Abstract
Background
Coronaviruses may lead to invasion of the central nervous system.
Purpose
To investigate the effects of COVID-19 infection on smell using cranial magnetic resonance imaging (MRI).
Material and Methods
Cranial MRI scans of 23 patients with COVID-19 (patient group [PG]) and 23 healthy controls (HCs) were evaluated. Peripheric (olfactory bulb [OB] volume and olfactory sulcus [OS] depth) and central (insular gyrus and corpus amygdala areas) smell regions were measured.
Results
Smell loss was present in nine patients (39.1%) in the PG. The means of the disease duration and antiviral treatment were 3.00 ± 2.35 and 5.65 ± 1.72 days, respectively. OB volumes of the PG were significantly lower than those of the HCs bilaterally. However, no significant differences were observed between the OS depth, insular gyrus, and corpus amygdala areas of both groups. The left corpus amygdala areas were both increased with the increased disease (P = 0.035, r = 0.442) and treatment durations (P = 0.037, r = 0.438). In the PG, longer treatment duration, increase in C-reactive protein (CRP), lymphocyte count decrease, and positive thoracic computed tomography (CT) involvement were related to OS depth decrease. Right corpus amygdala areas increased in patients with COVID-19 with increased D-dimer values, and thoracic CT involvement was detected.
Conclusion
COVID-19 disease affects the peripheric smell region of OBs and does not affect the central smell regions of the insular gyrus and corpus amygdala areas. The importance of our study is to detect MRI findings in patients with COVID-19 leading to odor disorders. These findings may help in diagnosing the disease at an early stage.
Keywords: COVID-19, olfactory bulb volume, olfactory sulcus depth, insular gyrus area, corpus amygdala area
Introduction
Coronaviruses are large-enveloped RNA viruses that often cause respiratory and enteric diseases in animals and humans (1). High pathogenic coronavirus, called SARS-CoV-2 (formerly known as 2019-nCoV), appeared in Wuhan, China, in December 2019, after “severe acute respiratory syndrome coronavirus” (SARS-CoV) and “Middle Eastern respiratory syndrome coronavirus” (MERS-CoV). This virus spread rapidly around the world and has a homological sequence with SARS-CoV, causing acute, highly lethal pneumonia. This virus, called coronavirus disease 2019 (COVID-19), has clinical symptoms like those of SARS-CoV and MERS-CoV (2).
In patients with COVID-19, respiratory distress is the most characteristic symptom, and most patients admitted to intensive care units lacked spontaneous breathing. In addition, some patients with COVID-19 also showed neurological symptoms, such as headaches, nausea, and vomiting. Coronaviruses do not always invade the respiratory tract, and invasion of the central nervous system induces neurological diseases (2).
Loss of smell is a significant symptom in patients with mild-to-moderate COVID-19 and is not associated with rhinorrhea and nasal obstruction. In 37.5% of patients, the loss of smell persists for at least seven days after treating the disease (3). It was reported that acute anosmia or ageusia was observed in 15.3% of patients in the early stage of COVID-19, which seems as important for the diagnosis (4). Mullol et al. (5) reported that without upper airway inflammatory disease, isolated loss of smell and/or taste (sudden and severe) may alert the physicians for COVID-19.
The aim of the present study was to investigate the effect of COVID-19 infection on the peripheral (olfactory bulb [OB] volume and olfactory sulcus [OS] depth) and central (insular gyrus and corpus amygdala) smell regions of the patients. Patients with COVID-19 (the patient group [PG] were compared with healthy controls (HCs) using cranial magnetic resonance imaging (MRI).
Material and Methods
This retrospective study was conducted at the Van Training and Research Hospital, Infectious Diseases and Radiology Departments, alongside Kırıkkale University Faculty of Medicine, Otolaryngology Department, following the principles of the Declaration of Helsinki. Cranial MRI value images were obtained from a database at the Van Training and Research Hospital Radiology Department. Approval was obtained first from the TR Ministry of Health, COVID-19 Scientific Research Evaluation Commission (2020–05–04T06_41_57), followed by Ethics Committee approval from the Van Training and Research Hospital, Clinical Research Ethics Committee (Date: 22 May 2020, Number: 2020/09). Informed consent was not required due to the retrospective nature of the study.
Participants
The present study was performed retrospectively. Cranial MRI scans of 46 adult patients were screened and selected from the database of Van Training and Research Hospital. The PG comprised 23 patients (13 men, 10 women; mean age = 37.08 ± 14.41 years; age range = 19–73 years) with a positive COVID-19 real-time polymerase chain reaction (RT–PCR) assay. The diagnosis of COVID-19 was detected by SARS-CoV-2RT–PCR (Qiagen Rotor GeneQ) using examples of the oropharyngeal and nasopharyngeal sweep. Cranial MRI was taken because of headaches in the patients who tested positive for COVID-19. The mean duration of the disease was 3.00 ± 2.35 days (range = 1–10 days), while the mean duration of treatment was 5.65 ± 1.72 days (range = 5–10 days).
Inclusion criteria were as follows: patients with a positive COVID-19 real-time polymerase chain reaction (RT–PCR) assay; and age ≥18 years of age. Individuals were excluded from the study if they had previous trauma, sinonasal or cranial surgery, sinonasal polyposis, sinonasal CSF leak, marked facial and/or nasal septal deformity, multiple sclerosis, Alzheimer’s disease, Parkinson’s disease, or epilepsy.
In the PG, loss of smell, nasal obstruction, and the presence of nasal discharge were evaluated from the hospital data. A thoracic computed tomography (CT) scan was obtained from all patients with COVID-19 and involvement was evaluated. Durations of disease and treatment were also noted. Leukocyte count and lymphocyte count were measured for all patients, and lymphopenia (lymphocyte count ≤800/mm3) was evaluated. Moreover, CRP (mg/L), D-dimer (ng/mL), and ferritin (ng/mL) values were also detected.
The HCs comprised 23 patients without COVID-19 (14 men, 9 women; mean age = 36.82 ± 13.10 years; age range = 18–71 years) with normal cranial MRI results. They were selected from the Van Training and Research Hospital’s database of Radiology Clinics.
Data acquisition
Cranial MRI measurements
MRI examinations were performed using 1.5-T MRI (General Electric MRI Systems, Signa 12/2010; General Electric Company, Milwaukee, WI, USA) with a cranial coil (eight-channel array head coil). Fluid-attenuated inversion recovery (FLAIR) images in the axial plane (TR/TE = 9002/155 ms, field of view [FOV] = 190 × 190 mm, and matrix = 192 × 160 mm) and T2-weighted (T2W) fast spin echo (FSE) images in the coronal plane (TR/TE = 4680/95 ms, FOV = 190 × 190 mm, and matrix 384 × 256 mm) were obtained using a slice thickness of 5 mm and intersection gap of 1 mm; a total of 20–25 sections were obtained.
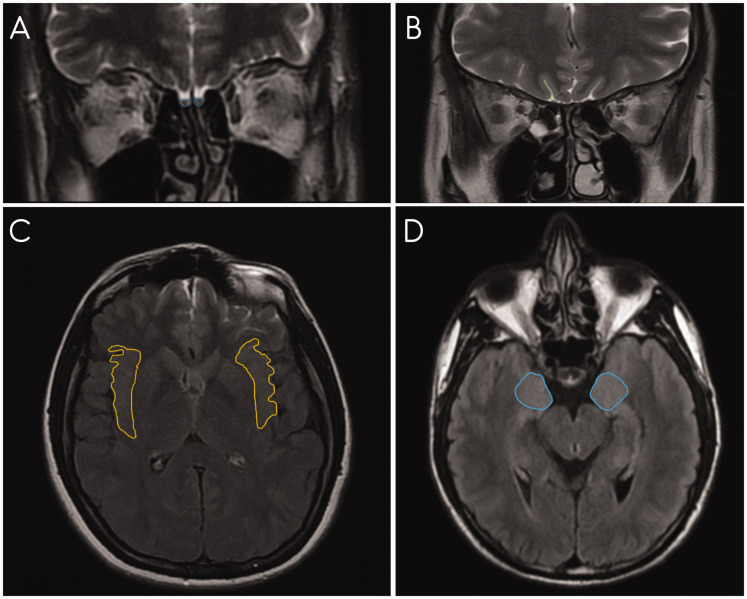
Measurement results were obtained from coronal T2W FSE images for OB volume and OS depth, and axial FLAIR images for the insular gyrus area and the corpus amygdala area (Fig. 1) (3). All measurements were evaluated by a single radiologist (V.B.).
Fig. 1.
(a) On coronal T2W FSE MRI images, OB volume measurements are shown. (b) On coronal T2W FSE MRI scans, OS depth measurements are shown. (c) On axial FLAIR MRI scans, insular gyrus area measurements are shown. (d) On axial FLAIR MRI scans, corpus amygdala area measurements are shown. FLAIR, fluid-attenuated inversion recovery; FSE, fast spin echo; MRI, magnetic resonance imaging; OB, olfactory bulb; OS, olfactory sulcus; T2W, T2-weighted.
Peripheral regions of smell
Measurement of OB volume was performed at the coronal T2W FSE sequence. From anterior to posterior screening, as the OB image was seen clearly, the surface of the OB was measured manually by an electronic cursor (as mm2) and the volume was calculated by multiplying this value with the slice thickness (as mm3) (3,6,7).
For the measurement of OS depth, in the coronal T2W FSE sequence, a virtual tangent line was drawn from the inferior orbital gyrus to the gyrus recti in the posterior plane of the orbit. The new perpendicular line was drawn from this tangent line to the deepest point of OS. The depth of this line gives the OS depth (as mm) (6–8).
Central regions of the smell
The insular gyrus area (mm2) was measured in the sections in which it was observed as the maximum. Measurements were performed on images in which the head of the caudate nucleus and the putamen were seen (9,10).
The corpus amygdala area (mm2) was measured in the sections in which it was observed as the maximum (9,10).
Statistical analysis
SPSS version 16.0 (SPSS Inc, IBM Corp., Chicago, IL, USA) was used. Chi-square test, Mann–Whitney U test, Wilcoxon-Signed Ranks test, and Spearman’s correlation rho efficient test were used.
A chi-square test was used to analyze the difference in the genders of the PG and HCs.
Mann–Whitney U test was used for the analysis of the difference (for OB volume, OS depth, insular gyrus area, and corpus amygdala area) between the PG and HCs.
In each of the PG and HCs, the difference between right and left sides (for OB volume, OS depth, insular gyrus area, and corpus amygdala area) was analyzed by the Wilcoxon-Signed Rank test.
Correlation analysis was performed by Spearman’s correlation rho efficient test.
A P < 0.05 was considered statistically significant.
Results
There were 13 men (56.5%) and 10 women (43.5%) in the PG, and 14 men (60.9%) and 9 women (39.1%) in the HCs (P = 0.765, χ2 = 0.090). No significant differences existed between the ages of the groups (P > 0.05) (Table 1).
Table 1.
Measurement results for OB volume, OS depth, insular gyrus area, and corpus amygdala area.
|
Patient group (COVID-19 group) (n = 23) |
Healthy controls (n = 23) |
P* | ||||||
|---|---|---|---|---|---|---|---|---|
| Mean | Median | SD | Mean | Median | SD | |||
| Age (years) | 37.08 | 38.00 | 14.41 | 36.82 | 38.00 | 13.10 | 0.974 | |
| Measurement results | ||||||||
| OB volume (mm3) | R | 33.72 | 33.15 | 4.18 | 35.58 | 36.94 | 4.74 | 0.040 |
| L | 33.29 | 32.43 | 4.84 | 35.94 | 36.61 | 4.21 | 0.030 | |
| P † | 0.574 | 0.563 | ||||||
| OS depth (mm) | R | 7.48 | 7.41 | 0.72 | 7.33 | 7.10 | 0.89 | 0.448 |
| L | 7.15 | 7.22 | 0.69 | 7.17 | 7.09 | 0.72 | 0.852 | |
| P † | 0.003 | 0.346 | ||||||
| Insular gyrus area (mm2) | R | 349.13 | 349.00 | 48.16 | 341.91 | 336.00 | 46.71 | 0.583 |
| L | 347.04 | 347.00 | 47.31 | 340.73 | 342.00 | 55.07 | 0.575 | |
| P † | 0.456 | 0.749 | ||||||
| Corpus amygdala area (mm2) | R | 235.39 | 233.00 | 33.41 | 232.17 | 227.00 | 37.56 | 0.758 |
| L | 236.82 | 241.00 | 33.44 | 231.34 | 224.00 | 38.96 | 0.455 | |
| P † | 0.484 | 0.659 | ||||||
*P value shows the results of Mann–Whitney U test.
†P value shows the results of Wilcoxon Signed Rank test.
OB, olfactory bulb; OS, olfactory sulcus.
The mean age of the PG was 37.08 ± 14.41 years (age range = 19–73 years). The mean age of the HCs was 36.82 ± 13.10 years (age range = 18–71 years).
Features of the COVID-19 group
Loss of smell was present in nine patients (39.1%) and absent in 14 patients (60.9%) in the PG. None of the patients in the PG experienced nasal obstruction or nasal discharge.
Thoracic CT involvement was detected in 10 patients (43.5%) and absent in 13 (56.5%) of the patients with COVID-19.
The mean leukocyte count was 5605.65 ± 1656.73/mm3 (range = 1380–7990/mm3), while the mean lymphocyte count was 1404.34 ± 720.51/mm3 (range = 450–3350/mm3). Lymphopenia was present in five patients (21.7%) and absent in 18 patients (78.3%) in the PG. The mean level of CRP was 7.24 ± 9.32 mg/L (range = 0.21–43.10 mg/L). The mean level of D-dimer was 119.34 ± 102.55 ng/mL (range = 11–346 ng/mL). The mean ferritin level was 174.46 ± 220.87 ng/mL (range = 18.94–1094.00 ng/mL).
A signal increase was not detected in the olfactory regions we examined in the FLAIR and FSE T2W MRI sequences.
Table 1 shows the measurement results for OB volume, OS depth, insular gyrus area, and corpus amygdala area.
OB volume
The OB volume of the PG was significantly lower than that in HCs bilaterally (P < 0.05). In the PG, the OB volumes were 33.72 mm3 and 33.29 mm3 on the right and left sides, respectively. However, in HCs, these values were 35.58 mm3 and 35.94 mm3, respectively. In both groups separately, no significant differences existed between OB volumes of the right and left sides (P > 0.05) (Table 1).
OS depth
No significant differences existed between the OS depth of both groups bilaterally (P > 0.05). In the PG, the OS depth of the left side was significantly lower than that of the right side (P < 0.05). In HCs, significant differences were unobserved between OS depth values of the right and left sides (P > 0.05) (Table 1).
Insular gyrus area
No significant differences existed between the insular gyrus areas of the two groups bilaterally (P > 0.05). Considering the PG and HCs separately, no significant differences were observed between the insular gyrus areas of the right and left sides (P > 0.05) (Table 1).
Corpus amygdala area
Significant differences were unobserved between the corpus amygdala areas of both groups bilaterally (P > 0.05). Considering the PG and HCs separately, no significant differences were observed between corpus amygdala areas of the right and left sides (P > 0.05) (Table 1).
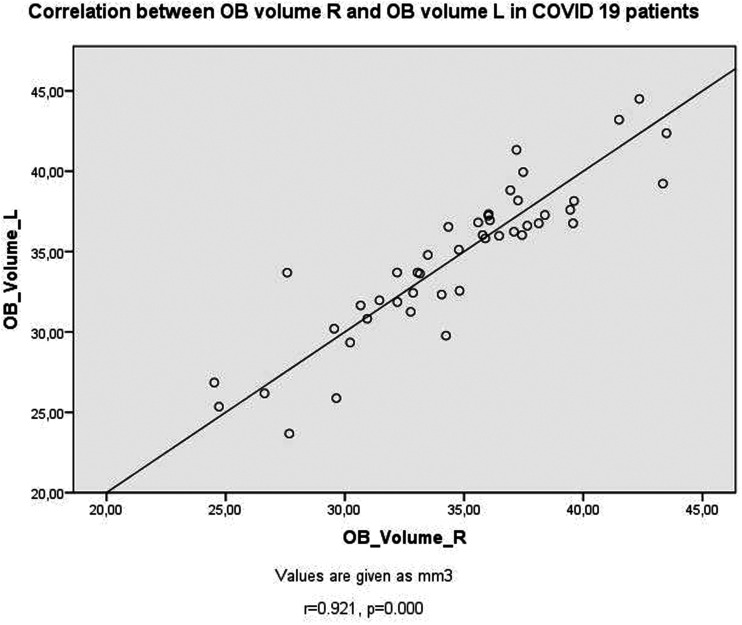
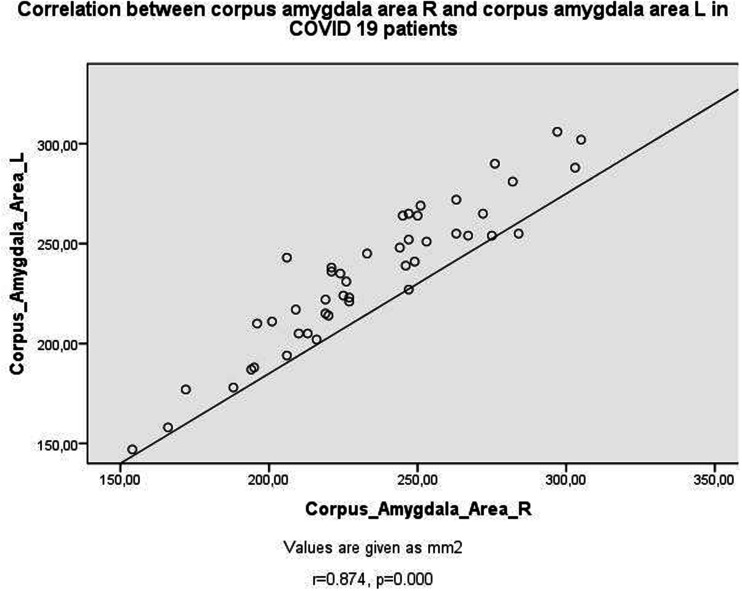
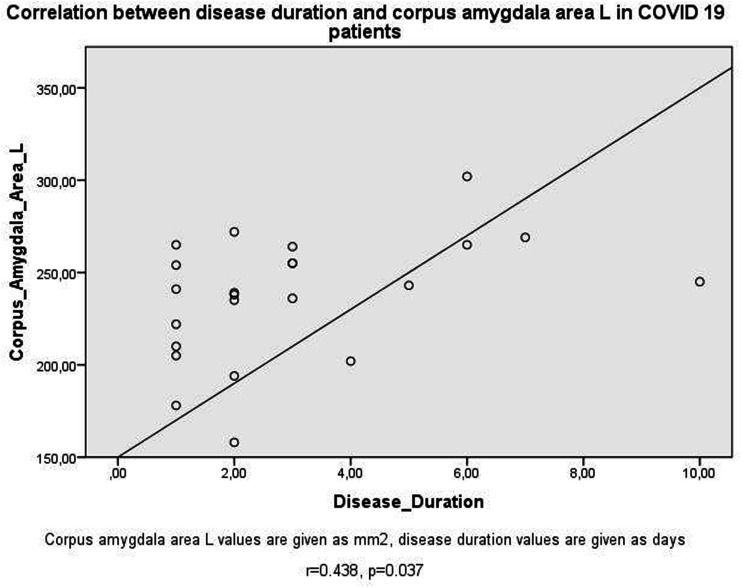
Table 2 shows the correlation test results in the PG. Positive correlations existed between OB volumes (Fig. 2), OS depths, insular gyrus areas, and corpus amygdala areas (Fig. 3) bilaterally (P < 0.05). As the left insular gyrus areas decreased, OB volumes increased bilaterally (P < 0.05). As the duration of the disease increased, the area of the left corpus amygdala increased (Fig. 4) (P < 0.05). As the duration of treatment increased, the depth of the left OS decreased and the area of the left corpus amygdala increased (P < 0.05). As CRP increased, OS depth values decreased bilaterally (P < 0.05). As D-dimer values increased, right corpus amygdala values increased (P < 0.05). As lymphocyte count decreased, right OS depth values decreased (P < 0.05). In patients with COVID-19 with thoracic CT involvement detected, left OS depth values decreased and right corpus amygdala areas increased (P < 0.05).
Table 2.
Correlation test results in the patient group (COVID-19 group).
|
OB volume (mm3) |
OS depth (mm) |
Insular gyrus area (mm2) |
Corpus amygdala area (mm2) |
|||||||
|---|---|---|---|---|---|---|---|---|---|---|
| R | L | R | L | R | L | R | L | |||
| OB volume (mm3) | R | r | 0.921 | 0.070 | –0.094 | –0.387 | –0.481 | –0.308 | –0.284 | |
| p | 0.000 | 0.752 | 0.670 | 0.068 | 0.020 | 0.153 | 0.189 | |||
| L | r | 0.921 | 0.148 | –0.010 | –0.394 | –0.484 | –0.294 | –0.284 | ||
| p | 0.000 | 0.500 | 0.964 | 0.063 | 0.019 | 0.173 | 0.189 | |||
| OS depth (mm) | R | r | 0.070 | 0.148 | 0.815 | –0.174 | –0.104 | 0.000 | 0.057 | |
| p | 0.752 | 0.500 | 0.000 | 0.427 | 0.638 | 1.000 | 0.797 | |||
| L | r | –0.094 | –0.010 | 0.815 | 0.027 | 0.096 | –0.001 | –0.024 | ||
| p | 0.670 | 0.964 | 0.000 | 0.902 | 0.662 | 0.996 | 0.913 | |||
| Insular gyrus area (mm2) | R | r | –0.387 | –0.394 | –0.174 | 0.027 | 0.978 | –0.051 | –0.051 | |
| p | 0.068 | 0.063 | 0.427 | 0.902 | 0.000 | 0.816 | 0.817 | |||
| L | R | –0.481 | –0.484 | –0.104 | 0.096 | 0.978 | –0.074 | –0.061 | ||
| p | 0.020 | 0.019 | 0.638 | 0.662 | 0.000 | 0.736 | 0.784 | |||
| Corpus amygdala area (mm2) | R | r | –0.308 | –0.294 | 0.000 | –0.001 | –0.051 | –0.074 | 0.874 | |
| p | 0.153 | 0.173 | 1.000 | 0.996 | 0.816 | 0.736 | 0.000 | |||
| L | r | –0.284 | –0.284 | 0.057 | –0.024 | –0.051 | –0.061 | 0.874 | ||
| p | 0.189 | 0.189 | 0.797 | 0.913 | 0.817 | 0.784 | 0.000 | |||
| Age | r | –0.030 | –0.077 | –0.401 | –0.304 | –0.103 | –0.152 | 0.209 | 0.000 | |
| p | 0.893 | 0.728 | 0.058 | 0.159 | 0.641 | 0.489 | 0.338 | 0.999 | ||
| Gender (Code 1: Male; Code 2: Female) | r | –0.264 | –0.317 | –0.020 | 0.000 | 0.291 | 0.317 | 0.007 | –0.020 | |
| p | 0.223 | 0.140 | 0.928 | 1.000 | 0.178 | 0.140 | 0.976 | 0.928 | ||
| Loss of smell (Code 1: Present; Code 0: Absent) | r | 0.107 | 0.087 | –0.114 | –0.215 | –0.208 | –0.222 | –0.195 | –0.316 | |
| p | 0.626 | 0.692 | 0.604 | 0.325 | 0.340 | 0.309 | 0.373 | 0.142 | ||
| Duration of disease (days) | r | 0.240 | 0.082 | –0.105 | –0.404 | –0.362 | –0.396 | 0.339 | 0.442 | |
| p | 0.270 | 0.709 | 0.633 | 0.056 | 0.090 | 0.061 | 0.114 | 0.035 | ||
| Duration of treatment (days) | r | –0.272 | –0.331 | –0.389 | –0.506 | –0.214 | –0.166 | 0.331 | 0.438 | |
| p | 0.208 | 0.123 | 0.066 | 0.014 | 0.326 | 0.450 | 0.123 | 0.037 | ||
| Leukocyte count (/mm3) | r | 0.080 | 0.087 | 0.143 | –0.124 | 0.057 | 0.094 | –0.148 | –0.333 | |
| p | 0.717 | 0.691 | 0.514 | 0.573 | 0.795 | 0.668 | 0.501 | 0.121 | ||
| CRP (mg/L) | r | –0.231 | –0.345 | –0.447 | –0.414 | 0.070 | 0.072 | 0.202 | 0.322 | |
| p | 0.288 | 0.106 | 0.033 | 0.050 | 0.752 | 0.743 | 0.355 | 0.134 | ||
| D-dimer (ng/mL) | r | –0.357 | –0.364 | –0.155 | 0.027 | –0.100 | –0.096 | 0.502 | 0.403 | |
| p | 0.094 | 0.088 | 0.481 | 0.902 | 0.648 | 0.661 | 0.015 | 0.057 | ||
| Ferritin (ng/mL) | r | 0.234 | 0.148 | –0.236 | –0.317 | –0.269 | –0.341 | 0.258 | 0.204 | |
| p | 0.282 | 0.500 | 0.279 | 0.140 | 0.215 | 0.111 | 0.234 | 0.350 | ||
| Lymphocyte count (/mm3) | r | –0.040 | 0.111 | 0.469 | 0.375 | 0.125 | 0.129 | –0.073 | –0.106 | |
| p | 0.858 | 0.613 | 0.024 | 0.078 | 0.570 | 0.557 | 0.740 | 0.631 | ||
| Lymphopenia (Lymphocyte count ≤ 800/mm3) (Code 1: Present; Code 0: Absent | r | –0.048 | –0.175 | –0.381 | –0.365 | 0.191 | 0.135 | 0.151 | 0.215 | |
| p | 0.829 | 0.425 | 0.072 | 0.086 | 0.383 | 0.539 | 0.491 | 0.325 | ||
| Thoracic CT involvement (Code 1: Present; Code 0: Absent) | r | –0.264 | –0.231 | –0.357 | –0.516 | –0.185 | –0.165 | 0.437 | 0.384 | |
| p | 0.223 | 0.288 | 0.094 | 0.012 | 0.397 | 0.451 | 0.037 | 0.071 | ||
*P value shows the results of Spearman’s correlation rho efficient test
CRP, C-reactive protein; CT, computed tomography; OB, olfactory bulb; OS, olfactory sulcus
Fig. 2.
Scatter plots of correlation between OB volume R and OB volume L in patients with COVID-19. OB, olfactory bulb.
Fig. 3.
Scatter plots of correlation between corpus amygdala area R and corpus amygdala area L in patients with COVID-19.
Fig. 4.
Scatter plots of correlation between duration of the disease and corpus amygdala area L in patients with COVID-19.
Discussion
The main symptoms of COVID-19 are dry cough, fever, and fatigue. However, in Shandong, China, a subset of patients experienced neurological symptoms and lacked respiratory symptoms (11). The most common symptoms of COVID-19 are headaches in 70.3% of patients, loss of smell in 70.2% of patients, nasal obstruction in 67.8% of patients, and cough in 63.2% of patients. In female patients, “smell loss, headache, nasal obstruction, and fatigue” are more common (3).
Viruses access host cells through the angiotensin-converting enzyme (ACE) 2. This enzyme is mostly located in type II alveolar cells. However, in the central nervous system, glial cells and neurons have been reported to express ACE 2 receptors. Therefore, the brain is also a potential target of the virus (12). Information about the transneuronal transport of SARS-CoV with an OB supports this hypothesis (13). However, viral invasion of OB should be evaluated as a neurobiological background in infected patients for smell and taste disorders (11,14). Anosmia is not one of the possible symptoms of COVID-19 (15,16).
In the present study, the effects of COVID-19 infection on peripheral (OB volume and OS depth) and central (insular gyrus and corpus amygdala) smell regions of the patients were investigated. Nasal obstruction or nasal discharge was lacking regarding patients with COVID-19 and loss of smell was present in 9 (39.1%) of them. The mean durations of the disease and treatment were 3.00 ± 2.35 days and 5.65 ± 1.72 days, respectively.
Our measurements showed that OB volumes in the PG were significantly lower than those in HCs bilaterally. However, significant differences were unobserved between the OS depth, insular gyrus, and corpus amygdala areas of both groups. In PG, the OS depth of the left side was significantly lower than that of the right side. COVID-19–related inflammation and edema (17) and neuropathic damage in the olfactory pathway (18–20) may cause a decrease in OB volume. Olfactory and taste disorders may be associated with the neuroinvasive potential of SARS-CoV-2 (1), which may be more common in European patients due to higher ACE-2 expression in the nasal mucosa (21).
In the PG, positive correlations existed between OB volumes, OS depths, insular gyrus areas, and corpus amygdala areas bilaterally. As disease and treatment durations increased, the left corpus amygdala area increased. OS depth decreased in patients with COVID-19 with longer treatment, with increased CRP values, decreased lymphocyte count, and thoracic CT involvement was detected. Right corpus amygdala areas increased in patients with COVID-19 with increased D-dimer values and thoracic CT involvement was detected.
Postviral anosmia is the most common underlying cause of anosmia, comprising up to 40% of cases. The underlying cause is primarily mucosal congestion, which induces nasal obstruction and conductive olfactory loss (22). While anosmia in the majority of cases resolves once the clinical symptoms and obstruction subside, some patients are left with permanent anosmia due to virus-induced sensory neuronal damage, known as postviral olfactory loss (23). Cellular factors for introducing SARS-CoV-2 (i.e. ACE 2 receptors and transmembrane serine protease 2) are expressed in the olfactory epithelium and olfactory sensory neurons. This indicates that the olfactory epithelium is an entry place for SARS-CoV-2 (24).
Galougahi et al. (25) reported the findings on OB MRI in a patient presenting with isolated anosmia secondary to COVID-19, which was confirmed by RT–PCR assay. From the sudden onset of complete loss of olfactory function, they found OB volume as normal. Abnormal signal density in the OB and tract was not detected, and signs of nasal congestion were unobserved.
Gilani et al. (26) reported that, with the onset of COVID-19, they noticed an increase in the number of patients admitted with a new beginning of full smell loss. They identified patients with complete loss of sense of smell over a two-week period after the COVID-19 outbreak. They hypothesized that the injury mechanism is like other coronavirus infections, causing peripheral and central neurological deficits.
SARS-CoV-2 is mainly effective on lymphocytes, especially T lymphocytes. In most patients, the absolute value of lymphocytes has decreased. Virus particles emitted from the respiratory mucosa and transmitted to other cells form a cytokine storm in the body and generate an immune response. It causes changes in peripheral white blood cells and immune cells, such as lymphocytes. Also, lymphopenia increases the risk of mortality in an early stage of COVID-19 disease (27). In the present study, lymphopenia was present in five patients (21.7%) in the PG. In the PG, the mean level of CRP was 7.24 ± 9.32 mg/L, the mean D-dimer level was 119.34 ± 102.55 mL, and the mean level of ferritin was 174.46 ± 220.87 ng/mL. While inflammatory biomarkers such as CRP and erythrocyte sedimentation rate have increased, neutrophils and procalcitonin have not changed significantly in mild forms of the COVID-19 disease. The pathogenesis of severe SARS-CoV-2 infection is associated with inflammatory response disorders. D-dimer increases significantly at the critical stage of the disease. However, not all these biomarkers are specific to SARS-CoV-2 and can similarly be seen in other viral infections (28). A significant reduction in lymphocyte count causes SARS-CoV-2 to consume immune cells and inhibit the body’s cellular immune function (27). An increase in CRP and lymphopenia is also detected in the critical group of patients (29). In our patients, lymphopenia may be attributed to endothelial damage related to an increase in CRP and D-dimer levels.
It has been reported that women are less prone to complications related to viral infections due to factors related to different innate immunity, steroid hormones, and sex chromosome-related factors (30). In particular, immune-regulating genes encoded by the female X chromosome can cause lower viral load levels and less inflammation in women than men (28). However, Lechien et al. (3) said that women are more susceptible to developing post-infectious odor dysfunctions in viral infections (i.e. Epstein–Barr virus, parainfluenza, or a previous form of coronavirus) (31).
In the present study, we investigated peripheric and central smell regions in patients with COVID-19. In the literature, we could not find any studies on the central smell regions of the insular gyrus and corpus amygdala in patients with COVID-19. In addition, no studies exist for OS depth in patients with COVID-19. Our study is the first report of all smell regions in patients with COVID-19 by MRI. In the literature, normal OB volume size and abnormal signal intensity have been reported in patients with COVID-19 (24). The signal increase was not detected in the olfactory regions we examined in the FLAIR and FSE T2W MRI sequences.
The present results showed that OB volumes were bilaterally lower in the PG. Moreover, OS depth was lower in the left side of the PG compared to the right side. OS depth, insular gyrus, and corpus amygdala areas did not differ in both groups. We concluded that COVID-19 disease affects the peripheric smell region of OBs and does not affect the central smell regions of the insular gyrus and corpus amygdala. In cases of longer treatment, increased CRP values, decreased lymphocyte count, and thoracic CT involvement, doctors should be aware of the possibility of decreased OS depth values.
The present study has some limitations. First, there was a small number of individuals in both groups as the number of patients in the PG was not high; therefore, the HCs were selected retrospectively and there were no clinical data for the HCs. In addition, the measurements are performed by a single radiologist three times to achieve the most accurate results in all measurements and their average values were taken.
In conclusion, the importance of the present study was to detect MRI findings in patients with COVID-19 leading to odor disorders. These findings may help in diagnosing the disease. Early recognition of the disease will help clinicians with early isolation and treatment. Early diagnosis will also significantly reduce the patient’s risk of virus insemination.
Footnotes
Declaration of conflicting interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) received no financial support for the research, authorship, and/or publication of this article.
ORCID iD: Nuray Bayar Muluk https://orcid.org/0000-0003-3602-9289
References
- 1.Glass WG, Subbarao K, Murphy B, et al. Mechanisms of host defense following severe acute respiratory syndrome‐coronavirus (SARS‐CoV) pulmonary infection of mice. J Immunol 2004; 173:4030–4039. [DOI] [PubMed] [Google Scholar]
- 2.Li YC, Bai WZ, Hashikawa T.The neuroinvasive potential of SARS-CoV2 may play a role in the respiratory failure of COVID-19 patients. J Med Virol 2020; 92:552–555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lechien JR, Chiesa-Estomba CM, Place S, et al. Clinical and epidemiological characteristics of 1420 European patients with mild-to-moderate coronavirus disease 2019. J Intern Med 2020;288:335–344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lee Y, Min P, Lee S, et al. Prevalence and duration of acute loss of smell or taste in COVID-19 patients. J Korean Med Sci 2020; 35:e174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mullol J, Alobid I, Mariño-Sánchez F, et al. The loss of smell and taste in the COVID-19 outbreak: a tale of many countries. Curr Allergy Asthma Rep 2020; 20:61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kandemir S, Muluk NB, Melikoglu B, et al. Smell functions in patients with multiple sclerosis: a prospective case-control study. B-ENT 2016; 12: 323–31. [PubMed]
- 7.Duprez TP, Rombaux P. Imaging the olfactory tract (cranial nerve #1). Eur J Radiol 2010; 74: 288–98. [DOI] [PubMed] [Google Scholar]
- 8.Buschhüter D, Smitka M, Puschmann S, et al. Correlation between olfactory bulb volume and olfactory function. Neuroimage 2008; 42:498–502. [DOI] [PubMed] [Google Scholar]
- 9.Hastings RS, Parsey RV, Oquendo MA, et al. Volumetric analysis of the prefrontal cortex, amygdala, and hippocampus in major depression. Neuropsychopharmacology 2004; 29:952–959. [DOI] [PubMed] [Google Scholar]
- 10.Rombaux P, Duprez T, Hummel T.Olfactory bulb volume in the clinical assessment of olfactory dysfunction. Rhinology 2009; 47:3–9. [PubMed] [Google Scholar]
- 11.Sellner J, Taba P, Öztürk S, et al. The need for neurologists in the care of COVID-19 patients. Eur J Neurol 2020;27:e31–e32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Baig AM, Khaleeq A, Ali U, et al. Evidence of the COVID-19 virus targeting the CNS: tissue distribution, host-virus interaction, and proposed neurotropic mechanisms. ACS Chem Neurosci 2020; 11:995–998. [DOI] [PubMed] [Google Scholar]
- 13.Netland J, Meyerholz DK, Moore S, et al. Severe acute respiratory syndrome coronavirus infection causes neuronal death in the absence of encephalitis in mice transgenic for human ACE2. J Virol 2008; 82:7264–7275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Giacomelli A, Pezzati L, Conti F, et al. Self-reported olfactory and taste disorders in SARS-CoV-2 patients: a cross-sectional study. Clin Infect Dis 2020;71:889–890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hummel T, Whitcroft KL, Andrews P, et al. Position paper on olfactory dysfunction. Rhinol Suppl 2017; 54:1–30. [DOI] [PubMed] [Google Scholar]
- 16.Vaira LA, Salzano G, Deiana G, et al. Anosmia and ageusia: common findings in COVID-19 patients. Laryngoscope 2020;130:1787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sarbu N, Shih RY, Jones RV, et al. White matter diseases with radiologic pathologic correlation. Radiographics 2016; 36:1426–1447. [DOI] [PubMed] [Google Scholar]
- 18.Yazdanpanah N, Saghazadeh A, Rezaei N.Anosmia: a missing link in the neuroimmunology of coronavirus disease 2019 (COVID-19). Rev Neurosci 2020; 31:691–701. [DOI] [PubMed] [Google Scholar]
- 19.Chetrit A, Lechien JR, Ammar A, et al. Magnetic resonance imaging of COVID-19 anosmic patients reveals abnormalities of the olfactory bulb: Preliminary prospective study. J Infect 2020; 81:816–846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mao L, Jin H, Wang M, et al. Neurologic manifestations of hospitalized patients with coronavirus disease 2019 in Wuhan, China. JAMA Neurol 2020; 77:683–690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Long C, Xu H, Shen Q, et al. Diagnosis of the Coronavirus disease (COVID-19): rRT-PCR or CT? Eur J Radiol 2020;126:108961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Welge-Lüssen A, Wolfensberger M.Olfactory disorders following upper respiratory tract infections. Adv Otorhinolaryngol 2006;26:125–132. [DOI] [PubMed]
- 23.Seiden AM.Postviral olfactory loss. Otolaryng Clin N Am 2004; 37:1159–1166. [DOI] [PubMed] [Google Scholar]
- 24.Bagheri SHR, Asghari AM, Farhadi M, et al. Coincidence of COVID-19 epidemic and olfactory dysfunction outbreak. Med J Islam Repub Iran 2020;34:62 [DOI] [PMC free article] [PubMed]
- 25.Galougahi MK, Ghorbani J, Bakhshayeshkaram M, et al. Olfactory Bulb Magnetic Resonance Imaging in SARS-CoV-2-Induced Anosmia: The First Report. Acad Radiol 2020;27:892–893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Gilani S, Roditi R, Naraghi M.COVID-19 and anosmia in Tehran, Iran. Med Hypotheses 2020;141:109757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Chen N, Zhou M, Dong X, et al. Epidemiological and clinical characteristics of 99 cases of 2019 novel coronavirus pneumonia in Wuhan, China: a descriptive study. Lancet 2020; 395:507–513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wang F, Hou H, Lou Y, et al. The laboratory tests and host immunity of COVID-19 patients with different severity of illness. JCI Insight 2020; 5:e137799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zhou Y, Fu B, Zheng X, et al. Pathogenic T cells and inflammatory monocytes incite inflammatory storm in severe COVID-19 patients. Natl Sci Rev 2020. doi: 10.1093/nsr/nwaa041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Conti P, Younes A.Coronavirus COV-19/SARS-CoV-2 affects women less than men: clinical response to viral infection. J Biol Regul Homeost Agents 2020; 34:339–343. [DOI] [PubMed] [Google Scholar]
- 31.Suzuki M, Saito K, Min WP, et al. Identification of viruses in patients with postviral olfactory dysfunction. Laryngoscope 2007; 117:272–277. [DOI] [PMC free article] [PubMed] [Google Scholar]