Abstract
The role of the 20,922-Da RecX protein and its interference with RecA activity were analyzed in Streptomyces lividans. The recX gene is located 220 bp downstream of recA. Transcriptional analysis by reverse transcriptase PCR demonstrated that recX and recA constitute an operon. While recA was transcribed at a basal level even under noninducing conditions, a recA-recX cotranscript was only detectable after induction of recA following DNA damage. The recA-recX cotranscript was less abundant than the recA transcript alone. The recX gene was inactivated by gene replacement. The resulting mutant had a clearly diminished colony size, but was not impaired in recombination activity, genetic instability, and resistance against UV irradiation. Expression of an extra copy of the S. lividans recA gene under control of the thiostrepton-inducible tipA promoter was lethal to the recX mutant, demonstrating that RecX is required to overcome the toxic effects of recA overexpression. Since inactivation of the recX gene did not influence transcription of recA, the putative function of the RecX protein might be the downregulation of RecA activity by interaction with the RecA protein or filament.
RecA is a multifunctional protein that is involved in homologous recombination, DNA repair, and the induction of the SOS response (13, 35). The protein is highly conserved among all prokaryotes (12, 23), and homologues of RecA are also found in eukaryotes (2). Transcriptional regulation of recA by the SOS repressor LexA has been well studied in Escherichia coli and Bacillus subtilis (18, 36). Under normal growth conditions, the LexA protein binds to a specific DNA sequence, the SOS box, upstream of the promoter region and inhibits transcription. Following DNA damage, autocleavage of the LexA repressor results in the induction of the respective genes. The SOS box of gram-positive bacteria, GAAC-N4-GTTC/T, differs from the binding site for LexA, CTGT-N8-ACAG, in gram-negative organisms (6, 34).
In streptomycetes, the RecA protein is assumed to be involved in genetic instability, which is a remarkable feature of these mycelium-forming and antibiotic-producing bacteria. Their chromosome is highly unstable under laboratory conditions and can suffer from very large deletions at rates higher than 0.1% (33). Genetic instability affects different phenotypical properties, including morphological differentiation, production of secondary metabolites, such as pigments and antibiotics, antibiotic resistance, secretion of extracellular enzymes, and, sometimes, genes for primary metabolism. A plausible model for a specific role of RecA in ensuring viability has been suggested by Volff and Altenbuchner (32): the occurrence of single-stranded breaks within the chromosome might cause the replication fork to collapse, as was described for E. coli (14). Due to the linearity of the Streptomyces chromosome (15, 16), this would result in the loss of a chromosomal end, and mutants containing large deletions would be segregated. If the cell is recombination proficient, these breaks can be repaired and the chromosomal ends are rescued. In a completely recombination-deficient mutant, the high frequency of deletions might interfere with the viability of the cell.
In many organisms, a gene termed recX was identified downstream of recA (7). In mycobacteria, the recX gene overlaps with the coding region of recA, and the two genes are cotranscribed (24). Overexpression of the wild-type recA gene in a Pseudomonas aeruginosa recA mutant (rec-2) was only tolerated if the recX gene was simultaneously expressed. Therefore, a regulatory role for recX in RecA activity was suggested (26). However, it was not clear whether it controls the expression of the recA gene or interacts directly with the RecA protein (26).
Various attempts have been made to generate recA deletion mutants in streptomycetes. It was only possible to isolate disruption mutants with residual RecA activity (1, 21). Therefore, a crucial role of the recA gene in ensuring the viability of streptomycetes was suggested. However, it could not conclusively be excluded in these experiments that a polar effect on downstream genes (e.g., recX) was responsible for the failure to generate recA-deficient Streptomyces lividans mutants. Such polar effects on recX have also been discussed by Papavinasasundaram et al. (24) to explain the inability to inactivate recA of Mycobacterium smegmatis.
In this paper, we report the transcriptional analysis of the S. lividans recX gene and the construction of a recX gene replacement mutant. The phenotypic characterization of the mutant suggested that RecX downregulates RecA activity by protein-protein interaction to overcome the toxic effects of RecA overexpression.
MATERIALS AND METHODS
Bacterial strains, plasmids, and media.
The E. coli strain used for subcloning and DNA sequencing was XL1-Blue (4). The parental Streptomyces strain was S. lividans TK64. E. coli cells were grown at 37°C in Luria-Bertani (LB) medium (25). Streptomyces strains were cultured as described previously (8). Antibiotics were supplemented, where appropriate, at the following concentrations: ampicillin, 150 μg ml−1; kanamycin, 50 μg ml−1; thiostrepton, 25 μg ml−1; gentamicin, 5 μg ml−1; chloramphenicol, 10 μg ml−1. The plasmids used in this work are listed in Table 1.
TABLE 1.
Plasmids used in this work
| Plasmid | Description | Reference or source |
|---|---|---|
| pUC18 | lacZ bla | 31 |
| pGM11 | aphII; temperature-sensitive Streptomyces vector | 37 |
| pJF293.2 | bla; PtipA | J. Altenbuchner, Stuttgart, Germany |
| pSVX1 | recX replacement plasmid; pGM11 derivative carrying the 1,550-bp PstI-NcoI fragment; tsr insertion within the BclI site | This study |
| pSVQ1 | Recombination test plasmid; pGM11 derivative carrying a 1,316-bp recQ PCR fragment, disrupted by the tsr gene | This study |
| pSVX2 | pGM11 derivative carrying the 1,685-bp SalI fragment encoding RecX and the C-terminal half of RecA | This study |
| pSVAX2 | recX complementation plasmid; pGM11 derivative carrying a 2,346-bp PCR fragment containing the complete recA-recX operon | This study |
| pIJ4123 | Streptomyces His tag expression vector; tsr kan PtipA redD | 30 |
| pSVX-his | pIJ4123 derivative carrying a PCR fragment containing the recX gene | This study |
| p2001/41 | Bifunctional SCP2 derivative, p15a E. coli ori, tsr cat | Unpublished data |
| pEXrecA | recA expression plasmid; p2001/41 derivative; PtipA recA tsr aacC1 | This study |
| pEXR169-H | recA expression plasmid; p2001/41 derivative; PtipA recA(R169-H) | This study |
DNA manipulations.
Standard procedures were performed as described by Hopwood et al. (8) and Sambrook et al. (25). Hybridization used digoxigenin-labeled dUTP and a digoxigenin detection kit (Boehringer, Mannheim, Germany). Gene replacement mutants were selected as described by Wohlleben and Muth (37).
Expression of recX.
The S. lividans recX gene was amplified by PCR with primers 5recX and 3recX (Table 2). Following restriction with NdeI and BamHI, the fragment was inserted into the Streptomyces expression vector pIJ4123 (30), yielding plasmid pSVX-his. In pSVX-his, the recX gene is expressed with an N-terminal His tag under the control of the thiostrepton-inducible tipA promoter (PtipA) (20).
TABLE 2.
Oligonucleotides used for RT-PCR analysis
| Primer | Strand | Oligonucleotide sequence (5′→3′) |
|---|---|---|
| recA1 | + | ATCGAGGTCATCCCGACCGGGTCT |
| recA2 | − | ATGTCGATCAGACCGCCCTCGC |
| recA3 | + | ATCAAGCAGAAGCTGGGCGTCGG |
| recX1 | + | TCCTCGTCGAGGGCCGAGAAGG |
| recX2 | − | CCGTGTCCTCGCCCTCTTCCTCC |
| glnA1 | + | GGCGAGCAGTACTCCCGCGACC |
| glnA2 | − | GTACACCAGGTTCACCGGCGCCTC |
| 5recX | + | GGAATTCCATATGGAGCCGTCCGCCGAGGA |
| 3recX | − | CGGGATCCTCGAGAACCCCTCGTCGCCGAGG |
Preparation of S. lividans RNA.
S. lividans was cultivated in 50 ml of YEME/LB (4+1) (YEME composition given in reference 8) for 2 to 3 days. The culture was induced with methyl methanesulfonate (MMS; 25 μg ml−1) for 20, 40, and 60 min. Cells were harvested and shock frozen at −70°C. An aliquot was resuspended in 100 μl of P-buffer containing 0.33 mg of lysozyme and incubated for 7 min at 37°C. RNA was extracted from uninduced and MMS-induced cultures by using the RNeasy Mini Kit (Qiagen, Hilden, Germany) according to the manufacturer's instructions.
RT-PCR analysis.
RNA prepared from S. lividans was treated with 3 U of RNase-free DNase I (Promega, Mannheim, Germany) and precipitated according to standard protocols (25). The RNA concentration was photometrically determined by using a Genequant fixed-wavelength photometer (Pharmacia, Freiburg, Germany). The reverse transcription reaction was carried out with an enhanced avian reverse transcriptase PCR (RT-PCR) kit (SIGMA, Germany) according to the manufacturer's instructions. A 5-μl aliquot of the RT reaction product was used as a template and amplified with Taq DNA polymerase (Qiagen). The PCR was carried out in a programmable Thermal Controller (MJ Research, Inc.) with the following profile: 1 cycle at 94°C for 120 s and 25 cycles at 94°C for 75 s, 60°C for 90 s, and 72°C for 110 s. The final step was an elongation reaction for 10 min at 72°C. The oligonucleotide primers are listed in Table 2. The PCR products were analyzed by agarose gel electrophoresis (1.0%).
Assay for UV sensitivity.
Spore dilutions of the recX mutant and the corresponding parental strain were plated onto LB agar and irradiated with UV light (Vilber Lourmat, VL115c; 254 nm, 730 μW/cm2) at a distance of 10 cm for various periods (2, 5, 10, 15, and 20 s), followed by incubation in the dark at 30°C for 3 days. Colonies were counted, and the percentage of survival was determined.
Assay for genetic instability.
Chloramphenicol-resistant cultures from the wild type and the recX mutant were incubated for several sporulation cycles. Subsequently, serial dilutions of spores were plated on LB medium without antibiotic at 30°C for 3 days. To determine the frequency of chloramphenicol-sensitive cultures, 1,000 colonies from the wild type and the recX mutant, respectively, were picked in parallel on LB agar without and with chloramphenicol (10 μg ml−1).
Assay for determining recombination activity.
To analyze recombination activity, plasmid pSVQ1, a pGM11 derivative carrying an S. lividans recQ gene fragment disrupted by a thiostrepton resistance cassette, was used. In the recX mutant, S. lividans SVX1, pSVQ1 can integrate into the chromosome by homologous recombination between the recQ fragments (720 and 596 bp) or the thiostrepton resistance gene (1,060 bp). In S. lividans TK64, the plasmid can only integrate within the recQ fragment. pSVQ1 was transferred into S. lividans TK64 and into the mutant SVX1 by polyethylene glycol-mediated protoplast transformation. Equal amounts of transformants were inoculated in liquid culture for 1 day at 28°C and 3 days at 39°C to eliminate the temperature-sensitive plasmid. Subsequently, mycelium was homogenized, and serial dilutions were spread on kanamycin-containing plates and nonselective agar and incubated at 39°C. The titer on kanamycin-containing plates, which indicates plasmid integration in relation to the titer on nonselective agar, gives the integration frequency.
RESULTS
Identification of the S. lividans recX gene.
At 220 bp downstream of the recA stop codon in Streptomyces coelicolor A(3)2 (EMBL accession no. AL020958), an open reading frame with significant similarity to the recX genes of Mycobacterium leprae (43.5% identity, 161-amino-acid [aa] overlap) and P. aeruginosa (28.5%, 147 aa) was identified. To prove the presence of the recX homologue and the conservation of the gene organization in S. lividans, we amplified the corresponding S. lividans fragment with primers deduced from the S. coelicolor sequence. Partial sequence analysis (data not shown) resulted in sequences identical to that of S. coelicolor. The recA-recX intergenic region contains several direct repeats and has the potential to form secondary structures. A hairpin structure with 20 bases in the stem and 7 in the loop (ΔE = −26.2 kJ/mol) which could act as a transcriptional terminator of recA transcription is located 64 bp downstream of the recA stop codon. This putative termination structure is also present downstream of the Streptomyces ambofaciens recA gene (1).
recX is cotranscribed with recA after induction with the DNA-damaging agent MMS.
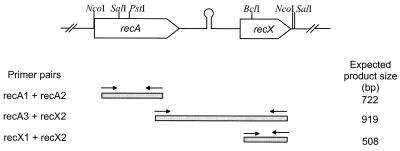
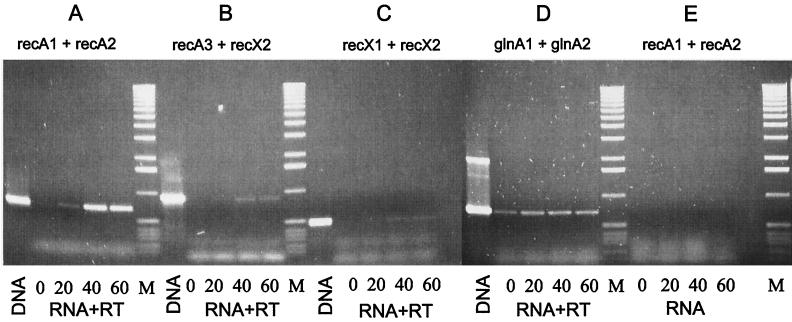
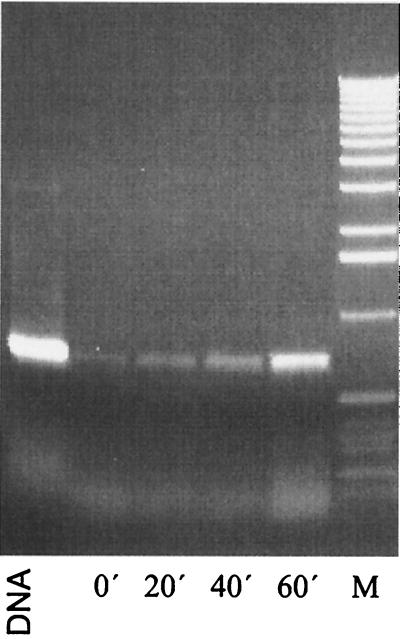
The distance of 220 bp between recA and recX and the putative termination structure downstream of recA suggested that these two genes were transcribed independently in S. lividans. An RT-PCR analysis was performed to assess whether both genes were cotranscribed. Since recA of S. lividans is regulated by the SOS repressor LexA (unpublished results), RNA was isolated after induction with the DNA-damaging agent MMS (11). Primer pairs within recA (recA1 and recA2) and recX (recX1 and recX2) were used to detect the independent transcription of each gene. In order to prove the presence of recA-recX cotranscripts, primers corresponding to the 3′ region of recA (recA3) and recX (recX2) were chosen (Table 2 and Fig. 1). The functionality of the primers for RT-PCR was demonstrated by PCR on genomic DNA as the template (Fig. 2, lane DNA). The absence of contaminating DNA in the RT-PCR was confirmed by a control PCR with RNA as a template (Fig. 2E). From uninduced cultures, no recX transcript and only a weak band indicating basal expression of the recA gene were detected (Fig. 2A to C, lane 1). Twenty minutes after induction with DNA-damaging MMS, the intensity of the recA-specific band increased, demonstrating induction of the recA gene during the SOS response. Transcription of the recX gene, however, was not detectable even 20 min after induction. A recA-recX cotranscript appeared only 40 and 60 min after induction (Fig. 2B, lanes 3 to 4), when expression of recA reached its maximum (Fig. 2A, lanes 3 and 4). This demonstrated that recX was cotranscribed with recA after induction of the SOS response. Probably due to the termination structure between recA and recX, the recA-recX cotranscript was produced only at a low level (less than 10%) compared to the recA transcript.
FIG. 1.
Organization of the S. lividans recA-recX operon. recA and recX are separated by a 220-bp fragment containing a hairpin structure (ΔE = −26.2 kJ/mol). Only relevant restriction sites are given. The primers used in the RT-PCR to identify recA and recX transcripts or recA-recX cotranscripts are indicated by arrows. The expected product sizes formed by the different primer combinations are listed on the right.
FIG. 2.
Transcriptional analysis of the recA-recX operon by RT-PCR. After induction with 25 μg of MMS ml−1, RNA was isolated from S. lividans TK64 at different intervals and used for RT-PCR with different primer combinations. (A) recA-specific primers. (B) recA-recX cotranscript-specific primers. (C) recX-specific primers. (D) Control reaction using glnA-specific primers. (E) Control reaction without RT. Lanes: DNA, control PCR with genomic DNA as template; 0, without induction; 20, 40, and 60, 20, 40, and 60 min after induction, respectively; M, size standard (1-kb ladder [Boehringer]: 12,216, 11,198, 10,180, 9,162, 8,144, 7,126, 6,108, 5,090, 4,072, 3,054, 2,036, 1,636, 1,018, 517, 506, 396, 344, 298, 220, 201, 154, 134, and 75 bp).
As a control for the quality of the RNA preparation, RT-PCR was also performed with a primer pair deduced from the glnA gene (glutamine synthetase I) which is not induced by DNA damage. In contrast to the recA and recA-recX message, the intensity of glnA transcription did not significantly change during MMS induction (Fig. 2D).
Construction of a recX gene replacement mutant in S. lividans TK64.
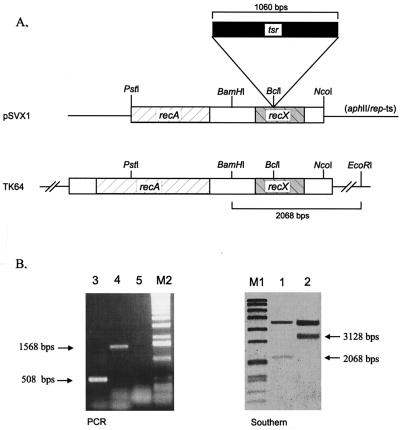
To analyze the role of RecX, we intended to inactivate the recX gene. Therefore, the cloned recX gene was disrupted by the insertion of the thiostrepton resistance gene into the single BclI site located in the N-terminal half of recX. The temperature-sensitive replacement plasmid pSVX1 (Fig. 3A), which carries the disrupted recX gene, was transferred into S. lividans TK64, and colonies were selected with the thiostrepton resistance marker integrated into the chromosome. Subsequently, the colonies were picked on LB agar containing thiostrepton (25 μg ml−1) or kanamycin (50 μg ml−1). One out of 600 colonies was found to be thiostrepton resistant and kanamycin sensitive, indicating gene replacement and plasmid loss.
FIG. 3.
Construction of an S. lividans recX mutant. (A) The recX gene was disrupted by the insertion of the thiostrepton resistance gene tsr into the single BclI site and cloned into the temperature-sensitive pGM11 plasmid, yielding pSVX1. The chromosomal recX copy was replaced via a double-crossover resulting in the mutant SVX1. (B) Using the 1,550-bp PstI-NcoI fragment as a probe and BamHI-EcoRI-digested total DNA of S. lividans TK64 (lane 1) and the mutant SVX1 (lane 2), the correct gene replacement was confirmed by Southern blotting. For PCR, the primers recX1 and recX2 (Fig. 1) which flank the BclI site (lane 3, TK64; lane 4, SVX1) were used. The absence of the replacement plasmid was demonstrated by using internal aphII primers (lane 5). M1, Bio-VII-Marker (Boehringer) (8,576, 7,427, 6,106, 4,899, 3,639, 2,799, 1,953, 1,882, 1,515, 1,482, 1,164, 992, 710, 492, and 359 bp). M2, 1-kb ladder (Boehringer) (12,216, 11,198, 10,180, 9,162, 8,144, 7,126, 6,108, 5,090, 4,072, 3,054, 2,036, 1,636, 1,018, 517, 506, 396, 344, 298, 220, 201, 154, 134, and 75 bp).
To verify the gene replacement, genomic DNA of the mutant was isolated and subjected to Southern blot analysis with a probe encoding the C-terminal part of RecA and the complete RecX. The probe hybridized with a 3,168-bp fragment that is 1,060 bp larger than the fragment in the wild type (Fig. 3B). This increase in size was due to the insertion of the tsr cassette. In addition, the recX genotype was further confirmed by PCR. With primers (recX1 and recX2) flanking the insertion site within recX, a fragment from the mutant was amplified which was 1,060 bp larger than the corresponding wild-type fragment (Fig. 3B).
Phenotype of the S. lividans SVX1 mutant.
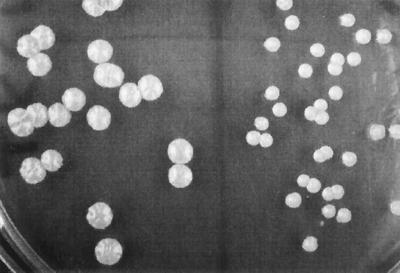
The recX mutant showed normal wild-type morphology on agar plates and in liquid culture. Only when spores were plated on solid medium was the colony size of the mutant clearly reduced (about 30% of the wild-type area) compared to that of S. lividans TK64 (Fig. 4). In order to investigate the effect on RecA-related functions, the UV sensitivity, the ability to undergo homologous recombination, and the genetic instability of the mutant SVX1 were analyzed.
FIG. 4.
Colony size of the S. lividans recX mutant SVX1 compared to that of TK64. Spores were plated on LB agar (left side, S. lividans TK64; right side, SVX1) and incubated at 30°C for 4 days.
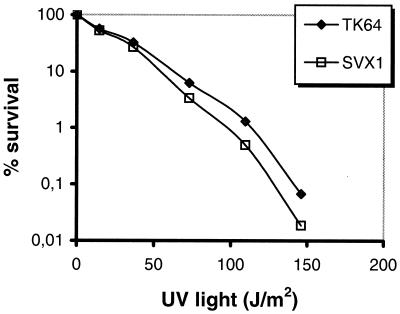
As demonstrated in Fig. 5, the UV sensitivity of SVX1 was not significantly affected, indicating that the mutant is still proficient in recombinational DNA repair and able to induce the SOS response.
FIG. 5.
UV sensitivity of the S. lividans SVX1 mutant. Spores of S. lividans TK64 and the recX mutant SVX1 were plated on LB agar and irradiated with UV light (254 nm, 730 μW/cm2) for various periods.
To test the recombination activity of the SVX1 mutant, the ability to integrate the temperature-sensitive plasmid pSVQ1 (Table 1) into the chromosome was studied as described in Materials and Methods. The plasmid integrated into the SVX1 chromosome with an efficiency of 2 to 10%, similar to that in the parental TK64 chromosome, demonstrating that the recX mutant pSVX1 is recombination proficient and that inactivation of recX does not significantly affect the recombination activity of S. lividans.
The genetic instability of the recX mutant SVX1 was assessed by analyzing the segregation of chloramphenicol-sensitive colonies, which arise by the loss of the chromosomal end containing the chloramphenicol resistance gene (cml). The mutant SVX1 segregated chloramphenicol-sensitive mutants at wild-type frequency (0.8%), thus indicating that genetic instability was also not affected by the inactivation of recX.
The only visible effect of recX inactivation was the small colony size after plating spores on solid medium. To confirm that this phenotype was due to the inactivation of recX and was not caused by an additional mutation, it was necessary to show phenotypic reversal. The S. lividans recX gene was amplified by PCR and cloned into the high-copy expression vector pIJ4123 (30) under control of the thiostrepton-inducible tipA promoter (PtipA) (pSVX-his). On thiostrepton-containing agar, the wild-type colony size of SVX1 carrying pSVX-his was fully restored.
Mutant SVX1 could also be complemented by plasmid pSVAX2 containing the complete recA-recX operon, including the promoter region (Fig. 1). In contrast, plasmid pSVX2, which encoded the complete RecX and the C-terminal half of RecA but lacked a functional promoter sequence, was not sufficient to restore wild-type size.
Overexpression of RecA is toxic in the absence of RecX.
To analyze the effects of recA overexpression in the recX mutant SVX1, the recA expression plasmid pEXrecA, an SCP2 (3) derivative which carried recA under control of the thiostrepton-inducible tipA promoter (20), was constructed. Transformants of S. lividans TK64(pEXrecA) and SVX1(pEXrecA) were grown for 2 days in liquid culture under uninduced conditions. Subsequently the cultures were homogenized, and the mycelial fragments were plated on medium containing thiostrepton (20 μg ml−1) and gentamicin (5 μg ml−1), respectively, to compare the colony titers under induced and noninduced conditions.
For S. lividans TK64(pEXrecA), the colony titer on thiostrepton was about 60% of that on gentamicin-containing plates, indicating the inhibitory effect of recA overexpression. For the recX mutant, however, no single colony could grow on thiostrepton-containing medium. This demonstrated that induction of the tipA promoter resulting in the overexpression of recA was lethal in the absence of RecX in S. lividans.
In contrast, if the recX gene was concomitantly expressed with recA in the recX mutant SVX1(pEXrecA pSVX-his), the colony titers under induced and noninduced conditions were the same (100%). Overexpression of recX allowed the cell to survive recA overexpression. This indicated that the function of RecX might be to counteract the toxicity of RecA.
To confirm that the toxic effects were caused by the biochemical activity of RecA and not by unspecific effects of protein overexpression, we expressed an inactive RecA (R-169H) protein in the mutant. In this mutant, arginine in position 169, which is one of the most conserved amino acid residues in bacterial RecA proteins and which is essential for the function (10), was replaced by a histidine residue (unpublished results). The inactive recA(R-169H) gene was inserted into the expression plasmid 2001/41 under control of the tipA promoter, and the resulting plasmid, pEXR169-H, was transferred into the mutant SVX1. After induction with thiostrepton, 95% of the titer compared to that under noninduced conditions was observed.
Transcription of recA is not influenced in the recX mutant SVX1.
In order to analyze the mode of action of RecX, the influence of RecX on transcription of recA was investigated. RT-PCR with RNA isolated from the mutant SVX1 was performed. Only a faint band indicating the basal transcription of recA was detected without induction. Twenty minutes after administration of MMS, the intensity of the recA-specific band increased, and the maximum of recA transcription was reached after 60 min (Fig. 6). This clearly demonstrated that recA transcription is not significantly enhanced in the absence of RecX. Therefore, a role of RecX in repressing recA transcription is very unlikely.
FIG. 6.
recA transcription in the recX mutant SVX1. Following induction with MMS, the transcription of recA was analyzed by RT-PCR using primers recA1 and recA2 (Fig. 1). Lanes: DNA, control PCR using genomic DNA as a template; 0′, without induction; 20′, 40′, and 60′, 20, 40, and 60 min after induction, respectively; lane M, size standard (1-kb ladder [Boehringer]).
DISCUSSION
The role of RecA in homologous recombination and in the induction of the SOS response has been elucidated in great detail (2, 13). However, only very little is known about the recX gene, which often is cotranscribed with recA and is supposed to be involved in modulating RecA activity (24, 26). By RT-PCR analysis, we showed that, as in other bacteria (7), the S. lividans recX gene is cotranscribed with recA following DNA damage, although the gene organization of the Streptomyces recA region suggested an independent transcription of recX. In contrast to E. coli, in which induction of the SOS response starts 1 min after UV irradiation and is completed after 4 to 5 min (28), expression of the S. lividans recA gene remained unchanged for the first 20 min. Only 40 min after induction, recA transcription reached its maximum. This delay in the induction of the SOS response in Streptomyces is difficult to understand. However, in M. smegmatis and Mycobacterium tuberculosis, the induction of the SOS response was also very slow, and the maximum levels of recA transcription were obtained 5 and 6 h after induction with DNA-damaging agents (19, 24). Simultaneously with the induction of recA, a recA-recX cotranscript appeared that was not detectable without induction. Two distinct transcripts are also formed in the recA-recX operon of P. aeruginosa. By Northern blotting, a 1.2-kb transcript representing the recA message and a 1.4-kb transcript comprising recA and recX were identified (9).
Although recA and recX form an operon in S. lividans, the transcription rates of both genes differ drastically. This is in contrast to M. smegmatis, in which both genes are transcribed with the same efficiency (24). Since the coding region of the M. smegmatis recX overlaps with the 3′ coding region of recA, it makes sense that the recX gene is always transcribed at the same level as recA. In S. lividans (and probably other Streptomyces strains), the weak termination structure between recA and recX might be responsible for transcription of recA without recX in the uninduced state and for the low level of recA-recX cotranscript in comparison to the level of recA transcript alone.
To elucidate the possible function of RecX, a recX mutant was constructed. Our ability to construct a recX mutant clearly showed that the recX gene is dispensable in Streptomyces. Therefore, the failure to inactivate recA (21) must be due to the recA gene itself and is not due to a polar effect on the downstream recX gene, as was discussed for M. smegmatis (24).
Only a very few data are available about the phenotypic effects of recX inactivation. The only published recX mutant represents a recA recX double mutant of M. smegmatis (24). Therefore, this mutant is not appropriate to analyze the function of RecX and its interference with RecA or RecA-related functions. For P. aeruginosa, a recX mutant was generated: in order to determine the coding region of the P. aeruginosa recA gene, several deletion mutants affecting not only recA but also the downstream regions were generated in the chromosome (9). As can now be deduced from the nucleotide sequence (26, 27), a recX-containing fragment has been deleted in mutant PDO7 recAΔ34. This deletion had only very slight effects on UV resistance. The recombination activity of PDO7 recAΔ34 was not analyzed (9).
The S. lividans recX mutant SVX1 was not affected in any of the classical recA-related functions, but the small colony size in comparison to that of the wild type showed that RecX deficiency interferes with normal growth. A more drastic phenotype was observed when recA was overexpressed. Whereas induction of recA expression resulted in the reduction of the colony titer to about 60% in the wild type, indicating the toxic effect of recA overexpression, growth of the recX mutant was completely inhibited. A similar observation was previously published by Sano (26) for P. aeruginosa and Papavinasasundaram et al. (24) for M. smegmatis. In these experiments, however, the authors intended to complement a recA (26) or a recA recX (24) double mutant and showed that recA could only be overexpressed if recX was coexpressed.
Since we could show that recA overexpression is lethal in a recX mutant, one would expect an impaired viability of the recX mutant after UV irradiation. DNA damage caused by UV irradiation should result in the induction of the SOS response and in overexpression of recA (17). Therefore, the recX mutant should not be inhibited directly by the UV irradiation, but due to the toxic action of RecA. However, the recX mutant was not significantly affected under these conditions. Probably, other SOS-induced genes are also involved in protecting the cell from RecA.
The nature of the toxic effect of recA overexpression is unknown. Since the recX mutant tolerated the overexpression of a mutated recA gene encoding an inactive protein, the toxic effects of RecA must be caused by one of the biochemical activities of RecA. The expression of several heterologous RecA proteins, e.g., from P. aeruginosa, B. subtilis, and Deinococcus radiodurans, and RAD51 from Saccharomyces cerevisiae has also been shown to be toxic to E. coli. In these cases, an enhanced affinity for DNA was suggested to be responsible for the toxicity (38). A mutant E. coli RecA(E-96D) protein that was toxic has been shown to prevent proper chromosome segregation (5).
Overexpression of recA was only tolerated in the mutant SVX1 when recX was simultanously highly expressed. In addition, the small colony size of the mutant was also complemented to the wild-type size. Obviously, the N-terminal 20-aa elongation containing the His tag that results from the Streptomyces expression vector pIJ4123 (30) did not substantially interfere with the activity of RecX.
About the mode of action of the RecX protein in controlling RecA activity, only speculations exist. Due to the basic character of the RecX proteins (pI value of about 9 to 11) and the weak similarity to resolvases, a possible function of the P. aeruginosa RecX as a transcriptional repressor of RecA has been discussed (7, 26). However, transcription of recA was not affected in the S. lividans recX mutant. Following induction with MMS, the transcription rate after 60 min was the same as in the wild type. This demonstrates that RecX does not repress transcription of recA. Furthermore, it was shown by immunoblotting that the same amount of RecA protein was produced in the recX mutant as in the wild type (unpublished results). A very similar result was described for P. aeruginosa. Deletion of the recX-containing DNA fragment also did not influence recA transcription or production of RecA protein in P. aeruginosa PDO7 recAΔ34 (9). Because RecX does not affect expression of recA, we propose an interaction of RecX with the RecA protein. This interaction could result in the inhibition of RecA activity to accelerate the shutdown of the SOS response.
Recently, it was suggested by Zaitsev and Kowalczykowski (38) that the function of RecA proteins from distinct bacteria is adapted to the specific needs of a given organism by the modulation of monomer-monomer interaction strength. Since all of the biochemical functions of RecA are directly affected by the DNA binding, an alteration of the binding characteristics might efficiently modulate the specific activity of RecA. The RecX protein might be a candidate protein for controlling RecA. RecX could interact with the highly variable and species-specific C terminus (12) of RecA, which is located at the outer site of the RecA filaments (29), explaining why the RecX proteins from the different bacteria show only low sequence conservation (20 to 43% identity).
ACKNOWLEDGMENTS
We thank J. Altenbuchner for providing plasmids and S. Tropf for reading the manuscript.
This research was supported by the Deutsche Forschungsgemeinschaft (SFB-323) and VCI (163607).
REFERENCES
- 1.Aigle B, Holl A C, Angulo J F, Leblond P, Decaris B. Characterization of two Streptomyces ambofaciens recA mutants: identification of the RecA protein by immunoblotting. FEMS Microbiol Lett. 1997;149:181–187. doi: 10.1111/j.1574-6968.1997.tb10326.x. [DOI] [PubMed] [Google Scholar]
- 2.Baumann P, West S C. Role of the human RAD51 protein in homologous recombination and double-stranded break repair. Trends Biochem Sci. 1998;23:247–251. doi: 10.1016/s0968-0004(98)01232-8. [DOI] [PubMed] [Google Scholar]
- 3.Bibb M J, Hopwood D A. Genetic studies of the fertility plasmid SCP2 and its SCP2* variants in Streptomyces coelicolor A3(2) J Gen Microbiol. 1981;126:427–442. [Google Scholar]
- 4.Bullock W O, Fernandez J M, Short J M. XL1-Blue, a high efficiency plasmid transforming recA Escherichia coli strain with beta galactosidase selection. BioTechniques. 1987;5:376–378. [Google Scholar]
- 5.Campbell M J, Davis R W. Toxic mutations in the recA gene of E. coli prevent proper chromosome segregation. J Mol Biol. 1999;286:417–435. doi: 10.1006/jmbi.1998.2456. [DOI] [PubMed] [Google Scholar]
- 6.Cheo D L, Bayles K W, Yasbin R E. Molecular characterization of regulatory elements controlling expression of the Bacillus subtilis recA+ gene. Biochimie. 1992;74:755–762. doi: 10.1016/0300-9084(92)90148-8. [DOI] [PubMed] [Google Scholar]
- 7.De Mot R, Schoofs G, Vanderleyden J. A putative regulatory gene downstream of recA is conserved in Gram-negative and Gram-positive bacteria. Nucleic Acids Res. 1994;22:1313–1314. doi: 10.1093/nar/22.7.1313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hopwood D A, Bibb M J, Chater K F, Kieser T, Bruton C, Kieser H M, Lydiate D J, Smith C P, Ward J M, Schrempf H. Genetic manipulation of Streptomyces: a laboratory manual. Norwich, United Kingdom: John Innes Foundation; 1985. [Google Scholar]
- 9.Horn J M, Ohman D E. Transcriptional and translational analyses of recA mutant alleles in Pseudomonas aeruginosa. J Bacteriol. 1988;170:1637–1650. doi: 10.1128/jb.170.4.1637-1650.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ishimori K, Sommer S, Bailone A, Takahashi M, Cox M M, Devoret R. Characterization of a mutant RecA protein that facilitates homologous genetic recombination but not recombinational DNA repair: RecA423. J Mol Biol. 1996;264:696–712. doi: 10.1006/jmbi.1996.0670. [DOI] [PubMed] [Google Scholar]
- 11.Janion C, Plewako S, Bebenek K, Sledziewska-Gojs E. Influence of dam and mismatch repair system on mutagenic and SOS-inducing activity of methyl methanesulfonate in Escherichia coli. Mutat Res. 1989;210:15–22. doi: 10.1016/0027-5107(89)90039-0. [DOI] [PubMed] [Google Scholar]
- 12.Karlin S, Brocchieri L. Evolutionary conservation of recA genes in relation to protein structure and function. J Bacteriol. 1996;178:1881–1894. doi: 10.1128/jb.178.7.1881-1894.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kowalczykowski S C, Dixon D A, Eggleston A K, Lauder S D, Rehrauer W M. Biochemistry of homologous recombination in Escherichia coli. Microbiol Rev. 1994;58:401–465. doi: 10.1128/mr.58.3.401-465.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kuzminov A. Collapse and repair of replication forks in Escherichia coli. Mol Microbiol. 1995;16:373–384. doi: 10.1111/j.1365-2958.1995.tb02403.x. [DOI] [PubMed] [Google Scholar]
- 15.Leblond P, Redenbach M, Cullum J. Physical map of the Streptomyces lividans 66 genome and comparison with that of the related strain Streptomyces coelicolor A3(2) J Bacteriol. 1993;175:3422–3429. doi: 10.1128/jb.175.11.3422-3429.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lin Y-S, Kieser H M, Hopwood D A, Chen C W. The chromosomal DNA of Streptomyces lividans 66 is linear. Mol Microbiol. 1993;10:923–933. doi: 10.1111/j.1365-2958.1993.tb00964.x. [DOI] [PubMed] [Google Scholar]
- 17.Little J W. Autodigestion of LexA and phage λ repressors. Proc Natl Acad Sci USA. 1984;81:1375–1379. doi: 10.1073/pnas.81.5.1375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Little J W, Edmiston S H, Pacelli L Z, Mount D W. Cleavage of the Escherichia coli LexA protein by the RecA protease. Proc Natl Acad Sci USA. 1980;77:3225–3229. doi: 10.1073/pnas.77.6.3225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Movahedzadeh F, Colston M J, Davis E O. Determination of DNA sequences required for regulated Mycobacterium tuberculosis RecA expression in response to DNA-damaging agents suggests that two modes of regulation exist. J Bacteriol. 1997;179:3509–3518. doi: 10.1128/jb.179.11.3509-3518.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Murakami T, Holt T G, Thompson C J. Thiostrepton-induced gene expression in Streptomyces lividans. J Bacteriol. 1989;171:1459–1466. doi: 10.1128/jb.171.3.1459-1466.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Muth G, Frese D, Kleber A, Wohlleben W. Mutational analysis of the Streptomyces lividans recA gene suggests that only mutants with residual activity remain viable. Mol Gen Genet. 1997;255:420–428. doi: 10.1007/s004380050514. [DOI] [PubMed] [Google Scholar]
- 22.Muth G, Nußbaumer B, Wohlleben W, Pühler A. A vector system with temperature-sensitive replication for gene disruption and mutational cloning in streptomycetes. Mol Gen Genet. 1989;219:341–348. [Google Scholar]
- 23.Nußbaumer B, Wohlleben W. Identification, isolation and sequencing of the recA gene of Streptomyces lividans TK24. FEMS Microbiol Lett. 1994;118:57–64. doi: 10.1111/j.1574-6968.1994.tb06803.x. [DOI] [PubMed] [Google Scholar]
- 24.Papavinasasundaram K G, Movahedzadeh F, Keer J T, Stoker N G, Colston M J, Davis E O. Mycobacterial recA is cotranscribed with a potential regulatory gene called recX. Mol Microbiol. 1997;24:141–153. doi: 10.1046/j.1365-2958.1997.3441697.x. [DOI] [PubMed] [Google Scholar]
- 25.Sambrook J, Fritsch E F, Maniatis T. Molecular cloning: a laboratory manual. 2nd ed. Cold Spring Harbor, N.Y: Cold Spring Harbor Laboratory Press; 1989. [Google Scholar]
- 26.Sano Y. Role of the recA-related gene adjacent to the recA gene in Pseudomonas aeruginosa. J Bacteriol. 1993;175:2451–2454. doi: 10.1128/jb.175.8.2451-2454.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sano Y, Kageyama M. The sequence and function of the recA gene and its protein in Pseudomonas aeruginosa PAO. Mol Gen Genet. 1987;208:412–419. doi: 10.1007/BF00328132. [DOI] [PubMed] [Google Scholar]
- 28.Sassanfar M, Roberts J W. Nature of the SOS-inducing signal in Escherichia coli. The involvement of DNA replication. J Mol Biol. 1990;212:79–96. doi: 10.1016/0022-2836(90)90306-7. [DOI] [PubMed] [Google Scholar]
- 29.Story R M, Weber I T, Steitz T A. The structure of the E. coli RecA protein monomer and polymer. Nature. 1992;355:318–325. doi: 10.1038/355318a0. [DOI] [PubMed] [Google Scholar]
- 30.Takano E, White J, Thompson C J, Bibb M J. Construction of thiostrepton-inducible, high-copy-number expression vectors for use in Streptomyces spp. Gene. 1995;166:133–137. doi: 10.1016/0378-1119(95)00545-2. [DOI] [PubMed] [Google Scholar]
- 31.Vieira J, Messing J. The pUC plasmids, a M13mp7-derived system for insertion mutagenesis and sequencing with synthetic universal primers. Gene. 1982;19:259–268. doi: 10.1016/0378-1119(82)90015-4. [DOI] [PubMed] [Google Scholar]
- 32.Volff J-N, Altenbuchner J. Influence of disruption of the recA gene on genetic instability and genome rearrangement in Streptomyces lividans. J Bacteriol. 1997;179:2440–2445. doi: 10.1128/jb.179.7.2440-2445.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Volff J N, Altenbuchner J. Genetic instability of the Streptomyces chromosome. Mol Microbiol. 1998;27:239–246. doi: 10.1046/j.1365-2958.1998.00652.x. [DOI] [PubMed] [Google Scholar]
- 34.Walker G C, Marsh L, Dodson L. Cellular responses to DNA damage. Environ Health Perspect. 1985;62:115–117. doi: 10.1289/ehp.8562115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.West S. Enzymes and molecular mechanism of genetic recombination. Annu Rev Biochem. 1992;61:603–640. doi: 10.1146/annurev.bi.61.070192.003131. [DOI] [PubMed] [Google Scholar]
- 36.Winterling K W, Levine A S, Yasbin R E, Woodgate R. Characterization of DinR, the Bacillus subtilis SOS repressor. J Bacteriol. 1997;179:1698–1703. doi: 10.1128/jb.179.5.1698-1703.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Wohlleben W, Muth G. Streptomyces plasmid vectors. In: Hardy K G, editor. Plasmids—a practical approach. 2nd ed. New York, N.Y: Oxford University Press; 1993. pp. 147–175. [Google Scholar]
- 38.Zaitsev E N, Kowalczykowski S C. Enhanced monomer-monomer interactions can suppress the recombination deficiency of the recA142 allele. Mol Microbiol. 1999;34:1–10. doi: 10.1046/j.1365-2958.1999.01552.x. [DOI] [PubMed] [Google Scholar]