Abstract
Immunotherapies seek to unleash the immune system against cancer cells. While a variety of immunotherapies exist, one of the most commonly used is immune checkpoint blockade, which refers to the use of antibodies to interfere with immunosuppressive signaling through immune checkpoint molecules. Therapies against various checkpoints have had success in the clinic across cancer types. However, the efficacy of checkpoint inhibitors has varied across different cancer types and non-responsive patient populations have emerged. Non-responders to these therapies have highlighted the importance of understanding underlying mechanisms of resistance in order to predict which patients will respond and to tailor individual treatment paradigms. In this review we discuss the literature surrounding tumor mediated mechanisms of immune checkpoint resistance. We also describe efforts to overcome resistance and combine checkpoint inhibitors with additional immunotherapies. Finally, we provide insight into the future of immune checkpoint blockade, including the need for improved preclinical modeling and predictive biomarkers to facilitate personalized cancer treatments for patients.
Keywords: Immunotherapy, Checkpoint inhibitors, Resistance, Biomarkers, Cancer
1. Introduction
Cancer immunotherapy seeks to target the immune system against tumor cells and has garnered significant attention as an alternative to traditional chemotherapies. While a variety of immunotherapies exist, monoclonal antibodies against immune checkpoint molecules are among the most successful and popular to date. Two traditional checkpoint molecules, PD-1 and CTLA-4, serve as ideal examples of how these immunotherapies function in patients.
An understanding of the role these molecules play in tumorigenic immunosuppression began with insight into the importance of T cells in immune surveillance of cancer and molecular events mediating T cell responses to antigen. Hints of the potential impact of the immune system on cancer progression were first seen in clinical reports tying the spontaneous regression of cancers in patients with autoimmune diseases [1]. Indeed, early exploratory studies in human patients, such as Halliday et al., suggested the role of T cells in the spontaneous regression of skin cancers [2]. This was followed by mechanistic studies further demonstrating the importance of the immune system in cancer biology, as exemplified by Shankaran et al. who highlighted the role of IFN-gamma and T cells in cancer immune surveillance [3]. Indeed, Shankaran et al. demonstrated the ability for T cells and IFN-gamma to protect against the development of carcinogen-induced sarcomas and epithelial carcinomas [3]. However, research into progressive tumors highlighted the high incidence of dysfunctional T cells in many cancers [4,5]. This demonstrated the importance of T cell activation and maintenance biology in immune mediated cancer killing.
The induction of a T cell response includes the recognition and binding of the TCR to an antigen presented in the major histocompatibility complex (MHC), as well as a second signal, as mentioned above, which frequently occurs as the binding of B7-1 (CD80) or B7-2 (CD86) to CD28 [6,7]. Subsequent studies demonstrated that activation of the T cell receptor (TCR) alone is inadequate for the generation of a robust immune response to an antigen. Rather, second and third signals in the form of costimulation and cytokine support were discovered to be required for adequate T cell activation [6,7]. Once activated, it became clear that T cells expressed checkpoint molecules, such as CTLA-4 and PD-1, which work in the induction and effector phases of a T cell mediated immune response, respectively, to support normal physiology and avoid issues of autoimmunity [8]. In normal physiology, CTLA-4 plays an important role in the negative regulation of T cell responses through a number of potential pathways, including the competitive binding of CD80 and CD86 and their trans-endocytosis [9]. Indeed, the value of CTLA-4 in normal physiology is highlighted by the lymphoproliferative disorders seen in mice deficient for CTLA-4 as a result of uncontrolled T cell activation [10]. After activation, T cells commonly upregulate PD-1 expression which acts as an inhibitory signal when binding its cognate ligand, programmed cell death 1 ligand 1 (PD-L1) [11]. Similarly, to CTLA-4, PD-1 plays an important role in the prevention of autoimmunity, with PD-1 receptor deficient mice developing an number of autoimmune conditions, including autoimmune dilated cardiomyopathy [12].
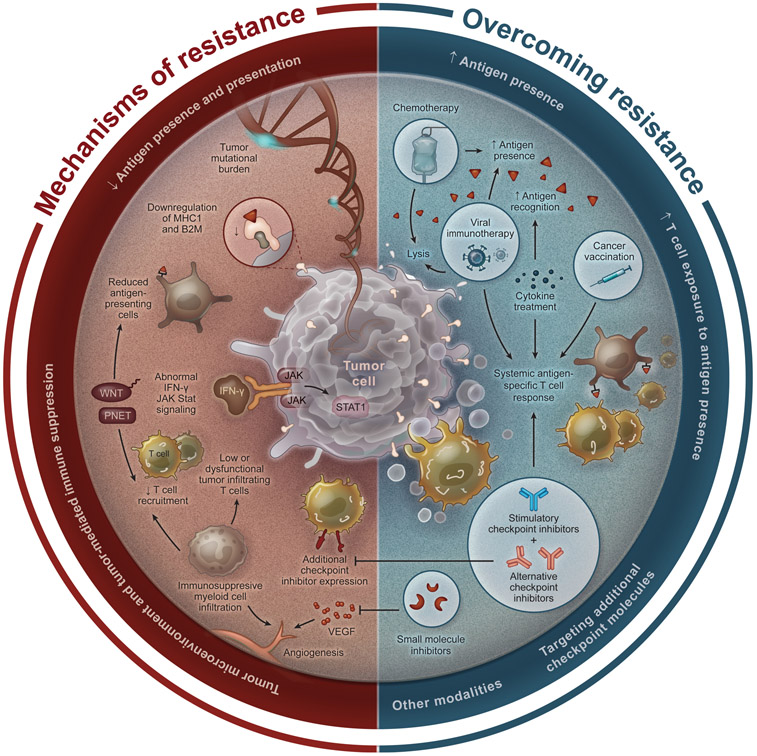
In cancer, however, these checkpoint molecules are pathologically co-opted by tumor cells and immunosuppressive tumor infiltrating immune cells to prevent immune mediated cancer cell clearance. The expression of checkpoint molecules is seen in multiple cancer types, including brain cancers, breast cancers, melanoma, and lung cancers [13]. Indeed, checkpoint molecule expression has been correlated with tumor tolerance in a number of preclinical cancer models [14]. This led to the development of checkpoint inhibitors (CPIs) in the form of monoclonal antibodies to block these inhibitory interactions and allow for the induction or continuation of an anti-tumor immune response [15]. CPIs have shown great promise in the clinical setting, with the potential to provide a durable anti-tumor immune response, leading to a number of clinical trials and use in a variety of cancers. However, while CPIs have seen incredible and durable success in the clinic as a potential alternative to traditional chemotherapy and revolutionized the treatment of multiple different malignancies, their limitations have also become clear as clinicians gain experience with their use. Indeed, despite the promise of CPIs, a significant number of patients with approved cancers do not respond to CPI treatment or respond briefly before developing resistance, with high variability between cancer types [16]. This has been an area of incredible frustration for both clinicians and patients hoping to utilize these treatments, spurring active research into CPI resistance. In this review, we provide a summary of key mechanisms of CPI resistance, methods to predict responders, overcoming resistance, and new and upcoming technologies to improve and augment traditional CPIs (Fig. 1).
Fig. 1. Diagram illustrating mechanisms of resistance to checkpoint blockade and strategies to overcome this resistance.
Shown are mechaisms of resistance to checkpoint blockade identified in cancers (left) and strategies to overcome this resistance that have been proposed and explored to date (right).
2. Mechanisms of resistance
The generation of a robust anti-tumor immune response is a complex process whose mechanisms are still not entirely understood. Anti-tumor immunity likely requires adequate tumor associated antigens, antigen presentation, co-stimulation and appropriate cytokine expression, and the dampening of immunosuppressive signaling pathways in the tumor microenvironment [17]. Perturbations of any of these steps can contribute to CPI resistance.
2.1. Antigen presence and presentation
As previously mentioned, the generation of a robust T cell mediated immune response requires antigen presentation via the major histocompatibility complexes of antigen presenting cells. As a result, it is not surprising that the success of CPIs has been correlated with tumor mutational burden, as an increased mutational burden is also likely indicative of overall tumor antigen number, providing more “targets” for the immune system [18-22]. This is highlighted well by Samstein et al. who assessed the tumor mutational burden of 1662 patients with CPI treatment and 5371 without CPI; they found that, for most cancer histologies, patients with a higher mutational burden had better responses to CPI treatment, despite inconsistent mutational cut-offs between cancer types. These findings were replicated by Valero, et al. who performed a similar study using data from 10,223 patients (80 % with CPI, 20 % without) [20]. Finally, in a recent study by Litchfield et al., whole-exome and transcriptomic data for >1000 CPI-treated patients across seven tumor types were collected and analyzed to assess markers of CPI responsiveness. They found that clonal tumor mutational burden followed by total tumor mutational burden best predicted response to CPI, underscoring the relevance of this topic. [23].
While a higher tumor mutational burden may provide more potential neoantigens for the immune system to hone in on, there is also evidence that cancers can escape the immune system though the removal of neoantigens from the tumor microenvironment [24,25]. In a study of 88 early and untreated non-small cell lung cancers, Rosenthal et al. found evidence of immunoediting of tumors, with a reduced number of neoantigens overall and reduced neoantigen load in sparsely infiltrated tumors. When assessing patients who had received immunotherapy, Anagnostou et al. also identified neoantigen loss in response to treatment as a potential escape mechanism, highlighting the evolution of tumors to a lower immunogenicity phenotype that is more resistant to CPI treatment. Interestingly, Gromeier et al. found that patients with recurrent glioblastoma who had a lower mutational burden had longer survival after viral or CPI therapy. In this study, tumors with a lower mutational burden had increased inflammatory gene signals, possibly indicating that even though ongoing immune neoantigen-editing is occurring in the tumor, this process may provide more immune cell substrate for CPI therapy [26]. Additional research in gliomas has demonstrated the development of hypermutated tumors in response to temozolomide treatment. However, these hypermutated tumors demonstrated low T cell infiltration, high intratumoral heterogeneity, and a poor response to anti-PD1 therapy, in contrast to other cancer types [27]. These results highlight the need for additional research to determine the relationship between tumor mutational burden and CPI response, including differences in that relationship between cancer types and over treatment time.
As would be expected given the previous studies highlighting the importance of tumor mutational burden on CPI response, antigen presentation also plays a critical role in treatment response. The expression of major histocompatibility complexes, which are vital in antigen presentation to T cells have been correlated with response to immunotherapy [28,29]. The beta-2-microglobulin (B2M), which classically stabilizes MHC I in particular and thus plays an important role in antigen presentation, has been identified as a potential mediator of CPI treatment outcomes [30-32]. Indeed, Sade-Feldman et al. demonstrated B2M loss of heterozygosity as being enriched in patients with metastatic melanoma who were non-responders to CPI, relative to those patients who responded to treatment [32]. However, a study of microsatellite instability-high (MSI-H) colorectal carcinomas, which have a high rate of response to immunotherapies but are also associated with B2M mutations, demonstrated good responses to immunotherapy despite the presence of B2M mutations, including those leading to loss of expression, highlighting the need for additional investigation into the role and prognostic value of this gene [33].
2.2. Tumor microenvironment and tumor mediated immune suppression
Tumor intrinsic signaling pathways have also been implicated in resistance to CPIs through immunosuppressive changes to the tumor microenvironment [34]. Signaling pathways that have been implicated in tumor evasion of immunotherapies include the WNT and PTEN pathways, amongst others [34]. In vitro studies have suggested the WNT signaling can induce immune suppression through a variety of mechanisms, including increased expression of the immunosuppressive cytokine IL-10 as well as suppression of dendritic cells [35]. In addition, the soluble WNT5a ligand has been shown to support the formation of immunosuppressive dendritic cells, supporting tumor progression [36]. Clinically, WNT signaling has also been shown to reduce the recruitment of T cells to the tumor microenvironment, further promoting resistance to CPIs [37,38]. The PTEN signaling pathway has similarly been implicated as contributing to CPI resistance. Peng et al. demonstrated that PTEN loss in both preclinical models and in patients was associated with decreased T cell infiltration and recruitment to the tumor microenvironment. Interestingly, in patients, PTEN loss was also associated with a reduced response to anti-PD-1 therapy, highlighting the clinical impact of this genetic alteration [39]. Zhao et al. reported similar findings in 66 patients with glioblastoma; they found an enrichment of PTEN mutations associated with immunosuppressive gene signatures in non-responders to CPI treatment, further implicating PTEN mutations in CPI response.
A major cytokine mediator of anti-tumor immune responses is interferon-gamma (IFN-y) signaling pathways. In a normal anti-tumor immune response, activated T cells secrete IFN-y, leading to JAK-STAT mediated signaling in tumor cells and the upregulation of both MHC I and PD-L1 as well as anti-tumor and proapoptotic effects [40]. Indeed, preclinical studies have highlighted the importance of IFN-y in immune mediated rejection of tumor cells [41]. These pathways have also been identified on multiple CRISPR screens, including Manguso et al. who utilized an in vivo CRISPR screen to identify mediators of PD-1 CPI resistance [42,43]. They highlighted loss of the gene PTPN2, which regulates the downstream anti-tumor effects of IFN-y as enhancing response to CPI, further highlighting the importance of IFN-y signaling [42]. Using cell lines from melanoma patients, Sucker et al. demonstrated that inactivating JAK1/JAK2 mutations, which can frequently arise in response to immunotherapy, confer resistance to the proapoptotic effects of IFN-y and reduce MHC I expression [44]. Clinical studies have also demonstrated these findings in patients receiving immunotherapies, with JAK1/JAK2 mutations repeatedly identified [30,45,46]. Indeed, Gao et al. identified copy number alterations in IFN-y signaling pathways as being significantly increased in both animal models and patients with resistance to anti-CTLA-4 therapy [46]. Nevertheless, additional investigation into IFN-y perturbations on the treatment efficacy of CPIs is needed, as well as ways to counteract immunosuppressive mutations.
Another consideration when discussing mechanisms of tumor escape from CPI is the tumor microenvironment (TME). The TME consists of the immune and non-immune cells, vessels, and extracellular environment surrounding a tumor. Immune cells in the TME can contribute to lack of CPI response through multiple modalities, including effector cell dysfunction and immunosuppressive cell phenotypes. A number of studies have shown a positive relationship between T cell infiltration and response to CPI, highlighting this as a potential mechanism of resistance in tumors with minimal T cell infiltration [47,48]. Cancers can also induce severe T cell dysfunction with the expression of multiple immunosuppressive molecules, adapting to a single checkpoint molecule. Indeed, a study by Koyama et al. demonstrated an upregulation of TIM-3, an alternative checkpoint molecule in mice with lung adenocarcinoma treated with anti-PD-1 therapy; blockade of TIM-3 resulted in improved survival, demonstrating the functional importance of alternative checkpoint molecule expression. The relatively inflexible epigenetics of exhausted T cells in the TME has also been implicated in the failure to generate a memory response following CPI [49]. Infiltration of immunosuppressive immune cells, including myeloid derived suppressor cells (MDSCs) and tumor associated macrophages (TAMs), in the TME can correspond with T cell infiltration and can also contribute to a CPI resistant environment [50-56]. Indeed, Peranzoni et al. discovered that in human lung squamous cell cancers macrophages prevented the infiltration of T cells into the tumor microenvironment via long lasting trapping [56]. In mice, depletion of macrophages with CSF-1R blockade increased T cell infiltration into tumors and potentiated anti-PD-1 treatment [56]. As mentioned, additional myeloid cell populations in the TME also mediate CPI resistance through a variety of mechanisms. Interestingly, in a cohort of 46 patients with metastatic melanoma undergoing anti-PD-1 therapy, Chen et al. identified a significantly closer proximity of CD68+ myeloid cells in non-responders at pre-treatment and on-treatment timepoints, highlighting an additional potential role for myeloid cells in CPI resistance [54]. In addition, Lo Russo et al. demonstrated an enrichment of CD163+CD33+PD-L1+ macrophages with epithelioid morphology in patients with hyperprogressive disease following CPI treatment, suggesting myeloid cells may be implicated in this clinical phenomena [52]. In addition to immune cell infiltration, VEGF expression in the TME has also been identified as associated with resistance to anti-PD-1 treatment in patients [54]. In fact, blockade of VEGF in combination with anti-PD-L1 CPI has been shown to increase antigen-specific T-cell migration in metastatic renal cell carcinoma [57]. This was associated with increased expression of MHC-I, T-effector markers, and CX3CL1, a potent attractor of T cells [57].
3. Overcoming resistance
Treatments to overcome resistance to CPIs work to generate a robust anti-tumor immune response by targeting many of the aforementioned mechanisms of tumor treatment escape. Indeed, combining CPIs with other forms of immunotherapy or chemotherapy holds great promise for the future of cancer treatment. Current combination strategies include combinations with chemotherapies, cancer vaccines, oncolytic viruses, alternative CPIs, and small molecule inhibitors.
3.1. Increasing T cell exposure to antigens
As previously mentioned, T cell exposure to antigens is a critical aspect of generating an anti-cancer immune response. Therapies that increase T cell exposure to tumor associated antigens in a favorable manner include cancer vaccinations, oncolytic viruses, and chemotherapies. Cancer vaccines have been shown to induce systemic antigen specific T cell responses and increase the infiltration of tumor specific T cells in the TME, making them attractive candidates for combination with CPIs as they increase the intratumoral substrate that can be acted upon [58]. Studies investigating the combination of cancer vaccines with CPI treatment have been promising thus far, demonstrating good safety profiles and some durable treatment responses in patients [59, 60]. Personalized vaccinations specific to a patients tumor and with the ability to generate better prime the immune system against a cancer also represent an exciting future area of investigation in combination with CPIs [61]. An additional method of increasing T cell antigen exposure is through the use of oncolytic viruses. Oncolytic viruses infect and lyse tumor cells, leading to local inflammation, stimulation of the immune system, and tumor associated antigen release. As a result, many consider oncolytic viruses as a form of local cancer vaccination [62]. Studies into the combination of oncolytic viruses with CPIs have been promising thus far. A phase II clinical trial of the oncolytic virus talimogene laherparepvec plus ipilimumab versus ipilimumab alone for the treatment of advanced melanoma demonstrated improved survival in the combination group, with no difference in adverse events; this highlights the safety, efficacy, and promise of such treatments in patients [63]. Chemotherapies can also lead to tumor lyses and antigen release, providing additional targets for T cells to hone in on, with some exciting results in the literature when combined with CPIs. Combining CPIs with traditional chemotherapies may also allow for doses of both treatments to be lowered, potentially reducing treatment associated side effects. In fact, a phase III clinical trial of patients with non-small cell lung cancer comparing pembrolizumab and chemotherapy to standard chemotherapy alone demonstrated significantly increased survival in patients with combination therapy [64]. Interestingly, preclinical studies have also indicated a potential negative impact of systemic chemotherapy on the efficacy of CPIs, due to harmful effects on the bone marrow and subsequent lymphodepletion [65]. This raises the question of which chemotherapies to combine with CPI treatment and the potential benefit of using local chemotherapies instead of systemic treatments with CPIs, when appropriate.
3.2. Targeting additional checkpoint molecules
While CTLA-4 and PD-1 were the first checkpoint molecules to be identified and are the most commonly targeted and studied, additional checkpoint molecules have since been discovered; these include both inhibitory and stimulatory checkpoints. Additional inhibitory checkpoint molecules that have been investigated include: LAG-3 (Lymphocyte activation gene 3), TIM-3 (T-cell immunoglobulin domain and mucin domain 3), and TIGIT (T cell immunoglobulin and ITIM domain), which can frequently be co-expressed with PD1 [66,67]. Blockade of these alternative inhibitory checkpoint molecules has shown significant promise in preclinical models as a monotherapy and in combination with PD-1 or CTLA-4 treatment. In addition, blocking the cognate ligand of PD-1, PD-L1 (Programmed death-ligand 1, inhibitory molecule), is a target of monoclonal antibodies, some of which have been approved by the FDA (Table 1) [68]. Clinical trials involving LAG-3, TIM-3, and TIGIT as mono- and combination therapies are underway [66,67]. Addition inhibitor checkpoint molecules that have been identified include IDO (Indoleamine 2,3-dioxygenase), KIR (Killer Ig-like receptors), and VISTA (V-domain Ig suppressor of T cell activation). Stimulatory checkpoints that have also been identified include 4-1BB, OX40, GITR (Glucocorticoid-Induced TNFR-Related), CD40, CD27, CD80, and ICOS (inducible T cell co-stimulator). Similarly to alternative inhibitory molecules, activation of these stimulatory checkpoint molecules has seen success in preclinical studies as monotherapies and in combination with traditional CPIs [69,70]. In fact, using the poorly immunogenic B16F10 melanoma model, Chen et al. demonstrated an improved treatment response to anti-PD-1 treatment combined with anti-4-1BB therapy relative to an anti-PD-1 and anti-LAG-3 combination, highlighting the promise of these therapies [70]. However, the addition of a second checkpoint inhibitor has been shown to increase the rate of serious autoimmune side effects in some cancers, highlighting the need to balance treatment efficacy with patient safety [71-73]. Additional investigation into the utility of agonistic treatments with or without another CPI are underway [69] (Tables 2 and 3).
Table 1.
Approved Checkpoint Inhibitors.
| Name & Cancer Type | Target | Year of FDA Approval |
Comment |
|---|---|---|---|
| Nivolumab | PD-1 | ||
| Melanoma | 2014 | For advanced or unresectable disease following prior treatment | |
| Squamous Non-Small Cell Lung Cancer | 2015 | For metastatic disease with progression on or after platinum-based chemotherapy | |
| Renal Cell Carcinoma | 2015 | For metastatic disease with prior anti-angiogenic therapy | |
| Classical Hodgkin Lymphoma | 2016 | For patients who relapsed or progressed after autologous hematopoietic stem cell transplantation and post-transplantation brentuximab vedotin | |
| Head and Neck Squamous Cell Carcinoma | 2016 | For recurrent or metastatic disease following prior treatment | |
| Urothelial Cancer | 2017 | For locally advanced or metastatic disease with progression on or after platinum-containing chemotherapy | |
| MSI-H or dMMR Colorectal Cancer | 2017 | For disease with progression following fluoropyrimidine, oxaliplatin, and irinotecan | |
| Hepatocellular Carcinoma | 2017 | For patients previously treated with sorafenib | |
| Small Cell Lung Cancer | 2018 | For metastatic disease with progression after platinum-based chemotherapy and at lease one other line of therapy | |
| Esophageal Squamous Cell Carcinoma | 2020 | For patients previously treated with fluoropyrimidine- and platinum-containing chemotherapy | |
| Gastric Cancer, Gastroesophageal Junction Cancer, and Esophageal Adenocarcinoma | 2021 | In combination with fluoropyrimidine- and platinum-containing chemotherapy for advanced or metastatic disease | |
| Pembrolizumab | PD-1 | ||
| Melanoma | 2014 | For advanced or unresectable disease following prior treatment | |
| Non-Small Cell Lung Cancer | 2015 | For metastatic disease following prior treatment | |
| Head and Neck Squamous Cell Carcinoma | 2016 | For recurrent or metastatic disease with progression on or after platinum-containing chemotherapy | |
| Solid Tumor with MSI-H or dMMR | 2017 | For unresectable or metastatic disease following prior treatment | |
| Classical Hodgkin Lymphoma | 2017 | For patients with refractory disease or who relapsed after three or more prior lines of therapy | |
| Urothelial Cancer | 2017 | For locally advanced or metastatic disease following prior treatment | |
| Gastric or Gastroesophageal Junction Cancer | 2017 | For locally advanced or metastatic disease following prior treatment | |
| Cervical Cancer | 2018 | For recurrent or metastatic disease with progression on or after chemotherapy | |
| Large B-Cell Lymphoma | 2018 | For refractory primary mediastinal disease following prior treatment | |
| Hepatocellular Carcinoma | 2018 | For patients previously treated with sorafenib | |
| Renal Cell Carcinoma | 2019 | For first-line treatment in combination with axitinib | |
| Small Cell Lung Cancer | 2019 | For metastatic disease with progression after platinum-based chemotherapy and at least one other line of therapy | |
| Esophageal Squamous Cell Carcinoma | 2019 | For locally advanced or metastatic disease following prior treatment | |
| Endometrial Carcinoma | 2019 | In combination with lenvatinib for advanced disease that is not MSI-H or dMMR following prior treatment | |
| Bladder Cancer | 2020 | For BCG-unresponsive, non-muscle invasive disease with carcinoma in situ | |
| Unresectable or TMB-H Solid Tumors | 2020 | For disease that progressed following prior treatment | |
| Cutaneous Squamous Cell Carcinoma | 2020 | For recurrent or metastatic disease not curable by surgery or radiation | |
| MSI-H or dMMR Colorectal Cancer | 2020 | For first-line treatment in unresectable or metastatic disease | |
| Triple Negative Breast Cancer | 2020 | In combination with chemotherapy for locally recurrent, unresectable, or metastatic disease | |
| Atezolizumab | PD-L1 | ||
| Urothelial Cancer | 2016 | For locally advanced or metastatic disease with progression on or after platinum-based chemotherapy | |
| Non-Small Cell Lung Cancer | 2016 | For metastatic disease with progression on or after platinum-based chemotherapy | |
| Bladder Cancer | 2017 | For locally advanced or metastatic disease | |
| Non-Squamous Non-Small Cell Lung Cancer | 2018 | In combination with bevacizumab, paclitaxel, and carboplatin for first-line treatment of metastatic disease | |
| Triple Negative Breast Cancer | 2019 | In combination with nab-paclitaxel chemotherapy for unresectable locally advanced or metastatic disease | |
| Small Cell Lung Cancer | 2019 | In combination with carboplatin and etoposide for first-line treatment of extensive-stage disease | |
| Hepatocellular Carcinoma | 2020 | In combination with bevacizumab for unresectable or metastatic disease in patients who have not received prior systemic therapy | |
| Melanoma | 2020 | In combination with cobimetinib and vemurafenib for BRAF V600-positive advanced disease | |
| Avelumab | PD-L1 | ||
| Merkel Cell Carcinoma | 2017 | For metastatic disease | |
| Urothelial Cancer | 2017 | For locally advanced or metastatic disease with progression on or after platinum-based chemotherapy | |
| Renal Cell Carcinoma | 2019 | In combination with axitinib for first-line treatment for advanced disease | |
| Durvalumab | PD-L1 | ||
| Non-Small Cell Lung Cancer | 2018 | For stage III unresectable disease that has not progressed after chemoradiation | |
| Small Cell Lung Cancer | 2020 | In combination with etoposide plus either carboplatin or cisplatin for extensive-stage disease | |
| Cemiplimab | PD-1 | ||
| Cutaneous Squamous Cell Carcinoma | 2018 | For locally advanced or metastatic disease not curable by surgery or radiation | |
| Basal Cell Carcinoma | 2021 | For advanced disease previously treated with hedgehog-pathway inhibitor or when HHI is not appropriate | |
| Non-Small Cell Lung Cancer | 2021 | For first-line treatment of locally advanced or metastatic disease | |
| Ipilimumab | CTLA-4 | ||
| Melanoma | 2011 | For late-stage metastatic disease | |
| Nivolumab and Ipilimumab | PD-1 and CTLA-4 | ||
| Melanoma | 2016 | For unresectable or metastatic disease | |
| Renal Cell Carcinoma | 2018 | For intermediate- and poor-risk advanced disease | |
| MSI-H or dMMR Colorectal Cancer | 2018 | For metastatic disease that has progressed following fluoropyrimidine, oxaliplatin and irinotecan | |
| Hepatocellular Carcinoma | 2020 | For patients previously treated with sorafenib | |
| Non-Small Cell Lung Cancer | 2020 | For first-line treatment of metastatic disease | |
| Malignant Pleural Mesothelioma | 2020 | For first-line treatment of unresectable, malignant, previously-untreated disease |
Table 2.
Common Checkpoint Molecules.
| Checkpoint Role |
Molecule | Description |
|---|---|---|
| Inhibitor | PD-1 (CD279) | Programmed cell death protein 1 (PD-1) is found on B cells and T cells and has a role in immune self-tolerance. Ligands are PD-L1 and PD-L2. PD-1 activation can reduce T cell receptor (TCR) signaling, trigger apoptosis of T cells, and reduce apoptosis of regulatory T-cells (Tregs). PD-1 has been implicated in other roles including regulation of CD8+ T cell exhaustion [89-91]. |
| CTLA-4 (CD152) | Cytotoxic T-lymphocyte-associated protein 4 (CTLA-4) is expressed by activated T cells and Tregs. CTLA-4 likely binds to ligands CD80 and CD86 on antigen-presenting cells (APCs), outcompeting the stimulatory molecule CD28, regulating the activation of T cells. CTLA-4 may promote T-cell anergy through activation of the PI3K/Akt pathway [9,92]. | |
| LAG-3 (CD223) | Lymphocyte-activation gene 3 (LAG-3) is expressed on several immune cells including B-cells, activated T cells, and natural killer (NK) cells. Ligands include Gal-3 and MHC II, the latter of which LAG-3 binds to with higher affinity than CD4. LAG-3 may regulate proliferation, granzyme production, and cytokine expression of T cells. LAG-3 may also increase differentiation of lymphocytes into Tregs and reduce CD8+ T cell function [93,94]. | |
| TIM-3 | T-cell immunoglobulin and mucin domain-3 (TIM-3) is a transmembrane protein expressed on CD4+ T cells, CD8+ T cells, and myeloid cells. The most common ligand activating TIM-3 is soluble Gal-9, with this interaction shown to induce T cell apoptosis. TIM-3 has also been implicated in CD8+ T cell exhaustion and the expression of Th1 and Th17 cytokines [95]. | |
| TIGIT | T cell immunoreceptor with Ig and ITIM domains (TIGIT) is expressed primarily on activated T cells, Tregs, and NK cells. Ligands include CD155 and CD112. Interaction with CD155 has been found to downregulate T cell and NK cell functions. TIGIT has been implicated in T-cell cytokine expression and regulation as well as the regulation of dendritic cells (DCs) [96,97]. | |
| IDO | Indoleamine-pyrrole 2,3-dioxygenase (IDO) is a rate-limiting enzyme in the kynurenine pathway, metabolizing tryptophan. IDO has been implicated in the suppression of effector T cells, suppression of NK cells, activation of Tregs, and activation of myeloid-derived suppressor cells (MDSCs). Trp depletion and kynurenine accumulation may be the mechanism of immunosuppressive IDO, as well as potential enzyme-independent effects [98,99]. | |
| KIR | Killer-cell immunoglobulin-like receptors (KIRs) are transmembrane proteins expressed by NK cells and a subset of T cells. KIR ligands are HLA Class I molecules (MHC I) on nucleated cells. KIR genes have high allelic diversity, with some haplotypes such as KIR2DL1-3 being implicated as checkpoint inhibitors [100,101]. | |
| VISTA | V-domain Ig suppressor of T cell activation (VISTA) is transmembrane immune regulator expressed on tumor-infiltrating lymphocytes, Tregs, MDSCs, and APCs. VISTA has been show to act as a co-inhibitory receptor and as a ligand on T cells. VISTA has also been implicated in suppressing macrophage and activated T cell activity as well as differentiation of Tregs [102]. | |
| Stimulator | OX40 (CD134) | Tumor necrosis factor receptor superfamily member 4, also known as OX40, is expressed on activated T cells as well as a number of other cell types. OX40 binds to OX40 ligand (OX40L), which is expressed by APCs and other cell types. OX40 has been implicated in extending the duration of effective immune responses and in immune memory response, as well as having a critical role in inflammatory response. OX40 has been show to be a prominent costimulatory receptor for activity, clonal expansion, and activation of T cells. OX40 is a member of the tumor necrosis factor (TNF) receptor superfamily along with CD27, CD40, 4-1BB, and GTIR [103,104]. |
| GITR | TNF receptor superfamily member 18 is also known as Glucocorticoid-induced TNFR-related protein (GITR). GTIR is expressed on most immune cells with higher levels on Tregs and potentially naïve T cells. GTIR ligand (GTIRL) is found on endothelial cells and APCs. GTIR has been implicated in allowing CD4+ T-effector cells to avoid suppression by Tregs. It may also increase proliferation of all CD4+ T-cell populations. It may also be critical to inflammatory response and play a role in CD8+ T cell activation [105,106]. | |
| CD28 | CD28 is expressed on CD4+ and CD8+ T cells. CD28 binds to CD80 and CD86, with these ligands mostly found on APCs. The co-stimulatory signals of CD28 have been shown to be critical for T cell activation and survival. CD28 binding is also linked to upregulation of specific lymphokines and cytokines in T cells. CD28 is a member of the CD28 family of receptors along with the positive regulator ICOS and the negative regulators PD-1, CTLA-4, and BTLA [107,108]. | |
| CD80 | CD80 is present primarily on APCs, but can be expressed by activated B cells, T cells, and monocytes. CD80 exhibits costimulatory activity in binding to CD28. Stimulation by CD80 may cause CD8+ cell to activate, or CD4+ cells to preferentially differentiate into Type 1 helper T cells. Interactions of MHC II on DCs with CD4+ T cells may lead to dendritic cell licensing and upregulation of CD80. CD80 can also bind to CTLA-4 for attenuation of immune response [109-111]. | |
| ICOS (CD278) | Inducible T-cell Costimulator (ICOS) is expressed on the surface of activated T cells and is CD28 family receptor. ICOS expression has been shown to have a strong role in T cell cytokine secretion. ICOS also likely has a role in the activity of memory T cells and effector T cells during immune response. ICOS ligand (ICOS-L) is expressed on professional APCs such as DCs, macrophages, and B cells. ICOS may also have a role in interactions between T cells and B cells through the CD40/CD40L pathway [112-115]. | |
| CD40 | TNF receptor superfamily member 5, also known as CD40, is a costimulatory protein expressed on the surface of a wide range of cells including B cells, DCs, macrophages, and other APCs. The cognate ligand, CD40L (CD154), is expressed primarily on the surface of CD4+ T-cells, as well as activated B cells. CD40 has been implicated in a variety of downstream responses including T-cell dependent immunoglobulin class switching and memory B cell development. CD40 also plays a role in cellular and adaptive immunity [116]. | |
| 4-1BB (CD137) | TNF receptor superfamily member 9, also known as 4-1BB, is expressed on activated CD4+ and activated CD8+ T cells. 4-1BB ligand (4-1BBL) is expressed on APCs. Activation of 4-1BB has been linked to proliferation and survival of CD8+ T cells. 4-1BB has also been implicated in increases of T cell cytolytic activity and changes in T cell cytokine expression. 4-1BB activation may also play a role in CD4+ T cell activation [117-119]. |
Table 3.
Combination Therapies.
| Combination Category |
Specific Combination |
Example | ClinicalTrials. gov Identifier |
|---|---|---|---|
| Antigen exposure | Cancer Vaccine + CPI | RO7198457 + Anti-PD-L1 [120] | NCT03289962 |
| Oncolytic Virus + CPI | Ad-RTS-hIL-12 + Anti-PD-1 [121] | NCT03636477 | |
| Chemotherapy + CPI | Pemetrexed and Platinum Chemotherapy + Anti-PD-1 [122] | NCT02578680 | |
| Additional Checkpoint Molecules | Inhibitory Checkpoint + CPI | Anti-TIM-3 + Anti-PD-1 [123] | NCT03961971 |
| Stimulatory Checkpoint + CPI | Anti-GITR + Anti-PD-1 [124] | NCT04225039 | |
| Other Modalities | |||
| Cytokine Treatment + CPI | IL-15 + Anti-PD-1 [125] | NCT03388632 | |
| Small Molecule Inhibitor + CPI | ALX148 (CD47 Blockade) + Anti-PD-1 [126] | NCT03013218 | |
| Adoptive Cell Therapy + CPI | CD19 CAR-T Expressing IL7 and CCL19 + Anti- PD-1 [127] | NCT04381741 |
3.3. Combining CPIs with other therapeutic modalities to overcome resistance
In addition to the aforementioned lines of investigation, research into a number of additional mechanisms to overcome CPI resistance are underway but still in their relative infancy. These include: reducing intratumoral MDSC populations [74], increasing immunosurveillance of tumors with low immune cell infiltration [75], targeting therapy-interfering TAMs [76], and cytokine therapies which can increase T cell activation, infiltration, and proliferation within tumors [67]. Small molecule inhibitors have also been explored in combination with CPIs. In a mouse model of non-small cell lung cancer, Douguet et al. investigated the use of a small molecule modulator of the purinergic P2RX7 receptor in combination with anti-PD-1 treatment, demonstrating a synergistic treatment response when the two were combined [77]. Similarly, De Henau et al. demonstrated the efficacy of a PI3Kγ inhibitor when combined with a CPI, due to a reduction in immunosuppressive myeloid cell [78]. Given these promising results, the combination of this small molecule inhibitor with anti-PD-1 treatment is currently underway in a Phase I clinical trial involving advanced solid tumors [79]. Cytokine therapies such as IL-15 or IL-2, that can support effector cell function have also been shown to work synergistically with CPI treatment [80]. For example, the IL-15 superagonist RLI has been shown to work synergistically with anti-PD1 therapy in a preclinical model of colorectal cancer, leading to increased survival relative to either treatment alone [81]. Promising preclinical data has led to IL-15 being explored in clinical trials as a combination treatment with nivolumab [82]. As the popularity of CPIs grows, further lines of investigation into mechanisms to overcome resistance will undoubtedly be opened. Combination treatments attacking the immune system in multiple ways and preventing or reversing resistance will likely continue to see a rise in their usage and are the future of CPI treatment.
4. Future directions
The future of cancer treatment with CPIs is bright and holds great promise for patients afflicted by cancer. The field has progressed quickly, and there have been a number of incredible advancements. However, most patients treated with CPI at this time will either be resistant to or develop resistance to these therapies, highlighting the need for additional investigation into this topic. Future research in CPI immunotherapy should include improved preclinical modeling systems as well as a movement towards personalized immunotherapy.
Currently, many preclinical cancer models do not accurately recapitulate the immune-tumor interface or clinical behavior of cancer in humans. Preclinical models are critical in quickly identifying the efficacy and pitfalls of treatments. As a result, it is vital that preclinical models are as close to human cancer as possible. Effort should be focused on producing accurate preclinical models and validating model accuracy in replicating human cancer through strict validation modalities. This is highlighted by Pérez-Guijarro et al. who developed multiple novel preclinical models for melanoma and validated them by assessing mutational landscapes, transcriptomes and tumor-infiltrating immune cell profiles while also cross-validation using clinical datasets. This in depth analysis allowed them to uncover a melanocytic plasticity signature that was predictive of response to CPI [83]. Patient derived organoid models created directly from biopsies of human cancer tissue can now also be created with an intact immune system, allowing for the assessment of immunotherapies [84]. These organoid models can facilitate high-throughput evaluation of CPIs, allowing for the identification of ideal combinations in an expedient fashion. In silico modeling of the tumor-immune interface may also allow for preliminary investigations into molecular relationships and ideal treatment combinations prior to proceeding with additional preclinical investigations. Indeed, Kather et al. demonstrated the promise of such modeling in colorectal cancer [85]. As the number of CPIs and potential combinations increases, higher throughput methods of testing treatment efficacy in specific will become increasingly valuable.
In addition to improved preclinical modeling of CPI treatment, it is also critical to identify those patients that will benefit the most from a specific treatment. As discussed previously, there are a number of resistance mechanisms that can differ between patients. As a result, personalized immunotherapy that is targeted against a specific patient’s tumor and resistance predispositions is critical. Current biomarkers to predict treatment responders consist of tumor mutational burden, checkpoint molecule expression levels, and profile scoring of the tumor microenvironment [86]. An interesting recent development in predicting responses to CPI has been the integration of omics and clinical data to create scoring systems that predict CPI response [87,88]. This is highlighted by Auslander et al. who created a score based on transcriptomic data to predict response to metastatic melanoma with an excellent area under the curve of 0.83 [87]. As larger omics datasets become available, the development of improved scoring systems utilizing machine learning techniques and artificial intelligence will likely continue. Additional, more accurate, predictive, and accessible biomarkers are needed. Ideally, biomarkers would help to inform initial treatment selection and could then be periodically checked throughout treatment to provide insight into treatment success or the development of resistance and the need to change or add therapies. Achieving these advances would enable CPIs to be employed as part of a precision medicine approach in which treatments are tailored for an individual patient to achieve personalized cancer care.
5. Conclusion
Checkpoint inhibitors represent an exciting treatment for patients with cancer. However, resistance to checkpoint inhibitor treatment occurs in the majority of patients. Key mechanisms of resistance include antigen presence and presentation, interferon-gamma signaling, and the tumor microenvironment and tumor mediated immunosuppression. Methods to overcome resistance are in development. More accurate preclinical modeling and improved biomarkers are critical in providing personalized cancer care to patients.
Acknowledgements
M.K.A. was supported by the NIH (1R01CA227136, 2R01NS079697, and 1R01CA260443), and by an Innovation Award from the Laboratory for Genomics Research.
References
- [1].Smith JL Jr., Stehlin JS Jr., Spontaneous regression of primary malignant melanomas with regional metastases, Cancer 18 (11) (1965) 1399–1415, . [DOI] [PubMed] [Google Scholar]
- [2].Halliday GM, Patel A, Hunt MJ, Tefany FJ, Barnetson RSC, Spontaneous regression of human melanoma/nonmelanoma skin cancer: association with infiltrating CD4+ T cells, World J. Surg 19 (3) (1995) 352–358, 10.1007/BF00299157. [DOI] [PubMed] [Google Scholar]
- [3].Shankaran V, Ikeda H, Bruce AT, et al. , IFNγ and lymphocytes prevent primary tumour development and shape tumour immunogenicity, Nature 410 (6832) (2001) 1107–1111, 10.1038/35074122. [DOI] [PubMed] [Google Scholar]
- [4].Mortarini R, Piris A, Maurichi A, et al. , Lack of terminally differentiated tumor-specific CD8+ T cells at tumor site in spite of antitumor immunity to self-antigens in human metastatic melanoma, Cancer Res. 63 (10) (2003) 2535–2545. http://europepmc.org/abstract/MED/12750277. [PubMed] [Google Scholar]
- [5].Zhao Y, Shao Q, Peng G, Exhaustion and senescence: two crucial dysfunctional states of T cells in the tumor microenvironment, Cell. Mol. Immunol 17 (1) (2020) 27–35, 10.1038/s41423-019-0344-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Chen L, Flies DB, Molecular mechanisms of T cell co-stimulation and co-inhibition, Nat. Rev. Immunol 13 (4) (2013) 227–242, 10.1038/nri3405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Shahinian A, Pfefer K, Lee KP, et al. , Differential T cell costimulatory requirements in CD28-deficient mice, Science (80-) 261 (5121) (1993) 609–612, 10.1126/science.7688139. [DOI] [PubMed] [Google Scholar]
- [8].Buchbinder EI, Desai A, CTLA-4 and PD-1 pathways, Am. J. Clin. Oncol 39 (1) (2016) 98–106, 10.1097/COC.0000000000000239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Qureshi OS, Zheng Y, Nakamura K, et al. , Trans-endocytosis of CD80 and CD86: a molecular basis for the cell-extrinsic function of CTLA-4, Science (80-) 332 (6029) (2011) 600–603, 10.1126/SCIENCE.1202947/SUPPL_FILE/QURESHI-SOM.PDF. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Waterhouse P, Penninger JM, Timms E, et al. , Lymphoproliferative disorders with early lethality in mice deficient in ctla-4, Science (80-) 270 (5238) (1995) 985–988, 10.1126/SCIENCE.270.5238.985. [DOI] [PubMed] [Google Scholar]
- [11].Sharpe AH, Pauken KE, The diverse functions of the PD1 inhibitory pathway, Nat. Rev. Immunol 18 (3) (2017) 153–167, 10.1038/nri.2017.108, 2017 183. [DOI] [PubMed] [Google Scholar]
- [12].Nishimura H, Okazaki T, Tanaka Y, et al. , Autoimmune dilated cardiomyopathy in PD-1 receptor-deficient mice, Science (80-) 291 (5502) (2001) 319–322, 10.1126/SCIENCE.291.5502.319. [DOI] [PubMed] [Google Scholar]
- [13].Tu L, Guan R, Yang H, et al. , Assessment of the expression of the immune checkpoint molecules PD-1, CTLA4, TIM-3 and LAG-3 across different cancers in relation to treatment response, tumor-infiltrating immune cells and survival, Int. J. Cancer 147 (2) (2020) 423–439, 10.1002/IJC.32785. [DOI] [PubMed] [Google Scholar]
- [14].Nirschl CJ, Drake CG, Molecular pathways: coexpression of immune checkpoint molecules: signaling pathways and implications for cancer immunotherapy, Clin. Cancer Res 19 (18) (2013) 4917–4924, 10.1158/1078-0432.CCR-12-1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Hargadon KM, Johnson CE, Williams CJ, Immune checkpoint blockade therapy for cancer: an overview of FDA-approved immune checkpoint inhibitors, Int. Immunopharmacol 62 (2018) 29–39, 10.1016/j.intimp.2018.06.001. [DOI] [PubMed] [Google Scholar]
- [16].Schoenfeld AJ, Hellmann MD, Acquired resistance to immune checkpoint inhibitors, Cancer Cell 37 (4) (2020) 443–455, 10.1016/j.ccell.2020.03.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Egen JG, Ouyang W, Wu LC, Human anti-tumor immunity: insights from immunotherapy clinical trials, Immunity 52 (1) (2020) 36–54, 10.1016/J.IMMUNI.2019.12.010. [DOI] [PubMed] [Google Scholar]
- [18].Gubin MM, Schreiber RD, The odds of immunotherapy success mutation load correlates with the response of melanomas to immunotherapy, Science (80-) 350 (6257) (2015) 158–159, 10.1126/science.aad4140. [DOI] [PubMed] [Google Scholar]
- [19].Van Allen EM, Miao D, Schilling B, et al. , Genomic correlates of response to CTLA-4 blockade in metastatic melanoma, Science (80-) 350 (6257) (2015) 207–211, 10.1126/science.aad0095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Valero C, Lee M, Hoen D, et al. , The association between tumor mutational burden and prognosis is dependent on treatment context, Nat. Genet 53 (1) (2021) 11–15, 10.1038/s41588-020-00752-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Yarchoan M, Hopkins A, Jaffee EM, Tumor mutational burden and response rate to PD-1 inhibition, N. Engl. J. Med 377 (25) (2017) 2500–2501, 10.1056/nejmc1713444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Samstein RM, Lee C-H, Shoushtari AN, et al. , Tumor mutational load predicts survival after immunotherapy across multiple cancer types, Nat. Genet 51 (2) (2019) 202–206, 10.1038/s41588-018-0312-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Litchfield K, Reading JL, Puttick C, et al. , Meta-analysis of tumor- and T cell-intrinsic mechanisms of sensitization to checkpoint inhibition, Cell 184 (3) (2021), 10.1016/j.cell.2021.01.002, 596–614.e14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Rosenthal R, Cadieux EL, Salgado R, et al. , Neoantigen-directed immune escape in lung cancer evolution, Nature 567 (7749) (2019) 479–485, 10.1038/S41586-019-1032-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Anagnostou V, Smith KN, Forde PM, et al. , Evolution of neoantigen landscape during immune checkpoint blockade in non-small cell lung cancer, Cancer Discov. 7 (3) (2017) 264–276, 10.1158/2159-8290.CD-16-0828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Gromeier M, Brown MC, Zhang G, et al. , Very low mutation burden is a feature of inflamed recurrent glioblastomas responsive to cancer immunotherapy, Nat Commun. 12 (1) (2021) 1–7, 10.1038/s41467-020-20469-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Touat M, Li YY, Boynton AN, et al. , Mechanisms and therapeutic implications of hypermutation in gliomas, Nature 580 (7804) (2020) 517–523, 10.1038/S41586-020-2209-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Garrido F, Aptsiauri N, Doorduijn EM, Garcia Lora AM, van Hall T, The urgent need to recover MHC class I in cancers for effective immunotherapy, Curr. Opin. Immunol 39 (2016) 44–51, 10.1016/j.coi.2015.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Gettinger S, Choi J, Hastings K, et al. , Impaired HLA class I antigen processing and presentation as a mechanism of acquired resistance to immune checkpoint inhibitors in lung cancer, Cancer Discov. 7 (12) (2017) 1420–1435, 10.1158/2159-8290.CD-17-0593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Zaretsky JM, Garcia-Diaz A, Shin DS, et al. , Mutations associated with acquired resistance to PD-1 blockade in melanoma, N. Engl. J. Med 375 (9) (2016) 819–829, 10.1056/NEJMoa1604958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Pereira C, Gimenez-Xavier P, Pros E, et al. , Genomic profiling of patient-derived xenografts for lung cancer identifies B2M inactivation impairing immunorecognition, Clin. Cancer Res 23 (12) (2017) 3203–3213, 10.1158/1078-0432.CCR-16-1946. [DOI] [PubMed] [Google Scholar]
- [32].Sade-Feldman M, Jiao YJ, Chen JH, et al. , Resistance to checkpoint blockade therapy through inactivation of antigen presentation, Nat. Commun 8 (1) (2017), 10.1038/s41467-017-01062-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Middha S, Yaeger R, Shia J, et al. , Majority of B2M -mutant and -deficient colorectal carcinomas achieve clinical benefit from immune checkpoint inhibitor therapy and are microsatellite instability-high, JCO Precis. Oncol. (3) (2019) 1–14, 10.1200/PO.18.00321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Spranger S, Gajewski TF, Impact of oncogenic pathways on evasion of antitumour immune responses, Nat. Rev. Cancer 18 (3) (2018) 139–147, 10.1038/nrc.2017.117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Yaguchi T, Goto Y, Kido K, et al. , Immune suppression and resistance mediated by constitutive activation of wnt/β-catenin signaling in human melanoma cells, J. Immunol 189 (5) (2012) 2110–2117, 10.4049/jimmunol.1102282. [DOI] [PubMed] [Google Scholar]
- [36].Holtzhausen A, Zhao F, Evans KS, et al. , Melanoma-derived Wnt5a promotes local dendritic-cell expression of IDO and immunotolerance: opportunities for pharmacologic enhancement of immunotherapy, Cancer Immunol. Res 3 (9) (2015) 1082–1095, 10.1158/2326-6066.CIR-14-0167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Sweis RF, Spranger S, Bao R, et al. , Molecular drivers of the non- T-cell-inflamed tumor microenvironment in urothelial bladder cancer, Cancer Immunol. Res 4 (7) (2016) 563–568, 10.1158/2326-6066.CIR-15-0274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Seiwert TY, Zuo Z, Keck MK, et al. , Integrative and comparative genomic analysis of HPV-positive and HPV-negative head and neck squamous cell carcinomas, Clin. Cancer Res 21 (3) (2015) 632–641, 10.1158/1078-0432.CCR-13-3310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Peng W, Chen JQ, Liu C, et al., Loss of PTEN promotes resistance to T cell–mediated immunotherapy, Cancer Discov. 6 (2) (2016) 202–216, 10.1158/2159-8290.CD-15-0283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Castro F, Cardoso AP, Gonçalves RM, Serre K, Oliveira MJ, Interferon-gamma at the crossroads of tumor immune surveillance or evasion, Front. Immunol 9 (May) (2018) 1, 10.3389/fimmu.2018.00847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Kaplan DH, Shankaran V, Dighe AS, et al. , Demonstration of an interferon γ-dependent tumor surveillance system in immunocompetent mice, Proc. Natl. Acad. Sci. U. S. A 95 (13) (1998) 7556–7561, 10.1073/pnas.95.13.7556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Manguso RT, Pope HW, Zimmer MD, et al. , In vivo CRISPR screening identifies Ptpn2 as a cancer immunotherapy target, Nature 547 (7664) (2017) 413–418, 10.1038/nature23270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].Patel SJ, Sanjana NE, Kishton RJ, et al. , Identification of essential genes for cancer immunotherapy, Nature 548 (7669) (2017) 537–542, 10.1038/nature23477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].Sucker A, Zhao F, Pieper N, et al. , Acquired IFNγ 3 resistance impairs antitumor immunity and gives rise to T-cell-resistant melanoma lesions, Nat. Commun 8 (1) (2017) 1–15, 10.1038/ncomms15440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [45].Shin DS, Zaretsky JM, Escuin-Ordinas H, et al. , Primary resistance to PD-1 blockade mediated by JAK1/2 mutations, Cancer Discov. 7 (2) (2017) 188–201, 10.1158/2159-8290.CD-16-1223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [46].Gao J, Shi LZ, Zhao H, et al. , Loss of IFN-γ pathway genes in tumor cells as a mechanism of resistance to anti-CTLA-4 therapy, Cell 167 (2) (2016), 10.1016/j.cell.2016.08.069, 397–404.e9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [47].Tumeh PC, Harview CL, Yearley JH, et al. , PD-1 blockade induces responses by inhibiting adaptive immune resistance, Nature 515 (7528) (2014) 568–571, 10.1038/nature13954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [48].Kümpers C, Jokic M, Haase O, et al. , Immune cell infiltration of the primary tumor, not PD-L1 status, is associated with improved response to checkpoint inhibition in metastatic melanoma, Front. Med 6 (2019) 27, 10.3389/fmed.2019.00027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [49].Pauken KE, Sammons MA, Odorizzi PM, et al. , Epigenetic stability of exhausted T cells limits durability of reinvigoration by PD-1 blockade, Science (80-) 354 (6316) (2016) 1160–1165, 10.1126/science.aaf2807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [50].Sundi D, Duggan MC, Savardekar H, et al. , Association of myeloid suppressor cells with resistance to checkpoint blockade immunotherapy in urothelial carcinoma, J. Clin. Oncol 38 (6_suppl) (2020) 559, 10.1200/jco.2020.38.6_suppl.559. [DOI] [Google Scholar]
- [51].Zhu Y, Knolhoff BL, Meyer MA, et al. , CSF1/CSF1R blockade reprograms tumor-infiltrating macrophages and improves response to T-cell checkpoint immunotherapy in pancreatic cancer models, Cancer Res. 74 (18) (2014) 5057–5069, 10.1158/0008-5472.CAN-13-3723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [52].Lo Russo G, Moro M, Sommariva M, et al. , Antibody-Fc/FcR interaction on macrophages as a mechanism for hyperprogressive disease in non-small cell lung cancer subsequent to PD-1/PD-L1 blockade, Clin. Cancer Res 25 (3) (2019) 989–999, 10.1158/1078-0432.CCR-18-1390. [DOI] [PubMed] [Google Scholar]
- [53].Ruffell B, Coussens LM, Macrophages and therapeutic resistance in cancer, Cancer Cell 27 (4) (2015) 462–472, 10.1016/j.ccell.2015.02.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [54].Chen PL, Roh W, Reuben A, et al. , Analysis of immune signatures in longitudinal tumor samples yields insight into biomarkers of response and mechanisms of resistance to immune checkpoint blockade, Cancer Discov. 6 (8) (2016) 827–837, 10.1158/2159-8290.CD-15-1545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [55].Haddad AF, Chen JS, Oh T, Pereira MP, Joshi RS, Aghi MK, Higher cytolytic score correlates with an immunosuppressive tumor microenvironment and reduced survival in glioblastoma, Sci. Rep 10 (1) (2020), 10.1038/S41598-020-73793-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [56].Peranzoni E, Lemoine J, Vimeux L, et al. , Macrophages impede CD8 T cells from reaching tumor cells and limit the efficacy of anti–PD-1 treatment, Proc. Natl. Acad. Sci. U. S. A 115 (17) (2018) E4041–E4050, 10.1073/pnas.1720948115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [57].Wallin JJ, Bendell JC, Funke R, et al. , Atezolizumab in combination with bevacizumab enhances antigen-specific T-cell migration in metastatic renal cell carcinoma, Nat. Commun 7 (1) (2016) 1–8, 10.1038/ncomms12624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [58].Fong L, Carroll P, Weinberg V, et al. , Activated lymphocyte recruitment into the tumor microenvironment following preoperative sipuleucel-T for localized prostate cancer, J. Natl. Cancer Inst 106 (11) (2014), 10.1093/jnci/dju268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [59].Wilgenhof S, Corthals J, Heirman C, et al. , Phase II study of autologous monocyte-derived mRNA electroporated dendritic cells (TriMixDC-MEL) plus ipilimumab in patientswith pretreated advanced melanoma, J. Clin. Oncol 34 (12) (2016) 1330–1338, 10.1200/JCO.2015.63.4121. [DOI] [PubMed] [Google Scholar]
- [60].Zhao J, Chen Y, Ding ZY, Liu JY, Safety and efficacy of therapeutic cancer vaccines alone or in combination with immune checkpoint inhibitors in cancer treatment, Front. Pharmacol 10 (2019), 10.3389/fphar.2019.01184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [61].Sahin U, Türeci Ö, Personalized vaccines for cancer immunotherapy, Science (80-) 359 (6382) (2018) 1355–1360, 10.1126/science.aar7112. [DOI] [PubMed] [Google Scholar]
- [62].Bommareddy PK, Shettigar M, Kaufman HL, Integrating oncolytic viruses in combination cancer immunotherapy, Nat. Rev. Immunol 18 (8) (2018) 498–513, 10.1038/s41577-018-0014-6, 2018 188. [DOI] [PubMed] [Google Scholar]
- [63].Chesney J, Puzanov I, Collichio F, et al. , Randomized, open-label phase II study evaluating the efficacy and safety of talimogene laherparepvec in combination with ipilimumab versus ipilimumab alone in patients with advanced, unresectable melanoma, J. Clin. Oncol 36 (17) (2018) 1658–1667, 10.1200/JCO.2017.73.7379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [64].Gandhi L, Rodríguez-Abreu D, Gadgeel S, et al. , Pembrolizumab plus chemotherapy in metastatic non–small-cell lung cancer, N. Engl. J. Med 378 (22) (2018) 2078–2092, 10.1056/nejmoa1801005. [DOI] [PubMed] [Google Scholar]
- [65].Mathios D, Kim JE, Mangraviti A, et al. , Anti-PD-1 antitumor immunity is enhanced by local and abrogated by systemic chemotherapy in GBM, Sci. Transl. Med 8 (370) (2016), 10.1126/scitranslmed.aag2942, 370ra180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [66].Qin S, Xu L, Yi M, Yu S, Wu K, Luo S, Novel immune checkpoint targets: moving beyond PD-1 and CTLA-4, Mol. Cancer 18 (1) (2019) 1–14, 10.1186/s12943-019-1091-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [67].Braun DA, Bakouny Z, Hirsch L, et al. , Beyond conventional immune-checkpoint inhibition — novel immunotherapies for renal cell carcinoma, Nat. Rev. Clin. Oncol (January) (2021) 1–16, 10.1038/s41571-020-00455-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [68].Reiss KA, Forde PM, Brahmer JR, Harnessing the power of the immune system via blockade of PD-1 and PD-L1: a promising new anticancer strategy, Immunotherapy 6 (4) (2014) 459, 10.2217/IMT.14.9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [69].Mayes PA, Hance KW, Hoos A, The promise and challenges of immune agonist antibody development in cancer, Nat. Rev. Drug Discov 17 (7) (2018) 509–527, 10.1038/nrd.2018.75. [DOI] [PubMed] [Google Scholar]
- [70].Chen S, Lee LF, Fisher TS, et al. , Combination of 4–1BB agonist and PD-1 antagonist promotes antitumor effector/memory CD8 T cells in a Poorly Immunogenic Tumor Model, Cancer Immunol. Res 3 (2) (2015) 149–160, 10.1158/2326-6066.CIR-14-0118. [DOI] [PubMed] [Google Scholar]
- [71].Hellmann MD, Ciuleanu T-E, Pluzanski A, et al. , Nivolumab plus ipilimumab in lung cancer with a high tumor mutational burden, N. Engl. J. Med 378 (22) (2018) 2093–2104, 10.1056/nejmoa1801946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [72].Martins F, Sofiya L, Sykiotis GP, et al. , Adverse effects of immune-checkpoint inhibitors: epidemiology, management and surveillance, Nat. Rev. Clin. Oncol 16 (9) (2019) 563–580, 10.1038/s41571-019-0218-0. [DOI] [PubMed] [Google Scholar]
- [73].Wang DY, Salem JE, Cohen JV, et al. , Fatal toxic effects associated with immune checkpoint inhibitors: a systematic review and meta-analysis, JAMA Oncol. 4 (12) (2018) 1721–1728, 10.1001/jamaoncol.2018.3923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [74].Davis RJ, Moore EC, Clavijo PE, et al. , Anti-PD-L1 efficacy can be enhanced by inhibition of myeloid-derived suppressor cells with a selective inhibitor of PI3Kd/g, Cancer Res. 77 (10) (2017) 2607–2619, 10.1158/0008-5472.CAN-16-2534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [75].Song E, Mao T, Dong H, et al. , VEGF-C-driven lymphatic drainage enables immunosurveillance of brain tumours, Nature 577 (7792) (2020) 689–694, 10.1038/s41586-019-1912-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [76].Arlauckas SP, Garris CS, Kohler RH, et al. , In vivo imaging reveals a tumor-associated macrophage-mediated resistance pathway in anti-PD-1 therapy, Sci. Transl. Med 9 (389) (2017), 10.1126/scitranslmed.aa13604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [77].Douguet L, Janho dit Hreich S, Benzaquen J, et al. , A small-molecule P2RX7 activator promotes anti-tumor immune responses and sensitizes lung tumor to immunotherapy, Nat. Commun 12 (1) (2021) 1–17, 10.1038/S41467-021-20912-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [78].De Henau O, Rausch M, Winkler D, et al. , Overcoming resistance to checkpoint blockade therapy by targeting PI3Kγ in myeloid cells, Nature 539 (7629) (2016) 443–447, 10.1038/nature20554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [79].Sullivan RJ, Hong DS, Tolcher AW, et al. , Initial results from first-in-human study of IPI-549, a tumor macrophage-targeting agent, combined with nivolumab in advanced solid tumors, J. Clin. Oncol 36 (15_suppl) (2018) 3013, 10.1200/jco.2018.36.15_suppl.3013. [DOI] [Google Scholar]
- [80].Berraondo P, Sanmamed MF, Ochoa MC, et al. , Cytokines in clinical cancer immunotherapy, Br. J. Cancer 120 (1) (2019) 6–15, 10.1038/s41416-018-0328-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [81].Desbois M, Le Vu P, Coutzac C, et al. , IL-15 trans -signaling with the superagonist RLI promotes effector/memory CD8 + T cell responses and enhances antitumor activity of PD-1 antagonists, J. Immunol 197 (1) (2016) 168–178, 10.4049/jimmunol.1600019. [DOI] [PubMed] [Google Scholar]
- [82].Wrangle JM, Velcheti V, Patel MR, et al. , ALT-803, an IL-15 superagonist, in combination with nivolumab in patients with metastatic non-small cell lung cancer: a non-randomised, open-label, phase 1b trial, Lancet Oncol. 19 (5) (2018) 694–704, 10.1016/S1470-2045(18)30148-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [83].Pérez-Guijarro E, Yang HH, Araya RE, et al. , Multimodel preclinical platform predicts clinical response of melanoma to immunotherapy, Nat. Med 26 (5) (2020) 781–791, 10.1038/s41591-020-0818-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [84].Neal JT, Li X, Zhu J, et al. , Organoid modeling of the tumor immune microenvironment, Cell 175 (7) (2018), 10.1016/j.cell.2018.11.021, 1972–1988.e16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [85].Kather JN, Poleszczuk J, Suarez-Carmona M, et al. , In silico modeling of immunotherapy and stroma-targeting therapies in human colorectal cancer, Cancer Res. 77 (22) (2017) 6442–6452, 10.1158/0008-5472.CAN-17-2006. [DOI] [PubMed] [Google Scholar]
- [86].Bai R, Lv Z, Xu D, Cui J, Predictive biomarkers for cancer immunotherapy with immune checkpoint inhibitors, Biomark. Res 8 (1) (2020) 34, 10.1186/S40364-020-00209-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [87].Auslander N, Zhang G, Lee JS, et al. , Robust prediction of response to immune checkpoint blockade therapy in metastatic melanoma, Nat. Med 24 (10) (2018) 1545–1549, 10.1038/s41591-018-0157-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [88].Liu D, Schilling B, Liu D, et al. , Integrative molecular and clinical modeling of clinical outcomes to PD1 blockade in patients with metastatic melanoma, Nat. Med 25 (12) (2019) 1916–1927, 10.1038/s41591-019-0654-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [89].Riley JL, PD-1 signaling in primary T cells, Immunol. Rev 229 (1) (2009) 114, 10.1111/J.1600-065X.2009.00767.X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [90].Kamada T, Togashi Y, Tay C, et al. , PD-1+ regulatory T cells amplified by PD-1 blockade promote hyperprogression of cancer, Proc. Natl. Acad. Sci. U. S. A 116 (20) (2019) 9999–10008, 10.1073/PNAS.1822001116/-/DCSUPPLEMENTAL. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [91].Haymaker C, Wu R, Bernatchez C, Radvanyi L, PD-1 and BTLA and CD8+ T-cell “exhaustion” in cancer: “exercising” an alternative viewpoint, Oncoimmunology 1 (5) (2012) 735, 10.4161/ONCI.20823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [92].Rudd CE, Taylor A, Schneider H, CD28 and CTLA-4 coreceptor expression and signal transduction, Immunol. Rev 229 (1) (2009) 12, 10.1111/J.1600-065X.2009.00770.X.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [93].Kisielow M, Kisielow J, Capoferri-Sollami G, Karjalainen K, Expression of lymphocyte activation gene 3 (LAG-3) on B cells is induced by T cells, Eur. J. Immunol 35 (7) (2005) 2081–2088, 10.1002/EJI.200526090. [DOI] [PubMed] [Google Scholar]
- [94].Kouo T, Huang L, Pucsek AB, et al. , Galectin-3 shapes antitumor immune responses by suppressing CD8+ T cells via LAG-3 and inhibiting expansion of plasmacytoid dendritic cells, Cancer Immunol. Res 3 (4) (2015) 412–423, 10.1158/2326-6066.CIR-14-0150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [95].Wolf Y, Anderson AC, Kuchroo VK, TIM3 comes of age as an inhibitory receptor, Nat. Rev. Immunol 20 (3) (2020) 173–185, 10.1038/S41577-019-0224-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [96].Chauvin JM, Zarour HM, TIGIT in cancer immunotherapy, J. Immunother Cancer 8 (2) (2020) 957, 10.1136/JITC-2020-000957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [97].Lozano E, Dominguez-Villar M, Kuchroo V, Hafler DA, The TIGIT/CD226 axis regulates human T cell function, J. Immunol 188 (8) (2012) 3869, 10.4049/JIMMUNOL.1103627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [98].Wang D, Saga Y, Mizukami H, et al. , Indoleamine-2,3-dioxygenase, an immunosuppressive enzyme that inhibits natural killer cell function, as a useful target for ovarian cancer therapy, Int. J. Oncol 40 (4) (2012) 929, 10.3892/IJO.2011.1295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [99].Zhai L, Ladomersky E, Lenzen A, et al. , IDO1 in cancer: a Gemini of immune checkpoints, Cell. Mol. Immunol 15 (5) (2018) 447–457, 10.1038/CMI.2017.143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [100].Cao Y, Wang X, Jin T, et al. , Immune checkpoint molecules in natural killer cells as potential targets for cancer immunotherapy, Signal Transduct. Target. Ther 5 (1) (2020), 10.1038/S41392-020-00348-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [101].Moretta L, Moretta A, Killer immunoglobulin-like receptors, Curr. Opin. Immunol 16 (5) (2004) 626–633, 10.1016/J.COI.2004.07.010. [DOI] [PubMed] [Google Scholar]
- [102].Le Mercier I, Chen W, Lines JL, et al. , VISTA regulates the development of protective anti-tumor immunity, Cancer Res. 74 (7) (2014) 1933, 10.1158/0008-5472.CAN-13-1506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [103].Croft M, So T, Duan W, Soroosh P, The significance of OX40 and OX40L to t cell biology and immune disease, Immunol. Rev 229 (1) (2009) 173, 10.1111/J.1600-065X.2009.00766.X.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [104].Redmond WL, Ruby CE, Weinberg AD, The role of OX40-mediated co-stimulation in T cell activation and survival, Crit. Rev. Immunol 29 (3) (2009) 187, 10.1615/critrevimmunol.v29.i3.10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [105].Knee DA, Hewes B, Brogdon JL, Rationale for anti-GITR cancer immunotherapy, Eur. J. Cancer 67 (2016) 1–10, 10.1016/J.EJCA.2016.06.028. [DOI] [PubMed] [Google Scholar]
- [106].van Olffen RW, Koning N, van Gisbergen KPJM, et al. , GITR triggering induces expansion of both effector and regulatory CD4+ T cells in vivo, J. Immunol 182 (12) (2009) 7490–7500, 10.4049/JIMMUNOL.0802751. [DOI] [PubMed] [Google Scholar]
- [107].Linsley PS, Ledbetter JA, The role of the CD28 receptor during T cell responses to antigen, Annu. Rev. Immunol 11 (1993) 191–212, 10.1146/ANNUREV.IY.11.040193.001203. [DOI] [PubMed] [Google Scholar]
- [108].Thompson CB, Lindsten T, Ledbetter JA, et al. , CD28 activation pathway regulates the production of multiple T-cell-derived lymphokines/cytokines, Proc. Natl. Acad. Sci. U. S. A 86 (4) (1989) 1333–1337, 10.1073/PNAS.86.4.1333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [109].Lang TJ, Nguyen P, Peach R, Gause WC, Via CS, In vivo CD86 blockade inhibits CD4+ T cell activation, whereas CD80 blockade potentiates CD8+ T cell activation and CTL effector function, J. Immunol 168 (8) (2002) 3786–3792, 10.4049/JIMMUNOL.168.8.3786. [DOI] [PubMed] [Google Scholar]
- [110].Thaiss CA, Semmling V, Franken L, Wagner H, Kurts C, Chemokines: a new dendritic cell signal for T cell activation, Front. Immunol 2 (August) (2011), 10.3389/FIMMU.2011.00031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [111].Mir MA, Introduction to costimulation and costimulatory molecules. Dev. Costimulatory Mol. Immunother. Dis, 2015, pp. 1–43, 10.1016/B978-0-12-802585-7.00001-7. January. [DOI] [Google Scholar]
- [112].Dong C, Juedes AE, Temann UA, et al. , ICOS co-stimulatory receptor is essential for T-cell activation and function, Nature 409 (6816) (2001) 97–101, 10.1038/35051100. [DOI] [PubMed] [Google Scholar]
- [113].Takahashi N, Matsumoto K, Saito H, et al. , Impaired CD4 and CD8 effector function and decreased memory T cell populations in ICOS-deficient patients, J. Immunol 182 (9) (2009) 5515–5527, 10.4049/JIMMUNOL.0803256. [DOI] [PubMed] [Google Scholar]
- [114].Wikenheiser DJ, Stumhofer JS, ICOS Co-stimulation: friend or foe? Front. Immunol 7 (August) (2016) 10.3389/FIMMU.2016.00304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [115].McAdam AJ, Greenwald RJ, Levin MA, et al. , ICOS is critical for CD40-mediated antibody class switching, Nature 409 (6816) (2001) 102–105, 10.1038/35051107. [DOI] [PubMed] [Google Scholar]
- [116].Elgueta R, Benson MJ, De Vries VC, Wasiuk A, Guo Y, Noelle RJ, Molecular mechanism and function of CD40/CD40L engagement in the immune system, Immunol. Rev 229 (1) (2009) 152–172, 10.1111/J.1600-065X.2009.00782.X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [117].Dawicki W, Watts TH, Expression and function of 4–1BB during CD4 versus CD8 T cell responses in vivo, Eur. J. Immunol 34 (3) (2004) 743–751, 10.1002/EJI.200324278. [DOI] [PubMed] [Google Scholar]
- [118].Cheuk ATC, Mufti GJ, Guinn BA, Role of 4-1BB:4-1BB ligand in cancer immunotherapy, Cancer Gene Ther. 11 (3) (2004) 215–226, 10.1038/SJ.CGT.7700670. [DOI] [PubMed] [Google Scholar]
- [119].Shuford WW, Klussman K, Tritchler DD, et al. , 4–1BB costimulatory signals preferentially induce CD8+ T cell proliferation and lead to the amplification in vivo of cytotoxic T cell responses, J. Exp. Med 186 (1) (1997) 47, 10.1084/JEM.186.1.47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [120].A Study of RO7198457 as a Single Agent and in Combination With Atezolizumab in Participants With Locally Advanced or Metastatic Tumors - Full Text View - ClinicalTrials.gov. https://clinicaltrials.gov/ct2/show/NCT03289962, (accessed 13.02.2021) [Google Scholar]
- [121].A Study of Ad-RTS-hIL-12 With Veledimex in Combination With Nivolumab in Subjects With Glioblastoma; a Substudy to ATI001–102 - Full Text View - ClinicalTrials.gov. https://clinicaltrials.gov/ct2/show/NCT03636477, (accessed 26.02.2021) [Google Scholar]
- [122].Gadgeel S, Rodríguez-Abreu D, Speranza G, et al. , Updated analysis from KEYNOTE-189: pembrolizumab or placebo plus pemetrexed and platinum for previously untreated metastatic nonsquamous non-small-cell lung cancer, J. Clin. Oncol 38 (14) (2020) 1505–1517, 10.1200/JCO.19.03136. [DOI] [PubMed] [Google Scholar]
- [123].Trial of Anti-Tim-3 in Combination With Anti-PD-1 and SRS in Recurrent GBM - Full Text View - ClinicalTrials.gov. https://clinicaltrials.gov/ct2/show/NCT03961971, (accessed 26.02.2021). [Google Scholar]
- [124].Anti-GITR/Anti-PD1/Stereotactic Radiosurgery, in Recurrent Glioblastoma - Full Text View - ClinicalTrials.gov. https://clinicaltrials.gov/ct2/show/NCT04225039, (accessed 26.02.2021). [Google Scholar]
- [125].Recombinant Interleukin-15 in Combination With Checkpoint Inhibitors Nivolumab and Ipilimumab in People With Refractory Cancers - Full Text View - ClinicalTrials.gov. https://clinicaltrials.gov/ct2/show/NCT03388632, (accessed 26.02.2021) [Google Scholar]
- [126].A Study of ALX148 in Patients With Advanced Solid Tumors and Lymphoma (ASPEN-01) - Full Text View - ClinicalTrials.gov. https://clinicaltrials.gov/ct2/show/NCT03013218, (accessed 26.02.2021). [Google Scholar]
- [127].CD19 CAR-T Expressing IL7 and CCL19 Combined With PD1 mAb for Relapsed or Refractory Diffuse Large B Cell Lymphoma - Full Text View - ClinicalTrials.gov. https://clinicaltrials.gov/ct2/show/NCT04381741, (accessed 26.02.2021). [Google Scholar]