Abstract
Salvia is a potentially valuable aromatic herb that has been used since ancient times. The present work studied the chemical profile of three Salvia species essential oils (EO): S. officinalis, S. virgata and S. sclarea, as well as assessing their antioxidant and enzyme inhibitory activities. A total of 144 compounds were detected by GC-MS analysis, representing 91.1, 84.7 and 78.1% in S. officinalis, S. virgata and S. sclarea EOs, respectively. The major constituents were cis-thujone, 2,4-hexadienal and 9-octadecenoic acid, respectively. The principal component analysis (PCA) score plot revealed significant discrimination between the three species. The antioxidant activity of the EOs was evaluated using in vitro assays. Only S. virgata EO showed antioxidant activity in the 2,2-diphenyl-1-picryl-hydrazyl (DPPH) assay (26.6 ± 1.60 mg Trolox equivalent (TE)/g oil). Moreover, this oil exhibited the highest antioxidant activity in 2,2-azino bis (3-ethylbenzothiazoline-6-sulphonic acid) (ABTS), cupric-reducing antioxidant capacity (CUPRAC) and ferric-reducing power (FRAP) assays in comparison with the other two EOs (190.1 ± 2.04 vs. 275.2 ± 8.50 and 155.9 ± 1.33 mg TE/g oil, respectively). However, S. virgata oil did not show any effect in the chelating ability assay, while in the PBD assay, S. officinalis had the best antioxidant activity (26.4 ± 0.16 mmol TE/g oil). Enzyme inhibitory effect of the EOs was assessed against acetylcholinesterase (AChE) and butyrylcholinesterase (BChE), tyrosinase, α-glucosidase and α-amylase. AChE enzyme was more sensitive to S. officinalis EO (4.2 ± 0.01 mg galantamine equivalent (GALAE)/g oil), rather than S. virgata EO, which was ineffective. However, S. virgata had the highest BChE effect (12.1 ± 0.16 mg GALAE/g oil). All studied oils showed good tyrosinase inhibitory activity, ranging between 66.1 ± 0.61 and 128.4 ± 4.35 mg kojic acid equivalent (KAE)/g oil). Moreover, the EOs did not exhibit any glucosidase inhibition and were weak or inefficient on amylase enzyme. Partial least squares regression (PLS-R) models showed that there is an excellent correlation between the antioxidant activity and the volatile profile when being compared to that of enzyme inhibitory activity. Thus, the studied Salvia essential oils are interesting candidates that could be used in drug discovery for the management of Alzheimer’s and hyperpigmentation conditions.
Keywords: antioxidants, chemical profile, chemometric analysis, enzyme inhibition, GC-MS, Salvia
1. Introduction
Salvia, a popular aromatic plant known as sage, is an evergreen perennial subshrub native to the Mediterranean region and cultivated in several parts of the world [1]. Genus Salvia L. is dominant in family Lamiaceae and comprises around 900 species [2]. The word Salvia in Latin means “healthy” or “to heal”, which indicates the plethora of notable uses due to the variety of biologically active metabolites present in this plant.
Sage was popular in Egyptian, Greek and Roman medicine [3]. Ancient Egyptians used the leaf to enhance fertility, while the Greeks used it to treat cough, enhance memory, and heal ulcers, sores and wounds. The plant is widespread in many cultures due to its culinary, medical and psychological effects. It is usually used as herbal tea, oil, flavor in cosmetics, perfumery and pharmaceutical products. Traditionally, it has been used in treating malaria, microbial infections, as a home disinfectant, mood elevator, to enhance cognitive performance and in managing some gastrointestinal disorders such as dyspepsia, spasms and flatulence [4,5].
Numerous studies have reported the essential oil yield and composition of different Salvia species. The variation of yield and composition is attributed to several factors, mainly environmental and agronomic conditions [6]. The essential oil has shown cytotoxic [7], antimutagenic [8], antimicrobial [9], hepatoprotective [10] and neuroprotective effects in addition to the treatment of some neurodegenerative disorders such as Alzheimer’s disease [11].
S. officinalis is the most popular species within the Salvia genus. It has traditionally been used to improve cognitive function and skin care [12]. Similarly, S. virgata has also been used in treating some skin diseases and for wound healing [13]. On the other hand, S. sclarea has been used as herbal tea as a tranquillizer and to improve circulation. Its oil has been used as an antimicrobial and food preservative [14].
The present study aimed to investigate the chemical profile of the essential oils of three Salvia species growing in Uzbekistan, S. officinalis L. (local Uzbek name is Dorivor marmarak), S. virgata Jacq. (Zig’irak marmarak) and S. sclarea L. (Mavrak). Principal component analysis (PCA) and hierarchical cluster analysis (HCA) were employed to differentiate between the three species based on the chemical profile of their essential oils. Additionally, the antioxidant activity of the oils was assessed using in vitro assays, as well as the enzyme-inhibitory activities against five enzymes that are crucial in certain diseases such as diabetes, Alzheimer’s and hyperpigmentation.
2. Results and Discussion
2.1. GC-MS Analysis of the Essential Oils of Salvia Species
GC-MS analysis of the oils could detect 144 compounds in the three oils, representing 91.1, 84.7and 78.1% of S. officinalis, S. virgata and S. sclarea EOs, respectively (Table 1) supplementary materials Figure S1 in the Supplementary Material. In S. officinalis oil, the major compounds were cis-thujone (18.6%), camphor (12.2%), 1,8-cineole (8.9%), α-humulene (6.1%) and n-butyl octadecenoate (5.6%). S. virgata EO was characterized by 2,4-hexadienal (9.4%), limonene (6.2%), γ-terpinene (5.2%) and p-cymene (4.5%), while in S. sclarea, 9-octadecenoic acid was the main constituent (6.9%), followed by n-butyl octadecenoate (5.7%) and linalyl acetate (4.7%). Two phenylpropanoids, eugenol and methyl eugenol, were detected with varying percentages in S. officinalis and S. sclarea oils.
Table 1.
Chemical profile of the aerial parts of S. officinalis, S. virgata and S. sclarea essential oils (n = 3 ± SD).
| No | KI† | Compound | Relative Abundance % | |||
|---|---|---|---|---|---|---|
| Cal. | Rep. | S. officinalis | S. virgata | S. sclarea | ||
| 1 | 1067 | 1067 | Camphene | 1.9 | 2.6 | - |
| 2 | 1130 | 1133 | β-Thujene | 0.9 | - | - |
| 3 | 1134 | 1137 | β-Pinene | 0.4 | - | - |
| 4 | 1148 | 1149 | δ-3-Carene | - | 0.6 | - |
| 5 | 1180 | 1184 | β-Myrcene | 0.9 | 1.2 | 0.8 |
| 6 | 1193 | 1195 | α-Terpinene | - | 3.0 | - |
| 7 | 1201 | 1203 | Limonene | 0.5 | 6.2 | 0.4 |
| 8 | 1207 | 1208 | 1,8-Cineole | 8.9 | - | 1.2 |
| 9 | 1215 | 1216 | (E)-2-Hexenal | 0.2 | 1.6 | 0.4 |
| 10 | 1230 | 1232 | γ-Terpinene | 0.6 | 5.2 | 0.3 |
| 11 | 1238 | 1240 | β-trans-Ocimene | - | - | 0.5 |
| 12 | 1267 | 1268 | p-Cymene | 0.5 | 4.5 | 0.2 |
| 13 | 1274 | 1276 | α-Terpinolene | 0.3 | 3.0 | tr |
| 14 | 1296 | 1298 | 1-Octen-3-one | tr | - | - |
| 15 | 1304 | 1305 | 2,4-Nonadienal | - | 3.3 | - |
| 16 | 1340 | 1342 | 6-Methyl-5-heptene-2-one | - | 0.2 | - |
| 17 | 1357 | 1359 | 1-Hexanol | - | 0.2 | - |
| 18 | 1370 | 1372 | allo-Ocimene | - | - | 0.2 |
| 19 | 1390 | 1390 | Nonanal | - | - | 0.2 |
| 20 | 1391 | 1391 | (Z)-Hex-3-en-1-ol | 0.4 | - | - |
| 21 | 1394 | 1395 | 2,4-Hexadienal | - | 9.4 | - |
| 22 | 1413 | 1414 | Butyl hexanoate | - | tr | 0.7 |
| 23 | 1427 | 1427 | trans-2-Octenal | - | tr | - |
| 24 | 1431 | 1435 | cis-Thujone | 18.6 | 0.9 | 0.7 |
| 25 | 1445 | 1445 | cis-Linalool oxide | - | - | 1.3 |
| 26 | 1447 | 1448 | trans-Thujone | 3.3 | 0.2 | tr |
| 27 | 1452 | 1453 | 1-Octen-3-ol | 0.0 | - | - |
| 28 | 1455 | 1456 | 1-Heptanol | 0.2 | - | 0.3 |
| 29 | 1464 | 1466 | trans-Linalool oxide | 0.4 | 0.2 | 1.1 |
| 30 | 1470 | 1470 | Fenchyl acetate | - | tr | - |
| 31 | 1484 | 1485 | α-Campholenal | - | 1.1 | 1.0 |
| 32 | 1493 | 1493 | α-Copaene | - | 0.8 | 1.3 |
| 33 | 1498 | 1498 | n-Decanal | tr | 0.3 | 0.2 |
| 34 | 1506 | 1505 | Camphor | 12.2 | - | 0.4 |
| 35 | 1521 | 1520 | Benzaldehyde | - | 0.4 | 0.7 |
| 36 | 1532 | 1532 | (Z)-2-Nonenal | - | 0.2 | - |
| 37 | 1553 | 1554 | Linalool | 0.4 | 0.2 | - |
| 38 | 1562 | 1564 | Linalyl acetate | 0.0 | 0.2 | 4.7 |
| 39 | 1565 | 1566 | trans-Pinocamphone | tr | tr | 0.3 |
| 40 | 1568 | 1569 | (E,E)-3,5-Octadien-2-one | - | 0.2 | - |
| 41 | 1573 | 1574 | Iso pulegone | 0.9 | tr | 0.2 |
| 42 | 1574 | 1574 | Pinocarvone | 0.2 | 0.2 | - |
| 43 | 1579 | 1579 | Bornyl acetate | 0.0 | 0.2 | 0.2 |
| 44 | 1581 | 1582 | 6-Methyl-3,5-heptadien-2-one | - | tr | 0.3 |
| 45 | 1588 | 1589 | trans-β-Caryophyllene | 3.2 | - | 0.9 |
| 46 | 1599 | 1598 | Terpinen-4-ol | 0.9 | 1.3 | tr |
| 47 | 1608 | 1608 | Aromadendrene | - | 0.3 | tr |
| 48 | 1617 | 1619 | Butyl octanoate | tr | - | 0.9 |
| 49 | 1623 | 1624 | β-Cyclocitral | tr | - | tr |
| 50 | 1629 | 1628 | 1-Terpineol | tr | tr | 0.2 |
| 51 | 1643 | 1644 | Pulegone | - | 0.5 | 0.2 |
| 52 | 1649 | 1650 | Alloaromadendrene | 1.0 | 0.2 | 0.3 |
| 53 | 1655 | 1658 | Sabinyl acetate | 0.3 | - | tr |
| 54 | 1672 | 1670 | 4-Vinylanisole | - | 0.2 | 0.2 |
| 55 | 1677 | 1679 | β-Citral | 0.2 | 0.5 | tr |
| 56 | 1680 | 1681 | α-Humulene | 6.1 | 1.2 | 0.3 |
| 57 | 1684 | 1684 | δ-Terpineol | 0.2 | - | 0.5 |
| 58 | 1702 | 1703 | γ-Muurolene | 0.6 | 1.7 | - |
| 59 | 1711 | 1712 | α-Terpineol | 1.6 | 1.0 | 2.5 |
| 60 | 1714 | 1715 | Borneol | 4.0 | 0.3 | 0.2 |
| 61 | 1720 | 1722 | Dodecanal | - | tr | 0.2 |
| 62 | 1723 | 1725 | Butyl nonanoate | - | 1.0 | 0.3 |
| 63 | 1728 | 1729 | Piperitone | 0.5 | 1.7 | 0.2 |
| 64 | 1733 | 1733 | Neryl acetate | tr | - | 0.7 |
| 65 | 1742 | 1746 | Carvyl acetate | tr | 0.3 | tr |
| 66 | 1750 | 1750 | Epoxylinalool | tr | - | 0.2 |
| 67 | 1752 | 1752 | δ-Cadinene | tr | - | 1.4 |
| 68 | 1761 | 1763 | 1-Decanol | - | 3.2 | - |
| 69 | 1782 | 1783 | Cubenene | 0.7 | 0.3 | 0.3 |
| 70 | 1784 | 1785 | α-Cadinene | - | 0.9 | tr |
| 71 | 1792 | 1793 | Myrtenol | 0.6 | - | tr |
| 72 | 1796 | 1797 | 2,4-Decadienal | - | tr | - |
| 73 | 1803 | 1805 | 2-Tridecanone | - | tr | 0.8 |
| 74 | 1814 | 1815 | β-Damascenone | 0.4 | - | - |
| 75 | 1822 | 1824 | β-Damascone | - | 2.2 | 0.3 |
| 76 | 1844 | 1845 | trans-Calamenene | - | 0.9 | 0.3 |
| 77 | 1855 | 1856 | cis-Carveol | 0.6 | tr | - |
| 78 | 1857 | 1857 | trans-Carveol | tr | 0.4 | 0.3 |
| 79 | 1867 | 1868 | (Z)-Geranyl acetone | - | 0.2 | 2.0 |
| 80 | 1869 | 1870 | exo-2-Hydroxycineole | tr | tr | 0.2 |
| 81 | 1884 | 1885 | Benzyl alcohol | tr | 0.2 | 0.4 |
| 82 | 1887 | 1887 | (E)-2-Dodecenal | - | 0.5 | - |
| 83 | 1915 | 1916 | α-Calacorene | - | 0.8 | - |
| 84 | 1917 | 1918 | Piperitenone | tr | 0.8 | 1.5 |
| 85 | 1920 | 1921 | Tetradecanal | 0.2 | - | - |
| 86 | 1926 | 1927 | Phenylethyl alcohol | 0.1 | 0.7 | 0.6 |
| 87 | 1930 | 1931 | trans-β-Ionone | tr | 0.3 | 0.3 |
| 88 | 1937 | 1938 | cis-Jasmone | tr | 0.2 | - |
| 89 | 1945 | 1945 | 2,6-Dimethyl-3,7-octadiene-2,6-diol | tr | 0.2 | 0.3 |
| 90 | 1949 | 1951 | (2E)-Hexenoic acid | 0.4 | 0.2 | 0.6 |
| 91 | 1953 | 1955 | cis-Caryophyllene oxide | 0.2 | tr | 2.3 |
| 92 | 1954 | 1954 | 2-Ethyl-hexanoic acid | - | tr | 0.4 |
| 93 | 1966 | 1967 | β-Ionone epoxide | tr | 0.5 | 0.3 |
| 94 | 1992 | 1993 | trans-β-Ionone-5,6-epoxide | 0.6 | 0.2 | 2.1 |
| 95 | 2000 | 2000 | Eicosane | - | 0.6 | 0.8 |
| 96 | 2000 | 2203 | 2-Methoxy-4-vinylphenol | tr | 0.5 | 0.7 |
| 97 | 2001 | 2003 | Methyl eugenol | 0.5 | - | 0.3 |
| 98 | 2012 | 2014 | Methyl tetradecanoate | 0.2 | tr | - |
| 99 | 2022 | 2024 | Glubulol | tr | 0.4 | tr |
| 100 | 2030 | 2032 | Cinnamaldehyde | 1.2 | tr | 0.7 |
| 101 | 2034 | 2035 | Nerolidol | tr | 0.4 | 0.2 |
| 102 | 2041 | 2042 | Pentadecanal | 0.3 | 0.3 | 0.4 |
| 103 | 2051 | 2052 | Octanoic acid | 0.7 | 0.2 | 0.4 |
| 104 | 2080 | 2081 | Viridiflorol | 4.3 | 0.5 | 0.4 |
| 105 | 2095 | 2099 | β-Elemenone | tr | 0.9 | tr |
| 106 | 2121 | 2121 | Spatulenol | tr | 1.0 | 2.5 |
| 107 | 2130 | 2131 | Hexahydrofarnesyl acetone | tr | 0.5 | 0.4 |
| 108 | 2133 | 2135 | 2-Hydroxy-4-methoxy-benzaldehyde | - | 0.4 | 0.2 |
| 109 | 2178 | 2179 | γ-Eudesmol | - | 0.5 | 0.2 |
| 110 | 2185 | 2186 | Eugenol | tr | - | 1.1 |
| 111 | 2192 | 2192 | Nonanoic acid | 0.3 | 0.7 | 0.6 |
| 112 | 2197 | 2198 | Thymol | 0.6 | 0.3 | 0.4 |
| 113 | 2205 | 2206 | Carvacrol | 0.7 | 0.5 | 0.2 |
| 114 | 2210 | 2210 | Methyl hexadecanoate | - | 0.3 | 0.5 |
| 115 | 2213 | 2215 | β-Eudesmol | - | 1.2 | 1.3 |
| 116 | 2217 | 2219 | Ledene oxide-(I) | - | tr | 0.7 |
| 117 | 2220 | 2223 | Sclareoloxide | 1.3 | - | 1.5 |
| 118 | 2240 | 2241 | Ethyl hexadecanoate | 0.4 | 0.5 | 2.4 |
| 119 | 2262 | 2264 | n-Decanoic acid | tr | 0.3 | 0.7 |
| 120 | 2300 | 2300 | n-Tricosane | 0.5 | 0.6 | 0.4 |
| 121 | 2321 | 2324 | Dihydroactinolide | - | 0.6 | 0.4 |
| 122 | 2330 | 2331 | (6R)-(β)-Caryophyllene oxide | - | 0.4 | 0.6 |
| 123 | 2340 | 2343 | Octadecanal | 0.5 | - | 3.4 |
| 124 | 2378 | 2379 | 4-Vinylphenol | tr | 0.4 | 1.5 |
| 125 | 2389 | 2390 | Isoelemicin | 0.5 | - | 0.7 |
| 126 | 2394 | 2396 | Tetracosane | tr | - | 0.2 |
| 127 | 2416 | 2419 | Butyl hexadecanoate | - | - | 0.2 |
| 128 | 2450 | 2451 | Dodecanoic acid | - | 0.3 | 0.2 |
| 129 | 2465 | 2469 | Penatcosane | tr | tr | 0.3 |
| 130 | 2541 | 2545 | Vanillin | - | 0.9 | 0.3 |
| 131 | 2547 | 2550 | 9,12,15-Octadecatrienoic acid, methyl ester | - | tr | tr |
| 132 | 2595 | 2597 | n-Hexacosane | - | - | 0.3 |
| 133 | 2650 | 2650 | Benzyl benzoate | tr | - | tr |
| 134 | 2654 | 2655 | n-Butyl octadecanoate | 5.6 | 0.2 | 5.7 |
| 135 | 2700 | 2712 | n-Heptacosane | tr | 0.5 | 0.4 |
| 136 | 2819 | 2819 | Pentadecanoic acid | - | - | - |
| 137 | 2826 | 2828 | n-Octacosane | - | tr | - |
| 138 | 2896 | 2899 | n-Hexadecanoic acid | - | 1.7 | 0.4 |
| 139 | 3000 | 3000 | n-Triacontane | - | tr | 0.4 |
| 140 | 3102 | 3100 | n-Hentriacontane | - | tr | tr |
| 141 | 3103 | 3104 | Octadecanoic acid | - | tr | - |
| 142 | 3153 | 3157 | 9-Octadecenoic acid | - | 0.2 | 6.9 |
| 143 | 3165 | 3168 | 9,12-Octadecadienoic acid | - | 0.2 | tr |
| 144 | 3192 | 3193 | 9,12,15-Octadecatrienoic acid | - | 0.4 | tr |
| Total % of identified compounds | 91.1 | 84.7 | 78.1 | |||
Compounds were identified based on the compounds’ mass spectral data and retention indices compared with those of the NIST Mass Spectral Library (December 2011), the Wiley Registry of Mass Spectral Data, 8th edition, and many authentic standards. The content (%) was calculated in triplicate using the normalization method based on the GC-MS data. The presented data are the average of three replicas, tr—trace concentration less than 0.1%. Standard deviation did not exceed 3% for all values. KI†: Kovats index calculated on VF-Wax CP 9205column.
Several factors may affect essential oil composition, such as geographical origin, harvesting season, method of oil extraction and growing conditions [15]. Iranian S. officinalis evidenced α-thujone (37.2%) as the main constituent, followed by 1,8-cineole (12.7%) and β-thujone (9.1%) [16]. Tunisian S. officinalis EO was characterized by camphor (33.6%), 1,8-cineole (22.2%) and α-thujone (21.4%) [17]. Romanian Salvia collected from cultivated and commercial samples showed α-thujone as the major compound in all analyzed oil samples (31.2–52.8%), followed by camphor and viridiflorol [18]. All the cited compounds were present in the herein studied S. officinalis EO. α-Thujone was usually common as one of the major identified compounds in S. officinalis oil. This compound showed potent antioxidant activity in several in silico and in vitro assays, comparable to standard antioxidant agents such as ascorbic acid and Trolox [19]. It also significantly adjusted cholesterol and triglyceride levels in diabetic rat models [20].
Pentacosane (20.1%), caryophyllene oxide (6.9%), phytol (6.8%), spathulenol (6.1%) and nonacosane (5.2%) were chief compounds in Turkish S. virgata EO [21]. The Iranian S. virgata’s EO is typified by β-caryophyllene, caryophyllene oxide and spathulenol [22,23]. The reported studies were significantly different from our results.
This divergence was also noted in the chemical composition of the essential oil of S. sclarea, where it is reported that in the Polish species, linalool (42.3%), α-terpineol (13.4%), geraniol (6.3%) and geranyl acetate (5.4%) were prevailing compounds [24]. However, linalyl acetate (19.7−31.0%), linalool (18.5−30.4%), geranyl acetate (4.4−12.1%) and α-terpineol (5.1−7.6%) were major components in different samples collected from Greece. Leaf EO was characterized by sclareoloxide (27.3%), thymol (20.6%) and caryophyllene oxide (9.9%), while sclareol (33.9%), linalool acetate (10.6%) and manoyl oxide (9.6%) were identified as the main components in flower essential oil from Egyptian plants [25]. However, in the present study, sclareol was not detected, only its derivative sclareoloxide (1.5%).
2.2. Antioxidant Effect of the Essential Oils of Salvia Species
Six in vitro assays were employed to evaluate the antioxidant activity of the three Salvia EOs. These were radical scavenging activity using 2,2-diphenyl-1-picryl-hydrazyl (DPPH), 2,2′-azino-bis(3-ethylbenzothiazoline-6-sulfonic acid) (ABTS) radical cation-based assay, total antioxidant capacity using cupric-reducing antioxidant capacity assay (CUPRAC), ferric-reducing antioxidant power assay (FRAP), EDTA chelating activity and phosphomolybdenum (PBD) assay. Only S. virgata oil showed antioxidant activity in DPPH assay (26.6 ± 1.60 mg TE/g oil, IC50: 1.98 ± 0.23 mg/mL). Moreover, the same oil exhibited the highest antioxidant activity in ABTS, CUPRAC and FRAP assays with the lowest IC50 values (0.75 ± 0.02, 0.39 ± 0.02 and 0.28 ± 0.01 mg/mL, respectively) in comparison with the others. In addition, the essential oil was more active than Trolox (IC50: 0.44 ± 0.02 mg/mL) in CUPRAC. However, S. virgata oil did not show any effect in the metal chelating assay, while in PBD assay, S. officinalis had the best antioxidant activity (26.4 ± 0.16 mmol TE/g oil, IC50: 0.10 ± 0.01 mg/mL) (Table 2). All tested essential oils exhibited stronger abilities in PBD assay compared to Trolox (IC50: 0.68 ± 0.01 mg/mL).
Table 2.
Antioxidant activity of Salvia essential oils *.
| Samples | DPPH | ABTS | CUPRAC | FRAP | Chelating | PBD | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| (mg TE/g oil) | IC50 | (mg TE/g oil) | IC50 | (mg TE/g oil) | IC50 | (mg TE/g oil) | IC50 | (mg EDTAE/g oil) | IC50 | (mmol TE/g oil) | IC50 | |
| S. officinalis | NA | NA | 55.7 ± 2.99 | 2.56 ± 0.19 | 74.3 ± 1.78 | 1.44 ± 0.01 | 55.3 ± 0.89 | 0.80 ± 0.02 | 62.3 ± 1.42 | 0.60 ± 0.01 | 26.4 ± 0.16 | 0.10 ± 0.01 |
| S. virgata | 26.6 ± 1.60 | 1.98 ± 0.23 | 190.1 ± 2.04 | 0.75 ± 0.01 | 275.2 ± 8.50 | 0.39 ± 0.02 | 155.9 ± 1.33 | 0.28 ± 0.01 | NA | NA | 15.1 ± 0.03 | 0.18 ± 0.01 |
| S. sclarea | NA | NA | 42.6 ± 0.51 | 3.34 ± 0.06 | 83.6 ± 0.72 | 1.28 ± 0.01 | 69.1 ± 2.02 | 0.64 ± 0.03 | 65.8 ± 2.85 | 0.58 ± 0.02 | 14.6 ± 0.26 | 0.19 ± 0.01 |
| Trolox | - | 0.22 ± 0.01 | - | 0.65 ± 0.09 | - | 0.44 ± 0.02 | - | 0.17 ± 0.01 | - | - | - | 0.68 ± 0.01 |
| EDTA | - | - | - | - | - | - | - | - | 0.04 ± 0.01 | - | - | |
* Values are reported as mean ± S.D. of three parallel measurements. IC50 values reported as mg/mL. TE: Trolox equivalent; EDTAE: EDTA equivalent; NA: not active.
It has been noted that natural products with antioxidant potential represent promising therapies for various diseases since excessive production of free radicals and lipid peroxidation of cell membranes are involved in the mechanistic pathophysiology of certain ailments, especially cardiovascular diseases, diabetes, Alzheimer’s, various types of cancers and others [26]. It is always recommended to assess the antioxidant activity of natural products by different methods with different mechanisms due to the complex nature of natural compounds [27]. Antioxidant activity of Salvia essential oils may be attributed to their volatile components. In the present study, it was found that S. officinalis oil is rich in oxygenated monoterpenes, which have been proven to possess the strongest antioxidant capacity relative to other classes of volatile compounds [28]. The major identified compound in this oil, α-thujone, showed good to moderate antioxidant capacity in a concentration-dependent manner in various assays such as DPPH, FRAP and hydroxyl, superoxide and nitric oxide radical scavenging activity [29]. A study showed that the antioxidant capacity of S. officinalis oil (with major compounds camphor and 1,8-cineole) was influenced by the time of hydro-distillation. The highest DPPH radical scavenging activity was observed for oil distilled in 2 h, while the highest activity in the TBARS assay was for oil distilled in 30 min. [30]. Regarding S. virgata, its flower oil showed better DPPH radical scavenging activity than its leaf oil, with activity equal to the standard butylated hydroxyanisole (BHA) [31]. Moreover, it was observed that oil isolated from aerial parts of S. virgata had better antioxidant activity in DPPH and FRAP assays when using the oil of full flowering rather than pre-flowering stage [32]. To the best of our knowledge, no previous extensive evaluation of the antioxidant activity of S. sclarea oil has been performed. However, its antioxidant capacity may also be attributed to some of its volatile constituents such as linalyl acetate, which has previously proven antioxidant potential in different assays, either in pure form or in oils where it is found as a major compound [33]. In addition to the different levels of different chemical components in the tested essential oils, the interactions between these components, namely antagonistic and synergetic, could affect the observed antioxidant properties [34,35,36].
2.3. Enzyme Inhibitory Effects of the Essential Oils of Salvia Species
The enzyme inhibitory effect of the oils was assessed against five enzymes which play a crucial step in certain medical conditions. Highest AChE inhibitory activity was recorded for S. officinalis (4.3 ± 0.01 mg galantamine equivalent (GALAE)/g oil; IC50: 0.68 ± 0.01 mg/mL), while S. virgata showed no effect at all. However, S. virgata had the highest BChE effect (12.1 ± 0.16 mg GALAE/g oil; IC50: 0.60 ± 0.01 mg/mL). All studied oils showed good tyrosinase inhibitory activity ranging between 66.1 ± 0.61 using S. sclarea EO to 128.4 ± 4.35 mg kojic acid equivalent ((KAE)/g oil) with S. officinalis EO. In addition, S. officinalis (IC50: 0.73 ± 0.01 mg/mL) exhibited stronger tyrosinase ability than standard inhibitor, kojic acid (IC50: 0.75 ± 0.01 mg/mL). Moreover, the oils did not exhibit any glucosidase inhibition, and exhibited weak or no activity as amylase inhibitors (Table 3).
Table 3.
Enzyme inhibitory properties of the Salvia essential oils *.
| Samples | AChE İnhibition | BChE İnhibition | Tyrosinase İnhibition | Amylase İnhibition |
Glucosidase İnhibition |
|||||
|---|---|---|---|---|---|---|---|---|---|---|
| (mg GALAE/g oil) | IC50 | (mg GALAE/g oil) | IC50 | (mg KAE/g oil) | IC50 | (mmol ACAE/g oil) | IC50 | (mmol ACAE/g oil) | IC50 | |
| S. officinalis | 4.3 ± 0.01 | 0.68 ± 0.01 | 12.0 ± 0.53 | 0.61 ± 0.03 | 128.4 ± 4.35 | 0.73 ± 0.01 | 0.7 ± 0.05 | 1.27 ± 0.07 | NA | NA |
| S. virgata | NA | NA | 12.1 ± 0.16 | 0.60 ± 0.01 | 94.0 ± 1.75 | 0.90 ± 0.01 | 0.1 ± 0.01 | >5 | NA | NA |
| S. sclarea | 2.9 ± 0.01 | 1.01 ± 0.01 | 11.5 ± 0.10 | 0.63 ± 0.01 | 66.1 ± 0.61 | 1.27 ± 0.07 | 1.1 ± 0.03 | 0.96 ± 0.01 | NA | NA |
| Galantamine | - | 0.01 ± 0.001 | - | 0.02 ± 0.01 | - | - | - | - | - | - |
| Kojic acid | - | - | - | - | - | 0.75 ± 0.01 | - | - | - | - |
| Acarbose | - | - | - | - | - | - | - | 0.66 ± 0.01 | - | 0.58 ± 0.01 |
* Values are reported as mean ± S.D. of three parallel measurements. IC50 values reported as mg/mL. GALAE: galantamine equivalent; KAE: kojic acid equivalent; ACAE: acarbose equivalent; NA: not active.
Inhibition of AChE leads to the accumulation of acetylcholine, leading to better communication between nerve cells, and thus eases the symptoms in Alzheimer’s patients [37]. BChE is also a co-regulator of acetylcholine. Therefore, its inhibition leads to better symptoms and prognosis in Alzheimer’s [38]. Previous clinical studies showed that administration of sage oil and herbal teas improved mental and cognitive function in Alzheimer’s individuals [39]. Alcoholic extracts of S. officinalis exhibited in vitro inhibition of AChE and BChE, with higher inhibition observed against BChE [40], which is in accordance with the present results, but regarding the essential oil.
Tyrosinase is a rate-limiting enzyme in melanin biosynthesis, as it oxidizes the amino acid tyrosine into melanin [41]. Its inhibitors, such as kojic acid, ellagic acid and hydroquinone, are used in the treatment of hyperpigmentation conditions and in skin-whitening cosmetics. A study on 19 essential oils showed that S. officinalis oil had moderate tyrosinase inhibitory activity with IC50 99.8 ± 1.750 μg/mL relative to kojic acid with IC50 2.3 ± 0.054 μg/mL [42]. Regarding S. virgata and S. sclarea oils, no previous data on their tyrosinase inhibitory activity were reported.
Both α-glucosidase and α-amylase digest carbohydrates, which leads to increasing levels of postprandial blood glucose, and their inhibition would lead to controlling postprandial hyperglycemia in diabetic patients, as well as reducing the risk for developing diabetes [43]. Although the studied Salvia oils showed no α-glucosidase inhibition and weak or no activity as α-amylase inhibitors, however, previous reports regarding their alcoholic and aqueous extracts recorded inhibitory activity for those enzymes [44]. Thus, their antidiabetic activity may be attributed to other active constituents not present in their essential oils, such as phenolic compounds.
Taken together, the observed enzyme inhibitory effects of the Salvia essential oils could have great potential for further pharmaceutical, nutraceutical and cosmeceutical applications. However, due to the complex nature of essential oils, interactions between chemical components should not be forgotten [45,46,47].
2.4. Chemometric Analysis
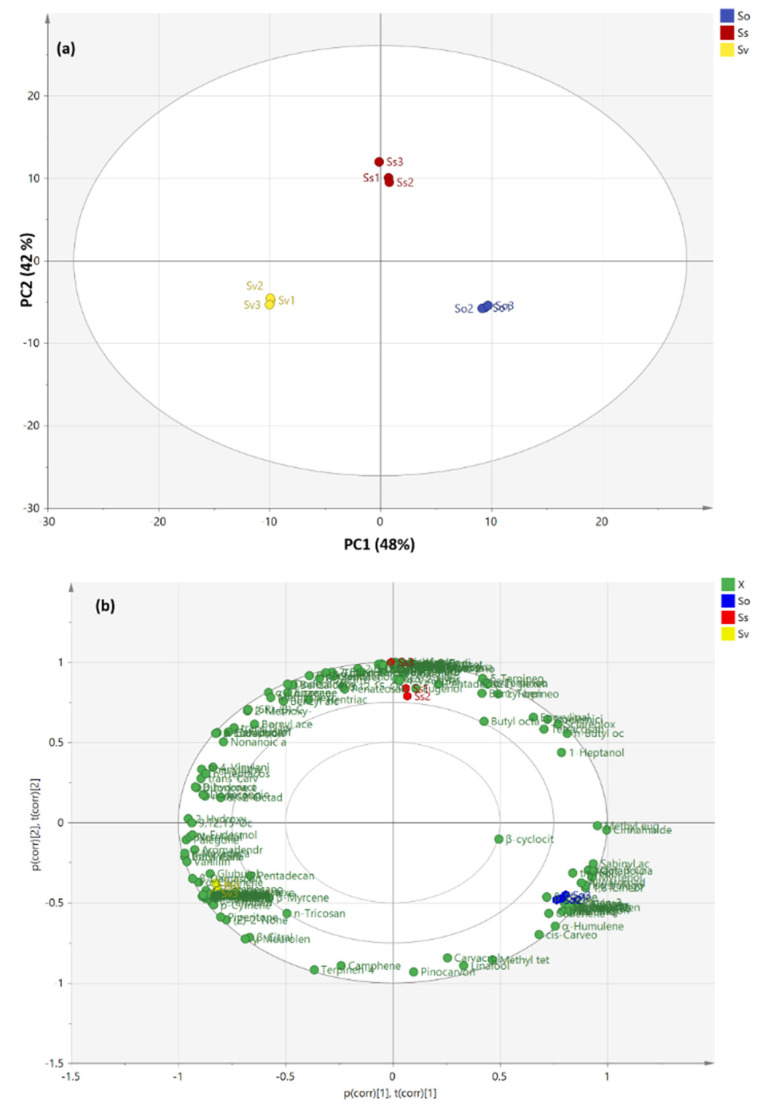
The GC-MS-based chemical profile of essential oils included both qualitative and quantitative discrepancies among different Salvia species; chemometric analysis was applied using principal component analysis (PCA) and hierarchal cluster analysis (HCA) to segregate closely related species, as well as to recognize any significant association between them [48]. A matrix of the total number of samples and their replicates (9 samples) multiplied by 144 variables (GC-MS peak area %) was constructed in MS Excel®, then subjected to chemometric analysis (PCA and HCA). Due to the large number of variables, PCA was first used to reduce the dimensionality of the multiple dataset, followed by removing the redundancy in the variables and utilizing raw data (peak area % for each compound as in Table 1). The PCA score plot accounting for 90% of the variation in the dataset (Figure 1a) highlights that the first principal component (48%) discriminates between S. virgata (Sv) (PC1 negative values on the lower quadrant) and the other two species (PC1 positive values), while the second principal component (42%) discriminates between S. sclarea (Ss) (positive loading along PC2) and the others (negative loading along the same axis).
Figure 1.
PCA score plot: (a) biplot; (b) based on GC-MS chemical profile of the essential oils of different Salvia species as displayed in Table 1. S. officinalis (So), S. virgata (Sv) and S. sclarea (Ss).
Figure 1b displays the biplot for both scores and loading; the plot enabled the visualization of similarities and difference among different species in terms of their chemical profiles. The species sited near different metabolites are patterned in the score plot on the bases of these metabolites. The biplot shows that there is no specific marker (compound) accounting for the discrimination between Salvia species, proving the significant importance of the whole chemical profile of the essential oils in the discrimination between different species, not solely the compounds existing in high percentage.
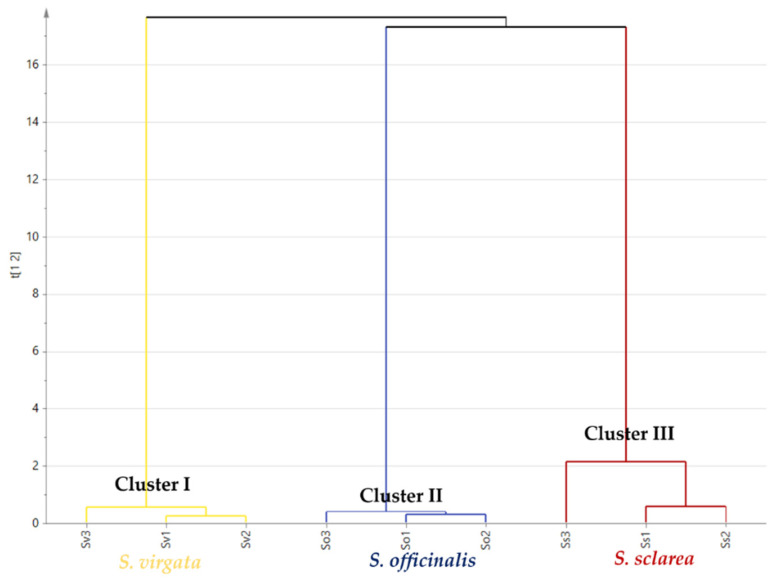
Additionally, HCA was applied as an unsupervised pattern recognition method to support results obtained by PCA. Figure 2 shows the HCA dendrogram, which displays segregation of different Salvia species in three main clusters. Cluster I, II and III present S. virgata (Sv), S. officinalis (So) and S. sclarea (Ss), respectively. The HCA dendrogram reveals the closeness of S. officinalis (So) and S. sclarea (Ss). HCA results endorse that of PCA.
Figure 2.
HCA dendrogram based on GC-MS chemical profile of the essential oils of different Salvia species as displayed in Table 1. S. officinalis (So), S. virgata (Sv) and S. sclarea (Ss).
Partial least squares (PLS) was applied to find a correlation between the volatile compounds and their antioxidant and enzyme inhibitory activities. PLS-R1 and PLS-R2 models were constricted by the data matrix X containing the peak area of the GC/MS and the response y vectors containing the antioxidant and enzyme inhibitory activities data, respectively. The model performance was estimated by the parameters of root mean square error of calibration (RMSEC), root mean square error of validation (RMSEV) and correlation (R2). PLS-R1 model parameters, including slope, offset, RMSEC, RMSEV and R2, are shown in Table 4, indicating the strong prediction ability of the PLS regression model. PLS-R1 models showed excellent linearity and accuracy, with R2 > 0.99 and slope close to 1 (a value close to 1 means the predicted values are close to the reference), with low differences between RMSEC and root mean square error of validation (RMSEV) revealing the robustness of the model. It was observed that both DPPH and PBD data displayed the lowest RMSEV values (0.5325 and 0.6550), respectively, suggesting that they are more representative than other techniques to measure the antioxidant activity. The prediction performance for the developed models is shown in Table 5. The results show that the antioxidant activity is correctly predicted with ±5% accuracy.
Table 4.
PLS-R1 model parameters used for prediction.
| Antioxidant Activity | Data Type | PLS-R1 | |||
|---|---|---|---|---|---|
| Slope | Offset | RMSE | R2 | ||
| DPPH | Cal. | 0.9992 | 0.0066 | 0.3428 | 0.9992 |
| Val. | 0.9959 | 0.0432 | 0.5325 | 0.9985 | |
| ABTS | Cal. | 0.9998 | 0.0121 | 0.7539 | 0.9998 |
| Val. | 0.9969 | 0.3342 | 1.1553 | 0.9997 | |
| FRAP | Cal. | 0.9988 | 0.1088 | 1.5135 | 0.9988 |
| Val. | 0.9959 | 0.4324 | 2.2877 | 0.9978 | |
| CUPRAC | Cal. | 0.9996 | 0.0525 | 1.7693 | 0.9996 |
| Val. | 0.9968 | 0.5267 | 2.6944 | 0.9993 | |
| EDTA | Cal. | 0.9990 | 0.0414 | 0.9411 | 0.9990 |
| Val. | 0.9959 | 0.1521 | 1.4157 | 0.9982 | |
| PBD | Cal. | 0.9937 | 0.1166 | 0.4353 | 0.9937 |
| Val. | 0.9874 | 0.2277 | 0.6550 | 0.9887 | |
RMSE: root mean squared error. R2: correlation. Cal: calibration. Val: validation.
Table 5.
Results of calibration and predictive ability of the PLS-R1 model. (S. officinalis (So), S. virgata (Sv) and S. sclarea (Ss)).
| DPPH | ABTS | FRAP | ||||
|---|---|---|---|---|---|---|
| Y Reference | Y Predicted | Y Reference | Y Predicted | Y Reference | Y Predicted | |
| So1 | 0.00 | 0.00 | 55.70 | 56.22 | 55.30 | 55.08 |
| So2 | 0.00 | 0.00 | 56.80 | 56.52 | 54.70 | 55.17 |
| So3 | 0.00 | 0.00 | 57.10 | 56.87 | 55.90 | 55.67 |
| Sv1 | 25.90 | 26.72 | 191.60 | 191.62 | 155.90 | 155.87 |
| Sv2 | 26.60 | 26.13 | 190.10 | 188.53 | 156.70 | 153.84 |
| Sv3 | 27.5 | 27.13 | 192.40 | 193.87 | 154.50 | 157.27 |
| Ss1 | 0.00 | 0.00 | 42.60 | 42.47 | 71.10 | 69.58 |
| Ss2 | 0.00 | 0.00 | 42.90 | 43.14 | 68.50 | 69.95 |
| Ss3 | 0.00 | 0.00 | 41.80 | 41.72 | 69.10 | 69.23 |
| CUPRAC | EDTA | PBD | ||||
| Y Reference | Y Predicted | Y Reference | Y Predicted | Y Reference | Y Predicted | |
| So1 | 74.30 | 73.88 | 63.50 | 62.61 | 26.40 | 26.38 |
| So2 | 75.20 | 74.17 | 62.30 | 62.49 | 25.80 | 26.42 |
| So3 | 73.50 | 74.99 | 61.60 | 62.29 | 26.90 | 26.27 |
| Sv1 | 274.88 | 275.83 | 0.00 | 0.00 | 14.80 | 15.08 |
| Sv2 | 275.20 | 271.55 | 0.00 | 0.00 | 15.10 | 15.19 |
| Sv3 | 276.30 | 278.85 | 0.00 | 0.00 | 15.40 | 15.03 |
| Ss1 | 82.50 | 83.46 | 65.80 | 65.54 | 14.60 | 14.41 |
| Ss2 | 83.60 | 84.30 | 66.50 | 65.25 | 14.90 | 14.42 |
| Ss3 | 84.20 | 82.60 | 64.40 | 65.86 | 13.70 | 14.36 |
Concerning PLS-R2, model parameters, including slope, offset, RMSEC, RMSEV and R2, are shown in Table 6, indicating the moderate prediction ability of the PLS regression model. PLS-R2 models showed good linearity and accuracy with R2 > 0.97, except for BChE inhibition, which exhibited much lower values. The prediction performance for the developed models is shown in Table 7.
Table 6.
PLS-R2 model parameters used for prediction.
| Enzyme Inhibition | Data Type | PLS-R2 | |||
|---|---|---|---|---|---|
| Slope | Offset | RMSE | R2 | ||
| AChE Inhibition | Cal. | 0.9983 | 0.0039 | 0.0717 | 0.9983 |
| Val. | 0.9948 | 0.0095 | 0.1062 | 0.9970 | |
| BChE Inhibition | Cal. | 0.5769 | 5.0525 | 0.3346 | 0.5769 |
| Val. | 0.3626 | 7.6161 | 0.5017 | 0.2483 | |
| Tyrosinase Inhibition | Cal. | 0.9990 | 0.0894 | 0.7721 | 0.9990 |
| Val. | 0.9947 | 0.4708 | 1.1165 | 0.9984 | |
| Amylase Inhibition | Cal. | 0.9885 | 0.0076 | 0.0484 | 0.9885 |
| Val. | 0.9792 | 0.0134 | 0.0732 | 0.9792 | |
RMSE: root mean squared error. R2: correlation. Cal: calibration. Val: validation.
Table 7.
Results of calibration and predictive ability of the PLS-R2 model.
| AChE Inhibition | BChE Inhibition | |||
|---|---|---|---|---|
| Y Reference | Y Predicted | Y Reference | Y Predicted | |
| So1 | 0.00 | 0.00 | 55.70 | 56.22 |
| So2 | 0.00 | 0.00 | 56.80 | 56.52 |
| So3 | 0.00 | 0.00 | 57.10 | 56.87 |
| Sv1 | 25.90 | 26.72 | 191.60 | 191.62 |
| Sv2 | 26.60 | 26.13 | 190.10 | 188.53 |
| Sv3 | 27.5 | 27.13 | 192.40 | 193.87 |
| Ss1 | 0.00 | 0.00 | 42.60 | 42.47 |
| Ss2 | 0.00 | 0.00 | 42.90 | 43.14 |
| Ss3 | 0.00 | 0.00 | 41.80 | 41.72 |
| Tyrosinase Inhibition | Amylase Inhibition | |||
| Y Reference | Y Predicted | Y Reference | Y Predicted | |
| So1 | 74.30 | 73.88 | 63.50 | 62.61 |
| So2 | 75.20 | 74.17 | 62.30 | 62.49 |
| So3 | 73.50 | 74.99 | 61.60 | 62.29 |
| Sv1 | 274.88 | 275.83 | 0.00 | 0.00 |
| Sv2 | 275.20 | 271.55 | 0.00 | 0.00 |
| Sv3 | 276.30 | 278.85 | 0.00 | 0.00 |
| Ss1 | 82.50 | 83.46 | 65.80 | 65.54 |
| Ss2 | 83.60 | 84.30 | 66.50 | 65.25 |
| Ss3 | 84.20 | 82.60 | 64.40 | 65.86 |
3. Materials and Methods
3.1. Plant Material
The S. officinalis L. (LRR № 017; 14 May 2020) was cultivated in Uzbekistan and collected from the botanical field of the Institute of the Chemistry of Plant Substances (41°20′12.42″ N 69°20′06.07″ E, Tashkent, Uzbekistan). S. virgata Jacq. (LRR № 153; 25 June 2020) and S. sclarea L. (LRR № 095; 18 June 2020) were collected from Qizilsoy (41°12′11.6″ N 69°45′45.4″ E Tashkent region). The plants were identified by Olim Khojimatov and the voucher samples have been deposited at the National Herbarium of the Institute of Botany, Academy of Sciences of Uzbekistan.
3.2. Extraction of Essential Oils of Salvia Species
Aerial parts of Salvia samples were air-dried in the shade. Essential oils were hydro-distilled (400 g dry powder in 1 L distilled water) using a Clevenger-type apparatus for 3 h. The yields were 0.8% w/w for S. officinalis, 0.2% w/w for S. virgata and 0.3% w/w for S. sclarea. The recovered oils were dried over anhydrous sodium sulphate and kept in sealed dark vials at 4 °C until analysis.
3.3. GC-MS Analysis of Essential Oils of Salvia Species
GC-MS of Salvia essential oils was carried out using an Agilent 7890 B gas chromatograph (Agilent Technologies, Rotterdam, The Netherlands). The column used was a VF-Wax CP 9205 fused silica (30 m × 0.25 mm, ID 0.25 µm). Helium was used as carrier gas at a flow rate of 0.9 mL/min. An Agilent 5977A mass selective detector was used, with a scan range of 45–950 atomic mass units with a detector temperature of 270 °C and split mode injection at a split ratio of 1:20. An autosampler was used for sample injection (0.5 µL) with an injector temperature of 250 °C. The interface temperature was 280 °C, the source temperature was 230 °C, and the ionization energy was 70 eV. The initial oven temperature was 50 °C for 5 min., which was then raised to 280 °C at a rate of 5 °C/min, then kept isothermal at 280 °C for 15 min. Standard alkanes (C7-C40) obtained from Sigma-Aldrich (Darmstadt, Germany) were used to calculate the Kovats index (KI). Chromatograms were generated using enhanced ChemStation software (Agilent Technologies, Waldbronn, Germany). Volatile compounds were identified by comparing their mass spectra and KI was calculated with the 9th edition of Wiley Registry of mass spectral data and NIST library.
3.4. Antioxidant Assays
In vitro assays were employed to evaluate the antioxidant activity of the three Salvia EOs using the 2,2-diphenyl-1-picryl-hydrazyl (DPPH), 2,2′-azino-bis(3-ethylbenzothiazoline-6-sulfonic acid) (ABTS) radical-cation-based assay, total antioxidant capacity using cupric-reducing antioxidant capacity assay (CUPRAC), ferric-reducing antioxidant power assay (FRAP), EDTA chelating activity and phosphomolybdenum (PBD) assay. These assays were performed according to previously described standard procedures, and values are expressed as Trolox or EDTA equivalent [49,50]. The experimental procedures are given in supplemental materials. To provide a comparison with standard compounds, IC50 values (the half inhibitory concentration) were also calculated for DPPH, ABTS and metal chelating assays. IC50 values for other assays (reducing power and phosphomolybdenum) reflect that the concentration at which absorbance occurs is 0.5.
3.5. Enzyme Inhibitory Assays
The enzyme inhibitory effect of the oils was assessed against five enzymes which play a crucial step in certain medical conditions. These included AChE, BChE, tyrosinase, α-glucosidase and α-amylase. Assays were carried out according to standard procedures, with values expressed as galantamine, kojic acid and acarbose equivalent for cholinesterase, tyrosinase and α-glucosidase/α-amylase inhibitory activities, respectively [50,51]. The experimental procedures are given in supplemental materials. IC50 values (the half inhibitory concentration) for each oil and standard inhibitors were also calculated for enzyme inhibitory assays.
3.6. Statistical Analysis
All analyses were conducted in triplicate. Values are expressed as means ± SD. Statistical significance was determined using one-way ANOVA followed by Tukey’s post hoc test (significance level at p < 0.05).
3.7. Chemometric Analysis
The data obtained from GC-MS were subjected to chemometric analysis. Principal component analysis (PCA) was applied as an initial step for data investigation to present an overview of all species divergences and to recognize markers responsible for this dissimilarity [52]. Hierarchal cluster analysis (HCA) was then applied to allow the clustering of different species. The clustering pattern was constructed by the single linkage method. PCA and HCA were accomplished using the SIMCA-P version 13.0 software package (Umetrics, Umeå, Sweden). A quantitative calibration model, partial least squares (PLS), was designed to find a correlation between the volatile compounds (GC/MS peak areas) (X) matrix and their antioxidant, enzyme inhibitory activities (Y) matrices. In this state, there was no division of data into model and test set, as only nine samples for each model were assessed (small dataset). PLS was performed using CAMO’s Unscrambler® X 10.4 software (Computer-Aided Modeling, AS, Oslo, Norway).
4. Conclusions
Salvia species are aromatic plants that have been widely used in various cultures since ancient times. In the present work, the chemical profile of three Salvia species essential oils was investigated. The studied species were S. officinalis, S. virgata and S. sclarea. Their major identified compounds were cis-thujone, 2,4-hexadienal and 9-octadecenoic acid in S. officinalis, S. virgata and S. sclarea EOs, respectively. The PCA score plot revealed significant discrimination of the three species even though its biplot was unable to identify the compounds responsible for these differences. The three Salvia species EOs exhibited moderate antioxidant activities. Highest AChE inhibitory activity was recorded for S. officinalis, while S. virgata had the highest BChE effect. All studied oils showed good tyrosinase inhibitory activity. Moreover, the oils did not exhibit any glucosidase inhibition, and exhibited weak or no activity as amylase inhibitors. Thus, the studied Salvia essential oils are interesting candidates that could be used in drug discovery for the management of Alzheimer’s and hyperpigmentation conditions.
Acknowledgments
The authors would like to thank the King Saud University Researchers Supporting Project (number RSP-2021/294) (Riyadh, Saudi Arabia) and Ministry of Innovative Development of the Republic of Uzbekistan (project number A-FA-2021-144). The author N.Z.M. thanks the Alexander von Humboldt Foundation for providing the opportunity to perform work in Germany.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/molecules27175365/s1, Figure S1: GC-chromatograms of the essential oils obtained from (A): Salvia officnalis, (B): S. sclarea, and (C): S. virgata aerial parts using the VF-Wax CP 9205 column.
Author Contributions
N.Z.M. and R.Z.M. were responsible for conceptualization, isolation of the volatiles, performing GC-MS analysis and revising the manuscript; H.A.G. and N.K. identified the volatile compounds, conducted the chemometric analysis, wrote the manuscript and revised the first draft; G.Z. performed the biological studies; B.N. revised the statistics and manuscript; O.K.K. was responsible for collection and identification of the plant species; N.M.A.M. and M.L.A. obtained funding and supported the writing and revised of the manuscript. All authors have read and agreed to the published version of the manuscript.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Data are available upon request from the first author.
Conflicts of Interest
The authors declare no conflict of interest.
Sample Availability
Samples of the compounds are available from the authors.
Funding Statement
This work was funded by King Saud University Researchers Supporting Project (number RSP-2021/294) (Riyadh, Saudi Arabia).
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Bagchi G.D., Srivastava G.N. Spices and Flavoring (Flavoring) crops|Leaf and Floral Structures. In: Caballero B., editor. Encyclopedia of Food Sciences and Nutrition. 2nd ed. Academic Press; Oxford, UK: 2003. pp. 5477–5486. [Google Scholar]
- 2.Tamokou J.D.D., Mbaveng A.T., Kuete V. Chapter 8—Antimicrobial Activities of African Medicinal Spices and Vegetables. In: Kuete V., editor. Medicinal Spices and Vegetables from Africa. Academic Press; Oxford, UK: 2017. pp. 207–237. [Google Scholar]
- 3.Andrews A.C. Sage as a Condiment in the Graeco-Roman Era. Econ. Bot. 1956;10:263–266. doi: 10.1007/BF02899005. [DOI] [Google Scholar]
- 4.Khalil R., Li Z. Antimicrobial activity of essential oil of Salvia officinalis L. collected in Syria. Afr. J. Biotechnol. 2011;10:8397–8402. [Google Scholar]
- 5.Walch S.G., Tinzoh L.N., Zimmermann B.F., Stühlinger W., Lachenmeier D.W. Antioxidant Capacity and Polyphenolic Composition as Quality Indicators for Aqueous Infusions of Salvia officinalis L. (sage tea) Front. Pharmacol. 2011;2:79. doi: 10.3389/fphar.2011.00079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Tuttolomondo T., Iapichino G., Licata M., Virga G., Leto C., La Bella S. Agronomic Evaluation and Chemical Characterization of Sicilian Salvia sclarea L. Accessions. Agronomy. 2020;10:1114. doi: 10.3390/agronomy10081114. [DOI] [Google Scholar]
- 7.Loizzo M.R., Tundis R., Menichini F., Saab A.M., Statti G.A., Menichini F. Cytotoxic activity of essential oils from Labiatae and Lauraceae families against in vitro human tumor models. Anticancer Res. 2007;27:3293–3299. [PubMed] [Google Scholar]
- 8.Vuković-Gacić B., Nikcević S., Berić-Bjedov T., Knezević-Vukcević J., Simić D. Antimutagenic effect of essential oil of sage (Salvia officinalis L.) and its monoterpenes against UV-induced mutations in Escherichia coli and Saccharomyces cerevisiae. Food Chem. Toxicol. Int. J. Publ. Br. Ind. Biol. Res. Assoc. 2006;44:1730–1738. doi: 10.1016/j.fct.2006.05.011. [DOI] [PubMed] [Google Scholar]
- 9.Ghavam M., Manca M.L., Manconi M., Bacchetta G. Chemical composition and antimicrobial activity of essential oils obtained from leaves and flowers of Salvia hydrangea DC. ex Benth. Sci. Rep. 2020;10:15647. doi: 10.1038/s41598-020-73193-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Koubaa F.G., Chaâbane M., Turki M., Ayadi F.M., El Feki A. Anti-oxidant and hepatoprotective effects of Salvia officinalis essential oil against vanadium-induced oxidative stress and histological changes in the rat liver. Environ. Sci. Pollut. Res. Int. 2021;28:11001–11015. doi: 10.1007/s11356-020-11303-z. [DOI] [PubMed] [Google Scholar]
- 11.Benny A., Thomas J. Essential Oils as Treatment Strategy for Alzheimer’s Disease: Current and Future Perspectives. Planta Med. 2019;85:239–248. doi: 10.1055/a-0758-0188. [DOI] [PubMed] [Google Scholar]
- 12.Ghorbani A., Esmaeilizadeh M. Pharmacological properties of Salvia officinalis and its components. J. Tradit. Complement. Med. 2017;7:433–440. doi: 10.1016/j.jtcme.2016.12.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Akkol E.K., Göger F., Koşar M., Başer K.H.C. Phenolic composition and biological activities of Salvia halophila and Salvia virgata from Turkey. Food Chem. 2008;108:942–949. doi: 10.1016/j.foodchem.2007.11.071. [DOI] [PubMed] [Google Scholar]
- 14.Kuźma L., Kalemba D., Rózalski M., Rózalska B., Wieckowska-Szakiel M., Krajewska U., Wysokińska H. Chemical composition and biological activities of essential oil from Salvia sclarea plants regenerated in vitro. Molecules. 2009;14:1438–1447. doi: 10.3390/molecules14041438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Barra A. Factors Affecting Chemical Variability of Essential Oils: A Review of Recent Developments. Nat. Prod. Commun. 2009;4:1147–1154. doi: 10.1177/1934578X0900400827. [DOI] [PubMed] [Google Scholar]
- 16.Golparvar A.R., Hadipanah A., Gheisari M.M., Naderi D., Rahmaniyan S., Khorrami M. Chemical composition and antimicrobial activity of essential oil of Salvia officinalis L. and Salvia virgata Jacq. J. Herb. Drugs. 2017;08:71–78. doi: 10.18869/JHD.2017.71. [DOI] [Google Scholar]
- 17.El Euch S.K., Hassine D.B., Cazaux S., Bouzouita N., Bouajila J. Salvia officinalis essential oil: Chemical analysis and evaluation of anti-enzymatic and antioxidant bioactivities. S. Afr. J. Bot. 2018;120:253–260. doi: 10.1016/j.sajb.2018.07.010. [DOI] [Google Scholar]
- 18.Oniga I., Oprean R., Toiu A., Benedec D. Chemical composition of the essential oil of Salvia officinalis L. from Romania. Rev. Med.-Chir. Soc. Med. Nat. Din Iasi. 2010;114:593–595. [PubMed] [Google Scholar]
- 19.Németh É.Z., Nguyen H.T. Thujone, a widely debated volatile compound: What do we know about it? Phytochem. Rev. 2020;19:405–423. doi: 10.1007/s11101-020-09671-y. [DOI] [Google Scholar]
- 20.Baddar N.W., Aburjai T.A., Taha M.O., Disi A.M. Thujone corrects cholesterol and triglyceride profiles in diabetic rat model. Nat. Prod. Res. 2011;25:1180–1184. doi: 10.1080/14786419.2010.496116. [DOI] [PubMed] [Google Scholar]
- 21.Coşge S.B., Uskutoğlu T., Cesur C., Ozavcı V., Doğan H. Determination of essential oil components, mineral matter, and heavy metal content of Salvia virgata Jacq. Grown in culture conditions. Turk. J. Agric. For. 2019;43:395–404. doi: 10.3906/tar-1812-84. [DOI] [Google Scholar]
- 22.Morteza-Semnani K., Saeedi M., Sh C., Vosoughi M. Essential Oil Composition of Salvia virgata Jacq. from Iran. J. Essent. Oil-Bear. Plants. 2005;8:330–333. doi: 10.1080/0972060X.2005.10643461. [DOI] [Google Scholar]
- 23.Rajabi Z., Ebrahimi M., Farajpour M., Mirza M., Ramshini H. Compositions and yield variation of essential oils among and within nine Salvia species from various areas of Iran. Ind. Crops Prod. 2014;61:233–239. doi: 10.1016/j.indcrop.2014.06.038. [DOI] [Google Scholar]
- 24.Acimovic M., Kiprovski B., Rat M., Sikora V., Popovic V., Koren A., Brdar-Jokanovic M. Salvia sclarea: Chemical composition and biological activity. J. Agron. Technol. Eng. Manag. 2018;1:18–28. [Google Scholar]
- 25.El-Gohary A.E., Amer H.M., Salama A.B., Wahba H.E., Khalid K.A. Characterization of the Essential Oil Components of Adapted Salvia sclarea L. (Clary sage) Plant Under Egyptian Environmental Conditions. J. Essent. Oil Bear. Plants. 2020;23:788–794. doi: 10.1080/0972060X.2020.1818635. [DOI] [Google Scholar]
- 26.Kurutas E.B. The importance of antioxidants which play the role in cellular response against oxidative/nitrosative stress: Current state. Nutr. J. 2016;15:71. doi: 10.1186/s12937-016-0186-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Munteanu I.G., Apetrei C. Analytical Methods Used in Determining Antioxidant Activity: A Review. Int. J. Mol. Sci. 2021;22:3380. doi: 10.3390/ijms22073380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Zengin H., Baysal A.H. Antibacterial and antioxidant activity of essential oil terpenes against pathogenic and spoilage-forming bacteria and cell structure-activity relationships evaluated by SEM microscopy. Molecules. 2014;19:17773–17798. doi: 10.3390/molecules191117773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Srinivasan R., Aruna A., Lee J.S., Kim M., Shivakumar M.S., Natarajan D. Antioxidant and Antiproliferative Potential of Bioactive Molecules Ursolic Acid and Thujone Isolated from Memecylon edule and Elaeagnus indica and Their Inhibitory Effect on Topoisomerase II by Molecular Docking Approach. Biomed. Res. Int. 2020;2020:8716927. doi: 10.1155/2020/8716927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Miguel G., Cruz C., Faleiro M.L., Simões M.T.F., Figueiredo A.C., Barroso J.G., Pedro L.G. Salvia officinalis L. essential oils: Effect of hydrodistillation time on the chemical composition, antioxidant and antimicrobial activities. Nat. Prod. Res. 2011;25:526–541. doi: 10.1080/14786419.2010.499513. [DOI] [PubMed] [Google Scholar]
- 31.Sarbanha S., Masoomi F., Kamalinejad M., Yassa N. Chemical Composition and Antioxidant Activity of Salvia virgata Jacq. and S. verticillata L. Volatile Oils from Iran. Planta Med. 2011;77:PE19. doi: 10.1055/s-0031-1282350. [DOI] [Google Scholar]
- 32.Alizadeh A. Essential Oil Constituents, Antioxidant and Antimicrobial Activities of Salvia virgata Jacq. from Iran. J. Essent. Oil Bear. Plants. 2013;16:172–182. doi: 10.1080/0972060X.2013.793974. [DOI] [Google Scholar]
- 33.Blažeković B., Yang W., Wang Y., Li C., Kindl M., Pepeljnjak S., Vladimir-Knežević S. Chemical composition, antimicrobial and antioxidant activities of essential oils of Lavandula intermedia ‘Budrovka’ and L. angustifolia cultivated in Croatia. Ind. Crops Prod. 2018;123:173–182. doi: 10.1016/j.indcrop.2018.06.041. [DOI] [Google Scholar]
- 34.Tsimogiannis D., Bimpilas A., Oreopoulou V. DPPH radical scavenging and mixture effects of plant o-diphenols and essential oil constituents. Eur. J. Lipid Sci. Technol. 2017;119:16003473. doi: 10.1002/ejlt.2016003473. [DOI] [Google Scholar]
- 35.Ciesla L.M., Wojtunik-Kulesza K.A., Oniszczuk A., Waksmundzka-Hajnos M. Antioxidant synergism and antagonism between selected monoterpenes using the 2,2-diphenyl-1-picrylhydrazyl method. Flavour Fragr. J. 2016;31:412–419. doi: 10.1002/ffj.3330. [DOI] [Google Scholar]
- 36.Meziane-Assami D., Ghouila Z., Assami K., Meklati B.Y., Chemat F. The deep impacting microwave irradiation on the quality and antioxidant capacity of rosemary essential oils obtained by solvent-free microwave extraction. J. Essent. Oil Res. 2022;34:12–20. doi: 10.1080/10412905.2021.2008028. [DOI] [Google Scholar]
- 37.Rees T.M., Brimijoin S. The role of acetylcholinesterase in the pathogenesis of Alzheimer’s disease. Drugs Today. 2003;39:75–83. doi: 10.1358/dot.2003.39.1.740206. [DOI] [PubMed] [Google Scholar]
- 38.Greig N.H., Lahiri D.K., Sambamurti K. Butyrylcholinesterase: An important new target in Alzheimer’s disease therapy. Int. Psychogeriatr. 2002;14((Suppl. 1)):77–91. doi: 10.1017/S1041610203008676. [DOI] [PubMed] [Google Scholar]
- 39.Babault N., Noureddine A., Amiez N., Guillemet D., Cometti C. Acute Effects of Salvia Supplementation on Cognitive Function in Athletes During a Fatiguing Cycling Exercise: A Randomized Cross-Over, Placebo-Controlled, and Double-Blind Study. Front. Nutr. 2021;8:949. doi: 10.3389/fnut.2021.771518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kennedy D.O., Pace S., Haskell C., Okello E.J., Milne A., Scholey A.B. Effects of cholinesterase inhibiting sage (Salvia officinalis) on mood, anxiety and performance on a psychological stressor battery. Neuropsychopharmacol. Off. Publ. Am. Coll. Neuropsychopharmacol. 2006;31:845–852. doi: 10.1038/sj.npp.1300907. [DOI] [PubMed] [Google Scholar]
- 41.Iozumi K., Hoganson G.E., Pennella R., Everett M.A., Fuller B.B. Role of Tyrosinase as the Determinant of Pigmentation in Cultured Human Melanocytes. J. Investig. Dermatol. 1993;100:806–811. doi: 10.1111/1523-1747.ep12476630. [DOI] [PubMed] [Google Scholar]
- 42.Aumeeruddy-Elalfi Z., Gurib-Fakim A., Mahomoodally M.F. Kinetic studies of tyrosinase inhibitory activity of 19 essential oils extracted from endemic and exotic medicinal plants. S. Afr. J. Bot. 2016;103:89–94. doi: 10.1016/j.sajb.2015.09.010. [DOI] [Google Scholar]
- 43.Poovitha S., Parani M. In vitro and in vivo α-amylase and α-glucosidase inhibiting activities of the protein extracts from two varieties of bitter gourd (Momordica charantia L.) BMC Complement. Altern. Med. 2016;16:185. doi: 10.1186/s12906-016-1085-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Rashed A.A., Rathi D.-N.G. Bioactive Components of Salvia and Their Potential Antidiabetic Properties: A Review. Molecules. 2021;26:3042. doi: 10.3390/molecules26103042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Savelev S., Okello E., Perry N.S.L., Wilkins R.M., Perry E.K. Synergistic and antagonistic interactions of anticholinesterase terpenoids in Salvia lavandulaefolia essential oil. Pharmacol. Biochem. Behav. 2003;75:661–668. doi: 10.1016/S0091-3057(03)00125-4. [DOI] [PubMed] [Google Scholar]
- 46.Yakoubi R., Megateli S., Sadok T.H., Bensouici C., Bağci E. A synergistic interactions of Algerian essential oils of Laurus nobilis L., Lavandula stoechas L. and Mentha pulegium L. on anticholinesterase and antioxidant activities. Biocatal. Agric. Biotechnol. 2021;31:101891. doi: 10.1016/j.bcab.2020.101891. [DOI] [Google Scholar]
- 47.Bektašević M., Politeo O. Biological Application of Essential Oils and Essential Oils Components in Terms of Antioxidant Activity and Inhibition of Cholinesterase Enzymes. In: de Oliveira M.A., de Aguiar Andrade E.E., editors. Essential Oils—Advances in Extractions and Biological Applications. IntechOpen; London, UK: 2022. [Google Scholar]
- 48.Kammoun A.K., Altyar A.E., Gad H.A. Comparative metabolic study of Citrus sinensis leaves cultivars based on GC–MS and their cytotoxic activity. J. Pharm. Biomed. Anal. 2021;198:113991. doi: 10.1016/j.jpba.2021.113991. [DOI] [PubMed] [Google Scholar]
- 49.Zengin G., Aktumsek A. Investigation of antioxidant potentials of solvent extracts from different anatomical parts of Asphodeline anatolica E. Tuzlaci: An endemic plant to Turkey. Afr. J. Tradit. Complement. Altern. Med. 2014;11:481–488. doi: 10.4314/ajtcam.v11i2.37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Mamadalieva N.Z., Böhmdorfer S., Zengin G., Bacher M., Potthast A., Akramov D.K., Janibekov A., Rosenau T. Phytochemical and biological activities of Silene viridiflora extractives. Development and validation of a HPTLC method for quantification of 20-hydroxyecdysone. Ind. Crops Prod. 2019;129:542–548. doi: 10.1016/j.indcrop.2018.12.041. [DOI] [Google Scholar]
- 51.Zengin G. A study on in vitro enzyme inhibitory properties of Asphodeline anatolica: New sources of natural inhibitors for public health problems. Ind. Crops Prod. 2016;83:39–43. doi: 10.1016/j.indcrop.2015.12.033. [DOI] [Google Scholar]
- 52.Brereton R.G. Chemometrics, Data Analysis for the Laboratory and Chemical Plant. John Wiley & Sons, Ltd.; Hoboken, NJ, USA: 2003. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data are available upon request from the first author.