To the Editor:
When evaluating an indeterminate pulmonary nodule (IPN) with radiologically normal lymph nodes, the interventional pulmonologist must decide which procedure should be performed first. Endobronchial ultrasound (EBUS) is simpler, faster, and less invasive, and is associated with a lower rate of adverse events. A positive EBUS lymph node evaluation will establish diagnosis and staging, and typically will provide enough material for ancillary testing, obviating the need for navigational bronchoscopy (NB). Conversely, if EBUS is negative, undertaking NB after comprehensive mediastinal staging may be associated with decreased yield owing to the development of atelectasis, and accumulation of bronchial secretions and bleeding. The yield of EBUS, however, is unlikely to be affected when performed after NB. Thus, the balance of risk and benefit should dictate the sequence of biopsies during bronchoscopy: when mediastinal or hilar lymph nodes are abnormal by imaging (chest CT or PET imaging), EBUS is typically performed first. When lymph nodes are radiologically normal, however, the sequence of biopsies remains controversial.
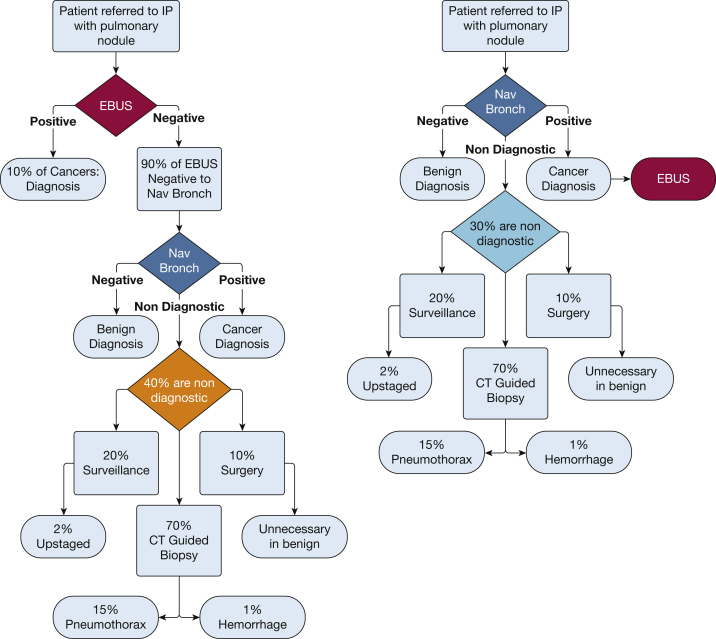
A malignant diagnosis after NB is almost always followed by EBUS interrogation of mediastinal and hilar lymph nodes for staging purposes. Conversely, EBUS is not typically pursued if a benign diagnosis is convincingly established by rapid onsite evaluation (ROSE), as for example in the case of granulomatous inflammation. The key decision of EBUS vs NB first hinges on whether the lower likelihood of requiring NB following EBUS is outweighed by the lower diagnostic yield and increased risk of adverse outcomes of NB following nondiagnostic EBUS. To determine which strategy would in theory yield the highest number of diagnostic bronchoscopies, we performed a Monte Carlo simulation to model the rates of adverse events following either an EBUS-first or an NB-first approach. Assuming the availability of ROSE and the other following assumptions, decision trees for an EBUS-first vs an NB-first approach can be illustrated as in Figure 1. Other important assumptions include the following: (1) a rate of N1, N2, or N3 disease of 10% in the absence of radiologically abnormal lymph nodes1; (2) a diagnostic yield of 70% for navigational bronchoscopy, decreased to 60% when performed after EBUS2, 3, 4, 5, 6; and (3) complication rates for navigational and EBUS bronchoscopy of 5% and 0.5%, respectively.
Figure 1.
Decision trees for an endobronchial ultrasound-first approach vs a navigational bronchoscopy-first approach. Percentage rates represent the probabilities used in the Monte Carlo simulation. EBUS = endobronchial ultrasound; IP = interventional pulmonologist; Nav Bronch = navigational bronchoscopy.
Methods
A Monte Carlo simulation was performed with MATLAB (MathWorks). A total of 1,000 patients were simulated, with a 50% prevalence of cancer. To achieve a 50% prevalence of cancer within the simulated population, each patient was randomly assigned “cancer” or “no cancer” status. Each simulated patient was then sent through both the EBUS-first and the NB-first decision trees in Figure 1, where at each step a random number was generated to determine the outcome. Any patient who went to simulated EBUS first had a 10% chance of returning a positive EBUS result. Thus, in the EBUS-first group, any EBUS-negative patient had a 90% chance to undergo NB. The rate of diagnostic NB was 70% in the NB-first group and 60% in the EBUS-first group. The rate of adverse events was set to 0.5%8 for the EBUS procedure and to 5%2,3 for the NB procedure. The rate at which bronchoscopy yielded a diagnosis was the primary end point of the study. We also looked at possible consequences after nondiagnostic bronchoscopy. We assume, based on data from our institution, that 70% of patients with nondiagnostic bronchoscopy results will undergo CT imaging-guided biopsy, and the remainder will undergo surveillance CT imaging (20%) and surgical resection (10%). Within this population, there are higher rates of adverse outcomes: CT-guided biopsy has a rate of pneumothorax of 15% and a 1% chance of hemorrhage.9 Waiting for a follow-up CT scan after a false negative bronchoscopy results in a chance of stage shift (cancer progression) in some patients, which we estimated at 2% after 90 days.10 Last, 12% of patients who undergo surgical resection are diagnosed with a benign process.7 The simulation was run 10,000 times, and the results were averaged over the 10,000 runs.
Results
The total numbers of EBUS and NB procedures, and of adverse events, are presented in Table 1. In this simulation of 1,000 patients, an NB-first approach resulted in 12% fewer nondiagnostic bronchoscopies than the EBUS-first approach (300 for NB-first vs 342 for EBUS-first), even though there were 17% more NBs overall (1,000 vs 855). The lower rate of nondiagnostic NB led to 12% fewer pneumothoraces (32 vs 36), 9% fewer stage shifts (0.6 vs 0.7), and 17% fewer surgeries on benign disease (15 vs 18). In conclusion, despite more NBs performed overall in the NB-first approach, the wait time following negative EBUS resulted in higher rates of all adverse outcomes in the EBUS-first approach.
Table 1.
Results of Monte Carlo Simulationa
| Parameter Measured | EBUS First | NB First | Difference | % Difference |
|---|---|---|---|---|
| Total No. of EBUS | 1,000 | 315.2 ± 14.4 | –684.8 | –68.5 |
| Total No. of NB | 854.9 ± 11.3 | 1,000 | 145.1 | 17.0 |
| First procedure positive | 50.0 ± 6.9 | 350.1 ± 14.8 | 300.1 | 600.3 |
| NB nondiagnostic | 341.9 ± 14.9 | 299.9 ± 12.8 | –42.0 | –12.3 |
| NB adverse events | 42.8 ± 6.4 | 50.1 ± 6.9 | 7.3 | 17.2 |
| EBUS adverse events | 5.0 ± 2.2 | 1.6 ± 1.3 | –3.4 | –68.3 |
| CT scan-guided biopsies | 239.3 ± 13.4 | 210.0 ± 12.8 | –29.3 | –12.2 |
| Pneumothorax from CT scan-guided biopsy | 35.9 ± 5.8 | 31.5 ± 5.5 | –4.4 | –12.2 |
| Hemorrhaging from CT scan-guided biopsy | 2.4 ± 1.5 | 2.1 ± 1.4 | –0.3 | –12.5 |
| Serial CT imaging | 67.6 ± 7.9 | 59.3 ± 7.5 | –8.3 | –12.3 |
| Stage shifts | 0.7 ± 0.8 | 0.6 ± 0.8 | –0.1 | –8.8 |
| Surgery | 35.0 ± 5.8 | 30.6 ± 5.4 | –4.4 | –12.5 |
| Surgery on benign disease | 18.4 ± 4.2 | 15.3 ± 3.8 | –3.2 | –17.1 |
EBUS = endobronchial ultrasound; NB = navigational bronchoscopy.
Total number of patients simulated, 1,000; simulated cancers (50% prevalence), 500 ± 15.7. Results are presented as average ± SD of 10,000 simulations.
Discussion
When evaluating a patient with an indeterminate pulmonary nodule referred to bronchoscopy, the decision to perform EBUS before NB is attractive because identification of malignant lymph nodes on ROSE may obviate the need for NB, resulting in a simpler, faster, and less invasive procedure associated with a lower rate of adverse events. A crucial consideration, however, is the plausible decrease in diagnostic yield associated with delayed NB. The increasing use of cone beam CT imaging and digital tomosynthesis-assisted NB has revealed the impact of atelectasis during bronchoscopic procedures, which interferes with NB and likely decreases its yield. As atelectasis was convincingly shown to be highly correlated with duration of procedure, it stands to reason that delaying NB after a comprehensive EBUS examination could result in a substantial decrease in diagnostic yield. Our estimate of 10% decreased diagnostic yield is aligned with current evidence on intraprocedural atelectasis.4 Conversely, our 10% rate of occult metastases in radiologically normal lymph node is likely overestimated, which could strengthen our conclusions further.
Our simulation data suggest that, somewhat counterintuitively, an NB-first approach to diagnosis of IPN with radiologically normal mediastinum may ultimately result in more diagnostic bronchoscopies and thus a lesser need for additional interventions with a net complication rate that favors NB. Although the Monte Carlo simulation presented here is simple in its construction, and the individual rates and assumptions are likely imperfect approximations, these results suggest that, at the very least, there is clinical equipoise regarding the optimal sequence of biopsies during bronchoscopy for IPNs without evidence of radiologically abnormal lymph nodes.
Acknowledgments
Author contributions: M. N. K. and F. M. performed study design and data analysis. M. N. K., B. E. H., and F. M. wrote and edited the manuscript.
Funding/support: This study was supported by the NIH National Cancer Institute [grant 1R01CA253923 to F. M.].
Financial/nonfinancial disclosures: The authors have reported to CHEST the following: F. M. is a consultant for Medtronic. None declared (M. N. K., B. E. H.).
Role of sponsors: The sponsor had no role in the design of the study, the collection and analysis of the data, or the preparation of the manuscript.
References
- 1.Martinez-Zayas G., Almeida F.A., Simoff M.J., et al. A Prediction Model to Help with Oncologic Mediastinal Evaluation for Radiation: HOMER. Am J Respir Crit Care Med. 2020;201(2):212–223. doi: 10.1164/rccm.201904-0831OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Aboudara M., Roller L., Rickman O., et al. Improved diagnostic yield for lung nodules with digital tomosynthesis-corrected navigational bronchoscopy: initial experience with a novel adjunct. Respirology. 2020;25(2):206–213. doi: 10.1111/resp.13609. [DOI] [PubMed] [Google Scholar]
- 3.Katsis J., Roller L., Aboudara M., et al. Diagnostic yield of digital tomosynthesis-assisted navigational bronchoscopy for indeterminate lung nodules. J Bronchology Interv Pulmonol. 2021;28(4):255–261. doi: 10.1097/LBR.0000000000000766. [DOI] [PubMed] [Google Scholar]
- 4.Sagar A.S., Sabath B.F., Eapen G.A., et al. Incidence and Location of Atelectasis Developed During Bronchoscopy Under General Anesthesia: the I-LOCATE trial. Chest. 2020;158(6):2658–2666. doi: 10.1016/j.chest.2020.05.565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Casal R.F., Sarkiss M., Jones A.K., et al. Cone beam computed tomography-guided thin/ultrathin bronchoscopy for diagnosis of peripheral lung nodules: a prospective pilot study. J Thorac Dis. 2018;10(12):6950–6959. doi: 10.21037/jtd.2018.11.21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bhadra K., Setser R.M., Condra W., Pritchett M.A. Lung navigation ventilation protocol to optimize biopsy of peripheral lung lesions. J Bronchology Interv Pulmonol. 2022;29(1):7–17. doi: 10.1097/LBR.0000000000000756. [DOI] [PubMed] [Google Scholar]
- 7.Polcz M.E., Maiga A.W., Brown L.B., et al. The impact of an interventional pulmonary program on nontherapeutic lung resections. J Bronchology Interv Pulmonol. 2019;26(4):287–289. doi: 10.1097/LBR.0000000000000592. [DOI] [PubMed] [Google Scholar]
- 8.Vaidya P.J., Munavvar M., Leuppi J.D., Mehta A.C., Chhajed P.N. Endobronchial ultrasound-guided transbronchial needle aspiration: safe as it sounds. Respirology. 2017;22(6):1093–1101. doi: 10.1111/resp.13094. [DOI] [PubMed] [Google Scholar]
- 9.Wiener R.S., Schwartz L.M., Woloshin S., Welch H.G. Population-based risk for complications after transthoracic needle lung biopsy of a pulmonary nodule: an analysis of discharge records. Ann Intern Med. 2011;155(3):137–144. doi: 10.1059/0003-4819-155-3-201108020-00003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Degeling K., Baxter N.N., Emery J., et al. An inverse stage-shift model to estimate the excess mortality and health economic impact of delayed access to cancer services due to the COVID-19 pandemic. Asia Pac J Clin Oncol. 2021;17(4):359–367. doi: 10.1111/ajco.13505. [DOI] [PMC free article] [PubMed] [Google Scholar]