Abstract
Purpose
Endovascular stenting has been used to manage superior vena cava syndrome for several decades and has become standard firstline practice. This study aims to investigate the outcomes of endovascular stenting in the management of superior vena cava syndrome (SVCS).
Methods
MEDLINE, EMBASE and PUBMED online databases were searched, with studies involving more than ten adult patients included. Studies identified spanned 27 years, from 1993 to 2020. Meta-analyses were performed based on Clopper–Pearson estimation.
Results
Fifty-four studies were identified, for a total of 2249 patients, of which 2015 had malignant SVCS and 222 benign SVCS. Pooled technical success and clinical success rates were 96.8% (95% CI 96.0–97.5%) and 92.8% (95% CI 91.7–93.8%). Technical success and clinical success rates for studies investigating benign SVCS alone were identical at 88.8% (95% CI 83.0–93.1%). Pooled patency remained above 90% for the first year. Average complication and re-intervention rates were 5.78% (SD = 9.3182) and 9.11% (SD = 11.190).
Conclusions
This review confirms the effectiveness of endovascular stenting in managing SVCS. Further directions of research may include specific outcomes of endovascular stenting in benign SVCS, and the impact of procedural characteristics, such as the use of anticoagulation and type of stent used, on outcomes.
Level of Evidence
Level III, systematic review of retrospective cohort studies.
Keywords: Endovascular stenting, Superior vena cava syndrome, Systematic review, Meta-analysis
Introduction
Superior vena cava syndrome (SVCS) arises when the superior vena cava (SVC) becomes partially or completely obstructed. Depending on the speed of onset, allowing the development of venous collaterals over time, the symptomology of SVCS ranges from asymptomatic to minor symptoms (e.g. headache, cough or neck vein distension), to acute respiratory compromise and rarely, mortality from laryngeal or cerebral oedema [1–3].
The aetiology of SVCS is predominantly due to malignant obstruction, with either primary malignancies or lymph node metastases extrinsically compressing or directly invading the SVC [1, 4]. SVCS is however increasingly caused by benign pathologies. Indwelling intravascular catheters or cardiac device leads have replaced rarer pathologies such as fibrosing mediastinitis to become the commonest benign cause of SVCS [4–10].
Traditional treatment modalities for malignant SVCS include radiotherapy, chemotherapy and surgical bypass [1, 4, 11]. The use of stenting as first-line therapy has gathered popularity to become standard practice in the past two decades [4, 10]. Endovascular intervention has been associated with more rapid, complete symptom relief and lower complication rates [1, 4, 12]. It also provides greater flexibility, as attempting subsequent alternative therapies is not precluded [10, 12]. Evidence is however limited primarily to single-centre studies and the impact of procedural characteristics such as stent type or use of anticoagulation has not been thoroughly explored.
This study aims to consolidate and summarise the published literature about the outcomes of endovascular stenting in SVCS via means of a systematic review and meta-analysis. We aim to synthesize the current evidence regarding outcomes including technical success of the procedure, clinical symptom resolution and reported recurrence and complications, as well as provide a comprehensive overview of the impact of procedural characteristics on these outcomes.
Methods
This systematic review and meta-analysis was designed and performed according to the Preferred Reporting Items of Systematic Reviews and Meta-Analyses (PRISMA) standards [13]. Study methodology was specified prior to data extraction and registered with PROSPERO (CRD 42021191795).
Literature Search
Two authors (EA, MK) performed the search of MEDLINE, EMBASE and PUBMED online databases to identify articles related to the outcomes of endovascular stenting in the treatment of SVCS. The following search terms were used, alone and in combinations; “superior vena cava syndrome”, “superior vena cava obstruction”, “superior vena cava” and “stent”. All retrieved studies were first screened on title and abstract, then screened studies read in full by both authors to determine eligibility for inclusion. The final search was on 14th November 2020.
Study Selection
Study selection was performed independently by two authors (EA, MK). The selection criteria were as follows: (1) Full text of the study had to be available in English. (2) Studies had to include 10 or more adult human patients. (3) Where studies concerned interventions in the SVC as well as other vessels, only studies with identified data for technical and clinical outcomes of SVC interventions, with or without involvement of brachiocephalic veins, were included.
Data Collection and Quality Assessment
The following data were extracted from each included study: (1) details of the study—first author, year, study type (prospective/retrospective), journal of publication, conflict of interests; (2) population demographic data—size of study population, mean age, gender, benign or malignant pathology, pre-intervention chemotherapy or radiotherapy; (3) procedural data—type and make of stent, use of anticoagulation or thrombolysis, technical and clinical success, complications, and (4) follow-up data—primary and secondary patencies, recurrence of symptoms, re-interventions and survival. Data were extracted independently by two authors (EA, MK). Where the reviewers had any disagreement, this was resolved by discussion and where necessary, consensus with the senior author (MH). The methodological quality of the included studies was assessed for risk of bias using the Newcastle–Ottawa scale [14].
Statistical Analysis
Meta-analyses were performed to report technical and clinical success of stenting to relieve SVCS, as well as recurrence of symptoms at 1, 3, 6 and 12 months. Using a random effects model, individual and pooled proportions and 95% confidence intervals were calculated by the Clopper–Pearson estimation method based on the exact binomial distribution. Statistical heterogeneity was assessed using the I2 (inconsistency) statistic. SAS software version 9.4 was used for analysis and production of the graphs.
Results
Study Selection
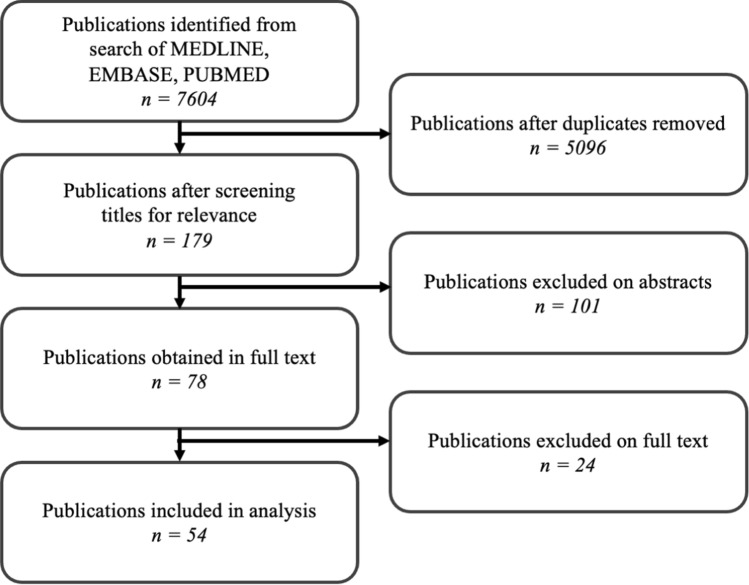
The initial search resulted in 7604 studies (Fig. 1). After removal of duplicates and screening on title and abstract, 78 studies were obtained and read in full text, of which 54 met all inclusion criteria, for a total of 2249 patients. Data extraction and study quality assessment were subsequently performed. The most frequent reasons for exclusion were insufficient sample sizes or undifferentiated reporting of outcomes in the SVC.
Fig. 1.
PRISMA flowchart showing selection of studies for analysis. Selection criteria were as follows: (1) Full text of the study had to be available in English. (2) Studies had to include 10 or more adult human patients. (3) Where studies concerned interventions in the SVC as well as other vessels, only studies with identified data for technical and clinical outcomes of SVC interventions, with or without involvement of brachiocephalic veins, were included
Patient Demographics and Study Characteristics
The total number of patients reported was 2249. The cumulative mean age of all patients was 58.7 years and the sex ratio (males/females) was 2.6 (1605/612). One study presented no data on patient demographics [15], while two did not present mean age [16, 17].
Characteristics of included studies are summarised in Table 1. Of the 54 studies included, 34/54 were retrospective, 17/54 were prospective, and 3/54 had both prospective and retrospective arms. No randomised controlled trials or multi-centre studies were identified. The risk of conflict of interests in all studies was low. Risk of bias, assessed via the Newcastle–Ottawa scale, is shown in Table 2. All papers scored between 7 and 9, indicating high quality.
Table 1.
Characteristic of the 54 included studies, including number of patients, mean age (years), pathology of SVCS studied, whether patient groups had received previous therapies for malignant SVCS, vessels involved, technical success rate, clinical success rate, pre-operative assessment in diagnosing SVCS, stent details and brands, use of procedural anticoagulation or antiplatelet therapy and follow-up protocol
| No. of patients (n) | Mean age (years) | Patient characteristics | Vessels involved | Tech. success (%) | Clin. success (%) | Pre-operative assessment | Stent details | Procedural details | Follow-up Protocol | |
|---|---|---|---|---|---|---|---|---|---|---|
| Dyet et al. [23] | 17 | 63.4 |
Malignant: 17 (100%) CRT: 0, C: 0, R: 14 |
SVC: 17 (100%) + BCV: 6 (35%) + IVC: 1 (6%) |
100 | 100 |
CT thorax Histology Venography |
Uncovered: 17 (100%) Wallstent |
Anticoag: Heparin 5000 IU Warfarin 3 m |
Imaging: Venogram at 1 m, 3 m Clinical: Patient reported Mean F/U: NR |
| Gaines et al. [15] | 20 | NR |
Malignant: 20 (100%) CRT: 0, C: 5, R: 11 |
NR | 90 | 90 | Venography |
Uncovered: 20 (100%) Gianturco-Z |
Anticoag: Heparin 5d |
Imaging: NR Clinical: Patient reported Mean F/U: NR |
| Crowe et al. [35] | 13 | 55.5 |
Malignant: 12 (92%) CRT: 0, C: 1, R: 10 Benign: 1 (8%) |
SVC: 13 (100%) + BCV: 11 (85%) |
84.6 | 84.6 | Venography |
Uncovered: 11 (100%) Gianturco-Z, Wallstent, Palmaz |
Anticoag: Heparin 5d |
Imaging: NR Clinical: Patient reported Mean F/U: NR |
| Hennequin et al. [57] | 14 | 60 |
Malignant: 13 (93%) CRT: 8, C: 5, R: 0 Benign: 1 (7%) |
SVC: 14 (100%) + BCV: 9 (64%) |
100 | 92.9 |
CT thorax Venography |
Uncovered: 14 (100%) Wallstent |
Anticoag: Heparin 5000 IU Heparin 24 h, LMWH 1 m |
Imaging: CT at 3 m, 6 m Clinical: Patient reported Mean F/U: 4.1 m |
| Shah et al. [58] | 13 | 60 |
Malignant: 13 (100%) CRT: 0, C: 0, R: 2 |
NR | 92.3 | 84.6 |
Histology Venography |
Uncovered: 12 (100%) Gianturco-Z, Wallstent |
Anticoag: Heparin 5000 IU Heparin 2d |
Imaging: NR Clinical: Patient reported Mean F/U: 3.7 m |
| Stock et al. [51] | 14 | 62 |
Malignant: 14 (100%) CRT: 1, C: 7, R: 3 |
SVC: 14 (100%) + BCV: 9 (64%) |
85.7 | 85.7 | Venography |
Uncovered: 12 (100%) Wallstent |
Anticoag: Heparin 5000 IU |
Imaging: NR Clinical: Patient reported Median F/U: 171d |
| Oudkerk et al. [18] | 30 | 60.4 |
Malignant: 30 (100%) CRT: 0, C: 12, R: 22 |
NR | 100 | 96.7 | Venography |
Uncovered: 30 (100%) Wallstent |
Anticoag: Heparin |
Imaging: Venogram at 2w Clinical: Patient reported Mean F/U: 2.5 m |
| Gross et al. [32] | 13 | 60.2 |
Malignant: 13 (100%) CRT: 6, C: 0, R: 5 |
SCV: 13 (100%) + BCV: 4 (31%) |
100 | 100 | Venography |
Uncovered: 13 (100%) Wallstent |
Anticoag: Heparin Dual antiplatelets 4w |
Imaging: NR Clinical: Patient reported Mean F/U: NR |
| Nicholson et al. [12] | 81 | 62.2 |
Malignant: 81 (100%) CRT: 0, C: 8, R: 11 |
NR | 93.8 | 93.8 | Venography |
Uncovered: 76 (100%) Wallstent |
Anticoag: NR |
Imaging: NR Clinical: Patient reported Mean F/U: NR |
| Tanigawa et al. [36] | 23 | 61.2 |
Malignant: 23 (100%) CRT: 1, C: 0, R: 10 |
SVC: 23 (100%) + BCV: 6 (26%) |
100 | 78.3 |
CT angiogram Venography |
Uncovered: 23 (100%) Gianturco-Z |
Anticoag: Heparin 3d |
Imaging: NR Clinical: Patient reported Mean F/U: NR |
| Qanadli et al. [33] | 12 | 54 | Benign: 12 (100%) |
SVC: 12 (100%) + BCV: 5 (42%) |
100 | 100 |
CT thorax Venography |
Uncovered: 12 (100%) Wallstent |
Anticoag: Heparin 5000 IU Dual antiplatelets 4w |
Imaging: CT at 3 m Clinical: Patient reported Mean F/U: 11 m |
| Thony et al. [37] | 26 | 54 |
Malignant: 26 (100%) CRT: 5, C: 6, R: 0 |
SVC: 26 (100%) + BCV: 8 (30%) |
96.2 | 80.8 |
CT thorax Venography |
Uncovered: 25 (100%) Wallstent, Strecker |
Anticoag: Heparin 3000 IU Aspirin 3 m |
Imaging: CT at 6 m Clinical: Patient reported Mean F/U: NR |
| Marcy et al. [59] | 39 | 59 |
Malignant: 37 (95%) CRT: NR, C: NR, R: NR Benign: 2 (5%) |
NR | 97.4 | 92.3 | Venography |
Uncovered: 39 (100%) Gianturco-Z, Strecker, Memotherm |
Anticoag: Heparin 5000 IU Aspirin |
Imaging: NR Clinical: Patient reported Mean F/U: 24w |
| Miller et al. [60] | 23 | 64 |
Malignant: 23 (100%) CRT: 1, C: 0, R: 7 |
NR | 100 | 82.6 |
CT thorax Venography |
Uncovered: 23 (100%) Wallstent |
Anticoag: NR |
Imaging: NR Clinical: Patient reported Mean F/U: NR |
| Sasano et al. [29] | 11 | 60 |
Malignant: 11 (100%) CRT: NR, C: NR, R: NR |
SVC: 11 (100%) + BCV: 7 (64%) |
100 | 90.9 |
CT thorax Venography |
Uncovered: 11 (100%) Wallstent |
Anticoag: Heparin 5000 IU Warfarin 3 m |
Imaging: NR Clinical: Patient reported Mean F/U: NR |
| Lanciego et al. [19] | 52 | 63 |
Malignant: 52 (100%) Stenting as first line intervention |
SVC: 52 (100%) + BCV: 33 (63%) |
100 | 100 | Venography |
Uncovered: 52 (100%) Wallstent |
Anticoag: Heparin 1w Dual antiplatelets 6 m |
Imaging: NR Clinical: Patient reported Mean F/U: NR |
| Smayra et al. [38] | 30 | 61 |
Malignant: 16 (54%) CRT: 0, C: 0, R: 6 Benign: 14 (46%) |
NR | 100 | 100 | Venography |
Uncovered: 30 (100%) Memotherm, Wallstent, Symphony |
Anticoag: Heparin 5000 IU |
Imaging: NR Clinical: Patient reported Mean F/U: 10 m |
| Wilson et al. [70] | 18 | 65 |
Malignant: 18 (100%) CRT: 0, C: 0, R: 6 |
SVC: 18 (100%) + BCV: 6 (33%) |
100 | 100 |
Histology Venography |
Uncovered: 18 (100%) Gianturco-Z, Strecker, Wallstent |
Anticoag: NIL |
Imaging: NR Clinical: Patient reported Mean F/U: NR |
| de Gregorio Ariza et al. [53] | 82 | 57.8 |
Malignant: 68 (83%) CRT: NR, C: NR, R: NR Benign: 14 (17%) |
NR | 95.1 | 95.1 |
CT angiogram Venography |
Uncovered: 82 (100%) Wallstent, Palmaz |
Anticoag: Heparin 5000 IU |
Imaging/Clinical: CXR/USS + assessment at 1, 3, 6, 12 m Mean F/U: 7 m (M), 31 m (B) |
| Chatziioannou et al. [61] | 18 | 56.6 |
Malignant: 18 (100%) CRT: NR, C: NR, R: NR |
SVC: 18 (100%) + BCV: 8 (44%) |
100 | 100 |
CT thorax Histology Venography |
Uncovered: 18 (100%) Memotherm |
Anticoag: Heparin 5000 IU |
Imaging: Venogram at 25d Clinical: Daily for 25d Mean F/U: NR |
| Courtehoux et al. [62] | 20 | 58 |
Malignant: 20 (100%) CRT: 10, C: 9, R: 0 |
SVC: 20 (100%) + BCV: 5 (25%) |
100 | 90 | CT thorax |
Uncovered: 20 (100%) Wallstent |
Anticoag: Heparin 5000 IU Warfarin + aspirin |
Imaging: NR Clinical: Patient reported Mean F/U: NR |
| Dinkel et al. [49] | 84 | 64 |
Malignant: 84 (100%) CRT: 0, C: 54, R: 28 |
SVC: 84 (100%) + BCV: 71% |
98.8 | 89.3 |
CT thorax Venography |
Uncovered: 83 (100%) Wallstent |
Anticoag: Heparin 5000 IU Long term anticoagulation |
Imaging: NR Clinical: Patient reported Mean F/U: NR |
| Monaco (2003) | 44 | 55.6 |
Malignant: 40 (91%) CRT: 33, C: 0, R: 0 Benign: 4 (9%) |
SVC: 44 (100%) + BCV: 17 (39%) |
100 | 100 |
CT thorax Venography |
Uncovered: 44 (100%) Wallstent |
Anticoag: Heparin 5000 IU Dual antiplatelets |
Imaging: NR Clinical: Patient reported Mean F/U: NR |
| Kim et al. [63] | 10 | 54 |
Malignant: 10 (100%) CRT: 5, C: 2, R: 1 |
NR | 100 | 90 | Venography |
Uncovered: 10 (100%) Wallstent |
Anticoag: Warfarin + aspirin |
Imaging: NR Clinical: Patient reported Mean F/U: NR |
| Urreticoechea [30] | 52 | 57 |
Malignant: 52 (100%) CRT: 4, C: 14, R: 2 |
NR | 100 | 100 |
Histology Venography |
Uncovered: 52 (100%) Wallstent, Memotherm |
Anticoag: Heparin 5000 IU LMWH or warfarin 3 m |
Imaging: NR Clinical: Patient reported Mean F/U: NR |
| Bierdrager et al. [20] | 17 | 65 |
Malignant: 17 (100%) CRT: NR, C: NR, R: NR |
NR | 88.2 | 88.2 |
CT thorax Venography |
Uncovered: 15 (100%) Symphony |
Anticoag: NIL |
Imaging: NR Clinical: Patient reported Mean F/U: NR |
| Sheikh et al. [64] | 19 | 46.4 | Benign: 19 (100%) | NR | 100 | 100 | NR |
Uncovered: 19 (100%) Wallstent, Memotherm, Palmaz, Gianturco-Z |
Anticoag: Long term anticoagulation |
Imaging: NR Clinical: Patient reported Mean F/U: 28.8 m |
| Barshes et al. [52] | 56 | 62.6 |
Malignant: 40 (71%) CRT: NR, C: NR, R: NR Benign: 16 (29%) |
NR | 100 | 96.4 | Venography |
Uncovered: 56 (100%) Palmaz, Wallstent |
Anticoag: Heparin 5000 IU Warfarin or clopidogrel |
Imaging/Clinical: CXR/USS + assessment at 1, 3, 6, 12 m Mean F/U: NR |
| Nagata et al. [31] | 71 | 63.4 |
Malignant: 71 (100%) CRT: NR, C: NR, R: NR |
SVC: 71 (100%) + BCV: 17 (24%) |
100 | 87.3 |
CT thorax Histology Venography |
Uncovered: 71 (100%) Spiral-Z, Gianturco-Z, Rosch-Z, Wallstent |
Anticoag: Heparin 5000 IU Warfarin 3 m |
Imaging: NR Clinical: Patient reported Mean F/U: NR |
| Lanciego et al. [21] | 149 | 65 |
Malignant: 149 (100%) CRT: 9, C: 24, R: 4 |
SVC: 149 (100%) + BCV: 77 (52%) |
100 | 82.6 | Venography |
Uncovered: 149 (100%) Wallstent |
Anticoag: Heparin 5000 IU Oral anticoagulants 6 m |
Imaging: NR Clinical: Patient reported Mean F/U: NR |
| Cho et al. [34] | 17 | 59 |
Malignant: 17 (100%) CRT: NR, C: NR, R: NR |
SVC: 17 (100%) + BCV: 7 (41%) + IJV: 1 (6%) |
100 | 100 |
CT thorax Venography |
Uncovered: 17 (100%) Memotherm, Wallstent, Absolute, Luminexx, Symphony |
Anticoag: NIL |
Imaging: NR Clinical: Patient reported Mean F/U: NR |
| Fagedet et al. [39] | 164 | 59.9 |
Malignant: 164 (100%) CRT: 0, C: 6, R: 3 |
SVC: 164 (100%) + BCV: 88 (54%) |
91.5 | 90.9 |
CT angiogram Venography |
Uncov/Covered: NR Wallstent, Memotherm, Cordis, Protégé, Strecker |
Anticoag: Heparin 3000 IU Aspirin 6 m |
Imaging: CT at 6 m, 12 m Clinical: Patient reported Mean F/U: 355.2d |
| Gwon et al. [25] | 73 | 61.3 |
Malignant: 73 (100%) CRT: 7, C: 48, R: 1 |
SVC: 73 (100%) + BCV: 47 (64%) |
100 | 93.2 |
CT thorax Histology—bronchoscopy, percutaneous needle, excision Venography |
Uncovered: 36 (49%) Covered: 37 (51%) ComVi, Zilver |
Anticoag: Heparin 5000 IU Aspirin or warfarin 3 m |
Imaging: CT at 1 m, 6 m Clinical: Assessment at 1, 3, 6, 9, 12 m Mean F/U: 150d |
| Maleux et al. [23] | 78 | 64.1 |
Malignant: 78 (100%) Stenting as first line intervention |
SVC: 78 (100%) + BCV: 9 (12%) |
100 | 100 |
CT thorax Venography |
Uncovered: 78 (100%) Zilver |
Anticoag: Heparin 5000 IU LMWH 1 m + aspirin |
Imaging: NR Clinical: Patient reported Mean F/U: NR |
| Andersen et al. [44] | 25 | 65 |
Malignant: 25 (100%) CRT: 25, C: 0, R: 0 |
NR | 96 | 96 |
CT thorax Venography |
Uncovered: 25 (100%) E-Luminexx, Zilver, Sinus-XL |
Anticoag: Heparin 5000 IU Aspirin |
Imaging: CT at 3 m Clinical: Patient reported Mean F/U: NR |
| Cho et al. [24] | 40 | 61.4 |
Malignant: 40 (100%) CRT: 9, C: 24, R: 1 |
SVC: 40 (100%) + BCV: 25 (63%) |
100 | 85 |
CT thorax Histology—bronchoscopy, biopsy Venography |
Covered: 40 (100%) ComVi |
Anticoag: NR |
Imaging: NR Clinical: Patient reported Mean F/U: 175d |
| Sobrinho and Aguiar [40] | 56 | 59.3 |
Malignant: 56 (100%) Stenting as first line intervention |
NR | 87.5 | 87.5 |
CT thorax Venography |
Uncovered: 49 (100%) Sinus-XL, Smartstent, Wallstent, Express |
Anticoag: Heparin 5000 IU LMWH + aspirin |
Imaging: NR Clinical: Patient reported Mean F/U: NR |
| Andersen et al. [44] | 12 | 69 |
Malignant: 12 (100%) CRT: 12, C: 0, R: 0 |
NR | 91.7 | 91.7 |
CT thorax Venography |
Uncovered: 12 (100%) Zilver |
Anticoag: Heparin 5000 IU |
Imaging: CT at 1 m, 3 m Clinical: Patient reported Mean F/U: 2 m |
| Breault et al. [65] | 44 | 56 | Benign: 44 (100%) | NR | 88.6 | 88.6 |
CT thorax Venography |
Uncovered: 40 (100%) Wallstent, Sinus-XL, Luminexx, Smartstent, Express |
Anticoag: Heparin 5000 IU |
Imaging: NR Clinical: Assessment at 3 m Mean F/U: 1275d |
| Leung et al. [41] | 56 | 64 |
Malignant: 56 (100%) CRT: NR, C: NR, R: NR |
SVC: 56 (100%) + BCV: 31 (55%) |
96.4 | 91.1 |
CT thorax Venography |
Uncovered: 54 (100%) Wallstent |
Anticoag: Heparin |
Imaging: NR Clinical: Patient reported Mean F/U: NR |
| Miazga et al. [66] | 112 | 64 |
Malignant: 109 (97%) CRT: NR, C: NR, R: NR Benign: 3 (3%) |
NR | 98.2 | 98.2 |
CT thorax Histology Venography |
Uncovered: 110 (100%) Epic, Smartstent |
Anticoag: NR |
Imaging: NR Clinical: Patient reported Mean F/U: NR |
| Mokry et al. [47] | 23 | 62.5 |
Malignant: 23 (100%) CRT: 15, C: 3, R: 1 |
NR | 100 | 95.7 |
CT thorax Venography |
Uncovered: 23 (100%) Sinus-XL |
Anticoag: Heparin 2000 IU Heparin 1w |
Imaging: NR Clinical: Patient reported Mean F/U: 66d |
| Büstgens et al. [69] | 141 | 64.6 |
Malignant: 141 (100%) CRT: 0, C: 57, R: 31 |
NR | 97.9 | 96.5 |
CT thorax Histology Venography |
Uncov/covered: NR Smartstent, Wallstent, Zilver, Epic |
Anticoag: Heparin 5000 IU |
Imaging: NR Clinical: Patient reported Mean F/U: NR |
| Massara et al. [71] | 25 | 65.5 | Benign: 25 (100%) | NR | 100 | 100 | Venography |
Uncovered: 25 (100%) Wallstent, Wallgraft, Express |
Anticoag: Dual antiplatelets |
Imaging/Clinical: USS + assessment at 1, 3, 6, 12, 18 m Mean F/U: NR |
| Anton et al. [46] | 31 | 67 |
Malignant: 31 (100%) CRT: 7, C: 11, R: 0 |
SVC: 31 (100%) + BCV: 10 (32%) |
100 | 100 |
CT thorax Venography |
Uncovered: 31 (100%) Sinus-XL, Protégé Everflex |
Anticoag: Heparin 3000 IU |
Imaging: CT Clinical: Patient reported Mean F/U: 184d |
| Calsina Juscafresa et al. [67] | 33 | 57.6 |
Malignant: 33 (100%) CRT: NR, C: NR, R: NR |
SVC: 33 (100%) + BCV: 20 (61%) |
100 | 84.8 |
CT angiogram Histology Venography |
Uncov/covered: NR Protégé, Wallstent, Express |
Anticoag: Heparin 4000 IU |
Imaging: NR Clinical: Patient reported Mean F/U: NR |
| Kuo et al. [68] | 12 | 58.4 |
Malignant: 12 (100%) CRT: 7, C: 5, R: 0 |
NR | 100 | 100 |
CT thorax Histology Venography |
Uncovered: 12 (100%) Wallstent |
Anticoag: Heparin 3000 IU Clopidogrel |
Imaging: CT at 3 m, 6 m, 1y Clinical: Patient reported Median F/U: 11.5 m |
| Niu et al. [16] | 47 | NR |
Malignant: 47 (100%) CRT: NR, C: NR, R: NR |
SVC: 47 (100%) + BCV: 27 (57%) |
100 | 97.9 |
CT thorax Histology—bronchoscopy, biopsy, oesophageal endoscopy, surgery Venography |
Uncovered: 47 (100%) Sinus-XL, Zilver, Luminexx, Smartstent |
Anticoag: Heparin 5000 IU Warfarin lifelong |
Imaging: CT at 1 m, 3 m, 6 m Clinical: Assessment every 2 m Mean F/U: 6 m |
| Haddad et al. [26] | 59 | 47 | Benign: 59 (100%) | NR | 79.7 | 79.7 |
CT thorax Venography |
Uncov/covered: NR Wallstent, Protégé, Smartstent, Gore Viabahn, iCast |
Anticoag: Heparin 5000 IU |
Imaging/Clinical: CT + assessment at 3 m, 6 m, 1y Mean F/U: 2.7y (C), 1.8y (U) |
| Majumdar et al. [22] | 10 | 42.2 | Benign: 10 (100%) | NR | 80 | 80 |
CT thorax Histology Venography |
Uncovered: 10 (100%) Wallstent, Palmaz, Cordis, EV3 |
Anticoag: NR |
Imaging: NR Clinical: Patient reported Mean F/U: 3.6y |
| Karakhanian et al. [72] | 28 | 52.5 |
Malignant: 18 (64%) CRT: NR, C: NR, R: NR Benign: 10 (36%) |
NR | 96.4 | 96.4 |
CT thorax Venography |
Uncov/covered: NR Wallstent, Sinus-XL, Sioxx |
Anticoag: Heparin 5000 IU |
Imaging: NR Clinical: Assessment for 90d Mean F/U: 90d |
| Ren et al. [42] | 12 | 64.3 |
Malignant: 12 (100%) CRT: 1, C: 5, R: 1 |
NR | 100 | 100 |
CT thorax Histology Venography |
Uncovered: 12 (100%) Sinus-XL, Zilver, Smartstent |
Anticoag: Heparin 5000 IU Warfarin |
Imaging: CT at 1 m, 3 m, 6 m Clinical: Assessment every 2 m Mean F/U: 4.9 m |
| Wang et al. [27] | 64 | 61.4 |
Malignant: 64 (100%) CRT: NR, C: NR, R: NR |
SVC: 64 (100%) + BCV: 21 (C), 20 (U) (64%) |
100 | 100 |
CT thorax Histology—percutaneous biopsy, bronchoscopy, endoscopy Venography |
Uncovered: 34 (53%) Covered: 30 (47%) Fluency, Luminexx |
Anticoag: Heparin 3d |
Imaging/Clinical: Assessment at 1, 3, 6 m Mean F/U: 6.2 m |
| Wei et al. [17] | 16 | NR |
Malignant: 16 (100%) Stenting as first line intervention |
SVC: 16 (100%) + BCV: 4 (25%) |
100 | 100 |
CT thorax Histology—CT-guided percutaneous biopsy Venography |
Uncovered: 16 (100%) Wallstent |
Anticoag: Long term anticoagulation |
Imaging: NR Clinical: Patient reported Mean F/U: NR |
CRT, Previous chemoradiotherapy; C, previous chemotherapy; R, previous radiotherapy; SVC, superior vena cava; BCV, brachiocephalic veins; IVC, inferior vena cava; NR, not recorded; LMWH, low molecular weight heparin; M, malignant; B, benign
Table 2.
Risk-of-bias quality assessment of the 54 included studies according to Newcastle–Ottawa Scale
| Study | Comparability | Outcome | Follow-up | Quality | ||||
|---|---|---|---|---|---|---|---|---|
| Representativeness | Selection | Outcome absence pre-intervention | Comparability of cohorts | Assessment of outcome | Appropriate follow-up period | Cohort follow-up achieved | Total (/9) | |
| Dyet et al. [23] | * | * | * | * | * | * | * | 9 |
| Gaines et al. [15] | * | * | * | * | * | * | * | 9 |
| Crowe et al. [35] | * | * | * | * | * | * | 8 | |
| Hennequin et al. [57] | * | * | * | * | * | * | * | 9 |
| Shah et al. [58] | * | * | * | * | * | * | * | 9 |
| Stock et al. [51] | * | * | * | * | * | * | * | 9 |
| Oudkerk et al. [18] | * | * | * | * | * | * | 8 | |
| Gross et al. [32] | * | * | * | * | * | * | * | 9 |
| Nicholson et al. [12] | * | * | * | * | * | * | 8 | |
| Tanigawa et al. [36] | * | * | * | * | * | * | * | 9 |
| Qanadli et al. [33] | * | * | * | * | * | * | 8 | |
| Thony et al. [37] | * | * | * | * | * | * | 9 | |
| Marcy et al. [59] | * | * | * | * | * | * | * | 9 |
| Miller et al. [60] | * | * | * | * | * | * | 8 | |
| Sasano et al. [29] | * | * | * | * | * | * | * | 9 |
| Lanciego et al. [19] | * | * | * | * | * | * | * | 9 |
| Smayra et al. [38] | * | * | * | * | * | 7 | ||
| Wilson et al. [70] | * | * | * | * | * | * | * | 9 |
| de Gregorio Ariza et al. [53] | * | * | * | * | * | * | * | 9 |
| Chatziioannou et al. [61] | * | * | * | * | * | * | * | 9 |
| Courtehoux et al. [62] | * | * | * | * | * | * | * | 9 |
| Dinkel et al. [49] | * | * | * | * | * | * | * | 9 |
| Monaco (2003) | * | * | * | * | * | * | * | 9 |
| Kim et al. [63] | * | * | * | * | * | * | * | 9 |
| Urreticoechea [30] | * | * | * | * | * | * | * | 9 |
| Bierdrager et al. [20] | * | * | * | * | * | * | * | 9 |
| Sheikh et al. [64] | * | * | * | * | * | * | * | 9 |
| Barshes et al. [52] | * | * | * | * | * | * | 8 | |
| Nagata et al. [31] | * | * | * | * | * | * | * | 9 |
| Lanciego et al. [21] | * | * | * | * | * | * | * | 9 |
| Cho et al. [34] | * | * | * | * | * | * | * | 9 |
| Fagedet et al. [39] | * | * | * | * | * | * | * | 9 |
| Gwon et al. [25] | * | * | * | * | * | * | * | 9 |
| Maleux et al. [23] | * | * | * | * | * | * | * | 9 |
| Andersen et al. [44] | * | * | * | * | * | * | * | 9 |
| Cho et al. [24] | * | * | * | * | * | * | * | 9 |
| Sobrinho and Aguiar [40] | * | * | * | * | * | * | * | 9 |
| Andersen et al. [44] | * | * | * | * | * | * | * | 9 |
| Breault et al. [65] | * | * | * | * | * | * | * | 9 |
| Leung et al. [41] | * | * | * | * | * | * | * | 9 |
| Miazga et al. [66] | * | * | * | * | * | * | * | 9 |
| Mokry et al. [47] | * | * | * | * | * | * | * | 9 |
| Büstgens et al. [69] | * | * | * | * | * | * | * | 9 |
| Massara et al. [71] | * | * | * | * | * | * | * | 9 |
| Anton et al. [46] | * | * | * | * | * | * | * | 9 |
| Calsina Juscafresa et al. [67] | * | * | * | * | * | * | 8 | |
| Kuo et al. [68] | * | * | * | * | * | * | * | 9 |
| Niu et al. [16] | * | * | * | * | * | * | * | 9 |
| Haddad et al. [26] | * | * | * | * | * | * | 8 | |
| Majumdar et al. [22] | * | * | * | * | * | * | 8 | |
| Karakhanian et al. [72] | * | * | * | * | * | * | * | 9 |
| Ren et al. [42] | * | * | * | * | * | * | * | 9 |
| Wang et al. [27] | * | * | * | * | * | * | * | 9 |
| Wei et al. [17] | * | * | * | * | * | 7 | ||
All studies scored between 7 and 9, indicating low risk of bias and high quality
All studies relied on clinical criteria to determine need for intervention, as well as pre-operative imaging to denote the nature of the obstruction. Five papers selected only patients presenting with significant stenosis, ranging between 75 and 90% [18–22]. One study categorised patient population by level of stenosis into high (> 80%), moderate (50–80%), and low grade (30–50%) [23].
Follow-up protocol was heterogenous among the included studies. Where prospectively specified, it involved regular imaging and clinical assessment in 9/54 studies or imaging only in 10/54 studies. The remaining studies relied on patients self-reporting symptoms. Length of follow-up was variable, with mean follow-up lengths ranging from 2 months to 3 years. All studies followed patients up where possible until death or study endpoint.
SVCS Pathology
Malignant or benign pathology was the exclusive cause of SVCS in 39/54 studies and 6/54 studies respectively. The remaining 9 studies did not discriminate by pathology. Of the 2237 patients in which SVCS pathology was reported, 222 had benign and 2015 had malignant pathology. The most frequent malignant pathologies included non-small cell and small cell bronchial carcinoma, as well as lymphadenopathies or invasion from extra-mediastinal primary tumours. Of the 6 studies that reported outcomes in benign SVCS, the primary pathology in 5 of these studies was indwelling medical devices, of which 1 included patients on dialysis. The primary pathology in the remaining study was fibrosing mediastinitis.
Stent Type
The type of stent used was reported in 50/54 studies, comprising 1795 patients. Uncovered stents were exclusively used in 47/50 studies. One study exclusively used covered stents [24], and three studies used both covered and uncovered stents in direct subgroup comparison [25–27]. The rationale for using covered stents in these papers was their anecdotal use in several published case reports of recurrent SVCS after uncovered stent placement or iatrogenic injury of the SVC [24–27]. The total number of patients receiving uncovered or covered stents was 1688/1795 and 107/1795.
Intra-procedural Anticoagulation
The use of intra-procedural anticoagulation was documented in 42/54 studies, comprising 1861 patients. Fixed doses of heparin injected before stenting were documented in 34/42 studies, with doses of 2000–5000 IU used. The most common dose used was 5000 IU in 28/34 studies. Use of heparin in the post-operative period at unspecified doses or frequency was documented in 8/42 studies.
Long-Term Anticoagulation
The use of long-term anticoagulation was documented in 27/54 studies, comprising 1197 patients. Regimens used were heterogenous in medication and duration of treatment. Where specified, regimens used more than once included 3 months of warfarin in 4 studies [28–31] or 1 month of dual antiplatelet therapy in 2 studies [32, 33]. Anticoagulation medications used included warfarin, aspirin, heparin, antiplatelets and their combinations. Three studies comprising 142 patients stated that neither intra-procedural nor long-term anticoagulation was attempted [3, 20, 34].
Intra-procedural Thrombolysis
The use of intra-procedural thrombolysis before stenting was documented in 19/54 studies, comprising 727 patients. In 16/19 studies, thrombolysis was only used for cases in which thrombosis above the stent was too severe to navigate across. In 3/19 studies, thrombolysis was used for all patients to prevent intra-stent thrombosis in follow-up [18, 35, 36]. The pharmacological agent used was specified in 16/19 studies, with urokinase, recombinant tissue plasminogen activator and streptokinase used in 8, 5 and 3 studies respectively. Mechanical thrombolysis, relying on thromboaspiration, fragmentation or crushing the thrombus against the vessel wall, was used in 3/19 studies [37–39].
Previous Treatments Attempted
Stenting was attempted as the first-line procedure for treatment of malignant SVCS in 4/54 studies, comprising 202 patients [17, 20, 22, 40]. One further study retrospectively investigated a cohort of 56 malignant SVCS patients, with 33 patients undergoing stenting at initial presentation before chemoradiotherapy and 23 only after the failure of chemoradiotherapy [41].
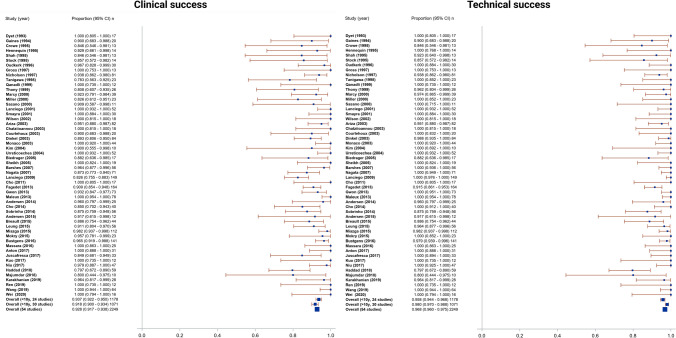
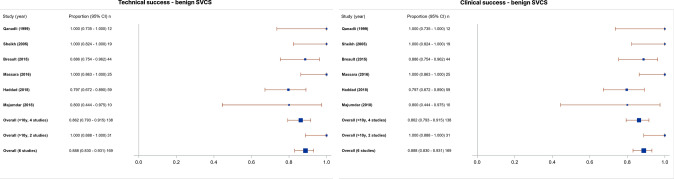
Technical Success
The technical success rate was 96.8% (95% CI 96.0–97.5%), with a range of 79.7–100% and I2 = 0 indicating no heterogeneity (Fig. 2). The technical success rate for studies investigating benign SVCS alone was 88.8% (95% CI 83.0–93.1%) (Fig. 3). Most studies described technical success as navigation and successful deployment and expansion of the stent across the obstruction or stenosis, with evidence of flow restoration on post-intervention venography. Further requisites for technical success where specified included a final pressure gradient < 10 mmHg in 4 studies [16, 24, 25, 42] and < 50% residual stenosis in 3 studies [22, 43, 44]. Two studies used the Society of Interventional Radiologists (SIR) definition of technical success; complete coverage of the obstruction, with overlapping margins of 1 cm on either side and residual stenosis < 30% [45–47].
Fig. 2.
Forest plots showing a technical and b clinical success plots. Pooled technical success rate was 96.8% (95% CI 96.0–97.5%, range 79.7–100%, I2 = 0). Pooled clinical success rate was 92.8% (95% CI 91.7–93.8%, range 78.3–100%, I2 = 0)
Fig. 3.
Forest plots showing a technical and b clinical success plots for 6 studies investigating benign SVCS alone. Pooled technical success rate and clinical success rate were 88.8% (95% CI 83.0–93.1%)
Clinical Success
The clinical success rate was 92.8% (95% CI 91.7–93.8%), with a range of 78.3–100% and I2 = 0 indicating no heterogeneity (Fig. 2). The clinical success rate for studies investigating benign SVCS alone was 88.8% (95% CI 83.0–93.1%) (Fig. 3). All studies except two described clinical success as acute improvement in symptoms, whether partial or complete, measured through patient description of symptoms or the Kishi scoring system [48]. One study further defined clinical failure as persistence of at least 2 of the cardinal symptoms of SVCS; prominent veins, facial oedema, plethora, dizziness, headaches and dyspnoea [49]. Dyspnoea was exempted from consideration as a symptom of clinical improvement in most studies, as it is a common symptom of underlying pulmonary disease and is frequently found in patients presenting with tumour invasion into the bronchus or pulmonary vessels. The other study defined clinical success as < 10 mmHg pressure gradient between ends of the stent after insertion [27].
Complications
Following the CIRSE complication [50], complications are presented in Table 3. The average complication rate was 5.78% (SD = 9.3182), with a range of 0–53.8%. No complications were reported in 25/54 studies. The overall 24-h mortality rate was 0.006%. The most frequent cause of mortality was rupture of the SVC leading to cardiac tamponade. The most frequent complications above Grade 3 reported were bleeding events while on long-term anticoagulation or antiplatelet therapy, pulmonary oedema and thromboembolic events. The most frequent complications below Grade 3 reported were stent migration, localized pain and puncture site haematoma.
Table 3.
Minor and major complications by Cardiovascular and Interventional Radiological Society of Europe (CIRSE) classification
| Grade 1 | Grade 3 | ||
| Localised pain | 12 | Haemopericardium | 4 |
| Puncture site haematoma | 11 | Sepsis | 3 |
| Fever | 7 | Arterial injury | 2 |
| Tachypnoea | 2 | Lower limb cellulitis/phlebitis | 2 |
| Grade 2 | Grade 4 | 2 | |
| Stent migration | 17 | Bleeding event on anticoagulation | 19 |
| Arrhythmia—SVT (4), VT (1), bradycardia (1) | 6 | Pulmonary embolism/DVT | 8 |
| Haemoptysis/haematemesis | 6 | Hoarseness due to laryngeal nerve damage | 3 |
| Transiently impaired venous drainage | 1 | Grade 6 | 3 |
| Grade 3 | Mortality in 24 h—tamponade (5), unknown (4), MI (1), PE (1), HF (1), haemopericardium (1) | 13 | |
| Pulmonary oedema | 10 | ||
| Cardiac tamponade due to iatrogenic SVC perforation | 7 |
SVT, supraventricular tachycardia; VT, ventricular tachycardia; DVT, deep venous thrombosis; MI, myocardial infarction; PE, pulmonary embolism; HF, heart failure
Recurrence and Re-interventions
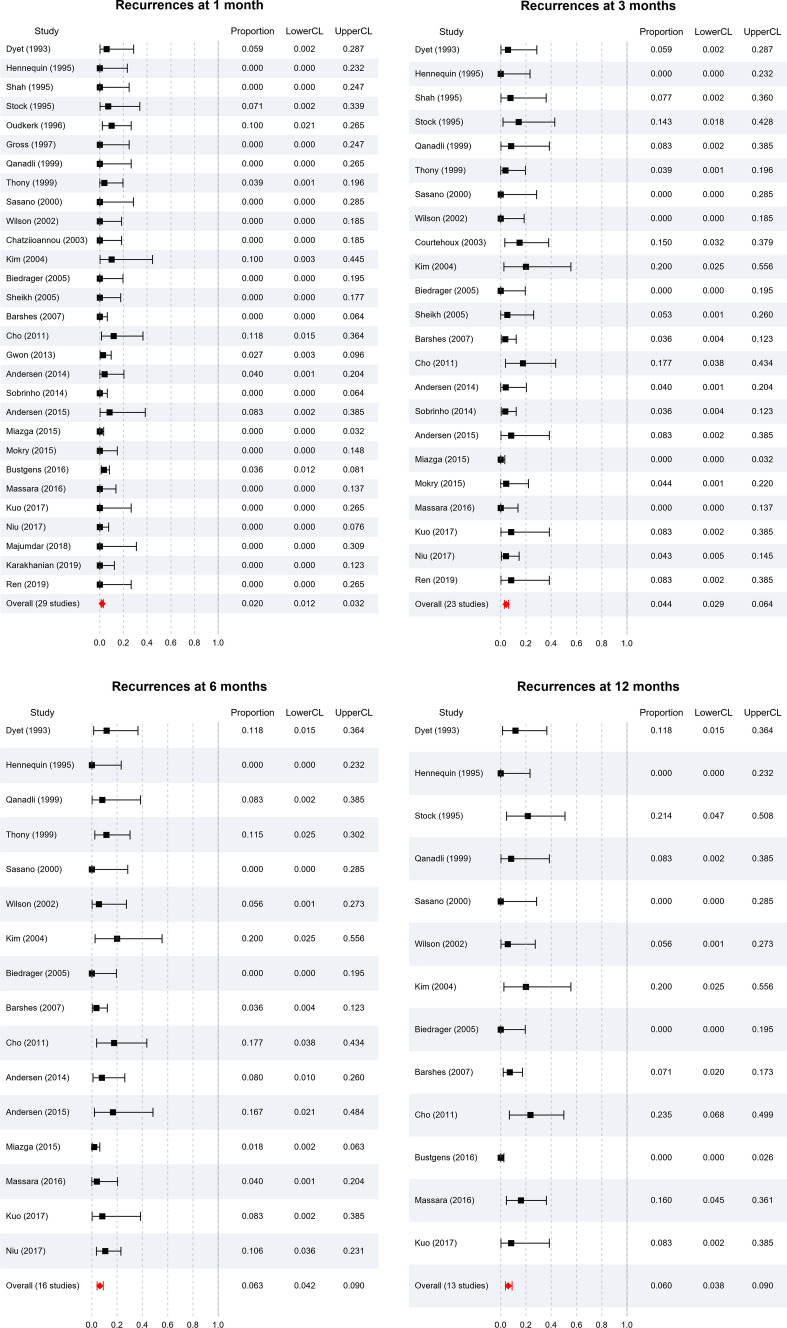
Primary patency was reported in 19/54 studies, comprising 906 patients, while secondary patency was reported in 20/54 studies, comprising 1117 patients. Primary and secondary patency ranged from 65 to 92% and 75–100% respectively. Primary patency was defined as continued stent patency without re-intervention at study endpoint, while secondary patency included those requiring re-interventions. Four studies separately defined primary patency as the time interval from procedure to re-intervention, ranging from 83 days to 31.3 months [16, 22, 27, 51].
Recurrence rate of symptoms in follow-up was reported in 29/54, 23/54, 16/54 and 13/54 studies at 1, 3, 6 and 12 months respectively (Fig. 4). From these studies, pooled patency for endovascular stenting in both benign and malignant SVCS remained above 90% for the first year (98.0%, 95.6%, 93.7% and 94.0% at 1, 3, 6 and 12 months respectively). At all timepoints, I2 = 0 indicating no heterogeneity. In order of frequency, the cause of recurrence was intra-stent thrombosis, tumour overgrowth above or below the stent, or tumour ingrowth through the stent. The average re-intervention rate was 9.11% (SD = 11.190), with a range from 0 to 60%. No re-interventions were required in 17/54 studies. Re-interventions performed included balloon dilatation, thrombolysis and further stenting.
Fig. 4.
Forest plots showing recurrence rates at a 1 month, b 3 months, c 6 months and d 12 months. Pooled patency remained above 90% for the first year (98.0%, 95.6%, 93.7% and 94.0% at 1, 3, 6 and 12 months respectively. At all timepoints, I2 = 0
One study found primary patency to be significantly longer in patients with malignant SVCS compared to SVCS secondary to haemodialysis [38]. Three studies found covered stent use to be significantly associated with lower rates of stent occlusion in follow-up, lower rates of symptom recurrence and longer primary and secondary patencies [25–27].
Survival
Median and mean survival were reported in 12/54 studies and 10/54 studies respectively, of which all but one study comprised patients with malignant pathology. The average median survival was 4.74 months (SD = 3.3602), with a range of 1–13 months. The average mean survival was 4.71 months (SD = 1.4235), with a range of 2.49–6.70 months. One study found survival was significantly longer in patients in whom stenting was attempted as a first-line procedure [41]. Another study found survival was significantly longer in patients who underwent subsequent chemotherapy, or chemoradiotherapy, as compared to patients who did not receive further treatment [21].
Discussion
This systematic review and meta-analysis confirm the efficacy and safety of endovascular intervention in SVCS, with high technical and clinical success rates of 96.8% and 92.8% respectively, patency remaining above 90% for the first year, and low complication and re-intervention rates. These results parallel current perceptions of SVCS among clinicians and correspond with the existing literature, which posits a technical success rate above 80% and clinical success rate above 90% [4, 7].
There is a relative paucity in research into benign SVCS, and this is reflected in the balance of studies investigating benign SVCS in this review. Of the studies investigating benign SVCS in this review, pooled technical and clinical success rates were identical at 88.8%. Several studies report differences in patency between patients with benign and malignant SVCS [39, 52, 53]. These results are however mixed and did not reach statistical significance, aside from a study by Smayra et al. which found primary patency to be significantly longer in malignant SVCS compared to haemodialysis-associated SVCS. Given the shift in aetiology of benign SVCS towards indwelling medical devices, and the resultant predicted increase in benign SVCS incidence [5], it is critical more research is directed into these pathologies.
Patients with benign SVCS also have higher life expectancies and survival [5, 10, 54]. The impact of declining stent patency with time, subsequent risk for re-intervention and need for long-term anticoagulation may hence be greater. Sfyroeras and colleagues performed a systematic review of 9 studies investigating benign SVCS and found pooled patencies of 90.7%, 71.2% and 48% at 1 month, 12 months and 36 months respectively, with 26.9% of patients requiring re-intervention [7]. The risks and benefits of stenting as a palliative procedure in malignant SVCS or therapeutic procedure in benign SVCS should be considered separately.
The use of covered stents was found in three studies to significantly improve outcomes [25–27]. Gwon et al. investigated 73 patients with malignant SVCS and reported significantly higher cumulative patencies across the first year between covered and uncovered stents [25]. This was corroborated in further studies of both benign and malignant SVCS [26, 27]. These three studies were however three of only four studies in this review to use covered stents, emphasizing the need for further research into the role of covered stents in the future.
Despite the growing body of evidence for stenting in SVCS, there is little evidence for a standardised anticoagulation regimen, both intra-procedurally and in follow-up. It has further not been proven that anticoagulation leads to improved outcomes. Ratzon et al. retrospectively investigated 183 malignant SVCS patients and found no statistically significant difference in intra-stent thrombosis in follow-up or survival associated with anticoagulation [55]. Similarly, Haddad et al. did not find a statistically significant difference in symptom recurrence, mean percent stenosis in follow-up, time to return of symptoms or primary patency in a population of 58 benign SVCS patients [56]. Given that adverse events on anticoagulation represent a significant proportion of complications post-procedure, further research is needed to identify if anticoagulation is necessary, and the ideal regimen.
The role of intra-procedural thrombolysis also requires further exploration. Fagedet et al. identified thrombosis as a risk factor significantly doubling the risk of symptom recurrence (HR 2.60), with this risk removed by using intra-procedural thrombolysis [39]. There is however little research published examining the impact of thrombolysis on long-term outcomes, or whether it should be used as a prophylactic measure against stent thrombosis in follow-up.
Stenting has largely replaced radiation therapy as the first line procedure for managing malignant SVCS, due to its immediate nature, high success rate, and the retained possibility to attempt alternative therapies [4, 10, 12]. The only study to specifically examine the impact of stenting as a first-line procedure was a retrospective study of 56 malignant SVCS patients by Leung et al. [41]. They found that patients who received stenting at initial presentation had significantly increased survival over patients who received stenting after the failure of traditional treatments, but found no significant difference in success rate, procedure time, symptom relief, complication rate or re-intervention rate. There are furthermore no published studies comparing endovascular intervention directly with chemotherapy, radiotherapy or surgical intervention alone in a randomized controlled trial.
A key limitation of this review is the lack of randomised controlled trials or prospectively designed studies with clearly specified follow-up strategies. Most of the studies included were retrospective, single-centre studies, raising the risk of selection or publication bias and possible overestimation of results. The inconsistency in following up patients and proportion of patients lost to follow-up limit review of long-term outcomes. This is further compounded by the short life expectancies or survival of malignant SVCS patients. Definitions of technical success, clinical success and primary and secondary patency varied across studies and guidelines of learned societies were not strictly followed, limiting the utility of meta-analysis. Further subgroup meta-analysis by SVCS pathology or additional therapies given was not performed due to the small proportion of data on such patients, limiting the applicability of these findings.
In conclusion, this systematic review and meta-analysis confirm endovascular stenting as a safe and effective therapeutic option for SVCS of all pathologies, with high technical and clinical success rates, as well as low complication and recurrence rates. Our study also consolidates current evidence for the impact of procedural considerations, such as stent type, use of anticoagulation and intra-stent thrombolysis. More research of higher methodological quality, such as randomised controlled trials or larger multi-centre studies, is needed to better elucidate the scope of efficacy of stenting, as well as the patients to which stenting could most benefit.
Author Contributions
This study was conceptualised by MH and UR, with a preliminary data search performed by UR. The final literature searches and data analysis were performed by EA and MK. NW contributed to the statistical data analysis. The manuscript draft was written by EA with critical revisions from MH. All authors read and approved the final manuscript.
Funding
This study was not supported by any funding.
Declarations
Conflict of interest
The authors declare that they have no conflict of interest. This study was supported by Imperial College London Healthcare Biomedical Research Centre.
Consent for Publication
For this type of study consent for publication is not required.
Ethical Approval
The article does not contain any studies with human participants or animals performed by any of the authors.
Informed Consent
For this type of study informed consent is not required.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Friedman T, Quencer K, Kishore S, Winokur R, Madoff D. Malignant venous obstruction: superior vena cava syndrome and beyond. Semin Interv Radiol. 2017;34(04):398–408. doi: 10.1055/s-0037-1608863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Cheng S. Superior vena cava syndrome. Cardiol Rev. 2009;17(1):16–23. doi: 10.1097/crd.0b013e318188033c. [DOI] [PubMed] [Google Scholar]
- 3.Wilson L, Detterbeck F, Yahalom J. Superior vena cava syndrome with malignant causes. N Engl J Med. 2007;356(18):1862–1869. doi: 10.1056/nejmcp067190. [DOI] [PubMed] [Google Scholar]
- 4.Azizi A, Shafi I, Shah N, Rosenfield K, Schainfeld R, Sista A, Bashir R. Superior vena cava syndrome. JACC Cardiovasc Interv. 2020;13(24):2896–2910. doi: 10.1016/j.jcin.2020.08.038. [DOI] [PubMed] [Google Scholar]
- 5.Rice T, Rodriguez R, Light R. The superior vena cava syndrome. Medicine. 2006;85(1):37–42. doi: 10.1097/01.md.0000198474.99876.f0. [DOI] [PubMed] [Google Scholar]
- 6.Deshwal H, Ghosh S, Magruder K, Bartholomew J, Montgomery J, Mehta A. A review of endovascular stenting for superior vena cava syndrome in fibrosing mediastinitis. Vasc Med. 2019;25(2):174–183. doi: 10.1177/1358863x19884130. [DOI] [PubMed] [Google Scholar]
- 7.Sfyroeras G, Antonopoulos C, Mantas G, Moulakakis K, Kakisis J, Brountzos E, et al. A review of open and endovascular treatment of superior vena cava syndrome of benign aetiology. Eur J Vasc Endovasc Surg. 2017;53(2):238–254. doi: 10.1016/j.ejvs.2016.11.013. [DOI] [PubMed] [Google Scholar]
- 8.Chee C, Bjarnason H, Prasad A. Superior vena cava syndrome: an increasingly frequent complication of cardiac procedures. Nat Clin Pract Cardiovasc Med. 2007;4(4):226–230. doi: 10.1038/ncpcardio0850. [DOI] [PubMed] [Google Scholar]
- 9.Butty S, Johnson M, Stevens D. Superior vena cava rupture and cardiac tamponade complicating the endovascular treatment of malignant superior vena cava syndrome: a case report and literature review. Semin Interv Radiol. 2015;32(04):439–444. doi: 10.1055/s-0035-1564795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Rizvi A, Kalra M, Bjarnason H, Bower T, Schleck C, Gloviczki P. Benign superior vena cava syndrome: stenting is now the first line of treatment. J Vasc Surg. 2008;47(2):372–380. doi: 10.1016/j.jvs.2007.09.071. [DOI] [PubMed] [Google Scholar]
- 11.Rachapalli V, Boucher L. Superior vena cava syndrome: role of the interventionalist. Can Assoc Radiol J. 2014;65(2):168–176. doi: 10.1016/j.carj.2012.09.003. [DOI] [PubMed] [Google Scholar]
- 12.Nicholson A, Ettles D, Arnold A, Greenstone M, Dyet J. Treatment of malignant superior vena cava obstruction: metal stents or radiation therapy. J Vasc Interv Radiol. 1997;8(5):781–788. doi: 10.1016/s1051-0443(97)70660-2. [DOI] [PubMed] [Google Scholar]
- 13.McInnes MDF, Moher D, Thombs BD, et al. Preferred reporting items for a systematic review and meta-analysis of diagnostic test accuracy studies: the PRISMA-DTA statement. JAMA. 2018;319:388–396. doi: 10.1001/jama.2017.19163. [DOI] [PubMed] [Google Scholar]
- 14.Wells G, Shea B, O'Connell D, Peterson J, Welch V, Losos M, Tugwell P. The Newcastle-Ottawa Scale (NOS) for assessing the quality of nonrandomised studies in meta-analyses. http://www.ohri.ca/programs/clinical_epidemiology/oxford.asp (2021). Retrieved 17 May 2021
- 15.Gaines P, Belli A, Anderson P, McBride K, Hemingway A. Superior vena caval obstruction managed by the gianturco Z stent. Clin Radiol. 1994;49(3):202–208. doi: 10.1016/s0009-9260(05)81778-7. [DOI] [PubMed] [Google Scholar]
- 16.Niu S, Xu Y, Cheng L, Cao C. Stent insertion for malignant superior vena cava syndrome: effectiveness and long-term outcome. Radiol Med (Torino) 2017;122(8):633–638. doi: 10.1007/s11547-017-0767-1. [DOI] [PubMed] [Google Scholar]
- 17.Wei S, Liu J, Li X, Song Z, Dong M, Zhao H, et al. A retrospective stenting study on superior vena cava syndrome caused by lung cancer. Thorac Cancer. 2020;11(7):1835–1839. doi: 10.1111/1759-7714.13461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Oudkerk M, Kuijpers T, Schmitz P, Loosveld O, de Wit R. Self-expanding metal stents for palliative treatment of superior vena caval syndrome. Cardiovasc Interv Radiol. 1996;19(3):146–151. doi: 10.1007/s002709900032. [DOI] [PubMed] [Google Scholar]
- 19.Lanciego C, Chacón J, Julián A, Andrade J, López L, Martinez B, et al. Stenting as first option for endovascular treatment of malignant superior vena cava syndrome. Am J Roentgenol. 2001;177(3):585–593. doi: 10.2214/ajr.177.3.1770585. [DOI] [PubMed] [Google Scholar]
- 20.Bierdrager E, Lampmann LE, Lohle PN, Schoemaker CM, Schijen JH, Palmen FM, van der Heul C. Endovascular stenting in neoplastic superior vena cava syndrome prior to chemotherapy or radiotherapy. Neth J Med. 2005;63(1):20–23. [PubMed] [Google Scholar]
- 21.Lanciego C, Pangua C, Chacón J, Velasco J, Boy R, Viana A, et al. Endovascular stenting as the first step in the overall management of malignant superior vena cava syndrome. Am J Roentgenol. 2009;193(2):549–558. doi: 10.2214/ajr.08.1904. [DOI] [PubMed] [Google Scholar]
- 22.Majumdar S, Shoela R, Kim D, Ramaswamy R, Mani N, Salter A, Akinwande O. Endovascular management of SVC syndrome due to fibrosing mediastinitis—a feasibility and safety analysis. Vasc Endovasc Surg. 2018;52(3):202–206. doi: 10.1177/1538574418757401. [DOI] [PubMed] [Google Scholar]
- 23.Maleux G, Gillardin P, Fieuws S, Heye S, Vaninbroukx J, Nackaerts K. Large-bore nitinol stents for malignant superior vena cava syndrome: factors influencing outcome. Am J Roentgenol. 2013;201(3):667–674. doi: 10.2214/ajr.12.9582. [DOI] [PubMed] [Google Scholar]
- 24.Cho Y, Gwon D, Ko G, Ko H, Kim J, Shin J, et al. Covered stent placement for the treatment of malignant superior vena cava syndrome: is unilateral covered stenting safe and effective? Korean J Radiol. 2014;15(1):87. doi: 10.3348/kjr.2014.15.1.87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gwon D, Ko G, Kim J, Shin J, Yoon H, Sung K. Malignant superior vena cava syndrome: a comparative cohort study of treatment with covered stents versus uncovered stents. Radiology. 2013;266(3):979–987. doi: 10.1148/radiol.12120517. [DOI] [PubMed] [Google Scholar]
- 26.Haddad M, Simmons B, McPhail I, Kalra M, Neisen M, Johnson M, et al. Comparison of covered versus uncovered stents for benign superior vena cava (SVC) obstruction. Cardiovasc Interv Radiol. 2018;41(5):712–717. doi: 10.1007/s00270-018-1906-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wang Z, Li C, Li J, Wu W, Li Y, Shi J. Covered versus uncovered stent insertion for malignant superior vena cava obstruction. Minim Invasive Ther Allied Technol. 2019;29(6):353–358. doi: 10.1080/13645706.2019.1653925. [DOI] [PubMed] [Google Scholar]
- 28.Dyet J, Nicholson A, Cook A. The use of the wallstent endovascular prosthesis in the treatment of malignant obstruction of the superior vena cava. Clin Radiol. 1993;48(6):381–385. doi: 10.1016/s0009-9260(05)81105-5. [DOI] [PubMed] [Google Scholar]
- 29.Sasano S, Onuki T, Mae M, Oyama K, Sakuraba M, Nitta S. Wallstent endovascular prosthesis for the treatment of superior vena cava syndrome. Jpn J Thorac Cardiovasc Surg. 2001;49(3):165–170. doi: 10.1007/bf02913595. [DOI] [PubMed] [Google Scholar]
- 30.Urruticoechea A. Treatment of malignant superior vena cava syndrome by endovascular stent insertion experience on 52 patients with lung cancer. Lung Cancer. 2004;43(2):209–214. doi: 10.1016/s0169-5002(03)00361-1. [DOI] [PubMed] [Google Scholar]
- 31.Nagata T, Makutani S, Uchida H, Kichikawa K, Maeda M, Yoshioka T, et al. Follow-up results of 71 patients undergoing metallic stent placement for the treatment of a malignant obstruction of the superior vena cava. Cardiovasc Interv Radiol. 2007;30(5):959–967. doi: 10.1007/s00270-007-9088-4. [DOI] [PubMed] [Google Scholar]
- 32.Gross C, Krämer J, Waigand J, Uhlich F, Schröder G, Thalhammer C, et al. Stent implantation in patients with superior vena cava syndrome. Am J Roentgenol. 1997;169(2):429–432. doi: 10.2214/ajr.169.2.9242747. [DOI] [PubMed] [Google Scholar]
- 33.Qanadli S, El Hajjam M, Mignon F, de Kerviler E, Rocha P, Barré O, et al. Subacute and chronic benign superior vena cava obstructions: endovascular treatment with self-expanding metallic stents. Am J Roentgenol. 1999;173(1):159–164. doi: 10.2214/ajr.173.1.10397119. [DOI] [PubMed] [Google Scholar]
- 34.Cho T, Janho K, Mohan I. The role of stenting the superior vena cava syndrome in patients with malignant disease. Angiology. 2010;62(3):248–252. doi: 10.1177/0003319710382772. [DOI] [PubMed] [Google Scholar]
- 35.Crowe M, Davies C, Gaines P. Percutaneous management of superior vena cava occlusions. Cardiovasc Interv Radiol. 1995;18(6):367–372. doi: 10.1007/bf00338303. [DOI] [PubMed] [Google Scholar]
- 36.Tanigawa N, Sawada S, Mishima K, Okuda Y, Mizukawa K, Ohmura N, et al. Clinical outcome of stenting in superior vena cava syndrome associated with malignant tumors. Acta Radiol. 1998;39(6):669–674. doi: 10.3109/02841859809175494. [DOI] [PubMed] [Google Scholar]
- 37.Thony F, Moro D, Witmeyer P, Angiolini S, Brambilla C, Coulomb M, Ferretti G. Endovascular treatment of superior vena cava obstruction in patients with malignancies. Eur Radiol. 1999;9(5):965–971. doi: 10.1007/s003300050777. [DOI] [PubMed] [Google Scholar]
- 38.Smayra T, Otal P, Chabbert V, Chemla P, Romero M, Joffre F, Rousseau H. Long-term results of endovascular stent placement in the superior caval venous system. Cardiovasc Interv Radiol. 2001;24(6):388–394. doi: 10.1007/s00270-001-0055-1. [DOI] [PubMed] [Google Scholar]
- 39.Fagedet D, Thony F, Timsit J, Rodiere M, Monnin-Bares V, Ferretti G, et al. Endovascular treatment of malignant superior vena cava syndrome: results and predictive factors of clinical efficacy. Cardiovasc Interv Radiol. 2011;36(1):140–149. doi: 10.1007/s00270-011-0310-z. [DOI] [PubMed] [Google Scholar]
- 40.Sobrinho G, Aguiar P. Stent placement for the treatment of malignant superior vena cava syndrome—a single-center series of 56 patients. Arch Bronconeumol (Engl Ed) 2014;50(4):135–140. doi: 10.1016/j.arbr.2014.03.001. [DOI] [PubMed] [Google Scholar]
- 41.Leung S, Sung T, Wan A, Leung K, Kan W. Endovascular stenting in the management of malignant superior vena cava obstruction: comparing safety, effectiveness, and outcomes between primary stenting and salvage stenting. Hong Kong Med J. 2015 doi: 10.12809/hkmj144363. [DOI] [PubMed] [Google Scholar]
- 42.Ren J, Cao C, Fu Y, Du H. Double stent insertion for combined malignant airway and superior vena cava obstruction. Medicine. 2019;98(21):e15777. doi: 10.1097/md.0000000000015777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Duvnjak S, Andersen P. Palliative treatment of superior vena cava syndrome with nitinol stents. Int J Angiol. 2014;23(04):255–262. doi: 10.1055/s-0034-1383432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Andersen P, Midtgaard A, Brenoe A, Elle B, Duvnjak S. A new nitinol stent for use in superior vena cava syndrome. Initial clinical experience. J Cardiovasc Surg. 2015;56(6):877–881. [PubMed] [Google Scholar]
- 45.Sacks D, McClenny T, Cardella J, Lewis C. Society of interventional radiology clinical practice guidelines. J Vasc Interv Radiol. 2003;14(9):S199–S202. doi: 10.1097/01.rvi.0000094584.83406.3e. [DOI] [PubMed] [Google Scholar]
- 46.Anton S, Oechtering T, Stahlberg E, Jacob F, Kleemann M, Barkhausen J, Goltz J. Endovascular stent-based revascularization of malignant superior vena cava syndrome with concomitant implantation of a port device using a dual venous approach. Support Care Cancer. 2017;26(6):1881–1888. doi: 10.1007/s00520-017-3997-9. [DOI] [PubMed] [Google Scholar]
- 47.Mokry T, Bellemann N, Sommer C, Heussel C, Bozorgmehr F, Gnutzmann D, et al. Retrospective study in 23 patients of the self-expanding sinus-XL stent for treatment of malignant superior vena cava obstruction caused by non-small cell lung cancer. J Vasc Interv Radiol. 2015;26(3):357–365. doi: 10.1016/j.jvir.2014.11.019. [DOI] [PubMed] [Google Scholar]
- 48.Kishi K, Sonomura T, Mitsuzane K, Nishida N, Yang R, Sato M, et al. Self-expandable metallic stent therapy for superior vena cava syndrome: clinical observations. Radiology. 1993;189(2):531–535. doi: 10.1148/radiology.189.2.8210386. [DOI] [PubMed] [Google Scholar]
- 49.Dinkel H, Mettke B, Schmid F, Baumgartner I, Triller J, Do D. Endovascular treatment of malignant superior vena cava syndrome: is bilateral wallstent placement superior to unilateral placement? J Endovasc Ther. 2003;10(4):788–797. doi: 10.1177/152660280301000416. [DOI] [PubMed] [Google Scholar]
- 50.Filippiadis DK, Binkert C, Pellerin O, Hoffmann RT, Krajina A, Pereira PL. Cirse quality assurance document and standards for classification of complications: the CIRSE classification system. Cardiovasc Intervent Radiol. 2017;40(8):1141–1146. doi: 10.1007/s00270-017-1703-4. [DOI] [PubMed] [Google Scholar]
- 51.Stock K, Jacob A, Proske M, Bolliger C, Rochlitz C, Steinbrich W. Treatment of malignant obstruction of the superior vena cava with the self-expanding Wallstent. Thorax. 1995;50(11):1151–1156. doi: 10.1136/thx.50.11.1151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Barshes N, Annambhotla S, El Sayed H, Huynh T, Kougias P, Dardik A, Lin P. Percutaneous stenting of superior vena cava syndrome: treatment outcome in patients with benign and malignant etiology. Vascular. 2007;15(5):314–321. doi: 10.2310/6670.2007.00067. [DOI] [PubMed] [Google Scholar]
- 53.de Gregorio Ariza M, Gamboa P, Gimeno M, Alfonso E, Mainar A, Medrano J, et al. Percutaneous treatment of superior vena cava syndrome using metallic stents. Eur Radiol. 2003;13(4):853–862. doi: 10.1007/s00330-002-1489-9. [DOI] [PubMed] [Google Scholar]
- 54.Mónaco R. Use of self-expanding vascular endoprostheses in superior vena cava syndrome. Eur J Cardiothorac Surg. 2003;24(2):208–211. doi: 10.1016/s1010-7940(03)00293-8. [DOI] [PubMed] [Google Scholar]
- 55.Ratzon R, Tamir S, Friehmann T, Livneh N, Dudnik E, Rozental A, et al. Thrombosis, anticoagulation and outcomes in malignant superior vena cava syndrome. J Thromb Thrombol. 2018;47(1):121–128. doi: 10.1007/s11239-018-1747-6. [DOI] [PubMed] [Google Scholar]
- 56.Haddad M, Thompson S, McPhail I, Bendel E, Kalra M, Stockland A, et al. Is long-term anticoagulation required after stent placement for benign superior vena cava syndrome? J Vasc Interv Radiol. 2018;29(12):1741–1747. doi: 10.1016/j.jvir.2018.07.020. [DOI] [PubMed] [Google Scholar]
- 57.Hennequin L, Fade O, Fays J, Bic J, Jaafar S, Bertal A, et al. Superior vena cava stent placement: results with the wallstent endoprosthesis. Radiology. 1995;196(2):353–361. doi: 10.1148/radiology.196.2.7617844. [DOI] [PubMed] [Google Scholar]
- 58.Shah R, Sabanathan S, Lowe R, Mearns A. Stenting in malignant obstruction of superior vena cava. J Thorac Cardiovasc Surg. 1996;112(2):335–340. doi: 10.1016/s0022-5223(96)70259-3. [DOI] [PubMed] [Google Scholar]
- 59.Marcy P, Magné N, Bentolila F, Drouillard J, Bruneton J, Descamps B. Superior vena cava obstruction: is stenting necessary? Support Care Cancer. 2001;9(2):103–107. doi: 10.1007/s005200000173. [DOI] [PubMed] [Google Scholar]
- 60.Miller J, McBride K, Little F, Price A. Malignant superior vena cava obstruction: stent placement via the subclavian route. Cardiovasc Interv Radiol. 2000;23(2):155–158. doi: 10.1007/s002709910033. [DOI] [PubMed] [Google Scholar]
- 61.Chatziioannou A, Alexopoulos T, Mourikis D, Dardoufas K, Katsenis K, Lazarou S, et al. Stent therapy for malignant superior vena cava syndrome. Eur J Radiol. 2003;47(3):247–250. doi: 10.1016/s0720-048x(02)00207-3. [DOI] [PubMed] [Google Scholar]
- 62.Courtheoux P, Alkofer B, Al Refaï M, Gervais R, Le Rochais J, Icard P. Stent placement in superior vena cava syndrome. Ann Thorac Surg. 2003;75(1):158–161. doi: 10.1016/s0003-4975(02)04293-5. [DOI] [PubMed] [Google Scholar]
- 63.Kim Y, Kim K, Ko Y, Park C, Lim S, Kim Y, et al. Endovascular stenting as a first choice for the palliation of superior vena cava syndrome. J Korean Med Sci. 2004;19(4):519. doi: 10.3346/jkms.2004.19.4.519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Sheikh M, Fernandez B, Gray B, Graham L, Carman T. Endovascular stenting of nonmalignant superior vena cava syndrome. Catheter Cardiovasc Interv. 2005;65(3):405–411. doi: 10.1002/ccd.20458. [DOI] [PubMed] [Google Scholar]
- 65.Breault S, Doenz F, Jouannic A, Qanadli S. Percutaneous endovascular management of chronic superior vena cava syndrome of benign causes: long-term follow-up. Eur Radiol. 2016;27(1):97–104. doi: 10.1007/s00330-016-4354-y. [DOI] [PubMed] [Google Scholar]
- 66.Miazga M, Jargiełło T, Drelich-Zbroja A, Karska K, Pyra K, Sojka M, et al. Endovascular treatment for superior vena cava obstruction. Postępy Nauk Medycznych. 2015;28(2):124–128. doi: 10.5604/08606196.1150855. [DOI] [Google Scholar]
- 67.Calsina Juscafresa L, Gil Bazo I, Grochowicz L, Páramo Alfaro M, López-Picazo González J, Moreno Jiménez M, Bilbao Jaureguizar J. Endovascular treatment of malignant superior vena cava syndrome secondary to lung cancer. Hosp Pract. 2017;45(3):70–75. doi: 10.1080/21548331.2017.1342507. [DOI] [PubMed] [Google Scholar]
- 68.Kuo T, Chen P, Shih C, Chen I. Endovascular stenting for end-stage lung cancer patients with superior vena cava syndrome post first-line treatments—a single-center experience and literature review. J Chin Med Assoc. 2017;80(8):482–486. doi: 10.1016/j.jcma.2017.04.005. [DOI] [PubMed] [Google Scholar]
- 69.Büstgens Felix, Loose Reinhard, Ficker Joachim, Wucherer Michael, Uder Michael, Adamus Ralf. Stent Implantation for Superior Vena Cava Syndrome of Malignant Cause. RöFo - Fortschritte auf dem Gebiet der Röntgenstrahlen und der bildgebenden Verfahren. 2017;189(05):423–430. doi: 10.1055/s-0042-122147. [DOI] [PubMed] [Google Scholar]
- 70.Wilson Lynn D., Detterbeck Frank C., Yahalom Joachim. Superior Vena Cava Syndrome with Malignant Causes. N Engl J Med. 2007;356(18):1862–1869. doi: 10.1056/NEJMcp067190. [DOI] [PubMed] [Google Scholar]
- 71.Massara Mafalda, De Caridi Giovanni, Alberti Antonino, Volpe Pietro, Spinelli Francesco. Symptomatic superior vena cava syndrome in hemodialysis patients: mid-term results of primary stenting. Semin Vasc Surg. 2016;29(4):186–191. doi: 10.1053/j.semvascsurg.2017.05.001. [DOI] [PubMed] [Google Scholar]
- 72.Karakhanian Walter Kegham, Karakhanian Walter Zavem, Belczak Sergio Quilici. Superior vena cava syndrome: endovascular management. J Vasc Bras. 2019;18:e20180062. doi: 10.1590/1677-5449.180062. [DOI] [PMC free article] [PubMed] [Google Scholar]