Abstract
The DNA binding protein Ssh10b, a member of the Sac10b family, has been purified from the hyperthermophilic archaeon Sulfolobus shibatae. Ssh10b constitutes about 4% of the cellular protein. Electrophoretic mobility shift assays showed that Ssh10b first bound a double-stranded DNA fragment with an estimated binding size of ∼∼12 bp, forming distinct shifts, until the DNA was coated with the protein. Binding of more Ssh10b resulted in the formation of smears of lower mobilities. The migration pattern of the smearing Ssh10b-DNA complexes was affected by temperature, whereas that of complexes associated with the distinct shifts was not. Interestingly, Ssh10b was capable of constraining negative DNA supercoils in a temperature-dependent fashion. While the ability of the protein to constrain supercoils was weak at 25°C, it was enhanced substantially at 45°C or higher temperatures (up to 80°C). Taken together, our data suggest that archaeal proteins of the Sac10b family may affect the topology of chromosomal DNA in thermophilic archaea at their growth temperatures.
Thermoacidophilic archaea of the genus Sulfolobus synthesize mixtures of small, abundant, and basic DNA binding proteins, which are grouped into three classes according to their molecular masses (7, 8, and 10 kDa) (8, 11). These proteins were first characterized by Reinhardt and colleagues in the 1980s (4, 5, 8, 11, 12). While the 7-kDa proteins, the most abundant of these proteins, have since been extensively studied (18), the 8- and 10-kDa proteins have received little attention. Recently, Forterre et al. (10) identified a Sulfolobus shibatae gene which encodes a protein homologous to Sac10b, one of the two 10-kDa proteins from Sulfolobus acidocaldarius. Interestingly, this gene, denoted ssh10b, has at least one homologue in each of all archaeal species whose genomes have been completely sequenced but not in species of either bacteria or eukarya. These homologues form the Sac10b family. Sac10b is the second most abundant protein in S. acidocaldarius (11). Previous studies suggest that Sac10b exists as dimers in solution and has a greater affinity for DNA than the 7- and 8-kDa proteins (8, 11). It has also been shown by electron microscopy that Sac10b binds cooperatively to DNA and, depending on protein concentration, forms different protein-DNA complexes (8, 15). Interestingly, the protein can envelop two double-stranded DNA helices into a helical protein structure at relatively low protein concentrations. Sac10b, however, does not induce DNA supercoiling or compact DNA. Given their ubiquity in archaea, members of the Sac10b family may play an important role in the organization and accessibility of genetic information in these organisms.
In this paper, we report the isolation of a small abundant DNA binding protein from the hyperthermophilic archaeon S. shibatae. We show that the protein is encoded by ssh10b and, therefore, is a member of the Sac10b protein family. The Ssh10b protein affects DNA supercoiling in a temperature-dependent fashion. While the protein has a weak ability to constrain DNA in negative supercoils at 25°C, it becomes highly capable of constraining negative supercoils at elevated temperatures (≥45°C). These results may help us to understand the structural basis of the adaptation of the chromosome in thermophilic archaea to high growth temperatures.
MATERIALS AND METHODS
Growth of S. shibatae.
S. shibatae ATCC 51178 was purchased from the American Type Culture Collection. A large-scale culture of S. shibatae was grown to an optical density at 600 nm of 1.5 at 75°C in Brock's medium (1) supplemented with 0.2% tryptone and 0.1% yeast extract in a 5-liter Bioflo fermentor (New Brunswick Scientific Co., Inc., Edison, N.J.) with occasional additions of H2SO4 to keep the pH of the culture below 4.5.
Enzymes and chemicals.
Pfu DNA ligase was from Stratagene. T4 DNA ligase, T4 polynucleotide kinase, and a nick translation kit were from Promega. SP Sepharose, Polybuffer exchanger 94, and Polybuffer 96 were from Pharmacia. Dithio-bis[succinimidyl propionate] (DSP), 1-ethyl-3-(3-dimethylaminopropyl)carbodiimide (EDC), and N-hydroxylsulfosuccinimide were from Pierce. Chloroquine phosphate was from Sigma.
Purification of Ssh10b.
S. shibatae cells were harvested from the fermentor culture (3,500 × g, 4°C, 20 min) and resuspended in 5 volumes of 20 mM Tris-HCl (pH 7.6)–0.1 mM EDTA–1 mM dithiothreitol (DTT). The cells were sonicated on ice at 40 W for 15 to 30 min. The lysate was centrifuged at 150,000 × g for 2.5 h at 4°C. Ammonium sulfate was added to the supernatant to 70%. The precipitate was dissolved in two original volumes of buffer A (30 mM potassium phosphate [pH 6.6], 0.1 mM EDTA, 0.1 mM DTT). The sample was applied to an SP Sepharose column (5 ml) which had been equilibrated in buffer A. Proteins were eluted with a 50-ml KCl gradient (0 to 0.75 M) in buffer A. Fractions containing Ssh10b were determined as described previously (15), pooled, and concentrated by ultrafiltration through a PM-10 membrane in an Amicon ultrafiltration unit. The concentrated sample was dialyzed against buffer B (20 mM ethanolamine-HCl [pH 10.2], 0.1 mM EDTA, 0.1 mM DTT) and applied to a Polybuffer exchanger 94 column (20 ml) which had been equilibrated in the same buffer. The column was washed with buffer B. Ssh10b eluted from the column in the flowthrough and was concentrated by ultrafiltration and stored at −70°C in storage buffer (20 mM Tris-HCl [pH 7.6], 1 mM DTT, 1 mM EDTA, 10% glycerol). All the column chromatography steps were carried out at 4°C. Ssh10b concentrations were determined by the Lowry method (14) using bovine serum albumin (BSA) as the standard.
Chemical cross-linking.
Ssh10b (8 μg) alone or in complex with pUC18 DNA (2 μg) was cross-linked for 1 h at 25 or 45°C in 20 mM HEPES–KOH (pH 7.6)–50 mM KCl with either 3 mM DSP or 20 mM EDC–10 mM N-hydroxylsulfosuccinimide in a final volume of 20 μl. Cross-linking reactions were stopped by the addition of the sample loading buffer for sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) (mecaptoethanol was omitted from the buffer for reactions with DSP). Samples were subjected to SDS-PAGE (15% polyacrylamide).
Electrophoretic mobility shift assays (EMSA).
An arbitrarily chosen 60-bp double-stranded DNA fragment was used in this study. The sequence of one strand of the fragment was as follows: GATCCCCCAA TGCTTCGTTT CGTATCACAC ACCCCAAAGC CTTCTGCTTT GAATGCTGCC. The fragment was labeled at the 5′ ends with [γ-32P]ATP. The labeled fragment (0.5 to 1 ng) was incubated with Ssh10b (0 to 20 μg) for 10 min at either 20 or 45°C in 20 mM Tris-HCl (pH 7.6)–10 mM MgCl2–1 mM DTT–100 μg of BSA per ml in a total volume of 20 μl. Protein-DNA complexes were electrophoresed in 0.1× TBE (17) in an 8% polyacrylamide gel which had been equilibrated to a constant current and maintained at either 20 or 45°C using a circulating water bath connected to the electrophoresis apparatus. Following electrophoresis, the gel was dried and exposed to X-ray film.
Nick closure assays.
The single-nick plasmid pUC18 was prepared as described previously (6). The nicked plasmid (2 μg) was incubated with Ssh10b at various mass ratios for 5 min at 25, 45, 60, or 80°C and ligated as described previously (16). T4 DNA ligase (3 Weiss units) was added to mixtures incubated at 25 and 45°C, whereas Pfu DNA ligase (4 Weiss units) was added to reaction mixtures at 60 and 80°C. An aliquot of each sample (0.5 μg of DNA) was subjected to two-dimensional electrophoresis in 1.2% agarose in 0.5× TPE (17). Gels were run at 2.75 V/cm in the first dimension, equilibrated for 2 h in 0.5× TPE containing chloroquine (3 μg/ml), and run at 1 V/cm in the second dimension in the presence of chloroquine (3 μg/ml). Topoisomers were detected by in-gel hybridization using as a probe 32P-labeled pUC18 prepared by nick translation (21).
CD measurements.
Circular dichroism (CD) spectra were obtained at various temperatures on a JASCO J-715 spectropolarimeter. Ssh10b was dialyzed in 10 mM potassium phosphate buffer, pH 7.0, and used at 100 μg/ml.
RESULTS AND DISCUSSION
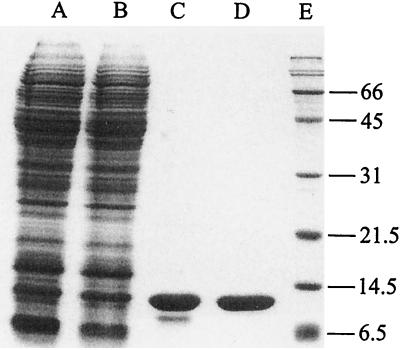
S. shibatae synthesizes copious amounts of small DNA binding proteins. In a previous study, we showed that the postribosomal fraction of the S. shibatae lysate contains two major DNA binding activities (16). One of them comprises members of the 7-kDa DNA binding protein family of Sulfolobus and has been purified and characterized previously (16). In the present study, we purified the protein responsible for the other DNA binding activity using a simple purification protocol including two column steps (Fig. 1). Typically, approximately 1 mg of the pure protein was obtained from 4 g (wet weight) of cells. The purified protein has a molecular mass of approximately 10.5 kDa, as determined by SDS-PAGE.
FIG. 1.
Purification of Ssh10b. Samples taken at various stages during the purification of Ssh10b were subjected to SDS-PAGE (15% polyacrylamide), and the gel was stained with Coomassie brilliant blue. Lane A, cell lysate; lane B, ammonium sulfate fraction; lane C, SP Sepharose peak fractions; lane D, chromatofocusing peak fractions; lane E, molecular weight standards with molecular weights (in thousands) indicated.
In order to identify the gene encoding the protein, we sought to determine the partial amino acid sequence of the purified protein. Automated amino acid sequence analysis showed that the protein is blocked at the N terminus. To obtain an internal peptide for sequencing, we treated the purified protein with trypsin, chymotrypsin, and thermolysin. However, none of these treatments resulted in significant cleavage. We then denatured the protein at 100°C in the presence of 0.1% SDS, digested the denatured protein in gel with the Staphylococcus V8 protease, and resolved the resulting proteolytic fragments by SDS-PAGE as described by Cleveland et al. (7). Two proteolytic fragments were obtained. N-terminal microsequencing of the smaller fragment produced the following sequence: IRVGSQVVTSQDGRQ. A search of sequences in GenBank revealed that an S. shibatae gene (GenBank accession number X98420) codes for a protein containing the above amino acid sequence. This protein was recently identified as a homologue of Sac10b and denoted Ssh10b (10). The Ssh10b protein has 97 amino acid residues and a molecular weight of 10,586, which agrees with the estimated size of the purified protein. The protein appears highly basic, with a calculated isoelectric point of ∼11, which is also consistent with the observation that the purified protein eluted in the flowthrough from the chromatofocusing column equilibrated to pH 10.2. In addition, the recombinant Ssh10b protein overproduced in Escherichia coli was specifically recognized by an antiserum raised against the purified protein (data not shown). Therefore, we conclude that the purified protein is Ssh10b.
Forterre et al. have presented an alignment of homologues of Sac10b from six archaeal species (10). A BLAST search of the currently available sequence databases using Ssh10b as a query revealed four additional Sac10b homologues from three other thermophilic archaea: Sulfolobus solfataricus (Sso10b; http://www.niji.imb.nrc.ca), Pyrococcus abyssi (Pab10b; GenBank accession number AJ248284), and Aeropyrum pernix (Ape10b-1 and Ape10b-2; GenBank accession number AP000062). The 10 known Sac10b homologues share about 35 to 66% identity and 55 to 82% similarity at the amino acid sequence level. S. shibatae also contains a distant paralogue of ssh10b which overlaps the 3′ region of the reverse gyrase topR gene (10). This gene (ssh10b2) encodes a putative protein which shares 32% identity with Ssh10b. It may be speculated that the divergence of the ssh10b2 gene results from its coevolution with the topR gene, functional diversification, or nonfunctional gene decaying. The product of the ssh10b2 gene was not found in our search for major DNA binding activities in S. shibatae (16). It has also been noticed that the ssh10b gene is located immediately upstream of the 5′ end of the topR gene (10). The location of ssh10b is intriguing and has been suggested to indicate a functional relationship between Ssh10b and reverse gyrase. This gene arrangement, however, is not conserved among the sequenced archaeal genomes except for those of the two Sulfolobus species.
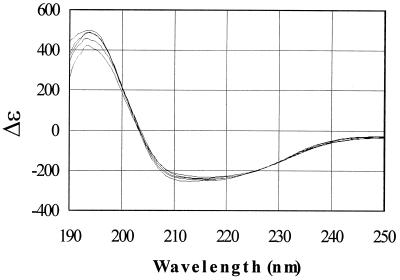
The CD spectra of the Ssh10b protein in 10 mM potassium phosphate buffer (pH 7.0) were obtained at temperatures ranging from 25 to 90°C (Fig. 2). The CD spectrum of Ssh10b remained largely unaffected by the change in temperature from 25 to 80°C and was slightly altered toward 90°C, suggesting that the protein is highly thermostable. The Ssh10b protein appears to have an ordered structure dominated by α-helices. Based on the method of Yang et al. (22), Ssh10b is 51.4% α-helix at 25°C and 55.2% α-helix at 80°C. The CD spectra of the complexes of Ssh10b with salmon testis DNA were approximately the sum of those of the protein and DNA alone at wavelengths above 200 nm and at temperatures between 25 and 90°C (data not shown).
FIG. 2.
CD spectra of Ssh10b at various temperatures. The CD spectra of Ssh10b in 0.02 M KH2PO4 (pH 7.0) were obtained at 25, 45, 60, 80, and 90°C. A curve with a smaller peak at 194 nm corresponds to a CD spectrum obtained at a higher temperature.
To estimate the cellular content of Ssh10b, we determined the protein concentrations of an S. shibatae lysate and a pure Ssh10b sample by the trichloroacetic acid-Lowry assay (18) using BSA as the standard, and subjected dilutions of the lysate and pure protein to electrophoresis on a tricine-SDS-polyacrylamide gel (20). After electrophoresis, the gel was stained with Coomassie brilliant blue R-250. The Ssh10b band in each pure protein lane and a well-resolved band having the mobility of Ssh10b in each lysate lane were scanned with a Hoefer densitometer, and the staining intensities of the bands were analyzed with SigmaGel software (Jandel). We calculate that the Ssh10b protein constitutes ∼4% of the total cellular protein. Thus, Ssh10b is nearly as abundant as Ssh7 in the cell (16).
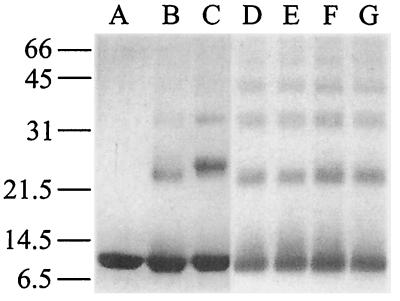
Intermolecular contacts between Ssh10b molecules were examined in chemical cross-linking experiments using either DSP, a 1.2-nm cross-linker reactive toward amino groups, or EDC, a zero-length cross-linker capable of forming isopeptide bonds between carboxyl and amino groups in protein (Fig. 3). We found that Ssh10b was readily cross-linked with DSP into dimers, trimers, and larger oligomers either at 25 or 45°C, and the protein appeared to be cross-linked equally well in solution and in complex with DNA. When EDC was used, Ssh10b was cross-linked more efficiently in a DNA-bound form than in solution. Moreover, the cross-linked products (e.g., dimers) formed in the presence and absence of DNA migrated with different mobilities. Presumably, the EDC-cross-linked contacts between Ssh10b molecules free in solution and those bound to DNA were different. As a control, neither BSA nor Ssh7 was cross-linked under our assay conditions. These results suggest that Ssh10b readily forms oligomers but does not exist predominantly in any specific oligomeric form. Our data differ from the previous report of Grote et al. (11) which states that the similar Sac10b protein appeared to exist as dimers in solution as determined by gel filtration. This discrepancy remains to be understood. Since the concentration of Ssh10b in our cross-linking reactions (0.4 mg/ml) is substantially lower than that in the cell (presumably >10 mg/ml based on the cellular content of the protein), formation of higher-order Ssh10b complexes is probably favored under the physiological conditions.
FIG. 3.
Chemical cross-linking of Ssh10b. Ssh10b was cross-linked alone or in complex with pUC18 DNA with either EDC (lanes B and C) or DSP (lanes D to G). After 1 h at 25 or 45°C, reactions were terminated and analyzed by SDS-PAGE. Lane A, control; lane B, Ssh10b alone at 25°C; lane C, Ssh10b bound to DNA at 25°C; lane D, Ssh10b alone at 25°C; lane E, Ssh10b bound to DNA at 25°C; lane F, Ssh10b alone at 45°C; lane G, Ssh10b bound to DNA at 45°C. Molecular weight standards (in thousands) are noted at the left.
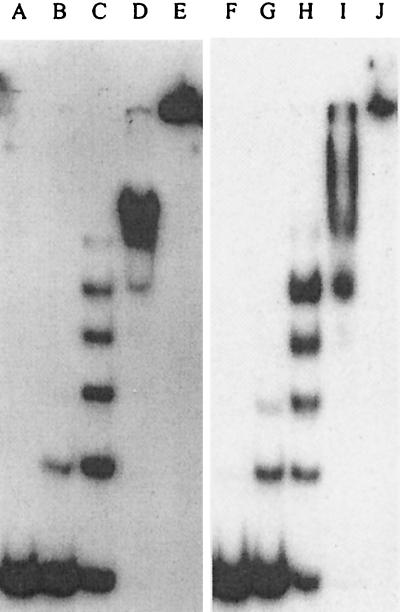
Interaction of Ssh10b with DNA was studied using an EMSA. We found that, as a 32P-labeled 60-bp double-stranded DNA fragment was titrated with Ssh10b, five well-resolved bands were formed at either 25 or 45°C (Fig. 4). Based on their mobilities, Ssh10b-DNA complexes corresponding to these shifts were formed by successive binding of Ssh10b molecules to the 60-mer. Whether the protein bound DNA as monomers, dimers, or larger oligomers remains to be determined. The number of resolvable shifts formed by Ssh10b on the 60-mer suggests that the protein has a binding size of approximately 12 bp. In addition, judging from the amount of Ssh10b required to retard 50% of the input DNA, the protein appeared to bind the DNA duplex with similar affinities (apparent Kd of ∼2 × 10−6 M, assuming that Ssh10b exists as monomers) at the two temperatures. At higher protein concentrations, Ssh10b-DNA complexes became unresolvable and, eventually, could no longer enter the gel. We noticed that the migration patterns of the Ssh10b-DNA complexes larger than that associated with the fifth shift were reproducibly different at the two temperatures. It appeared that the gel retardation pattern of Ssh10b was biphasic, with a low-binding-density phase and a high-binding-density one. In the low-binding-density phase, Ssh10b probably bound DNA in a regularly spaced fashion with a binding size of ∼12 bp. In the high-binding-density phase, Ssh10b-DNA complexes presumably had a different structure. Our data suggest that the migration patterns of Ssh10b–60-mer complexes at 25 and 45°C were similar in the low-binding-density phase but detectably different in the high-binding-density phase. Lurz et al. (15) have shown by electron microscopy that, upon binding to double-stranded DNA, Sac10b initially encloses two duplex DNA helices into a helical protein structure. At higher protein/DNA ratios (>5, wt/wt), the two DNA helices are separated and each duplex is coated with Sac10b. Because of its small size, the DNA fragment used in this study could not be folded by Ssh10b to form a protein-DNA complex containing two strands of duplex DNA. To determine if Ssh10b is capable of binding simultaneously to two DNA fragments, we included an unlabeled 108-bp duplex DNA fragment in the standard assay. No new shifts were observed, at least not in the low-binding-density phase (data not shown), suggesting that Ssh10b binds to one double-stranded DNA helix under our experimental conditions. The structural details of the Ssh10b-DNA complexes formed in the high-binding-density phase remain to be understood.
FIG. 4.
Binding of Ssh10b to a double-stranded DNA fragment. A 32P-labeled 60-bp double-stranded DNA fragment was titrated with Ssh10b. After incubation for 10 min at either 25°C (lanes A to E) or 45°C (lanes F to J), mixtures were loaded onto a polyacrylamide gel that had been equilibrated to a constant current and warmed to 25 or 45°C. Electrophoresis was carried out in 0.1× TBE at either 25 or 45°C. Ssh10b concentrations were 0 (lanes A and F), 1.2 μM (lanes B and G), 2.4 μM (lanes C and H), 4.8 μM (lanes D and I), and 9.6 μM (lanes E and J).
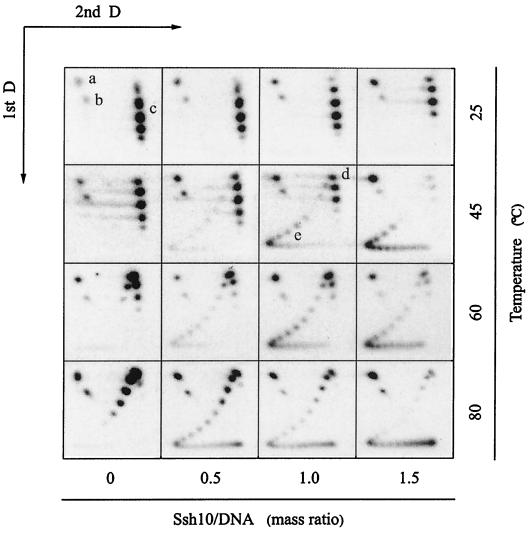
Most chromosomal DNA binding proteins, such as eukaryal histones, bacterial HU, archaeal histones, and 7-kDa proteins, are capable of constraining DNA in supercoils, a property consistent with the proposed roles of these proteins in chromosomal organization (9, 13, 16, 19). In this study, we wished to learn how the Ssh10b protein might affect the topology of DNA. We employed an assay in which a single-nick plasmid was ligated either with or without bound Ssh10b, and the linking change of the plasmid as a result of Ssh10b binding was examined (Fig. 5). T4 and Pfu DNA ligases were used so that the assay could be performed at temperatures ranging from 25 to 80°C. In our control experiments, identical results were obtained when the single-nick plasmid was ligated in the presence or absence of Ssh10b using either ligase at temperatures where both enzymes were active. When the nick plasmid was ligated at 25°C in the absence of Ssh10b, the resulting closed circular molecule contained about two positive supercoils. Addition of Ssh10b to the reaction mixture had a weak, but detectable, effect on the linking number of the ligated plasmid. An increase in the protein/DNA ratio was accompanied by a linear decrease in the average linking number of the plasmid. This relationship was found to exist in a range of Ssh10b/DNA mass ratios from 0 to at least 6 (data not shown). When the assay was carried out at 45°C or higher temperatures, however, the ability of Ssh10b to introduce negative supercoils into the plasmid increased drastically. As a control, the linking number of the plasmid ligated in the absence of Ssh10b decreased linearly with an increase in temperature, as expected from the known effect of temperature on the helix rotation angle of DNA (2). The plasmid ligated at 80°C had about four more negative supercoils than that ligated at 25°C. The weak effect of Ssh10b binding on DNA supercoiling at 25°C is consistent with the electron microscopic observation that formation of a complex between the Sac10b protein and a covalently closed circular DNA did not introduce supercoils into regions of DNA unbound by the protein (15). Interestingly, nick closure products obtained at ≥45°C appeared to be distributed in two clusters: one with no detectable linking deficit and another with a large negative linking deficit on average. The relaxed cluster had an average linking number close to that of the plasmid ligated at the same temperature in the absence of bound protein. The average linking changes of the negatively supercoiled clusters were estimated to be approximately −11 to approximately −12 at 45 and 60°C and slightly more at 80°C, compared to those of the plasmid topoisomers in the controls at the same temperatures. A linking change of −12 corresponds to a superhelical density of −0.04 for the plasmid. The relative abundance of the two clusters appeared to depend on the Ssh10b/DNA ratio. As the ratio increased, more DNA was converted from the relaxed form into the negatively supercoiled form, but the average linking changes of the two clusters were not influenced as dramatically by the Ssh10b/DNA ratio. To interpret these data, we suggest that the change in the ability of Ssh10b to introduce negative supercoils into the plasmid at ≥45°C occurs only when the number of bound Ssh10b molecules per plasmid circle exceeds a critical point. So, at a given Ssh10b/DNA ratio, only those of the plasmid molecules that were bound by Ssh10b to densities greater than the critical point formed the highly negatively supercoiled cluster. As the protein/DNA ratio increased, a larger fraction of nicked plasmid molecules would be bound by more Ssh10b than the critical amount and, therefore, become highly negatively supercoiled when ligated at the elevated temperatures. The critical point, if present, has yet to be determined. A possibility exists, however, that the Ssh10b/DNA ratio at which binding of the protein to DNA switches from the low-binding-density phase to the high-binding-density phase represents the critical point since the electrophoretic behavior of Ssh10b-DNA complexes formed at greater ratios is affected by temperature, as revealed in our EMSA.
FIG. 5.
Nick closure analysis of the effect of Ssh10b on DNA supercoiling. Ssh10b and the single-nick pUC18 plasmid were mixed at various protein/DNA mass ratios: 0, 0.5, 1.0, and 1.5. Following incubation at an assay temperature (25, 45, 60, or 80°C), T4 DNA ligase (for reactions at 25 and 45°C) or Pfu DNA ligase (for reactions at 60 and 80°C) was added. Incubation was continued for 20 min. Reactions were terminated by the addition of SDS and EDTA. An aliquot of each reaction mixture was subjected to two-dimensional electrophoresis in 1.2% agarose. The gel was processed for in-gel hybridization, dried, and exposed to X-ray film. a, nicked pUC18; b, linear pUC18; c, positively supercoiled pUV18 topoisomers; d, relaxed pUC18; e, negatively supercoiled pUC18 topoisomers. 1st D, first dimension; 2nd D, second dimension.
The omnipresence of the 10-kDa proteins in thermophilic archaea is a strong indication of the physiological importance of this protein family to these organisms. However, the in vivo role of these proteins remains to be elucidated. Previous studies suggest that the Sac10b protein does not induce DNA supercoiling and is not involved in DNA packaging (8, 15). However, the conclusions may require reevaluation since the influence of experimental conditions (e.g., temperature) was not carefully examined in these studies. In the present study, we have shown that the ability of Ssh10b to affect DNA topology is influenced by temperature in a fashion that has not been reported for any other DNA binding protein. This highly abundant protein is capable of significantly affecting DNA supercoiling at the growth temperature of S. shibatae, suggesting that the 10-kDa proteins may function in chromosomal organization and accessibility. It is conceivable that the Ssh10b protein may affect DNA supercoiling by wrapping the DNA around a protein bead or core and/or altering the number of base pairs per helical turn of the DNA. If binding of Ssh10b results in wrapping of DNA, the protein may be involved in DNA compaction. Since Sac10b was shown to lack the ability to compact DNA, presumably, at room temperature (15), it is of interest to investigate if the 10-kDa proteins are capable of compacting DNA at the growth temperatures of the organisms. Equally plausible is the possibility that, upon binding, Ssh10b changes the helical periodicity of DNA in such a way that the protein-bound DNA exists in an underwound form. The underwound DNA would presumably permit access by various DNA-dependent cellular processes in a context of general linking excess (3).
ACKNOWLEDGMENT
This work was supported by grant 39770006 from the National Science Foundation of China to L.H.
REFERENCES
- 1.Brock T D, Brock K M, Belly R T, Weiss R L. Sulfolobus: a new genus of sulfur-oxidizing bacteria living at low pH and high temperature. Arch Mikrobiol. 1972;84:54–68. doi: 10.1007/BF00408082. [DOI] [PubMed] [Google Scholar]
- 2.Charbonnier F, Erauso G, Barbeyron T, Prieur D, Forterre P. Evidence that a plasmid from a hyperthermophilic archaebacterium is relaxed at physiological temperatures. J Bacteriol. 1992;174:6103–6108. doi: 10.1128/jb.174.19.6103-6108.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Charbonnier F, Forterre P. Comparison of plasmid DNA topology among mesophilic and thermophilic eubacteria and archaebacteria. J Bacteriol. 1994;176:1251–1259. doi: 10.1128/jb.176.5.1251-1259.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Choli T, Henning P, Wittmann-Liebold B, Reinhardt R. Isolation, characterization, and microsequence analysis of a small basic methylated DNA binding protein from the archaebacterium Sulfolobus solfataricus. Biochim Biophys Acta. 1988;950:193–203. doi: 10.1016/0167-4781(88)90011-5. [DOI] [PubMed] [Google Scholar]
- 5.Choli T, Wittmann-Liebold B, Reinhardt R. Microsequence analysis of DNA-binding proteins 7a, 7b, and 7e from the archaebacterium Sulfolobus acidocaldarius. J Biol Chem. 1988;263:7087–7093. [PubMed] [Google Scholar]
- 6.Clark D J, Felsenfeld G. Formation of nucleosomes on positively supercoiled DNA. EMBO J. 1991;10:387–395. doi: 10.1002/j.1460-2075.1991.tb07960.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cleveland D W, Fischer S G, Kirschner M W, Laemmli U K. Peptide mapping by limited proteolysis in sodium dodecyl sulfate and analysis by gel electrophoresis. J Biol Chem. 1977;252:1102–1106. [PubMed] [Google Scholar]
- 8.Dick J, Reinhardt R. The structure of DNA-binding proteins from eu- and archaebacteria. In: Gualerzi C O, Pon C L, editors. Bacterial chromatin. New York, N.Y: Springer-Verlag; 1986. pp. 185–218. [Google Scholar]
- 9.Drlica K, Rouviere-Yaniv J. Histonelike proteins of bacteria. Microbiol Rev. 1987;51:301–319. doi: 10.1128/mr.51.3.301-319.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Forterre P, Confalonieri F, Knapp S. Identification of the gene encoding archaeal-specific DNA-binding proteins of the Sac10b family. Mol Microbiol. 1999;32:669–670. doi: 10.1046/j.1365-2958.1999.01366.x. [DOI] [PubMed] [Google Scholar]
- 11.Grote M, Dijk J, Reinhardt R. Ribosomal and DNA binding proteins of the thermoacidophilic archaebacterium Sulfolobus acidocaldarius. Biochim Biophys Acta. 1986;873:405–413. [Google Scholar]
- 12.Kimura M, Kimura J, Davie P, Reinhardt R, Dijk J. The amino acid sequence of a small DNA binding protein from the archaebacterium Sulfolobus solfataricus. FEBS Lett. 1984;176:176–178. doi: 10.1016/0014-5793(84)80935-7. [DOI] [PubMed] [Google Scholar]
- 13.Lopez-Garcia, Knapp P S, Ladenstein R, Forterre P. In vitro DNA binding of the archaeal protein Sso7d induces negative supercoiling at temperatures typical for thermophilic growth. Nucleic Acids Res. 1998;26:2322–2328. doi: 10.1093/nar/26.10.2322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lowry O H, Rosebrogh N J, Farr A L, Randall R J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951;193:265–275. [PubMed] [Google Scholar]
- 15.Lurz R, Grote M, Dijk J, Reinhardt R, Dobrinski B. Electron microscopic study of DNA complexes with proteins from archaebacterium Sulfolobus acidocaldarius. EMBO J. 1986;5:3715–3721. doi: 10.1002/j.1460-2075.1986.tb04705.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mai V Q, Chen X, Hong R, Huang L. Small abundant DNA binding proteins from the thermophilic archaeon Sulfolobus shibatae constrains negative DNA supercoils. J Bacteriol. 1998;180:2560–2563. doi: 10.1128/jb.180.9.2560-2563.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Maniatis T, Fritsch E F, Sambrook J. Molecular cloning: a laboratory manual. Cold Spring Harbor, N.Y: Cold Spring Harbor Laboratory; 1982. [Google Scholar]
- 18.Peterson G L. A simplification of the protein assay method of Lowry et al. which is more generally applicable. Anal Biochem. 1977;83:346–356. doi: 10.1016/0003-2697(77)90043-4. [DOI] [PubMed] [Google Scholar]
- 19.Sandman K, Pereira S L, Reeve J N. Diversity of prokaryotic chromosomal proteins and the origin of the nucleosome. Cell Mol Life Sci. 1998;54:1350–1364. doi: 10.1007/s000180050259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Schagger H, Von Jagow G. Tricine-sodium dodecyl sulfate-polyacrylamide gel electrophoresis for the separation of proteins in the range from 1 to 100 kDa. Anal Biochem. 1987;166:368–379. doi: 10.1016/0003-2697(87)90587-2. [DOI] [PubMed] [Google Scholar]
- 21.Wu H-Y, Shyy S, Wang J C, Liu L F. Transcription generates positively and negatively supercoiled domains in the template. Cell. 1988;53:433–440. doi: 10.1016/0092-8674(88)90163-8. [DOI] [PubMed] [Google Scholar]
- 22.Yang J T, Wu C S, Martinez H M. Calculation of protein conformation from circular dichroism. Methods Enzymol. 1986;130:208–269. doi: 10.1016/0076-6879(86)30013-2. [DOI] [PubMed] [Google Scholar]