Abstract
Objectives
The purpose of this study was to compare the effects of two acute doses of Capsiate (CAP; 6 vs. 12 mg) on upper body resistance exercise performance in trained men.
Methods
Using a randomized, crossover and double-blind design, 20 resistance-trained males were supplemented with low-dose CAP (6 mg), high-dose CAP (12 mg) or placebo 45 minutes before exercise. Subjects performed 4 sets of bench press with repetitions to failure at 70% 1 repetition maximum (1RM) and 2 minutes of rest between each set. The ratings of perceived exertion (RPE) and blood lactate were analyzed at baseline and after exercise.
Results
Total weight lifted was greater in the low CAP (2,454.6 ± 448.6 kg) compared to placebo (2,354.7 ± 458.6 kg, p = 0.039) and high CAP (2,309.3 ± 428.1 kg, p = 0.001). There was no significant difference between conditions for RPE (p = 0.155) and blood lactate (p = 0.434).
Conclusion
In summary, 6 mg CAP increased total weight lifted and repetitions to failure on bench press exercise in trained men, while 12 mg did not present any effect.
Keywords: Strength exercise, pre-workout, weightlifters
INTRODUCTION
Capsiate (CAP) is an analogue of capsaicin naturally present in the fruit of CH-19 sweet peppers. CAP exerts physiological and biochemical effects binding to the transient receptor potential vanilloid subfamily member 1 receptors (TRPV1), present in the sarcoplasmic reticulum and plasma membrane of several cells, including the skeletal muscle. In contrast to capsaicin, CAP is not pungent in taste, as it is hydrolyzed during permeation through mucosa (9, 18). Supplementation with CAP has been documented to induce significant beneficial changes in the muscle energy metabolism, impacting the utilization of substrates and ATP production (11, 13).
The ergogenic benefits of CAP have been shown to occur in both acute and chronic supplementation conditions (6). Chronic adaptations to CAP supplementation are likely linked to the acute effects of CAP, therefore, a better understanding of the acute effects may lead to a better prediction of the chronic results, especially given the limited body of information available regarding the chronic adaptations to CAP supplementation (5). In regard to the acute effects, capsaicin treatment during 24h in C2C12 muscle cells has been demonstrated to enhance intracellular calcium concentrations leading to enhanced TRVP1 protein expression, increased mitochondrial content and enhanced basal ATP levels. In a preclinical study performed in mice, Kazuya et al. (11) demonstrated that acute supplementation of CAP (2h) following a high dose (100 mg/kg body wt.) increased peak muscle force generation and total force generation during isometric contractions provoked by electro stimulation in-situ. In this study, the authors suggested that the ergogenic effects of CAP were based on decreased ATP cost for twitch force generation and an increased Ca+ sensitivity of the contractile apparatus. Interestingly, both low and high doses (10 or 100mg/kg body wt.) reduced the ATP cost of twitch force generation, which opens the possibility for a dose-effect, at least in rodents.
In humans, CAP supplementation has been studied, with positive effects on performance in both aerobic and resistance training modalities. As an example, 12 mg of CAP supplementation improved total back squat load lifted by young men with a lower rating of perceived exertion (RPE) after four sets to muscular failure (70% of 1RM) (3). The same capsaicin dosage, resulted in significant benefits on five sets of 10-seconds knee extension maximal isometric contractions (7). Additionally, Cross et al. (4) observed higher knee extensor peak torque after acute supplementation with capsaicin fruit gummy at 1.2 mg compared to isocaloric placebo, although this result should be interpreted with caution, mainly because the lack of pungency in the chewable placebo supplement. Lastly, Opheim and Rankin (15) did not find significant benefits of 28.5 mg of capsaicin ingested via 3 g of powdered cayenne pepper on repeat sprint ability (15 × 30-m sprints with 35-second intervals) for 7 days.
Traditionally, chronic low doses of CAP, such as 6 mg/d (17), 2 mg CAP or 4 mg CAP (16) have been used for treatment of overweight or obese humans, and have been shown to decrease fat mass and increase fat oxidation, however, whether acute low-dose (6 mg CAP) improves resistance exercise performance is unknown. For example, previous work from Kazuya et al. (11) have demonstrated a dose-effects relationship between CAP and twitch force-generating capacity in mice. A single dose of CAP (at 10 or 100 mg/kg body wt) during 6 min of maximal repeated isometric contractions reduced the amount of ATP produced from glycolysis and oxidative phosphorylation but increased the relative contribution of oxidative phosphorylation to total energy turnover (+28 and +21% in the 10mg and 100 mg groups, respectively), and ATP cost of twitch force generation was further reduced in the 10 mg (−35%) and 100 mg (−45%). Although the highest CAP dose also increased the twitch force-generating capacity, this question has not been addressed yet in humans. Important differences between studies such as the exercise test employed and form of administration in addition to different dosages of capsinoids make it difficult to compare previous studies. Furthermore, all studies conducted with humans applied lower body exercise, thus it is not clear if CAP could be also effective to increase upper body resistance exercise performance. Hence, the purpose of this study was to compare two acute doses of CAP, low and high (6 vs. 12 mg), on upper body muscle performance and to investigate if a dose-response relationship exists for humans.
METHODS
Participants
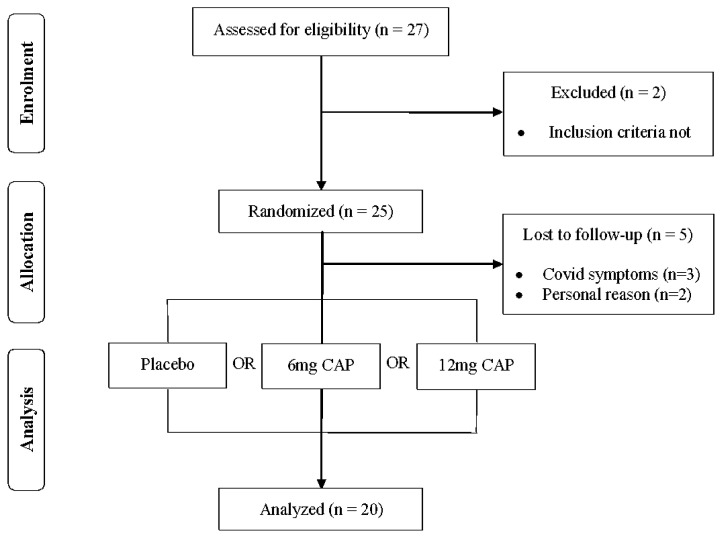
Initially, the sample size was calculated using an effect size (0.46) for total repetitions to failure from a previous study conducted by Williams et al. (19), which verified the effects of acute beetroot juice supplementation on repetitions to failure on the bench press exercise. Using an α of 0.05 and power of 0.80 via G*Power 3.1 software, it was estimated that we would need 10 participants, therefore, we over-recruited the number of participants. Out of a total of 27 men who participated in the first screening, twenty-five resistance-trained males met all the inclusion criteria and agreed to participate in the study protocol. During the intervention, five participants dropped out due to flu symptoms during the study (n = 3), or personal reasons (n = 2). Thus, 20 resistance-trained males successfully completed all study visits. The figure 1 shows the recruitment procedures (CONSORT flowchart).
Figure 1.
CONSORT Flowchart.
The inclusion criteria for the present study were: 1) males between 18 to 35 years of age, with at least 1 year of resistance training experience, at a minimum frequency of 3 days per week and 60 minutes per day; 2) be able to lift at least 100% of their body mass in the bench press exercise and a relative 1RM of 1.0 to 1.5 kg/body weight; 2) without any existing contraindications involving the cardiovascular system, muscles, joints, or bones of the upper limbs that could limit exercise; 3) who did not consumed chili peppers frequently (maximum once per month); 4) free from consumption of anabolic steroids or any other illegal agents known to increase performance for the previous year; 4) did not smoke or drink alcohol within of testing visits. Subjects were instructed to avoid taking any performance-enhancing supplements during the study period.
The project was approved by the Ethics Research Group of the Federal University of Piaui, Terezina, Piaui, Brazil (Protocol number: 3.169.545), and the research was conducted according to the 2013 Revision of the Declaration of Helsinki. Written informed consent was obtained from all subjects after they had been informed about the purpose and risks of the study. This research was carried out fully in accordance with the ethical standards of the International Journal of Exercise Science (14).
Protocol
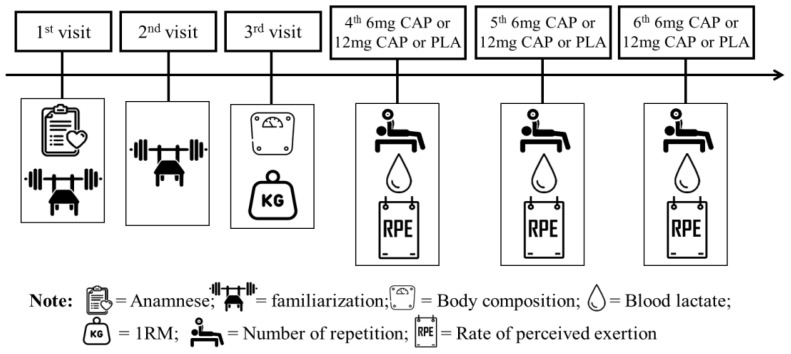
The study was conducted using a randomized, cross-over, placebo-controlled and double-blind study. There were 6 visits including: 2 familiarization visits, one repetition maximum test (1 RM) visit, and three experimental trial visits. Firstly, the volunteers participated in an interview to verify the inclusion criteria and completed one familiarization session to become acquainted with the 1RM test procedures. 48 h later the participants went to the laboratory for the second familiarization session. After 72 h, the participants were assessed for anthropometrics and body composition and performed the 1RM. On the following three visits, a simple randomization technique was used for allocation using randomizer.com and each subject consumed the placebo (starch) or two different doses of CAP supplement: low CAP (6 mg) or high CAP (12 mg) 45 minutes before workout in a randomized, counter-balanced, double-blind fashion. All trials were performed at the same time (between 8 to 11 AM) at the laboratory and separated by a minimum of 72 hours. The experimental design is illustrated in Figure 2.
Figure 2.
Experimental design.
Initially, one week prior to the 1RM test, the participants performed two familiarization sessions to acclimate to the equipment and cadence of movement on the bench press. The grip width of the bar was recorded during the measurement of 1RM and repeated in the other sessions. A general warm-up at 5.5 km/h was performed on the treadmill for 5 minutes, followed by a specific warm-up with bench press on the Smith machine, using only the weight of the bar for ten repetitions, since the weight of the bar is about 20 kg.
Body weight was measured using a Filizola electronic scale, accurate to 0.1 kg and maximum capacity of 150 kg. Height was measured using a portable stadiometer (Caprice 2060, Sanny), with an accuracy of 0.1. The adjustable set square was positioned over the head, while the individual stood in an upright, barefoot position, with heels together, arms extended at the side of the body, looking forward. The fat-free mass (FFM) and fat mass (FM) in kilograms, and percentage of fat mass (FM%) were assessed using spectral bioelectrical impedance analysis and accompanying software (Biodynamics model 310e), with the volunteer lying in the supine position with arms and legs extended and slightly abducted to avoid contact between the upper limbs and the trunk and between the lower limbs. During the collection, all metallic objects were removed from the body. Based on results of a small pilot study (n = 8), the test retest intraclass correlation coefficient (ICC) from our lab was FM (0.97), %BF (0.96) and FFM (0.99).
For the 1RM test, the participants reported to the laboratory having refrained from any upper body exercise for at least 72 hours prior to 1RM and experimental trials. The participants completed a warm-up protocol, which consisted of 5 minutes of walking on treadmill (5.5 km.h-1) and subsequent 1 set of 10 repetitions at approximately 50% of the 1RM on the bench press performed on the Smith machine. Next, the subjects were allowed up to 5 attempts to determine the 1RM. When they performed two correct maximum repetitions, the load was progressively increased until the volunteer was able to perform only one maximum repetition correctly. Between each attempt, a rest of 3 to 5 minutes was given, according to the National Strength and Conditioning Association (1).
Next, the participant performed three random sessions separated by at least 72 hours: low CAP (6 mg), high CAP (12 mg) or placebo. The participants completed four sets of bench press on the Smith machine until momentary muscular failure with a load corresponding to 70% of the 1RM with the eccentric phase controlled for two seconds and the concentric phase performed at the highest possible voluntary speed and 2 minutes of rest between each set. During all tests, verbal incentives were given for maximum concentric speed and maximum effort. Each valid repetition was recorded when the volunteer started from the full elbow extension, touched the bar on the chest and returned to the starting position. In addition, the shoulder blades were required to be kept on the bench (avoiding scapular protraction at the end of the concentric phase). The participants were encouraged to allow a brief pause at the end of the concentric phase of the movement to avoid transferring power to the eccentric phase. The use of the chest to take impulse during the transition of the eccentric phase to the concentric phase of the movement was not allowed. The volunteers were free to place their feet on the floor during the exercise; however, the participants were required to adopt the same foot position in all situations. The total number of repetitions performed was recorded for each set of each condition and intensity and used to analyze total weight lifted (repetition × load × sets).
The participants were advised to maintain their customary nutritional regimen and to avoid taking any supplements, chili peppers or other spicy foods during the study, as well as coffee, tea, alcohol and/or stimulant drinks for a period of 12 hours prior to the visits. Participants were instructed to eat their breakfast 1:30 h before each trial and replicate the same meal intake as the first day in the subsequent trials. All food intakes were analyzed for total kilocalorie and macronutrient intakes using MyFitnessPal.com (http://www.myfitnesspal.com) and shown in the Table 1. Each participant consumed 2 capsules of placebo (starch), low dose of CAP (1 capsule of 6 mg of CAP + 1 placebo) or high dose of CAP (2 capsules of 6 mg of CAP = 12 mg), which were identical in appearance to ensure a double-blind design, 45 minutes before each trial. The product is standardized to 30–50% capsinoids (Capsicum annuum L. Lepuge-Pharmaceutical manufacturing Ltda, São Bernardo do Campo, SP, Brazil) and the final product contains 45.2% Capsiate (Harmonia Pharmacy, São Paulo, SP, Brazil). Study products were delivered to each individual subject by a researcher who was not directly involved in the data collection to ensure double-blinding.
Table 1.
General characteristics of the sample and dietary intake pre-exercise.
| Variables | Mean ± SD |
|---|---|
| Age (y) | 28 ± 4.1 |
| Height (cm) | 176.0 ± 6.4 |
| Weight (kg) | 81.7 ± 7.2 |
| 1 RM (kg) | 98.4 ± 10.8 |
| Relative 1-RM | 1.2 ± 0.1 |
| Body Composition | |
| Body fat (%) | 17.7 ± 3.3 |
| Fat Mass (kg) | 14.5 ± 3.0 |
| Fat Free Mass (kg) | 67.3 ± 6.1 |
| Dietary Intake Pre-exercise | |
| Carbohydrate (g) | 61.3 ± 26.5 |
| Protein (g) | 26.7 ± 15.4 |
| Lipids (g) | 21.1 ± 12.8 |
| Total Intake (kcal) | 541.7.2 ± 242.1 |
The Accutrend Lactate portable lactate analyzer (Roche Diagnostics, Mannheim, Germany) was used to measure whole blood at rest and 3 minutes after exercise collected via finger stick. Coded reagent strips fill by capillary action directly from the sample site and whole-blood lactate concentrations are determined by reflectance photometry in 60 seconds. For whole-blood the analyzer's range is between 0.8 and 22.0 mmol/L (Roche Diagnostics, 2007). The rating of perceived exertion (RPE) was recorded immediately after each set using the OMNI Perceived Exertion Scale for Resistance Exercise (OMNI-RES) (12).
Statistical Analysis
The Shapiro-Wilk test was used to verify the normality of the data set and the data are shown as mean and standard deviation. The estimated sphericity was verified according to Mauchly’s W test, and the Greenhouse–Geisser correction was used when necessary. Two-way repeated measures analysis of variance with the Bonferroni adjustment for multiple comparisons were used to compare total repetition, total weight lifted and RPE. A condition (Placebo, low CAP, and high CAP) × time (4 sets) repeated measures analysis of variance with the Bonferroni adjustment for multiple comparisons was used to compare the number of repetitions during four sets between three conditions. A condition (Placebo, low CAP, and high CAP) × time (rest vs. post-exercise) repeated measures analysis of variance with the Bonferroni adjustment for multiple comparisons was used to compare blood lactate. The partial eta squared (η2) was reported for condition, time, and interaction effects. The effect size was calculated via Cohen’s d, which describes the difference between the means normalized to the pooled standard deviation (SD) of the placebo vs. low CAP or placebo vs. high CAP, whereby a value of > 0.20 was considered small, > 0.50 moderate, and > 0.80 large. Statistical significance was set at p < 0.05. The data were analyzed using the Statistical Package for Social Sciences 17.0 (SPSS Inc. Chicago. IL. USA).
RESULTS
Table 1 presents the mean and standard deviation (SD) values for age, height, body weight, experience, resistance training frequency, body composition and dietary intake pre-exercise.
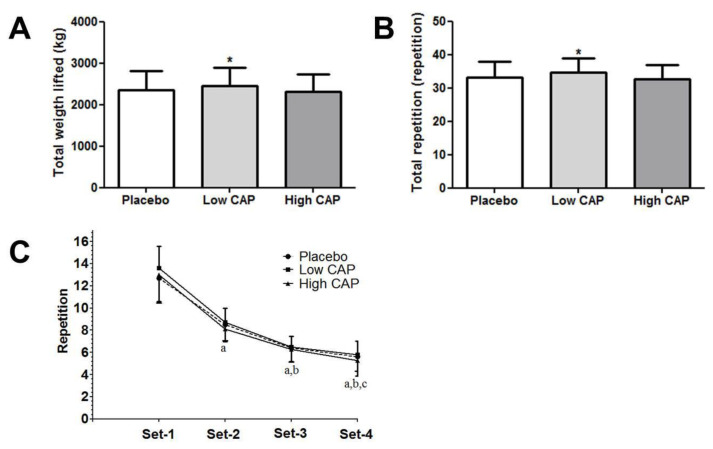
Figure 3 shows the comparison between three experimental conditions on the upper body resistance exercise performance.
Figure 3.
Comparison between conditions on the upper body resistance exercise performance.
Note: A = Total weight lifted; B = Total repetition; C = Number of repetition in each set; Low CAP = 6 mg; High CAP = 12 mg; * = significant difference between placebo and high CAP; a = significant difference between set-1; b = significant difference between set-2; c = significant difference between set-3.
There was a significant difference between conditions for total repetitions (F = 6.569, p = 0.004, η2 = 0.26) with higher number of repetitions in the low CAP compared to the placebo (p = 0.043) and high CAP (p = 0.001) (Figure 3A). The effect size was small for low CAP (d = 0.31) and trivial for high CAP (d = 0.13).
For total weight lifted, there was a significant difference between conditions (F = 7.075, p = 0.002, η2 = 0.27) with greater total weight lifted in the low CAP compared to placebo (p = 0.039) and high CAP (p = 0.001) (Figure 3B). The effect size was small for low CAP (d = 0.23) and trivial for high CAP (d = 0.10).
There was a significant decrease across set for all conditions (F = 235.013, p < 0.001, η2 = 0.92) (Figure 3C) but no supplement x set interaction was observed (F = 1.294, p = 0.266, η2 = 0.06).
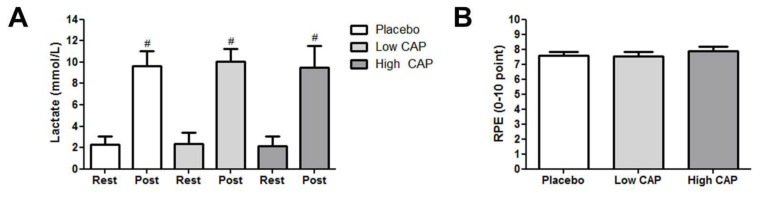
The lactate concentration increased post-exercise in relation to rest for all conditions (F = 503.119, p < 0.001, η2 = 0.96), but there were no significant differences between conditions (F = 2.270, p = 0.117, η2 = 0.11), or supplement x set interaction interactions (F = 0.779, p = 0.434, η2 = 0.04) (Figure 4A). The effect size was small for low CAP (d = 0.38) and trivial in the high CAP (d = 0.05).
Figure 4.
Comparison between conditions on the lactate concentrations (A) and RPE= rate of perceived exertion (B).
Note: Low CAP= 6 mg; High CAP= 12 mg; #= significant difference between rest.
There was no significant difference between conditions for RPE (F = 1.959, p = 0.155, η2 = 0.09) (Figure 4B). Effect size were trivial for low CAP (d = 0.10) but small in the high CAP (d = 0.29).
DISCUSSION
Traditionally, chronic low doses of CAP (2mg, 4mg or 6mg) have been used for overweight or obesity treatment in humans to decrease fat mass and increase fat oxidation at rest (16, 17). However, to our knowledge, this was the first study to compare the potential benefits of 6 mg compared to 12 mg of CAP supplementation on upper body resistance exercise in trained men. The results of the present study support that 6 mg CAP increased total weight lifted on the bench press in trained men. However, 12 mg CAP had no effect and was no different from placebo.
A preclinical study by Kazuya et al. (11) demonstrated that both low and high doses of CAP (10 or 100mg/kg body wt) reduced the ATP cost of twitch force generation, thus opening the possibility for a dose effect. In this sense, the low dose used in this study (6 mg CAP) also demonstrated improved performance on upper body exercise in trained men, however, the higher dose of 12 mg did not show any effect. Although we couldn’t find data in humans based on capsinoid pharmacokinetics (i.e., rate of appearance, uptake for the skeletal muscles and excretion) for different doses, such differences may be related to a dose response relationship where 12 mg may be situated in the descendent part of the dose-performance relationship (we hypothesize an inverted U-shaped relationship), at least for the upper body, thus making 6 mg but not 12 mg optimal for the upper body muscles. Thus, we would suggest that there is a minimum amount of CAP needed to consume but after exceeding that threshold would not have any further benefits.
On the other hand, previous studies of de Freitas et al. (3) demonstrated 12mg CAP improved total weight lifted with lower RPE after 4 sets to muscular failure, with 70% of 1RM in the squat exercise in trained men. Employing the same dosage of 12mg, Gomes et al. (7) found significant benefits of CAP on five 10-seconds knee extension maximal isometric contractions. In contrast to the previous studies, there was no benefit of 12 mg CAP on the bench press in the current study. Possible explanations for the discrepancy between the above studies and ours may be due to the exercise test employed (free weight vs. smith machine), type of contraction (isotonic vs. isometric muscle contraction) and type of muscle mass involved (lower body vs. upper body), which may change the pattern of recruitment of different muscle fibers and muscle bioenergetics.
In this respect, Gejl et al. (8) compared myosin heavy chain 2 (MHC) fibers from muscle triceps brachii with MHC I fibers of vastus lateralis muscle and they verified greater Ca2+ sensitivity and increased Ca2+ concentration and higher force production, at a submaximal effort in the arm muscle compared to leg muscle. According to the authors, the higher Ca2+ sensitivity may have meant a lower threshold for neural stimulation, and hence a reduced sense of effort to develop a certain submaximal force (8). This, summed to the ergogenic effect of CAP in low doses in skeletal muscle tissue, as a consequence of a decreased ATP cost for twitch force generation and an increased Ca+ sensitivity of the contractile apparatus (11) may have caused ergogenic effects for the upper body limbs. Therefore, the minimal and optimal dose to improve performance in humans should be investigated and future research is required to assess the effect of low dose and high doses in different body parts, since as mentioned above, skeletal muscles from different body parts may respond differently to capsinoids based on their specific fiber composition and biochemistry.
In a chronic study, Hsu et al. (10) verified dose-dependent effects when they compared control, 205 mg/kg capsaicin, 410 mg/kg capsaicin, and 1025 mg/kg capsaicin once a day for 28 consecutive days and demonstrated that grip strength and swimming time were greater in the higher dose. It is important to note, however, that the dose used in animals is much greater than the usual human equivalent dose on which relative body mass relationships between species are not possible. For humans, however, the literature suggests that a single oral dose of 15 or 30 mg CAP is safe and well tolerated in healthy males (2). Thus, we hypothesized that 12 mg CAP would result in greater total weight lifted when compared to 6 mg CAP, however, we reject this hypothesis, at least for the upper body performance and conditions herein studied. A limitation to this study was our inability to measure Ca2+ release and muscle activation. Therefore, more research is necessary to investigate the mechanism by which CAP could induce acute performance and the potential difference between doses in different exercises, muscles involved, different types of muscle contraction and population.
Conclusions
In summary, acute low-dose of CAP (6 mg) increased total weight lifted and repetitions to failure on bench press exercise in trained men, while 12 mg did not present any effect, although the underlying mechanism of diminishing benefits of high-dose CAP supplementation is currently unknown. Therefore, the present study suggests that 6 mg can be an effective strategy to enhance muscle performance during the bench press exercise in trained men. The results of this study may be applied by coaches, weightlifters and sport nutritionist looking to improve upper body performance in resistance exercise.
REFERENCES
- 1.Baechle TR, Earle RW. Essentials of strength training and conditioning. Human kinetics; 2008. [Google Scholar]
- 2.Bernard BK, Watanabe E, Kodama T, Tsubuku S, Otabe A, Nakajima M, Masumori S, Shimada S, Tanaka J, Masuyama T. Studies of the toxicological potential of capsinoids: V. Genotoxicity studies of dihydrocapsiate. Int J Toxicol. 2008;27(Suppl 3):59–72. doi: 10.1080/10915810802513536. [DOI] [PubMed] [Google Scholar]
- 3.Conrado de Freitas M, Cholewa JM, Freire RV, Carmo BA, Bottan J, Bratfich M, Della Bandeira MP, Gonçalves DC, Caperuto EC, Lira FS, Rossi FE. Acute capsaicin supplementation improves resistance training performance in trained men. J Strength Cond Res. 2018;32:2227–2232. doi: 10.1519/JSC.0000000000002109. [DOI] [PubMed] [Google Scholar]
- 4.Cross BL, Parker D, Langan SP, Grosicki GJ. Effect of a commercially available low-dose capsaicin supplement on knee extensor contractile function. International Journal of Exercise Science. 2020;13:312–318. doi: 10.70252/ELCT8391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.de Moura ESVEL, Cholewa JM, Billaut F, Jäger R, de Freitas MC, Lira FS, Rossi FE. Capsaicinoid and capsinoids as an ergogenic aid: A systematic review and the potential mechanisms involved. Journal of the International Society of Sports Nutrition. 2021;16:464–473. doi: 10.1123/ijspp.2020-0677. [DOI] [PubMed] [Google Scholar]
- 6.de Moura ESVEL, Cholewa JM, Jäger R, Zanchi NE, de Freitas MC, de Moura RC, Barros EML, Antunes BM, Caperuto EC, Ribeiro SLG, Lira FS, Pereira Dos Santos MA, Rossi FE. Chronic capsiate supplementation increases fat-free mass and upper body strength but not the inflammatory response to resistance exercise in young untrained men: a randomized. placebo-controlled and double-blind study. 2021;18:50. doi: 10.1186/s12970-021-00446-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.dos Santos Gomes W, de Freitas MC, Dutra YM, Rossi F, Estanislau TB, Gonçalves DC, Campos EZ. Effects of capsiate supplementation on maximal voluntary contraction in healthy men. International Journal of Sports Medicine. :2021. doi: 10.1055/a-1502-6563. [DOI] [PubMed] [Google Scholar]
- 8.Gejl KD, Hvid LG, Andersson EP, Jensen R, Holmberg HC, Ørtenblad N. Contractile properties of MHC I and II fibers from highly trained arm and leg muscles of cross-country skiers. Frontiers in Physiology. 2021;12:682943. doi: 10.3389/fphys.2021.682943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gupta R, Kapoor B, Gulati M, Kumar B, Gupta M, Singh SK, Awasthi A. Sweet pepper and its principle constituent capsiate: Functional properties and health benefits. Critical Reviews in Food Science and Nutrition. 2021:1–25. doi: 10.1080/10408398.2021.1913989. [DOI] [PubMed] [Google Scholar]
- 10.Hsu YJ, Huang WC, Chiu CC, Liu YL, Chiu WC, Chiu CH, Chiu YS, Huang CC. Capsaicin supplementation reduces physical fatigue and improves exercise performance in mice. Nutrients. 2016;8 doi: 10.3390/nu8100648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kazuya Y, Tonson A, Pecchi E, Dalmasso C, Vilmen C, Fur YL, Bernard M, Bendahan D, Giannesini B. A single intake of capsiate improves mechanical performance and bioenergetics efficiency in contracting mouse skeletal muscle. American Journal of Physiology Endocrinology and Metabolism. 2014;306:E1110–1119. doi: 10.1152/ajpendo.00520.2013. [DOI] [PubMed] [Google Scholar]
- 12.Lagally KM, Robertson RJ. Construct validity of the OMNI resistance exercise scale. J Strength Cond Res. 2006;20:252–256. doi: 10.1519/R-17224.1. [DOI] [PubMed] [Google Scholar]
- 13.Luo Z, Ma L, Zhao Z, He H, Yang D, Feng X, Ma S, Chen X, Zhu T, Cao T, Liu D, Nilius B, Huang Y, Yan Z, Zhu Z. TRPV1 activation improves exercise endurance and energy metabolism through PGC-1α upregulation in mice. Cell Res. 2012;22:551–564. doi: 10.1038/cr.2011.205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Navalta JW, Stone WJ, Lyons TS. Ethical issues relating to scientific discovery in exercise science. International Journal of Exercise Science. 2019;12:1. doi: 10.70252/EYCD6235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Opheim MN, Rankin JW. Effect of capsaicin supplementation on repeated sprinting performance. J Strength Cond Res. 2012;26:319–326. doi: 10.1519/JSC.0b013e3182429ae5. [DOI] [PubMed] [Google Scholar]
- 16.Rogers J, Urbina SL, Taylor LW, Wilborn CD, Purpura M, Jäger R, Juturu V. Capsaicinoids supplementation decreases percent body fat and fat mass: Adjustment using covariates in a post hoc analysis. BMC obesity. 2018;5:22. doi: 10.1186/s40608-018-0197-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Snitker S, Fujishima Y, Shen H, Ott S, Pi-Sunyer X, Furuhata Y, Sato H, Takahashi M. Effects of novel capsinoid treatment on fatness and energy metabolism in humans: possible pharmacogenetic implications. The American Journal of Clinical Nutrition. 2009;89:45–50. doi: 10.3945/ajcn.2008.26561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Tominaga M. Molecular mechanisms of trigeminal nociception and sensation of pungency. Chemical Senses. 2005;30(Suppl 1):i191–192. doi: 10.1093/chemse/bjh179. [DOI] [PubMed] [Google Scholar]
- 19.Williams TD, Martin MP, Mintz JA, Rogers RR, Ballmann CG. Effect of acute beetroot juice supplementation on bench press power, velocity, and repetition volume. J Strength Cond Res. 2020;34:924–928. doi: 10.1519/JSC.0000000000003509. [DOI] [PubMed] [Google Scholar]