Abstract
There is an urgent need for development of drugs that are able to reverse the adverse effects of opioids on breathing and arterial blood-gas (ABG) chemistry while preserving opioid analgesia. The present study describes the effects of bolus injections of N-acetyl-L-cysteine (L-NAC, 500 μmol/kg, IV) on ventilatory parameters, ABG chemistry, Alveolar-arterial (A-a) gradient, sedation (righting reflex) and analgesia status (tail-flick latency assay) in unanesthetized adult male Sprague Dawley rats receiving a continuous infusion of fentanyl (1 μg/kg/min, IV). Fentanyl infusion elicited pronounced disturbances in (1) ventilatory parameters (e.g., decreases in frequency of breathing, tidal volume and minute ventilation), (2) ABG chemistry (decreases in pH, pO2, sO2 with increases in pCO2), (3) A-a gradient (increases that were consistent with reduced alveolar gas exchange), and (4) sedation and analgesia. Bolus injections of L-NAC given 60 and 90 min after start of fentanyl infusion elicited rapid and sustained reversal of the deleterious effects of fentanyl infusion on ventilatory parameters and ABG chemistry, whereas they did not affect the sedative or analgesic effects of fentanyl. Systemic L-NAC is approved for human use, and thus our findings raise the possibility that this biologically active thiol may be an effective compound to combat opioid-induced respiratory depression in human subjects.
Keywords: Fentanyl infusion, Opioid-induced respiratory depression, N-acetyl-L-cysteine, Rats
1. Introduction
The clinical effectiveness of opioid analgesics are often compromised by their adverse effects on breathing [1–6]. Opioid-induced respiratory depression (OIRD) in many cases can be reversed by opioid receptor antagonists, including naloxone, but these antagonists also block the analgesic actions of opioids, which may not be problematic in unexpected overdose situations, however is unwanted when analgesia is required, such as during and immediately following surgery (2–4). Several classes of non-opioid receptor antagonist agents have been investigated as potential OIRD reversal drugs [2–8]. The majority of these OIRD reversal agents did not enter into human clinical trials or have successful outcomes in such trials because of lack of efficacy and/or high degrees of toxicity/side effects [4,5,7,8]. Accordingly, there remains an urgent unmet need to introduce drugs that effectively reverse OIRD by mechanisms independent of opioid receptor blockade. We are currently reporting on the potencies/efficacies of bolus injections of L- and D-thiolesters, such as L-cysteine ethyl ester [9], L-glutathione ethyl ester [10], D-cystine dimethyl ester and D-cystine diethyl ester (11, 12), D-cysteine ethyl ester [12,13],and L-cysteine methyl ester [14] to prevent or reverse the adverse effects of bolus injections of the opioids-morphine and fentanyl-on ventilatory parameters, arterial blood-gas chemistry (pH, pCO2, pO2, sO2), and Alveolar-arterial gradient (i.e., the index of active alveolar gas exchange) in rats while minimally impacting the sedative or analgesic action of the opioids. In all of these studies, the administration of the parent thiols (i.e., L, D-cysteine, L-glutathione, D-cystine) were minimally effective [9–14]. Moreover, our findings that bolus injections of N-acetyl-L-cysteine methyl ester (L-NACme) were unable to reverse the adverse effects of morphine on breathing in unanesthetized rats [11], suggests that the thiolester forms of L- and D-cysteine are active, whereas modifications to the L,D-cysteine backbone prevents activity. Moreover, we have unpublished findings that bolus injections of L-NAC itself (500 μmol/kg, IV) given before or 5 min after bolus injections of morphine (10 mg/kg, IV) or fentanyl (75 μg/kg, IV) have no effects on the ventilatory depressant effects of the opioids. Consistent with our findings, we have found no published evidence that L-NAC beneficially effects opioid-induced respiratory depression (OIRD), whereas it is evident that L-NAC has numerous beneficial effects in experimental animals/animal cell preparations [15–22] and in humans/human cell preparations [23–29]. In truth, we are using L-NAC, which readily enters central and peripheral cells upon systemic/oral ingestion, to (1) provide reducing equivalents, (2) increase the intracellular concentrations of L-cysteine and L-glutathione in order to exert numerous downstream intracellular actions [19,22,30–35], (3) provide a negative control for our active L, D-thiolesters, and (4) discount the above mechanisms of action activated by L-NAC.
Our lab is focused on studying the ability of L,D-thiolester drugs to overcome the ventilatory depressant effects elicited by continuous intravenous infusions of fentanyl in unanesthetized rats. Currently opioid infusions are used in adult and pediatric human patients [36–41] but their ability to provide pain relief is often compromised by their propensity to cause respiratory depression [42–47]. We now report our unexpected findings that intravenous injections of L-NAC reverse the deleterious effects of continuous fentanyl infusion on ventilatory parameters and ABG chemistry, whereas they did not affect the sedative or analgesic effects of the opioid. It therefore appears that continuous infusion of fentanyl somehow sets up a scenario that allows for L-NAC to modulate intracellular signaling cascades the mediated fentanyl-induced OIRD. Systemic/oral administration of L-NAC is approved for human use for a variety of conditions [21,23–26,28–30, 48–51], and our findings raise the possibility that L-NAC could be readily evaluated for potential reversal of OIRD elicited by the infusion of fentanyl in human subjects.
2. Methods
2.1. Permissions, rats, and surgical procedures
All studies were carried out in strict accordance with the NIH Guide for Care and Use of Laboratory Animals (NIH Publication No. 80–23) revised in 1996, and in strict compliance with the ARRIVE (Animal Research: Reporting of In Vivo Experiments) guidelines. All protocols involving the use of rats were approved by the Animal Care and Use Committees of Galleon Pharmaceuticals, the University of Virginia, and Case Western Reserve University. Adult male Sprague Dawley rats were purchased from Harlan Industries (Madison, WI, USA). After five days of recovery from transportation, the rats received two jugular vein catheters placed in the same vein under 2–3% isoflurane anesthesia [10–14, 52]. One of the jugular catheters was to allow for continuous infusion of fentanyl and the other allowed for bolus injections of vehicle or L-NAC. Another group of rats received the jugular vein catheters and a femoral artery catheter as described previously [10–14,52]. The rats were given five days to recover from surgery before use in experiments. All femoral arterial catheters were flushed daily with a heparin solution (50 units of heparin in 0.1 M, pH 7.4 phosphate-buffered saline). The femoral arterial and jugular vein catheters were flushed with 0.3 ml of phosphate-buffered saline (0.1 M, pH 7.4) 2–3 h before the start of the study. The pH of all of the stock solutions of vehicle and L-NAC were adjusted to pH of 7.2 with 0.125 M NaOH. All studies were done in a quiet laboratory room with a relative humidity of 51 ± 2% and room temperature of 21.4 ± 0.2 °C. The ventilatory, ABG chemistry studies, and the antinociception experiments were performed in separate groups of rats so as to not compromise the ventilatory recordings. The plethysmography recordings, antinociception recording sessions, and arterial blood sampling studies (ABG assays) were done by an investigator who injected the opioid, and vehicle or L-NAC. The syringes with vehicle or L-NAC were filled by another investigator, such that the studies were blinded. In addition, the data files resulting from each experiment were collated and analyzed by another investigator in the group.
2.2. Protocols for whole body plethysmography measurement of ventilatory parameters
Ventilatory parameters were recorded continuously in unrestrained freely-moving rats by a whole body plethysmography system (PLY3223; Data Sciences International, St. Paul, MN) as described previously (9–14). The directly recorded and derived parameters [53–56] are defined in Supplemental Table 1 and Supplemental Fig. 1 These parameters and their abbreviations are as follows: frequency of breathing (Freq), tidal volume (TV), minute ventilation (MV), inspiratory time (Ti), expiratory time (Te), Ti/Te, end inspiratory pause (EIP), end expiratory pause (EEP), peak inspiratory flow (PIF), peak expiratory flow (PEF), PIF/PEF, expiratory flow at 50% expired TV (EF50), relaxation time (RT), inspiratory drive (TV/Ti), expiratory drive (TV/Te), apneic pause [(Te/RT) 1] and non-eupneic breathing index (NEBI). On the day of study, each rat was placed in an individual plethysmography chamber and given 60–75 min to acclimatize so that baseline (Pre) ventilatory parameter values could be accurately defined. Two groups of rats (n = 6 per group) received a continuous infusion of fentanyl (1 μg/kg/min, IV) and after 60 min and 90 min, one group of rats received bolus injections of vehicle (saline, IV), whereas the other group of rats received injections of L-NAC (500 μmol/kg, IV). Ventilatory parameters were monitored for a further 30 min following the second injection of vehicle or L-NAC at timepoint 90 min. The body weights of the two groups were similar to one another (Supplemental Table 2) and as such, ventilatory parameters related to volumes such as TV, PIF, PEF and EF50 are presented without correcting for body weight. FinePointe (DSI) software constantly corrected digitized ventilatory values originating from the respiratory waveforms for alterations in chamber humidity and temperature. Corrections of ventilatory parameters for alterations in body temperature were not necessary because temperatures recorded in other groups of rats changed minimally during the study and because these changes were virtually identical in the two groups (Supplemental Table 3). Pressure changes associated with the respiratory waveforms were converted to volumes (e.g., TV, PIF, PEF, EF50) employing the algorithms of Epstein and colleagues [57,58]. Specifically, factoring in chamber humidity and temperature, cycle analyzers filtered the acquired signals, and FinePointe algorithms generated an array of box flow data that identified a waveform segment as an acceptable breath. Flows at this point were “box flow” signals and from this, minimum and maximum box flow values were determined and multiplied by a compensation factor provided by the selected algorithm [57,58] thus producing TV, PIF, PEF and EF50 values that were used to determine non-eupneic breathing events expressed as the non-eupneic breathing index (NEBI), reported as the percentage of non-eupneic breathing events per individual epoch [11–14,59]. Apneic pause was determined by the formula, (Expiratory Time/Relaxation Time) − 1 [11–14,59].
2.3. Protocols for blood gas measurements and determination of Arterial-alveolar gradient
ABG chemistry parameters (pH, pCO2, pO2 and sO2) and A-a gradients were recorded before (Pre), 60 min after continuous infusion of fentanyl (1 μg/kg/min, IV), 30 min after an injection vehicle (n = 9 rats, 83.1 ± 0.4 days of age; 337 ± 3 g) or L-NAC (500 μmol/kg, IV; 83.5 ± 0.3 days; 340 ± 3 g), and again 30 min after a second injection of vehicle or L-NAC (500 μmol/kg, IV) as previously described [10–14,58]. The samples of arterial blood (100 μL) taken at the above times were injected into a Radiometer blood-gas analyzer (ABL800 FLEX) to obtain ABG values. The A-a gradient defines differences between alveolar O2 and arterial blood O2 concentrations (10–14). A reduction in PaO2, without a concomitant alteration in A-a gradient is due to hypo-ventilation, whereas a decrease in PaO2 with a concomitant elevation in A-a gradient indicates an on-going mismatch in ventilation-perfusion in the alveoli [10–14]. A-a gradient = PAO2 − PaO2, where PAO2 is the partial pressure (p) of alveolar O2 and PaO2 is pO2 in the sampled arterial blood. PAO2 = [(FiO2 × (Patm - PH2O) - (PaCO2/respiratory quotient)], where FiO2 is the fraction of O2 in inspired air; Patm is atmospheric pressure; PH2O is the partial pressure of H2O in inspired air; PaCO2 is pCO2 in arterial blood; and respiratory quotient (RQ) is the ratio of CO2 eliminated/O2 consumed. We took FiO2 of room air to be 21% = 0.21, Patm to be 760 mmHg, and PH2O to be 47 mmHg [11]. We took the RQ value of our adult male rats to be 0.9 [10–14,52,60,61].
2.4. Antinociception assessment by tail-flick latency assay
We recorded TFL values before (Pre), 15, 30, 45 and 60 min during continuous infusion of fentanyl (1 μg/kg/min, IV), 15 and 30 min after an injection vehicle (n = 9 rats, 82.2 ± 0.3 days of age; 331 ± 3 g) or L-NAC (500 μmol/kg, IV; 82.7 ± 0.4 days; 333 ± 3 g), and again 15 and 30 min after a second injection of vehicle or L-NAC (500 μmol/kg, IV) via the use of a Tail-Flick Analgesia Meter (IITC Life Science Inc., USA) as detailed previously [10–14,58]. The TFL procedure involved a minor degree of manual restraint while positioning the tail to apply a thermal beam sufficient to induce a latency of tail withdrawal of approximately 2.5 s [10–14,58]. In brief, prior to the infusion of fentanyl, the rats were allowed to crawl inside a canvas garden glove and were lightly restrained within the glove to allow for the thermal withdrawal latencies to be determined. During the infusion of fentanyl, placement of the rat within the glove was helped by the investigator. The 9 points on the tail (Pre and 8 subsequent points) to apply the heat beam were marked by a marker pen ahead of time and were placed in a concentric circle about 1 mm apart from one another to avoid any potential tissue damage. TFL data are shown as actual TFL (sec) and as maximum possible effect (% MPE) determined by the formula, %MPE = [(post-injection TFL − baseline TFL)/(12 − baseline TFL)] × 100 [10–14].
2.5. Body temperatures, and sedation as determined by the modified righting reflex test
We recorded rat body temperatures before (Pre), 30 and 60 min during continuous infusion of fentanyl (1 μg/kg/min, IV), 15 and 30 min after an injection vehicle (n = 9 rats, 83.4 ± 0.4 days of age; 336 ± 3 g) or L-NAC (500 μmol/kg, IV; 83.9 ± 0.4 days; 339 ± 3 g), and again 15 and 30 min after a second injection of vehicle or L-NAC (500 μmol/kg, IV) via the use of a rectal thermometer (DSI Inc., USA) as detailed previously [10–14,58]. The rats were placed in separate open plastic boxes and allowed 45–60 min to acclimatize. A thermistor probe was inserted 5–6 cm into the rectum to allow for regular recordings of body temperature. A 2–3 in. length of the probe cable connected to a tele-thermometer (YellowSprings Instruments, South Burlington, Vermont), was taped to the tail. Note: these rats, along with those rats used in the plethysmography and ABG experiments, were not fasted prior to their use. The ability of the rats to regain their feet and remain on all four paws without immediately returning to their original immobile position (e.g., on their side or splayed out on the bellies) was determined and taken as the modified righting reflex (i.e., no stimulus was provided for them to initiate rising to their four paws).
2.6. Data analyses
Directly recorded and arithmetically-derived ventilatory parameters were grouped into 1 min bins to be taken for statistical analyses. Pre-drug 1 min bins excluded occasional marked deviations from resting values due to abrupt rat movements, such as scratching. All ventilatory, ABG chemistry, A-a gradient, TFL, body temperature, and righting reflex data are presented as mean ± SEM and were evaluated using one-way and two-way ANOVA followed by Bonferroni corrections for multiple comparisons between means using the error mean square terms from each ANOVA analysis [62–65] as detailed previously [10–14]. A P < 0.05 value denoted the initial level of statistical significance that was modified according to the number of comparisons between means as described by Wallenstein et al. [64]. The modified t-statistic is t = (mean group 1 - mean group 2)/[s × (1/n1 + 1/n2)1/2] where s2 = the mean square within groups term from the ANOVA (the square root of this value is used in the modified t-statistic formula) and n1 and n2 are the number of rats in each group under comparison. Based on elementary inequality called Bonferroni’s inequality, a conservative critical value for modified t-statistics obtained from tables of t-distribution using a significance level of P/m, where m is the number of comparisons between groups to be performed [65]. The degrees of freedom are those for the mean square for within group variation from the ANOVA table. The critical Bonferroni value cannot be found in conventional tables of the t-distribution but can be approximated from tables of the normal curve by t * = z + (z + z3)/4 n, with n being the degrees of freedom and z being the critical normal curve value for P/m [62–65]. Wallenstein et al. [64] first demonstrated that the Bonferroni procedure is preferable for general use since it has the widest range of applications, and because it provides critical values that are lower than those of other procedures when the investigator can limit the number of comparisons and will be slightly larger than other procedures if many comparisons are made. As mentioned, a value of P < 0.05 was taken as the initial level of statistical significance [62–64] and statistical analyses were performed with the aid of GraphPad Prism software (GraphPad Software, Inc., La Jolla, CA).
3. Results
3.1. Baseline ventilatory parameters
Descriptions of the ventilatory parameters used in this study are provided in Supplemental Table 1 and Supplemental Fig. 1. There were no differences in baseline (Pre) parameters between the two groups of rats (Supplemental Table 2). Note that the infusion of fentanyl (1 μg/kg/min, IV) and subsequent injections of vehicle or L-NAC (500 umol/kg, IV) exerted minor changes in body temperature (Supplemental Table 3). Therefore, body temperature was not a factor in the expression of the ventilatory responses described below (see Methods for explanation).
3.2. Ventilatory responses
3.2.1. Frequency of breathing, tidal volume and minute ventilation
Actual values of frequency of breathing (Freq), tidal volume (TV) and minute ventilation (MV) before (Pre), and during the infusion of fentanyl (1 μg/kg/min, IV) and subsequent injections of vehicle (VEH) or L-NAC (500 umol/kg, IV) given at the 60 and 90 min infusion timepoints are shown in Fig. 1. The infusion of fentanyl elicited pronounced decreases in Freq, TV and MV that were equal in the two groups. The two injections of vehicle elicited minor immediate responses, and presumably did not affect the temporal effects of fentanyl infusion. In contrast, the first injection of L-NAC (500 μmol/kg, IV) caused immediate and sustained increases in these parameters to levels approaching (Freq and MV) and actually reaching (TV) pre-fentanyl infusion levels. The second injection of L-NAC did not further boost Freq, whereas it did boost TV and therefore MV.
Fig. 1.
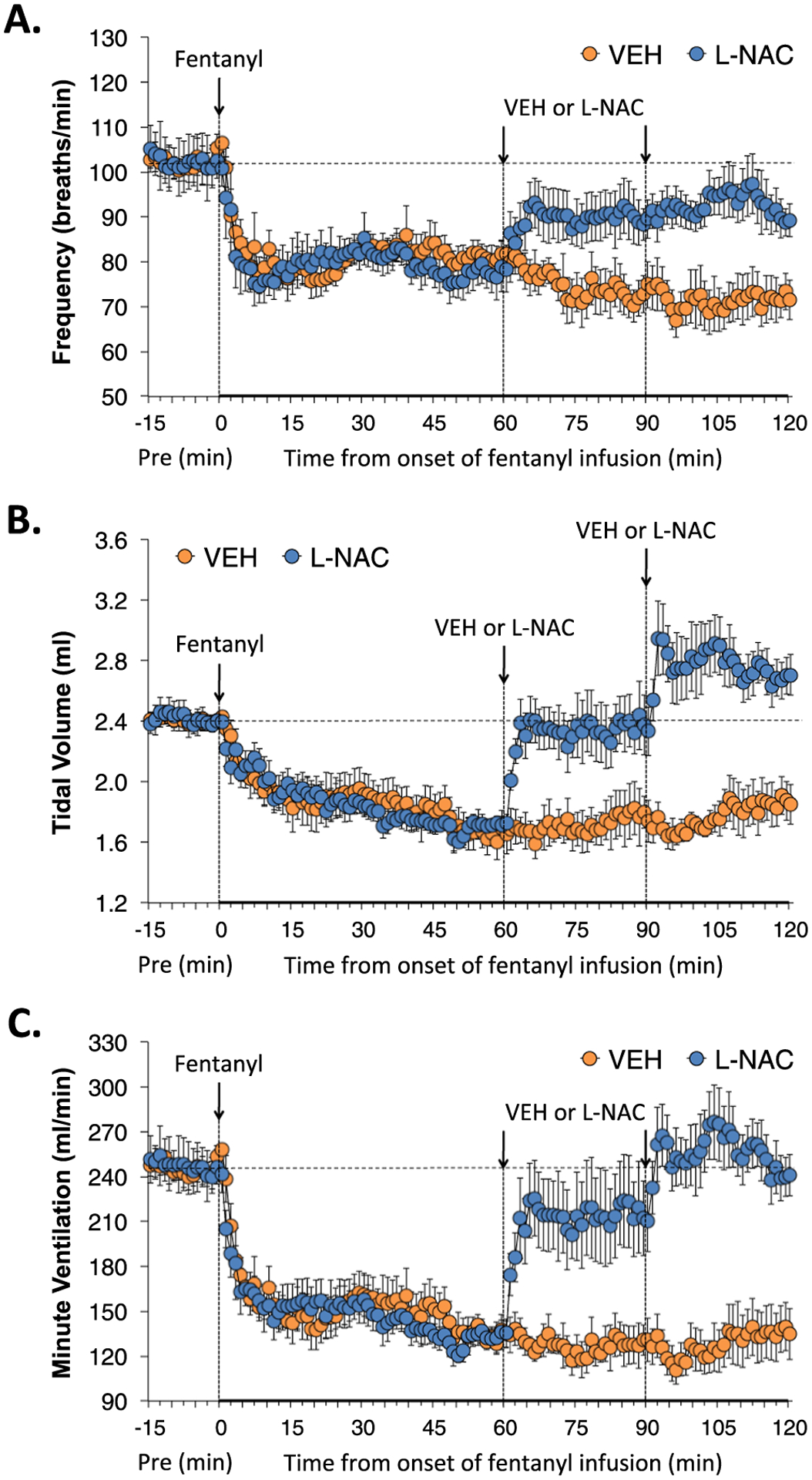
L-NAC reverses the adverse effects of fentanyl infusion on Freq, TV and MV. Actual values of frequency of breathing (Panel A), tidal volume (Panel B) and minute ventilation (Panel C) before (Pre), and during the continuous intravenous infusion of fentanyl (1 μg/kg/min) and following two bolus intravenous injections of vehicle (VEH) or N-acetyl-L-cysteine (L-NAC, 500 μmol/kg) given at 60 and 90 min after start of fentanyl infusion in two groups of unanesthetized rats. There were 6 rats in the VEH group and 6 rats in the L-NAC group. The data are presented as mean ± SEM.
3.2.2. Inspiratory time, expiratory time and inspiratory quotient
Inspiratory time (Ti), expiratory time (Te) and Ti/Te (inspiratory quotient) during various stages of the study are summarized in Fig. 2. The infusion of fentanyl elicited substantial and sustained increases in Ti that were only minimally affected by injections of vehicle or L-NAC (Panel A). As seen in Panel B, the infusion of fentanyl elicited a relatively minor decrease in Te in the VEH group that reversed into an increase in Te at about 45–50 min and was sustained at the latter stages of infusion in the VEH group. The infusion of fentanyl also elicited a relatively minor decrease in Te in the L-NAC group that began to reverse into an increase in Te at about 45–50 min however the L-NAC injections prevented this increase in Te such that the slow downward trajectory of Te during fentanyl infusion was sustained. As such, L-NAC caused a sustained elevation in Ti/Te compared to vehicle-injected rats (Panel C).
Fig. 2.
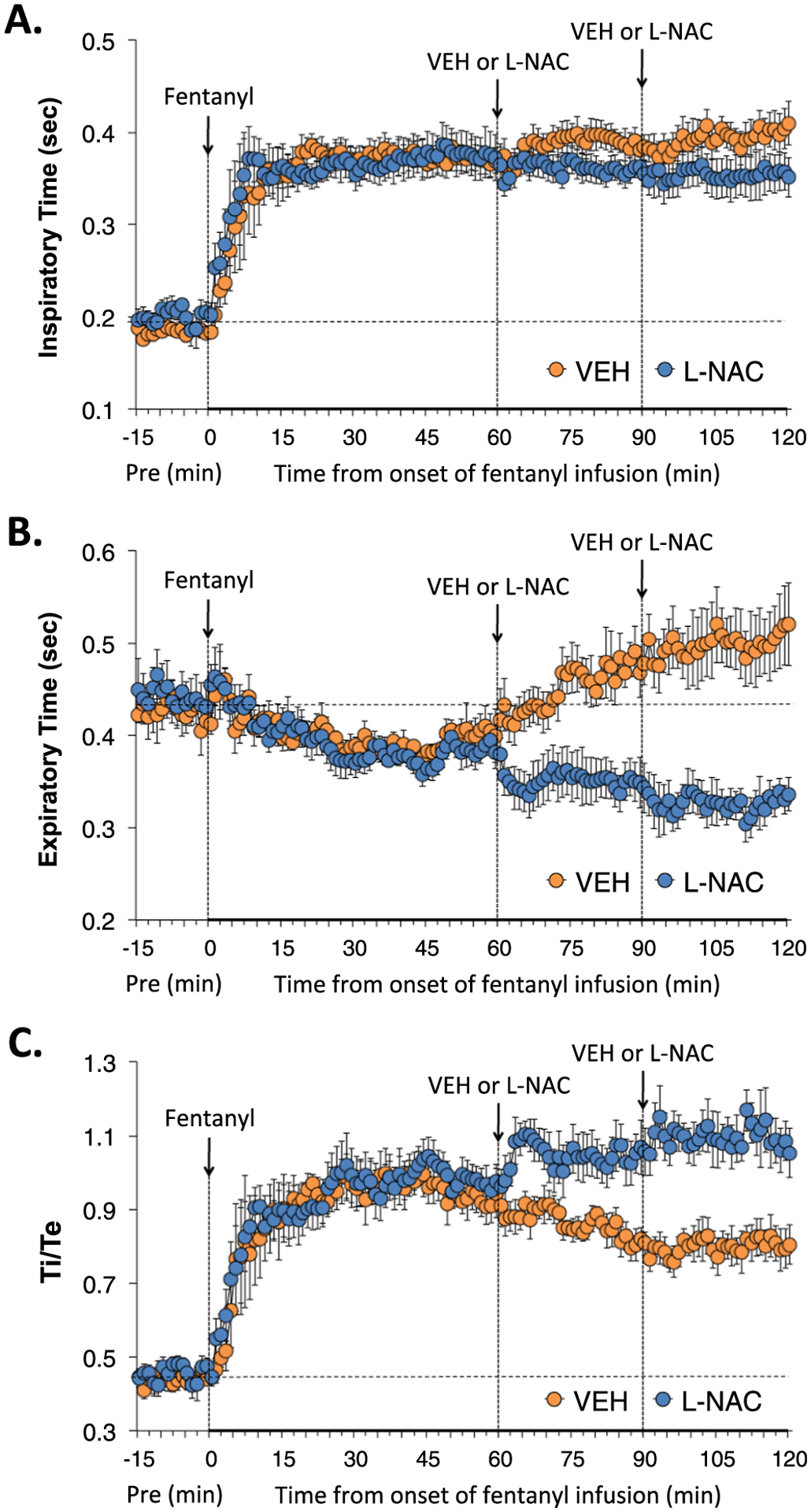
L-NAC reverses the adverse effects of fentanyl infusion on Ti, Te and Ti/Te. Actual values of inspiratory time (Ti) (Panel A), expiratory time (Te) (Panel B) and Ti/Te (Panel C) before (Pre), and during continuous intravenous infusion of fentanyl (1 μg/kg/min) and following two bolus intravenous injections of vehicle (VEH) or N-acetyl-L-cysteine (L-NAC, 500 μmol/kg) given at 60 and 90 min after start of fentanyl infusion in two groups of unanesthetized rats. There were 6 rats in the VEH group and 6 rats in the L-NAC group. The data are presented as mean ± SEM.
3.2.3. End inspiratory pause and end expiratory pause
End inspiratory pause (EIP), and end expiratory pause (EEP) during various stages of the study are summarized in Fig. 3. The infusion of fentanyl elicited a relatively prompt and sustained increase in EIP that was unaffected by the two injections of L-NAC. The infusion of fentanyl did not immediately affect EEP, but rather remarkably EEP rose quickly and markedly beginning about 50 min into the continuous infusion of fentanyl. The first injection of L-NAC elicited a prompt and sustained decrease in EEP, whereas the second injection had no discernible further effect.
Fig. 3.
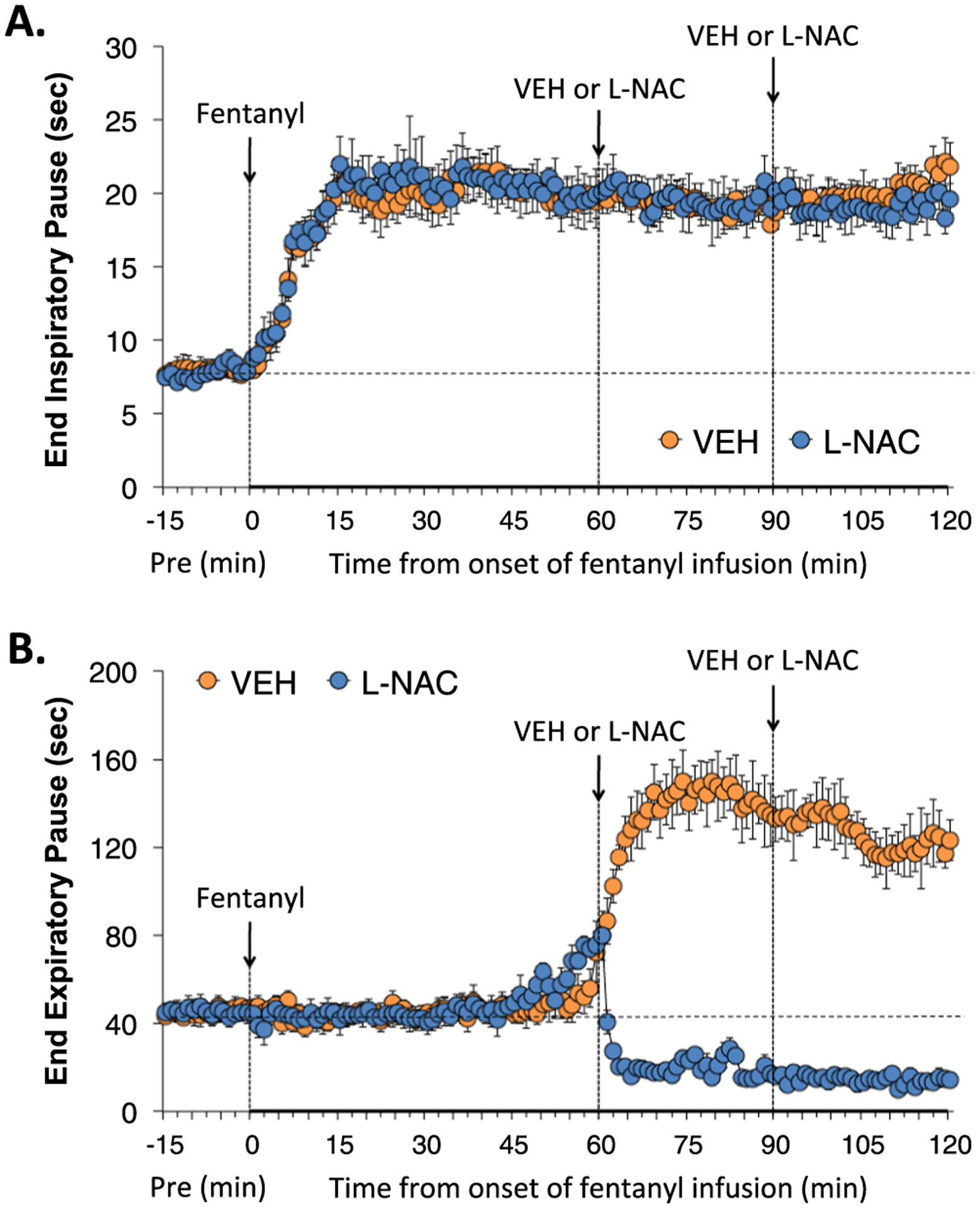
L-NAC reverses the adverse effects of fentanyl infusion on EEP, but not EIP. Actual values of end inspiratory pause (Panel A) and end expiratory pause (Panel B) before (Pre), and during continuous intravenous infusion of fentanyl (1 μg/kg/min) and following two bolus intravenous injections of vehicle (VEH) or N-acetyl-L-cysteine (L-NAC, 500 μmol/kg) given at 60 and 90 min after start of fentanyl infusion in two groups of unanesthetized rats. There were 6 rats in the VEH group and 6 rats in the L-NAC group. The data are presented as mean ± SEM.
3.2.4. Peak inspiratory flow, peak expiratory flow and flow balance
Peak inspiratory flow (PIF), peak expiratory flow (PEF) and PIF/PEF (flow balance) during various stages of the experiment are shown in Fig. 4. The fentanyl infusion elicited a pronounced and sustained decrease in PIF that was unaffected by injections of vehicle, whereas the injections of L-NAC elicited a sustained partial recover of PIF (Panel A). The infusion of fentanyl elicited minor effects on PEF that were unchanged with injections of vehicle. The injections of L-NAC elicited substantial and sustained increases in PEF (Panel B). As a result, the substantial decrease in PIF/PEF during infusion of fentanyl was not affected by the injections of vehicle or L-NAC (Panel C).
Fig. 4.
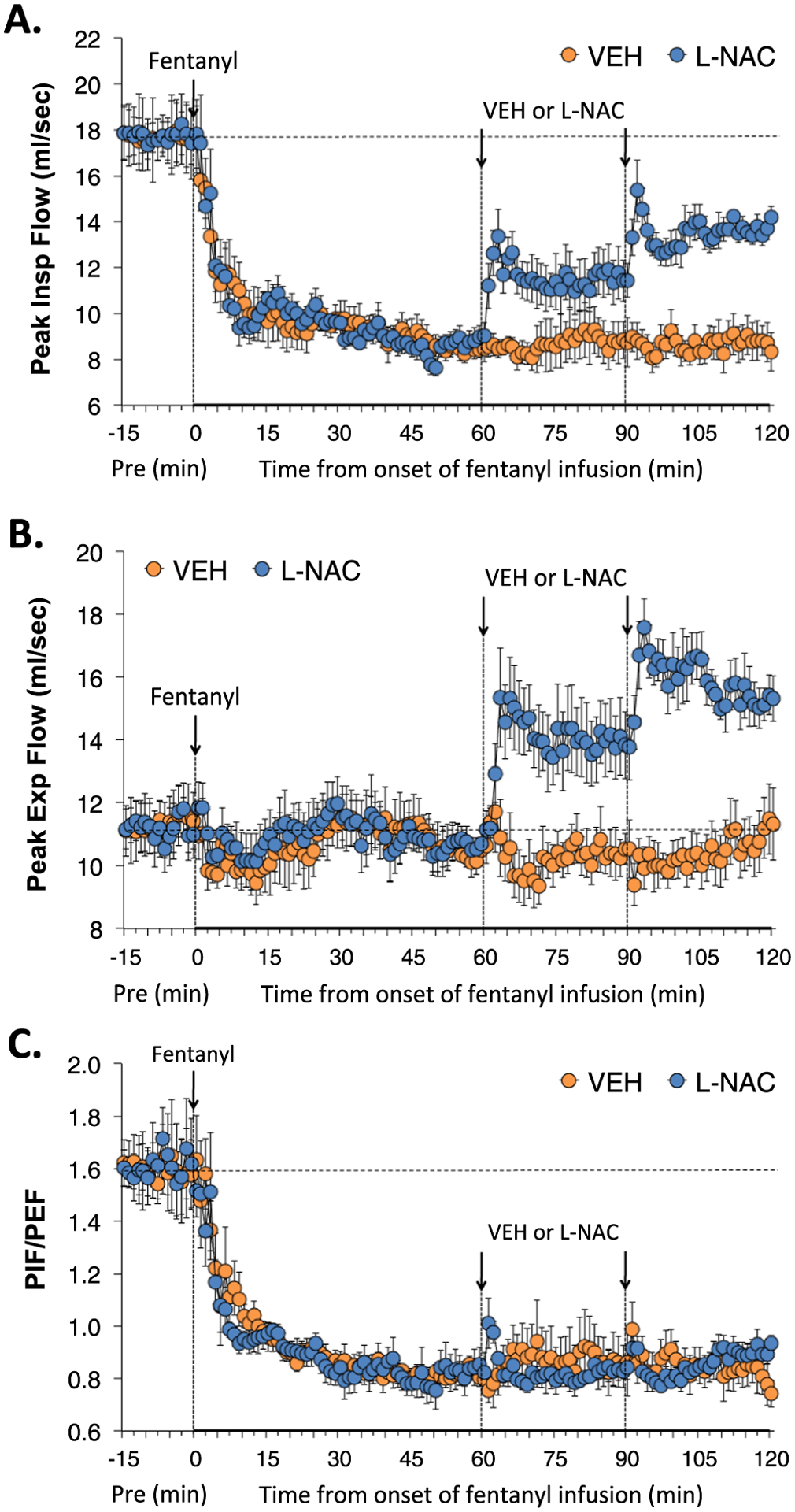
L-NAC reverses the adverse effects of fentanyl infusion on PIF and PEF. Actual values of peak inspiratory flow (PIF) (Panel A), peak expiratory flow (PEF) (Panel B) and PIF/PEF (Panel C) before (Pre), and during continuous intravenous infusion of fentanyl (1 μg/kg/min) and following two bolus intravenous injections of vehicle (VEH) or N-acetyl-L-cysteine (L-NAC, 500 μmol/kg) given at 60 and 90 min after start of fentanyl infusion in two groups of unanesthetized rats. There were 6 rats in the VEH group and 6 rats in the L-NAC group. The data are presented as mean ± SEM.
3.2.5. Inspiratory drive and expiratory drive
Inspiratory (TV/Ti) and expiratory (TV/Te) drives during various stages of the experiment are shown in Fig. 5. The infusion of fentanyl elicited pronounced and sustained decreases in inspiratory drive that were partially reversed by injections of L-NAC (Panel A). The fentanyl infusion elicited gradual and eventually substantial decreases in expiratory drive in the rats that received injections of vehicle, whereas the injections of L-NAC elicited immediate, substantial and sustained increases in expiratory drive that surpassed baseline pre-fentanyl infusion values (Panel B).
Fig. 5.
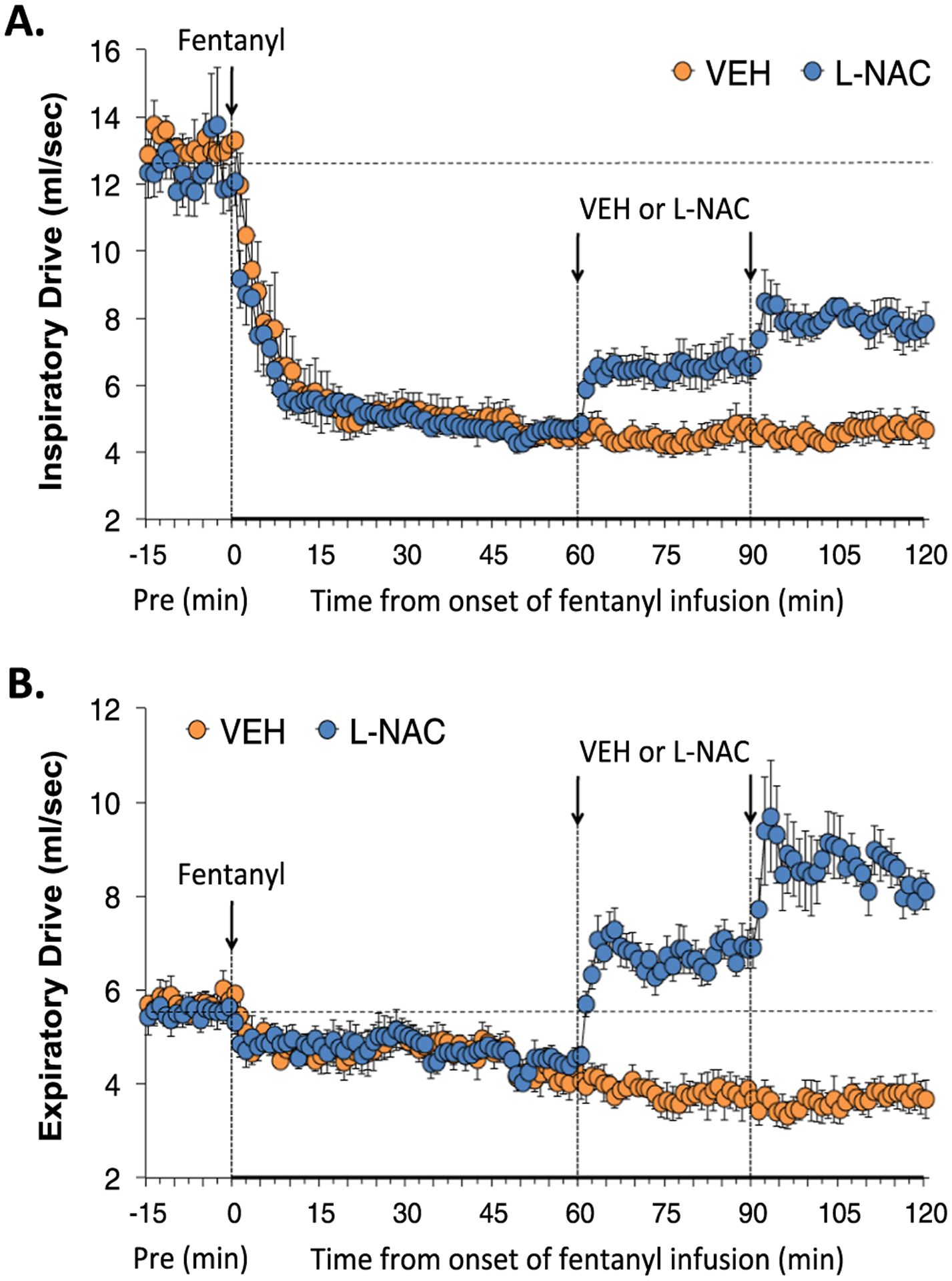
L-NAC reverses the adverse effects of fentanyl infusion on inspiratory and expiratory drives. Acutal values of inspiratory drive (TV/Ti) (Panel A) and expiratory drive (TV/Te) (Panel B) before (Pre), and during continuous intravenous infusion of fentanyl (1 μg/kg/min) and following two bolus intravenous injections of vehicle (VEH) or N-acetyl-L-cysteine (L-NAC, 500 μmol/kg) given at 60 and 90 min after start of fentanyl infusion in two groups of unanesthetized rats. There were 6 rats in the VEH group and 6 rats in the L-NAC group. The data are presented as mean ± SEM.
3.2.6. Relaxation time, expiratory delay, apneic pause, EF50 and NEBI
Relaxation time (RT), expiratory delay (Te-RT) and apneic pause [(Te/RT)−1] during various stages of the experiment are shown in Fig. 6. The infusion of fentanyl elicited a substantial and sustained decrease in RT that was not affected by the injections of vehicle or L-NAC (Panel A). The infusion of fentanyl also caused a sustained increase in expiratory delay (Panel B), and apneic pause (Panel C) that was markedly diminished by the injections of L-NAC. Expiratory flow at 50% tidal volume (EF50) and non-eupneic breathing index (NEBI) during various stages of the study are shown in Supplemental Fig. 2. The fentanyl infusion elicited substantial increases in EF50 (Panel A) that were only minimally affected by injections of L-NAC. The fentanyl infusion did not clearly affect the low level of non-eupneic breathing (Panel B). The first L-NAC injection caused a noticeable decrease in NEBI, whereas the level of NEBI after the second injection of L-NAC was not noticeably different from those that received the second injection of vehicle.
Fig. 6.
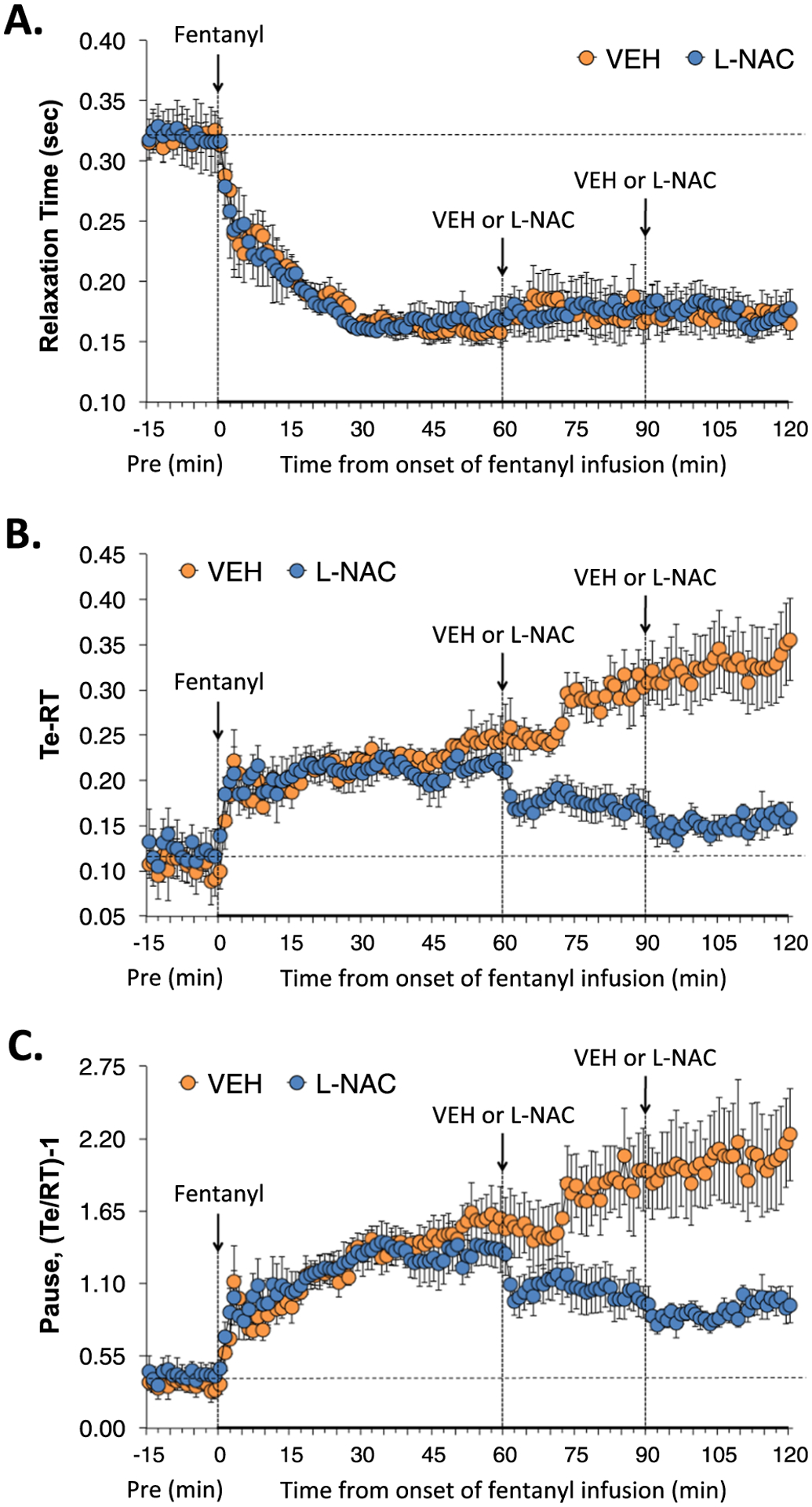
L-NAC reverses adverse effects of fentanyl infusion on expiratory delay and apneic pause. Actual values of relaxation time (RT) (Panel A), expiratory delay (Te-RT) (Panel B) and apneic pause [((Te/RT)−1) (Panel C) before (Pre), and during continuous intravenous infusion of fentanyl (1 μg/kg/min) and after two bolus intravenous injections of vehicle (VEH) or N-acetyl-L-cysteine (L-NAC, 500 μmol/kg) given at 60 and 90 min after start of fentanyl infusion in two groups of unanesthetized rats. There were 6 rats in the VEH group and 6 rats in the L-NAC group. The data are presented as mean ± SEM.
3.2.7. Cumulative ventilatory responses
A summary of the total (i.e., cumulative changes in ventilatory parameters recorded over the first 60 min of fentanyl infusion (1 μg/kg/min, IV)) in the two experimental groups are summarized in Supplemental Table 4. As described above, the infusion of fentanyl elicited pronounced decreases in Freq, TV, MV, PIF, PIF/PEF, RT, inspiratory drive, expiratory drive and NEBI, substantial increases in Ti, Ti/Te, EIP, EF50, apneic pause and RT-Te, and minimal changes in Te, EEP or PEF. Ventilatory parameters at key timepoints in the studies are shown in Supplemental Tables 5 and 6. The timepoints are Pre-values, values at 60 min of infusion (i.e., immediately prior to the first injection of vehicle or L-NAC), values at 90 min of infusion (i.e., 30 min post-injection one and immediately prior to injection two) and at 120 min of fentanyl infusion (i.e., 30 min post-injection two). These tables provide summary data and statistics to support the conclusions above concerning the effects of the infusion of fentanyl on baseline ventilatory parameters and the responses by the two injections of vehicle or L-NAC. A summary of the total (cumulative) changes in ventilatory parameters elicited by the first injection of vehicle or L-NAC (500 μmol/kg, IV) in rats receiving a continuous infusion of fentanyl (1 μg/kg/min, IV) are summarized in Fig. 7. These values are expressed as % changes from Pre-values so as to determine the degree of reversal by L-NAC. As seen in Panel A, the first L-NAC injection reversed the adverse effects of fentanyl on Freq, TV, MV, Te and EEP, but did not affect the fentanyl-induced increases in Ti, Ti/Te or EIP. As seen in Panel B, the first injection of L-NAC only partially reversed the fentanyl-induced decrease in inspiratory drive (InspD), whereas it converted the decrease in expiratory drive (ExpD) to an increase. Additionally, L-NAC elicited a pronounced increase in PEF and significantly decreased NEBI, but did not effect the fentanyl-induced changes in PIF, PIF/PEF and RT. As can be seen in Panel C, the first injection of L-NAC caused a marked reduction in the fentanyl-induced increase in apneic pause and relative expiratory delay (Te-RT). A summary of the total (cumulative) changes in ventilatory parameters elicited by injection two of vehicle or L-NAC (500 μmol/kg, IV) in the rats receiving continuous infusion of fentanyl (1 μg/kg/min, IV) are summarized in Fig. 8 Again these values are expressed as % changes of Pre-values. As seen in Panel A, the second injection of L-NAC reversed the deleterious effects of fentanyl on Freq, TV, MV, Te and EEP, but did not affect the fentanyl-induced changes in Ti, Ti/Te or EIP. As seen in Panel B, the second injection of L-NAC reversed the effects of fentanyl on PIF, PEF, but not PIF/PEF. Moreover, the second injection of L-NAC reversed the effects of fentanyl on inspiratory (InspD) and expiratory (ExpD) drives, but not EF50, RT or NEBI. As seen in Panel C, the second injection of L-NAC caused a substantial reduction in the fentanyl-induced increase in apneic pause and relative expiratory delay (Te-RT).
Fig. 7.
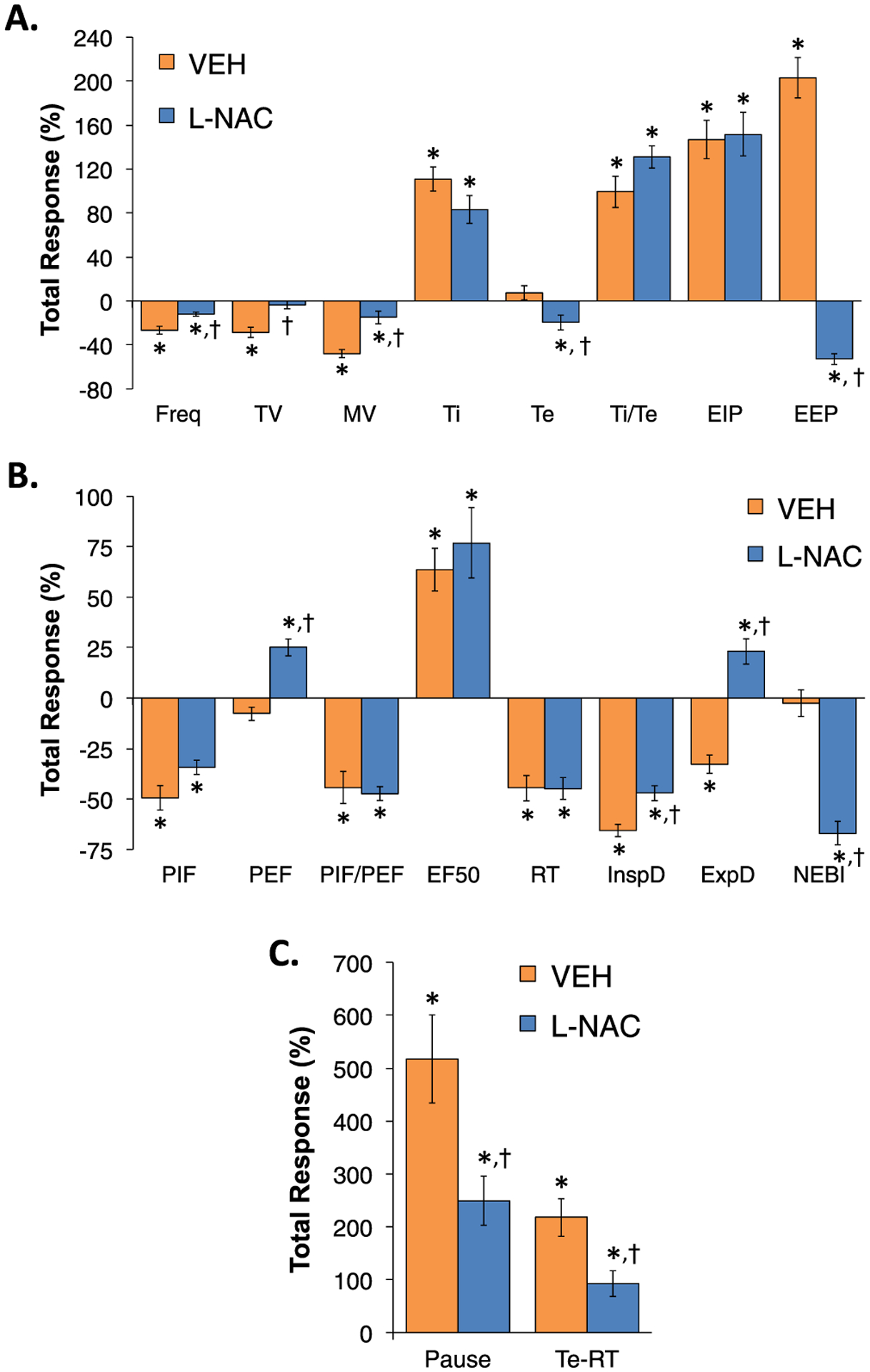
The first injection of L-NAC reverses many adverse effects of continuous fentanyl infusion on ventilatory parameters. A summary of total (cumulative) changes in Freq, TV, MV, Ti, Te, Ti/Te, EIP, EEP (Panel A), PIF, PEF, PIF/PEF, EF50, InspD, ExpD, NEBI (Panel B), and apneic pause and Te-RT (Panel C) during the 30 min period after the first IV injection of vehicle or N-acetyl-L-cysteine (L-NAC, 500 μmol/kg) in rats receiving a continuous infusion of fentanyl (1 μg/kg/min, IV). There were 6 rats in each group. Data are presented as mean ± SEM. *P < 0.05, significant change from Pre-values. †P < 0.05, L-NAC versus Pre.
Fig. 8.
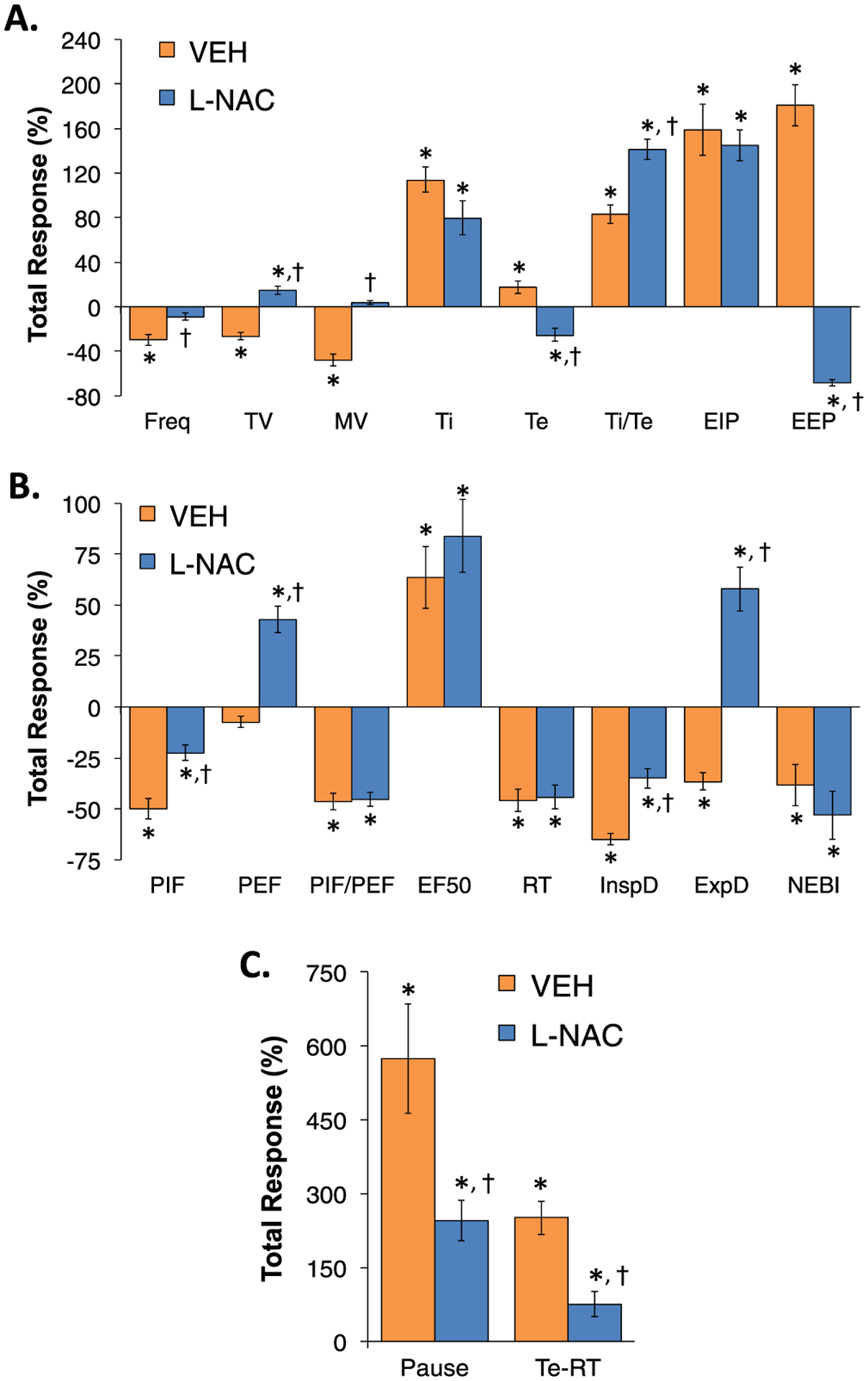
The second injection of L-NAC reverses many adverse effects of continuous fentanyl infusion on ventilatory parameters. A summary of total (cumulative) changes in Freq, TV, MV, Ti, Te, Ti/Te, EIP, EEP (Panel A), PIF, PEF, PIF/PEF, EF50, InspD, ExpD, NEBI (Panel B), and apneic pause and Te-RT (Panel C) during the 30 min period after the second IV injection of vehicle or N-acetyl-L-cysteine (L-NAC, 500 μmol/kg) in rats receiving a continuous infusion of fentanyl (1 μg/kg/min, IV). There were 6 rats in each group. Data are presented as mean ± SEM. *P < 0.05, significant change from Pre-values. †P < 0.05, L-NAC versus Pre.
3.3. Arterial blood gas chemistry
The values for pH, pCO2, pO2, sO2 and A-a gradient at various timepoints during the experiment in the two groups of rats are summarized in Supplemental Table 7. The arithmetic changes in these parameters that occurred at the 60 min timepoint of infusion of fentanyl (1 μg/kg/min, IV) in the two groups of rats, 30 min after the first injection of vehicle or L-NAC (500 μmol/kg, IV), and 30 min after the second injection of vehicle or L-NAC (500 μmol/kg, IV) are summarized in Fig. 9. The infusion of fentanyl elicited profound decreases in pH, pO2 and sO2 after 60 min of infusion that were accompanied by equally pronounced increases in pCO2 and A-a gradient. The two injections of vehicle given 30 min apart did not elicit appreciable changes in these values. In contrast, the first injection of L-NAC elicited a pronounced reversal of the negative effects of fentanyl on all ABG chemistry and A-a gradient values as recorded 30 min after injection. The beneficial effects of L-NAC were still evident 30 min after the second injection of L-NAC.
Fig. 9.
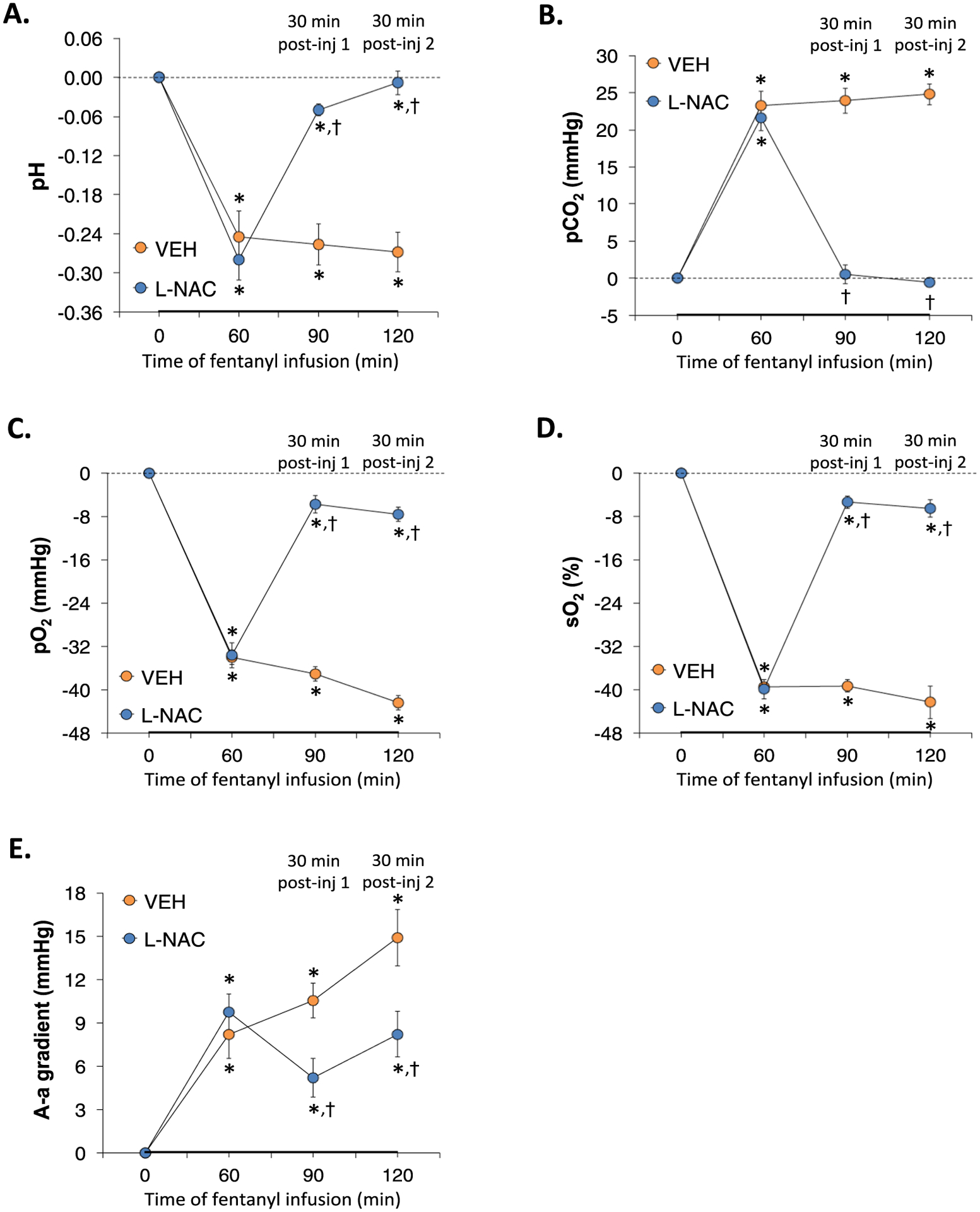
L-NAC reverses the adverse effects of continuous fentanyl infusion on ABG chemistry and A-a gradient. A summary of the arithmetic changes in pH (Panel A), pCO2 (Panel B), pO2 (Panel C), sO2 (Panel D), and Alveolar-arterial (A-a) gradient (Panel E) elicited by continuous infusion of fentanyl (1 μg/kg/min, IV) and bolus IV injections of vehicle or N-acetyl-L-cysteine (L-NAC, 500 μmol/kg) in unanesthetized rats. There were 6 rats in each group. The data are presented as mean ± SEM. *P < 0.05, significant change from Pre-values. †P < 0.05, L-NAC versus Pre.
3.4. Tail-flick latencies
TFL values recorded at various timepoints during the experiments are shown in Table 1. As can be seen, the infusion of fentanyl (1 μg/kg/min, IV) elicited a sustained increase in TFL in the two groups of rats that was evident within 15 min. The IV injections of vehicle or L-NAC (500 μmol/kg) did not affect the antinociceptive effects of the continuous fentanyl infusion.
Table 1.
Antinociceptive status during various timepoints of the experiments.
| Parameters | Study Phase | Injection Phase | Study Group | |
|---|---|---|---|---|
| Vehicle | L-NAC | |||
| Actual TFL values, sec | Pre | 2.2 ± 0.1 | 2.1 ± 0.1 | |
| Post-infusion 15 min | 11.6 ± 0.2 | 11.5 ± 0.1 | ||
| Post-infusion 30 min | 11.9 ± 0.1 | 12.0 ± 0.0 | ||
| Post-infusion 45 min | 11.9 ± 0.1 | 11.9 ± 0.1 | ||
| Post-infusion 60 min | 12 ± 0.0 | 11.9 ± 0.1 | ||
| Post-infusion 75 min | Post-drug injection 1–15 min | 12 ± 0.0 | 11.9 ± 0.1 | |
| Post-infusion 90 min | Post-drug injection 1–30 min | 11.9 ± 0.0 | 12.0 ± 0.0 | |
| Post-infusion 105 min | Post-drug injection 2–15 min | 11.9 ± 0.0 | 11.8 ± 0.1 | |
| Post-infusion 120 min | Post-drug injection 2–30 min | 11.9 ± 0.0 | 11.9 ± 0.1 | |
| Delta changes from Pre, sec | Post-infusion 15 min | 95.7 ± 1.5 * | 95.0 ± 0.9* | |
| Post-infusion 30 min | 99.2 ± 0.8 * | 100 ± 0* | ||
| Post-infusion 45 min | 99.4 ± 0.8 * | 99.1 ± 0.8* | ||
| Post-infusion 60 min | 99.5 ± 0.8 * | 99.0 ± 0.7* | ||
| Post-infusion 75 min | Post-drug injection 1–15 min | 100 ± 0 * | 99.3 ± 0.7* | |
| Post-infusion 90 min | Post-drug injection 1–30 min | 98.7 ± 0.9 * | 100 ± 0* | |
| Post-infusion 105 min | Post-drug injection 2–15 min | 99.2 ± 0.8 * | 98.4 ± 1.1* | |
| Post-infusion 120 min | Post-drug injection 2–30 min | 99.3 ± 0.7 * | 99.2 ± 0.8* | |
TFL, tail-flick latency. L-NAC, N-acetyl-L-cysteine (500 umol/kg, IV). The data are presented as mean ± SEM. There were 9 rats in each group.
P < 0.05, significant change from Pre-values. There were no between group differences at any timepoint (P > 0.05, for all comparisons).
3.5. Righting reflex
Prior to starting the fentanyl infusion (1 μg/kg/min, IV), it was impossible to determine the righting reflex because the rats refused to be put on their side. Upon commencement of the fentanyl infusion, the rats were obviously sedated and it was easy to place them on their side, and at 5 min post start of fentanyl infusion the two groups of rats (group 1 received bolus IV injections of vehicle, group 2 received bolus IV injections of L-NAC) got back on all four paws at 4.2 ± 1.8 min and 4.6 ± 1.5 min, respectively. From 15 min onward, none of the fentanyl-infused rats got back on their paws after being placed on their side (30 min cut-off testing time), regardless of whether they received injections of vehicle or L-NAC.
4. . Discussion
The present study shows that continuous intravenous infusion of fentanyl at 1 μg/kg/min elicited pronounced sedative and anti-nociceptive effects in unanesthetized male Sprague Dawley rats that were not affected by two bolus injections of L-NAC (2 × 500 μmol/kg, IV) given at 60 min and 90 min timepoints during infusion. As such, it is evident that these doses of L-NAC do not sufficiently rise to levels that may directly block opioid receptors or the signaling pathways that mediate these actions of fentanyl [66–68]. In contrast, the findings that injections of L-NAC elicit a pronounced reversal of the adverse effects of continuous fentanyl infusion on ventilatory parameters, ABG chemistry and A-a gradient provide direct evidence that L-NAC interferes with opioid-receptor signaling pathways that elicit the depression of breathing and alveolar gas exchange during continuous exposure to fentanyl. We propose that L-NAC ameliorates these effects by its propensity to alter the redox status or activities of intracellular signaling proteins [19,69,70] rather than direct blockade of opioid receptors, for which there is no direct evidence either way. Of vital interest to us is that the administration of bolus injections of L-NAC either immediately before or immediately after administrations of bolus injections of fentanyl or morphine were completely without effect (unpublished observation). As such, we are surprised at the efficacy of L-NAC in rats receiving the continuous infusion of fentanyl and suspect that the infusion causes intracellular signaling events, including formation of protein complexes, that are susceptible to L-NAC or downstream products, which are not sustained by bolus injections of opioids, and therefore are not affected by bolus injections of L-NAC given minutes afterward.
The present study demonstrates that the continuous infusion of fentanyl elicited marked changes in ventilatory parameters in unanesthetized rats. The pronounced and sustained effects of the 1.0 μg/kg/min fentanyl infusion, which were established within 5 min of starting the infusion, were somewhat surprising since these responses were approximately equal in magnitude to a bolus dose of 75 μg/kg fentanyl in freely-moving rats [10]. We have no detailed explanation for why the effects of the fentanyl infusion were so robust, but it may be possible that continuous occupation of opioid receptors is somehow able to increase the strength of the response noting that the pharmacokinetics of fentanyl, including access to brain sites, would most likely differ remarkably between continuous infusion and bolus injection. Our data shows that fentanyl infusion elicited substantial and sustained decreases in Freq and TV and therefore pronounced reductions in MV. The fentanyl-induced decrease in Freq was associated with sustained increase in Ti whereas Te, which was decreased in the early stages of infusion, gradually reverted to a sustained increase in Te. EIP rose rapidly upon commencement of the fentanyl infusion, whereas EEP abruptly began to rise at about 55 min of infusion. We do not understand any of the reasons for or mechanisms behind this pattern of responses, but it is obvious that fentanyl differentially affected the signaling pathways that control inspiration compared to those controlling expiration. Similarly, the infusion of fentanyl elicited a substantial and sustained decrease in PIF, whereas it minimally affected PEF. The infusion of fentanyl did increase EF50 substantially, thereby promoting this aspect of expiratory mechanics. In addition, whereas fentanyl infusion decreased RT (i.e., it reduced the time to expire 64% of TV), it lengthened the difference in time between the decay of RT and Te, as expressed by greater Te-RT and apneic pause [(RT/Te)−1] values. As would be expected from the changes in TV, Ti and Te, the infusion of fentanyl elicited pronounced decreases in inspiratory drive (TV/Ti) and expiratory drive (TV/Te) most likely because of actions in the brainstem [66, 67]. We have reported that bolus injections of fentanyl (75 μg/kg, IV) markedly increase non-eupneic breathing events (NEBI) in unanesthetized rats due primarily to enhancing the incidence of apneas (10). In contrast, it is evident that the continuous infusion of fentanyl had relatively minimal effects on NEBI. We assume that the levels of fentanyl and potential bioactive metabolites [71] that are reached during the infusion do not obtain those reached upon bolus injection of 75 μg/kg of fentanyl and therefore do not affect central and/or peripheral structures underlying the adverse effects of the synthetic opioid, fentanyl, on NEBI. The changes in ABG chemistry elicited by the fentanyl infusion are consistent with hypoventilation, whereas the increased A-a gradient is also consistent with the opioid causing a decrease in alveolar-gas exchange by mechanisms that likely involve atelectasis (10, 52).
Key findings of the present study include that the bolus injections of L-NAC elicit a rapid and sustained reversal of many of the adverse effects of the fentanyl infusion on breathing. For example, the L-NAC injections reversed the fentanyl-induced depression of Freq, TV and MV, with the enhancement of Freq associated with a profound reversal of the negative effects of fentanyl on Te and EEP, but with minimal effects on the fentanyl-induced increases in Ti or EIP. As such, it appears that L-NAC acts on the systems by which fentanyl infusion depresses TV and specifically affects the fentanyl-sensitive central/peripheral systems that regulate expiratory timing, but not the fentanyl-sensitive systems that control inspiratory timing. As would be expected from the above findings, it was evident that L-NAC reversed the adverse effects of fentanyl infusion on both inspiratory and expiratory drives most likely by acting in brainstem sites expected to participate in the adverse effects of fentanyl on the drives to inhale and exhale. The findings that the injections of L-NAC reversed the negative effects of fentanyl infusion on PIF certainly suggests that L-NAC can reverse the deleterious effects of the fentanyl infusion on ventilatory mechanics associated with inspiration, and perhaps those ventilatory mechanisms involved with the external intercostal muscles. Nonetheless, the pronounced increases in PEF following bolus injections of L-NAC, suggest that L-NAC can drive ventilatory mechanics associated with expiration as well, including, perhaps, the activity of the internal intercostal muscles. In addition, although the injections of L-NAC did not influence the fentanyl-induced decrease in RT, they did produce substantial reductions in the fentanyl-induced increases in Te-RT and apneic pause [(RT/Te) 1] values by specifically diminishing the fentanyl-induced increases in the duration of Te. Although the first injection of L-NAC reduced NEBI in the fentanyl-infused rats, it is difficult to interpret these data because of the relatively minor changes that are involved. Even so, it could be said that L-NAC promotes somewhat the stability of eupneic breathing in the presence of the fentanyl infusion. Finally, the ability of L-NAC to rapidly overcome the adverse effects of the fentanyl infusion on arterial blood pH, pCO2, pO2 and sO2, and A-a gradient is consistent with the L-NAC-induced reversal of the adverse effects of the fentanyl infusion on breathing, and may speak to undefined actions of L-NAC within the lungs if the fentanyl-induced increase in A-a gradient is not simply due to hypoventilation-induced atelectasis.
In summary, this study raises the possibility that L-NAC could be employed to reverse OIRD in human subjects receiving a continuous intravenous infusion of fentanyl, while maintaining the analgesic and sedative actions of the powerful synthetic opioid. L-NAC has been used in numerous clinical trial with various levels of success (48–51). Obviously, the efficacy of L-NAC upon oral or intravenous administration will depend upon numerous factors that affects its bioavailability (e.g., rate of degradation in the blood, liver and/or kidneys, and formation of mixed disulfides), and the rate of entry into cells. Accordingly, we are planning follow-up studies with N-acetyl cysteine amide [72] and L-NAC methyl ester [73], which rapidly and readily enter cells, and with L-D-thiolesters, such as L-cysteine ethyl ester [9], L-glutathione ethyl ester [10], D-cystine dimethyl ester, D-cystine diethyl ester [11,12], D-cysteine ethyl ester [12,13] and L-cysteine methyl ester [14] to see whether these compounds provide an enhanced activity profile compared to L-NAC.
Supplementary Material
Acknowledgements
The authors wish to thank the staff at the animal care facilities at Galleon Pharmaceuticals, Inc., the University of Virginia and Case Western Reserve University for their expert and most caring technical assistance. The authors also wish to thank David Kalergis (CEO, Atelerix Life Sciences) for providing perspectives related to clinical importance of our findings. These studies were funded partially by an NIH/NIDA grant (U01DA051373, Optimization of Novel Thiolesters as a Therapeutic Strategy for Combating Opioid Overdoses and Abuse) to SJL. SJL also received funding from Galleon Pharmaceuticals, Inc. Address correspondence to Stephen J. Lewis, Department of Pediatrics, Division of Pulmonology, Allergy and Immunology. School of Medicine. Case Western Reserve University, 10900 Euclid Avenue, Cleveland, OH 44106-4984. Phone: 843-422-7639. Email: sjl78@case.edu.
Funding
This work was supported by NIH, USA grants 1P01HL101871, 1R61HL154136-01 and U01DA051373.
Footnotes
CRediT authorship contribution statement
Paulina M. Getsy: Conceptualization of study, Analyses of data, Writing - original draft and editing, Writing - review and editing. James N. Bates: Conceptualization of study, Analyses of data, Writing - original draft and editing. Walter J. May: Conceptualization of study, reduction to practice. Investigation - performance of the experiments, Writing - review and editing. Santhosh M. Baby: Conceptualization: reduction to practice. Investigation - performance of experiments, Writing - review and editing. Tristan H.J. Lewis. Investigation - performance of the experiments, Analyses of data, Writing - review and editing. Yee-Hsee Hsieh: Conceptualization of the study, Writing - review and editing. Benjamin Gaston: Conceptualization Writing - original draft and editing Writing - review and editing. Stephen J. Lewis: Conceptualization of the study, Investigation - performance of experiments, Analyses of data, Writing - original draft and editing. Writing - review and editing.
Conflict of interest statement
The leadership of Galleon Pharmaceuticals were not directly involved in this study as a commercial entity. Only the principal scientists of Galleon Pharmaceuticals were involved in study design, collection, analysis, interpretation of data, writing of this article and the decision to submit it for publication. The remaining authors declare that the research described in this manuscript was performed in the absence of commercial or financial relationships that could be construed as a potential conflict of interest. All authors declare no other competing interests.
Appendix A. Supporting information
Supplementary data associated with this article can be found in the online version at doi:10.1016/j.biopha.2022.113277.
Data availability
Data will be made available on request.
References
- [1].van Dorp E, et al. , Naloxone treatment in opioid addiction: the risks and benefits, Expert Opin. Drug Saf 6 (2007) 125–132, 10.1517/14740338.6.2.125. [DOI] [PubMed] [Google Scholar]
- [2].Boom M, et al. , Non-analgesic effects of opioids: opioid-induced respiratory depression, Curr. Pharm. Des 18 (2012) 5994–6004, 10.2174/138161212803582469. [DOI] [PubMed] [Google Scholar]
- [3].Dahan A, et al. , Incidence, reversal, and prevention of opioid-induced respiratory depression, Anesthesiology 112 (2010) 226–338, 10.1097/ALN.0b013e3181c38c25. [DOI] [PubMed] [Google Scholar]
- [4].Dahan A, et al. , Averting opioid-induced respiratory depression without affecting analgesia, Anesthesiology 128 (2018) 1027–1037, 10.1097/ALN.0000000000002184. [DOI] [PubMed] [Google Scholar]
- [5].Algera MH, et al. , Opioid-induced respiratory depression in humans: a review of pharmacokinetic-pharmacodynamic modelling of reversal, Br. J. Anaesth 122 (2019) e168–e179, 10.1016/j.bja.2018.12.023. [DOI] [PubMed] [Google Scholar]
- [6].Arendt F, The opioid-overdose crisis and fentanyl: the role of online information seeking via internet search engines, Health Commun. 36 (2021) 1148–1154, 10.1080/10410236.2020.1748820. [DOI] [PubMed] [Google Scholar]
- [7].van der Schier R, et al. , Opioid-induced respiratory depression: reversal by non-opioid drugs, F1000Prime Rep. 6 (2014) 79, 10.12703/P6-79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Imam MZ, et al. , Countering opioid-induced respiratory depression by non-opioids that are respiratory stimulants, F1000Res. 9:F1000 Fac. Rev (2020) 91, 10.12688/f1000research.21738.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Mendoza J, et al. , L-Cysteine ethyl ester reverses the deleterious effects of morphine on, arterial blood-gas chemistry in tracheotomized rats, Respir. Physiol. Neurobiol 189 (2013) 136–143, 10.1016/j.resp.2013.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Jenkins MW, et al. , Glutathione ethyl ester reverses the deleterious effects of fentanyl on ventilation and arterial blood-gas chemistry while prolonging fentanyl-induced analgesia, Sci. Rep 11 (2021) 6985, 10.1038/s41598-021-86458-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Gaston B, et al. , D-Cystine di(m)ethyl ester reverses the deleterious effects of morphine on ventilation and arterial blood gas chemistry while promoting antinociception, Sci. Rep 11 (2021) 10038, 10.1038/s41598-021-89455-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Getsy PM, et al. , D-Cysteine ethylester and D-cystine dimethylester reverse the deleterious effects of morphine on arterial blood-gas chemistry and Alveolar-arterial gradient in anesthetized rats. Resp. Physiol. Neurobiol 302 (2022) 103912, 10.1016/j.resp.2022.103912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Getsy PM, et al. , D-Cysteine ethyl ester reverses the deleterious effects of morphine on breathing in freely-moving rats while not negating the antinociceptive effects of the opioid, Front Pharmacol. (2022). In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Getsy PM, et al. , L-cysteine methyl ester reverses the deleterious effects of morphine on ventilatory parameters and arterial blood-gas chemistry in unanesthetized rats, Front Pharm. (2022). Submitted for publication. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Bhat RS, et al. , Morphine-induced macrophage apoptosis: oxidative stress and strategies for modulation, J. Leukoc. Biol 75 (2004) 1131–1138, 10.1189/jlb.1203639. [DOI] [PubMed] [Google Scholar]
- [16].Zhou W, Kalivas PW, N-acetylcysteine reduces extinction responding and induces enduring reductions in cue- and heroin-induced drug-seeking, Biol. Psychiatry 63 (2008) 338–340, 10.1016/j.biopsych.2007.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Trivedi MS, Deth R, Redox-based epigenetic status in drug addiction: a potential contributor to gene priming and a mechanistic rationale for metabolic intervention, Front Neurosci. 8 (2015) 444, 10.3389/fnins.2014.00444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Estrada JA, et al. , Opioid receptor (DOR) signaling and reactive oxygen species (ROS) mediate intermittent hypoxia induced protection of canine myocardium, Basic Res Cardiol. 111 (2016) 17, 10.1007/s00395-016-0538-5. [DOI] [PubMed] [Google Scholar]
- [19].Liu Y, et al. , N-acetyl-cysteine attenuates remifentanil-induced postoperative hyperalgesia via inhibiting matrix metalloproteinase-9 in dorsal root ganglia, Oncotarget 8 (2017) 16988–17001, 10.18632/oncotarget.15217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Hodebourg R, et al. , Heroin seeking becomes dependent on dorsal striatal dopaminergic mechanisms and can be decreased by N-acetylcysteine, Eur. J. Neurosci 50 (2019) 2036–2044, 10.1111/ejn.13894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Famitafreshi H, Karimian M, Reduction of anxiety level is associated with an oxidative-stress imbalance in the hippocampus in morphine administration period in male rats, J. Addict. Dis 38 (2020) 64–70, 10.1080/10550887.2020.1717281. [DOI] [PubMed] [Google Scholar]
- [22].Ali M, et al. , N-acetyl-L-cysteine ameliorates mitochondrial dysfunction in ischemia/reperfusion injury via attenuating Drp-1 mediated mitochondrial autophagy, Life Sci. 293 (2022), 120338, 10.1016/j.lfs.2022.120338. [DOI] [PubMed] [Google Scholar]
- [23].Grant JE, et al. , A double-blind, placebo-controlled study of N-acetyl cysteine plus naltrexone for methamphetamine dependence, Eur. Neuropsychopharmacol 20 (2010) 823–828, 10.1016/j.euroneuro.2010.06.018. [DOI] [PubMed] [Google Scholar]
- [24].Saify K, et al. , Down-regulation of antioxidant genes in human SH-SY5Y cells after treatment with morphine, Life Sci. 144 (2016) 26–29, 10.1016/j.lfs.2015.11.014. [DOI] [PubMed] [Google Scholar]
- [25].Duailibi MS, et al. , N-acetylcysteine in the treatment of craving in substance use disorders: Systematic review and meta-analysis, Am. J. Addict 26 (2017) 660–666, 10.1111/ajad.12620. [DOI] [PubMed] [Google Scholar]
- [26].Khalefa HG, et al. , Evaluation of the effect of N-acetylcysteine on the prevention and amelioration of paclitaxel-induced peripheral neuropathy in breast cancer patients: a randomized controlled study, Breast Cancer Res. Treat 183 (2020) 117–125, 10.1007/s10549-020-05762-8. [DOI] [PubMed] [Google Scholar]
- [27].Mulkens CE, et al. , Postoperative pain reduction by pre-emptive N-acetylcysteine: an exploratory randomized controlled clinical trial, Reg. Anesth. Pain. Med 46 (2021) 960–964, 10.1136/rapm-2021-102884. [DOI] [PubMed] [Google Scholar]
- [28].Smaga I, et al. , N-acetylcysteine in substance use disorder: a lesson from preclinical and clinical research, Pharm. Rep 73 (2021) 1205–1219, 10.1007/s43440-021-00283-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Martinez-Banaclocha M, N-acetyl-cysteine: modulating the cysteine redox proteome in neurodegenerative diseases, Antioxid. (Basel) 11 (2022) 416, 10.3390/antiox11020416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Atkuri KR, et al. , N-Acetylcysteine - a safe antidote for cysteine/glutathione deficiency, Curr. Opin. Pharm 7 (2007) 355–359, 10.1016/j.coph.2007.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Dodd S, et al. , N-acetylcysteine for antioxidant therapy: pharmacology and clinical utility, Expert Opin. Biol. Ther 8 (2008) 1955–1962, 10.1517/14728220802517901. [DOI] [PubMed] [Google Scholar]
- [32].Yolland COB, et al. , Improvement of cognitive function in schizophrenia with N-acetylcysteine: a theoretical review, Nutr. Neurosci 23 (2020) 139–148, 10.1080/1028415X.2018.1478766. [DOI] [PubMed] [Google Scholar]
- [33].Bridgeman MM, et al. , Cysteine and glutathione concentrations in plasma and bronchoalveolar lavage fluid after treatment with N-acetylcysteine, Thorax 46 (1991) 39–42, 10.1136/thx.46.1.39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Alnahdi A, et al. , N-acetyl cysteine attenuates oxidative stress and glutathione-dependent redox imbalance caused by high glucose/high palmitic acid treatment in pancreatic Rin-5F cells, PLoS One 14 (2019), e0226696, 10.1371/journal.pone.0226696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Aldini G, et al. , N-Acetylcysteine as an antioxidant and disulphide breaking agent: the reasons why, Free Radic. Res 52 (2018) 751–762, 10.1080/10715762.2018.1468564. [DOI] [PubMed] [Google Scholar]
- [36].Scholz J, et al. , Clinical pharmacokinetics of alfentanil, fentanyl and sufentanil. An update, Clin. Pharm 31 (1996) 275–292, 10.2165/00003088-199631040-00004. [DOI] [PubMed] [Google Scholar]
- [37].Feltracco P, et al. , Pain control after liver transplantation surgery, Transpl. Proc 46 (2014) 2300–2307, 10.1016/j.transproceed.2014.07.023. [DOI] [PubMed] [Google Scholar]
- [38].Srinivasan V, et al. , Conversion from prolonged intravenous fentanyl infusion to enteral methadone in critically ill children, World J. Clin. Pedia 6 (2017) 110–117, 10.5409/wjcp.v6.i2.110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Diwan S, Nair A, A retrospective study comparing analgesic efficacy of ultrasound-guided serratus anterior plane block versus intravenous fentanyl infusion in patients with multiple rib fractures, J. Anaesthesiol. Clin. Pharm 37 (2021) 411–415, 10.4103/joacp.JOACP_349_19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Venkatraman R, et al. , Comparison of low dose intravenous fentanyl and morphine infusion for postoperative analgesia in spine fusion surgeries - a randomized control trial, Braz. J. Anesthesiol 71 (2021) 339–344, 10.1016/j.bjane.2020.12.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Krancevich NM, et al. , Impact of opioid administration in the intensive care unit and subsequent use in opioid-naive patients, Ann. Pharm 56 (2022) 52–59, 10.1177/10600280211016856. [DOI] [PubMed] [Google Scholar]
- [42].Holley FO, van Steennis C, Postoperative analgesia with fentanyl: pharmacokinetics and pharmacodynamics of constant-rate i.v. and transdermal delivery, Br. J. Anaesth 60 (1988) 608–613, 10.1093/bja/60.6.608. [DOI] [PubMed] [Google Scholar]
- [43].Ellis DJ, Millar WL, Reisner LS, A randomized double-blind comparison of epidural versus intravenous fentanyl infusion for analgesia after cesarean section, Anesthesiology 72 (1990) 981–986, 10.1097/00000542-199006000-00006. [DOI] [PubMed] [Google Scholar]
- [44].Vaughn PR, Townsend SF, Thilo EH, McKenzie S, Moreland S, Denver KK, Comparison of continuous infusion of fentanyl to bolus dosing in neonates after surgery, J. Pediatr. Surg 31 (1996) 1616–1623, 10.1016/s0022-3468(96)90033-0. [DOI] [PubMed] [Google Scholar]
- [45].Joshi GP, et al. , A comparison of the remifentanil and fentanyl adverse effect profile in a multicenter phase IV study, J. Clin. Anesth 14 (2002) 494–499, 10.1016/s0952-8180(02)00404-x. [DOI] [PubMed] [Google Scholar]
- [46].Kurihara Y, et al. , Efficacy and complications of fentanyl intravenous infusions in postoperative pediatric patients, Masui 57 (2008) 1414–1420. [PubMed] [Google Scholar]
- [47].Cao X, et al. , Fentanyl-induced respiratory depression is attenuated in pregnant patients, Drug Des. Devel Ther 11 (2017) 3325–3332, 10.2147/DDDT.S147304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [48].Millea PJ, N-acetylcysteine: multiple clinical applications, Am. Fam. Physician 80 (2009) 265–269. [PubMed] [Google Scholar]
- [49].Samuni Y, et al. , The chemistry and biological activities of N-acetylcysteine, Biochim Biophys. Acta 1830 (2013) 4117–4129, 10.1016/j.bbagen.2013.04.016. [DOI] [PubMed] [Google Scholar]
- [50].Bavarsad Shahripour R, et al. , N-acetylcysteine (NAC) in neurological disorders: mechanisms of action and therapeutic opportunities, Brain Behav. 4 (2014) 108–122, 10.1002/brb3.208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [51].Tenório MCDS, et al. , N-acetylcysteine (NAC): impacts on human health, Antioxid. (Basel) 10 (2021) 967, 10.3390/antiox10060967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [52].Henderson F, et al. , Role of central and peripheral opiate receptors in the effects of fentanyl on analgesia, ventilation and arterial blood-gas chemistry in conscious rats, Respir. Physiol. Neurobiol 191 (2014) 95–105, 10.1016/j.resp.2013.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [53].Hamelmann E, et al. , Noninvasive measurement of airway responsiveness in allergic mice using barometric plethysmography, Am. J. Respir. Crit. Care Med 156 (1997) 766–775, 10.1164/ajrccm.156.3.9606031. [DOI] [PubMed] [Google Scholar]
- [54].Lomask M, Further exploration of the Penh parameter, Exp. Toxicol. Pathol 57 (Suppl 2) (2006) 13–20, 10.1016/j.etp.2006.02.014. [DOI] [PubMed] [Google Scholar]
- [55].Tsumuro T, et al. , Nasal congestion model in Brown Norway rats and the effects of some H1-antagonists, Int Immunopharmacol. 6 (2006) 759–763, 10.1016/j.intimp.2005.11.009. [DOI] [PubMed] [Google Scholar]
- [56].Quindry JC, et al. , Plethysmography measurements of respiratory function in conscious unrestrained mice, J. Physiol. Sci 66 (2016) 157–164, 10.1007/s12576-015-0408-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [57].Epstein MA, Epstein RA, A theoretical analysis of the barometric method for measurement of tidal volume, Respir. Physiol 32 (1978) 105–120, 10.1016/0034-5687(78)90103-2. [DOI] [PubMed] [Google Scholar]
- [58].Epstein RA, et al. , Practical implementation of the barometric method for measurement of tidal volume, J. Appl. Physiol 49 (1980) 1107–1115, 10.1152/jappl.1980.49.6.1107. [DOI] [PubMed] [Google Scholar]
- [59].Getsy PM, et al. , Enhanced non-eupneic breathing following hypoxic, hypercapnic or hypoxic-hypercapnic gas challenges in conscious mice, Respir. Physiol. Neurobiol 204 (2014) 147–159, 10.1016/j.resp.2014.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [60].Stengel A, et al. , Central injection of the stable somatostatin analog ODT8-SST induces a somatostatin2 receptor-mediated orexigenic effect: role of neuropeptide Y and opioid signaling pathways in rats, Endocrinology 151 (2010) 4224–4235, 10.1210/en.2010-0195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [61].Chapman CD, et al. , Paraventricular nucleus anandamide signaling alters eating and substrate oxidation, Neuroreport 23 (2012) 425–429, 10.1097/WNR.0b013e32835271d1. [DOI] [PubMed] [Google Scholar]
- [62].Ludbrook J, Multiple comparison procedures updated, Clin. Exp. Pharm. Physiol 25 (1998) 1032–1037, 10.1111/j.1440-1681.1998.tb02179.x. [DOI] [PubMed] [Google Scholar]
- [63].McHugh ML, Multiple comparison analysis testing in ANOVA, Biochem. Med. (Zagreb) 21 (2011) 203–209, 10.11613/bm.2011.029. [DOI] [PubMed] [Google Scholar]
- [64].Wallenstein S, et al. , Some statistical methods useful in circulation research, Circ. Res 47 (1980) 1–9, 10.1161/01.res.47.1.1. [DOI] [PubMed] [Google Scholar]
- [65].Winer BJ, Statistical principles of experimental design, McGraw-Hill Book Co, 1971, pp. 752–809. [Google Scholar]
- [66].Dahan A, et al. , Incidence, reversal, and prevention of opioid-induced respiratory depression, Anesthesiology 112 (2010) 226–338, 10.1097/ALN.0b013e3181c38c25. [DOI] [PubMed] [Google Scholar]
- [67].Bateman JT, et al. , Understanding and countering opioid-induced respiratory depression, Br. J. Pharm (2022), 10.1111/bph.15580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [68].Williams JT, et al. , Regulation of μ-opioid receptors: desensitization, phosphorylation, internalization, and tolerance, Pharm. Rev 65 (2013) 223–254, 10.1124/pr.112.00594243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [69].Lee J, et al. , Morphine prevents glutamate-induced death of primary rat neonatal astrocytes through modulation of intracellular redox, Immunopharmacol. Immunotoxicol 26 (2004) 17–28, 10.1081/iph-120029941. [DOI] [PubMed] [Google Scholar]
- [70].Blanco-Ayala T, et al. , N-acetylcysteine inhibits kynurenine aminotransferase II, Neuroscience 444 (2020) 160–169, 10.1016/j.neuroscience.2020.07.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [71].Patel JC, Parveen S, In vitro and in vivo analysis of fentanyl and fentalog metabolites using hyphenated chromatographic techniques: a review, Chem. Res. Toxicol 35 (2022) 30–42, 10.1021/acs.chemrestox.1c00225. [DOI] [PubMed] [Google Scholar]
- [72].Sunitha K, et al. , N-Acetylcysteine amide: a derivative to fulfill the promises of N-acetylcysteine, Free Radic. Res 47 (2013) 357–367, 10.3109/10715762.2013.781595. [DOI] [PubMed] [Google Scholar]
- [73].Tsikas D, et al. , S-Nitroso-N-acetyl-L-cysteine ethyl ester (SNACET) and N-acetyl-L-cysteine ethyl ester (NACET)-Cysteine-based drug candidates with unique pharmacological profiles for oral use as NO, H2S and GSH suppliers and as antioxidants: Results and overview, J. Pharm. Anal 8 (2018) 1–9, 10.1016/j.jpha.2017.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data will be made available on request.


