Dear Editor,
Hagiya and colleagues recently reported a poor humoral immune response towards third dose of SARS-CoV-2 mRNA vaccine in the older Japanese population.1 This observation highlights that generalisation of results obtained from vaccine trials to specific subpopulations may be hazardous. People living with HIV (PLWH) represent another population poorly represented in large-scale vaccine trials. Despite the fact that PLWH are at higher risk of severe coronavirus disease 2019,2 immunological data following vaccination in this population remain sparse.3, 4, 5, 6
We prospectively evaluated humoral and T-cell immune responses before (T0) and after (T1) administration of a third dose of SARS-CoV-2 vaccine, either BNT162b2 or mRNA-1273, in PLWH followed-up at the University Hospital of Liège (Belgium) and in HIV-negative healthcare workers (HCWs). Biological analyses included quantification of anti-trimeric spike protein specific IgG (anti-S IgG), 50% neutralising antibody titres (NT50) against wild-type (WT) and Omicron (BA.1/B.1.1.529) strains, and SARS-CoV-2-specific interferon-gamma (IFN-γ) release using the QuantiFERON SARS-CoV-2 assay which contains two different pools (Ag1 and Ag2) of spike-embedded peptides (Appendix p1-2). We compared immune parameters at both timepoints between PLWH and HCWs using linear regression models on log10-transformed variables. Evolution of the immune parameters was analysed using signed-rank test for paired observations. Results were contrasted according to participants’ prior SARS-CoV-2 infection. All models were adjusted for participants and process-related characteristics that had a significant univariate impact on at least one variable of interest (Appendix p2).
119 PLWH and 79 HCWs were enrolled in the study and constituted the study cohort for analysis at T0. Among them, 80 PLWH and 51 HCWs completed the whole study and constituted the study cohort for T1 (Fig. S1). Participants’ characteristics are displayed in Table 1 . 84% PLWH and all HCWs received BNT162b2 as first two doses of vaccine. For the third dose, all HCWs and 52.5% PLWH received BNT162b2 and the remaining 47.5% received mRNA-1273. All PLWH except one were infected with HIV-1, with a median time since diagnosis of 11 years. All were on antiretroviral therapy. Among PLWH initially included at T0, median CD4+ T cell count was 680/μL (IQR 546-898) and 7 patients had a viral load over 50 copies/mL.
Table 1.
Background characteristics of PLWH and HCWs individuals at T0 and T1.
| Variable | PLWH at T0 | HCWs at T0 | p-value | PLWH at T1 | HCWs at T1 | p-value |
|---|---|---|---|---|---|---|
| (n=119) | (n=79) | (n=80) | (n=51) | |||
| Male sex | 59 (49.6) | 13 (16.5) | <0.0001 | 43 (53.8) | 11 (21.6) | 0.0003 |
| Age (Years) | 45.2 ± 10.6 | 43.7 ± 11.5 | 0.36 | 45.6 ± 10.7 | 43.0 ± 10.0 | 0.18 |
| 18-29 | 6 (5.0) | 7 (8.9) | 4 (5.0) | 2 (3.9) | ||
| 30-39 | 36 (30.2) | 27 (34.2) | 24 (30.0) | 22 (43.1) | ||
| 40-49 | 36 (30.2) | 19 (24.0) | 21 (26.2) | 13 (25.5) | ||
| 50-59 | 29 (24.4) | 17 (21.5) | 22 (27.5) | 10 (19.6) | ||
| ≥60 | 12 (10.1) | 9 (11.4) | 9 (11.3) | 4 (7.8) | ||
| BMI (kg/m²) | 28.0 ± 5.1 | 25.1 ± 6.2, n=76 | 0.0006 | 27.5 ± 5.6 | 25.9 ± 6.9, n=50 | 0.13 |
| Underweight (<18.5) | 0 (0.0) | 2 (2.6) | 0 (0.0) | 2 (4.0) | ||
| Normal range (18.5-24.9) | 34 (28.6) | 38 (50.0) | 29 (36.2) | 22 (44.0) | ||
| Overweight (25-29.9) | 50 (42.0) | 24 (31.6) | 34 (42.5) | 17 (34.0) | ||
| Obese (≥30) | 35 (29.4) | 12 (15.8) | 17 (21.3) | 9 (18.0) | ||
| Ethnicity | - | - | ||||
| Caucasian | 45 (37.8) | - | 34 (42.5) | - | ||
| African | 69 (58.0) | - | 41 (51.3) | - | ||
| Other | 5 (4.2) | - | 5 (6.2) | - | ||
| Medical history | ||||||
| Diabetes mellitus | 8 (6.7) | 3 (3.8) | 0.53 | 5 (6.2) | 1 (2.0) | 0.40 |
| Hypertension | 32 (26.9) | 14 (17.7) | 0.13 | 18 (22.5) | 7 (13.7) | 0.21 |
| Heart failure coronary artery disease | 2 (1.7) | 1 (1.3) | - | 2 (2.5) | 0 (0.0) | - |
| Stroke | 2 (1.7) | 0 (0.0) | - | 1 (1.2) | 0 (0.0) | - |
| Liver disease | 1 (0.8) | 0 (0.0) | - | 1 (1.2) | 0 (0.0) | - |
| Kidney disease | 0 (0.0) | 0 (0.0) | - | 0 (0.0) | 0 (0.0) | - |
| Chronic lung disease | 1 (0.8) | 0 (0.0) | - | 1 (1.2) | 0 (0.0) | - |
| Asthma | 0 (0.0) | 6 (7.6) | 0.0036 | 0 (0.0) | 3 (5.9) | 0.0028 |
| Autoimmune disease | 1 (0.8) | 4 (5.1) | 0.083 | 0 (0.0) | 2 (3.9) | - |
| Hematological cancer | 0 (0.0) | 4 (5.1) | - | 0 (0.0) | 1 (2.0) | - |
| Non hematological cancer | 9 (7.6) | 4 (5.1) | 0.74 | 7 (8.8) | 4 (7.8) | 1.0 |
| Solid-organ/cell transplantation | 0 (0.0) | 0 (0.0) | - | 0 (0.0) | 0 (0.0) | - |
| Immunosuppressive drugs | - | - | ||||
| Corticosteroids | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | ||
| Other | 1 (0.8) | 1 (1.3) | 0 (0.0) | 0 (0.0) | ||
| Previous SARS-CoV-2 infection (before T0) | ||||||
| Questionnaire | 26 (21.9) | 19 (24.0) | 0.72 | 14 (17.5) | 15 (29.4) | 0.11 |
| Positive anti-N antibody | 50 (42.0) | 13 (16.9), n=77 | 0.0002 | 30 (37.5) | 10 (20.0), n=50 | 0.035 |
| SARS-CoV-2 experienced* | 55 (46.2) | 21 (26.6) | 0.0054 | 32 (40.0) | 16 (31.4) | 0.32 |
| Previous SARS-CoV-2 infection (before T1) | - | - | - | |||
| Questionnaire | - | - | - | 15 (18.8) | 18 (35.3) | 0.033 |
| Positive anti-N antibody | - | - | - | 40 (50.0) | 17 (34.0), n=50 | 0.074 |
| SARS-CoV-2 experienced* | - | - | - | 41 (51.2) | 22 (43.1) | 0.37 |
| Experienced (between T0 and T1) | - | - | - | 9 (11.2) | 6 (11.7) | - |
| First vaccine dose | - | - | ||||
| BNT162b2 mRNA (Pfizer) | 101 (84.9) | 79 (100.0) | 69 (86.2) | 51 (100.0) | ||
| mRNA-1273 (Moderna) | 8 (6.7) | 0 (0.0) | 4 (5.0) | 0 (0.0) | ||
| ChAdOx1-S (Astra Zeneca) | 10 (8.4) | 0 (0.0) | 7 (8.8) | 0 (0.0) | ||
| Second vaccine dose | - | - | ||||
| BNT162b2 mRNA (Pfizer) | 100 (84.0) | 79 (100.0) | 69 (86.2) | 51 (100.0) | ||
| mRNA-1273 (Moderna) | 9 (7.6) | 0 (0.0) | 4 (5.0) | 0 (0.0) | ||
| ChAdOx1-S (Astra Zeneca) | 10 (8.4) | 0 (0.0) | 7 (8.8) | 0 (0.0) | ||
| Third vaccine dose | - | - | ||||
| BNT162b2 mRNA (Pfizer) | - | - | 42 (52.5) | 51 (100.0) | ||
| mRNA-1273 (Moderna) | - | - | 38 (47.5) | 0 (0.0) | ||
| Time between first and second vaccine dose (weeks) | 5.0 (4.0-5.0) | 3.0 (3.0-3.1) | <0.0001 | 5.0 (4.4-5.0) | 3.0 (3.0-3.1) | <0.0001 |
| Time between second vaccine dose and sample at T0 (weeks) | 25 (23-28) | 24 (24-24) | 0.025 | 25 (23-27) | 24 (24-24) | 0.014 |
| Time between second and third vaccine dose (weeks) | - | - | - | 27 (25-31) | 38 (35-39) | <0.0001 |
| Time between third vaccine dose and sample at T1 (weeks) | - | - | - | 2.4 (3.1-3.9) | 4.7 (4.0-8.0) | <0.0001 |
| Time between T0 and T1 (weeks) | - | - | - | 5 (4-6) | 19 (18-19) | <0.0001 |
| HIV infection | - | - | ||||
| HIV-1 | 118 (99.2) | - | 79 (98.8) | - | ||
| HIV-2 | 1 (0.8) | - | 1 (1.2) | - | ||
| Prior AIDS diagnosis | 45 (37.8) | - | 27 (33.8) | - | ||
| Time at T0 since HIV diagnosis (years) | 11 (6-18) | - | - | 11 (6.5-18) | - | - |
| <1 | 1 (0.8) | - | 1 (1.2) | - | ||
| 1-5 | 27 (22.7) | - | 17 (21.3) | - | ||
| 6-10 | 26 (21.9) | - | 17 (21.3) | - | ||
| >10 | 65 (54.6) | - | 45 (56.2) | - | ||
| Nadir CD4+T cell count per μL | 259 (163-462) | - | - | 292 (166-502) | - | - |
| <200 | 39 (32.8) | - | 25 (31.2) | - | ||
| ≥200 | 80 (67.2) | - | 55 (68.8) | - | ||
| Last CD4+T cell count per μL (2021 or 2022) | 680 (546-898) | - | - | 743 (592-940) | - | - |
| <350 | 8 (6.7) | - | 3 (3.7) | - | ||
| 350-499 | 17 (14.3) | - | 11 (13.8) | - | ||
| ≥500 | 94 (79.0) | - | 66 (82.5) | - | ||
| CD4/CD8 ratio, n=117 | 1.03 ± 0.57 | - | - | 1.1 ± 0.57 | - | - |
| <0.6 | 25 (21.4) | - | 16 (20.0) | - | ||
| 0.6-1 | 40 (34.2) | - | 26 (32.5) | - | ||
| >1 | 52 (44.4) | - | 38 (47.5) | - | ||
| Last plasma viral load copies/mL | <20 (<20-<20) | - | - | <20 (<20-<20) | - | - |
| <50 | 112 (94.1) | - | 75 (93.8) | - | ||
| Time on ART (years) | 10.7 ± 6.6 | - | 10.7 ± 6.9 | - |
Results are expressed as n (%), mean ± SD, or Median (Q1-Q3) as appropriate and p-values of Chi-square or Fisher exact test, ANOVA, or Kruskal-Wallis test respectively.
: Previous SARS-CoV-2 infection if ‘Yes’ at questionnaire or positive anti-nucleocapsid antibodies.
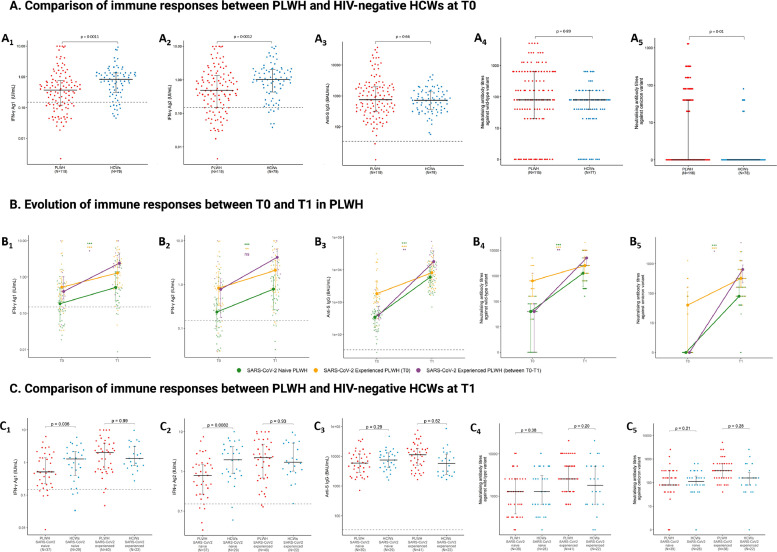
Overall, before the third vaccine dose (T0), SARS-Cov-2 specific IFN-ɣ production was significantly lower in PLWH than in HCWs (p < 0.01) (Fig. 1A , Table S1). In contrast, neutralising antibody titres (nAbTs) against Omicron were higher in PLWH (p = 0.01). Anti-S IgG levels and nAbTs against WT were similar between the two groups. Considering participants’ history of SARS-CoV-2 infection, IFN-ɣ production was lower only among SARS-CoV-2 naïve PLWH (p < 0.01) (Table S1). Also, nAbTs against Omicron were increased only among SARS-CoV-2 experienced PLWH (p < 0.01). It is worth noting that sampling at T0 had been performed earlier for HCWs, before the emergence of Omicron, preventing our HIV-negative population from being infected by this specific variant. Administration of a third dose of the SARS-CoV-2 vaccine elicited a significant increase in every parameter reflecting immune response among both HCWs and PLWH (p < 0.001) (Fig. 1B, Table S2). Evolution between T0 and T1 of any of the parameters was not significantly different between PLWH and HCWs. The proportion of PLWH with detectable Omicron nAbTs rose from 27.3% to 87.4% but median nAbT against Omicron remained 8-fold lower than median nAbT against WT (p < 0.001). Furthermore, nAbTs against Omicron and WT were both significantly lower among SARS-CoV-2 naïve PLWH compared to those previously infected (p < 0.001). After three doses of vaccine, we did not find a significant difference between PLWH and HCWs in any of the immune parameters investigated (Table S3). However, considering participants’ history of SARS-CoV-2 infection, IFN-ɣ production was still lower among SARS-CoV-2 naïve PLWH compared to naïve HCWs (p < 0.05 and p < 0.01 for Ag1 and Ag2, respectively), whereas it was similar between SARS-CoV-2 experienced PLWH and HCWs (Fig. 1C, Table S3). Subgroups analyses found no significant difference between immune responses of HIV-infected individuals according to their CD4+ T cell count or CD4+/CD8+ T cell ratio (Table S4, S5).
Fig. 1A.
Comparison of cellular and humoral immune responses between people living with HIV and HIV-negative healthcare workers before administration of the third dose of SARS-CoV-2 mRNA vaccine (T0).
SARS-CoV-2-specific IFN-γ release for Ag1 (A1), SARS-CoV-2-specific IFN-γ release for Ag2 (A2), Anti-S IgG (A3), neutralising antibody titres against Wild-type variant (A4), and neutralising antibody titres against Omicron variant (A5) were measured and compared between PLWH (n=119) and HCWs (n=79) who had received two doses of the SARS-CoV-2 vaccine. Dots represent subjects, whiskers represent median and IQR, and horizontal dashed line corresponds to the positivity cutoff (IFN-γ > 0.15 IU/mL and anti-S IgG ≥ 33.8 BAU/mL were considered positive). Statistics were calculated using adjusted linear regression models on log10-transformed variables. Exact number of participants in each group is indicated in Table S1.
Fig 1B. Evolution of cellular and humoral immune responses following the third dose of SARS-CoV-2 mRNA vaccine in SARS-CoV-2 naïve and experienced PLWH.
SARS-CoV-2-specific IFN-γ release for Ag1 (B1), SARS-CoV-2-specific IFN-γ release for Ag2 (B2), Anti-S IgG (B3), neutralising antibody titres against Wild-type variant (B4), and neutralising antibody titres against Omicron variant (B5) were measured and compared before (T0) and after a third dose (T1) of the SARS-CoV-2 mRNA vaccine among PLWH (n=80), divided into 3 subgroups according to history of SARS-CoV-2 infection (naïve, experienced before T0, and experienced between T0 and T1). Dots represent subjects, whiskers represent median and IQR, and horizontal dashed line corresponds to the positivity cutoff (IFN-γ > 0.15 IU/mL and anti-S IgG ≥ 33.8 BAU/mL were considered positive). Statistics were calculated using linear regression models on log10-transformed variables. Exact number of participants for each group is indicated in Table S5.
Fig 1C. Comparison of cellular and humoral immune responses between people living with HIV and healthcare workers after administration of the third dose of SARS-CoV-2 mRNA vaccine (T1).
SARS-CoV-2-specific IFN-γ release for Ag1 (C1), SARS-CoV-2-specific IFN-γ release for Ag2 (C2), Anti-S IgG (C3), neutralising antibody titres against Wild-type variant (C4), and neutralising antibody titres against Omicron variant (C5) were measured and compared between SARS-CoV-2 experienced and naïve PLWH (n=80) and HCWs (n=51) two to eight weeks after administration of a third dose of the SARS-CoV-2 mRNA vaccine. Dots represent subjects, whiskers represent median and IQR, and horizontal dashed line corresponds to the positivity cutoff (IFN-γ > 0.15 IU/mL and anti-S IgG ≥ 33.8 BAU/mL were considered positive). Statistics were calculated using adjusted linear regression models on log10-transformed variables. Exact number of participants for each group is indicated in Table S6.
Factors impacting the magnitude of immune responses in PLWH are displayed in Appendix (Table S6to S10). Of interest, SARS-CoV-2 infection either before T0 (p < 0.001) or between T0 and T1 (p < 0.05) was associated with higher anti-S IgG titres and nAbTs against both variants after three doses. Among PLWH specifically, the magnitude of T-cell-mediated response elicited by the mRNA-1273 vaccine was more important than that elicited by BNT162b2 vaccine (p < 0.01).
Administration of a third dose of SARS-CoV-2 vaccine induced robust humoral and T-cell immune responses against SARS-CoV-2 in almost all participants. Humoral immune responses were similar between PLWH and HCWs, both before and after the third dose, which is in line with recently published data.4 , 7 Although SARS-CoV-2 specific IFN-ɣ production increased after the third dose, it remained significantly lower among SARS-CoV-2 naïve PLWH compared to HCWs. Our data suggest that dysfunction of virus-specific T cell immunity, which is even found among HIV-positive patients with undetectable viral load, might lead to a suboptimal cell-mediated immune response following vaccination, especially in patients with no history of SARS-CoV-2 infection. In contrast, hybrid immunity conferred a similar T-cell immune response between PLWH and HIV-negative individuals. This observation could be attributed to the development of a distinct population of IFN-ɣ and IL-10-expressing memory SARS-CoV-2 spike-specific CD4+ T cells following vaccination of previously infected individuals.8
Our results suggest that vaccine boosting enables broad neutralising immunity.9 Indeed, the third dose elicited the production of anti-Omicron nAbTs in almost all participants. However, anti-Omicron nAbTs remained eight-fold lower compared to those against WT, which is in line with earlier reports and may reflect a less effective protection against this variant.4 , 10
In conclusion, a third dose of SARS-CoV-2 vaccine considerably enhanced SARS-CoV-2 specific humoral and cellular immunity in PLWH. Humoral immune responses were similar between PLWH and HIV-negative individuals. However, our data raise concerns about the vaccine's ability to induce protective T-cell immune response among PLWH with no history of SARS-CoV-2 infection. Further studies are needed to understand the clinical consequences of such observations and characterise the potential protective advantage of hybrid immunity in PLWH.
Fig. 1 was created using BioRender.com.
Funding
This work was supported by the Léon Fredericq Foundation (To GD and MM) and the FNRS (Fonds National de la Recherche Scientifique) (To SR, grant number PER/PGY H.P 030.20). M.E. and N.L. are FNRS doctoral clinical specialist candidates, AT is aspirant FNRS (PhD fellow), GD is an FNRS postdoctoral clinical master specialist and SR is an FNRS Senior Research Associate.
Role of the funder/sponsor
The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Ethic committee
Written informed consent was obtained from each participant and the study was approved by the Research Ethic Committee of the University Hospital of Liège (approval reference number: 2021-54).
CRediT authorship contribution statement
Majdouline El Moussaoui: Conceptualization, Methodology, Formal analysis, Investigation, Writing – original draft, Writing – review & editing. Salomé Desmecht: Investigation, Writing – review & editing. Aleksandr Tashkeev: Methodology, Formal analysis, Writing – review & editing. Nicolas Lambert: Methodology, Formal analysis, Investigation, Writing – review & editing. Nathalie Maes: Writing – review & editing. Joachim Braghini: Investigation, Writing – review & editing. Nicole Marechal: Methodology, Formal analysis, Writing – review & editing. Céline Quintana: Investigation, Writing – review & editing. Karine Briquet: Writing – review & editing. Stéphanie Gofflot: Investigation, Writing – review & editing. Françoise Toussaint: Investigation, Writing – review & editing. Marie-Pierre Hayette: Writing – review & editing, Supervision. Pieter Vermeersch: Investigation, Writing – review & editing. Laurence Lutteri: Investigation, Writing – review & editing. Céline Grégoire: Writing – review & editing. Yves Beguin: Writing – review & editing. Souad Rahmouni: Writing – review & editing. Michel Moutschen: Writing – review & editing, Supervision, Funding acquisition. Daniel Desmecht: Writing – review & editing, Supervision. Gilles Darcis: Conceptualization, Methodology, Formal analysis, Investigation, Writing – original draft, Writing – review & editing, Supervision, Funding acquisition.
Declaration of Competing Interest
All other authors declare no competing interests.
Acknowledgments
We thank the study participants for their voluntary contribution.
Footnotes
Supplementary material associated with this article can be found, in the online version, at doi:10.1016/j.jinf.2022.09.006.
Appendix. Supplementary materials
References
- 1.Hagiya H., Hikita T., Habu T., Asada M., Yorifuji T., Toyooka S., et al. Poor vaccine responsiveness towards third-dose mRNA vaccine of COVID-19 in Japanese older people. J Infect. 2022;S0163-4453(22):00413. doi: 10.1016/j.jinf.2022.07.007. -3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Boffito M., Waters L. More evidence for worse COVID-19 outcomes in people with HIV. Lancet HIV. 2021;8(11):e661–e662. doi: 10.1016/S2352-3018(21)00272-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hassold N., Brichler S., Ouedraogo E., Leclerc D., Carroue S., Gater Y., et al. Impaired antibody response to COVID-19 vaccination in advanced HIV infection. AIDS. 2022;36(4):F1–F5. doi: 10.1097/QAD.0000000000003166. [DOI] [PubMed] [Google Scholar]
- 4.Lapointe H.R., Mwimanzi F., Cheung P.K., Sang Y., Yaseen F., Umviligihozo G., et al. People with HIV receiving suppressive antiretroviral therapy show typical antibody durability after dual COVID-19 vaccination, and strong third dose responses. J Infect Dis. 2022:jiac229. doi: 10.1093/infdis/jiac229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Tau L., Turner D., Adler A., Marom R., Ahsanov S., Matus N., et al. SARS-CoV-2 humoral and cellular immune responses of patients with HIV after vaccination with BNT162b2 mRNA COVID-19 vaccine in the Tel-Aviv medical center. Open Forum Infect Dis. 2022;9(4):ofac089. doi: 10.1093/ofid/ofac0896. Published 2022 Feb 23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Antinori A., Cicalini S., Meschi S., Bordoni V., Lorenzini P., Vergori A., et al. Humoral and cellular immune response elicited by mRNA vaccination against SARS-CoV-2 in people living with HIV (PLWH) receiving antiretroviral therapy (ART) according with current CD4 T-lymphocyte count [published online ahead of print, 2022 Apr 2] Clin Infect Dis. 2022:ciac238. doi: 10.1093/cid/ciac238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Brumme Z.L., Mwimanzi F., Lapointe H.R., Cheung P.K., Sang Y., Duncan M.C., et al. Humoral immune responses to COVID-19 vaccination in people living with HIV receiving suppressive antiretroviral therapy. NPJ Vaccines. 2022;7:28. doi: 10.1038/s41541-022-00452-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Rodda L.B., Morawski P.A., Pruner K.B., Fahning M.L., Howard C.A., Franko N., et al. Imprinted SARS-CoV-2-specific memory lymphocytes define hybrid immunity. Cell. 2022;185(9):1588–1601. doi: 10.1016/j.cell.2022.03.018. e14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Evans J.P., Zeng C., Carlin C., Lozanski G., Saif L.J., Oltz E.M., et al. Neutralizing antibody responses elicited by SARS-CoV-2 mRNA vaccination wane over time and are boosted by breakthrough infection. Sci Transl Med. 2022;14(637):eabn8057. doi: 10.1126/scitranslmed.abn805710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ogbe A., Pace M., Bittaye M., Tipoe T., Adele S., Alagaratnam J., et al. Durability of ChAdOx1 nCoV-19 vaccination in people living with HIV. JCI Insight. 2022;7(7) doi: 10.1172/jci.insight.157031. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.