Abstract
Outcomes of COrona VIrus Disease-19 (COVID-19) in patients with rheumatic diseases (RDs) reported in various studies are heterogenous owing to the influence of age and comorbidities which have a significant bearing on the infection risk, severity, morbidity, and mortality. Diabetes mellitus (DM) and RDs are closely linked with underlying pathobiology and treatment of RDs affecting the risk for DM as well as the glycemic control. Hence, we undertook this narrative review to study the influence of DM on outcomes of COVID-19 in patients with RDs. Additionally, aspects of patient attitudes and immune response to COVID-19 vaccination were also studied. The databases of MEDLINE/PubMed, Scopus, and Directory of Open Access Journals (DOAJ) were searched for relevant articles. Studies from mixed cohorts revealed insufficient data to comment on the influence of DM on the risk of infection, while most studies showed twice the odds for hospitalization and mortality with DM. Specific cohorts of rheumatoid arthritis and systemic lupus erythematosus revealed a similar association. Poor health was noted in patients with spondyloarthritis and DM during the pandemic. The presence of DM did not affect patient attitudes towards vaccination and did not predispose to additional vaccine-related adverse effects. Immune response to inactivated vaccines was reduced but mRNA vaccines were maintained in patients with DM. Detailed assessment of DM with its duration, end-organ damage, and glycemic control along with a focused association of DM with various aspects of COVID-19 like risk, hospitalization, severity, mortality, post-COVID sequelae, immune response to infection, and vaccination are needed in the future.
|
Key Points • Diabetes mellitus is associated with the severity of infection, COVID-19-related hospitalization, and mortality in rheumatic diseases across most studies but studies analyzing its specific role are lacking. • Poor outcomes of COVID-19 in RA and poor health in spondyloarthritis are strongly associated with diabetes mellitus. • Diabetes mellitus may negatively influence the humoral response to inactivated vaccines but does not seem to affect the immune responses to mRNA vaccines. • Diabetes mellitus does not influence the attitude towards vaccination or deviation from the prescribed medications during the pandemic. |
Keywords: Comorbidities, COVID-19, Diabetes mellitus, Rheumatic diseases, Rheumatoid arthritis, Systemic lupus erythematosus
Introduction
Over 2 years into the COrona VIrus Disease-19 (COVID-19) pandemic, the world has observed over 0.5 billion cases with 6.3 million deaths as of June 19, 2022. The maximum number of cases were observed in the USA followed by India and Brazil, whereas the USA, Brazil, and India observed the highest mortality in the same order [1].
Interestingly, these regions have a high prevalence of diabetes mellitus (DM) and obesity and not surprisingly, the earliest reports on outcomes of COVID-19 from China revealed an increased association of infection and severe outcomes with DM, hypertension (HTN), and cardiovascular diseases (CVDs) [2–7]. A nationwide study from Korea identified an increased association of risk of infection as well as severity with DM in addition to other comorbidities [8]. A systematic review and meta-analysis revealed poor outcomes like hospitalization and mortality associated with DM; however, there was no association with an increased risk of infection. A further meta-regression revealed the association of DM with mortality [9]. DM exacerbates the inflammatory response to SARS-CoV2 with an increase in cytokines, reactive oxygen species, and renin-angiotensin system leading to vascular endothelial damage, acute lung injury, lung fibrosis, and increased risk for thromboembolic complications [10, 11]. A reverse effect of the pandemic on DM was also observed with poor glycemic control and a higher rate of diabetic ketoacidosis as compared to the pre-pandemic period [12].
The association between DM and rheumatic diseases (RDs) is bidirectional. There is an increased risk of certain RDs like osteoarthritis, crystal-induced arthropathy, diffuse idiopathic skeletal hyperostosis, and tendinopathies with DM. It is also associated with poorer outcomes and treatment-related complications, especially infections, due to a combined effect of DM and immunosuppressant use [13].
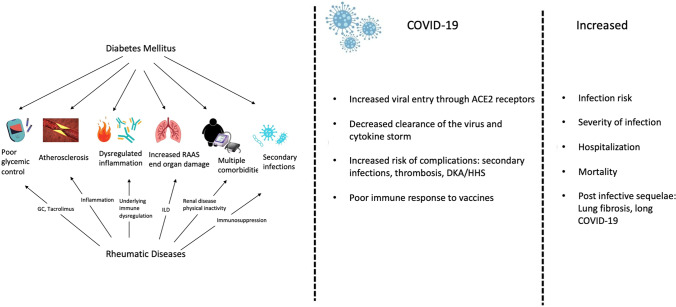
On the other hand, chronic inflammation characterized by elevated proinflammatory cytokines like tumor necrosis factor α and interleukin-6 in rheumatoid arthritis (RA) promotes insulin resistance and the development of DM [14]. The use of glucocorticoids (GCs) in RA negatively impacts insulin resistance and increases the risk of DM by 25–30% with every 5-mg increase in the dose of GC in prednisolone equivalents [15]. Control of disease activity using disease-modifying anti-rheumatoid drugs (DMARDs) effectively mitigates this risk [16]. Similarly, there is an increased risk of DM in systemic lupus erythematosus (SLE) owing to chronic inflammation as well as the presence of anti-insulin antibodies [17]. Furthermore, treatment of SLE requires higher doses and duration of GCs which adds to the risk of development of DM [18, 19]. This can be translated to other inflammatory arthritides and systemic rheumatic diseases which are characterized by chronic inflammation with an increased risk of obesity owing to poor physical activity secondary to pain and fatigue. There is a new school of thought wherein DM is considered to have autoimmune origins with the dysregulated production of proinflammatory adipokines by adipose tissue driven by obesity, unhealthy lifestyle, and advanced age. This eventually leads to insulin resistance and DM (Fig. 1) [20].
Fig. 1.
Interaction of diabetes mellitus, rheumatic diseases, and COVID-19. *DKA diabetic ketoacidosis, HHS hyperosmolar hyperglycemic syndrome, CVD cardiovascular disease, GC glucocorticoids, HTN hypertension, COVID-19 COrona VIrus Disease-2019
Patients with RDs form a vulnerable group owing to underlying immune dysregulation and immunosuppressant use for poor outcomes with COVID-19. The elevated risk of DM in this subgroup further adds to the complexity of the issue [21, 22]. Thus, it is imperative to study the effects of comorbid DM in patients with RDs on the outcomes of COVID-19. Reviews with a focused objective of studying the same are lacking and the original articles available are heterogenous in terms of methodology, ethnicity, and outcomes. Thus, we aimed to carry out a narrative review on the role of type 2 DM as a comorbidity and its effect on outcomes in patients with RDs infected with severe acute respiratory syndrome-corona virus 2 (SARS-CoV2), with a focus on hospitalization due to pneumonia, admission to intensive care unit, and death. We have also analyzed the influence of DM on patient attitudes and immune response to vaccination.
Search strategy
Searches through MEDLINE/PubMed and Scopus were performed in line with previously published recommendations [23]. Articles published till June 20, 2022, were reviewed using the following keywords: [(“COVID-19” AND “Diabetes Mellitus” AND “Rheumatic disease” OR “Immune-Mediated Inflammatory Disease), (“COVID-19” AND “Rheumatic Disease” AND “Vaccine”), (“COVID-19” AND “Diabetes Mellitus” AND “Rheumatoid Arthritis”), (“COVID-19” AND “Diabetes Mellitus” AND “Systemic Lupus Erythematosus”)] and similarly for specific diseases. Articles in languages other than English and reviews, conference proceedings, and editorials were excluded. Relevant articles searchable at the Directory of Open Access Journals (DOAJ) and references of included articles were also processed for eligibility and inclusion for this narrative review.
Studies with mixed cohorts
COVID-19 is usually a self-limiting illness but can have severe outcomes in the form of hospitalization, cytokine storm, acute respiratory distress syndrome, post-COVID-19 lung disease, long COVID, and mortality ranging from 0.5 to 2.7% [24, 25]. Most studies from the early phases of the pandemic showed an increased risk for adverse outcomes in patients with RDs. However, these patients also have other associated poor prognostic factors like age and comorbidities. These complicate the analysis and call for a guarded interpretation of data.
COVID-19 and RDs
Early reports from countrywide or global registries estimated an increased risk for hospitalization and mortality in patients with RDs and COVID-19 [26–28]. A Danish countrywide registry with 11,122 individuals with COVID-19 reported an increased association of RDs with hospitalization (odds ratio [OR] 2.1, confidence interval [CI] 1.8–2.5) and mortality (OR 1.5 95% CI 1.1–2), but when it was adjusted for comorbidities, this association became insignificant [26]. A subsequent study from the Danish national registry with 58,052 patients with RDs revealed an increased association of RA (OR 1.2, 95% CI 1.2–2.3) with hospitalization which persisted when adjusted for age, sex, and comorbidities; the event rate overall was low to identify specific risk factors in patients with RA [29]. Another study from the USA across 24 health organizations (TriNetX research network) examined 31,461 patients of which 681 had RDs. There was an increased risk for death associated with RDs (OR 2.05, 95% CI 1.54–2.72) as well as all other comorbidities on univariate analysis. However, on multivariate analysis, older age, male gender, non-white ethnicity, and underlying cardiac, pulmonary, liver, and kidney diseases remained significant associations. Thus, overall, there is an increased risk for hospitalization and adverse outcomes in patients with RDs, most of which can be attributed to associated comorbidities.
Risk of COVID-19 in RDs and DM
A study in India carried out during the first wave of COVID-19 in 2020 revealed an increased association of the risk of COVID-19 with the presence of DM in patients with RDs, but this association became insignificant in adjusted multivariate analysis [30]. Another study in Spain (2021) did not find any influence of DM on the risk of COVID-19 in patients with RDs [31]. Most studies have not specifically analyzed DM as a risk factor for COVID-19 in RDs (Table 1).
Table 1.
Characteristics of studies with mixed cohorts of RDs and COVID-19
| Study | Type | N (%)* | N (%), COVID-19 (confirmed and probable) | N (%), DM | Moderate to severe COVID-19 N (%) and its associations | Hospitalizations N (%) and its associations | Mortality N (%) and its associations | Other salient features |
|---|---|---|---|---|---|---|---|---|
| Santos et al., 2020, Spain [43] |
Observational, prospective (cross-sectional) |
38 RA 11 (39) |
38 | 15 (32) | - | 38 |
10 of 38 (26) UV: - Older age - DM (OR 33, 95% CI 3.4–314) - HTN - Dyslipidemia -ILD - CVD - High disease activity MV: - High disease activity - Dyslipidemia -CVD - ILD |
|
| Pablos et al., 2020, Spain [68] |
Observational, prospective (cohort) |
228 RA 65 (29), SpA 71 (30). CTDs 92 (40) |
228 | 39 (17) |
72 of 228 (31.6) UV: - Older age - M - Obesity - DM (OR 1.9, 95% CI 1.34–2.79) - HTN - Lung disease -CVD |
162 of 228 (71) ICU 15 of 228 (7) |
41 of 228 (18) | |
| Shobha et al., 2020, India [30] |
Observational, prospective (cohort) |
3807 RA 1964 (52.2), SLE 561 (14.8), SpA 313 (8.2), SSc 134 (3), vasculitis 107 (2.8), SS 69 (1.8), IIM 81 (2.1) |
23 (0.6) UV: - DM (3.67, 95% CI 1.5–8.98) - HTN - Lung disease - GC (OR 3, 95% CI 1.23–7.34) - ARB - Flu vaccine - MMF - CYC - Biologics MV: - Lung disease |
420 (11) |
15 of 23 (65.2) ICU 3 of 23 (13) |
- | ||
| Habermann et al., 2020, USA [69] | Observational, prospective (case series) |
86 RA 20 (23), SpA 30 (35), IBD 37 (43), psoriasis 14 (16) |
86 | - |
14 (16) - Older age - RA - HT - DM - Lung disease - GC - HCQ - MTX |
- | - | - |
| Nunez et al., 2020, Spain [41] |
Observational, prospective (cross-sectional) |
123 RA 50 (40.65), SpA 24 (21) |
123 | 17 (13.8) | - |
54 of 123 (51) ICU 2 of 123 (1.6) UV: - Older age - CTDs - HTN - DM (OR 5.15, 95% CI 1.5–16.8) - Lung disease - Heart disease Protective: - TNFi - F - NSAIDs |
12 of 123 (10) | |
| Montero et al., 2020, Spain [70] |
Observational, retrospective (cross-sectional) |
62 RA 20 (32), SpA 16 (26) |
62 | 12 (20) | - |
42 of 62 (68) UV: - Age > 70 - M - Lung disease - CVD - HTN - DM - GC MV: - M - Lung disease - GC |
10 of 62 (6) - Older age - M - DM - HTN |
|
| Gianfrancesco et al., 2020, Global [28] |
Prospective, observational (Global Rheumatology alliance-2019 registry) |
600 RA 230 (38), SLE 85 (14), SpA 122, vasculitis 44 (7), SS 28 (5), IIM (3), gout 19 (3), SSc 16 (3), PMR 12 (2), sarcoidosis 10 (2) |
600 | 69 (12) | - |
277 of 600 (46) UV: - Older age - HTN - Lung disease - DM - CVD - CKD - Smoking - SLE - Vasculitis - GC > 10 mg MV: - Age > 65 - HT/CVD - DM (OR 2.61, 95% CI 1.39–4.88) - CKD - GC > 10 (OR 2.1, 95% CI 1.1–3.9) (Protective) - b/tsDMARD |
55(9) | |
| Marques et al., 2021, Brazil *[39] |
Observational, prospective (ReumaCoV Registry Brasil) |
334 SLE 110 (32.9), RA 95 (28.4), SpA 68 (20), Ssc 23 (6.9), vasculitis 10 (3.3) |
334 | 39 (11.5) | - |
110 of 334 (33) ICU 50 of 334 (15) MV: - Age > 50 - No TNFi - GC (PR 1.82, 95% CI 1.2–2.7) - Pulse MPS (PR 2.9, 95% CI 1.7–4.9) |
28 of 334 (8.4) MV: - Age > 50 - No TNFi - Pulse MPS (PR 2.86, 95% CI 1.6–5.1) |
Presence of DM was a significant factor (PR 1.4, 95% CI 1.2–1.8) for the need for emergence care |
| Monroy et al., 2021, Spain [31] |
Observational, retrospective (cross-sectional) |
1090 RA 334 (31), vasculits 38 (3.5), SSc 43 (4), SLE 80 (7), IIM 17 (49) |
85 of 1090 (7.7) - Older age - HTN - Dyslipidemia |
- |
12 of 1090 (1.1) - GC - TCZ use |
58 of 1090 (5.3) Protective - F - TNFi - IBD |
4 of 1090 (0.3) | |
| 2021, France* [33] |
Observational, retrospective (French RMD cohort) |
694 RA 213 (30), SpA 235 (34), vasculitis 65(), SLE 46 (6), SS 17 (2.5), SSc 25 (3), IIM 12 (2) |
694 | - |
256 of 694 (37) UV: - Older age - Obesity - DM (aOR 2.14, 95% CI 1.12–4) - HTN - ILD - CKD - GC (aOR 2.3, 95% CI 1.3–3.8) - MMF - RTX MV: - Older age - BMI - HTN - GC (OR 1.9, 95% CI 1.1–3.5) - MMF - RTX |
256 of 694 (37) ICU 87 (12.5) UV: - Older age - CVD - DM (aOR 5.3 95% CI 2.6–10.85) - HTN - CKD - GC (aOR 2.8, 95% CI 1.2–6) Protective - TNFi MV: - Older age - DM (OR 4.3, 95% CI 2.1–9.1) - BMI - GC (OR 1.9, 95% CI 1.2–3) - Colchicine (MV) |
58 of 694 (8) UV: - Older age - CVD - ILD - DM (aOR 2.89, 95% CI 1.3–6) - HTN - CKD - CTD - GC (aOR 2.6, 95% CI 1.3–5) - MMF - RTX |
|
| Gracia et al., 2021, Spain [38] |
Observational, prospective (COVIDSER cohort) |
7782 |
462 (5.5) RA 154 (33) SpA 165 (36), SLE 61 (17) |
- | - |
106 of 462 (23) ICU 21 (4.5) UV: - Older age - BMI - GC - b use - Lung disease - DM - HTN Protective - TNFi MV: - Older age - Obesity - Liver disease Protective - TNFi |
19 of 462 (4.1) | - |
| Faye et al., USA, 2021 [42] |
Observational, retrospective (cross-sectional) |
62 RA 16, sarcoidosis 8 |
62 124 controls |
14 (22) | - |
Compared to controls - Race - Comorbidities - IS - GC |
Composite ICU, intubation, death, compared to controls UV: - Older age - Comorbidities - HTN - CVD |
Composite of ICU admission, intubation, and death compared to controls: - Older age - Comorbidities-HTN -CVD |
| Esatoglu et al., 2021, Turkey [71] |
Observational, prospective (cohort) |
165 RA 60 (36), SpA 42 (25), CTDs 29 (18), FMF 14, BD 15 (9) |
165 | 17 (10) | 165 |
141 of 165 (85) ICU 22 of 165 (13) |
16 of 165 (10) | - |
| Arleo et al., 2021, USA [72] |
Observational, retrospective (cross-sectional) |
70 RA 26 (37), SLE 8 (11) |
70 | - | - |
34 of 70 (50) ICU 17 of 70 (25) UV: - Older age - CKD - CHF - GC - GCA/PMR Protective - TNFi |
6 of 70 (18) | |
| Alzahrani et al., Saudi Arabia, 2021 [34] |
Observational, prospective (cross-sectional) |
47 RA 25 (53.2), SLE 10 (21) |
47 | 7 (15) |
9 of 47 (19) Older age |
24 of 47 (50) ICU 5 of 47 (10) |
- | - |
| Boekel et al., 2022, Netherlands [52] |
Observational, prospective (Web-based survey) |
3673 RA 1714 (56), SpA 1040 (28.3), SLE 175 (6), JIA 51 (2), vasculitis 81 (3), SS 190 (6), SSc 61 (2), MCTD 27 (0.9), PMR 125 (4); 1243 HCs |
347 of 3673 (9) | 183 (6) | - |
23 of 347 (7) ICU 3 of 347 (1) - Older age - DM - Lung disease - B-cell depleting therapy Protective - TNFi -HCQ |
1 of 347 (0.2) | |
| Esalatmanesh et al., 2022, Iran [40] |
Observational, prospective (cross-sectional) |
196 RA 113 (57.7), SpA 22 (11.2), SLE 30 (15.3), vasculitis 22 (11.2) |
196 | 28 (14.3) |
98 of 196 (50) UV: - Age > 65 - DM [6.3 (2.9–13.7)] - Obesity - CKD - NSAIDs MV: - NSAIDs - GC (OR 4.8, 95% CI 1.1–20) - DM (OR 5.4, 95% CI 2.1–13.5) - Lung disease |
73 of 196 (37.2) ICU 21 of 196 (10.7) UV: - Age > 65 - DM (OR 6.2, 95% CI 2.3–16.3) - Lung disease - CKD - GC (OR 6.6, 95% CI 2.5–17.5) - Azathioprine - Disease activity MV: - NSAIDs - GC (OR 5.3 (1.9–14.8)) - DM (OR 4.6, 95% CI 1.7–12.9) |
15 of 196 (7.6) |
Flare: 32 (16.3) - Active disease - CTDs - Hospitalization |
| Strangfeld et al., 2021, [36] |
Observational, prospective (Global Rheumatology alliance-2019 registry) |
3729 RA 1224 (36), SpA 836 (22), SLE 355 (10), vasculitis (258), other cTDs 473 (14), JIA 21 (0.6), |
3729 | 410 (12.4) | 1368 of 3729 (43) |
390 of 3729 (10) MV: - Age > 65 - M - Lung disease - CVD + HT - High disease activity - RTX - GC > 10 mg/D (OR 1.7, 95% 1.2–2.4) |
||
| Izadi et al., 2021 [37] |
Observational, prospective (Global Rheumatology alliance-2019 registry) |
3441 inflammatory arthritis | 3441 | 401 (12) | - |
939 of 3441 (27) - JAKi |
166 of 3441 (5) - RTX - JAKi |
Increased risk for hospitalization and death: MV: - Older age - Obesity - Lung disease-CVD, -DM (OR 1.54, 95% CI 1.1–2.21) - CKD -SSZ - Lef, - GC |
•Disease with numbers < 10 not mentioned
•COVID-19 Corona VIrus Disease-19, DM diabetes mellitus, RA rheumatoid arthritis, HTN hypertension, ILD interstitial lung disease, CVD cardiovascular disease, SpA spondyloarthritis, CTDs connective tissue diseases, M male sex, ICU intensive care unit, UV univariate, MV multivariate, GC glucocorticoids, SLE systemic lupus erythematosus, SSc systemic sclerosis, IIM idiopathic inflammatory myopathy, MMF mycophenolate mofetil, CYC cyclophosphamide, ARB angiotensin receptor blockers, HCQ hydroxychloroquine, MTX methotrexate, TNF tumor necrosis factor, NSAIDs non-steroidal anti-inflammatory drugs, PMR polymyalgia rheumatica, CKD0 Chronic Kidney Disease, b biologic, c conventional, ts targeted synthetic, DMARD disease-modifying anti-rheumatoid drug, MPS methyl prednisolone, IBD inflammatory bowel disease, SS Sjogren syndrome, RTX rituximab, BMI body mass index, IS immunosuppressant, FMF familial Mediterranean fever, CHF congestive heart failure, GCA giant cell arteritis, SSZ sulfasalazine, Lef leflunomide, TCZ tocilizumab, aOR adjusted odds ratio, PR prevalence ratio
Severity of COVID-19 in RDs and DM
Cohort studies: A French (French RMD cohort) and Spanish (COVIDSER) studies have reported an increased association of DM with moderate to severe disease on univariate analysis (OR 2.14, 95% CI 1.12–4 adjusted for age and sex and OR 1.9, 95% CI 1.34–2.79) respectively [32, 33]. However, the association did not persist on multivariate analysis [32, 33]. Age, obesity, glucocorticoid (GC) use, mycophenolate mofetil (MMF), and rituximab (RTX) were significantly associated with severe disease on multivariate analysis in the French study [33].
Cross-sectional studies: Studies in Spain and Saudi Arabia found no association between DM and the severity of infection [31, 34]. A nationwide medical records review of 902 patients admitted to ICU due to COVID-19 across Russia found ten patients with RDs, of which four had DM and two subsequently died. So, patients with RDs were not overrepresented in ICU, but those with comorbidities like DM had a higher risk for severe outcomes [35].
Hospitalization for COVID-19 in RDs and DM
The Global Rheumatology Alliance (GRA) is a physician-reported registry of COVID-19 in patients with RDs across 34 countries of different ethnicities. The first report of 600 patients revealed an increased association of DM with hospitalizations (OR 2.6, 95% CI 1.39–4.88) along with GC > 10 mg (OR 2.1, 95% CI 1.1–3.9) as an independent risk factor [28]. Subsequent examination of 3729 patients with RDs revealed an association with mortality with GC > 10 mg (OR 1.69, 95% CI 1.18–2.4) but not with DM [36]. Furthermore, in 3441 patients with inflammatory arthritis from the GRA, the composite outcome of hospitalization and mortality was strongly associated with DM (OR 1.54, 95% CI 1.1–2.21) as well as daily GC use (OR 1.07, 95% CI 1.05–1.08 per 1-mg increase in dose in prednisolone equivalents) [37].
Cohort studies: An association of DM with hospitalization was observed in the French RMD cohort (OR 5.3, 95% CI 2.6–10.85 adjusted for age and sex) on univariate analysis which persisted on multivariate analysis (OR 4.3, 95% CI 2.1–9.1) [33]. The Spanish COVIDSER cohort did not find an association of DM with hospitalization on multivariate analysis [38]. Similarly, data from the Brazil ReumaCoV registry did not find an association between hospitalization and DM; however, a strong association was seen with GC use (prevalence ratio [PR] 1.8, 95% CI 1.1–2.7) and/or recent pulse methyl prednisolone use (PR 2.9, 95% CI 1.7–4.87) [39].
Cross-sectional studies: Studies in Spain and Iran have found an association with DM and hospitalization in patients with RDs (OR 5.15, 95% CI 1.5–16.8 and OR 6.2, 95% CI 2.3–16.3) [40, 41]. Most studies have identified an association with GC independent of DM (Table 1).
Thus, even in patients without a history of established DM, GC-induced hyperglycemia can be a factor associated with severe disease and hospitalization.
COVID-19-related mortality in RDs and DM
GRA-19: DM was not associated with an increased risk of death in a study from the GRA [36].
Cohort studies: DM was significantly associated with death in the French RMD cohort study (OR 2.89, 95% CI 1.3–6 adjusted for age and sex) [33, 39]. In the ReumaCoV Brasil cohort, recent pulse MPS use was associated with mortality (PR 2.86, 95% CI 1.59–5.14) [39].
Cross-sectional studies: A study in Spain identified an increased risk of DM with mortality (OR 33, 95% CI 2.4–314) only on univariate analysis whereas another study in the USA reported an increased association with comorbidities but DM was not analyzed specifically [42, 43].
Thus, data on DM and the risk of COVID-19 is insufficient to comment on their association. Whereas most studies reveal an increased influence of DM on severity, hospitalization, and mortality, age, obesity, hypertension, CVD, and use of GC are frequently associated with DM; thus, unadjusted analysis can be misleading. There is a need for focused adjusted multivariate analysis with a specific objective of the influence of DM and GC-induced hyperglycemia on outcomes of COVID-19 for a definite association.
Specific diseases
Most studies across the world have analyzed outcomes of COVID-19 in mixed cohorts. Some cohorts of RA and SLE have specifically looked for outcomes in these two diseases concerning disease activity, drugs, and comorbidities.
Rheumatoid arthritis
Three studies from the USA and Iran have studied outcomes of COVID-19 and the influence of DM in patients with RA. A matched cohort study using national Veterans Affairs data from the USA was carried out by identifying COVID-19 in this cohort through December 2020. Of 33,886 patients with RA, 2007 (5.9%) developed COVID-19. The risk of infection was associated with the presence of DM [hazard ratio (HR), 1.29, 95% CI 1.13–1.44] along with Hispanic race, underlying cardiac, liver disease, obesity, and the number of hospitalizations in the previous year. Severe COVID-19 was observed in 388 (19%) and was associated with DM (HR 1.85, 95% 1.47–2.33), underlying lung, liver, kidney, and cardiac disease, malignancy, and the number of hospitalizations in the previous year. When compared with non-RA controls, patients with RA on conventional/biologic/targeted synthetic DMARDs and prednisolone use (HR 1.84, 95% CI 1.43–2.45) had significantly worse outcomes of hospitalization and/or death [44].
Similarly, a cross-sectional study in Iran analyzed 128 patients with RA and COVID-19 and found an increased risk of infection and hospitalization with DM on univariate and multivariate analysis [42]. Another comparative study from the TriNetX in the USA did not find an association between DM and hospitalization or death [45]. However, GC use was significantly associated with both of these poor outcomes. Additionally, on primary analysis, the risks for all complications and adverse outcomes were significantly higher in patients with RA [45]. But after propensity matching for age and comorbidities, the risks for adverse outcomes with RA did not differ as compared to non-RA patients except for sepsis and venous thromboembolism [46]. Overall, the increased risk for adverse outcomes in RA is related to age and associated comorbidities, with a specific association of DM with increased risk of infection, hospitalization, and death. This seems to be further compounded by GC use, especially at doses > 5 mg (Table 2).
Table 2.
Characteristics of studies with rheumatoid arthritis and COVID-19
| Study | Type | N (%) | N (%), COVID-19 and its associations | N (%), DM | Moderate to severe COVID-19 N (%) and its associations | Hospitalizations N (%) and its associations | Mortality N (%) and its associations | Other salient features |
|---|---|---|---|---|---|---|---|---|
| England et al., 2021, USA [44] |
Observational, retrospective (cohort) |
33,886 |
2007 (5.9) MV (1): - Hispanic race - DM (adjusted HR 1.29 [95% CI 1.13–1.44)] - Heart failure - Liver disease - BMI < 18..5 or > 30 - Hospitalizations in the past year MV (2): - b/tsDMARDs - GC |
- |
388 of 2007 (19) MV: - Lung disease - DM (adjusted HR 1.85, 95% CI 1.47–2.33) - Heart failure - Liver disease - Cancer - CKD - Hospitalizations in the past year |
345 of 2007 (17) c/b/tsDMARDs and GC compared to non-RA controls |
84 of 2007 (4) c/b/tsDMARDs and GC compared to non-RA controls |
|
| Mahdavi et al., 2021, Iran [45] |
Observational, prospective (cross-sectional) |
128 |
128 UV: - Obesity - DM (OR 2.19, 95% CI 1.29–3.71) - Lung disease - CKD - GC > 5 mg/D - SSZ - TNFi MV: - F - Obesity - DM (corrected OR1.77, 95% CI 1.01–3.12) - Lung disease - CKD - GC > 5 mg/D - TNFis |
24 of 128 (18.8) | - |
49 of 128 (38.3) ICU 14 of 128 (10.9) - Age > 65 - Obesity - DM - NSAIDs - GC |
11 of 128 (8.6) | |
| Raiker et al., 2022, USA [46] |
Observational, retrospective (cross-sectional) |
9730 | 9730 | 3156 (32.4) | - |
2334 of 9730 (23.9) ICU 366 of 9730 (4.79) - M - Black race - GC - RTX |
357 of 9730 (3.67) - M - Black race - GC |
Outcomes not worse in RA after propensity matching for age and comorbidities |
COVID-19 COrona VIrus Disease-19, DM diabetes mellitus, RA rheumatoid arthritis, HTN hypertension, ILD interstitial lung disease, CVD cardiovascular disease, CTD connective tissue disease, M male sex, ICU intensive care unit, UV univariate, MV multivariate, GC glucocorticoid, MMF mycophenolate mofetil, CYC cyclophosphamide, ARB angiotensin receptor blockers, HCQ hydroxychloroquine, MTX methotrexate, TNF tumor necrosis factor, NSAIDs non-steroidal anti-inflammatory drugs, b biologic, c conventional, ts targeted synthetic, DMARD disease-modifying anti-rheumatoid drug, MPS methyl prednisolone, RTX rituximab, BMI body mass index, IS immunosuppressant, CHF congestive heart failure, HR hazard ratio, OR odds ratio
All three studies have analyzed patients before the availability of COVID-19 vaccines and need to be interpreted accordingly as discussed below [44–46].
Systemic lupus erythematosus
Studies in Europe and the USA have revealed an increased association of DM with the severity of disease as well as worse outcomes like ICU admission and mortality [44, 45]. A study from the GRA with 1606 patients with SLE reported an increased association of poor outcomes with older age, male sex, GC dose, active disease, and the number of comorbidities [49]. Thus, the outcomes of COVID-19 in SLE are driven largely by demographic factors, comorbidities, and GC use (Table 3) [49].
Table 3.
Characteristics of studies with systemic lupus erythematosus and COVID-19
| Study | Type | N (%) | N (%), COVID-19 (confirmed and probable) | N (%), DM | Moderate to severe COVID-19 N (%) and its associations | Hospitalizations N (%) and its associations | Mortality N (%) and its associations | Other salient features |
|---|---|---|---|---|---|---|---|---|
| Fernandez Ruiz et al., 2020, USA [73] |
Observational, prospective (cohort, web-based survey) |
226 | 83 of 226 (7.5) | 6 (7) |
26 of 83 (31) - Older age - Non-white race - Comorbidity |
19 of 83 (23) - Non-white race - Comorbidity - High BMI |
4 of 83 (5) | |
| Cordtz et al., 2021, Denmark [48] |
Observational, prospective (cohort) |
2533 | 95(3.7) | - |
16 of 95 (17) UV: - DM - lung disease |
- | - | |
| Mageau et al., 2021, France [47] |
Observational, prospective (cohort) |
11,055 | 1411 (13) | 245 (17.3) | - |
1411 ICU 293 of 1411 (21) |
134 of 1411 (9.5) |
Poor outcome (ICU admission and mortality) UV: - Older age - Male - CKD - DM - HTN - CVD - LN - Organ transplantation |
COVID-19 COrona VIrus Disease-19, DM diabetes mellitus, RA rheumatoid arthritis, HTN hypertension, ILD interstitial lung disease, CVD cardiovascular disease, CTD connective tissue disease, M male sex, ICU intensive care unit, UV univariate, MV multivariate, GC glucocorticoid, MMF mycophenolate mofetil, CYC cyclophosphamide, ARB angiotensin receptor blockers, HCQ hydroxychloroquine, MTX methotrexate, TNF tumor necrosis factor, NSAIDs non-steroidal anti-inflammatory drugs, b biologic, c conventional, ts targeted synthetic, DMARD disease-modifying anti-rheumatoid drugs, MPS methyl prednisolone, RTX rituximab, BMI body mass index, IS immunosuppressant, CHF congestive heart failure
Other diseases
A French study of 199 patients with sarcoidosis did not find any influence of DM on the risk of COVID-19 [50]. Eight of them acquired the infection (4%) and the event rates were small for the assessment of the influence of DM on other adverse outcomes.
A Spanish web-based cross-sectional observational study (REUMAVID) evaluated the effects of COVID-19 on various aspects of healthcare for RDs [51]. An analysis of 587 patients with axial spondyloarthritis was carried out of which 4.3% had DM. This group of patients was stratified into good and poor health as per the Assessment of SpondyloArthritis International Society Health Index (ASAS HI). Poor health was associated with DM (10.9% versus 2%) in addition to smoking, high body mass index (BMI), psoriasis, hypercholesterolemia, kidney disease, lung disease, depression, greater pain killer, and GC use (17.7% versus 10%). DM was associated with poor health and function (OR 5.85, 95% CI 2.5–13.5) on univariate, (OR 4.85, 95% CI 1.35–17.65) in addition to smoking, unemployment, poor physical activity, pain killer, and GC use on multivariate analysis. Possibly, it forms a vicious cycle of poor physical activity, worsening disease activity, and inflammation, leading to poor control of DM. This may hold true for other inflammatory RDs if analyzed specifically in this fashion [51].
Immune response to infection and vaccination
The antibody response to COVID-19 in patients with RA and other RDs was lower than in HC but the difference was not significant [52, 53]. Coexistent comorbidities did not determine the antibody response after COVID-19 [50].
Studies have also analyzed the effects of DM in RDs on the humoral immune response to SARS-CoV-2 vaccines [55–57]. A study in India reported a negative association between anti-spike antibody titers and DM in addition to a shorter dosing interval of 4 weeks between the two doses of AZD1222 (AztraZenaca/ChAdOx1) vaccine in a cohort of 561 patients with RDs [54]. The presence of DM did not influence the response to BioNtech mRNA vaccine as reported in a study from Qatar in 100 patients with RDs [55]. Another study in Greece in a cohort of 605 patients with RDs revealed that 88% developed a humoral immune response to mRNA vaccines with age and comorbidities having no influence on the response. Drugs like RTX, MMF, and methotrexate negatively affected the response to vaccination [56]. An Austrian study of 82 patients with RDs and 82 healthy controls reported reduced seroconversion in patients with RDs with comorbidities, but there was no influence of comorbidities on the absolute antibody titers after two doses of the mRNA vaccine. Overall, DM negatively influences humoral response to inactivated vaccines but does not affect the immune responses to mRNA vaccines. Similarly, DM did not influence the T cell responses to mRNA and inactivated vaccines studied in 140 patients with RDs [57].
Furthermore, comorbidities are not risk factors for breakthrough infections. A study in India reported that post-vaccination antibody titers are the most important determinant for breakthrough infections [58]. On the other hand, many comorbidities were associated with breakthrough infections in Israel, but DM was not a significant factor [59].
Patient attitude and behaviors
Surveys to study the attitude towards vaccination, vaccine-related adverse events, and adherence to prescribed treatment have been conducted across various countries [60]. A Greek cohort study of 561 patients with RDs of which 441 (79%) were vaccinated found that DM was not associated with increased adverse events or flares [61]. However, avoidance of vaccination for the fear of flares and adverse events was associated with young age, unemployment, nocebo-prone behaviors, DM, and underlying lung disease. The association with DM did not persist on multivariate analysis [58]. Other web-based surveys from China and Egypt did not find an influence of DM on the attitude of patients towards vaccination [62, 63].
A Turkish survey of 229 patients with spondyloarthritis and 48 patients with RA found that 36% changed their treatment during the pandemic and that DM was a significant factor for deviation from prescribed drugs [64]. A similar US-based survey of 734 patients with RA found similar results with 30% reporting medication changes but with no influence of DM on their decision [65].
Long COVID
Long COVID refers to the presence of symptoms beyond 4 weeks of COVID-19 without an alternative diagnosis. DM has been associated with the persistence of symptoms for a longer duration in a large mobile application-based survey of 4182 patients with COVID-19 [66]. Furthermore, a recent study from the US Department of Veterans Affairs cohort found an increased risk of DM in patients suffering from long COVID [67]. However, studies on the influence of DM in patients with RDs and the development of long COVID are lacking and this needs to be explored in future studies.
Limitations and future studies
Focused studies of the specific association of DM as a comorbidity in patients with RDs with the severity of COVID-19 are lacking. Data from nationwide and global registries can be analyzed to clarify whether there is any association as frequently associated comorbidities with DM like older age, HTN, obesity, and CVDs make interpretation of univariate analysis difficult. Heterogenous methodologies, retrospective data collection, and lack of details about glycemic control are some of the reasons for conflicting associations of COVID-19 outcomes with DM. Furthermore, factors such as lockdowns in the initial period of the pandemic, vaccination, and emergence of newer variants in the latter half of it significantly confound the risk and severity of the infection and their associations which have not been factored in studies. Another aspect that future studies can delve into is the biological basis for poor outcomes with plausible alteration of cytokine pathways and the role of advanced glycosylation end products with premature aging as factors for poor outcomes in patients with both DM and RDs. On the therapeutics front, the role of drugs like metformin, anti-platelets, and statins which have pleiotropic effects can be explored in this setting to control the systemic hyperinflammatory state.
Conclusion
DM and GC usage seem to increase the risk for severe COVID-19, hospitalization, and mortality. Vaccine responses, especially to inactivated SARS-CoV-2 vaccines, are also reduced. Compliance with anti-diabetic medications with good glycemic control seems to abate the risks for adverse outcomes of COVID-19 in RDs. Patients with RDs with DM may be good candidates for booster vaccinations to enhance vaccine-induced immune response.
Author contribution
Conceptualization: Gasparyan AY, Yessirkepov M, and Zimba O. Methodology: Gasparyan AY, Kitas GD, and Yessirkepov M. Writing—original draft: Pankti M and Gasparyan AY. Writing—review and editing: Pankti M, Gasparyan AY, Zimba O, Kitas GD, and Yessirkepov M. All the authors have approved the final manuscript and take full responsibility for the integrity of all aspects of the work.
Declarations
Disclosures
None.
Footnotes
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.COVID Live - coronavirus statistics - Worldometer. https://www.worldometers.info/coronavirus/#countries. Accessed 19 Jun 2022
- 2.Diabetes rates by country 2022. https://worldpopulationreview.com/country-rankings/diabetes-rates-by-country. Accessed 19 Jun 2022
- 3.Obesity rates by country 2022. https://worldpopulationreview.com/country-rankings/obesity-rates-by-country. Accessed 19 Jun 2022
- 4.Zhou F, Yu T, Du R, et al. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study. The Lancet. 2020;395:1054–1062. doi: 10.1016/S0140-6736(20)30566-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Liu J, Zhang S, Wu Z, et al. Clinical outcomes of COVID-19 in Wuhan, China: a large cohort study. Ann Intensive Care. 2020;10:99. doi: 10.1186/s13613-020-00706-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ahmed S, Gasparyan AY, Zimba O (2021) Comorbidities in rheumatic diseases need special consideration during the COVID-19 pandemic. Rheumatol Int 41:243–256. 10.1007/s00296-020-04764-5 [DOI] [PMC free article] [PubMed]
- 7.Güler T, Yurdakul FG, Acar Sivas F, et al. Rehabilitative management of post-acute COVID-19: clinical pictures and outcomes. Rheumatol Int. 2021;41:2167–2175. doi: 10.1007/s00296-021-05003-1. [DOI] [PubMed] [Google Scholar]
- 8.Ji W, Huh K, Kang M, et al. Effect of underlying comorbidities on the infection and severity of COVID-19 in Korea: a nationwide case-control study. J Korean Med Sci. 2020;35:e237. doi: 10.3346/jkms.2020.35.e237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Akiyama S, Hamdeh S, Micic D, Sakuraba A. Prevalence and clinical outcomes of COVID-19 in patients with autoimmune diseases: a systematic review and meta-analysis. Ann Rheum Dis. 2021;80:384–391. doi: 10.1136/annrheumdis-2020-218946. [DOI] [PubMed] [Google Scholar]
- 10.Lim S, Bae JH, Kwon H-S, Nauck MA. COVID-19 and diabetes mellitus: from pathophysiology to clinical management. Nat Rev Endocrinol. 2021;17:11–30. doi: 10.1038/s41574-020-00435-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Apicella M, Campopiano MC, Mantuano M, et al. COVID-19 in people with diabetes: understanding the reasons for worse outcomes. Lancet Diabetes Endocrinol. 2020;8:782–792. doi: 10.1016/S2213-8587(20)30238-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lee Y, Kim M, Oh K, et al. Comparison of initial presentation of pediatric diabetes before and during the coronavirus disease 2019 pandemic era. J Korean Med Sci. 2022;37:e176. doi: 10.3346/jkms.2022.37.e176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Burner TW, Rosenthal AK. Diabetes and rheumatic diseases. Curr Opin Rheumatol. 2009;21:50–54. doi: 10.1097/BOR.0b013e32831bc0c4. [DOI] [PubMed] [Google Scholar]
- 14.Tian Z, Mclaughlin J, Verma A, et al. The relationship between rheumatoid arthritis and diabetes mellitus: a systematic review and meta-analysis. Cardiovasc Endocrinol Metab. 2021;10:125–131. doi: 10.1097/XCE.0000000000000244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Movahedi M, Beauchamp M-E, Abrahamowicz M, et al. Risk of incident diabetes mellitus associated with the dosage and duration of oral glucocorticoid therapy in patients with rheumatoid arthritis. Arthritis Rheumatol Hoboken NJ. 2016;68:1089–1098. doi: 10.1002/art.39537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Verma AK, Bhatt D, Goyal Y, et al. Association of rheumatoid arthritis with diabetic comorbidity: correlating accelerated insulin resistance to inflammatory responses in patients. J Multidiscip Healthc. 2021;14:809–820. doi: 10.2147/JMDH.S285469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lidar M, Braf A, Givol N, et al. Anti-insulin antibodies and the natural autoimmune response in systemic lupus erythematosus. Lupus. 2001;10:81–86. doi: 10.1191/096120301669081314. [DOI] [PubMed] [Google Scholar]
- 18.Bruce IN, Urowitz MB, Gladman DD, et al. Risk factors for coronary heart disease in women with systemic lupus erythematosus: the Toronto Risk Factor Study. Arthritis Rheum. 2003;48:3159–3167. doi: 10.1002/art.11296. [DOI] [PubMed] [Google Scholar]
- 19.El Magadmi M, Ahmad Y, Turkie W, et al. Hyperinsulinemia, insulin resistance, and circulating oxidized low density lipoprotein in women with systemic lupus erythematosus. J Rheumatol. 2006;33:50–56. [PubMed] [Google Scholar]
- 20.de Candia P, Prattichizzo F, Garavelli S, et al. Type 2 diabetes: how much of an autoimmune disease? Front Endocrinol. 2019;10:451. doi: 10.3389/fendo.2019.00451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.CDC (2022) People with certain medical conditions. In: Cent. Dis. Control Prev. https://www.cdc.gov/coronavirus/2019-ncov/need-extra-precautions/people-with-medical-conditions.html. Accessed 28 Apr 2022
- 22.Al-Adhoubi NK, Ali M, Wahshi HA, et al. COVID-19 mortality in patients with rheumatic diseases: a real concern. Curr Rheumatol Rev. 2022;18:234–242. doi: 10.2174/1573397118666220412114514. [DOI] [PubMed] [Google Scholar]
- 23.Gasparyan AY, Ayvazyan L, Blackmore H, Kitas GD. Writing a narrative biomedical review: considerations for authors, peer reviewers, and editors. Rheumatol Int. 2011;31:1409. doi: 10.1007/s00296-011-1999-3. [DOI] [PubMed] [Google Scholar]
- 24.Wiersinga WJ, Rhodes A, Cheng AC, et al. Pathophysiology, transmission, diagnosis, and treatment of coronavirus disease 2019 (COVID-19): a review. JAMA. 2020;324:782–793. doi: 10.1001/jama.2020.12839. [DOI] [PubMed] [Google Scholar]
- 25.Xiang G, Xie L, Chen Z, et al. Clinical risk factors for mortality of hospitalized patients with COVID-19: systematic review and meta-analysis. Ann Palliat Med. 2021;10:2723–2735. doi: 10.21037/apm-20-1278. [DOI] [PubMed] [Google Scholar]
- 26.Reilev M, Kristensen KB, Pottegård A, et al. Characteristics and predictors of hospitalization and death in the first 11 122 cases with a positive RT-PCR test for SARS-CoV-2 in Denmark: a nationwide cohort. Int J Epidemiol. 2020;49:1468–1481. doi: 10.1093/ije/dyaa140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Williamson EJ, Walker AJ, Bhaskaran K, et al. Factors associated with COVID-19-related death using OpenSAFELY. Nature. 2020;584:430–436. doi: 10.1038/s41586-020-2521-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Gianfrancesco M, Hyrich KL, Al-Adely S et al (2020) Characteristics associated with hospitalisation for COVID-19 in people with rheumatic disease: data from the COVID-19 Global Rheumatology Alliance physician-reported registry. Ann Rheum Dis 79:859–866. 10.1136/annrheumdis-2020-217871 [DOI] [PMC free article] [PubMed]
- 29.Cordtz R, Lindhardsen J, Soussi BG, et al. Incidence and severeness of COVID-19 hospitalization in patients with inflammatory rheumatic disease: a nationwide cohort study from Denmark. Rheumatology. 2021;60:SI59–SI67. doi: 10.1093/rheumatology/keaa897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Shobha V, Chanakya K, Haridas V, et al. Do all patients with rheumatic diseases have a higher risk of COVID 19? Initial results from the Karnataka Rheumatology Association COVID 19 Cohort Study (KRACC) Indian J Rheumatol. 2021;16:164–168. doi: 10.4103/injr.injr_261_20. [DOI] [Google Scholar]
- 31.Sarmiento-Monroy JC, Espinosa G, Londoño M-C, et al. A multidisciplinary registry of patients with autoimmune and immune-mediated diseases with symptomatic COVID-19 from a single center. J Autoimmun. 2021;117:102580. doi: 10.1016/j.jaut.2020.102580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Pablos JL, Galindo M, Carmona L, et al. Clinical outcomes of hospitalised patients with COVID-19 and chronic inflammatory and autoimmune rheumatic diseases: a multicentric matched cohort study. Ann Rheum Dis. 2020;79:1544–1549. doi: 10.1136/annrheumdis-2020-218296. [DOI] [PubMed] [Google Scholar]
- 33.Contributors F, SFR, SNFMI, SOFREMIP, CRI, IMIDIATE consortium and, Severity of COVID-19 and survival in patients with rheumatic and inflammatory diseases: data from the French RMD COVID-19 cohort of 694 patients. Ann Rheum Dis. 2021;80:527–538. doi: 10.1136/annrheumdis-2020-218310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Alzahrani ZA, Alghamdi KA, Almaqati AS. Clinical characteristics and outcome of COVID-19 in patients with rheumatic diseases. Rheumatol Int. 2021;41:1097–1103. doi: 10.1007/s00296-021-04857-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Moiseev S, Avdeev S, Brovko M, et al. Rheumatic diseases in intensive care unit patients with COVID-19. Ann Rheum Dis. 2021;80:e16. doi: 10.1136/annrheumdis-2020-217676. [DOI] [PubMed] [Google Scholar]
- 36.Strangfeld A, Schäfer M, Gianfrancesco MA, et al. Factors associated with COVID-19-related death in people with rheumatic diseases: results from the COVID-19 Global Rheumatology Alliance physician-reported registry. Ann Rheum Dis. 2021;80:930–942. doi: 10.1136/annrheumdis-2020-219498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Izadi Z, Brenner EJ, Mahil SK, et al. Association between tumor necrosis factor inhibitors and the risk of hospitalization or death among patients with immune-mediated inflammatory disease and COVID-19. JAMA Netw Open. 2021;4:e2129639. doi: 10.1001/jamanetworkopen.2021.29639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Gracia JMÁ, Sanchez-Piedra C, Manero J, et al. Role of targeted therapies in rheumatic patients on COVID-19 outcomes: results from the COVIDSER study. RMD Open. 2021;7:e001925. doi: 10.1136/rmdopen-2021-001925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Marques CDL, Kakehasi AM, Pinheiro MM et al (2021) High levels of immunosuppression are related to unfavourable outcomes in hospitalised patients with rheumatic diseases and COVID-19: first results of ReumaCoVBrasil registry. RMD Open 7:e001461. 10.1136/rmdopen-2020-001461 [DOI] [PMC free article] [PubMed]
- 40.Esalatmanesh K, Azadbakht J, Hajialilo M, et al. Clinical course, chest computed tomography severity score and outcome of coronavirus disease 2019 (COVID-19) in patients with rheumatic diseases. Egypt Rheumatol. 2022;44:245–250. doi: 10.1016/j.ejr.2021.12.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Freites Nuñez DD, Leon L, Mucientes A, et al. Risk factors for hospital admissions related to COVID-19 in patients with autoimmune inflammatory rheumatic diseases. Ann Rheum Dis. 2020;79:1393–1399. doi: 10.1136/annrheumdis-2020-217984. [DOI] [PubMed] [Google Scholar]
- 42.Faye AS, Lee KE, Laszkowska M, et al. Risk of adverse outcomes in hospitalized patients with autoimmune disease and COVID-19: a matched cohort study from New York city. J Rheumatol. 2021;48:454–462. doi: 10.3899/jrheum.200989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Santos CS, Morales CM, Álvarez ED, et al. Determinants of COVID-19 disease severity in patients with underlying rheumatic disease. Clin Rheumatol. 2020;39:2789–2796. doi: 10.1007/s10067-020-05301-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.England BR, Roul P, Yang Y, et al. Risk of COVID-19 in rheumatoid arthritis: a National Veterans Affairs matched cohort study in at-risk individuals. Arthritis Rheumatol Hoboken NJ. 2021;73:2179–2188. doi: 10.1002/art.41800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Malek Mahdavi A, Varshochi M, Hajialilo M, et al. Factors associated with COVID-19 and its outcome in patients with rheumatoid arthritis. Clin Rheumatol. 2021;40:4527–4531. doi: 10.1007/s10067-021-05830-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Raiker R, Pakhchanian H, DeYoung C, et al. Short term outcomes of COVID-19 in lupus: propensity score matched analysis from a nationwide multi-centric research network. J Autoimmun. 2021;125:102730. doi: 10.1016/j.jaut.2021.102730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Mageau A, Papo T, Ruckly S et al (2022) Survival after COVID-19-associated organ failure among inpatients with systemic lupus erythematosus in France: a nationwide study. Ann Rheum Dis 81:569–574. 10.1136/annrheumdis-2021-221599 [DOI] [PubMed]
- 48.Cordtz R, Kristensen S, Dalgaard LPH, et al. Incidence of COVID-19 hospitalisation in patients with systemic lupus erythematosus: a nationwide cohort study from Denmark. J Clin Med. 2021;10:3842. doi: 10.3390/jcm10173842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Ugarte-Gil MF, Alarcon GS, Izadi Z, et al. Characteristics associated with poor COVID-19 outcomes in individuals with systemic lupus erythematosus: data from the COVID-19 Global Rheumatology Alliance. Ann Rheum Dis. 2022;81:970–978. doi: 10.1136/annrheumdis-2021-221636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Desbois AC, Marques C, Lefèvre L, et al. Prevalence and clinical features of COVID-19 in a large cohort of 199 patients with sarcoidosis. Clin Exp Rheumatol. 2022;40:195–196. doi: 10.55563/clinexprheumatol/b7zd6b. [DOI] [PubMed] [Google Scholar]
- 51.Benavent D, Garrido-Cumbrera M, Plasencia-Rodríguez C et al (2022) Poor health and functioning in patients with axial spondyloarthritis during the COVID-19 pandemic and lockdown: REUMAVID study (phase 1). Ther Adv Musculoskelet Dis 14:1759720X211066685. 10.1177/1759720X211066685 [DOI] [PMC free article] [PubMed]
- 52.Boekel L, Hooijberg F, Vogelzang EH, et al. Antibody development and disease severity of COVID-19 in non-immunised patients with rheumatic immune-mediated inflammatory diseases: data from a prospective cohort study. RMD Open. 2022;8:e002035. doi: 10.1136/rmdopen-2021-002035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Shenoy P, Ahmed S, Shanoj KC, et al. Antibody responses after documented COVID-19 disease in patients with autoimmune rheumatic disease. Clin Rheumatol. 2021;40:4665–4670. doi: 10.1007/s10067-021-05801-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Mehta P, Paul A, Ahmed S et al (2022) Effectiveness of delayed second dose of AZD1222 vaccine in patients with autoimmune rheumatic disease. ClinRheumatol. 10.1007/s10067-022-06247-3 [DOI] [PMC free article] [PubMed]
- 55.Alsaed O, Emadi SA, Satti E et al (2022) Humoral response of patients with autoimmune rheumatic disease to BNT162b2 vaccine: a retrospective comparative study. Cureus 14. 10.7759/cureus.24585 [DOI] [PMC free article] [PubMed]
- 56.Tzioufas AG, Bakasis A-D, Goules AV, et al. A prospective multicenter study assessing humoral immunogenicity and safety of the mRNA SARS-CoV-2 vaccines in Greek patients with systemic autoimmune and autoinflammatory rheumatic diseases. J Autoimmun. 2021;125:102743. doi: 10.1016/j.jaut.2021.102743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Prendecki M, Clarke C, Edwards H, et al. Humoral and T-cell responses to SARS-CoV-2 vaccination in patients receiving immunosuppression. Ann Rheum Dis. 2021;80:1322–1329. doi: 10.1136/annrheumdis-2021-220626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Ahmed S, Mehta P, Paul A, et al. Postvaccination antibody titres predict protection against COVID-19 in patients with autoimmune diseases: survival analysis in a prospective cohort. Ann Rheum Dis. 2022;81:868–874. doi: 10.1136/annrheumdis-2021-221922. [DOI] [PubMed] [Google Scholar]
- 59.Bieber A, Sagy I, Novack L, et al. BNT162b2 mRNA COVID-19 vaccine and booster in patients with autoimmune rheumatic diseases: a national cohort study. Ann Rheum Dis. 2022;81:1028–1035. doi: 10.1136/annrheumdis-2021-221824. [DOI] [PubMed] [Google Scholar]
- 60.Li YK, Lui MPK, Yam LL, et al. COVID-19 vaccination in patients with rheumatic diseases: vaccination rates, patient perspectives, and side effects. Immun Inflamm Dis. 2022;10:e589. doi: 10.1002/iid3.589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Fragoulis GE, Bournia V-K, Mavrea E, et al. COVID-19 vaccine safety and nocebo-prone associated hesitancy in patients with systemic rheumatic diseases: a cross-sectional study. Rheumatol Int. 2022;42:31–39. doi: 10.1007/s00296-021-05039-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Chen J, Cai W, Liu T et al (2022) The COVID-19 vaccine: attitudes and vaccination in patients with autoimmune inflammatory rheumatic diseases. Rheumatol Autoimmun 2:82–91. 10.1002/rai2.12028 [DOI] [PMC free article] [PubMed]
- 63.Tharwat S, Abdelsalam HA, Abdelsalam A, Nassar MK. COVID-19 vaccination intention and vaccine hesitancy among patients with autoimmune and autoinflammatory rheumatological diseases: a survey. Int J Clin Pract. 2022;2022:5931506. doi: 10.1155/2022/5931506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Zateri C, Birtane M, Aktaş İ, et al. Attitudes of patients with spondylarthritis or rheumatoid arthritis regarding biological treatment during COVID-19 pandemic: a multi-center, phone-based, cross-sectional study. Arch Rheumatol. 2021;36:473–481. doi: 10.46497/ArchRheumatol.2021.8364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Michaud K, Pedro S, Wipfler K, et al. Changes in disease-modifying antirheumatic drug treatment for patients with rheumatoid arthritis in the US during the COVID-19 pandemic: a three-month observational study. Arthritis Care Res. 2021;73:1322–1331. doi: 10.1002/acr.24611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Sudre CH, Murray B, Varsavsky T, et al. Attributes and predictors of long COVID. Nat Med. 2021;27:626–631. doi: 10.1038/s41591-021-01292-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Xie Y, Al-Aly Z. Risks and burdens of incident diabetes in long COVID: a cohort study. Lancet Diabetes Endocrinol. 2022;10:311–321. doi: 10.1016/S2213-8587(22)00044-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Pablos JL, Abasolo L, Alvaro-Gracia JM, et al. Prevalence of hospital PCR-confirmed COVID-19 cases in patients with chronic inflammatory and autoimmune rheumatic diseases. Ann Rheum Dis. 2020;79:1170–1173. doi: 10.1136/annrheumdis-2020-217763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Haberman R, Axelrad J, Chen A, et al. Covid-19 in immune-mediated inflammatory diseases - case series from New York. N Engl J Med. 2020;383:85–88. doi: 10.1056/NEJMc2009567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Montero F, Martínez-Barrio J, Serrano-Benavente B, et al. Coronavirus disease 2019 (COVID-19) in autoimmune and inflammatory conditions: clinical characteristics of poor outcomes. Rheumatol Int. 2020;40:1593–1598. doi: 10.1007/s00296-020-04676-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Esatoglu SN, Tascilar K, Babaoğlu H et al (2021) COVID-19 among patients with inflammatory rheumatic diseases. Front Immunol 12:651715. 10.3389/fimmu.2021.6517 [DOI] [PMC free article] [PubMed]
- 72.Arleo T, Tong D, Shabto J, et al. Clinical course and outcomes of COVID-19 in rheumatic disease patients: a case cohort study with a diverse population. Clin Rheumatol. 2021;40:2633–2642. doi: 10.1007/s10067-021-05578-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Fernandez-Ruiz R, Masson M, Kim MY, et al. Leveraging the United States epicenter to provide insights on COVID-19 in patients with systemic lupus erythematosus. Arthritis Rheumatol. 2020;72:1971–1980. doi: 10.1002/art.41450. [DOI] [PMC free article] [PubMed] [Google Scholar]