial of an intervention for toddlers with autism: The Early Start Denver Model. Pediatrics. 2010;125:e17–e23. doi: 10.1542/peds.2009-0958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Divan G., Vajaratkar V., Cardozo P., Huzurbazar S., Verma M., Howarth E., Emsley R., Taylor C., Patel V., Green J. The feasibility and effectiveness of PASS Plus, a lay health worker delivered comprehensive intervention for autism spectrum disorders: Pilot RCT in a rural low and middle income country setting. Autism Res. 2019;12:328–339. doi: 10.1002/aur.1978. [DOI] [PubMed] [Google Scholar]
- 32.Drew A., Baird G., Baron-Cohen S., Cox A., Slonims V., Wheelwright S., Swettenham J., Berry B., Charman T. A pilot randomised control trial of a parent training intervention for pre-school children with autism. Eur. Child Adolesc. Psychiatry. 2002;11:266–272. doi: 10.1007/s00787-002-0299-6. [DOI] [PubMed] [Google Scholar]
- 33.Estes A., Munson J., Rogers S.J., Greenson J., Winter J., Dawson G. Long-term outcomes of early intervention in 6-year-old children with autism spectrum disorder. J. Am. Acad. Child Adolesc. Psychiatry. 2015;54:580–587. doi: 10.1016/j.jaac.2015.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Gengoux G.W., Abrams D.A., Schuck R., Millan M.E., Libove R., Ardel C.M., Phillips J.M., Fox M., Frazier T.W., Hardan A.Y. A pivotal response treatment package for children with autism spectrum disorder: An RCT. Pediatrics. 2019;144:e20190178. doi: 10.1542/peds.2019-0178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Green J., Charman T., McConachie H., Aldred C., Slonims V., Howlin P., Le Couteur A., Leadbitter K., Hudry K., Byford S., et al. Parent-mediated communication-focused treatment in children with autism (PACT): A randomised controlled trial. Lancet. 2010;375:2152–2160. doi: 10.1016/S0140-6736(10)60587-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Green J., Leadbitter K., Ellis C., Taylor L., Moore H.L., Carruthers S., James K., Taylor C., Balabanovska M., Langhorne S., et al. Combined social communication therapy at home and in education for young autistic children in England (PACT-G): A parallel, single-blind, randomised controlled trial. Lancet Psychiatry. 2022;9:307–320. doi: 10.1016/S2215-0366(22)00029-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hampton L.H., Kaiser A.P., Fuller E.A. Multi-component communication intervention for children with autism: A randomized controlled trial. Autism. 2020;24:2104–2116. doi: 10.1177/1362361320934558. [DOI] [PubMed] [Google Scholar]
- 38.Hardan A.Y., Gengoux G.W., Berquist K.L., Libove R.A., Ardel C.M., Phillips J., Frazier T.W., Minjarez M.B. A randomized controlled trial of Pivotal Response Treatment Group for parents of children with autism. J. Child Psychol. Psychiatry. 2015;56:884–892. doi: 10.1111/jcpp.12354. [DOI] [PubMed] [Google Scholar]
- 39.Kaale A., Fagerland M.W., Martinsen E.W., Smith L. Preschool-based social communication treatment for children with autism: 12-month follow-up of a randomized trial. J. Am. Acad. Child Adolesc. Psychiatry. 2014;53:188–198. doi: 10.1016/j.jaac.2013.09.019. [DOI] [PubMed] [Google Scholar]
- 40.Kasari C., Gulsrud A., Paparella T., Hellemann G., Berry K. Randomized comparative efficacy study of parent-mediated interventions for toddlers with autism. J. Consult. Clin. Psychol. 2015;83:554. doi: 10.1037/a0039080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Landa R.J., Holman K.C., O’Neill A.H., Stuart E.A. Intervention targeting development of socially synchronous engagement in toddlers with autism spectrum disorder: A randomized controlled trial. J. Child Psychol. Psychiatry. 2011;52:13–21. doi: 10.1111/j.1469-7610.2010.02288.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Oosterling I., Visser J., Swinkels S., Rommelse N., Donders R., Woudenberg T., Roos S., Van Der Gaag R.J., Buitelaar J.K. Randomized controlled trial of the focus parent training for toddlers with autism: 1-year outcome. J. Autism Dev. Disord. 2010;40:1447–1458. doi: 10.1007/s10803-010-1004-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Parsons D., Cordier R., Lee H., Falkmer T., Vaz S. A randomised controlled trial of an information communication technology delivered intervention for children with autism spectrum disorder living in regional Australia. J. Autism Dev. Disord. 2019;49:569–581. doi: 10.1007/s10803-018-3734-3. [DOI] [PubMed] [Google Scholar]
- 44.Rahman A., Divan G., Hamdani S.U., Vajaratkar V., Taylor C., Leadbitter K., Aldred C., Minhas A., Cardozo P., Emsley R., et al. Effectiveness of the parent-mediated intervention for children with autism spectrum disorder in south Asia in India and Pakistan (PASS): A randomised controlled trial. Lancet Psychiatry. 2016;3:128–136. doi: 10.1016/S2215-0366(15)00388-0. [DOI] [PubMed] [Google Scholar]
- 45.Reitzel J., Summers J., Lorv B., Szatmari P., Zwaigenbaum L., Georgiades S., Duku E. Pilot randomized controlled trial of a Functional Behavior Skills Training program for young children with autism spectrum disorder who have significant early learning skill impairments and their families. Res. Autism Spectr. Disord. 2013;7:1418–1432. doi: 10.1016/j.rasd.2013.07.025. [DOI] [Google Scholar]
- 46.Rickards A.L., Walstab J.E., Wright-Rossi R.A., Simpson J., Reddihough D.S. One-year follow-up of the outcome of a randomized controlled trial of a home-based intervention programme for children with autism and developmental delay and their families. Child Care Health Dev. 2009;35:593–602. doi: 10.1111/j.1365-2214.2009.00953.x. [DOI] [PubMed] [Google Scholar]
- 47.Roberts J., Williams K., Carter M., Evans D., Parmenter T., Silove N., Clark T., Warren A. A randomised controlled trial of two early intervention programs for young children with autism: Centre-based with parent program and home-based. Res. Autism Spectr. Disord. 2011;5:1553–1566. doi: 10.1016/j.rasd.2011.03.001. [DOI] [Google Scholar]
- 48.Rogers S.J., Estes A., Lord C., Vismara L., Winter J., Fitzpatrick A., Guo M., Dawson G. Effects of a brief Early Start Denver Model (ESDM)–based parent intervention on toddlers at risk for autism spectrum disorders: A randomized controlled trial. J. Am. Acad. Child Adolesc. Psychiatry. 2012;51:1052–1065. doi: 10.1016/j.jaac.2012.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Rogers S.J., Estes A., Lord C., Munson J., Rocha M., Winter J., Greenson J., Colombi C., Dawson G., Vismara L.A., et al. A multisite randomized controlled two-phase trial of the Early Start Denver Model compared to treatment as usual. J. Am. Acad. Child Adolesc. Psychiatry. 2019;58:853–865. doi: 10.1016/j.jaac.2019.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Scahill L., Bearss K., Lecavalier L., Smith T., Swiezy N., Aman M.G., Sukhodolsky D.G., McCracken C., Minshawi N., Turner K., et al. Effect of parent training on adaptive behavior in children with autism spectrum disorder and disruptive behavior: Results of a randomized trial. J. Am. Acad. Child Adolesc. Psychiatry. 2016;55:602–609. doi: 10.1016/j.jaac.2016.05.001. [DOI] [PubMed] [Google Scholar]
- 51.Schertz H.H., Odom S.L., Baggett K.M., Sideris J.H. Effects of joint attention mediated learning for toddlers with autism spectrum disorders: An initial randomized controlled study. Early Child. Res. Q. 2013;28:249–258. doi: 10.1016/j.ecresq.2012.06.006. [DOI] [Google Scholar]
- 52.Siller M., Hutman T., Sigman M. A parent-mediated intervention to increase responsive parental behaviors and child communication in children with ASD: A randomized clinical trial. J. Autism Dev. Disord. 2013;43:540–555. doi: 10.1007/s10803-012-1584-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Solomon R., Van Egeren L.A., Mahoney G., Huber M.S.Q., Zimmerman P. PLAY Project Home Consultation intervention program for young children with autism spectrum disorders: A randomized controlled trial. J. Dev. Behav. Pediatrics. 2014;35:475. doi: 10.1097/DBP.0000000000000096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Strain P.S., Bovey E.H. Randomized, controlled trial of the LEAP model of early intervention for young children with autism spectrum disorders. Top. Early Child. Spec. Educ. 2011;31:133–154. doi: 10.1177/0271121411408740. [DOI] [Google Scholar]
- 55.Tonge B., Brereton A., Kiomall M., Mackinnon A., Rinehart N.J. A randomised group comparison controlled trial of ‘preschoolers with autism’: A parent education and skills training intervention for young children with autistic disorder. Autism. 2014;18:166–177. doi: 10.1177/1362361312458186. [DOI] [PubMed] [Google Scholar]
- 56.Turner-Brown L., Hume K., Boyd B.A., Kainz K. Preliminary efficacy of family implemented TEACCH for toddlers: Effects on parents and their toddlers with autism spectrum disorder. J. Autism Dev. Disord. 2019;49:2685–2698. doi: 10.1007/s10803-016-2812-7. [DOI] [PubMed] [Google Scholar]
- 57.Valeri G., Casula L., Menghini D., Amendola F.A., Napoli E., Pasqualetti P., Vicari S. Cooperative parent-mediated therapy for Italian preschool children with autism spectrum disorder: A randomized controlled trial. Eur. Child Adolesc. Psychiatry. 2020;29:935–946. doi: 10.1007/s00787-019-01395-5. [DOI] [PubMed] [Google Scholar]
- 58.Vernon T.W., Holden A.N., Barrett A.C., Bradshaw J., Ko J.A., McGarry E.S., Horowitz E.J., Tagavi D.M., German T.C. A pilot randomized clinical trial of an enhanced pivotal response treatment approach for young children with autism: The PRISM model. J. Autism Dev. Disord. 2019;49:2358–2373. doi: 10.1007/s10803-019-03909-1. [DOI] [PubMed] [Google Scholar]
- 59.Welterlin A., Turner-Brown L.M., Harris S., Mesibov G., Delmolino L. The home TEACCHing program for toddlers with autism. J. Autism Dev. Disord. 2012;42:1827–1835. doi: 10.1007/s10803-011-1419-2. [DOI] [PubMed] [Google Scholar]
- 60.Sterne J.A., Savović J., Page M.J., Elbers R.G., Blencowe N.S., Boutron I., Cates C.J., Cheng H.Y., Corbett M.S., Eldridge S.M., et al. RoB 2: A revised tool for assessing risk of bias in randomised trials. BMJ. 2019;366:l4898. doi: 10.1136/bmj.l4898. [DOI] [PubMed] [Google Scholar]
- 61.Lakens D. Calculating and reporting effect sizes to facilitate cumulative science: A practical primer for t-tests and ANOVAs. Front. Psychol. 2013;4:863. doi: 10.3389/fpsyg.2013.00863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Borenstein M., Hedges L.V., Higgins J.P., Rothstein H.R. Introduction to Meta-Analysis. John Wiley & Sons; Hoboken, NJ, USA: 2021. [Google Scholar]
- 63.Higgins J.P., Thompson S.G., Deeks J.J., Altman D.G. Measuring inconsistency in meta-analyses. BMJ. 2003;327:557–560. doi: 10.1136/bmj.327.7414.557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Richardson M., Garner P., Donegan S. Interpretation of subgroup analyses in systematic reviews: A tutorial. Clin. Epidemiol. Glob. Health. 2019;7:192–198. doi: 10.1016/j.cegh.2018.05.005. [DOI] [Google Scholar]
- 65.Sullivan K., Stone W.L., Dawson G. Potential neural mechanisms underlying the effectiveness of early intervention for children with autism spectrum disorder. Res. Dev. Disabil. 2014;35:2921–2932. doi: 10.1016/j.ridd.2014.07.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Higgins J.P., Thomas J., Chandler J., Cumpston M., Li T., Page M.J., Welch V.A., editors. Cochrane Handbook for Systematic Reviews of Interventions. John Wiley & Sons; Hoboken, NJ, USA: 2019. [Google Scholar]
- 67.Austin Z., editor. Encyclopedia of Pharmacy Practice and Clinical Pharmacy: Pharmacy Practice Research Methods/Section Editor Zaheer-Ud Din Babar. Pharmacy Practice/Section Editors Zubin Austin, Zaheer-Ud-Din Babar. Elsevier; Amsterdam, The Netherlands: 2019. [Google Scholar]
- 68.Howlin P., Magiati I., Charman T. Systematic review of early intensive behavioral interventions for children with autism. Am. J. Intellect. Dev. Disabil. 2009;114:23–41. doi: 10.1352/2009.114:23-41. [DOI] [PubMed] [Google Scholar]
- 69.Peters-Scheffer N., Didden R., Korzilius H., Sturmey P. A meta-analytic study on the effectiveness of comprehensive ABA-based early intervention programs for children with autism spectrum disorders. Res. Autism Spectr. Disord. 2011;5:60–69. doi: 10.1016/j.rasd.2010.03.011. [DOI] [Google Scholar]
- 70.Fuller E.A., Oliver K., Vejnoska S.F., Rogers S.J. The effects of the Early Start Denver Model for children with autism spectrum disorder: A meta-analysis. Brain Sci. 2020;10:368. doi: 10.3390/brainsci10060368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Warren Z., McPheeters M.L., Sathe N., Foss-Feig J.H., Glasser A., Veenstra-VanderWeele J. A systematic review of early intensive intervention for autism spectrum disorders. Pediatrics. 2011;127:e1303–e1311. doi: 10.1542/peds.2011-0426. [DOI] [PubMed] [Google Scholar]
- 72.Alonso-Esteban Y., Alcantud-Marín F. Screening, Diagnosis and Early Intervention in Autism Spectrum Disorders. Children. 2022;9:153. doi: 10.3390/children9020153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Hadders-Algra M. Early diagnostics and early intervention in neurodevelopmental disorders—age-dependent challenges and opportunities. J. Clin. Med. 2021;10:861. doi: 10.3390/jcm10040861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Smith T., Iadarola S. Evidence base update for autism spectrum disorder. J. Clin. Child Adolesc. Psychol. 2015;44:897–922. doi: 10.1080/15374416.2015.1077448. [DOI] [PubMed] [Google Scholar]
- 75.Vivanti G., Prior M., Williams K., Dissanayake C. Predictors of outcomes in autism early intervention: Why don’t we know more? Front. Pediatrics. 2014;2:58. doi: 10.3389/fped.2014.00058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Di Rezze B., Duku E., Szatmari P., Volden J., Georgiades S., Zwaigenbaum L., Smith I.M., Vaillancourt T., Bennett T.A., Elsabbagh M., et al. Examining trajectories of daily living skills over the preschool years for children with autism spectrum disorder. J. Autism Dev. Disord. 2019;49:4390–4399. doi: 10.1007/s10803-019-04150-6. [DOI] [PubMed] [Google Scholar]
- 77.Bal V.H., Kim S.H., Cheong D., Lord C. Daily living skills in individuals with autism spectrum disorder from 2 to 21 years of age. Autism. 2015;19:774–784. doi: 10.1177/1362361315575840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Green S.A., Carter A.S. Predictors and course of daily living skills development in toddlers with autism spectrum disorders. J. Autism Dev. Disord. 2014;44:256–263. doi: 10.1007/s10803-011-1275-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Fulceri F., Grossi E., Contaldo A., Narzisi A., Apicella F., Parrini I., Tancredi R., Calderoni S., Muratori F. Motor skills as moderators of core symptoms in autism spectrum disorders: Preliminary data from an exploratory analysis with artificial neural networks. Front. Psychol. 2019;9:2683. doi: 10.3389/fpsyg.2018.02683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Zampella C.J., Wang L.A., Haley M., Hutchinson A.G., De Marchena A. Motor skill differences in autism spectrum disorder: A clinically focused review. Curr. Psychiatry Rep. 2021;23:64. doi: 10.1007/s11920-021-01280-6. [DOI] [PubMed] [Google Scholar]
- 81.Elliott L.K., Weiss J.A., Lloyd M. Beyond the motor domain: Exploring the secondary effects of a fundamental motor skill intervention for children with autism spectrum disorder. Adapt. Phys. Act. Q. 2021;38:195–214. doi: 10.1123/apaq.2020-0024. [DOI] [PubMed] [Google Scholar]
- 82.Magán-Maganto M., Bejarano-Martín Á., Fernández-Alvarez C., Narzisi A., García-Primo P., Kawa R., Posada M., Canal-Bedia R. Early detection and intervention of ASD: A European overview. Brain Sci. 2017;7:159. doi: 10.3390/brainsci7120159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Rojas-Torres L.P., Alonso-Esteban Y., Alcantud-Marín F. Early intervention with parents of children with Autism Spectrum Disorders: A review of programs. Children. 2020;7:294. doi: 10.3390/children7120294. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All data are available in the main text or the extended data. The search protocols and datasets generated and/or analyzed during the current study are available from the corresponding author on reasonable request.