Abstract
Plants are sources of sunscreen ingredients that prevent cellular mutations involved in skin cancer and aging. This study investigated the sunscreen properties of the extracts from some ornamental plants growing in Colombia. The UV filter capability of the flower extracts obtained from Rosa centifolia L., Posoqueria latifolia (Rudge) Schult, and Ipomoea horsfalliae Hook. was examined. Photoprotection efficacies were evaluated using in vitro indices such as sun protection factor and critical wavelength. UVB antigenotoxicity estimates measured with the SOS Chromotest were also obtained. Extract cytotoxicity and genotoxicity were studied in human fibroblasts using the trypan blue exclusion and Comet assays, respectively. Major compounds of the promising flower extracts were identified by UHPLC–ESI+–Orbitrap–MS. The studied extracts showed high photoprotection efficacy and antigenotoxicity against UVB radiation, but only the P. latifolia extract showed broad-spectrum photoprotection at non-cytotoxic concentrations. The P. latifolia extract appeared to be safer for human fibroblast cells and the R. centifolia extract was shown to be moderately cytotoxic and genotoxic at the highest assayed concentrations. The I. horsfalliae extract was unequivocally cytotoxic and genotoxic. The major constituents of the promising extracts were as follows: chlorogenic acid, ecdysterone 20E, rhamnetin-rutinoside, cis-resveratrol-diglucoside, trans-resveratrol-diglucoside in P. latifolia; quercetin, quercetin-glucoside, quercetin-3-rhamnoside, kaempferol, kaempferol-3-glucoside, and kaempferol-rhamnoside in R. centifolia. The potential of the ornamental plants as sources of sunscreen ingredients was discussed.
Keywords: ultraviolet light, photoprotection, antigenotoxicity, cytotoxicity, genotoxicity, human fibroblasts, ornamental plants
1. Introduction
Photoprotection is a preventive strategy to defend human skin against cancer and photoaging [1]. The sunlight UV rays that reach the Earth’s surface, such as ultraviolet A (320–400 nm) and ultraviolet B (280–320 nm), cause DNA damage (e.g., cyclobutane pyrimidine dimers), which initiates inflammatory processes and skin cancer [2,3]. The use of sunscreens is among the most popular strategies in photoprotection [4]. Sunscreens contain compounds that act like filters, either absorbing or refracting the UV radiation [5]. Relevant properties of sunscreens used commercially have been reviewed [6,7].
It has been indicated that active ingredients of commercial sunscreens can be toxic to humans and coral reefs [8,9,10,11], which has increased the interest in using phytochemicals in sunscreen formulations [12,13,14,15,16,17]. Phytochemicals are genoprotective and anticancer agents [18,19] that are considered to be non-toxic and pharmacologically safe for human consumption [13]. These phytochemicals can prevent cellular mutations involved in skin cancer and aging by regulating UV-induced mutability [20,21].
Among the wide plant diversity, ornamental species have been widely used as raw materials for the cosmetic industry due to both their fragrance and therapeutic properties [22]. Colombia is the second highest exporter of ornamental flowers worldwide [23]; however, until today, their use has been exclusively for decorative purposes. In the present study, we took advantage of the cosmetic and therapeutic properties of ornamental plants to find phytochemicals that can be used as sunscreen ingredients.
The work aims were as follows: (i) to evaluate the UV absorption capability of flower extracts obtained from some ornamental plants growing in Colombia by means of in vitro protection efficacies (SPFin vitro, and λc), (ii) to study the correlation between in vitro UVB protection efficacy (SPFin vitro) and complementary SOS Chromotest-based DNA protection using data from flower extracts, (iii) to evaluate in human fibroblast the cytotoxicity and genotoxicity of promising extracts, and (iv) to study the chemical composition of promising extracts as sources of sunscreens. We showed that the flower extracts isolated from ornamental plants, R. centifolia and P. latifolia, were rich in photoprotective compounds. P. latifolia extract, which is photostable and relatively safe, seems to be a good candidate for a sunscreen active ingredient.
2. Results
2.1. In Vitro Photoprotection Efficacy of the Flower Extracts
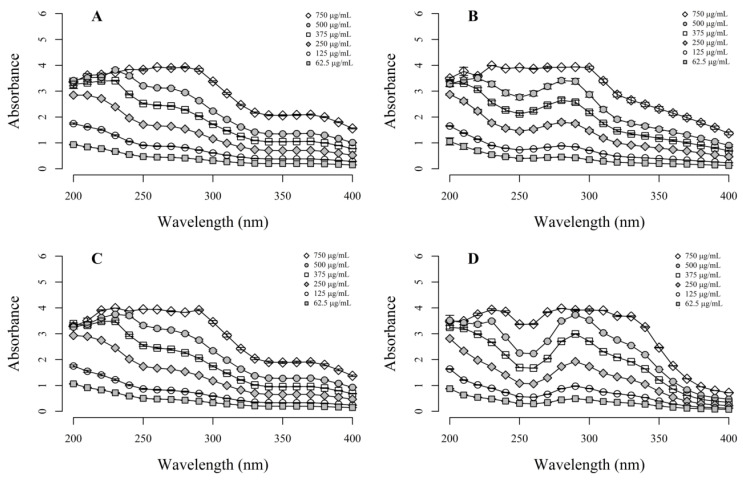
The UV absorbance spectrum of each flower extract is shown in Figure 1; high levels of absorbance across the UV spectrum were observed for all extracts. The extracts showed the highest absorbance peaks for λ between 225 and 320 nm. All the absorbance peaks depended on the extract concentrations.
Figure 1.
UV absorbance spectra of the flower extracts obtained from: (A) R. centifolia pink, commercial variety, (B) R. centifolia fuchsia, commercial variety, (C) P. latifolia, and (D) I. horsfalliae. Error bars indicate the standard error of the mean for at least three independent experiments (n = 3).
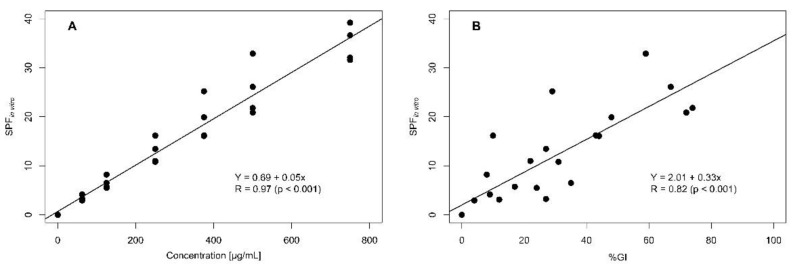
The SPFin vitro values calculated for each flower extract are presented in Table 1. According to the European Commission categories (see Section 4), the four flower extracts showed UVB photoprotection efficacy (SPFin vitro ≥ 6.0); the SPFin vitro values increased with concentration (R = 0.97, p ˂ 0.001, Figure 2A). Except for the I. horsfalliae extract, other extracts also showed broad-spectrum (UVA-UVB) protection efficacy (λc ≥ 370 nm) at a high extract concentration (750 µg/mL).
Table 1.
List of the studied plant species. For each plant extract, the following indices are shown: (i) UVB protection efficacy (SPFin vitro); (ii) critical wavelength (λc); (iii) genotoxicity inhibition percentage (%GI) obtained using the SOS Chromotest; and (iv) percentages of human fibroblast cell viability (%CV). At photoprotective and non-cytotoxic extract concentrations, the percentage of effectiveness (Eff) values was also estimated for the minimum erythema dose (MDE) according to the Fitzpatrick skin scale.
| Species (CNH Voucher) | Conc. (µg/mL) | SPFin vitro | λc | %GI | %CV | Eff I | Eff II | Eff III | Eff IV |
|---|---|---|---|---|---|---|---|---|---|
| Rosa centifolia pink, commercial variety | 0 | 0 ± 0 | 0 ± 0 | 0 ± 0 | 93 ± 2 | - | - | - | - |
| 62 | 3 ± 0 | 360 ± 0 | 4 ± 4 | 93 ± 2 | - | - | - | - | |
| 125 | 6 ± 0 | 360 ± 0 | 17 ± 6 | 91 ± 2 | - | - | - | - | |
| 250 | 11 ± 0 | 360 ± 0 | 31 ± 7 | 88 ± 2 | 95% | 98% | 100% | 97% | |
| LC30 = 363 | 15 ± 0 | 360 ± 0 | 44 ± 3 | 70 ± 0 | 93% | 100% | 100% | 100% | |
| LC50 = 492 | 21 ± 0 | 360 ± 0 | 50 ± 1 | 50 ± 0 | 83% | 96% | 87% | 84% | |
| 500 | 21 ± 0 | 360 ± 0 | 56 ± 2 | 49 ± 16 | - | - | - | - | |
| 750 | 32 ± 0 | 370 ± 0 | 72 ± 2 | 22 ± 10 | - | - | - | - | |
| Rosa centifolia fuchsia, commercial variety | 0 | 0 ± 0 | 0 ± 0 | 0 ± 0 | 94 ± 1 | - | - | - | - |
| 62 | 3 ± 0 | 357 ± 0 | 12 ± 8 | 93 ± 1 | - | - | - | - | |
| 125 | 5 ± 0 | 353 ± 0 | 24 ± 5 | 91 ± 1 | - | - | - | - | |
| 250 | 10 ± 0 | 360 ± 0 | 22 ± 7 | 87 ± 3 | 88% | 92% | 99% | 100% | |
| LC30 = 492 | 23 ± 0 | 360 ± 0 | 30 ± 5 | 70 ± 0 | 88% | 100% | 84% | 90% | |
| 500 | 25 ± 0 | 360 ± 0 | 43 ± 3 | 69 ± 13 | 73% | 84% | 80% | 79% | |
| LC50 = 702 | 36 ± 1 | 370 ± 0 | 55 ± 3 | 50 ± 0 | 91% | 94% | 89% | 94% | |
| 750 | 32 ± 1 | 370 ± 0 | 74 ± 3 | 45 ± 6 | - | - | - | - | |
| Posoqueria latifolia (COL512080) | 0 | 0 ± 0 | 0 ± 0 | 0 ± 0 | 90 ± 3 | - | - | - | - |
| 62 | 3 ± 0 | 357 ± 0 | 27 ± 2 | 90 ± 2 | - | - | - | - | |
| 125 | 6 ± 0 | 360 ± 0 | 27 ± 2 | 90 ± 1 | - | - | - | - | |
| 250 | 13 ± 1 | 360 ± 0 | 27 ± 2 | 88 ± 3 | 81% | 79% | 90% | 90% | |
| 375 | 19 ± 0 | 360 ± 0 | 48 ± 2 | 84 ± 4 | 86% | 83% | 88% | 90% | |
| 500 | 26 ± 2 | 360 ± 0 | 58 ± 2 | 82 ± 6 | 89% | 98% | 90% | 100% | |
| 750 | 35 ± 1 | 370 ± 0 | 67 ± 2 | 73 ± 6 | 91% | 93% | 92% | 99% | |
| Ipomoea horsfalliae (COL587134) | 0 | 0 ± 0 | 0 ± 0 | 0 ± 0 | 93 ± 1 | - | - | - | - |
| 62 | 4 ± 0 | 340 ± 0 | 9 ± 5 | 89 ± 0 | - | - | - | - | |
| 125 | 7 ± 0 | 340 ± 0 | 8 ± 6 | 76 ± 5 | 87% | 89% | 90% | 91% | |
| LC30 = 250 | 12 ± 0 | 340 ± 0 | 10 ± 4 | 70 ± 9 | 100% | 93% | 90% | 96% | |
| LC50 = 398 | 39 ± 0 | 350 ± 0 | 20 ± 6 | 50 ± 0 | 99% | 100% | 99% | 100% | |
| 500 | 39 ± 0 | 350 ± 0 | 29 ± 8 | 36 ± 12 | - | - | - | - | |
| 750 | 39 ± 0 | 350 ± 0 | 59 ± 3 | 16 ± 5 | - | - | - | - | |
| Commercial sunscreen | 0 | 0 ± 0 | 0 ± 0 | 0 ± 0 | 94 ± 0 | - | - | - | - |
| (Eau Thermale Avène SPF 50+) †,‡ | 465 | 27 ± 0 | 370 ± 0 | 0 ± 0 | 84 ± 1 | 100% | 100% | 100% | 100% |
| 930 | 30 ± 0 | 370 ± 0 | 4 ± 0 | 54 ± 6 | 100% | 100% | 100% | 100% | |
| 1870 | 40 ± 0 | 380 ± 0 | 14 ± 1 | 27 ± 5 | - | - | - | - | |
| 3750 | 40 ± 0 | 380 ± 0 | 32 ± 1 | 5 ± 3 | - | - | - | - | |
| 7500 | 40 ± 0 | 380 ± 0 | 42 ± 0 | 2 ± 0 | - | - | - | - | |
| 15,000 | 40 ± 0 | 380 ± 0 | 67 ± 1 | 0 ± 0 | - | - | - | - | |
| 30,000 | 40 ± 0 | 380 ± 0 | 82 ± 1 | 0 ± 0 | - | - | - | - | |
| Titanium dioxide † | 0 | 0 ± 0 | 0 ± 0 | 0 ± 0 | 88 ± 2 | - | - | - | - |
| 50 | 6 ± 0 | 380 ± 0 | 0 ± 0 | 58 ± 1 | 87% | 83% | 75% | 82% | |
| 62 | 6 ± 0 | 390 ± 0 | 12 ± 1 | 39 ± 6 | - | - | - | - | |
| 100 | 11 ± 0 | 390 ± 0 | 20 ± 4 | 19 ± 0 | - | - | - | - | |
| 125 | 12 ± 0 | 390 ± 0 | 32 ± 6 | 0 ± 0 | - | - | - | - | |
| 250 | 26 ± 0 | 390 ± 0 | 57 ± 7 | 0 ± 0 | - | - | - | - | |
| 500 | 40 ± 0 | 380 ± 0 | 82 ± 3 | 0 ± 0 | - | - | - | - | |
| 1000 | 40 ± 0 | 380 ± 0 | 100 ± 2 | 0 ± 0 | - | - | - | - | |
| 2000 | 40 ± 0 | 380 ± 0 | 93 ± 4 | 0 ± 0 | - | - | - | - |
CNH: Colombian National Herbarium. The SPFin vitro values were classified in categories according to the European Commission recommendation as follows: no protection (0.0 ≤ SPFin vitro ≤ 5.9), low protection (6.0 ≤ SPFin vitro ≤ 14.9), medium protection (15.0 ≤ SPFin vitro ≤ 29.9), high protection (30.0 ≤ SPFin vitro ≤ 59.9), and very high protection (SPFin vitro ≥ 60.0). A λc > 370 nm defines broad-spectrum protection. The MDE values were previously indicated by Valbuena et al. [24], and these are as follows: type I (0.035 J/cm2 = 350 J/m2), type II (0.056 J/cm2 = 560 J/m2), type III (0.070 J/cm2 = 700 J/m2), and type IV (0.084 J/cm2 = 840 J/m2). †, For the comparison, a widely used commercial sunscreen (Eau Thermale Avène SPF 50+) and sunscreen ingredient (titanium dioxide) were included. ‡, The higher sunscreen concentration (v/v) evaluated was 30 mg/mL, dissolved in distilled water.
Figure 2.
Correlation between UVB photoprotection efficacy (SPFin vitro) and extract concentration (A) and %GI estimates (B). A database containing 28 paired SPFin vitro and %GI values, corresponding to photoprotective flower extracts, was used.
2.2. Relations between SPFin vitro and %GI Estimates in Flower Extracts
None of the flower extracts had increased I values at concentrations assayed in the SOS Chromotest (data not shown); therefore, they were considered not genotoxic in the model E. coli PQ37 cells. All photoprotective extracts also showed antigenotoxic against UVB radiation (Table 1). The SPFin vitro and %GI values in flower extracts were highly correlated (R = 0.82, p ˂ 0.001, Figure 2B). That is, the greater the UVB photoprotective efficiency, the lower the genetic damage.
2.3. Extract Cytotoxicity in Human Fibroblast (MRC5) Cells
Cytotoxicity dose–response relations were studied for each flower extract at a concentration range between 62 and 750 µg/mL (Table 1). Next, lethal concentrations, 50% (LC50) and 30% (LC30), in human fibroblast (MRC5) cells were obtained by interpolation. Based on the LC50 values, extract cytotoxicity was as follows: I. horsfalliae (398 µg/mL) ˃ R. centifolia pink, commercial variety (492 µg/mL) ˃ R. centifolia fuchsia, commercial variety (702 µg/mL). The extracts were safe for fibroblast cells at concentrations lower than LC30 values as follows: I. horsfalliae (250 µg/mL); R. centifolia pink, commercial variety (363 µg/mL); and R. centifolia fuchsia, commercial variety (492 µg/mL). The P. latifolia extract was unique in that it was shown to be non-cytotoxic to fibroblast cells at the concentration range studied. All the extracts were relatively less cytotoxic than the commercial sunscreen (Eau Thermale Avène SPF 50+) and the sunscreen active ingredient (titanium dioxide) used for comparison.
2.4. Extract Genotoxicity in Human Fibroblast (MRC5) Cells
Genotoxicity dose–response relations were studied for each flower extract (Table 2). According to genotoxicity criteria, the extracts produced some degree of DNA damage at the following concentrations: I. horsfalliae (187.5 µg/mL) ˃ R. centifolia (375.0 µg/mL) ˃ P. latifolia (750 µg/mL). Evaluation of the equivalent solvent concentrations (dilutions) in extracts indicated that the solvent (methanol) was not genotoxic in human fibroblasts for the concentration range tested. Therefore, except for P. latifolia, the extracts showed low-to-moderate genotoxicity across the concentration range studied. Such genotoxicity showed a clear dose–response relationship (extract concentration–DNA damage), and demonstrates the importance of establishing the safe extract concentrations for potential use as sunscreen ingredients. These data also suggest a cytotoxic mode of action depending on the genotoxicity for all extracts.
Table 2.
Genotoxicity of the flower extracts in MRC5 human fibroblasts cells. The genetic damage index (GDI) values and their corresponding standard errors, calculated from at least three independent experiments, are given. Pearson correlation coefficients (R) showing the relationship between GDI extract concentrations are also presented.
| Conc. (µg/mL) | ± SE) † | ||||
|---|---|---|---|---|---|
| R. centifolia (Pink) | R. centifolia (Fuchsia) | P. latifolia | I. horsfalliae | SEC ‡ | |
| 0.0 | 0.12 ± 0.05 | 0.08 ± 0.02 | 0.20 ± 0.05 | 0.21 ± 0.04 | 0.19 ± 0.05 |
| 46.9 | 0.39 ± 0.04 | 0.21 ± 0.09 | 0.48 ± 0.03 | 0.51 ± 0.22 | 0.80 ± 0.28 |
| 93.7 | 0.46 ± 0.04 | 0.27 ± 0.07 | 0.65 ± 0.13 | 0.90 ± 0.25 | 0.84 ± 0.28 |
| 187.5 | 0.98 ± 0.09 | 0.74 ± 0.20 | 0.66 ± 0.08 | 2.20 ± 0.40 | 0.83 ± 0.28 |
| 375.0 | 1.30 ± 0.33 | 1.93 ± 0.28 | 0.99 ± 0.27 | 3.31 ± 0.05 | 0.85 ± 0.30 |
| 750.0 | 2.39 ± 0.52 | 3.55 ± 0.04 | 1.56 ± 0.09 | 3.82 ± 0.03 | 0.91 ± 0.28 |
| PC | 3.91 ± 0.02 | 3.91 ± 0.01 | 3.93 ± 0.02 | 3.93 ± 0.02 | 3.90 ± 0.08 |
| R= | 0.99 (p ˂ 0.05) | 0.99 (p ˂ 0.05) | 0.98 (p ˂ 0.05) | 0.92 (p ˂ 0.05) | 0.53 (p = 0.28) |
The standard mutagen 4-nitro-quinoline-1-oxide (0.89 µg/mL) was used as positive control (PC). †, The DNA damage criteria were as follows: (i) GDI values between 0 and 1 (no DNA damage); (ii) GDI values between 1 and 2 (little DNA damage); (iii) GDI values between 2 and 3 (moderate DNA damage); and (iv) GDI values between 3 and 4 (severe DNA damage). In addition, a clear dose–response relationship (concentration–DNA damage) must exist. ‡, SEC: GDI values for solvent (methanol) equivalent concentrations (between 39 and 618 mM) in the extracts.
2.5. In Vitro Photoprotection Efficacy and Photostability at Safe Extract Concentrations
At photoprotective and safe extract concentrations, namely, at non-cytotoxic (≤LC30) and non-genotoxic concentrations, only the P. latifolia extract showed high UVB and broad-spectrum (UVA-UVB) photoprotection efficacy values (Table 1). The other extracts demonstrated a reduction in their SPFin vitro values, which indicates their low photoprotection efficacy (6.0 ≤ SPFin vitro ≤ 15.0; λc ˂ 370 nm) values. Conversely, extract photostability or effectiveness (Eff) when they were irradiated at Fitzpatrick’s MDE was consistently high at photoprotective and safe extract concentrations. According to these results, the P. latifolia and R. centifolia extracts at non-cytotoxic concentrations could be good candidates for use as sunscreen active ingredients. Conversely, the I. horsfalliae extract at non-cytotoxic concentrations was shown to be genotoxic (Table 2) and poorly photoprotective (Table 1); therefore, this extract was excluded from further analyses.
2.6. Chemical Characterization of the Promising Flower Extracts by UHPLC–ESI+–Orbitrap–MS
The yields of the hydroalcoholic flower extracts were as follows: P. latifolia (41.2 ± 0.1%) ˃ R. centifolia fuchsia, commercial variety (30.9 ± 0.0%) ˃ R. centifolia pink, commercial variety (18.2 ± 0.1%). The UHPLC–ESI+–Orbitrap–MS analysis of the flower extracts from R. centifolia and P. latifolia plant species allowed us to identify presumptively several compounds on the basis of their mass spectra fragmentation patterns and exact mass measurements (Table 3).
Table 3.
Major constituents identified in flower extracts using UHPLC–ESI+–Orbitrap–MS. Extract constituents were dependent on their retention times (min) in chromatograms. The extracts were as follows: A—Rosa centifolia pink, commercial variety, B—Rosa centifolia fuchsia, commercial variety, and C—Posoqueria latifolia.
| No. | tR, min | Compounds | Formula | Calculated Mass | Experim. Mass. | ∆ppm | HCD, eV | Product Ions | mg/g of Extract | ||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ± SE, n = 3) | |||||||||||||
| [M]+ | [M + H]+ | Fragment Type | m/z (I, %) | A | B | C | |||||||
| 1 | 3.30 | Cyanidin-3,5-glucoside a,b,c | C27H31O16 | 611.1612 | - | 611.15987 | 1.37 | 20 | [M-C6H10O5]+ [M-2C6H10O5]+ [M-2C6H10O5-C8H6O3]+ |
449.10965 (100) 287.05522 (52) 137.02374 (21) |
- | 34 ± 1 | - |
| 2 | 3.61 | Chlorogenic acid a,b,c | C16H18O9 | - | 355.10235 | 355.10384 | 0.76 | 10 | [(M + H)-H2O]+ [(M + H)-C7H10O5]+ [(M + H)-C7H10O5-H2O]+ |
337.09030 (0.3) 181.04933 (2) 163.03917 (100) |
- | - | 35 ± 1 |
| 3 | 3.83 | Quercetin-rutinoside-rhamnoside a,b | C33H40O20 | - | 757.21856 | 757.21907 | 0.66 | 0 | [(M + H)-C6H10O4]+ [(M + H)-C6H10O4-H2O]+ [(M + H)-C6H10O4-C6H10O5]+ [(M + H)-C6H10O4-C6H10O5-C6H10O4]+ |
611.16151 (31) 593.1487 (0.1) 449.10834 (3) 303.05017 (100) |
- | - | 1.5 ± 0.1 |
| 4 | 3.96 | Kaempferol-rhamninoside a,b | C33H40O19 | - | 741.22365 | 741.22209 | 2.10 | 10 | [(M + H)-C6H10O4]+ [(M + H)-2C6H10O4]+ [(M + H)-2C6H10O4-C6H10O5]+ [(M + H)-2C6H10O4-C6H10O5-C8H6O2]+ |
595.16736 (13) 449.1091 (13) 287.05585 (100) 153.01669 (0.1) |
- | - | 3.7 ± 0.4 |
| 5 | 4.00 | Rhamnetin-rhamnoside a,b | C34H42O20 | - | 771.23421 | 771.23531 | 1.41 | 0 | [(M + H)-C6H10O4]+ [(M + H)-C6H10O4-H2O]+ [(M + H)-2C6H10O4]+ [(M + H)-2C6H10O4-C6H10O5]+ [(M + H)-2C6H10O4-C6H10O5-C8H6O3]+ |
625.17715 (34) 607.16452 (0.3) 479.11913 (17) 317.06532 (100) 167.03427 (0.1) |
- | - | 1.7 ± 0.1 |
| 6 | 4.10 | Ecdysterone a,b | C27H44O7 | - | 481.31598 | 481.31476 | 1.22 | 0 | [(M + H)-H2O]+ [(M + H)-2H2O]+ [(M + H)-3H2O]+ [(M + H)-4H2O]+ |
463.30446 (100) 445.29547 (64) 427.28415 (16) 409.27374 (1) |
- | - | 64 ± 8 |
| 7 | 4.10 | Quercetin-3-rutinoside a,b,c | C27H30O16 | - | 611.1612 | 611.16095 | 0.47 | 10 | [(M + H)-C6H10O4]+ [(M + H)-C6H10O4-C6H10O5]+ [(M + H)-C6H10O4-C6H10O5-C8H6O3]+ |
465.10226 (30) 303.04836 (100) 153.01828 (7) |
1.3 ± 0.1 | 4.5 ± 0.1 | 6.9 ± 0.4 |
| 8 | 4.20 | Quercetin-glucoside a,b,c | C21H20O12 | - | 465.10275 | 465.10321 | 0.98 | 10 | [(M + H)-C6H10O5]+ [(M + H)-C6H10O5-C8H6O3]+ |
303.04956 (100) 153.01776 (2) |
6.3 ± 0.7 | 17 ± 1 | 1.3 ± 0.1 |
| 9 | 4.31 | Kaempferol-neohesperidoside a,b | C27H30O15 | - | 595.16574 | 595.16379 | 3.29 | 10 | [(M + H)-H2O]+ [(M + H)-C6H10O4]+ [(M + H)-C6H10O4-C6H10O5]+ [(M + H)-C6H10O4-C6H10O5-C8H6O2]+ |
577.15659 (0.2) 449.10574 (33) 287.05545 (100) 153.01762 (1) |
- | - | 8.7 ± 0.5 |
| 10 | 4.31 | Rhamnetin-rutinoside a,b | C28H32O16 | - | 625.17631 | 625.17697 | 1.06 | 10 | [(M + H)-C6H10O4]+ [(M + H)-C6H10O4-C6H10O5]+ [(M + H)-C6H10O4-C6H10O5-C8H6O3]+ |
479.11914 (27) 317.06548 (100) 167.03214 (0.01) |
- | - | 17 ± 1 |
| 11 | 4.33 | Quercetin-arabinoside a,b | C20H19O11 | - | 435.09218 | 435.0923 | 0.11 | 10 | [(M + H)-C5H8O4]+ [(M + H)-C5H8O4-C8H6O3]+ |
303.04884 (100) 153.01845 (6) |
1.08 ± 0.04 | 1.4 ± 0.2 | - |
| 12 | 4.43 | Quercetin-3-rhamnoside a,b,c | C21H20O11 | - | 449.10838 | 449.10800 | 0.36 | 10 | [(M + H)-C6H10O4]+ [(M + H)-C6H10O4-C8H6O3]+ |
303.04836 (100) 153.01828 (4) |
49 ± 2 | 32 ± 1 | - |
| 13 | 4.43 | Kaempferol-3-glucoside a,b,c | C21H20O11 | - | 449.10783 | 449.10773 | 0.24 | 10 | [(M + H)-C6H10O5]+ [(M + H)-C6H10O5-C8H6O2]+ |
287.05415 (100) 153.01802 (1) |
70 ± 12 | 41 ± 1 | - |
| 14 | 4.57 | Kaempferol-arabinoside a,b | C20H18O10 | - | 419.09727 | 419.09756 | 0.69 | 0 | [(M + H)-C5H8O4]+ [(M + H)-C5H8O4-C8H6O2]+ |
287.05553 (85) 153.01845 (1) |
6.4 ± 0.5 | 2.0 ± 0.1 | |
| 15 | 4.60 | Rosmarinic acid a,b,c | C18H16O8 | - | 361.09179 | 361.09157 | 0.62 | 10 | [(M + H)-H2O]+ [(M + H)-C9H8O4]+ [(M + H)-C9H8O4-H2O]+ |
343.07965 (0.2) 181.04958 (10) 163.03841 (100) |
- | - | 0.5 ± 0.0 |
| 16 | 4.60 | cis-Resveratrol-diglucoside a,b | C26H32O13 | - | 553.19156 | 553.19199 | 0.41 | 10 | [(M + H)-H2O]+ [(M + H)-2H2O]+ [(M + H)-C6H10O5]+ [(M + H)-C6H10O5-H2O]+ [(M + H)-2C6H10O5]+ |
535.18141 (3) 517.17064 (0.4) 391.13762 (94) 373.12898 (100) 229.08403 (0.1) |
- | - | 140 ± 7 |
| 17 | 4.70 | Kaempferol-rhamnoside a,b | C21H21O10 | - | 433.11292 | 433.11453 | 1.39 | 10 | [(M + H)-C6H10O4]+ [(M + H)-C6H10O4-C8H6O2]+ |
287.05499 (100) 153.01749 (0.3) |
64 ± 8 | 23 ± 2 | - |
| 18 | 4.75 | trans-Resveratrol-diglucoside a,b | C26H32O13 | - | 553.19156 | 553.19199 | 0.41 | 10 | [(M + H)-H2O]+ [(M + H)-2H2O]+ [(M + H)-C6H10O5]+ [(M + H)-C6H10O5-H2O]+ [(M + H)-2C6H10O5]+ |
535.18141 (8) 517.17064 (0.2) 391.13762 (100) 373.12898 (62) 229.08403 (0.1) |
- | - | 280 ± 16 |
| 19 | 5.16 | Quercetin a,b,c | C15H10O7 | - | 303.05047 | 303.0499 | 0.10 | 10 | [(M + H)-C8H6O3]+ | 153.01776 (1) | 160 ± 26 | 130 ± 14 | - |
| 20 | 5.63 | Kaempferol a,b,c | C15H10O6 | - | 287.05501 | 287.05647 | 5.08 | 10 | [(M + H)-C8H6O2]+ | 153.01897 (0.2) | 146 ± 5 | 51 ± 4 | - |
a Tentative identification based on comparison with [M+] or [M + H]+ ions reported in the literature for Rosa spp. [25,26]. b Tentative identification based on comparison with molecule fragmentation pattern in mass spectra and on databases [27,28,29,30]. c Standard compounds used for the comparison of their mass spectra with those present in the extracts studied. HCD, higher-energy-collisional-dissociation cell.
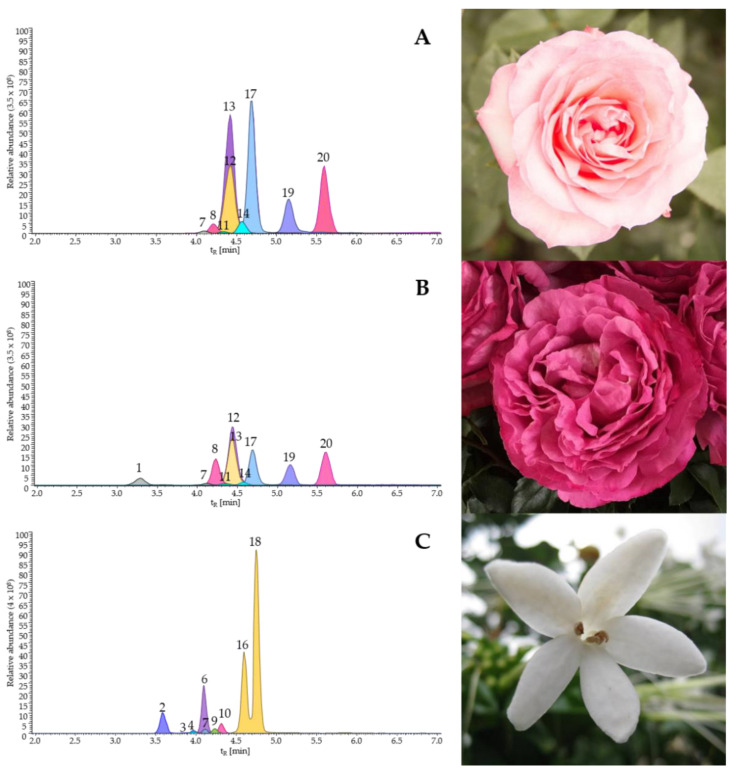
The major compounds in the extracts (≥17 mg ± SD/g of extracts) were as follows: quercetin-3-rhamnoside (49 ± 2), kaempferol-3-glucoside (70 ± 12), kaempferol-rhamnoside (64 ± 8), quercetin (160 ± 26), and kaempferol (146 ± 5) in R. centifolia pink, commercial variety; cyanidin-3,5-glucoside (34 ± 1), quercetin-glucoside (17 ± 1), quercetin-3-rhamnoside (32 ± 1), kaempferol-3-glucoside (41 ± 1), kaempferol-rhamnoside (23 ± 2), quercetin (130 ± 14), and kaempferol (51 ± 4) in R. centifolia fuchsia, commercial variety; chlorogenic acid (35 ± 1), ecdysterone (64 ± 8), rhamnetin-rutinoside (17 ± 1), cis-resveratrol-diglucoside (140 ± 7), and trans-resveratrol-diglucoside (280 ± 16) in P. latifolia. Other compounds were also detected in the extracts at a minor proportion (˂17 mg ± SD/g of extracts), as follows: quercetin-rutinoside-rhamnoside (1.5 ± 0.1), kaempferol-rhamninoside (3.7 ± 0.4), ramnetin-rhamnoside (1.7 ± 0.1), quercetin-3-rutinoside (6.9 ± 0.4), kaempferol-neohesperidoside (8.7 ± 0.5), and quercetin-arabinoside (1.4 ± 0.2). The chromatographic profiles obtained for the flower extracts studied, using the UHPLC–ESI+–Orbitrap–MS technique, are shown in Figure 3.
Figure 3.
The extracted ionic currents (EICs) of [M]+ or protonated molecules [M + H]+ present in the total ion current (TIC) obtained by UHPLC–ESI+–Orbitrap–MS for three flower extracts: (A) R. centifolia (pink variety), (B) R. centifolia (fuchsia variety), and (C) P. latifolia. The peak numbers correspond to major compounds as follows: 1—Cyanidin-3,5-glucoside; 2—Chlorogenic acid; 6—Ecdysterone; 8—Quercetin-glucoside; 10—Rhamnetin-rutinoside; 12—Quercetin-3-rhamnoside; 13—Kaempferol-3-glucoside; 16—cis-Resveratrol-diglucoside; 17—Kaempferol-rhamnoside; 18—trans-Resveratrol-diglucoside; 19—Quercetin; 20—Kaempferol.
3. Discussion
The present work evidenced that the flower extracts isolated from several ornamental plants cultivated in Colombia contained compounds that could be used as sunscreen ingredients. Among the eleven plant species studied in our project (data not shown), three plants (R. centifolia, P. latifolia, and I. horsfalliae) in particular showed good photoprotective properties. This finding provides new evidence on plant extract applicability as a source of solar filters (Table 4).
Table 4.
Some plant species with reported sunscreen properties †.
| Plant Family | Species Name | UV Protective Rank | References |
|---|---|---|---|
| Adoxaceae | Sambucus nigra | UVA | Jarzycka et al. [34] |
| Asteraceae | Achyrocline satureioides | UVB | Fuentes et al. [17] |
| Baccharis antioquensis | UVA-UVB | Mejía-Giraldo et al. [14,35,36] | |
| Chromolaena pellia | UVA-UVB | Fuentes et al. [17] | |
| Helichrysum arenarium | UVA | Jarzycka et al. [34] | |
| Pentacalia pulchella | UVA-UVB | Mejía-Giraldo et al. [14,35,37] | |
| Bromeliaceae | Neoglaziovia variegata | UVB | de Oliveira-Junior et al. [38] |
| Calophyllaceae | Calophyllum inophyllum | UVA-UVB | Ku et al. [39] |
| Convolvulaceae | Ipomoea horsfalliae | UVB | Sierra et al. [40] |
| Cucurbitaceae | Momordica charantia | UVB | Guimarães de Sousa et al. [41] |
| Clusiaceae | Garcinia brasiliensis | UVB | Figueiredo et al. [42] |
| Fabaceae | Bauhinia microstachya | UVB | Reis-Mansur et al. [43] |
| Dimorphandra gardneriana | UVB | Nunes et al. [15] | |
| Myricaceae | Morella parvifolia | UVA-UVB | Puertas-Mejía et al. [44] |
| Nycataginaceae | Boerhavia diffusa | UVB | Guimarães de Sousa et al. [41] |
| Rosaceae | Crataegus monogyna | UVA | Jarzycka et al. [34] |
| Verbenaceae | Lippia microphylla | UVB | Nunes et al. [15] |
| Lippia origanoides | UVB | Fuentes et al. [17] | |
| Vitaceae | Vitis vinifera | UVA-UVB | Hübner et al. [45] |
†, Modified from Fuentes et al. [17].
We also showed that the photoprotection efficacy depended on extract concentration. The photoprotective extracts were also antigenotoxic against UVB. This indicates that they acted as filters that absorbed or refracted the UV rays and reduced genotoxicity. As in the previous studies [17,31,32,33], our data support the use of DNA damage detection assay (in this case, the SOS Chromotest) as an effective complement that improves the efficacy of photoprotection measurement.
As we indicated in the Introduction, some organic ingredients of commercial sunscreens can be toxic to humans and coral reefs [8,9,10], with inorganic filters (i.e., titanium dioxide or zinc oxide) being a safer alternative [11]. At photoprotective concentrations, the extracts were relatively less cytotoxic than a commercial sunscreen (Eau Thermale Avène SPF 50+), and its active ingredient (titanium dioxide) was used for comparison. In this sense, the plants studied here could be new and safer sources of ingredients for the development of commercial sunscreens.
The P. latifolia extract was the most promising for use as a sunscreen, because it showed high photoprotective efficacy, antigenotoxicity, photostability, and relatively low cytotoxicity and genotoxicity in human fibroblasts. P. latifolia, commonly known as jasmine tree—mountain lily (azuceno)—monkey apple, is a native plant from Central and South America [46]. This tree possesses flowers with a very intense scent, whose main fragrance compound is (Z)-3-hexenyl acetate [47]. We found that P. latifolia ethanolic extract was rich in cis- and trans-resveratrol diglucosides, two stilbenoid isomers, which were previously identified in Vitis vinifera [48] and Glycine max [49]. Chlorogenic acid (35 ± 1 mg/g), ecdysterone (64 ± 8 mg/g), and rhamnetin-rutinoside (17 ± 1 mg/g) were present as well at relevant concentrations. Chlorogenic acid, resveratrol, and its derivatives have been reported as compounds with solar filter activity [50,51,52,53]. Resveratrol and its derivatives have also shown antigenotoxicity against UV radiation and skin cancer chemopreventive properties [54,55,56]. We hypothesized that some of these compounds, or their combinations, were responsible for the sunscreen/antigenotoxic properties of the extracts studied here. Among the plant species studied, the P. latifolia extract appeared to be the best candidate and the safest for use as a sunscreen active ingredient.
The flower extracts from R. centifolia had high UVB and broad-spectrum protection efficacies as well. However, these extracts showed between low and moderate cytotoxicity and genotoxicity in human fibroblasts measured at the highest concentrations compared with those Rosa species extracts previously studied [57]. This supported the importance of establishing safe extract concentrations for use of the extracts as sunscreen ingredients, as has been previously suggested [22]. Thus, these extracts should be used with caution until more details on their genotoxicity are known. The R. centifolia extracts were rich in kaempferol and quercetin and their derivatives (quercetin-glucoside, quercetin-3-rhamnoside, kaempferol-3-glucoside, kaempferol-rhamnoside), as has also been found for other Rosa species [26,58,59,60,61]. For R. centifolia species, the antimutagenic activity has been previously reported against ethyl methanesulfonate [62]. Kaempferol and quercetin showed solar filter activity [52,53,63]. These main compounds were possibly responsible for the sunscreen/antigenotoxic properties of the R. centifolia extracts studied in this work.
Conversely, the extract obtained from I. horsfalliae, a species with previously reported sunscreen properties [40], was shown to be cytotoxic and genotoxic to human fibroblast cells. Little is still known about the genotoxicity of the major compounds (chlorogenic acid, dicaffeoylquinic acid, and scopoletin) of this extract. Chlorogenic acid and scopoletin have shown clastogenic activity [64,65], which suggests that flower extract’s genotoxic activity could be related to these compounds, although, a synergistic effect of these compounds could also be the cause of their genotoxicity. Confirmation of this hypothesis requires further studies.
4. Materials and Methods
4.1. Plant Material and Extracts
The flowers from Rosa centifolia (Rosaceae), pink and fuchsia commercial varieties, were supplied by Flexport—Colombia S.A.S. (Bogotá, Cundinamarca, Colombia). The flowers from Posoqueria latifolia (Rubiaceae) and Ipomoea horsfalliae (Convolvulaceae) species were collected from experimental plots at the Agroindustrial Pilot Complex of the National Center for Agroindustrialization of Aromatic and Medicinal Tropical Vegetal Species (CENIVAM). The P. latifolia e I. horsfalliae species were identified at the Colombian National Herbarium, where their vouchers (COL; voucher number in parentheses) were placed (Table 1).
For each specimen, undamaged and fully developed flowers were dried in an Advantage Plus Tray Lyophilizer (Virtis Co., Gardiner, ME, USA) and were used for solvent extraction as indicated by Sierra et al. [40]. In brief, dried flowers (1 g) were mixed with acidified ethanol solution (20 mL, 0.5% HCl, 1:1 v/v) and put for 5 min in an S15H ultrasound bath (Elmasonic, Singen, Germany). The mixture was filtered, and the residue was extracted twice more. Extracts were roto-evaporated and then were dried as indicated above. Extract stock solutions were prepared from the dried powder (30 mg), which were dissolved in methanol (1 mL), vortexed (3 min), exposed to ultrasound (10 min, 40 °C), and centrifuged (5000× g, 10 min). The supernatant (1 mL) was then filtered and was stored at −80 °C in a Thermo Scientific® Series-86 DEG C ultra-low-temperature freezer (Thermo Scientific, Waltham, MA, USA). Before their use, the extract stock solutions were defrosted and refrigerated (5–8 °C) for 24 h.
4.2. Chemicals, Buffer, Enzymes, and Culture Media
The titanium dioxide, Luria–Bertani (LB) media, antibiotics (ampicillin and tetracycline), trypan blue solution (0.4%), Bioultra lyophilized proteinase K, and high-resolution agarose were obtained from Sigma-Aldrich Corp. (Milwaukee, WI, USA). The YOYO solution was purchased from Thermo Scientific (Waltham, MA, USA). The substrates for β-galactosidase (o-nitrophenyl-β-d-galactopyranoside) and alkaline phosphatase (p-nitrophenylphosphate) were purchased from Amresco (Slon, OH, USA). The Dulbecco’s modified Eagle medium (DMEM), Ham’s nutrient mixture F12 (F-12), fetal bovine serum (FBS), phosphate-buffered saline (PBS), trypsin EDTA solution, and antibiotics (penicillin–streptomycin mixture) were acquired from Gibco (Grand Island, NY, USA). Other reagents and solvents were obtained from commercial houses J.T. Baker (Phillipsburg, NJ, USA) or Merck (Kenilworth, NJ, USA).
4.3. UV Absorption Capability
Aqueous aliquots (1.5 mL) of each extract, prepared at different concentrations (between 62.5 and 750.0 μg/mL) using distilled water, were placed in a quartz cuvette (1 cm step length and 1.5 mm glass thickness), and their UV absorption spectra (λ = 200–400 nm) were recorded in triplicate using the Skanlt 3.2 function on a Multiskan GO UV spectrophotometer (Thermo Scientific, Waltham, MA, USA). Distilled water was used as a blank. A minimum of three independent experiments were carried out per extract dilution. The absorbance values were recorded for wavelength intervals of 10 nm. The average absorbance values and their corresponding standard errors were plotted using the program ggplot2 of the R platform [66].
4.4. In Vitro Photoprotection Efficacy
We estimated UVB photoprotection efficacy using the sun protection factor (SPFin vitro) described by Sayre et al. [67] and by further simplification to the UV spectrophotometric Mansur´s method [68]: SPFspectrophotometric = , where EE()—erythemal effect spectrum at wavelength λ, I()—solar intensity spectrum at wavelength λ, A()—absorbance of the extract solution determined by UV spectrophotometry at a wavelength (λ), and CF—correction factor (CF = 10). The values of EE (λ) × I were constant [67]. The broad-spectrum protection efficacy was determined by calculating critical wavelength [69]: λc = , where A(λ) was the absorbance at wavelength λ, λc is the critical wavelength (nm), and dλ is the wavelength step (1 nm). The SPFin vitro values were classified in categories according to European Commission recommendations [70], as follows: no protection (0.0 ≤ SPFin vitro ≤ 5.9), low protection (6.0 ≤ SPFin vitro ≤ 14.9), medium protection (15.0 ≤ SPFin vitro ≤ 29.9), high protection (30.0 ≤ SPFin vitro ≤ 59.9), and very high protection (SPFin vitro ≥ 60.0). The broad-spectrum protection sunscreens were those that showed protection efficacy values (SPF ≥ 15 and λc > 370 nm) according to the Food and Drug Administration (FDA) rule [71].
4.5. In Vitro Photostability
Extract aliquots (1 mL) were distributed into Petri plates with a 5 cm diameter for their irradiation. The Petri plates were irradiated in darkness using a UVA/UVB irradiation chamber BS-02 (Dr. Grobel UV-Elektronik GmbH, Etlingen, Germany) equipped with a radiation controller, UV-MAT, from the same commercial house. This radiation controller continuously measured the irradiance, calculated the irradiation dose, and switched the lamps after reaching the target dose. Operating at 100% intensity, the UVB lamps in the irradiation chamber had an irradiance value of 4 mW/cm2. The UVB radiation doses applied were those corresponding to minimum erythema dose (MDE) in humans according to the Fitzpatrick skin scale [24]. These were as follows: type I (0.035 J/cm2 = 350 J/m2), type II (0.056 J/cm2 = 560 J/m2), type III (0.070 J/cm2 = 700 J/m2), and type IV (0.084 J/cm2 = 840 J/m2). The relative photostability of the extracts was expressed as the percentage of effectiveness (Eff) of SPFin vitro after UV exposure and was calculated as follows: [14].
4.6. Antigenotoxicity against UVB Radiation Estimates Based on SOS Chromotest
Before extract antigenotoxic effects were assayed, their genotoxicities were investigated using the SOS Chromotest [72], as was described previously by Quintero et al. [73]. The antigenotoxicity assay was conducted using a co-incubation procedure as described by Fuentes et al. [74]. Briefly, the cells were simultaneously treated with extracts (between 62.5 and 750.0 μg/mL) and 10 J/m2 of UVB radiation, which largely induced the SOS function Escherichia coli PQ37 cells [75]. After, they were cultured for 2 h at 37 °C and shaken at 300 rpm in a Thermomixer apparatus (Eppendorf, Sao Paulo, Brazil). Negative (distilled water) and positive (10 J/m2 of UVB) controls were always included in each assay. A minimum of four independent experiments per treatment with two replicates were conducted. β-Galactosidase (βG) and alkaline phosphatase (AP) activities were assayed in 96-well plates (Brand-GMBH, Wertheim, Germany), as described by Fuentes et al. [74]. The antigenotoxicity (the ability of the plant extract to protect against UV-induced DNA damage) was measured as a significant reduction in the SOS induction factor (I) in E. coli PQ37 cells and was expressed as a percentage of the genotoxicity inhibition, as follows: %GI = 1 − ()/() × 100, where Ict was the SOS induction factor in the co-incubation procedure; Int was the SOS induction factor in non-treated cells, and IUVB was the SOS induction factor in UVB-treated cells. Negative values of %GI were considered as zero; therefore, this parameter ranged from 0% to 100%. The minimal concentration that produces a significant (p ≤ 0.05) genotoxicity inhibition (CGI) in PQ37 cells was used for comparison of the genoprotective potential of the tested samples.
4.7. Extract Cytotoxicity in Human Fibroblast (MRC5) Cells
Cytotoxicity of flower extracts in MRC-5 cells was evaluated using the trypan blue exclusion assay [76]. The lung embryo fibroblast (MRC5) cells [77] were grown on DMEM/F-12 medium (5 mL) supplemented with 10% heat-inactivated fetal bovine serum (FBS) and 1% of GIBCO Penicillin-Streptomycin, at 37 °C, and under CO2 (5%) conditions in a Midi 40 incubator (Thermo Scientific, Marietta, OH, USA). Every three days, cells were grown in fresh medium to reach a confluence of 80%. Cell cultures were mixed with each extract at final concentrations between 62.5 and 750.0 μg/mL, and kept at 37 °C (24 h) under CO2 (5%) atmosphere conditions. After 24 h, trypsin EDTA-treated cells were centrifuged (4000× g, 6 min), dissolved in PBS buffer (100 μL), and mixed (10 μL) with the same volume of trypan blue (0.4%) to assess cell viability. Live and dead cells were counted using a Neubauer chamber and Eclipse E200 optical microscope (Nikon Instruments Inc., NY, USA). At least three independent experiments were carried out for each treatment. The results are expressed as percentages of cell viability (%CV) per treatment, as follows: %CV = (Living cells)/(Total cells) × 100. Lethal concentrations 50% (LC50) and 30% (LC30) for each flower extract were calculated by interpolation using the graphic method [78]. LC50 and LC30 were considered as bordering cytotoxic and non-cytotoxic concentrations, respectively. That is, extracts were cytotoxic at values ≥ LC50 and non-cytotoxic at values ≤ LC30.
4.8. Extract Genotoxicity in Human Fibroblast (MRC5) Cells
Genotoxicity of flower extracts in MRC-5 cells was evaluated using the Comet assay. For this purpose, a high-throughput Trevigen CometChip® platform (Gaithersburg, MD, USA) was used as indicated by Sykora et al. [79], with some minor modifications. Firstly, a CometChip® slide, cleaned previously with ethanol, was covered with an agarose solution prepared in PBS at 1.3% and tempered at 45 °C; then, the agarose was solidified for 24 h at 4 °C. Trypsin-treated cells (3 mL) were collected by centrifugation (2000× g, 6 min), were washed twice using NaCl solution (0.75%), centrifuged and suspended in fresh NaCl solution (3 mL), and then were quantified using a Neubauer counting chamber. A cell suspension (3 mL) prepared at 4.4 × 104 cell/mL was mixed with an equal volume of low-melting-point agarose prepared in PBS at 1%, and the mix was poured on the CometChip® slide. The agarose was solidified for 15 min at 4 °C. Finally, the CometChip® chamber was ensembled by hermetically sealing to prevent mixing between the wells.
For cell treatments, 100 μL extract samples (prepared between 62.5 and 750.0 μg/mL) or standard mutagen used as a positive control (4-nitroquinoline 1-oxide prepared at 0.89 μg/mL) were loaded into wells of the CometChip® chamber. A sample of DMEM medium (100 μL) was considered as the negative control. The chamber with treatments was incubated for 30 min at 37 °C under CO2 (5%) atmosphere conditions. The solutions of each treatment were removed from the wells, and 30 μL of Bioultra Proteinase K (0.19 mg/mL) was loaded in each well for cell enzymatic lysis (1 h, 37 °C).
After that, the CometChip® slide was removed from the CometChip® chamber and submerged for 15 min at 4 °C in a Comet electrophoresis tank (Cleaver Scientific Ltd., Rugby, Warwickshire, UK), which contained alkaline buffer (0.3 N NaOH, 1 mM EDTA, pH 13). The electrophoresis was carried out for 30 min at 300 mA and 25 V. The CometChip® slide was submerged for 15 min in a tray containing neutralizing solution (0.4 M TRIS, pH 7.5); then, it was washed with distilled water and dried at 37 °C in a Midi 40 incubator. Finally, cell nuclei contained in each microgel were stained with 7 µL of YOYO solution (1 mM prepared in 5% DMSO) and were immediately scored using a Zeiss Axio Observer 7 fluorescence microscope (GmbH, Oberkochen, Germany).
DNA damage was expressed in arbitrary units based on the classification of Comets into five categories (0–4), as was proposed by Collins et al. [80]. A genetic damage index (GDI) was calculated for each treatment, as follows: GDI = (N0 × 0 + N1 × 1 + N2 × 2 + N3 × 3 + N4 × 4)/n, where Ni was the number of nuclei scored in each category and n was the number of scored cells per slide [81]. Two hundred cells per slide and two slides per treatment were analyzed. The results from at least three independent experiments were averaged to obtain the GDI values for each treatment.
4.9. UHPLC–ESI+–Orbitrap–MS Analysis
The flower extracts were analyzed by a UHPLC Dionex™ UltiMate™ 3000 (Thermo Fisher Scientific, Bremen, Germany) coupled to an Orbitrap™ mass detector (Exactive Plus, TFS, Bremen, Germany), using a heated-electrospray interface (HESI-II) operated in positive-ion acquisition mode (350 °C). The extract component separation was carried out on a Hypersil GOLD™ aQ column (TFS, Sunnyvale, CA, USA), of 100 mm × 2.1 mm id, ×1.9 μm particle size, at 30 °C. The mobile phase was as follows: A: water (0.2% formic acid) and B: acetonitrile (0.2% formic acid). Analysis started with 100% A and changed linearly up to 100% B in 8 min, remained for 4 min, and then returned to 100% A in 1 min; then, it remained in equilibrium for 3 min. The mobile phase flow was 0.3 mL/min, and the injection volume was 1 μL. Capillary voltage (3.5 kV, 320 °C) and higher-energy-collisional dissociation cell (HCD) were used in 10–40 eV range. Mass range in all experiments was set at m/z 80–1000. The data obtained were processed with the Thermo XCalibur™ Roadmap software, version 3.1.66.10. Compound identification was based on the extracted ion currents (EICs) of the protonated molecules, the exact masses of the ions, mass spectra fragmentation patterns, and by comparison of the experimental mass spectra with those of standards compounds.
4.10. Statistical Analysis
The SPFin vitro, λc, Eff, %GI, survival (%), and GDI values and their corresponding standard errors were calculated. In all cases, the data passed the Kolmogorov–Smirnov and F-maximum tests for normality and variance homogeneity, respectively; therefore, the parametric tests were used in subsequent data analyses. When a significant F-value was obtained in one-way analysis of variance (ANOVA), the groups were subsequently compared with Tukey’s test. The Pearson correlation analysis was used to examine the relationship between extract and compounds concentrations, SPFin vitro, and %GI estimates. For all statistical analyses, a value of p < 0.05 indicated significance. The R program [66] was used for all analyses.
5. Conclusions
Our findings show that flower extracts from ornamental plants were rich in photoprotective compounds. Several studied flower extracts showed solar filter activity and photoprotection, which was attributed to their major compounds or to their combinations. P. latifolia extract, which is photostable and safe, appeared to be the best candidate for use as a sunscreen active ingredient. R. centifolia extracts showed between low and moderate cytotoxicity and genotoxicity in human fibroblasts at the highest concentrations assayed, while the I. horsfalliae extract was unequivocally cytotoxic and genotoxic. Therefore, R. centifolia and I. horsfalliae extracts should be used with caution until more details on their toxicity and genotoxicity are obtained. In addition, it is necessary to test these phytochemicals using in vivo mammalian assays for practical use of these extracts in photoprotection. Sunscreen based on phytochemicals will require cost-effective processes that combine plant tissue culture and enriched-fraction extraction techniques, to guarantee a stable supply of these raw materials.
Acknowledgments
The authors thank Carlos Frederico Martins Menck from Universidade de Sao Paulo for supplying the human fibroblast (MRC5) cell line, and Flexport—Colombia S.A.S for supplying the Rosa centifolia flowers.
Author Contributions
Conceptualization, J.L.F.; methodology, J.L.F., R.E.O. and E.E.S.; validation, C.A.P.B., D.A.V.M., S.J.F.G. and L.J.S.; formal analysis, C.A.P.B., D.A.V.M., S.J.F.G. and L.J.S.; investigation, J.L.F. and E.E.S.; resources, J.L.F. and E.E.S.; data curation, J.L.F., C.A.P.B., D.A.V.M., S.J.F.G. and L.J.S.; writing—original draft preparation, J.L.F.; writing—review and editing, J.L.F. and E.E.S.; visualization, C.A.P.B., D.A.V.M., S.J.F.G. and L.J.S.; supervision, J.L.F., R.E.O. and E.E.S.; project administration, J.L.F. and E.E.S. All authors have read and agreed to the published version of the manuscript.
Institutional Review Board Statement
The Ministry of Environment and Sustainable Development of Colombia supported the Universidad Industrial de Santander through the permit to genetic resources and derivative access for bioprospecting (Contract No. 270). The project RC-FP44842-212-2018 was approved by the Scientific Research Ethical Committee (Record No. 15–2017, File No. 4110) from Universidad Industrial de Santander. The experiments and the chemical management were carried out according to national law (Resolution No. 008430-1993) from Ministry of Health of Colombia and Institutional Manual of Integrated Management and Processes (PGIR–PGGA.05).
Informed Consent Statement
Not applicable.
Data Availability Statement
All data, tables, and figures are original.
Conflicts of Interest
No potential conflicts of interest are reported by the authors.
Sample Availability
The extracts samples are available from J.L.F. and E.E.S.
Funding Statement
The authors are grateful for funding from the Ministry of Science, Technology and Innovation, the Ministry of Education, the Ministry of Industry, Commerce and Tourism, and ICETEX, Programme Ecosistema Científico-Colombia Científica, from the Francisco José de Caldas Fund, Grant RC-FP44842-212-2018.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Yeager D.G., Lim H.W. What’s new in photoprotection A review of new concepts and controversies. Dermatol. Clin. 2019;37:149–157. doi: 10.1016/j.det.2018.11.003. [DOI] [PubMed] [Google Scholar]
- 2.Ganesan A., Hanawalt P. Photobiological origins of the field of genomic maintenance. Photochem. Photobiol. 2016;92:52–60. doi: 10.1111/php.12542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Schuch A.P., Moreno N.C., Schuch N.J., Menck C.F.M., Garcia C.C.M. Sunlight damage to cellular DNA: Focus on oxidatively generated lesions. Free Radic. Biol. Med. 2017;107:110–124. doi: 10.1016/j.freeradbiomed.2017.01.029. [DOI] [PubMed] [Google Scholar]
- 4.Guan L.L., Lim H.W., Mohammad T.F. Sunscreens and photoaging: A review of current literature. Am. J. Clin. Dermatol. 2021;22:819–828. doi: 10.1007/s40257-021-00632-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Jansen R., Osterwalder U., Wang S.Q., Burnett M., Lim H.W. Photoprotection. Part II. Sunscreen: Development, efficacy, and controversies. J. Am. Acad. Dermatol. 2013;69:867.e1–867.e14. doi: 10.1016/j.jaad.2013.08.022. [DOI] [PubMed] [Google Scholar]
- 6.Osterwalder U., Sohn M., Herzog B. Global state of sunscreens. Photodermatol. Photoimmunol. Photomed. 2014;30:62–80. doi: 10.1111/phpp.12112. [DOI] [PubMed] [Google Scholar]
- 7.Young A.R., Claveau J., Rossi A.B. Ultraviolet radiation and the skin: Photobiology and sunscreen photoprotection. J. Am. Acad. Dermatol. 2017;76:100–109. doi: 10.1016/j.jaad.2016.09.038. [DOI] [PubMed] [Google Scholar]
- 8.Downs C.A., Kramarsky-Winter E., Segal R., Fauth J., Knutson S., Bronstein O., Ciner F.R., Jeger R., Lichtenfeld Y., Woodley C.M., et al. Toxicopathological effects of the sunscreen UV filter, oxybenzone (benzophenone-3), on coral planulae and cultured primary cells and its environmental contamination in Hawaii and the U.S. virgin islands. Arch. Environ. Contam. Toxicol. 2016;70:265–288. doi: 10.1007/s00244-015-0227-7. [DOI] [PubMed] [Google Scholar]
- 9.Ghazipura M., McGowan R., Arslan A., Hossain T. Exposure to benzophenone-3 and reproductive toxicity: A systematic review of human and animal studies. Reprod. Toxicol. 2017;73:175–183. doi: 10.1016/j.reprotox.2017.08.015. [DOI] [PubMed] [Google Scholar]
- 10.Schneider S.L., Lim H.W. Review of environmental effects of oxybenzone and other sunscreen active ingredients. J. Am. Acad. Dermatol. 2019;80:266–271. doi: 10.1016/j.jaad.2018.06.033. [DOI] [PubMed] [Google Scholar]
- 11.Narla S., Lim H.W. Sunscreen: FDA regulation, and environmental and health impact. Photochem. Photobiol. Sci. 2020;19:66–70. doi: 10.1039/C9PP00366E. [DOI] [PubMed] [Google Scholar]
- 12.Serafini M.R., Guimarães A.G., Quintans J.S.S., Araujo A.A.S., Nunes P.S., Quintans-Junior L.J. Natural compounds for solar photoprotection: A patent review. Expert Opin. Ther. Patents. 2015;25:467–478. doi: 10.1517/13543776.2014.1000863. [DOI] [PubMed] [Google Scholar]
- 13.Cefali L.C., Ataide J.A., Moriel P., Foglio M.A., Mazzola P.G. Plant-based active photoprotectants for sunscreens. Int. J. Cosmet. Sci. 2016;38:346–353. doi: 10.1111/ics.12316. [DOI] [PubMed] [Google Scholar]
- 14.Mejía-Giraldo J.C., Henao-Zuluaga K., Gallardo C., Atehortúa L., Puertas-Mejía M.A. Novel in vitro antioxidant and photoprotection capacity of plants from high altitude ecosystems of Colombia. Photochem. Photobiol. 2016;92:150–157. doi: 10.1111/php.12543. [DOI] [PubMed] [Google Scholar]
- 15.Nunes A.R., Rodrigues A.L.M., de Queiroz D.B., Vieira I.G.P., Neto J.F.C., Calixto J.T., Jr., Tintino S.R., Maia de Morais S., Coutinho H.D.M. Photoprotective potential of medicinal plants from Cerrado biome (Brazil) in relation to phenolic content and antioxidant activity. J. Photochem. Photobiol. B. 2018;189:119–123. doi: 10.1016/j.jphotobiol.2018.10.013. [DOI] [PubMed] [Google Scholar]
- 16.Morocho-Jácome A.L., Batello-Freire T., Costa de Oliveira A., Santos de Almeida T., Rosado C., Robles-Velasco M.V., Rolim-Baby A. In vivo SPF from multifunctional sunscreen systems developed with natural compounds—A review. J. Cosmet. Dermatol. 2021;20:729–737. doi: 10.1111/jocd.13609. [DOI] [PubMed] [Google Scholar]
- 17.Fuentes J.L., Villamizar-Mantilla D.A., Flores-González S.J., Núñez L.A., Stashenko E.E. Plants growing in Colombia as sources of active ingredients for sunscreens. Int. J. Radiat. Biol. 2021;97:1705–1715. doi: 10.1080/09553002.2021.1987564. [DOI] [PubMed] [Google Scholar]
- 18.Cavinato M., Waltenberger B., Baraldo G., Grade C.V.C., Stuppner H., Jansen-Durr P. Plant extracts and natural compounds used against UVB-induced photoaging. Biogerontology. 2017;18:499–516. doi: 10.1007/s10522-017-9715-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sajadimajd S., Bahramsoltani R., Iranpanah A., Kumar-Patra J., Das G., Gouda S., Rahimi R., Rezaeiamiri E., Cao H., Giampieri F., et al. Advances on natural polyphenols as anticancer agents for skin cancer. Pharmacol. Res. 2020;151:104584. doi: 10.1016/j.phrs.2019.104584. [DOI] [PubMed] [Google Scholar]
- 20.Chhabra G., Ndiaye M.A., Garcia-Peterson L.M., Ahmad N. Melanoma chemoprevention: Current status and future prospects. Photochem. Photobiol. 2017;93:975–989. doi: 10.1111/php.12749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Montes de Oca M.K., Pearlman R.L., McClees S.F., Strickland R., Afaq F. Phytochemicals for the prevention of photocarcinogenesis. Photochem. Photobiol. 2017;93:956–974. doi: 10.1111/php.12711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mileva M., Ilieva Y., Jovtchev G., Gateva S., Zaharieva M.M., Georgieva A., Dimitrova L., Dobreva A., Angelova T., Vilhelmova-Ilieva N., et al. Rose flowers—A delicate perfume or a natural healer? Biomolecules. 2021;11:127. doi: 10.3390/biom11010127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Desempeño del Sector Floricultor . EE-Estudio Sector de Flores-2017 09 28. Superintendencia de Sociedades; Bogota, Colombia: 2017. [(accessed on 30 March 2022)]. Available online: https://www.supersociedades.gov.co/SiteCollectionDocuments/2017/ [Google Scholar]
- 24.Valbuena M.C., Nova-Villanueva J.A., Sánchez-Vanegas G. Minimal Erythema Dose: Correlation with Fitzpatrick skin type and concordance between methods of erythema assessment in a patient sample in Colombia. Actas Dermosifiliogr. 2020;111:390–397. doi: 10.1016/j.adengl.2019.12.010. [DOI] [PubMed] [Google Scholar]
- 25.Harborne J. In: The Handbook of Natural Flavonoids. Harborne J., Baxter H., editors. Volume 2. John Wiley and Sons; Hoboken, NJ, USA: 1999. p. 879. [Google Scholar]
- 26.Fetni S., Bertella N., Ouahab A., Zapater J., Fernandez S. Composition and biological activity of the Algerian plant Rosa canina L. by HPLC-UV-MS. Arab. J. Chem. 2020;13:1105–1119. doi: 10.1016/j.arabjc.2017.09.013. [DOI] [Google Scholar]
- 27.Barnes J., Schug K. Structural characterization of cyanidin-3,5-diglucoside and pelargonidin-3,5-diglucoside anthocyanins: Multi-dimensional fragmentation pathways using high performance liquid chromatography-electrospray ionization ion-trap-time-of-flight mass spectrometry. Int. J. Mass Spectrom. 2011;308:71–80. doi: 10.1016/j.ijms.2011.07.026. [DOI] [Google Scholar]
- 28.March R., Miao X. A fragmentation study of kaempferol using electrospray quadrupole time-of-flight mass spectrometry at high mass resolution. Int. J. Mass Spectrom. 2004;231:157–167. doi: 10.1016/j.ijms.2003.10.008. [DOI] [Google Scholar]
- 29.Wang Y.H., Avula B., Jadhav A., Smillie T., Khan I. Structural characterization and identification of ecdysteroids from Sida rhombifolia L. in positive electrospray ionization by tandem mass spectrometry. Rapid Commun. Mass Spectrom. 2008;22:2413–2422. doi: 10.1002/rcm.3625. [DOI] [PubMed] [Google Scholar]
- 30.METLINTM Metabolomic Data Bases. [(accessed on 21 May 2019)]. Available online: https://metlin.scripps.edu.
- 31.Mouret S., Bogdanowicz P., Haure M.J., Castex-Rizzi N., Cadet J., Favier A., Douki T. Assessment of the photoprotection properties of sunscreens by chromatographic measurement of DNA damage in skin explants. Photochem. Photobiol. 2011;87:109–116. doi: 10.1111/j.1751-1097.2010.00834.x. [DOI] [PubMed] [Google Scholar]
- 32.Schuch A.P., Moraes M.C.S., Yagura T., Menck C.F.M. Highly sensitive biological assay for determining the photoprotective efficacy of sunscreen. Environ. Sci. Technol. 2014;48:11584–11590. doi: 10.1021/es503721a. [DOI] [PubMed] [Google Scholar]
- 33.Cediel-Becerra J.D.D., Suescún-Sepúlveda J.A., Fuentes J.L. Prodigiosin production and photoprotective/antigenotoxic properties in Serratia marcescens indigenous strains from eastern cordillera of Colombia. Photochem. Photobiol. 2022;98:254–261. doi: 10.1111/php.13507. [DOI] [PubMed] [Google Scholar]
- 34.Jarzycka A., Lewińska A., Gancarz R., Wilk K.A. Assessment of extracts of Helichrysum arenarium, Crataegus monogyna, Sambucus nigra in photoprotective UVA and UVB; photostability in cosmetic emulsions. J. Photochem. Photobiol. B. 2013;128:50–57. doi: 10.1016/j.jphotobiol.2013.07.029. [DOI] [PubMed] [Google Scholar]
- 35.Mejía-Giraldo J.C., Winkler R., Gallardo C., Sánchez-Zapata A.M., Puertas-Mejía M.A. Photoprotective potential of Baccharis antioquensis (Asteraceae) as natural sunscreen. Photochem. Photobiol. 2016;92:742–752. doi: 10.1111/php.12619. [DOI] [PubMed] [Google Scholar]
- 36.Mejía-Giraldo J.C., Scaiano J.C., Gallardo-Cabrera C., Puertas-Mejía M.A. Photoprotection and photostability of a new lignin-gelatin-Baccharis antioquensis-based hybrid biomaterial. Antioxidants. 2021;10:1904. doi: 10.3390/antiox10121904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Mejía-Giraldo J.C., Winkler R., Puertas-Mejía M. Novel UV filters from Pentacalia pulchella extracts with photoprotective properties and antioxidant activity. Photochem. Photobiol. Sci. 2021;20:1585–1597. doi: 10.1007/s43630-021-00120-z. [DOI] [PubMed] [Google Scholar]
- 38.de Oliveira-Junior R.G., Rocha-Souza G., Guimarães A.L., de Oliveira A.P., de Souza-Araújo C., Cabral-Silva J., Marques-Pacheco A.G., de Lima-Saraiva S.R.G., Rolim L.A., Rolim-Neto P.J., et al. Photoprotective, antibacterial activity and determination of phenolic compounds of Neoglaziovia variegata (Bromeliaceae) by high performance liquid chromatography-diode array detector (HPLC-DAD) analysis. Afr. J. Pharm. Pharmacol. 2015;9:576–584. [Google Scholar]
- 39.Ku W.J., Lin C.J., Lin P.H. UV-Protection performance of Calophyllum inophyllum seed extracts: A natural ultraviolet screening agent. Nat. Prod. Commun. 2021;16:1934578X20985650. doi: 10.1177/1934578X20985650. [DOI] [Google Scholar]
- 40.Sierra L.D., Córdoba Y., Mejía J.J., Quintero-Rueda E., Ocazionez R.E., Avila-Acevedo J.G., García-Bores A.M., Espinosa-González A.M., Benítez-Flores J.C., González-Valle M.R., et al. Photoprotective activity of Ipomoea horsfalliae flower extract. Rev. Bras. Farmacogn. 2020;30:69–79. doi: 10.1007/s43450-020-00024-6. [DOI] [Google Scholar]
- 41.Guimarães de Sousa R., da Silva Lima A.D., Neves de Lima E. Incremento da atividade fotoprotetora e antioxidante de cosméticos contendo extratos vegetais da Caatinga. Braz. J. Nat. Sci. 2020;3:225–230. [Google Scholar]
- 42.Figueiredo S.A., Pinto-Vilela F.M., da Silva C.A., Cunha T.M., dos Santos M.H., Vieira-Fonseca M.J. In vitro and in vivo photoprotective/photochemopreventive potential of Garcinia brasiliensis epicarp extract. J. Photochem. Photobiol. B. 2014;131:65–73. doi: 10.1016/j.jphotobiol.2014.01.004. [DOI] [PubMed] [Google Scholar]
- 43.Reis-Mansur M.C.P.P., Guimarães-Leitão S., Cerqueira-Coutinho C., Vermelho A.B., Silva R.S., Presgrave O.A.F., Leitão A.A.C., Leitão G.G., Ricci-Júnior E., Santos E.P. In vitro and in vivo evaluation of efficacy and safety of photoprotective formulations containing antioxidant extracts. Rev. Bras. Farmacogn. 2016;26:251–258. doi: 10.1016/j.bjp.2015.11.006. [DOI] [Google Scholar]
- 44.Puertas-Mejía M.A., Gutierrez-Villegas M.I., Mejía-Giraldo J.C., Winkler R., Rojano B. In vitro UV absorption properties and radical scavenging capacity of Morella parvifolia (Benth.) Parra-Os extracts. Braz. J. Pharm. Sci. 2018;54:e17498. doi: 10.1590/s2175-97902018000317498. [DOI] [Google Scholar]
- 45.Hübner A.A., Sarruf F.D., Oliveira C.A., Neto A.V., Fischer D.C.H., Kato E.T.M., Lourenço F.R., Baby R., Bacchi E.M. Safety and photoprotective efficacy of a sunscreen system based on grape pomace (Vitis vinifera L.) phenolics from winemaking. Pharmaceutics. 2020;12:1148. doi: 10.3390/pharmaceutics12121148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Delprete P.G. Taxonomic history, morphology, and reproductive biology of the tribe Posoquerieae (Rubiaceae, Ixoroideae) Ann. Missouri Bot. Gard. 2009;96:79–89. doi: 10.3417/2006192. [DOI] [Google Scholar]
- 47.Ariza O., Rueda E., Archila J., Martínez J., Stashenko E. Determinación mediante HS-SPME/GC-MS, de la composición química de la fragancia y el absoluto de las flores de Posoqueria latifolia. Scientia et Technica. 2007;1:59–61. [Google Scholar]
- 48.Romero-Pérez A.I., Lamuela-Raventós R.M., Waterhouse A.L., de la Torre-Boronat M.C. Levels of cis- and trans-resveratrol and their glucosides in white and rose Vitis vinifera wines from Spain. J. Agric. Food Chem. 1996;44:2124–2128. doi: 10.1021/jf9507654. [DOI] [Google Scholar]
- 49.Shimoda K., Hamada M., Hamada H., Takemoto M., Hamada H. Synthesis of resveratrol glycosides by cultured plant cells. Nat. Prod. Commun. 2013;8:907–909. doi: 10.1177/1934578X1300800713. [DOI] [PubMed] [Google Scholar]
- 50.Kitagawa S., Yoshii K., Morita S.Y., Teraoka R. Efficient topical delivery of chlorogenic acid by an oil-in-water microemulsion to protect skin against UV-induced damage. Chem. Pharm. Bull. 2011;59:793–796. doi: 10.1248/cpb.59.793. [DOI] [PubMed] [Google Scholar]
- 51.Polonini H.C., Lima L.L., Gonçalves K.M., Resende do Carmo A.M., da Silva A.D., Barbosa-Raposo N.R. Photoprotective activity of resveratrol analogues. Bioorg. Med. Chem. 2013;21:964–968. doi: 10.1016/j.bmc.2012.11.052. [DOI] [PubMed] [Google Scholar]
- 52.Stevanato R., Bertelle M., Fabris S. Photoprotective characteristics of natural antioxidant polyphenols. Regul. Toxicol. Pharmacol. 2014;69:71–77. doi: 10.1016/j.yrtph.2014.02.014. [DOI] [PubMed] [Google Scholar]
- 53.Kostyuk V., Potapovich A., Albuhaydar A.R., Mayer W., De Luca C., Korkina L. Natural substances for prevention of skin photoaging: Screening systems in the development of sunscreen and rejuvenation cosmetics. Rejuvenation Res. 2018;21:91–101. doi: 10.1089/rej.2017.1931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Baur J.A., Sinclair D.A. Therapeutic potential of resveratrol: The in vivo evidence. Nat. Rev. Drug Discov. 2006;5:493–506. doi: 10.1038/nrd2060. [DOI] [PubMed] [Google Scholar]
- 55.Lorencini M., Brohema C.A., Dieamant G.C., Zanchin N.I.T., Maibach H.I. Active ingredients against human epidermal aging. Ageing Res. Rev. 2014;15:100–115. doi: 10.1016/j.arr.2014.03.002. [DOI] [PubMed] [Google Scholar]
- 56.Hu S., Chen F., Wang M. Photoprotective effects of oxyresveratrol and kuwanon O on DNA damage induced by UVA in human epidermal keratinocytes. Chem. Res. Toxicol. 2015;28:541–548. doi: 10.1021/tx500497u. [DOI] [PubMed] [Google Scholar]
- 57.Shokrzadeh M., Habibi E., Modanloo M. Cytotoxic and genotoxic studies of essential oil from Rosa damascene Mill., Kashan, Iran. Med. Glas. 2017;14:152–157. doi: 10.17392/901-17. [DOI] [PubMed] [Google Scholar]
- 58.Mikanagi Y., Yokoi M., Ueda Y., Saito N. Flower flavonol and anthocyanin distribution in subgenus Rosa. Biochem. Syst. Ecol. 1995;23:183–200. doi: 10.1016/0305-1978(95)93849-X. [DOI] [Google Scholar]
- 59.Cai Y., Xing J., Sun M., Zhan Z., Corke H. Phenolic antioxidants (hydrolyzable tannins, flavonols, and anthocyanins) identified by LC-ESI-MS and MALDI-QIT-TOF-MS from Rosa chinensis flowers. J. Agric. Food Chem. 2005;53:9940–9948. doi: 10.1021/jf052137k. [DOI] [PubMed] [Google Scholar]
- 60.Kumar N., Bhandari P., Singh B., Bari S. Antioxidant activity and ultra-performance LC-electrospray ionization-quadrupole time-of-flight mass spectrometry for phenolics-based fingerprinting of Rose species: Rosa damascena, Rosa bourboniana and Rosa brunonii. Food Chem. Toxicol. 2009;47:361–367. doi: 10.1016/j.fct.2008.11.036. [DOI] [PubMed] [Google Scholar]
- 61.Nađpal J., Lesjak M., Šibul F., Anačkov G., Četojevič-Simin D., Mimica-Dukič N., Beara I. Comparative study of biological activities and phytochemical composition of two rose hips and their preserves: Rosa canina L. and Rosa arvensis Huds. Food Chem. 2016;192:907–914. doi: 10.1016/j.foodchem.2015.07.089. [DOI] [PubMed] [Google Scholar]
- 62.Kumar S., Gautam S., Sharma A. Identification of antimutagenic properties of anthocyanins and other polyphenols from rose (Rosa centifolia) petals and tea. J. Food Sci. 2013;78:H948–H954. doi: 10.1111/1750-3841.12135. [DOI] [PubMed] [Google Scholar]
- 63.Choquenet B., Couteau C., Paparis E., Coiffard L.J.M. Flavonoids and polyphenols, molecular families with sunscreen potential: Determining effectiveness with an in vitro method. Nat. Prod. Commun. 2008;4:227–230. doi: 10.1177/1934578X0900400212. [DOI] [PubMed] [Google Scholar]
- 64.Stich H.F., Rosin M.P., Wu C.H., Powrie W.D. A comparative genotoxicity study of chlorogenic acid (3-O-caffeoylquinic acid) Mutat. Res. 1981;90:201–212. doi: 10.1016/0165-1218(81)90001-X. [DOI] [PubMed] [Google Scholar]
- 65.Guardado-Yordi E., Matos M.J., Pérez-Martínez A., Tornes A.C., Santana L., Molina E., Uriarte E. In silico genotoxicity of coumarins: Application of Phenol-Explorer food database to functional food science. Food Funct. 2017;8:2958–2966. doi: 10.1039/C7FO00402H. [DOI] [PubMed] [Google Scholar]
- 66.R Core Team . R: A Language and Environment for Statistical Computing. R Foundation for Statistical Computing; Vienna, Austria: 2013. [(accessed on 31 March 2022)]. Available online: http://www.R-project.org. [Google Scholar]
- 67.Sayre R.M., Agin P.P., LeeVee G.J., Morlowe E. A comparison of in vivo and in vitro testing of sunscreens formulas. Photochem. Photobiol. 1979;29:559–566. doi: 10.1111/j.1751-1097.1979.tb07090.x. [DOI] [PubMed] [Google Scholar]
- 68.Mansur J.S., Breder M.N.R., Mansur M.C.A., Azulay R.D. Determinação do fator de proteção solar por espectrofotometria. An. Bras. Dermatol. Rio Janeiro. 1986;61:121–124. [Google Scholar]
- 69.Diffey B.L. A method for broad spectrum classification of sunscreens. Int. J. Cosmet. Sci. 1994;16:47–52. doi: 10.1111/j.1467-2494.1994.tb00082.x. [DOI] [PubMed] [Google Scholar]
- 70.Lionetti N., Rigano L. The new sunscreens among formulation strategy, stability issues, changing norms, safety and efficacy evaluations. Cosmetics. 2017;4:15. doi: 10.3390/cosmetics4020015. [DOI] [Google Scholar]
- 71.Department of Health and Human Services, Food and Drug Administration (FDA) Labeling and Effectiveness Testing; Sunscreen Drug Products for Over-the-Counter Human Use. Volume 76. Department of Health and Human Services, Food and Drug Administration (FDA); Silver Spring, MD, USA: 2011. pp. 35620–35665. Final Rule Federal Register, 21 CFR Parts 201 and 310 [Docket No. FDA–1978–N–0018-0698] [Google Scholar]
- 72.Quillardet P., Hofnung M. The SOS Chromotest, a colorimetric bacterial assay for genotoxins: Procedures. Mutat. Res. 1985;147:65–78. doi: 10.1016/0165-1161(85)90020-2. [DOI] [PubMed] [Google Scholar]
- 73.Quintero N., Stashenko E.E., Fuentes J.L. The influence of organic solvents on genotoxicity and antigenotoxicity estimates in the SOS Chromotest. Genet. Mol. Biol. 2012;35:503–514. doi: 10.1590/S1415-47572012000300018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Fuentes J.L., García-Forero A., Quintero-Ruiz N., Prada-Medina C.A., Rey-Castellanos N., Franco-Niño D.A., Contreras-García D.A., Cordoba-Campo Y., Stashenko E.E. The SOS Chromotest applied for screening plant antigenotoxic agents against ultraviolet radiation. Photochem. Photobiol. Sci. 2017;16:1424–1434. doi: 10.1039/C7PP00024C. [DOI] [PubMed] [Google Scholar]
- 75.Prada-Medina C.A., Aristizabal-Tessmer E.T., Quintero-Ruiz N., Serment-Guerrero J.H., Fuentes J.L. Survival and SOS response induction in ultraviolet B irradiated Escherichia coli cells with defective repair mechanisms. Int. J. Radiat. Biol. 2016;92:321–328. doi: 10.3109/09553002.2016.1152412. [DOI] [PubMed] [Google Scholar]
- 76.Strober W. Trypan blue exclusion test of cell viability. Curr. Protoc. Immunol. 2015;111:A3.B.1–A3.B.3. doi: 10.1002/0471142735.ima03bs111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Jacobs J.P., Jones C.M., Baille J.P. Characteristics of a human diploid cell designated MRC- smelly Emma 5. Nature. 1970;227:168–170. doi: 10.1038/227168a0. [DOI] [PubMed] [Google Scholar]
- 78.Ara N., Nur M.H., Amran M.S., Wahid M.I.I., Ahmed M. In vitro antimicrobial and cytotoxic activities of leaves and flowers extracts from Lippia alba. Pak. J. Biol. Sci. 2009;12:87–90. doi: 10.3923/pjbs.2009.87.90. [DOI] [PubMed] [Google Scholar]
- 79.Sykora P., Witt K.L., Revanna P., Smith-Roe S.L., Dismukes J., Lloyd D.G., Engelward B.P., Sobol R.W. Next generation high throughput DNA damage detection platform for genotoxic compound screening. Sci. Rep. 2018;8:2771. doi: 10.1038/s41598-018-20995-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Collins A., Dušinská M., Franklin M., Somorovská M., Petrovská H., Duthie S., Vaughan N. Comet assay in human biomonitoring studies: Reliability, validation, and applications. Environ. Mol. Mutagen. 1997;30:139–146. doi: 10.1002/(SICI)1098-2280(1997)30:2<139::AID-EM6>3.0.CO;2-I. [DOI] [PubMed] [Google Scholar]
- 81.Pitarque M., Creus A., Marcos R., Hughes J.A., Anderson D. Examination of various biomarkers measuring genotoxic endpoints from Barcelona airport personnel. Mutat. Res. 1999;440:195–204. doi: 10.1016/S1383-5718(99)00026-1. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All data, tables, and figures are original.