Abstract
BACKGROUND
Detection and elective repair of abdominal aortic aneurysms (AAA) guided by known risk-factor specific screening decrease AAA-related mortality. However, minimal epidemiologic data exist for AAA in female persons and racial minority groups. We established the contemporary risk of US AAA hospitalization across age, sex, and race.
METHODS
National Inpatient Sample and US Census (2012–2018) data were used to quantify age-, sex-, and race-specific incidences and adjusted odds ratios (aOR) of AAA hospitalizations (≥18 years), associated risk factors, and in-hospital mortality. Interaction terms evaluated subgroups.
RESULTS
Among 1,728,374,183 US residents during the study period (51.3% female; 78.4% White, 12.7% Black, 5.7% Asian), 211,501,703 were hospitalized (age, 57.56±0.04 years; 58.4% female; 64.9% White, 14.3% Black, 2.5% Asian) of which 291,850 were for AAA (age, 73.17±0.04 years; 22.6% female; 81.8% White, 5.6% Black, 1.6% Asian). An estimated 15.2 (95%CI, 15.1–15.3) and 1.7 (95%CI, 1.7–1.7) hospitalizations per 100,000 residents were for intact (iAAA) and ruptured (rAAA) AAA, respectively. 16.2% of iAAA (83.8% male; 79.1% White) and 18.4% of rAAA (86.4% male; 75.0% White) hospitalizations occurred in patients <65 years. For iAAA, female sex (aOR, 0.27 [95%CI, 0.27–0.28]) compared to male sex and both Black (0.47 [95%CI, 0.45–0.49]) and Asian (0.86 [95%CI, 0.83–0.93]) persons compared to White persons had reduced aOR for hospitalization. For rAAA, the reduced aOR persisted for female sex (0.33 [95%CI, 0.32–0.36]) and for Black persons (0.52 [95%CI, 0.46–0.58]). While female sex demonstrated an overall reduced odds of AAA hospitalization, female persons who were older, Black, or had peripheral vascular disease (P-interactions<0.001) had a relative increase in AAA hospitalization aOR. Female sex (aOR, 1.54 [95%CI, 1.38–1.70]), but not Black or Asian race, was associated with increased mortality which was more pronounced for iAAA (1.93 [95%CI, 1.66–2.25]) than rAAA (1.29 [95%CI, 1.13–1.48]; P-interaction<0.001).
CONCLUSIONS
We confirmed a substantially decreased adjusted risk of AAA hospitalization for female sex and racial minority groups; however, aging and comorbid peripheral vascular disease reduced these differences. The disparate risk of AAA hospitalization by sex and race highlights the importance of inclusivity in future AAA studies.
Keywords: Abdominal aortic aneurysm, social determinants of health, epidemiology, race, sex
Table of Contents Summary
Racial minority groups and female persons have a lower risk of AAA hospitalization; however, the risk approaches that of White male persons as age increases. The authors suggest race and sex must be included in future AAA studies.
Introduction
Abdominal aortic aneurysms (AAA) represent a degenerative process resulting in progressive dilation of the aorta. Most AAA remain asymptomatic until rupture. Upon rupture, only 50% survive to undergo repair with postoperative mortality nearing 40%.1 AAA is estimated to affect ~2% of the United States (US) population.2 Early detection through AAA screening and elective repair mitigate the risk of AAA-related death.3, 4 However, AAA repair is not without risk, especially among racial minority groups and female persons who experience increased perioperative complications and mortality compared to White male persons.5–7 While these differences in outcomes are likely multifactorial (i.e. access to care, testing of devices in White males, biology), epidemiological data surrounding AAA in females and racial minority groups are lacking.
AAA screening guidelines were developed to balance the morbidity and mortality of rupture with that of elective repair. For male persons age 65–75, the 2019 US Preventative Services Taskforce (USPSTF) recommended one-time AAA screening among ever smokers (Grade B) and selective screening among never smokers (Grade C).8 For female persons aged 65–75, the USPSTF recommended against screening never smokers without a family history of AAA (Grade D) and determined there was insufficient evidence to support screening even among ever smokers (Grade I).
The USPSTF, Society for Vascular Surgery, and American Heart Association screening guidelines for AAA were derived from high-quality, randomized controlled trials performed in primarily non-US populations and were extrapolated from 33 studies in 69 publications.8,9 The majority of these seminal investigations were conducted in predominantly White male persons and excluded female sex. Fewer than 20% reported patient race. When race was reported, >95% of participants were White persons and none were Asian persons.9
In this study, we established the contemporary incidence of age-, sex-, and race-specific AAA hospitalizations in the US using the National Inpatient Sample (NIS) and US Census data.
Methods
This retrospective cohort study was deemed exempt from human subjects’ review by the University of Pittsburgh’s Human Research Protection Office (STUDY20110441). Data were presented in accordance with the NIS, Healthcare Cost and Utilization Project (HCUP), Agency for Healthcare Research and Quality (AHRQ) use agreements, and the American Heart Association Disparities Research, CDC Health Equity Style Guide, and STROBE guidelines (Supplement).10–14
Data Source and Patient Cohort
We examined AAA hospitalizations in the US using NIS and US Census15 (2012–2018) data. The NIS samples 20% of discharges from non-federal, acute care hospitals in the US regardless of the expected payer, allowing for national population estimates of hospitalizations and outcomes. The NIS captures patient-level data including demographics, up to 15 diagnoses and procedure codes, hospital length of stay (LOS), in-hospital mortality, discharge disposition, and total hospitalization costs. Race and ethnicity are categorized as one data element in the NIS,16 whereas the US Census data categorize race and ethnicity independently.
We included all NIS hospitalizations and excluded children (<18 years). We defined AAA hospitalizations by International Classification of Disease, Clinical Modifications (ICD-CM) codes for primary diagnoses of intact AAA (iAAA; 441.4, I71.4) or ruptured AAA (rAAA; 441.3, I71.3). Comorbid conditions were categorized using secondary ICD-CM diagnoses in accordance with NIS guidance: i) AHRQ Severity File (2012–2015)16, ii) Elixhauser comorbidity measures (2015–2018)17, or as previously described iii) connective tissue disorders (i.e., Marfan’s, Ehlers-Danlos) and iv) current or prior tobacco use.18 In-hospital AAA interventions were identified by ICD, Procedure Coding System.19
Statistical Analysis
Data were reported as estimates of US hospitalizations, generated by the NIS sampling stratification weights with adjustments for year-based differences in trend analysis.11, 20 Estimated continuous variables were presented as mean ± standard error and categorical variables as proportion (%) with 95%CI. Missing data were reported if >0.1%.
The primary outcome was the estimated incidence and adjusted odds of both iAAA and rAAA hospitalization. Secondarily, we investigated in-hospital mortality. We calculated overall and category-specific (i.e., age-, sex-, and race-specific) incidences by synchronizing estimated AAA NIS hospitalizations (events: numerator) per 100,000 US residents (Census: denominator).20, 21 Among NIS hospitalizations, adjusted odds ratios (aOR) were generated using multivariable logistic regressions, including co-variates and subgroup analyses determined a priori. Interaction terms evaluated the associations between outcomes and subgroups with separate models to explore sex- and race-based associations. All modeled survey weighted data were referred to as adjusted odds. Data analyses and figures were completed in Stata 17.0 (Stata Corp) and PRISM 9 (GraphPad Software). Analysis code is publicly available (https://github.com/srli13/US_AAA_Sex_Race_Epi.git).
Sensitivity Analysis
We completed three sensitivity analyses. First, to account for ethnicity categorization discrepancies between the NIS and US Census, we reclassified hospitalizations identified as Hispanic in the NIS to White race. Second, we pursued two additional models excluding comorbid conditions potentially in the causal pathway to AAA development, including only i) age, sex, race and ii) age, sex, race, socioeconomic factors covariates. Third, we defined AAA hospitalization to include both an AAA primary diagnosis and in-hospital AAA repair code.
Results
Estimated US AAA Hospitalizations
Among 250,212,608 estimated hospitalizations in the NIS, 211,501,703 (84.5%) were for adults (age, 57.56±0.04 years; 58.4% female sex; 64.9% White, 14.3% Black, 2.5% Asian, 18.3% other races). AAA accounted for 291,850 hospitalizations (age, 73.17±0.04 years), of which 22.6% were female sex and 81.8% were White (5.6% Black, 1.6% Asian). Compared to the 262,532 iAAA hospitalizations (90.0%), patients hospitalized for rAAA (N=29,318 [10.0%]) were older (74.38±0.14 vs 73.04±0.04 years) with a higher proportion of female sex (27.4% vs 22.0%) and lower proportions of comorbid hypertension (64.4% vs 70.3%), PVD (29.4% vs 36.0%), and tobacco use (50.0% vs 61.8%; Table I). Additionally, patients hospitalized for rAAA underwent fewer AAA repair procedures (65.8% vs 75.8%), had longer LOS (8.18±0.15 vs 3.47±0.03 days), higher in-hospital mortality (35.0% vs 1.5%), more expensive hospitalizations ($181,730±2,784 vs 120,373±785), fewer discharges home (24.6% vs 77.2%), and lower proportion of Medicare (40.2% vs 43.4%) when compared to patients hospitalized for iAAA
Table I.
Characteristics of Hospitalized Patients Overall and by Abdominal Aortic Aneurysm Stratified Type
| All Hospitalizations (Estimated N*=211,501,703) | Intact AAA Hospitalizations (Estimated N*=262,532) | Ruptured AAA Hospitalizations (Estimated N*=29,318) | |
|---|---|---|---|
| Age Category†, % (95%CI) | |||
| <65 Years | 57.8 (57.6–58.0) | 16.2 (15.9–16.5) | 18.4 (17.4–19.4) |
| 65–75 Years | 17.8 (17.7–17.8) | 39.4 (39.0–39.8) | 31.3 (30.0–32.5) |
| >75 Years | 22.9 (22.7–23.0) | 40.5 (40.0–40.9) | 46.7 (45.4–48.1) |
| Female Sex‡, % (95%CI) | 58.4 (58.3–58.5) | 22.0 (21.6–22.4) | 27.4 (26.3–28.6) |
| Race‡,§, % (95%CI) | |||
| White | 64.9 (64.4–65.3) | 82.0 (81.4–82.7) | 79.6 (78.4–80.8) |
| Black | 14.3 (14.1–14.6) | 5.6 (5.3–5.8) | 5.5 (5.0–6.2) |
| Asian | 2.5 (2.4–2.6) | 1.6 (1.5–1.8) | 1.9 (1.5–2.3) |
| Other|| | 17.8 (17.5–18.1) | 7.8 (7.5–8.1) | 8.2 (7.5–8.9) |
| Missing# | 4.5 (4.2–4.8) | 4.6 (4.1–5.2) | 6.6 (5.8–7.5) |
| Region of United States, % (95%CI) | |||
| Northeast | 19.0 (18.3–19.7) | 17.8 (16.7–18.9) | 18.0 (16.6–19.6) |
| Midwest | 22.5 (21.8–23.3) | 25.6 (24.4–26.8) | 27.2 (25.6–28.9) |
| South | 39.2 (38.4–40.1) | 40.1 (38.8–41.5) | 36.1 (34.3–38.0) |
| West | 19.2 (18.6–19.9) | 16.5 (15.6–17.4) | 18.6 (17.3–20.0) |
| Comorbid Conditions, % (95%CI) | |||
| Diabetes Mellitus | 22.4 (22.3–22.6) | 17.6 (17.3–18.0) | 14.6 (13.7–15.5) |
| Hypertension | 45.8 (45.6–46.0) | 70.3 (69.7–70.9) | 64.4 (63.1–65.7) |
| Congestive Heart Failure | 11.0 (10.9–11.1) | 5.5 (5.3–5.8) | 9.1 (8.4–9.9) |
| Renal Failure | 12.5 (12.5–12.6) | 14.5 (14.2–14.9) | 19.8 (18.7–20.8) |
| Peripheral Vascular Disease | 5.3 (5.3–5.4) | 36.0 (35.4–36.5) | 29.4 (28.1–30.6) |
| Tobacco Use | 30.7 (30.5–30.9) | 61.8 (61.3–62.4) | 50.0 (48.4–51.0) |
| Chronic Lung Disease | 17.5 (17.4–17.6) | 30.5 (30.0–30.9) | 28.4 (27.3–29.6) |
| Connective Tissue Disorder | 0.04 (0.04–0.05) | 0.10 (0.08–0.13) | 0.14 (0.07–0.23) |
| Abdominal Aortic Aneurysm Repair Type, % (95%CI) | |||
| Endovascular | 1.0 (1.0–1.0) | 67.0 (66.4–67.6) | 39.1 (37.8–40.4) |
| Open | 0.2 (0.2–0.2) | 8.8 (8.5–9.2) | 26.7 (25.5–27.9) |
| None | 98.8 (98.8–98.8) | 24.2 (23.6–24.6) | 34.2 (33.0–35.5) |
| In-hospital Outcomes | |||
| Hospital Length of Stay, Mean±Standard Error, Days | 4.71±0.01 | 3.47±0.03 | 8.18±0.15 |
| In-hospital Mortality, % (95%CI) | 2.2 (2.2–2.2) | 1.5 (1.4–1.6) | 35.0 (33.7–36.2) |
| Total Hospital Charges**, Mean± Standard Error, $ | 47,971.0±256.6 | 120,373.0±785.4 | 181,730.1±2784.0 |
| Discharge Location, % (95%CI) | |||
| Home | 64.6 (64.5–64.8) | 77.2 (76.7–77.7) | 24.6 (23.5–25.7) |
| Skilled Nursing Facility | 16.2 (16.2–16.3) | 8.6 (8.4–8.9) | 25.8 (24.6–27.0) |
| Home Health Care | 13.4 (13.3–13.6) | 11.2 (10.8–11.6) | 11.2 (10.4–12.1) |
| Other Hospital | 2.0 (2.0–2.1) | 1.1 (1.0–1.2) | 2.9 (2.5–3.4) |
| Other†† | 0.05 (.04-.07) | 0.3 (0.3–0.4) | 0.4 (0.3–0.7) |
| Socioeconomic Factors | |||
| Income Quartile‡‡, % (95%CI) | |||
| Quartile 1 | 29.7 (29.3–30.1) | 26.2 (25.6–26.9) | 26.3 (25.0–27.5) |
| Quartile 2 | 25.6 (25.4–25.9) | 28.1 (27.6–28.6) | 28.4 (27.1–29.6) |
| Quartile 3 | 23.2 (22.9–23.4) | 24.7 (24.2–25.2) | 23.5 (22.4–24.7) |
| Quartile 4 | 19.5 (19.1–19.9) | 19.2 (18.6–19.8) | 20.0 (18.9–21.2) |
| Missing | 2.0 (2.0–2.1) | 1.7 (1.6–1.9) | 1.8 (1.5–2.2) |
| Payer Status, % (95%CI) | |||
| Medicare | 27.0 (26.7–27.4) | 43.4 (43.4–44.4) | 40.2 (38.6–41.7) |
| Medicaid | 10.5 (10.3–10.7) | 1.8 (1.6–1.9) | 2.3 (2.0–2.8) |
| Private | 15.5 (15.3–15.8) | 8.6 (8.2–8.9) | 8.8 (8.1–9.6) |
| Self-pay | 2.2 (2.2–2.3) | 0.4 (0.4–0.5) | 1.6 (1.3–1.9) |
| No Charge | 0.2 (0.2–0.2) | 0.1 (0.1–0.1) | 0.1 (0.1–0.2) |
| Other§§ | 1.6 (1.6–1.7) | 1.2 (1.1–1.3) | 1.6 (1.3–2.0) |
| Missing | 42.9 (42.2–43.5) | 44.6 (43.3–45.9) | 45.4 (43.6–47.1) |
National estimates obtained by applying stratified discharge weights provided by the NIS to unweighted NIS data.18
Age categorized in accordance with before (<65), during (65–75), and after (>75) the age of screening in accordance with US Preventive Services Task Force recommendations.5
Characteristics for abdominal aortic aneurysm hospitalizations stratified by sex are summarized in Supplemental Table II
Not all states provide information on race to Healthcare Cost and Utilization Program.13
Other includes Hispanic, Native American, and Other.
Reported if >0.1% of variables are missing.
Total charge estimates for intact ( 31,100,000,000±442,000,000) and ruptured ( 5,240,000,000±128,000,000) abdominal aortic aneurysm.
Other includes against medical advice and alive, destination unknown.
Classification derived from estimated median household income of residents in patient’s ZIP code.13
Includes Worker’s Compensation, Civilian Health and Medical Program of the Uniformed Services, Civilian Health and Medical Program of the Department of Veterans Affairs, Title V, and other government programs.13
AAA indicates abdominal aortic aneurysm; NIS, National Inpatient Sample; CI, Confidence Interval; No., Number.
Female persons were hospitalized for iAAA at older ages (75.2±0.1 vs. 72.6±0.1 years) and had a higher proportion of Black persons (8.7% [95%CI, 8.3–9.4%] vs. 4.6% [95%CI, 4.4–4.8%]) compared to male persons. Female persons were also more likely to forgo repair (30.8% [95%CI, 30.0–31.7%] vs. 23.5% [95%CI, 23.0–24.0%]) or undergo open repair (12.5% [95%CI, 11.9–13.1%] vs. 10.1% [95%CI, 9.8–10.5%]) compared to male persons. Black persons (71.2±0.2 years) were hospitalized at younger ages compared to White persons (73.3±0.1 years). Black (33.3% [95%CI, 31.7–35.0%]) and Asian persons (28.1% [95%CI, 25.3–31.0%]) were more likely to forgo repair compared to White persons (24.3% [95%CI, 23.8–24.8%]). Finally, White persons underwent a higher proportion of endovascular procedures (65.3% [95%CI, 64.7–65.9%]) compared to Black (56.5% [95%CI, 54.7–58.3%]) and Asian (60.0% [95%CI, 56.8–63.1%]) persons.
Estimated Incidence of AAA Hospitalization
Among 1,728,374,183 adult residents identified in the US Census (51.3% female sex; 78.4% White, 12.7% Black, 5.7% Asian; 17.5% Northeast, 21.2% Midwest, 37.7% South, 23.6% West)22, an estimated 15.2 (95%CI, 15.1–15.3) per 100,000 and 1.7 (95%CI, 1.7–1.7) per 100,000 were hospitalized with a primary diagnosis of iAAA and rAAA. The incidence of iAAA among male persons was nearly four-fold that of female persons (Table II). The incidence for iAAA among White persons was double that of Black persons and four-fold that of Asian persons. Similar trends were observed for rAAA. The incidence of rAAA in male persons was almost four-fold that of female persons and the incidence in White persons was nearly three-fold greater than for Black and Asian persons (Table II).
Table II.
Overall and Age-, Sex-, and Race-specific Incidences of Abdominal Aortic Aneurysm Hospitalizations
| US Census Resident Population No. | Intact AAA Hospitalization | Ruptured AAA Hospitalization | |||||
|---|---|---|---|---|---|---|---|
| NIS Estimated No. | Estimated Incidence per 100,000 (95%CI) | NIS Estimated No. | Estimated Incidence per 100,000 (95%CI) | ||||
| Category-specific Incidence* | Overall Incidence† | Category-specific Incidence* | Overall Incidence† | ||||
| Overall | 1,728,374,183 | 262,532 | 15.2 (15.1–15.3) | 29,318 | 1.7 (1.7–1.7) | ||
| Age Category‡ | |||||||
| <65 Years | 1,394,481,765 | 42,521 | 3.1 (3.0–3.1) | 2.5 (2.4–2.5) | 5,392 | 0.4 (0.4–0.4) | 0.3 (0.3–0.3) |
| 65–74 Years | 191,666,633 | 103,311 | 53.9 (53.6–54.2) | 6.0 (5.9–6.0) | 9,156 | 4.8 (4.7–4.9) | 0.5 (0.5–0.5) |
| ≥75 Years | 142,225,785 | 106,208 | 74.7 (74.2–75.1) | 6.1 (6.1–6.2) | 13,702 | 9.6 (9.5–9.8) | 0.8 (0.8–0.8) |
| Sex Category | |||||||
| Female | 887,125,727 | 57,724 | 6.5 (6.5–6.6) | 3.3 (3.3–3.4) | 8,035 | 0.9 (0.9–0.9) | 0.5 (0.5–0.5) |
| Male | 841,248,456 | 204,572 | 24.3 (24.2–24.4) | 11.8 (11.8–11.9) | 21,271 | 2.5 (2.5–2.6) | 1.2 (1.2–1.3) |
| Race Category|| | |||||||
| White | 1,355,650,200 | 215,165 | 15.9 (15.8–15.9) | 12.4 (12.4–12.5) | 23,322 | 1.7 (1.7–1.7) | 1.4 (1.3–1.4) |
| Black | 219,990,860 | 14,590 | 6.6 (6.5–6.7) | 0.8 (0.8–0.9) | 1,624 | 0.7 (0.7–0.8) | 0.1 (0.1–0.1) |
| Asian | 97,994,723 | 4,208 | 4.3 (4.2–4.4) | 0.2 (0.2–0.3) | 550 | 0.6 (0.5–0.6) | 0.03 (0.03–0.04) |
Incidence numerator is the National Inpatient Sample estimated number of hospitalizations and denominator is the category-specific United States resident population.
Incidence numerator is the National Inpatient Sample estimated number of hospitalizations and denominator is the overall United States resident population (Estimated N=1,728,374,183). Summation of incidences within each category equals the overall incidence.
Age categorized in accordance with before (<65), during (65–75), and after (>75) the age of screening in accordance with US Preventive Services Task Force recommendations5
The overall incidence within this category will not sum to the incidence for the full US Census resident population as not all races in the US are represented in the table.
AAA indicates abdominal aortic aneurysm; US, United States; NIS, National Inpatient Sample; CI, Confidence Interval, No., Number.
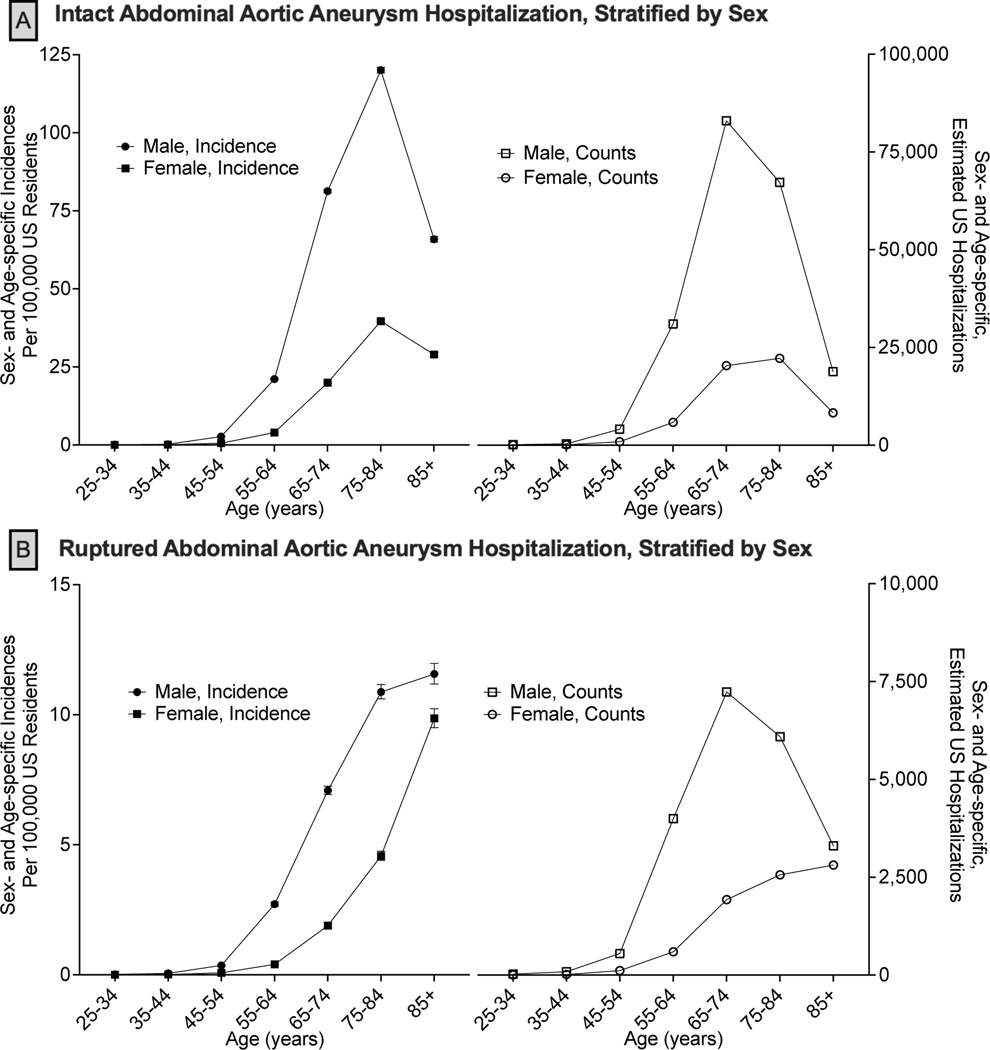
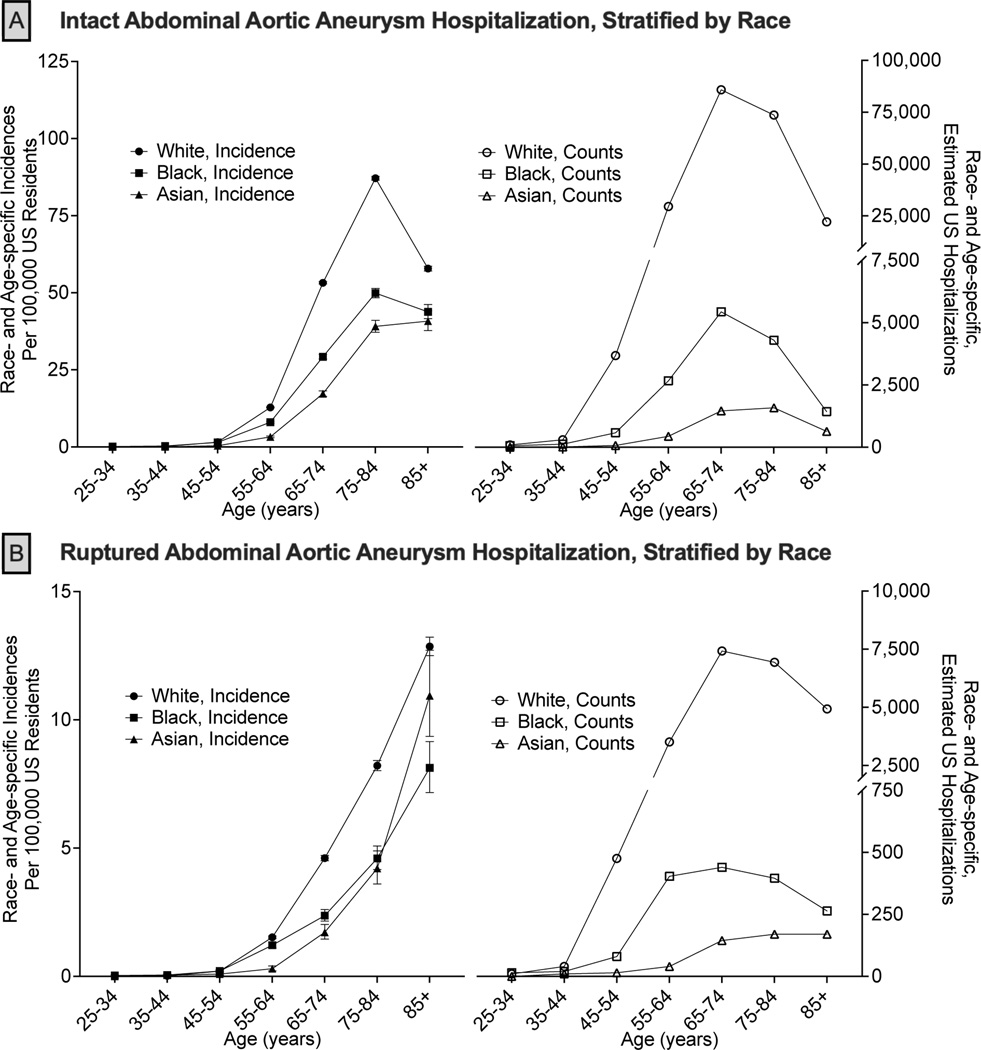
After 45 years of age, sex- and race-specific hospitalization events and incidences of AAA hospitalizations rapidly increased (Figures 1, 2). For iAAA, 42,540 (16.2%) hospitalizations occurred at <65 years of age, of which 83.8% were male sex and 79.1% were White persons (8.1% Black persons, 1.3% Asian persons). The peak decade of both sex- and race-specific hospitalization events for iAAA was between 65–74 years while peak incidence was between 75–84 years. Peak iAAA incidence was greater among male compared to female sex (120.1 [95%CI, 119.2–121.0] vs. 36.7 [95%CI, 39.2–40.2] per 100,000; Figure 1A). Peak race-specific incidence of iAAA hospitalizations was highest among White persons and lowest among Asian persons (White 87.1 [95%CI, 86.5–87.8], Black 49.9 [95%CI, 48.4–51.4], Asian 39.1 [95%CI, 37.2–41.1] per 100,000; Figure 2A).
Figure 1. Age-specific Estimated Incidence and Discharges of Abdominal Aortic Aneurysm Hospitalizations, Stratified by Sex.
National age-specific incidences with 95% confidence intervals (left) and number (events; right) of hospitalizations for intact (Panel A) and ruptured (Panel B) abdominal aortic aneurysms. National hospitalization estimates are generated from the National Inpatient Sample using the sex-specific population estimates from the United States Census. The gray boxes correspond to the United States Preventive Services Taskforce age guidelines for one-time abdominal aortic aneurysm screening.8
Figure 2. Age-specific Estimated Incidence and Discharges of Abdominal Aortic Aneurysm Hospitalizations, Stratified by Race.
National age-specific incidences with 95% confidence intervals (left) and number (events; right) of hospitalizations for intact (Panel A) and ruptured (Panel B) abdominal aortic aneurysms. National hospitalization estimates are generated from the National Inpatient Sample using the race-specific population estimates from the United States Census. The gray boxes correspond to the United States Preventive Services Taskforce age guidelines for one-time abdominal aortic aneurysm screening.8
rAAA hospitalization events mirrored iAAA in which 18.4% (N=5,390) occurred at <65 years of age, with the vast majority being male sex (86.4%) and White persons (75.0%; 9.7% Black persons, 1.2% Asian persons). In contrast to iAAA, the incidence of rAAA hospitalizations continually increased with age without peaking (Figure 1, 2). In older decades (>85 years), the incidence of rAAA hospitalizations for female sex approached that of male sex (9.9 [95%CI, 9.5–10.2] vs. 11.6 [95%CI, 11.2–12.0] per 100,000; Figure 1B) and the incidence among Asian persons (10.9 [95%CI, 9.4–12.7] per 100,000) outpaced Black persons (8.1 [95%CI, 7.2–9.2] per 100,000), approaching the incidence of White persons (12.9 [95%CI, 12.5–13.2] per 100,000; Figure 2B).
Adjusted Odds of AAA Hospitalization
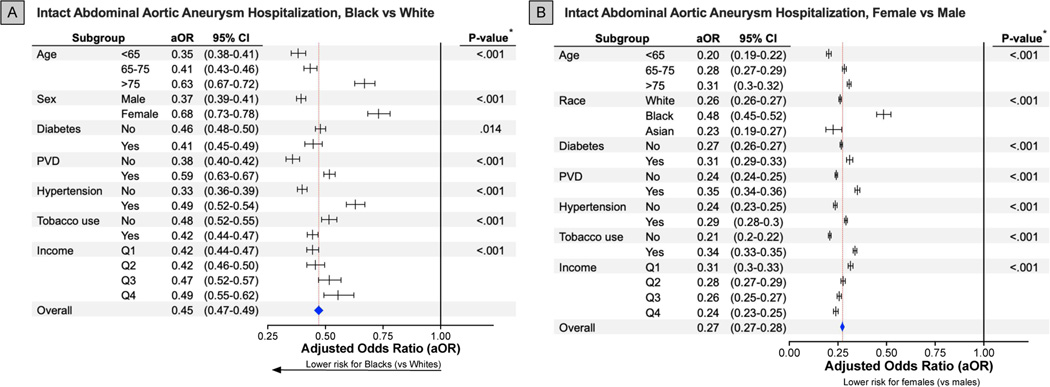
The adjusted relative risk for iAAA hospitalization was 73% lower for female sex compared to male sex. The adjusted relative risk was also 53% and 14% lower for Black and Asian persons, respectively, compared to the White persons (Supplemental Table I). The associations between adjusted odds of iAAA hospitalizations and sex/race subgroups were consistently observed. However, adjusted odds of iAAA hospitalization were moderated by age, race, sex, comorbid conditions, and socioeconomic factors (Figure 3, S1A). While female sex was associated an overall reduced adjusted odds of iAAA hospitalization compared to male sex, older female persons had a relatively increased adjusted odds of iAAA hospitalization compared to younger female persons (<65 years aOR=0.20 [95%CI, 0.19–0.22]; >75 years aOR=0.31 [95%CI, 0.30–0.32]). Black female persons similarly had a relatively increased adjusted odds of iAAA hospitalization compared to White and Asian female persons (Black aOR=0.48 [95%CI, 0.45–0.52]; White aOR=0.26 [95%CI, 0.26–0.27]; Asian aOR=0.23 [95%CI, 0.19–0.27]). Among Black and Asian persons who had an overall decreased adjusted odds of iAAA hospitalization compared to White persons, older compared to younger patients as well as female compared to male persons had relatively increased adjusted odds of iAAA hospitalizations.
Figure 3. Association Between Intact Abdominal Aortic Aneurysm Hospitalization and Race and Sex Among Subgroups.
* P-value of interaction term comparing between sex and the determined subgroups.
All forest plots visualize adjusted odds ratios and associated 95% confidence intervals for each specific subgroup (lines) and overall (blue diamond; red dashed line). The horizontal black line at an adjusted odds ratio of 1.00 represents an equivalent risk between Black and White (A) or females and males (B).
aOR indicates adjusted odds ratio; 95%CI, 95% confidence interval; PVD, peripheral vascular disease; Q, quartile.
A relative increase in adjusted odds of hospitalization was observed in female persons with comorbid diabetes, PVD, hypertension, tobacco use, and lower income (Figure 3B). Additionally, Black and Asian persons with comorbid PVD, hypertension, lack of tobacco use, and higher income demonstrated a relatively increased adjusted odds of iAAA hospitalization compared to Black and Asian persons without these factors (Figure 3A, S1A).
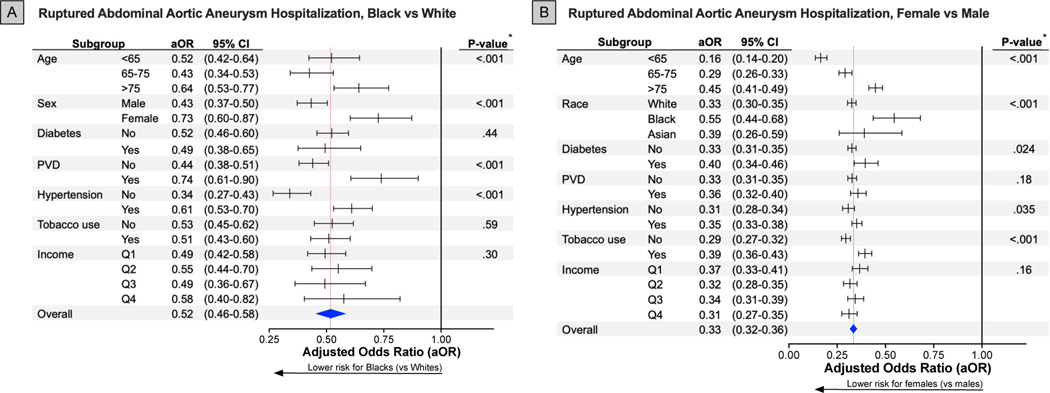
The adjusted relative risk for rAAA hospitalizations was 67% lower for female sex compared to male sex and 48% lower for Black, but not for Asian, compared to White persons (Supplemental Table I). Associations between rAAA hospitalization and race/sex subgroups paralleled that of iAAA hospitalizations with consistently observed reduced adjusted odds of rAAA hospitalization across subgroups. Importantly, these adjusted odds were also moderated by age, race, sex, and comorbid conditions. Among female persons, older versus younger patients (>75 years aOR=0.45 [95%CI, 0.41–0.49]; <65 years aOR=0.16 [95%CI, 0.14–0.20]), Black versus White or Asian persons (Black aOR=0.55 [95%CI, 0.44–0.68]; White aOR=0.33 [95%CI, 0.30–0.35]; Asian aOR=0.39 [95%CI, 0.26–0.59]), and tobacco users versus non-users (tobacco aOR=0.39 [95%CI, 0.36–0.43]; no tobacco aOR=0.29 [95%CI, 0.27–0.32]) had relatively increased adjusted odds for rAAA hospitalization (Figure 4B). Similarly, among Black persons, older versus younger and female sex versus male sex demonstrated relatively increased adjusted odds for rAAA hospitalization (Figure 4A).
Figure 4. Association Between Ruptured Abdominal Aortic Aneurysm Hospitalization and Race and Sex Among Subgroups.
* P-value of interaction term comparing between sex and the determined subgroups.
All forest plots visualize adjusted odds ratios and associated 95% confidence intervals for each specific subgroup (lines) and overall (blue diamond; red dashed line). The horizontal black line at an adjusted odds ratio of 1.00 represents an equivalent risk between Black and White (A) as well as females and males (B).
In-Hospital Mortality
The estimated in-hospital mortality rate was 4.8% (N=14,100) for all AAA hospitalizations, accounting for >2,000 deaths annually. While female sex was associated with an increased adjusted odds of in-hospital mortality (aOR=1.54 [95%CI, 1.38–1.70]), Black and Asian persons were not (Supplemental Table I). In subgroup analysis, the association between female sex and increased in-hospital mortality was ubiquitously observed but was moderated by race and AAA hospitalization type. White female persons (aOR=1.68 [95%CI, 1.50–1.88]) demonstrated increased adjusted odds of in-hospital mortality; this was not observed for Black and Asian female persons (Supplemental Figure 2). Additionally, the increased risk of in-hospital mortality among female persons compared to male persons was greater for iAAA (aOR=1.93 [95%CI, 1.66–2.25]) than for rAAA (aOR=1.29 [95%CI, 1.13–1.48]; P-interaction<0.001; Supplemental Figure 2) hospitalizations.
Sensitivity Analysis
Recategorization of Hispanic ethnicity to White race and both alternative models without comorbidities demonstrated consistently reduced adjusted odds for female sex (ranges: iAAA aOR=0.22–0.27; rAAA aOR=0.30–0.33) compared to male sex as well as Black (iAAA aOR=0.45–0.47; rAAA aOR=0.48–0.52) and Asian (iAAA aOR=0.58–0.69; rAAA aOR=0.56–0.78; Supplemental Table II) compared to White persons. When limiting hospitalizations to those with both an AAA primary diagnosis and in-hospital AAA procedure (iAAA N=199,030; rAAA N=29,310), the reduced sex- and race-specific adjusted odds of iAAA for female sex (aOR=0.27 [95%CI, 0.27–0.28]) and Black persons (aOR=0.52 [95%CI, 0.46–0.58]), but not Asian persons (aOR=0.95 [95%CI, 0.78–1.15]) were observed. However, the adjusted odds of rAAA hospitalizations (female aOR=0.96 [95%CI, 0.88–1.04]; Black aOR=1.10 [95%CI, 0.78–1.15]; Asian aOR=0.93 [95%CI, 0.79–1.09]), were not significantly different across race/sex subgroups (Supplemental Table II).
Discussion
We evaluated the risk of AAA hospitalizations using synchronized NIS and US Census data to inform existing age-, sex-, and race-specific knowledge gaps in the epidemiology of AAA care. An estimated total of 15 and 2 per 100,000 US residents were hospitalized annually for iAAA and rAAA, respectively, generating nearly 4.5 billion and 750 million in US healthcare charges each year. The reduced risk of AAA hospitalization for female sex and Black persons compared to male sex and White persons was consistent across subgroups. Importantly, increasing age was a great equalizer across sex and race, raising the risk of hospitalization for females and racial minority groups closer to that of males and White persons, especially for rAAA. Finally, White female persons demonstrated an increased risk of in-hospital mortality, which was most pronounced in iAAA compared to rAAA hospitalizations. These contemporary epidemiologic US data highlight sex- and race-specific risks and uniquely illustrate the need for the inclusion of female persons and racial minority groups in AAA research to better understand this disease in our diverse population.
Existing evidence demonstrating the benefit of AAA screening and elective repair was derived primarily from studies performed in non-US populations which often excluded female sex and did not report patient race.9, 23–27 These data, obtained from a homogenous population of White male persons, have shaped current AAA screening guidelines.3, 8, 28 In 2019, the USPSTF identified a need for epidemiologic studies to delineate AAA risk among aging, sex, and racial subpopulations.8 Defining contemporary AAA epidemiology and outcomes among female persons and racial minority groups is of particular importance as prior investigations demonstrated an increased risk of perioperative mortality and complications for female and Black persons undergoing AAA repair.5–7 We expanded this prior knowledge and showed the association between White female persons and increased in-hospital mortality was greatest among iAAA hospitalizations, presumably for elective procedures. However, we did not find increased mortality risk in Black persons, male or female, undergoing AAA repair. We also identified a four-fold and a two-fold lower female and Black persons-specific incidence, respectively, of iAAA hospitalizations compared to male and White persons. This knowledge, in combination with the established increased risk of AAA repair in White female persons, illustrates the need to consider both sex and race differences in screening and elective repair guidelines. In fact, screening and subsequent AAA repair in female persons groups may confer more harm than benefit from over diagnosis (i.e., diagnosis-related anxiety, invasive repair, and costly hospitalizations).29
The drastic differences in AAA hospitalizations across sex and race may, in part, represent patient preferences or provider implicit biases and not disease-related differences.30 Data suggest that female and Black persons are less likely to pursue elective AAA repair, tempering iAAA hospitalizations.31, 32 Further, Black persons more frequently die in-hospital, require intensive care, and pursue life sustaining measures, potentially culminating in elevated rAAA hospitalizations and the estimated lower rates of repair.33, 34 Yet, our findings were robust in subgroup and sensitivity analyses. Therefore, future retrospective as well as prospective investigations must include female sex and racial groups to more clearly inform the risk-benefit balance of AAA screening and elective repair.
The reduced incidence of AAA hospitalizations observed in female and Black persons was multifactorial and includes patient preferences as discussed above, as well as physiologic, social determinants of health, and genetic factors.30–34 The protective effects of estrogen, which reduces collagen destruction, remodeling, and immune cell migration, mitigate AAA development in females.35, 36 Interestingly, Black female persons had a risk of hospitalization closer to that of White male persons for both iAAA and rAAA. Estrogen exposure (i.e., age at menopause) is influenced by a complex relationship of socioeconomic and health-related factors.37 While race has not been shown to affect the onset of menopause, economic distress and lower education levels are associated with earlier onset of menopause and reduced estrogen exposure.37, 38 While these associations may not entirely explain the increased risk of AAA hospitalization for Black compared to White female persons, the lower incomes among Black persons in our analysis may contribute to reduced estrogen exposure and increased incidence of AAA hospitalizations. Additionally, our data and previous studies demonstrated that social determinants of health influence differences in healthcare access among racial minority groups.39 We observed an increasing risk of iAAA, but not rAAA, hospitalization among Black persons with increasing income. However, even in the highest income quartile, the risk of iAAA hospitalization was still nearly 50% lower than that of White persons. Thus, socioeconomic factors do not completely explain the observed racial differences.5, 6 Finally, as family history of AAA contributes significantly to an individual’s risk, the prevalence of susceptibility genes regulating inflammation, proteases, and smooth muscle phenotype may differ significantly by race.40 Further investigations into biologic factors and associated heritable and population-based differences (i.e., race and ethnicity) may elucidate the underlying mechanistic risk of AAA and guide the development of pharmacologic therapies for AAA.
Our analysis reiterates the substantially increased incidence and risk of AAA hospitalization in aging patients which varied by sex and race, particularly for rAAA.5, 7 Comparing the mean age of AAA hospitalization across sexes and race must be interpreted with caution as comparison of such data points may solely represent the age distribution and life expectancy of the US population (i.e., female and White persons outlive male and Black persons). Therefore, we synchronized NIS and US Census data to account for these sex- and race- specific differences in population distribution. Combining these databases allowed a more accurate understanding of the estimated incidence for each sex and race. Using this strategy, our data ubiquitously demonstrated a peak age-specific incidence of iAAA hospitalizations at 65–75 years, while the incidence of rAAA hospitalizations continued to increase into the ninth decade of life. Interestingly, nearly one in five rAAA hospitalizations occurred at an age younger than the recommended screening window of age 65–75 years. Of these hospitalizations, 66% occurred among White male persons. Since the studies that shaped the current guidelines did not delineate race, the recommended screening age of 65 may be skewed towards an older age by not accounting for the influence of race on the risk of AAA development. Our analysis accounted for sex-, race-, and age-specific risk estimates of US AAA hospitalizations, and supports the growing evidence to reduce the age for AAA screening among White male persons.41
In contrast to younger decades, after 85 years of age, the race- and sex-specific incidence and adjusted risk of AAA hospitalizations for racial minority groups and female persons approached that of White male persons. Multiple reasons may exist for the equalization of sex- and race-specific risk for AAA hospitalization with increasing age. Such differences may represent biological differences (i.e., decreasing estrogen exposure with age among women) and subsequent temporal development of AAA between these groups. Alternatively, the trend towards equalization of AAA hospitalization for female persons and racial minority groups with increasing age may arise from the existing treatment paradigms (i.e., preferential male screening and sex/race disparities in cardiovascular disease) leading to delayed AAA diagnosis in female persons and racial minority groups.8, 42 While such potential causes require further investigation, the latter represents a missed opportunity to identify AAAs in younger and more robust female and Black persons in whom treatment outcomes may be improved to levels comparable to White male persons.
Our data have limitations. First, the incidence of rAAA did not include those who died without hospitalization, thus limiting our epidemiologic understanding of AAA. Second, all administrative data were retrospectively abstracted relying upon hospital ICD coding which has regional variability.43 We accounted for such variability through the inclusion of these factors in our multivariable models; however, an on-going risk of hospitalization misclassification and unmeasured confounding may exist. Third, the NIS categorizes Hispanic ethnicity as a race, preventing evaluation of the effects of ethnicity on race and exact synchronization with US Census data. Lastly, the NIS does not capture medications, family history, AAA anatomic information, and access to care/screening, and thus cannot further inform our findings.
Conclusion
The contemporary incidence and risk of AAA hospitalization in the US varied significantly by age, sex, and race. Female sex as well as racial minority groups were associated with substantial decreases while older age and White race conferred significant increases in the incidence and adjusted odds of AAA hospitalizations. These distinct differences across age, sex, and race subgroups highlight the importance of inclusiveness in future studies.
Supplementary Material
ARTICLE HIGHLIGHTS.
Type of Research:
Retrospective cohort study of data synchronized from the National Inpatient Sample and US Census
Key Findings:
The risk of AAA hospitalizations were lower for Black compared to White persons (intact: OR=0.47 [95%CI, 0.45–0.49]; ruptured: OR=0.52 [95%CI, 0.46–0.58]) and female compared to male sex (intact: aOR, OR=0.27 [95%CI, 0.27–0.28; rupture: OR=0.33 [95%CI, 0.32–0.36]). The reduced risk by race and sex were minimized with aging and peripheral vascular disease.
Take home Message:
We confirmed a substantially decreased adjusted risk of AAA hospitalization for female sex and racial minority groups; however, aging and comorbid peripheral vascular disease reduced these differences.
Acknowledgements
The University of Pittsburgh holds a Physician-Scientist Institutional Award from the Burroughs Wellcome Fund (Li).
Funding/Support
This research was supported in part by the grant 5T32HL098036 from the National Heart, Lung, and Blood Institute (Li, Reitz, Phillips), L30 AG064730 National Institute on Aging (Reitz) and U01TR002393 (Shireman) National Center for Advancing Translational Sciences and the Office of the Director, National Institutes of Health.
Role of funders/sponsors
These funding sources had no role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; preparation, review, or approval of the manuscript; and decision to submit for publication.
Presentation: A subset of our work has been presented at the American Heart Association’s annual Scientific Sessions 2021 (Abstract Number: 10519)
Footnotes
Conflict of interest disclosure
No authors report disclosures, conflict of interest or relevant financial interests related to the content of the manuscript. The opinions expressed here are those of the authors and do not necessarily reflect the position of the Department of Veterans Affairs or the US government.
Publisher's Disclaimer: This is a PDF file of an article that has undergone enhancements after acceptance, such as the addition of a cover page and metadata, and formatting for readability, but it is not yet the definitive version of record. This version will undergo additional copyediting, typesetting and review before it is published in its final form, but we are providing this version to give early visibility of the article. Please note that, during the production process, errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Karthikesalingam A, Holt PJ, Vidal-Diez A, Ozdemir BA, Poloniecki JD, Hinchliffe RJ, et al. Mortality from ruptured abdominal aortic aneurysms: Clinical lessons from a comparison of outcomes in england and the USA. Lancet. 2014;383:963–969 [DOI] [PubMed] [Google Scholar]
- 2.Summers KL, Kerut EK, Sheahan CM, Sheahan MG, 3rd. Evaluating the prevalence of abdominal aortic aneurysms in the united states through a national screening database. J Vasc Surg. 2021;73:61–68 [DOI] [PubMed] [Google Scholar]
- 3.Chaikof EL, Dalman RL, Eskandari MK, Jackson BM, Lee WA, Mansour MA, et al. The society for vascular surgery practice guidelines on the care of patients with an abdominal aortic aneurysm. J Vasc Surg. 2018;67:2–77 e72 [DOI] [PubMed] [Google Scholar]
- 4.Lindholt JS, Norman PE. Meta-analysis of postoperative mortality after elective repair of abdominal aortic aneurysms detected by screening. Br J Surg. 2011;98:619–622 [DOI] [PubMed] [Google Scholar]
- 5.Osborne NH, Upchurch GR Jr., Mathur AK, Dimick JB. Explaining racial disparities in mortality after abdominal aortic aneurysm repair. J Vasc Surg. 2009;50:709–713 [DOI] [PubMed] [Google Scholar]
- 6.Vogel TR, Cantor JC, Dombrovskiy VY, Haser PB, Graham AM. Aaa repair: Sociodemographic disparities in management and outcomes. Vasc Endovascular Surg. 2008;42:555–560 [DOI] [PubMed] [Google Scholar]
- 7.McPhee JT, Hill JS, Eslami MH. The impact of gender on presentation, therapy, and mortality of abdominal aortic aneurysm in the united states, 2001–2004. J Vasc Surg. 2007;45:891–899 [DOI] [PubMed] [Google Scholar]
- 8.US Preventive Services Task Force. Screening for Abdominal Aortic Aneurysm: US Preventive Services Task Force Recommendation Statement. JAMA. 2019;322(22):2211– 2218. [DOI] [PubMed] [Google Scholar]
- 9.Guirguis-Blake JM, Beil TL, Senger CA, Coppola EL. Primary care screening for abdominal aortic aneurysm: Updated evidence report and systematic review for the us preventive services task force. JAMA. 2019;322:2219–2238 [DOI] [PubMed] [Google Scholar]
- 10.Ghaferi AA, Schwartz TA, Pawlik TM. Strobe reporting guidelines for observational studies. JAMA Surg. 2021 [DOI] [PubMed] [Google Scholar]
- 11.Khera R, Angraal S, Couch T, Welsh JW, Nallamothu BK, Girotra S, et al. Adherence to methodological standards in research using the national inpatient sample. JAMA. 2017;318:2011–2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Healthcare Cost and Utilization Project, Agency for Healthcare Research and Quality. Checklist for Working with the NIS. Rockville, MD; Updated March 2021. Accessed May 1, 2021. https://www.hcup-us.ahrq.gov/db/nation/nis/nischecklist.jsp [Google Scholar]
- 13.Breathett K, Spatz ES, Kramer DB, Essien UR, Wadhera RK, Peterson PN, et al. The Groundwater of Racial and Ethnic Disparities Research: A Statement From Circulation: Cardiovascular Quality and Outcomes. Circ Cardiovasc Qual Outcomes. 2021;14(2):e007868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Centers for Disease Control and Prevention. Preferred Terms for Select Population Groups & Communities. Updated October 2021. Accessed May 1, 2021. https://www.cdc.gov/healthcommunication/Preferred_Terms.html
- 15.Annual Estimates of the Resident Population by Sex, Age, Race, and Hispanic Origin for the United States: April 1, 2010 to July 1, 2019. (NC-EST2019-ASR6H); Updated June 2020. Accessed May 1, 2021. https://www.census.gov/newsroom/press-kits/2020/population-estimates-detailed.html
- 16.Healthcare Cost and Utilization Project, Agency for Healthcare Research and Quality. NIS Description of Data Elements. Rockville, MD; Updated September 2008. Accessed May 1, 2021. https://www.hcup-us.ahrq.gov/db/nation/nis/nisdde.jsp [Google Scholar]
- 17.Healthcare Cost and Utilization Project, Agency for Healthcare Research and Quality. Elixhauser Comorbidity Software Refined for ICD-10-CM. Updated October 2020. Accessed May 1, 2021. https://www.hcup-us.ahrq.gov/toolssoftware/comorbidityicd10/comorbidity_icd10.jsp
- 18.Desai RJ, Solomon DH, Shadick N, Iannaccone C, Kim SC. Identification of smoking using medicare data--a validation study of claims-based algorithms. Pharmacoepidemiol Drug Saf. 2016;25:472–475 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Columbo JA, Kang R, Trooboff SW, Jahn KS, Martinez CJ, Moore KO, et al. Validating publicly available crosswalks for translating icd-9 to icd-10 diagnosis codes for cardiovascular outcomes research. Circ Cardiovasc Qual Outcomes. 2018;11:e004782 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Healthcare Cost and Utilization Project, Agency for Healthcare Research and Quality. Producing National HCUP Estimates. Updated December 2018. Accessed May 1, 2021. www.hcup-us.ahrq.gov/tech_assist/nationalestimates/508_course/508course_2018.jsp
- 21.Healthcare Cost and Utilization Project, Agency for Healthcare Research and Quality. NIS Database Documentation. Updated April 2021. Accessed May 1, 2021. www.hcup-us.ahrq.gov/db/nation/nis/nisdbdocumentation.jsp
- 22.U.S. Census Bureau PD. Table 1. Annual Estimates of the Resident Population: April 1, 2010 to July 1, 2019 (PEPANNRES). Updated December 2019. Accessed May 1, 2021. https://data.census.gov/cedsci/table?q=population%20estimate%20&g=0100000US 0400000_0200000US1,2,3,4&tid=PEPPOP2019.PEPANNRES.
- 23.Ashton HA, Buxton MJ, Day NE, Kim LG, Marteau TM, Scott RA, et al. The multicentre aneurysm screening study (mass) into the effect of abdominal aortic aneurysm screening on mortality in men: A randomised controlled trial. Lancet. 2002;360:1531–1539 [DOI] [PubMed] [Google Scholar]
- 24.Norman PE, Jamrozik K, Lawrence-Brown MM, Le MT, Spencer CA, Tuohy RJ, et al. Population based randomised controlled trial on impact of screening on mortality from abdominal aortic aneurysm. BMJ. 2004;329:1259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Scott RA, Wilson NM, Ashton HA, Kay DN. Influence of screening on the incidence of ruptured abdominal aortic aneurysm: 5-year results of a randomized controlled study. Br J Surg. 1995;82:1066–1070 [DOI] [PubMed] [Google Scholar]
- 26.Lindholt JS, Sogaard R. Population screening and intervention for vascular disease in danish men (viva): A randomised controlled trial. Lancet. 2017;390:2256–2265 [DOI] [PubMed] [Google Scholar]
- 27.McCaul KA, Lawrence-Brown M, Dickinson JA, Norman PE. Long-term outcomes of the western australian trial of screening for abdominal aortic aneurysms: Secondary analysis of a randomized clinical trial. JAMA Intern Med. 2016;176:1761–1767 [DOI] [PubMed] [Google Scholar]
- 28.Hirsch AT, Haskal ZJ, Hertzer NR, Bakal CW, Creager MA, Halperin JL, et al. Acc/aha 2005 practice guidelines for the management of patients with peripheral arterial disease (lower extremity, renal, mesenteric, and abdominal aortic): A collaborative report from the american association for vascular surgery/society for vascular surgery, society for cardiovascular angiography and interventions, society for vascular medicine and biology, society of interventional radiology, and the acc/aha task force on practice guidelines (writing committee to develop guidelines for the management of patients with peripheral arterial disease): Endorsed by the american association of cardiovascular and pulmonary rehabilitation; national heart, lung, and blood institute; society for vascular nursing; transatlantic inter-society consensus; and vascular disease foundation. Circulation. 2006;113:e463–654 [DOI] [PubMed] [Google Scholar]
- 29.Berlin L Overdiagnosed: Making people sick in pursuit of health. JAMA : the journal of the American Medical Association. 2011;305:1356–1359 [Google Scholar]
- 30.Chapman EN, Kaatz A, Carnes M. Physicians and implicit bias: How doctors may unwittingly perpetuate health care disparities. J Gen Intern Med. 2013;28:1504–1510 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hawker GA, Wright JG, Coyte PC, Williams JI, Harvey B, Glazier R, et al. Differences between men and women in the rate of use of hip and knee arthroplasty. N Engl J Med. 2000;342:1016–1022 [DOI] [PubMed] [Google Scholar]
- 32.Farjah F, Wood DE, Yanez ND 3rd, Vaughan TL, Symons RG, Krishnadasan B, et al. Racial disparities among patients with lung cancer who were recommended operative therapy. Arch Surg. 2009;144:14–18 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Pritchard RS, Fisher ES, Teno JM, Sharp SM, Reding DJ, Knaus WA, et al. Influence of patient preferences and local health system characteristics on the place of death. Support investigators. Study to understand prognoses and preferences for risks and outcomes of treatment. J Am Geriatr Soc. 1998;46:1242–1250 [DOI] [PubMed] [Google Scholar]
- 34.Barnato AE, Berhane Z, Weissfeld LA, Chang CC, Linde-Zwirble WT, Angus DC, et al. Racial variation in end-of-life intensive care use: A race or hospital effect? Health Serv Res. 2006;41:2219–2237 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wu XF, Zhang J, Paskauskas S, Xin SJ, Duan ZQ. The role of estrogen in the formation of experimental abdominal aortic aneurysm. Am J Surg. 2009;197:49–54 [DOI] [PubMed] [Google Scholar]
- 36.Sinha I, Cho BS, Roelofs KJ, Stanley JC, Henke PK, Upchurch GR, Jr. Female gender attenuates cytokine and chemokine expression and leukocyte recruitment in experimental rodent abdominal aortic aneurysms. Ann N Y Acad Sci. 2006;1085:367–379 [DOI] [PubMed] [Google Scholar]
- 37.Gold EB, Crawford SL, Avis NE, Crandall CJ, Matthews KA, Waetjen LE, et al. Factors related to age at natural menopause: Longitudinal analyses from swan. Am J Epidemiol. 2013;178:70–83 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Wise LA, Krieger N, Zierler S, Harlow BL. Lifetime socioeconomic position in relation to onset of perimenopause. J Epidemiol Community Health. 2002;56:851–860 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Nasser SA, Senatore FF. Contemporary concepts in access to healthcare: Identification and elimination of disparities in care of minority patients. Prog Cardiovasc Dis. 2020;63:2–3 [DOI] [PubMed] [Google Scholar]
- 40.Nordon IM, Hinchliffe RJ, Loftus IM, Thompson MM. Pathophysiology and epidemiology of abdominal aortic aneurysms. Nat Rev Cardiol. 2011;8:92–102 [DOI] [PubMed] [Google Scholar]
- 41.Anjorin A, Vemulapalli S, Svetkey L, Greiner M, Southerland K, Smerek M, et al. Underutilization of Guideline-based Abdominal Aortic Aneurysm Screening in an Academic Health System. J Vasc Surg. 2021;74(3):e226–e226. [DOI] [PubMed] [Google Scholar]
- 42.Mensah GA, Mokdad AH, Ford ES, Greenlund KJ, Croft JB. State of disparities in cardiovascular health in the united states. Circulation. 2005;111:1233–1241 [DOI] [PubMed] [Google Scholar]
- 43.Lucyk K, Tang K, Quan H. Barriers to data quality resulting from the process of coding health information to administrative data: A qualitative study. BMC Health Serv Res. 2017;17:766. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.