Abstract
Background.
Despite advances in treatment, survival from acute lymphoblastic leukemia (ALL) remains lower among non-White children than White children in the US. We investigated the association of race/ethnicity and socioeconomic status (SES) with survival.
Procedures.
We analyzed 9,295 Californian children (3,251 Whites, 4,890 Hispanics, 796 Asians, and 358 Blacks) aged ≤ 19 years diagnosed with a first primary ALL during 1988–2011. We used the Kaplan–Meier method to estimate survival at 1, 5, and 10 years after diagnosis for three calendar periods. Hazard ratios of death for race/ethnicity, SES, and clinical factors were estimated by Cox regression models.
Results.
Median follow-up time was 7.4 years (range 0–25 years). Over time, survival after ALL improved steadily, but inequalities persisted across races/ethnicities. Five-year survival (95% confidence interval) was 85.0% (83.6–86.2) for White, 81.4% (78.3–84.0) for Asian, 79.0% (77.8–80.2) for Hispanic, and 74.4% (69.4–78.8) for Black children. In multivariable-adjusted models, the hazard of death was increased by 57% among Black, 38% among Hispanic, and 33% among Asian children compared with White children. Patients residing in the lowest SES neighborhoods at diagnosis had a 39% increased risk of death relative to those living in higher SES neighborhoods.
Conclusion.
Despite significant improvements in survival, non-White children and children residing in low SES neighborhoods experienced worse survival even after adjusting for potential confounders. Our findings highlight the need to capture specific information on disease biology, treatment, and treatment adherence to better understand the predictors of lower survival in minority and low SES groups.
Keywords: childhood, leukemia, population-based, race/ethnicity, SES, survival
INTRODUCTION
Acute lymphoblastic leukemia (ALL) is the most common pediatric neoplasm and the leading cause of death due to disease in children and adolescents aged 1–19 years in the United States (US).[1] Several studies have reported an increase in the incidence of childhood ALL in Europe [2] and the US.[3] Evidence suggests that there may be an inherited genetic predisposition to this disease among different races/ethnicities.[4] Strikingly, genetic factors that increase the susceptibility to ALL appear also to be associated with drug-resistant ALL phenotypes and might, in part, explain the poor survival in certain ethnic groups.[5]
Survival from childhood ALL represents one of the most successful advances in the history of science and medicine. ALL was consistently fatal until the 1950s; however, currently approximately 90% of children can be cured in developed countries.[6] This progress has been attributed largely to the use of effective chemotherapy regimens of variable intensities that are adapted to precise risk stratification and assessment of early treatment response.[6]
Despite the dramatic improvement in the survival of children with ALL in the last four decades, survival has varied widely by race/ethnicity in developed [7] and developing nations.[8] Non-adherence to treatment, lack of access to care, cultural influences, socioeconomic status (SES), and biologic features have been implicated in these variations.[9] However, the extent to which these factors contribute to survival inequalities remain unclear.
California has the largest and most racially and ethnically diverse population in the US [10] and it has maintained a statewide high-quality, population-based cancer surveillance system since 1988. In this study, we examined how survival after ALL varied by race/ethnicity, SES, and clinical factors in Californian children over a 24-year period. Our population-based study on childhood ALL simultaneously investigates the association of race/ethnicity, neighborhood SES, health insurance, type of treating facility, treatment, and secondary neoplasms as well as factors examined previously (e.g., age, gender, immunophenotype, and calendar period).
METHODS
Patients and Study Design
For this population-based observational study, data were retrieved for children and adolescents aged 0–19 years residing in California when diagnosed with a first, primary ALL from January 1, 1988 through December 31, 2011, and followed for vital status through December 31, 2012. Data were obtained from the California Cancer Registry (CCR), to which all new cases of cancer diagnoses must be reported by state law. The CCR contributes to approximately half of the data in the Surveillance, Epidemiology, and End Results (SEER) program of the National Cancer Institute (NCI) and is estimated to include more than 99% of all invasive cancers diagnosed in California. We included the following morphology codes from the International Classification of Diseases for Oncology, third edition (ICD-O-3):[11] 9,727, 9,728, 9,729, 9,811, 9,812, 9,813, 9,814, 9,815, 9,816, 9,817, 9,818, 9,835, 9,836, and 9,837. Among 9,429 eligible patients, 9,295 were included for survival analysis. The following patients were excluded from analysis: 7 reported by death certificate only (DCO), 5 reported by autopsy only, 51 for whom race/ethnicity was unknown, 60 of Non–Hispanic American Indian (NHAI) race/ethnicity for whom the small sample size precluded analysis, and 11 with inconsistent dates of diagnosis or follow-up and/or leukemia classification. ALL was morphologically verified in 99.8% of patients, and the percentage of cases with verified vital status on December 31, 2012, was 87.1%.
Institutional review board (IRB) approval—Ethics approval for human subjects research was obtained from the California Prevention Institute of California Institutional Review Board. As the analysis was based on state-mandated cancer registry data, the study was conducted in accordance with the waivers of individual informed consent and HIPPA authorization.
Covariates
Covariates included in the analysis were age at diagnosis (<1, 1–4, 5–9, 10–14, and 15–19 years); gender (male, female); race/ethnicity (Non-Hispanic White [White], Non-Hispanic Black [Black], Hispanic, and Non-Hispanic Asian/Pacific Islander [Asian]); immunophenotype (categorized as B-cell, T-cell, or not otherwise specified [NOS] according to the morphology codes); secondary neoplasms; and neighborhood SES. Secondary neoplasm was defined as a new malignancy registered in the CCR after the diagnosis of ALL, following the SEER’s multiple primaries rules for hematopoietic diseases.[12] Some types of malignant neoplasms have been associated with worse prognosis [13] and we have controlled for their occurrence in our analyses. Because information on SES at the individual level is not collected by the CCR, a previously developed neighborhood SES measure [14] was used. It is derived from principal components analysis of seven census indicator variables of SES (education level, proportion unemployed and with a blue collar job, proportion below 200% of federal poverty level, and median household income, rent, and home value). This index is based on data at the level of the census block groups and is considered adequate as a surrogate to SES at individual level,[15] and can capture neighborhood-level factors that may affect cancer incidence and outcomes.[16] SES was divided into quintiles based on the statewide distribution and assigned to patients on the basis of their residence at time of diagnosis. Other covariates included type of insurance at time of initial treatment (private, public, no insurance, or unknown) collected from 1996 onwards; calendar period (1988–1995, 1996–2003, 2004–2011); and type of treating hospital. Because the care provided by specialized pediatric oncologic centers may be different from that provided in general hospitals, we identified children’s hospitals and pediatric cancer centers in California by using listings from the Children’s Hospital Association [17] and the Children’s Oncology Group (COG).[18] These hospitals offer clinical trials sponsored by the COG, which is supported by the NCI. On the basis of the cancer reporting facility, patients were classified by whether they had received care at a pediatric cancer center (yes, no). Chemotherapy, radiotherapy, and time to chemotherapy were evaluated in descriptive analyses of treatment. They were not included in the statistical model because of changes in the use of central nervous system (CNS) radiation over time [19] and the widespread use of chemotherapy protocols. Inclusion of treatment in the model did not change the associations observed among race/ethnicity, SES, and survival.
Statistical Analyses
We used the χ2 test to compare frequency distributions of sociodemographic and clinical characteristics by race/ethnicity. Follow-up time was defined as the date of diagnosis to the date of death from any cause, or censoring at the end of the study period (December 31, 2012) or last known date of follow-up, whichever came first.
We estimated overall survival at 1, 5, and 10 years for each covariate (except chemotherapy and radiation) and calendar period by the Kaplan–Meier method. The log-rank test was used to compare differences in survival across strata. We used unadjusted and multivariable-adjusted Cox regression models to estimate the hazard ratios (HRs) of death with associated 95% confidence interval (CI).
We tested the proportional-hazards assumption by examining log–log survival plots and confirmed the results by using Schoenfeld residuals. There was evidence that age, immunophenotype, and secondary neoplasms violated the proportional hazard assumption, and these were therefore included as stratification variables in the models. Secondary neoplasm was analyzed as a time-dependent variable.
Because information on type of insurance was not routinely collected prior to 1996, we ran three Cox regression models: a model without insurance with all patients, a model without insurance but limited to patients diagnosed from 1996 onwards, and another model including insurance but limited to patients diagnosed from 1996 onwards. We investigated interactions between racial/ethnic groups and other covariates. Statistical analyses were performed by using the Stata 13 software and a two-sided P-value < 0.05 was considered statistically significant.
RESULTS
Sociodemographic and Clinical Characteristics
Table I shows patients and disease characteristics by race/ethnicity. In the 9,295 patients in our cohort, there was a higher percentage of males (58%) than females (42%). More than half the patients (52%) were Hispanic, followed by White (35%), Asian (9%), and Black (4%). The median age at diagnosis was 4 years for Asian, 5 years for White and Hispanic, and 7 years for Black children. By immunophenotype, 60% of patients had B-cell, 12% had T-cell, and approximately 28% had NOS ALL. The proportion of T-cell ALL was significantly higher in Black (23%) than in White (15%), Asian (13%), and Hispanic (10%) children. White and Asian children were more likely to have private insurance (80% and 74%, respectively) than Black and Hispanic children (53% and 40% respectively). Approximately 1.4% of children were diagnosed with secondary neoplasms, of which 58% were solid and 46% were hematopoietic. The use of CNS radiation decreased progressively from 24% in the first time period to 12% in the last period. Chemotherapy was administered to more than 98% of children, of whom at least 95% received chemotherapy within 2 weeks of diagnosis.
TABLE I.
Sociodemographic and Clinical Characteristics of Children (Aged 0–19 Years) With Acute Lymphoblastic Leukemia Diagnosed From 1988 to 2011 and Followed Up to 2012 in California, by Race/Ethnicity
| Covariates | Whites N (%) | Blacks N (%) | Hispanics N (%) | Asians N (%) | Total cohort N (%) | P a |
|---|---|---|---|---|---|---|
| Total | 3,251 (35) | 358 (4) | 4,890 (52) | 796 (9) | 9,295 (100) | |
| Age at diagnosis, years | ||||||
| <1 | 69 (2.1) | 9 (2.5) | 158 (3.2) | 29 (3.6) | 266 (2.9) | |
| 1–4 | 1,468 (45.2) | 117 (32.7) | 2,023 (41.4) | 382 (48.0) | 3,990 (42.9) | |
| 5–9 | 868 (26.7) | 102 (28.5) | 1,216 (24.9) | 194 (24.4) | 2,382 (25.6) | |
| 10–14 | 465 (14.3) | 74 (20.7) | 807 (16.5) | 101 (12.7) | 1,447 (15.5) | |
| 15–19 | 381 (11.7) | 56 (15.6) | 686 (14.0) | 90 (11.3) | 1,213 (13.1) | <0.0001 |
| Median | 5 | 7 | 5 | 4 | 5 | |
| Gender | ||||||
| Male | 1,911 (58.8) | 206 (57.5) | 2,815 (57.6) | 459 (57.7) | 5,391 (58.0) | |
| Female | 1,340 (41.2) | 152 (42.5) | 2,075 (42.4) | 337 (42.3) | 3,904 (42.0) | 0.738 |
| Chemotherapy | ||||||
| No | 44 (1.3) | 11 (3.1) | 79 (1.6) | 7 (0.9) | 141 (1.5) | |
| Yes | 3,207(98.7) | 347 (96.9) | 4,811 (98.4) | 789 (99.1) | 9,154 (98.5) | 0.031 |
| CNS radiation | ||||||
| No | 2,717 (83.6) | 275 (76.8) | 4,085 (83.5) | 687 (86.3) | 7,764 (83.5) | |
| Yes | 534 (16.4) | 83 (23.2) | 805 (16.5) | 109 (13.7) | 1,531 (16.5) | 0.001 |
| Treatment at a pediatric cancer center | ||||||
| No | 931 (28.6) | 131 (36.6) | 1,571 (32.1) | 240 (30.1) | 2,873 (30.9) | |
| Yes | 2,320 (71.4) | 227 (63.4) | 3,319 (67.9) | 556 (69.9) | 6,422 (69.1) | 0.001 |
| Leukemia immunophenotype | ||||||
| T-cell | 483 (14.9) | 84 (23.4) | 464 (9.5) | 102 (12.8) | 1,133 (12.2) | |
| B-cell | 1,736 (53.4) | 176 (49.2) | 3,183 (65.1) | 490 (61.6) | 5,585 (60.1) | |
| NOS | 1,032 (31.7) | 98 (27.4) | 1,243 (25.4) | 204 (25.6) | 2,581 (27.7) | <0.0001 |
| Secondary neoplasms | ||||||
| No | 3,209 (98.7) | 356 (99.4) | 4,838 (98.9) | 782 (98.2) | 9,185 (98.8) | |
| Yes | 42 (1.3) | 2 (0.6) | 52 (1.1) | 14 (1.8) | 110 (1.2) | 0.223 |
| Socioeconomic status | ||||||
| 1. Lowest 20% | 247 (7.6) | 96 (26.8) | 2,067(42.2) | 102 (12.8) | 2,513 (27.0) | |
| 2 | 532 (16.4) | 109 (30.5) | 1,256 (25.7) | 120 (15.1) | 2,020 (21.7) | |
| 3 | 683 (21.0) | 66 (18.4) | 831 (17.0) | 139 (17.5) | 1,723 (18.5) | |
| 4 | 847 (26.0) | 58 (16.2) | 479 (9.8) | 200 (25.1) | 1,585 (17.1) | |
| 5. Highest 20% | 942 (29.0) | 29 (8.1) | 257 (5.3) | 235 (29.5) | 1,463 (15.7) | <0.0001 |
| Calendar period | ||||||
| 1988–1995 | 1,169 (35.9) | 104 (29.0) | 1,162 (23.8) | 222 (27.9) | 2,657 (28.6) | |
| 1996–2003 | 1,093 (33.6) | 127 (35.5) | 1,670 (34.1) | 270 (33.9) | 3,160 (34.0) | |
| 2004–2011 | 989 (30.4) | 127 (35.5) | 2,058 (42.1) | 304 (38.2) | 3,478 (37.4) | <0.0001 |
| Type of health insurance: limited to cases diagnosed from 1996 onwards (N = 6638) | ||||||
| No insurance | 14 (0.7) | 9 (3.5) | 106 (2.9) | 4 (0.7) | 133 (2.0) | |
| Private insurance | 1,669 (80.1) | 135 (53.2) | 1,493 (40.0) | 425 (74.0) | 3,722 (56.1) | |
| Public insurance | 341 (16.4) | 101 (39.8) | 1,997 (53.6) | 128 (22.3) | 2,567 (38.7) | |
| Unknown | 58 (2.8) | 9 (3.5) | 132 (3.5) | 17 (3.0) | 216 (3.2) | <0.0001 |
CNS, central nervous system; NOS, not otherwise specified.
χ2 P-value.
Survival
Table II displays survival probabilities at 1, 5, and 10 years, by sociodemographic and clinical characteristics. Figures 1 and 2 show survival by race/ethnicity and SES, respectively. The median follow-up time was 7.4 years (range 0–25 years). By the end of the study period, 1,955 study patients died. Survival improved steadily over calendar time but was persistently lower for Black, Hispanic, and Asian children than for White children. Differences in survival were most striking between Black and White children.
TABLE II.
Overall Survival With 95% Confidence Intervals for Acute Lymphoblastic Leukemia at 1, 5, and 10 Years After Diagnosis in Children (0–19 Years Old) in California From 1988 to 2011, by Sociodemographic and Clinical Factors
| Covariates | 1-year survival (95%CI) | 5-year survival (95%CI) | 10-year survival (95%CI) |
|---|---|---|---|
| All children | 94.5 (94.0–95.0) | 81.2 (80.3–82.0) | 77.1 (76.1–78.0) |
| Age at diagnosis | |||
| <1 | 76.9 (71.3–81.6) | 50.2 (43.7–56.2) | 45.7 (39.1–52.1) |
| 1–4 | 97.9 (97.4–98.3) | 89.3 (88.2–90.3) | 86.3 (85.1–87.4) |
| 5–9 | 96.6 (95.8–97.3) | 86.2 (84.7–87.6) | 80.7 (78.8–82.4) |
| 10–14 | 91.8 (90.2–93.1) | 73.5 (71.0–75.7) | 69.0 (66.3–71.5) |
| 15–19 | 86.3 (84.2–88.1) | 60.2 (57.2–63.0) | 55.8 (52.6–58.8) |
| Log-rank test P-value<0.00001 | |||
| Race/ethnicity | |||
| White | 95.8 (95.0–96.4) | 85.0 (83.6–86.2) | 81.5 (80.0–82.9) |
| Black | 91.8 (88.4–94.2) | 74.4 (69.4–78.8) | 70.7 (65.3–75.4) |
| Hispanic | 93.9 (93.2–94.5) | 79.0 (77.8–80.2) | 74.4 (73.0–75.7) |
| Asian | 94.4 (92.6–95.8) | 81.4 (78.3–84.0) | 77.4 (74.0–80.4) |
| Log-rank test P-value<0.00001 | |||
| Gender | |||
| Male | 94.3 (93.7–94.9) | 79.5 (78.3–80.6) | 75.1 (73.8–76.3) |
| Female | 94.7 (94.0–95.4) | 83.5 (82.2–84.7) | 79.9 (78.4–81.2) |
| Log-rank test P-value<0.00001 | |||
| Leukemia immunophenotype | |||
| B-cell | 95.4 (94.8–95.9) | 82.7 (81.6–83.7) | 77.8 (76.5–79.0) |
| T-cell | 90.8 (88.9–92.3) | 73.8 (71.0–76.3) | 71.0 (68.0–73.7) |
| NOS | 94.3 (93.3–95.1) | 81.1 (79.5–82.6) | 77.8 (76.1–79.4) |
| Log-rank test P-value<0.00001 | |||
| Calendar period | |||
| 1988–1995 | 93.0 (91.9–93.9) | 76.9 (75.2–78.5) | 72.8 (71.1–74.5) |
| 1996–2003 | 94.8 (93.9–95.5) | 80.7 (79.3–82.1) | 76.7 (75.1–78.1) |
| 2004–2011 | 95.5 (94.7–96.1) | 85.7 (84.3–87.0) | N/A |
| Log-rank test P-value<0.00001 | |||
| Socioeconomic status | |||
| 1. Lowest 20% | 93.5 (92.4–94.4) | 77.0 (75.3–78.7) | 72.5 (70.5–74.3) |
| 2 | 94.5 (93.4–95.5) | 81.5 (79.6–83.2) | 77.8 (75.6–79.6) |
| 3 | 94.5 (93.3–95.4) | 82.3 (80.3–84.1) | 78.4 (76.2–80.5) |
| 4 | 95.3 (94.1–96.2) | 82.2 (80.1–84.1) | 78.2 (75.9–80.3) |
| 5. Highest 20% | 95.5 (94.3–96.4) | 85.4 (83.3–87.1) | 81.3 (78.9–81.6) |
| Log-rank test P-value<0.00001 | |||
| Treatment at a pediatric cancer center | |||
| No | 92.9 (91.9–93.8) | 77.0 (75.4–78.6) | 73.2 (71.4–74.9) |
| Yes | 95.2 (94.7–95.7) | 83.0 (82.0–84.0) | 78.9 (77.7–80.0) |
| Log-rank test P-value = 0.0014 | |||
| Type of health insurance: limited to cases diagnosed from 1996 onwards (N = 6638) | |||
| No insurance | 93.3 (88.1–96.9) | 77.6 (68.9–84.1) | 74.2 (64.4–81.6) |
| Private insurance | 96.6 (94.9–96.2) | 85.2 (83.9–86.4) | 81.8 (80.3–83.2) |
| Public insurance | 94.8 (93.9–96.5) | 81.5 (79.9–83.1) | 76.3 (74.3–78.3) |
| Unknown | 91.6 (87.0–94.6) | 66.2 (59.3–72.2) | 63.0 (55.8–69.3) |
| Log-rank test P-value<0.00001 | |||
CI, confidence interval; NOS, not otherwise specified; N/A, not applicable.
Fig 1.

Overall survival by race/ethnicity among children (0–19 years old) diagnosed with acute lymphoblastic leukemia in California, 1988–2011.
Fig 2.
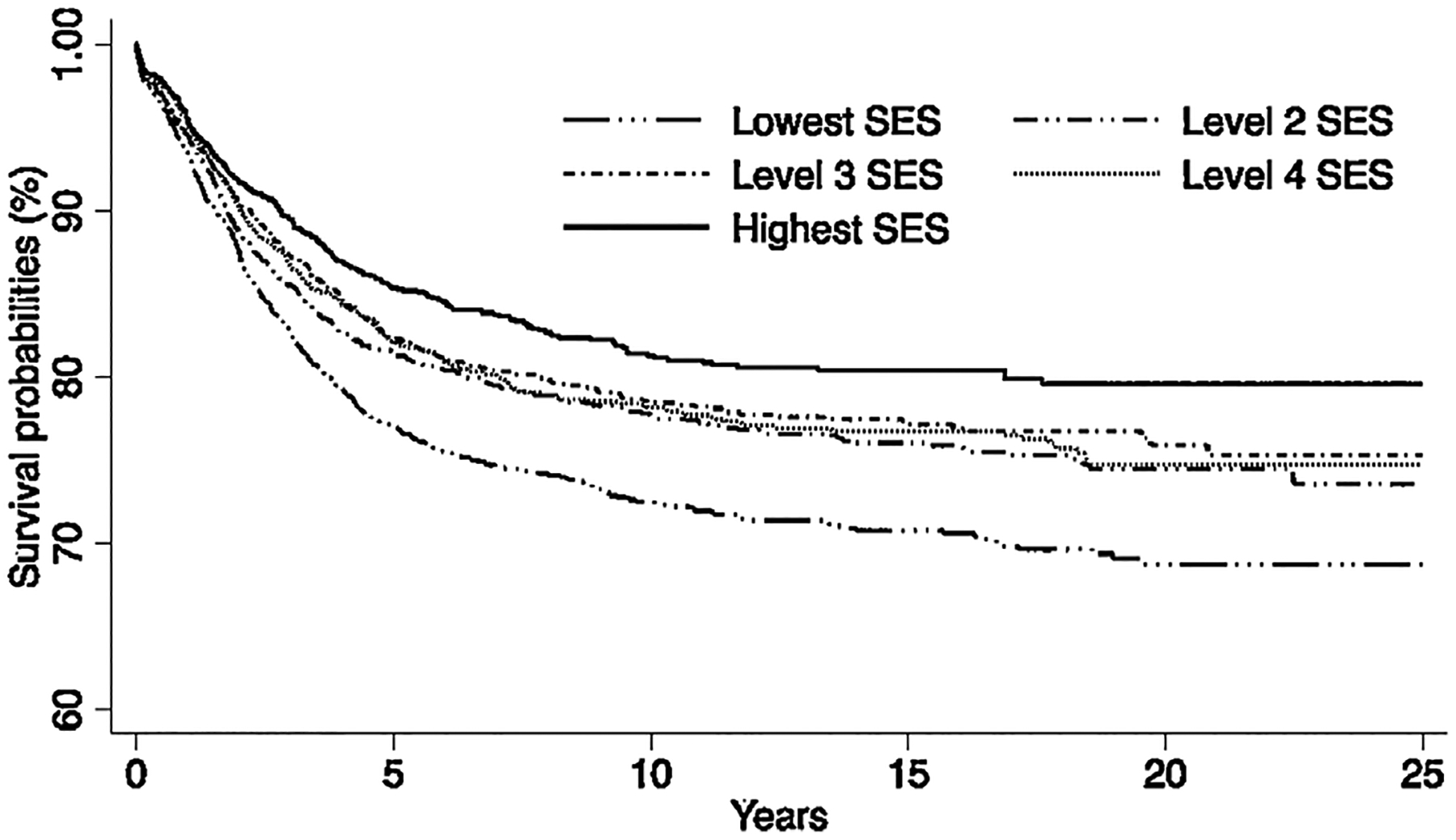
Overall survival by socioeconomic status among children (0–19 years old) diagnosed with acute lymphoblastic leukemia in California, 1988–2011.
Unadjusted and Multivariable Analyses
In the unadjusted model all variables were associated with significant increased hazard of death. After multivariable adjustment, our analysis revealed that the HRs of death were still significant for race/ethnicity and SES (Table III). The hazard of death was increased by 57% (HR = 1.57 [1.26–1.96]) among Black, 38% (HR = 1.38 [1.23–1.55]) among Hispanic, and 33% (HR = 1.33 [1.12–1.59]) among Asian children compared with White children. Patients residing in the lowest SES neighborhoods were at 39% (HR = 1.39 [1.18–1.64]) increased risk of death than those in the higher SES neighborhoods. After controlling for other covariates, the hazard of death was not associated with the type of hospital in which children were treated or with type of insurance for patients diagnosed from 1996 onwards. Insurance minimally attenuated the HRs for race/ethnicity and SES among patients diagnosed from 1996 onwards (Table III). In addition, the inclusion of SES in our model did not substantially change the racial/ethnic differences in survival that we observed. There were no significant interactions between race/ethnicity, SES, calendar period, and other study covariates.
TABLE III.
Unadjusted and Multivariable-Adjusted Hazard Ratios and 95% Confidence Intervals for Overall Survival in Children (0–19 Years Old) With Acute Lymphoblastic Leukemia in California.
| Covariates | Death N (%) | Unadjusted HR1 (1988–2011) (95%CI) | Adjusted HR2 (1988–2011) (95%CI) | Adjusted HR3 (1996–2011) (95%CI) | Adjusted HR4 (1996–2011) (95%CI) |
|---|---|---|---|---|---|
| Race/ethnicity | |||||
| White | 568 (29.1) | 1.00 (Reference) | 1.00 (Reference) | 1.00 (Reference) | 1.00 (Reference) |
| Black | 100 (5.1) | 1.78 (1.44–2.20) | 1.57 (1.26–1.96) | 1.74 (1.31–2.31) | 1.72 (1.29–2.28) |
| Hispanic | 1,123 (57.4) | 1.47 (1.33–1.62) | 1.38 (1.23–1.55) | 1.43 (1.22–1.68) | 1.37 (1.17–1.62) |
| Asian | 164 (8.4) | 1.26 (1.06–1.50) | 1.33 (1.12–1.59) | 1.42 (1.13–1.79) | 1.40 (1.11–1.76) |
| Gender | |||||
| Male | 1,237 (63.3) | 1.27 (1.16–1.39) | 1.19 (1.09–1.31) | 1.20 (1.06–1.35) | 1.19 (1.06–1.35) |
| Female | 718 (36.7) | 1.0 (Reference) | 1.0 (Reference) | 1.00 (Reference) | 1.00 (Reference) |
| Socioeconomic status | |||||
| 1.Lowest 20% | 623 (32.3) | 1.61 (1.39–1.87) | 1.39 (1.18–1.64) | 1.40 (1.12–1.75) | 1.30 (1.04–2.27) |
| 2. | 414 (21.2) | 1.29 (1.10–1.51) | 1.15 (0.97–1.35) | 1.20 (0.95–1.51) | 1.15 (0.91–1.44) |
| 3. | 339 (17.3) | 1.20 (1.02–1.41) | 1.13 (0.95–1.33) | 1.10 (0.87–1.38) | 1.06 (0.84–1.34) |
| 4. | 324 (16.6) | 1.23 (1.04–1.45) | 1.17 (0.99–1.39) | 1.22 (0.97–1.54) | 1.20 (0.95–1.51) |
| 5. Highest 20% | 246 (12.6) | 1.00 (Reference) | 1.00 (Reference) | 1.00 (Reference) | 1.00 (Reference) |
| Calendar period | |||||
| 1988–1995 | 781 (39.9) | 1.66 (1.47–1.87) | 1.97 (1.74–2.24) | N/A | N/A |
| 1996–2003 | 744 (38.1) | 1.38 (1.22–1.56) | 1.50 (1.33–1.70) | 1.52 (1.34–1.73) | 1.50 (1.33–1.71) |
| 2004–2011 | 430 (22.0) | 1.00 (Reference) | 1.00 (Reference) | 1.00 (Reference) | 1.00 (Reference) |
| Treatment at a pediatric cancer center | |||||
| No | 724 (37.0) | 1.35 (1.23–1.48) | 1.06 (0.96–1.16) | 1.05 (0.92–1.19) | 1.05 (0.92–1.19) |
| Yes | 1,231 (63.0) | 1.00 (Reference) | 1.00 (Reference) | 1.00 (Reference) | 1.00 (Reference) |
| Type of health insurance: model limited to cases diagnosed from 1996 onwards (N = 6638) | |||||
| No insurance | 29 (2.5) | 1.54 (1.06–2.23) | N/A | N/A | 1.22 (0.83–1.89) |
| Private insurance | 583 (49.6) | 1.00 (Reference) | N/A | N/A | 1.00 (Reference) |
| Public insurance | 487 (41.5) | 1.31 (1.16–1.47) | N/A | N/A | 1.15 (1.01–1.32) |
| Unknown | 75 (6.4) | 2.31 (1.82–2.94) | N/A | N/A | 1.77 (1.38–2.26) |
HR, hazard ratio; CI, confidence interval; NOS, not otherwise specified. The multivariable models were adjusted for all variables presented in the table and stratified by age, immunophenotype and secondary neoplasm. HR1, unadjusted model; Hr2, adjusted model without insurance, 1988–2011; Hr3, adjusted model without insurance, 1996–2011; Hr4, adjusted model with insurance, 1996–2011.
DISCUSSION
In our large population-based study of nearly 10,000 children with ALL, survival for Black, Hispanic, and Asian children was lower than that for White children. The survival differences we observed in our cohort persisted over time and were most marked between Black and White children. In contrast to previous studies reporting that survival of Asian children was similar to [20] or better [21] than for White, Hispanic, and Black children, our study showed that Asian children in California had lower survival than White children with ALL. Our results are consistent with a previous study [7] that also used US population-based data, but we extended their findings by additionally investigating neighborhood SES, secondary neoplasms, type of insurance, treatment, and treating facility.
Genetic and non-genetic factors help to explain disparities in cancer survival. Our population-based study allowed the investigation of non-genetic factors and found that neighborhood SES had a significant, independent association with survival, particularly when comparing children residing in the highest and lowest SES neighborhoods. The inclusion of SES in our statistical model did not substantially change the racial/ethnic differences in survival that we observed, suggesting that other factors underlie these survival disparities. Our SES finding is consistent with previous studies of poorer survival among financially deprived populations.[22]
White and Asian children were more likely than Hispanic and Black children to have private insurance, but the type of insurance did not significantly affect survival after ALL after adjustment for other variables. Insurance may have not been associated with survival because, in California, patients younger than 21 years are eligible for California Children’s Services (CCS), a state program that offers insurance for chronic and complex diseases and covers all children with cancer with or without insurance. Although the CCS program ensures that all children with ALL have access to care, this may not be sufficient in the long-term for children with low SES. Differences in relapse rates among children from different racial/ethnic groups have been observed. In a study on adherence to oral 6-mercaptopurine during the maintenance phase of ALL treatment, non-adherence was significantly higher among non-White children than White children and it considerably increased relapse rates. Sociodemographic characteristics also played a significant role in adherence to treatment.[22]
Although past evidence suggests that children with ALL treated at specialized pediatric cancer centers had better survival than those at general hospitals,[23] our study did not find survival differences by treating facility. Because the treating facility typically refers to the hospital that initially diagnosed and/or treated the patient, it is possible that some children admitted in non-specialized pediatric hospitals were later referred to pediatric cancer centers where standardized COG protocols were used, thus confounding our results.
ALL is a lethal disease if treatment is not started promptly. Although the lack of appropriate chemotherapy agents might contribute to the lower survival in Eastern Europe,[24] our examination of the proportion of children treated with chemotherapy and time from diagnosis to the start of treatment showed that the majority of study patients were treated within the first 2 weeks of diagnosis. However, late diagnosis might have had an adverse effect on outcome. Parents who are undocumented immigrants or of lower SES may wait longer to seek medical care for their children or may do so when the child is already severely sick. Late diagnosis may increase the risk of (early) death [25–27] because patients may develop severe infectious and/or metabolic complications prior to referral to a specialized cancer center.[28] However, we did not have sufficient information to evaluate this possibility.
Our data indicate that the use of prophylactic cranial irradiation has decreased markedly over time, suggesting protocol adherence to the new recommendations for using systemic and intrathecal therapy instead of radiation for children with high-risk CNS relapse. This recommendation aims to prevent late radiation-related complications such as second neoplasms.[29] Infants and older children had significant lower survival than did children aged 1–9 years, supporting findings in previous studies in Europe [30] in the US.[1]
The treatment of childhood leukemia is complex, expensive, and lengthy (2.5–3 years). With modern supportive care, fewer than 10% of deaths among children with ALL are due to therapy-associated toxicity,[31] and disease relapse remains the leading cause of death.[32] Although relapsed ALL is treated with curative intent in the US, the long-term survival of children who relapse is only approximately 25%, even when bone marrow transplant is available.[32] Multiple factors might affect the survival of children with ALL, and this can be a complex construct involving socioeconomic and cultural variables.[22]
Differences in disease biology may explain, in part, the persistent gap in survival by race/ethnicity. For example, in our study, survival differences were more marked between Black and White children (Fig. 1, Table II). Intrinsic biologic features may partially explain this observation. Previous studies reported that compared to White children, Black children with ALL had a higher incidence of unfavorable features, including high leukocyte count, higher proportion of T-cell leukemia, chromosome translocations [e.g. t(1,19)], and molecular abnormalities associated with an increased risk of relapse.[33] In contrast, approximately 50% of White children have ALL with favorable genetic features (B-cell ALL), which translate to excellent prognosis.[4] Pui et al.[34] reported that survival rate of Black children receiving intensive risk-based therapy and comprehensive supportive care can be similar to that of White children, thereby reducing the impact of these adverse factors. However, to our knowledge, these results found at a single institution, have not been replicated.
Intrinsic biologic differences may also play an important role in the poor prognosis of ALL among Hispanic children. A recent review [9] of the genomic profiling of ALL associated with susceptibility and outcome among Hispanic children identified a novel subtype of ALL called Philadelphia chromosome-like (Ph-like) ALL among these children. The incidence of Ph-like ALL in Hispanic children is significantly higher (35%) than in non-Hispanic children (7%). Approximately 50% of children with this subtype overexpress the somatic cytokine receptor-like factor 2 (CRLF2).[33] Furthermore, Perez-Andreu et al.[35] demonstrated that inherited GATA binding protein 3 (GATA3) variants are also overrepresented among Hispanics and increase the susceptibility to Ph-like ALL. The presence of both these variants is associated with a higher risk of relapse among Hispanic children with ALL and may in part explain their poor response to treatment.
Our study has some limitations. Data on specific genetic abnormalities have only been collected by the CCR since 2010. Because of the small size of this group, we could not compare the survival of children on the basis of genetic characteristics. However, this will be of interest in future studies. Most children and adolescents with ALL in California are treated at pediatric cancer centers that use COG protocols, but we do not have information about which patients are treated with these protocols and the intensity of treatment administered. We lacked data on relapse rates, as disease recurrence is not routinely collected by population-based cancer registries.
The strengths of our study include the use of a high-quality population-based dataset, a large sample of an ethnically and racially diverse population, and long period of post-diagnostic observation that allowed us to examine trends in outcome. Our study covered nearly the entire population of children and adolescents diagnosed with ALL in California and provided information on numerous factors such as neighborhood SES, insurance, treatment, treating facility, secondary neoplasm, and immunophenotype as well as age, gender, and calendar period.
In summary, despite the remarkable improvement in cure rates after ALL, non-White children and children in low SES neighborhoods have been disproportionally dying even when access to high-quality care is available and standardized protocols are followed. In the coming years, genomic findings will dramatically change the prognostic classification of ALL. In the era of precision medicine, the value of population-based cancer registries can be improved by collaborating with pediatric oncologists and cancer registries from COG-affiliated hospitals. Capturing specific biologic (e.g., ALL genomic signature, minimal residual disease, blast chromosomal abnormalities, presenting white counts, and NCI risk grouping), and socioeconomic (e.g., treatment adherence) information can help to identify predictors of racial/ethnic differences in treatment failure and guide the development of interventions aimed at improving survival for minority and low SES children with ALL.
ACKNOWLEDGEMENTS
The authors thank Michel P Coleman (LSHTM) for the early contribution to this project, Shawky Matta and Kathleen Davidson-Allen (CPIC) for their expertise with cancer registry data, and Vani Shanker (SJCRH) for manuscript editing.
Grant sponsor: Children with Cancer UK; Grant sponsor: Cancer Center Support (CORE), National Institutes of Health P30; Grant number: CA021765-30; Grant sponsor: American Lebanese Syrian Associated Charities (ALSAC); Grant sponsor: Stanford Cancer Institute (SLG); Grant sponsor: California Department of Public Health; Grant number: U58DP003862-01; Grant sponsor: California Health and Safety Code Section; Grant number: 103885; Grant sponsor: Surveillance, Epidemiology, and End Results (SEER); Grant sponsor: National Cancer Institute (NCI); Grant number: HHSN261201000140C; Grant sponsor: Cancer Prevention Institute; Grant number: N01-PC-35136; Grant sponsor: Public Health Institute; Grant numbers: N02-PC-15105; U55/CCR921930-02; HHSN261201000034C; Grant sponsor: University of Southern California; Grant number: HHSN261201000035C; Grant sponsor: Disease Control and Prevention’s National Program
Abbreviations:
- ALL
acute lymphoblastic leukemia
- CCR
California cancer registry
- CCS
California children’s services
- COG
children’s oncology group
- CI
confidence interval
- CNS
central nervous system
- CRLF2
cytokine receptor-like factor 2
- DCO
death certificate only
- HR
hazard ratio
- ICD-O-3
international classification of diseases for oncology, third edition
- NCI
national cancer institute
- NHAI
non-hispanic American Indian
- NOS
not otherwise specified
- SEER
surveillance, epidemiology, and end results
- SES
socioeconomic status
- US
United States
Footnotes
Conflict of interests: Nothing to declare.
References
- 1.Smith MA, Seibel NL, Altekruse SF, Ries LAG, Melbert DL, O’Leary M, Smith FO, Reaman GH. Outcomes for children and adolescents with cancer: Challenges for the twenty-first century. J Clin Oncol 2010;28:2625–2634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Steliarova-Foucher E, Stiller C, Kaatsch P, Berrino F, Coebergh JW, Lacour B, Parkin M. Geographical patterns and time trends of cancer incidence and survival among children and adolescents in Europe since the 1970s (the ACCIS project): An epidemiological study. Lancet 2004;364:2097–2105. [DOI] [PubMed] [Google Scholar]
- 3.Xie Y, Davies SM, Xiang Y, Robison LL, Ross JA. Trends in leukemia incidence and survival in the United States (1973–1998). Cancer 2003;97:2229–2235. [DOI] [PubMed] [Google Scholar]
- 4.Yang W, Trevino LR, Yang JJ, Scheet P, Pui CH, Evans WE, Relling MV. ARID5B snp rs10821936 is associated with risk of childhood acute lymphoblastic leukemia in Blacks and contributes to racial differences in leukemia incidence. Leukemia 2010;24:894–896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Xu H, Yang W, Perez-Andreu V, Devidas M, Fan Y, Cheng C, Pei D, Scheet P, Burchard EG, Eng C, Huntsman S, Torgerson DG, Dean M, Winick NJ, Martin PL, Camitta BM, Bowman WP, Willman CL, Carroll WL, Mullighan CG, Bhojwani D, Hunger SP, Pui CH, Evans WE, Relling MV, Loh ML, Yang JJ. Novel susceptibility variants at 10p12.31–12.2 for childhood acute lymphoblastic leukemia in ethnically diverse populations. J Natl Cancer Inst 2013;105:733–742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Pui CH, Evans WE. A 50-year journey to cure childhood acute lymphoblastic leukemia. Semin Hematol 2013;50:185–196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Goggins WB, Lo FFK. Racial and ethnic disparities in survival of US children with acute lymphoblastic leukemia: Evidence from the SEER database 1988–2008. Cancer Causes Control 2012;23:737–743. [DOI] [PubMed] [Google Scholar]
- 8.Macdougall LG, Jankowitz P, Cohn R, Bernstein R. Acute childhood leukemia in Johannesburg. Ethnic differences in incidence, cell type, and survival. Am J Pediatr Hematol Oncol 1986;8:43–51. [DOI] [PubMed] [Google Scholar]
- 9.Lim JY, Bhatia S, Robison LL, Yang JJ. Genomics of racial and ethnic disparities in childhood acute lymphoblastic leukemia. Cancer 2014;120:955–962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lee B, Iceland J, Sharp G. Racial and Ethnic Diversity Goes Local: Charting Change in American Communities Over Three Decades. Rhode Island: Brown University; 2012. [Google Scholar]
- 11.World Health Organization. International Classification of Disease for Oncology, third edition; 2000. [Google Scholar]
- 12.Ruhl J, Adamo M, Dickie L. Hematopoietic and Lymphoid Neoplasm Coding Manual. Bethesda, MD: National Cancer Institute; 2015. p. 25–29. [Google Scholar]
- 13.Schmiegelow K, Levinsen MF, Attarbaschi A, Baruchel A, Devidas M, Escherich G, Gibson B, Heydrich C, Horibe K, Ishida Y, Liang DC, Locatelli F, Michel G, Pieters R, Piette C, Pui CH, Raimondi S, Silverman L, Stanulla M, Stark B, Winick N, Valsecchi MG. Second malignant neoplasms after treatment of childhood acute lymphoblastic leukemia. J Clin Oncol 2013;31:2469–2476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Yost K, Perkins C, Cohen R, Morris C, Wright W. Socioeconomic status and breast cancer incidence in California for different race/ethnic groups. Cancer Causes Control 2001;12:703–711. [DOI] [PubMed] [Google Scholar]
- 15.Krieger N Overcoming the absence of socioeconomic data in medical records: Validation and application of a census-based methodology. Am J Public Health 1992;82:703–710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Tao L, Foran JM, Clarke CA, Gomez SL, Keegan TH. Socioeconomic disparities in mortality after diffuse large B-cell lymphoma in the modern treatment era. Blood 2014;123:3553–3562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Children’s Hospital Association. http://www.childrenshospitals.net. Accessed June 22, 2014.
- 18.Children’s Oncology Group (COG). http://www.childrensoncologygroup.org. Accessed July 10, 2014.
- 19.Pui CH, Campana D, Pei D, Bowman WP, Sandlund JT, Kaste SC, Ribeiro RC, Rubnitz JE, Raimondi SC, Onciu M, Coustan-Smith E, Kun LE, Jeha S, Cheng C, Howard SC, Simmons V, Bayles A, Metzger ML, Boyett JM, Leung W, Handgretinger R, Downing JR, Evans WE, Relling MV. Treating childhood acute lymphoblastic leukemia without cranial irradiation. N Engl J Med 2009;360:2730–2741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kadan-Lottick NS, Ness KK, Bhatia S, Gurney JG. Survival variability by race and ethnicity in childhood acute lymphoblastic leukemia. JAMA 2003;290:2008–2014. [DOI] [PubMed] [Google Scholar]
- 21.Bhatia S, Sather HN, Heerema NA, Trigg ME, Gaynon PS, Robison LL. Racial and ethnic differences in survival of children with acute lymphoblastic leukemia. Blood 2002;100:1957–1964. [DOI] [PubMed] [Google Scholar]
- 22.Bhatia S, Landier W, Hageman L, Kim H, Chen Y, Crews KR, Evans WE, Bostrom B, Casillas J, Dickens DS, Maloney KW, Neglia JP, Ravindranath Y, Ritchey AK, Wong FL, Relling MV. Adherence to oral 6-mercaptopurine in African, American and Asian children with acute lymphoblastic leukemia: A Children’s Oncology Group study. Blood 2014;2345–2353:23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Meadows AT, Kramer S, Hopson R, Lustbader E, Jarrett P, Evans AE. Survival in childhood acute lymphocytic leukemia: Effect of protocol and place of treatment. Cancer Invest 1983;1:49–55. [DOI] [PubMed] [Google Scholar]
- 24.Gatta G, Capocaccia R, Stiller CA, Kaatsch P, Berrino F, Terenziani M. Childhood cancer survival trends in Europe: A EUROCARE working group study. J Clin Oncol 2005;23:3742–3751. [DOI] [PubMed] [Google Scholar]
- 25.De Angelis C, Pacheco C, Lucchini G, Arguello M, Conter V, Flores A, Biondi A, Masera G, Baez F. The experience in Nicaragua: Childhood leukemia in low-income countries—the main cause of late diagnosis may be “medical delay”. Int J Pediatr 2012;2012:129707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Dang-Tan T, Trottier H, Mery LS, Morrison HI, Barr RD, Greenberg ML, Franco EL. Delays in diagnosis and treatment among children and adolescents with cancer in Canada. Pediatr Blood Cancer 2008;51:468–474. [DOI] [PubMed] [Google Scholar]
- 27.Fajardo-Gutierrez A, Sandoval-Mex AM, Mejia-Arangure JM, Rendon-Macias ME, Martinez-Garcia Mdel. Clinical and social factors that affect the time to diagnosis of Mexican children with cancer. Med Pediatr Oncol 2002;39:25–31. [DOI] [PubMed] [Google Scholar]
- 28.Bunin NJ, Pui CH. Differing complications of hyperleukocytosis in children with acute lymphoblastic or acute nonlymphoblastic leukemia. J Clin Oncol 1985;3:1590–1595. [DOI] [PubMed] [Google Scholar]
- 29.Hijiya N, Hudson MM, Lensing S, Zacher M, Onciu M, Behm FG, Razzouk BI, Ribeiro RC, Rubnitz JE, Sandlund JT, Rivera GK, Evans WE, Relling MV, Pui CH. Cumulative incidence of secondary neoplasms as a first event after childhood acute lymphoblastic leukemia. JAMA 2007;297:1207–1215. [DOI] [PubMed] [Google Scholar]
- 30.Gatta G, Botta L, Rossi S, Aareleid T, Bielska-Lasota M, Clavel J, Dimitrova N, Jakab Z, Kaatsch P, Lacour B, Mallone S, Marcos-Gragera R, Minicozzi P, Sanchez-Perez MJ, Sant M, Santaquilani M, Stiller C, Tavilla A, Trama A, Visser O, Peris-Bonet R. Childhood cancer survival in Europe 1999–2007: Results of EUROCARE-5—a population-based study. Lancet Oncol 2014;15:35–47. [DOI] [PubMed] [Google Scholar]
- 31.Pui CH, Pei D, Sandlund JT, Ribeiro RC, Rubnitz JE, Raimondi SC, Onciu M, Campana D, Kun LE, Jeha S, Cheng C, Howard SC, Metzger ML, Bhojwani D, Downing JR, Evans WE, Relling MV. Long-term results of St. Jude total therapy studies 11, 12, 13a, 13b, and 14 for childhood acute lymphoblastic leukemia. Leukemia 2010;24:371–382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Locatelli F, Moretta F, Rutella S. Management of relapsed acute lymphoblastic leukemia in childhood with conventional and innovative approaches. Curr Opin Oncol 2013;25:707–715. [DOI] [PubMed] [Google Scholar]
- 33.Harvey RC, Mullighan CG, Chen IM, Wharton W, Mikhail FM, Carroll AJ, Kang H, Liu W, Dobbin KK, Smith MA, Carroll WL, Devidas M, Bowman WP, Camitta BM, Reaman GH, Hunger SP, Downing JR, Willman CL. Rearrangement of CRLF2 is associated with mutation of JAK kinases, alteration of IKZF1, Hispanic/Latino ethnicity, and a poor outcome in pediatric B-progenitor acute lymphoblastic leukemia. Blood 2010;115:5312–5321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Pui CH, Pei D, Pappo AS, Howard SC, Cheng C, Sandlund JT, Furman WL, Ribeiro RC, Spunt SL, Rubnitz JE, Jeha S, Hudson MM, Kun LE, Merchant TE, Kocak M, Broniscer A, Metzger ML, Downing JR, Leung W, Evans WE, Gajjar A. Treatment outcomes in Black and White children with cancer: Results from the SEER database and St. Jude Children’s Research Hospital, 1992 through 2007. J Clin Oncol 2012;30:2005–2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Perez-Andreu V, Roberts KG, Harvey RC, Yang W, Cheng C, Pei D, Xu H, Gastier-Foster JES, Lim JY, Chen IM, Fan Y, Devidas M, Borowitz MJ, Smith C, Neale G, Burchard EG, Torgerson DG, Klussmann FA, Villagran CR, Winick NJ, Camitta BM, Raetz E, Wood B, Yue F, Carroll WL, Larsen E, Bowman WP, Loh ML, Dean M, Bhojwani D, Pui CH, Evans WE, Relling MV, Hunger SP, Willman CL, Mullighan CG, Yang JJ. Inherited GATA3 variants are associated with Ph-like childhood acute lymphoblastic leukemia and risk of relapse. Nat Genet 2013;45:1494–1498. [DOI] [PMC free article] [PubMed] [Google Scholar]


