Abstract
Intestinal parasitic infections are distributed virtually throughout the world, with high prevalence rates in tropical and sub-tropical parts of the world as well as in Ethiopia. Children between age groups of 5–10 years are at the highest risk of infection. The intestinal nematode Ascaris lumbricoides infects approximately 25% of the world’s population annually. Hence, this study was the first attempt to assess the prevalence and associated factors of A. lumbricoides infection among the school children from Offa district, Ethiopia. A cross-sectional study was conducted from January to April, 2020 in three selected elementary school. Data were collected through questionnaires and stool samples examination. The overall prevalence of A. lumbricoides was noted as 41.4% and was the leading cause of intestinal parasitoids followed by Schistosoma mansoni (27.6%), Trichuris trichiura (18.1%) and Strongyloides stercoralis (4.1%). Children age group between 5 and 10 years found more susceptible to the infection. Eating habits like unwashed raw vegetables (P = 0.035), absence of toilets (P = 0.000), children who defecate in open field (P = 0.041), drinking unprotected water (P = 0.034), toilet without cover (P = 0.027), lack of hand washing before meal and after defecation (P = 0.000), (P = 0.048) were the key factors significantly associated with A. lumbricoides infection. The present study showed that A. lumbricoides was a major health problem among school children and requires annual de-worming to control morbidity associated with intestinal parasites.
Keywords: Ascariasis, Ascaris lumbricoides, Neglected tropical diseases, School children, Ethiopia
Introduction
Sub-Saharan African countries have always been challenged by infectious diseases, some of which are caused by the helminth and protozoan parasites (Hotez et al. 2008). In fact, one-fourth of the known human infectious diseases are caused by the helminth group A.lumbricoidesis a nematode, or roundworm, which parasitizes the human gastrointestinal tract is one of the major human’s intestinal parasite, especially in the children (Shah and Shahidullah, 2018). It may be due to the fertilization capacity of female worms to deliver huge numbers of eggs that are characterized by being profoundly safe to natural conditions, as well as to the ease of contamination transmission among individuals due to the ingestion of eggs containing the hatchlings in its moment arrange with nourishment and water that are sullied with them (Leung et al. 2020). There’s no in susceptibility when re-infection happens (Shah et al. 2018).
Worldwide, A. lumbricoides is among the most common helminth human infections with an estimated 800 million to 1.2 billion people affected, and it causes more than 60,000 deaths annually (Shah et al. 2018; Dold and Holland 2011). Infection occurs in both male and female, but children are more susceptible to infection compare to adults, especially between the ages of 3 and 8 years (Scott 2008). This is primarily disseminated in zones with warm, sodden environments. Almost 150 countries across the globe are under the threat of Ascariasis prevalence. The distribution of A. lumbricoides shows that 8.3% of cases were in South America, Central America, and the Caribbean, 16.7% of cases were in Africa and the Middle East, and 75% of cases were in Central and Southeast Asia and the Oceanic region (Asaolu and Ofoezie 2018).
Ascaris lumbricoides is the “large roundworm” of humans, growing to a length up to 31 cm (12 inches in male) and 49 cm (19 inches in female). A. lumbricoides infection is a major public health problem especially in developing countries and infects the gastrointestinal tract of man (Tefera et al. 2017; Sowole et al. 2017). A. lumbricoides are soil transmitted helminth (STH), a group of human gastrointestinal nematodes transmitted through ingestion of embryonated eggs in raw vegetables, water or soil contaminated hand and through direct contact with eggs or larva in the soil environment (Asemoto et al. 2014; Iwu et al. 2016).
Ascaris lumbricoides parasite is one of the commonest and most prevalent infections worldwide (Abu et al. 2016; Yoseph and Beyene 2020). A. lumbricoides infections are among the most prevalent and widespread chronic human intestinal infections worldwide with the greatest public health burden occurring in developing countries, particularly in Sub-Saharan Africa (De Silva et al. 2003; Bethony et al. 2006). A. lumbricoides infection rate among children is more prevalent in countries with poor socioeconomic status, where living conditions are unhygienic and untreated wastewater, unsafe and inadequate provision of water, poor personal hygiene and environmental sanitation (Pal and Anberber 2014; WHO 2019).
Ascaris lumbricoides infections, as in many developing countries are also common in Ethiopia, are the second most predominant causes of outpatient morbidity, where children are the most affected group (Chala 2013). The highest infection rate of A. lumbricoides are usually observed in school aged children, due to their bad habits of playing or handling of infested soils, eating with soiled hands, unhygienic toilet practices, drinking and eating of contaminated water and food (WHO 2003). A. lumbricoides infection associated with socioeconomic status (poverty and sanitation), socio-demographic factors including source of drinking water at home (protected or unprotected), absence of toilet in the home, toilet type, poor personal hygiene and environmental sanitation, drinking polluted water, eating contaminated food with A. lumbricoides egg, eating unwashed or raw vegetables, lack of hand washing before meal and after defecation. The highest risk factors for contracting A. lumbricoides infection are poor sanitation and poor hygiene (Chen et al. 2007). Ascariasis, highly predominant among children in developing countries, is thought to cause widespread and important morbidity. The role of Ascariasis as a causative factor towards the etiology of childhood malnutrition has been demonstrated by clinical examination of patients with enormous infection; although, well-planned information are limited (Freij et al. 1979).
In Ethiopia, enteral parasitic infection’s area are the main causes of mortality and morbidity inflicting a series of public health issues like deficiency disease, anemia, and growth retardation still as higher status to alternative infections (Stephenson et al. 1993; Nokes and Bundy 1993; De Silva et al. 1997; Hadidjaja et al. 1998). Poor environmental sanitation, irrigation, overcrowding, relocation, and low altitude were prompt to be blame for high prevalence of internal organ parasitic infection within the country (Tedla 1981; Tedla and Jemaneh 1985). Unfortunately, data is lacking on the burden of intestinal parasites among school children from Offa district of Wolaita Zone, Ethiopia. Therefore, the proposed work was the first attempt to determine the prevalence and risk factors associated with the school children among selected schools from the studied area. The results of the proposed study is highlighted and discussed briefly below. Analysis of facts may provide evidence on the dissemination and generality of intestinal parasites and may aid in suggesting a new plan to combat those groups that might be at risk of infection.
Methods
Study area
Offa District is one of the 15 Districts of Wolaita Zone. It is located at 37° 71’E latitude and 6° 83’ N longitude having altitude ranging of 1200–2000 masl (Fig. 1). The total land area cover of the district is 38,537 ha which comprises a total of 21 rural villages and 4 municipal administrative sub-towns (Tibebu and Zinabu, 2016). It is found at about 29 km towards the west of zonal capital city i.e. Wolaita Soddo, 183 km far from the regional city of Hawassa, and 382 km apart from the capital city of Ethiopia i.e. Addis Ababa. Offa district is one of the foods insecure districts in the Southern National Nationality and Peoples Region (SNNPR). The district has a total population of 127, 387 of which, 74,455 are males and 52,932 are females (CSA 2007). In the District, there are 21 governmental elementary schools, 4 high schools and 5 health institutions. The main drinking water source of the district people is unsafe water such as river, spring, well and limited supply of pipe water.
Fig. 1.
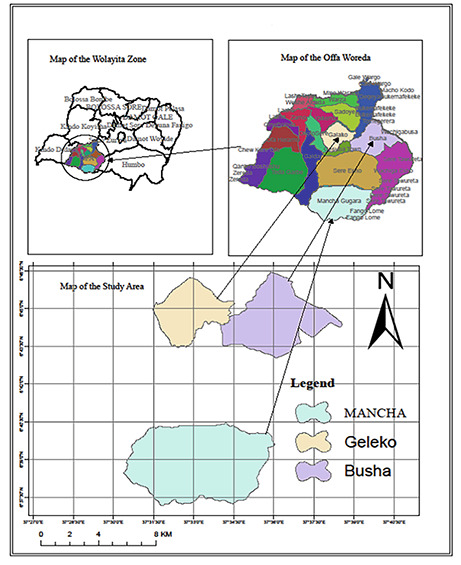
Location map of study area
Design of Study and Settings
Descriptive cross-sectional study was conducted between January to April 2020 and both quantitative and qualitative approaches were preferred to gather information. A total number of 391 school age children were selected as study participants from 3 schools among 21 elementary schools available in the proposed area by simple random sampling method. In order to obtain a more representative sample with the highest precision, students were stratified according to their age levels of 5–10 years, 11–15 years and <15 years. These three age groups are selected because they are old enough to possess sufficient ability and good knowledge to realize and fill the questionnaire. Age groups were first selected by stratified sampling technique. In the end, a quota was allocated for each age group by proportional allocation as per the number of the students present in each age group. Finally, the target students were picked by the systematic random sampling from age class based on their total population.
Sample Size Determination and Sampling Techniques
Sample size determination
The sample size (n) was determined by using the following statistical formula of Naing et al. (2006)
N= (Zα/2)2 P (1-p) E / (D) 2.
Where: N = number of the sample (participant students); P = expected prevalence of A. lumbricoides = 47.3% (0.473); D = precision error (margin of error) between sample and population standard value of (5%) (0.05); E = design effect which reflects the sampling design used in the study; Zα/2 = standard normal deviate (1.96) with 95% CI.
N= (1.96)2 *0.473(1–0.473) / (0.05)2.
N =383 participants for simple random sampling.
To minimize error from non-responsive study participants, 2% of the sample size was added as a contingency to the normal sample size (383+8). Therefore, three hundred ninety one (391) school children were selected to participate in the study.
Inclusion criteria
The study was consented and enrolled students from three different age groups of Geleko elementary school, Busha elementary school and Mancha elementary school, willing to participate in accordance with the written consent to participate, and able to give a stool sample and undergo a 30 min face to face interview.
Exclusion criteria
The participants were excluded from the study if they are unwilling to participate in accordance with the written consent to participate and were not ready to give a stool sample and undergo a 30 min face to face interview.
Sampling Procedure
After proper instruction, each participant was given a clean, dry, and leak-proof stool cup along with pieces of applicator sticks to bring proper fresh stool specimen. All participants were informed to bring approximately 5 g of stool samples so that no contaminates can be mixed. The unique code (sex, age and grade level) of the student was labeled on submission of the stool sample. A portion of the stool was preserved in 10% formalin. The stool specimens were immediately transported in an ice box to the Gesuba town primary hospital, Offa district, Ethiopia for laboratory analysis within 3 h of collection. Stool examination was carried out according to the techniques described previously using direct saline thin smear wet mount microscopy (Kamau et al. 2012). Briefly, two wet preparations of fresh stool collected from the same school children were prepared. 0.25 mg of stool sample emulsified in formal saline was kept at one end of a glass slide and Lugols iodine on the opposite side of the same slide. The slide was then covered with a cover slip and examined under the light microscope by using low and high power objective lenses to observe the presence of A. lumbricoides eggs and other parasites. (Chala 2013).
Data collection method
Data were collected by three well-trained peoples through semi-structured questionnaire by conducting face to face interview from elementary school children. Questionnaires involve sex of children, age of children, habit of hand washing before meal and after defecation, toilet type which the students used, drinking water source for students, educational level of parents, presence or absence of toilet at their home and eating habit of unwashed raw vegetables.
Data Analysis
Data were analyzed by using SPSS statistical software package version 20. Descriptive statistics were calculated to describe the study population characteristics and infection rate of A. lumbricoides in elementary school children. Chi-square was used to analyze association between A. lumbricoides infection and associated risk factors in Offa district elementary school. The dependent variables were the infection rate of A. lumbricoides (positive or negative status of A. lumbricoides). Independent variables were the grade level of children, age category of children, sex, washing habit of vegetables, fruits and hands, water handling practice, eating habit of raw vegetables, personal hygiene practice, parents income level, parents educational level, the habits of environmental sanitation and presence or absence of toilets. Finally, data were summarized in percentages and presented in the form of tables. P values < 0.05 were considered as statistically significant and P values > 0.05 considered as statistically insignificant.
Results
Distribution of A. lumbricoides infection
From the 391 children selected all 391 (100%) provided a stool sample for this study. 54.5% of study subjects were male and 45.4% of study subjects were females. One hundred sixty-two of the study subjects 162/391 (41.4%) had a stool sample positive for A. lumbricoides. Female participants (42.7%) showed a slightly higher infection rate than male participants (40.4%). About age groups, children aged 5–10 years had higher prevalence (50.5%) compare to children aged 11–15 and <15 years (33.8% and 30.8%) (Table 1).
Table 1.
Socio-demographic, and sanitary characteristics of the study participants
| Characters | Frequency | Percentage | ||||
|---|---|---|---|---|---|---|
| Socio-demographic factors | ||||||
| Sex | Male | 213 | 54.5 | |||
| Female | 178 | 45.5 | ||||
| Age | 5–10 | 190 | 48.6 | |||
| 11–15 | 136 | 34.8 | ||||
| >15 | 65 | 16.6 | ||||
|
Parents educational categories |
Illiterate | 122 | 31.2 | |||
| Elementary | 142 | 36.3 | ||||
| Secondary | 68 | 17.4 | ||||
| Diploma/degree | 59 | 15.1 | ||||
| Sanitary factors | ||||||
| Source of drinking water | Protected | 122 | 31.2 | |||
| Unprotected | 269 | 68.6 | ||||
|
status of latrine at home |
Presence | 318 | 81.3 | |||
| Absence | 73 | 23.0 | ||||
| Latrine type | Pit latrine with cover | 132 | 41.5 | |||
| Pit latrine without cover | 186 | 58.5 | ||||
| Place of defection | Open field | 58 | 79.5 | |||
| Neighbor latrine | 10 | 13.7 | ||||
| Public latrine | 5 | 6.8 | ||||
|
Hand washing with soup before meal |
Always | 115 | 29.4 | |||
| Sometimes | 194 | 49.6 | ||||
| Never | 82 | 21 | ||||
|
Hand washing with soup after defecation |
Always | 72 | 18.4 | |||
| Sometimes | 175 | 44.8 | ||||
| Never | 144 | 36.8 | ||||
|
Eating habit of unwashed raw vegetables |
Always | 146 | 37.3 | |||
| Sometimes | 177 | 45.3 | ||||
| Never | 68 | 17.4 | ||||
Socio-demographic characteristics
A total of 391 male and female students selected of different age groups and divided into three age groups (5–10 years, 11–15 years and >15 years). Out of 391 participants, 178 (45.5) were female students. About 190 (48.6%) of the children were in the age groups of 5–10 years, 136 (34.8%) of the sample study populations were in the age group of 11–15 years and 65 (16.6%) were in the age group of >15. 31.2% of the parents were found illiterate, and they didn’t know anything about an intestinal parasite. Regarding sanitary characteristic, use of unprotected water accounts 269 (68.6%), 23% children lack to access to toilets at home, 82 (21.0%) children never wash their hand before eating food and 36.8% participants never wash their hands with soap after defecation. In addition, 146 (37.3%) of the children always eat raw unwashed vegetables (Table 1).
Prevalence of intestinal parasites
From the 154 participants stool sample examined, 73/154 (47.7%) participants were found positive (+) for A. lumbricoides infection from Mancha elementary school children. Out of 128 participants from Geleko elementary school children, 36/128 (28.1%) participants found positive (+) for A. lumbricoides infection. From the 109 participants stool samples examined by microscopy, 53/109 (48.6%) participants were found positive (+) for the A. lumbricoides infection of Busha elementary school children. The highest prevalence of the A. lumbricoides infection 53/109 (48.6%) was observed in children of Busha and Mancha elementary school children 73/154 (47.4%) and the lowest prevalence of A. lumbricoides infection was reported in Geleko elementary school children 36/128 (28.1%) by using the microscopic examination of stool sample (Table 1; Fig. 2).
Fig. 2.
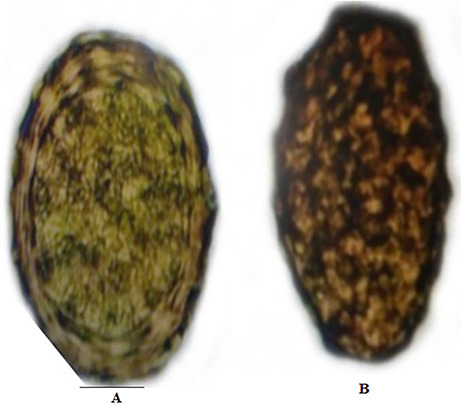
The diagnostic stage of Ascaris lumbricoides isolated from fresh stool samples of children. A: Fertilized eggs; B: Unfertilized eggs
Other helminthes observed during microscopic examination in three selected elementary school in Offa district
Four different helminth species were identified among the study subjects with the most dominant helminth parasite being A. lumbricoides 162/391 (41.4%) followed by Schistosoma mansoni 108/391 (27.6%), Trichuris trichura (71/391) (18.1%) and Strongyloides stercoralis 16/391 (4.1%). Distribution of intestinal helminth parasites infection in elementary school children surveyed showed that the highest infection rate of A. lumbricoides followed by Schistosoma mansoni in the three selected elementary school children of Offa district (Table 2).
Table 2.
Prevalence of Ascaris lumbricoides in three selected elementary schools of Offa district
| Helminths | Ascariasis (+) Or ( -) % | Over all infection (%) | |||
| Mancha (N=154) | Geleko (N=128) | Busha (N=109) | |||
| Ascaris lumbricoides | + | 73 (47.4%) | 36 (28.1%) | 53 (48.6%) | 162 (41.4%) |
| - | 81 (52.6%) | 92 (71.9%) | 56 (51.4%) | 229 (58.6%) | |
Prevalence of Ascaris lumbricoides in elementary school children by age and sex
The infection rate of A. lumbricoides parasite in male and female children was 40.4% and 42.7% respectively (Table 3). There was no statistical significant difference in infection of A. lumbricoides parasite between males and females (P > 0.05; χ2 = 0.643).
Table 3.
Distribution of other helminth infection observed during microscopic examination in school children stool samples Prevalence of Ascaris lumbricoides in elementary school children by age and sex
| Sr. No. | Types of helminth | Number of infected children (%) | Total infection (%) | |||
|---|---|---|---|---|---|---|
| Mancha (N=54) (%) | Geleko (N=128) (%) | Busha (N=109) (%) | ||||
| 1 | Ascaris lumbricoides | 73 (47.4) | 36 (28.1) | 53 (48.6) | 162 (41.4) | |
| 2 | Schisto somamansoni | 39 (25.3) | 40 (31.3) | 29 (26.6) | 108 (27.6) | |
| 3 | Trichuris trichiura | 23 (14.9) | 33 (25.8) | 15 (13.8) | 71 (18.1) | |
| 4 | Strongyloides stercoralis | 6 (3.9) | 3 (2.3) | 7 (6.4) | 16 (4.1) | |
| Total | 141 (36.1) | 112 (28.6) | 104 (26.6) | 357 (91.3) | ||
Major risk factors of infection Ascaris lumbricoides in school children
Eight factors were identified as associated with A. lumbricoides infections (Tables 4, 5 and 6). The study participants parents categorized under illiterate (50.0%), education at the level of elementary school (38.7%), high school (39.7%) and diploma/degree (32.2%) were positive for A. lumbricoides infection, respectively. However, there was no significant relation between A. lumbricoides infection and parents education level (χ2 = 6.272, P = 0.099). The A. lumbricoides infection and lack of toilet access at home have strong associations (χ2 = 121.014, P = 0.000). High infection rate was found among children who had no toilet at their home (98.6%) compared to those who had toilet at their home (28.3%). Associations between A. lumbricoides infections and pit toilet without cover was statistically significant (χ2 = 4.881, P = 0.027). Most of the children who have pit toilet without cover exposed to A. lumbricoides infection (33.3%) than those have pit toilet with cover (22.0). The relation between A. lumbricoides infection and children who were defecated at open field were statistically significant (χ2 = 6.388, P = 0.041). Children defected out of toilet or at open field and public toilet were mostly infected by A. lumbricoides (100%) than children who defect at neighbor toilet 90.0%. There was a strong association between A. lumbricoides infections and lack of hand washing before meal (χ2 = 88.032, P = 0.000). Hand washing before eating has also been shown to protect against infection with A. lumbricoides. A. lumbricoides infection was high in individuals who never wash their hand with soap before meal (86.6%) than those who sometimes and always wash their hand (31.4% and 26.1%). Furthermore, there was a significant association between A. lumbricoides infection and lack of hand washing after defecation (χ2 = 6.059, P = 0.048). A. lumbricoides infection was high in individuals who sometimes and never wash their hands with soap after defection (40.6% and 47.9%) than those who always wash their hand with soap after defecation (30.6%). Children who were eating raw unwashed vegetables were always highly affected by A. lumbricoides (49.3%) than those who were sometimes eating and never (38.4%, and 32.4%). These mean children who were eating unwashed raw vegetables were highly affected by Ascaris lumbricoides than children who were eating washed and cooked vegetables (Tables 4, 5 and 6).
Table 4.
Prevalence of Ascaris lumbricoides in elementary school children by age and sex
| Age and sex | Total No. of examined (%) | No. of Ascariasis Pos (+) (%) | No. of Ascariasis Neg (-) (%) |
χ2 | P value | ||
|---|---|---|---|---|---|---|---|
| Sex | Male | 213 (54.5) | 86 (40.4) | 127 (59.6) | 0.215 |
♣ 0.643 |
|
| Female | 178 (45.5) | 76 (42.7) | 102 (57.3) | ||||
| Age | 5–10 | Male | 101 (53.1) | 50 (49.5) | 51 (50.4) | 12.766 |
⎫ 0.002 |
| Female | 89 (46.8) | 46 (51.7) | 43 (48.3) | ||||
| 11–15 | Male | 72 (52.9) | 23 (31.9) | 49 (68.0) | |||
| Female | 64 (41.5) | 23 (35.9) | 41 (64.0) | ||||
| <15 | Male | 40 (61.5) | 13 (32.5) | 27 (67.5) | |||
| Female | 25 (38.5) | 7 (28.0) | 18 (72) | ||||
| All age group | Male | 213 (54.5) | 86 (40.4) | 127 (59.6) | |||
| Female | 178 (45.5) | 76 (42.7) | 102 (57.3) | ||||
| Total | 391 (100%) | 162 (41.4%) | 229 (58.6%) | ||||
Key: No. Pos = Number of Ascariasis Positive; No. Neg = Number of Ascariasis negative
⎫ Significant difference at P < 0.05
♣ Non significant at P > 0.05
Table 5.
Associations between Ascaris lumbricoides infection with parent’s educational level, and source of drinking water among study participants
| Risk factors (variables) | Total No. of Examined (%) |
No. of Ascariasis Pos. (+) (%) | No. of Ascariasis Neg. (-) (%) | χ2 | P value | |
|---|---|---|---|---|---|---|
| Parents educational level | Illiterate | 122 (31.2) | 61 (50.0) | 61 (15.6) | 6.272 |
♣ 0.099 |
| Elementary | 142 (36.3) | 55 (38.7) | 87 (61.3) | |||
| Secondary | 68 (17.4) | 27 (39.7) | 41 (60.3) | |||
| Diploma/degree | 59 (15.1) | 19 (32.2) | 40 (67.8) | |||
|
Source of drinking water |
Protected pipe water | 122 (31.2) | 41 (33.6) | 81 (66.4) | 4.475 |
⎫ 0.034 |
| Unprotected | 269 (68.8) | 121 (45) | 148 (55.0) | |||
Key: No. Pos = Number of Ascariasis Positive, No. Neg = Number of Ascariasis negative
⎫ Significant difference at P < 0.05
♣ Non significant at P > 0.05
Table 6.
Associations between Ascaris lumbricoides infection and presence or absence of latrine at home, type of latrine, place of defection, in the study area
| Risk factors(variables) | Total No. of Examined (%) |
No of Ascariasis Pos (+) (%) | No of Ascariasis Neg (-) (%) |
χ2 | P value | |
|---|---|---|---|---|---|---|
| Latrine facilities at home | Present | 318 (81.3) | 90 (28.8) | 228 (71.7) | 121.014 |
⎫ 0.000 |
| Absent | 73 (18.7) | 72 (98.6) | 1 (1.4) | |||
| Latrine type | With cover | 132 (41.5) | 29 (22.0) | 103 (78) | 4.881 |
⎫ 0.027 |
| Without cover | 186 (58.5) | 62 (33.3) | 124 (66.7) | |||
| Place of defection | Ppen field | 58 (79.5) | 58 (100) | 0 (0.0) | 6.388 |
⎫ 0.041 |
| Neighbor latrine | 10 (13.7) | 9 (90.0) | 1 (10.0) | |||
| Public latrine | 5 (6.8) | 5 (100) | 0 (0.00) | |||
Key: No. Pos = Number of Ascariasis Positive, No. Neg = Number of Ascariasis negative
⎫ Significant difference at P < 0.05
♣ Non significant at P > 0.05
Table 7.
Associations between Ascaris lumbricoides infection with habit of hand washing with soap before meal after defecation, and eating habit of unwashed raw vegetables
| Risk factors | Total No. of Examined (%) |
No. of Ascariasis Pos (+) (%) | No. of Ascariasis Neg (-) (%) |
χ2 | P value | |
|---|---|---|---|---|---|---|
| Hand washing with soap before meal | Every time | 115 (29.4) | 30 (26.1) | 85 (73.9) | 88.032 |
⎫ 0.000 |
| Sometimes | 194 (49.6) | 61 (31.4) | 133 (68.6) | |||
| Never | 82 (21.0) | 71 (86.6) | 11 (13.4) | |||
| Hand washing with soap after defecation | Every time | 72 (18.4) | 22 (30.6) | 50 (69.4) | 6.059 |
⎫ 0.048 |
| Sometimes | 175 (44.8) | 71 (40.6) | 104 (59.4) | |||
| Never | 144 (36.8) | 69 (47.9) | 75 (52.1) | |||
|
Eating habit of unwashed raw vegetables |
Always | 146 (37.3) | 72 (49.3) | 74 (50.7) | 6.711 |
⎫ 0.035 |
| Sometimes | 177 (45.3) | 68 (38.4) | 109 (61.6) | |||
| Never | 68 (17.4) | 22 (32.4) | 46 (67.6) | |||
Key: No. Pos = Number of Ascariasis Positive, No. Neg = Number of Ascariasis negative
⎫ Significant difference at P < 0.05
♣ Non significant at P > 0.05
Discussion
The three known soil-transmitted helminth infections, Ascariasis, Trichuriasis, and hookworm, are major clinical disorders in man, especially in children. The gastrointestinal tract of a child living in poverty in a less developed country is likely to be parasitized with at least one and in several cases these three soil-transmitted helminth, with resultant impairments in physical, intellectual, and psychological development (Bethony et al. 2006).
According to this study results, A. lumbricoides infection is a major health problem in primary school children in Offa district. The overall prevalence of A. lumbricoides in children was recorded 41.4% and such high burden of infection of intestinal parasites has been consistently reported by a number of studies conducted among school children live in vulnerable rural communities of Ethiopia. Results of the present study showed high rate of infection compared to studies conducted in different parts of Ethiopia, Hosanna Town (11.5%) (Baruda 2012), Wonago Town (37.2%) (Abera and Tafesse 2019), Jima Zone (39.5%) (Tadesse et al. 2008), Babile Town (4.3%) (Tadesse 2005), Teda Health Centre, Northwest Ethiopia (23.2%) (Abate et al. 2013).
On the other hand, this finding showed that the prevalence of intestinal parasite was lower than the studies conducted among children conducted in, Dawro Zone (47.3%) (Alemayehu and Tomas 2015), Chencha Town (60.5%) (Abossie and Seid 2014), Wondo-genet (83.4%) (Berhanu and Germany 2003).
These differences in prevalence of A. lumbricoides in different study areas may be due to the differences in soil types, geographic, environmental sanitation, economic and educational status of parents, accessibility of safe drinking water, personal hygiene socioeconomic conditions as well as differences in an altitude (Padmaja et al. 2014; Okyay et al. 2004). This finding indicates that the source of A. lumbricoides infection might be contaminated stool, vegetables, water and food by A. lumbricoides eggs. Transmission of A. lumbricoides is by the ingestion of the infective eggs from soiled hands and food contaminated with human stool (Bethony et al. 2006).
The prevalence of A. lumbricoides was higher in children’s aged from 5 to 10 years, because Children of this age period often spend most of their time in playing with sand and eating unclean food remains under the trees with unwashed hands, they eat unwashed raw vegetables; they do not wash their hands with soap before meal and after defecation may explain the results. Therefore, the possibility of exposure to the infective stage of A. lumbricoides parasites is higher in elementary school children compared to high school children in the study area. This finding was similar to the recent study conducted in northwest Ethiopia (Eyayu et al. 2021). The highest prevalence of the A. lumbricoides infection was reported in children attending Busha and Mancha elementary school 53/109 (48.6%) and 73/154 (47.7%). Most of the children in Busha and Mancha governmental school has no pipe water, and they drink water from well, ponds spring and streams. The finding is consistent and comparable with the finding of previous reports showing that children in lower grade had a greater prevalence of intestinal parasite diseases (De Silva et al. 1997; Asemoto et al. 2014; Tefera et al. 2017; Fentahun et al. 2019).
The odds of intestinal parasitic infection among school children that had a habit of ingesting raw vegetables is high when compare to the children who were eating washed and cooked vegetables. This finding agrees with finding from Baruda (2012). This might be due to the truth that raw vegetables can act as an attractive source of culture media for intestinal parasites.
The overall, prevalence of A. lumbricoides infection among school children was 41.4%. The prevalence of S. mansoni, T. trichiura and S. stercoralis was 27.6, 18.1 and 4.1% respectively. These parasites have been pursuing as leading intestinal human pathogenic protozoan among little ones. This finding substantiates previous studies that S. ansoni, T. trichiura and S. stercoralis were predominant intestinal protozoan effect school children (Worku et al. 2014; Abah and Arene 2015). This study argues that the prevalence of intestinal protozoan infections was ranging from 2.0 to 7.0% in developed countries and 20.0– 30.0% in most developing countries (Regmi et al. 2014). Another report from Nigeria and Tanzania has concluded that intestinal protozoa are major cause of public health burden (Benjamin et al. 2013; Lorina and Emeka 2013).
The lowest prevalence of A. lumbricoides infection was reported in Geleko elementary school children, 36/128 (28%). Most of the children in Geleko governmental elementary school had pit toilets with cover and they washed their hand with soap before meal and after defecation because their parents advise them. According to this study in Offa district elementary school children, females (42.7%) had slightly higher infection rate than males (40.4%) but this was not statistically significant (χ2 = 0. 215, P = 0.643) in all age groups. In the present study, A. lumbricoides was the most common species recovered from the children. This is consistent with the findings of similar studies conducted elsewhere (Kirwan et al. 2009; Kounnavong et al. 2011; Sowemimo and Asaolu 2011).
The prevalence of A. lumbricoides in elementary school children of several studies conducted in central Nigeria were found to be 28% (Gowon et al. 2018), in Sri Lanka 37.8% (Galgamuwa et al. 2016). There was no significant association in female and male children and education level of parents and prevalence of A. lumbricoides infection.
The overall prevalence of A. lumbricoides in the present study was 41.4%. According to WHO, soil-transmitted helminth endemic area classifications, there are three categories in line with the application of MDA: (i) high transmission (where prevalence is > 50%), (ii) moderate transmission (where prevalence is between 20 and 50%), and (iii) low transmission (where prevalence is < 20%) (WHO 2006; 2012). As such, the proposed area of work can be classified into moderate transmission group calling for deworming yearly.
Conclusions
In general, the current study showed a high prevalence of A. lumbricoides within the study area indicated that parasitic infections are a major public health issues. The current study has additionally discovered that A. lumbricoides a typical parasite species that causes infection among school children of 5–10 years old of study area. The study also highlights once more the considerably variation among schools, in the studied place even within small geographical area, which needs further attention. Therefore, there is an urgent need to improve socioeconomic status, enhancing sanitation services, instilling health education and to implement integrated control programs for A. lumbricoides infection prevention.
Acknowledgements
The authors would like to thanks Department of Biology, College of Natural and Computational Sciences, Dilla University, Dilla, Ethiopia for cooperating with the research work.
Authors Contribution
NMC, STH, FE conceived and designed the study. TZ analyzed the data. STH, NMC, FE performed microscopy analysis of parasites. NMC and VU wrote the paper. STH, TZ, FE edited the manuscript. All authors read and approved the manuscript for publication.
Funding
No specific funding has been received for the proposed work. Hence, declaration of funding is not applicable.
Availability of data and materials
Raw data can be obtained from the corresponding author upon reasonable request.
Declarations
Conflict of interest
The authors declare that they don’t have any conflict of interest.
Ethics approval and consent to participate
The study was reviewed and approved by the ethical committee of Biology department, Dilla University, Dilla, Ethiopia. Ethical considerations were addressed by treating positive intestinal protozoa by giving the drug of choice freely under the prescription and clinical supervision by an authorized health professionals at study sites. The permission has been obtained from Gesuba town primary hospital for sample collection. The questionnaires concerning the prevalence study were filled during sample collection. Written consent was obtained from participants working in different establishments. Apart from these, respondents were asked to fill the questionnaire and assist during sample collection. The information obtained during course of study was kept confidential. Paper data were kept in a locked cabinet confidentially and computer based data were secured with passwords. Except the research team members, no one could access patient data.
Consent for publication
Not applicable.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- Abah AE, Arene FO (2015) Status of Intestinal Parasitic Infections among Primary School Children in Rivers State, Nigeria.J Parasito Res937096 [DOI] [PMC free article] [PubMed]
- Abate A, Kibret B, Bekalu E, Abera S, Teklu T, Yalew A, Endris M, Worku L, Tekeste Z (2013) The Cross-Sectional Study on the Prevalence of intestinal Parasites and associated risk factors in Teda health centre, Northwest Ethiopia.ISRN Parasito5–8 [DOI] [PMC free article] [PubMed]
- Abera M, Tafesse G. Prevalence and Impact of Ascaris lumbricoides Infection on the Physical Growth of Primary School Age Students in Wonago Town, Gedeo Zone, Southern Ethiopia. Int J Curr Res Academic Rev. 2019;7(6):45–53. [Google Scholar]
- Abossie A, Seid M. Assessment of the Prevalence of Intestinal Parasitosis and Associated Risk Factors among Primary School Children in Chencha Town. South Ethiopia BMC Pub Health. 2014;14:166–178. doi: 10.1186/1471-2458-14-166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abu MA, Behnke JM, Boughattas S, Al-Thani A, Doiphode SH. A decade of intestinal Protozoan epidemiology among settled immigrants in Qatar. BMC Infect Dis. 2016;16:370. doi: 10.1186/s12879-016-1728-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alemayehu B, Tomas Z. Reports of prevalence of Intestinal Helminthiasis and Associated Risk Factors among Schoolchildren in Dawro Zone, Southern Ethiopia. Acta Parasitological Globalism. 2015;5(11):71–78. [Google Scholar]
- Asaolu SO, Ofoezie IE (2018) Ascaris spp. In: Global Water Pathogen Project. E. Lansing, MI: Michigan State University, UNESCO 3–39
- Asemoto O, Nmorsi O, Isaac C, Odoya E, Akinseye J, Isaac O. Chemokines’ responses to Ascaris lumbricoides sole infection and co-infection with hookworm among Nigerians. North Americ J Med Sci. 2014;6:84–88. doi: 10.4103/1947-2714.127750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baruda B (2012) Prevalence and intensity of intestinal protozoa and soil-transmitted helminth infections and their association with anthropometric measurements of children in primary school, Hossana town, southern Ethiopia. M.Sc. Thesis, Haramaya University. 1–88
- Benjamin S, Hanspeter M, Shaali M. Prevalence of intestinal protozoa infection among school-aged children on Pemba Island, Tanzania. BMC Infect Dis. 2013;6:1756–2330. [Google Scholar]
- Berhanu E, Germany M (2003) Human helminthiasis in WondoEth Med J41333–344
- Bethony J, Brooker S, Albonico M, Geiger SM, Loukas A. Soil-transmitted helminth infections: ascariasis, trichuriasis, and hookworm. Lancet. 2006;367:1521–1532. doi: 10.1016/S0140-6736(06)68653-4. [DOI] [PubMed] [Google Scholar]
- Chala B. Prevalence of intestinal parasitic infections in Mojo Health Center, Eastern Ethiopia: a 6-year (2005-2010) retrospective study. Epidemio. 2013;3:119. doi: 10.4172/2161-1165.1000119. [DOI] [Google Scholar]
- Chen Y, Zhang Y, Yang B, Qi T, Lu H. Short report: Seroprevalence of Entamoebahistolytica infection in HIV- infected patients in China. Ameri J Trop Med Hygiene. 2007;77(5):825–828. doi: 10.4269/ajtmh.2007.77.825. [DOI] [PubMed] [Google Scholar]
- CSA (2007) Summary and statistical report of the population and housing census results
- De Silva NR, Brooker S, Hotez PJ, Montresor A, Engles D, Savioli L. Soil-transmitted helminth infections. Updating the global picture Trends Parasito. 2003;19:547–551. doi: 10.1016/j.pt.2003.10.002. [DOI] [PubMed] [Google Scholar]
- De Silva NR, Guyatt HL, Bundy DA. Morbidity and mortality due to ascaris-induced intestinal obstruction. Trans R Soc Trop Med Hyg. 1997;91:31–36. doi: 10.1016/S0035-9203(97)90384-9. [DOI] [PubMed] [Google Scholar]
- Dold C, Holland CV. Ascaris and ascariasis. Microbes Infect. 2011;13(7):632–637. doi: 10.1016/j.micinf.2010.09.012. [DOI] [PubMed] [Google Scholar]
- Eyayu T, Kiros T, Workineh L, Sema M, Damtie S, Hailemichael W, et al. Prevalence of intestinal parasitic infections and associated factors among patients attending at Sanja Primary Hospital, Northwest Ethiopia: An institutional-based cross-sectional study. PLoS ONE. 2021;16(2):e0247075. doi: 10.1371/journal.pone.0247075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fentahun AA, Asrat A, Bitew A. Intestinal parasitic infections and associated factors among mentally disabled and non-disabled primary school students, Bahir Dar, Amhara regional state, Ethiopia, 2018: a comparative cross-sectional study. BMC Infect Dis. 2019;19:549. doi: 10.1186/s12879-019-4165-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freij L, Meeuwisse GW, Berg NO, Wall S, Gebre-Medhin M. Ascariasis and malnutrition. A study in urban Ethiopian children. Am J Clin Nutr. 1979;32(7):1545–1553. doi: 10.1093/ajcn/32.7.1545. [DOI] [PubMed] [Google Scholar]
- Galgamuwa LS, Iddawela D, Dharmaratne SD. Intestinal protozoa infections, associated risk factors and clinical features among children in a low-income tea plantation community in Sri Lanka. Int J Community Med Public Health. 2016;3:2452–2458. doi: 10.18203/2394-6040.ijcmph20163053. [DOI] [Google Scholar]
- Gowon AI, Baba OV, Baba OI, Akpu PA, Lynda AE. Ascaris lumbricoides infection using microscopy and IgG4 detection techniques in a school children population in Central Nigeria: An epidemiological study. J Infect Dis Treat. 2018;4(1):5. [Google Scholar]
- Hadidjaja P, Bonang E, Suyardi MA, Abidin SAN, Ismid IS, Margono SS. The effect of intervention methods on nutritional status and cognitive function of primary school children infected with Ascaris lumbricoides. Ameri J of Trop Med Hygiene. 1998;59:791–795. doi: 10.4269/ajtmh.1998.59.791. [DOI] [PubMed] [Google Scholar]
- Hotez PJ, Brindley PJ, Bethony JM, King CH, Pearce EJ, Jacobson J. Helminth infections: the great neglected tropical diseases. J Clin Invest. 2008;118:1311–1321. doi: 10.1172/JCI34261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iwu RU, Ikeanumba M, Azoro AV. Hookworm and Ascaris infections among School-aged Children in Ehime Mbano Local government area of Imo State, Nigeria. J Bacteriol Parasito. 2016;7:278. [Google Scholar]
- Kamau P, Aloo-Obudho P, Kabiru E, Ombacho K, Langat B, Mucheru O, Ireri L. Prevalence of intestinal parasitic infections in certified food-handlers working in food establishments in the City of Nairobi, Kenya. J Biomed Res. 2012;26(2):84–89. doi: 10.1016/S1674-8301(12)60016-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirwan P, Asaolu SO, Molloy SF. Patterns of soil-transmitted helminth infection and impact of four-monthly albendazole treatments in preschool children from semi-urban communities in Nigeria: a double-blind placebo-controlled randomised trial. BMC Infect Dis. 2009;9:20. doi: 10.1186/1471-2334-9-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kounnavong S, Vonglokham M, Houamboun H, Odermatt P, Boupha B. Soil-transmitted helminth infections and risk factors in preschool children in southern rural Lao People’s Democratic Republic. Trans R Soc Trop Med Hyg. 2011;105:160–166. doi: 10.1016/j.trstmh.2010.11.011. [DOI] [PubMed] [Google Scholar]
- Leung AKC, Leung AAM, Wong AHC, Hon KL. Human Ascariasis: An Updated Review. Recent Pat Inflamm Allergy Drug Discov. 2020;14(2):133–145. doi: 10.2174/22122710MTA3eOTIl5. [DOI] [PubMed] [Google Scholar]
- Lorina I, Emeka E. Prevalence of Intestinal Helminthic Infection among School Children in Rural and Semi Urban Communities in Nigeria. IOSR J Dental Med Sci. 2013;6:0853. [Google Scholar]
- Naing L, Winn T, Rusli BN. Practical issues in calculating the sample size for prevalence studies. Med Stat Arch Orofacial Sci. 2006;1:9–14. [Google Scholar]
- Nokes C, Bundy DA. Compliance and absenteeism in school children: implications for helminth control. Trans R Soc Trop Med Hyg. 1993;87:148–152. doi: 10.1016/0035-9203(93)90464-2. [DOI] [PubMed] [Google Scholar]
- Okyay P, Ertug S, Gultekin B. Intestinal parasites prevalence and related factors in school children, a western city sample-Turkey. BMC Pub Health. 2004;4:64. doi: 10.1186/1471-2458-4-64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Padmaja N, Swaroop PS, Nageswararao P. Prevalence of Intestinal Parasitic Infections among School Children in and around Amalapuram. J Pub Health Med Res. 2014;2(2):36–38. [Google Scholar]
- Pal M, Anberber M (2014) Monograph on Microbiology of Drinking Water. First Edn. (LAP) Lambert Academic Publishers: Saarbruchen Germany 1–54
- Regmi P, Rai K, Mukhiya R, Tamang Y, Gurung P, Mandal P, Kumar S. Prevalence of Intestinal Parasites and Associated Risk Factors among School Children of Kalaiya in Bara District, Nepal. JSM Microbiol. 2014;2:1009. [Google Scholar]
- Scott ME. Ascaris lumbricoides: A review of its epidemiology and relationship to other infections. Ann Nestle. 2008;66:7–22. [Google Scholar]
- Shah J, Shahidullah A. Ascaris lumbricoides: A startling discovery during screening colonoscopy. Case Rep Gastroenterol. 2018;12:224–229. doi: 10.1159/000489486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sowemimo OA, Asaolu SO. Current status of soil-transmitted helminthiases among pre-school and school-aged children from Ile-Ife, Osun State, Nigeria. J Helminthol. 2011;85:234–238. doi: 10.1017/S0022149X10000489. [DOI] [PubMed] [Google Scholar]
- Sowole AR, Agbolade OM, Adebayo RO. Ascariasis among children from two primary schools in Ijebu North-East, South-West Nigeria. FUW Trends Sci Tech J. 2017;2:46–48. [Google Scholar]
- Stephenson LS, Latham MC, Adams EJ, Kinoti SN, Pertet A. Physical fitness, growth and appetite of Kenyan school boys with hookworm, Trichuris trichiura and Ascaris lumbricoides infections are improved four months after a single dose of albendazole. J Nutrit. 1993;123:1036–1046. doi: 10.1093/jn/123.6.1036. [DOI] [PubMed] [Google Scholar]
- Tadesse G. The prevalence of intestinal helminthic infections and associated risk factors among school children in Babile town, eastern Ethiopia. Eth J Health Develop. 2005;19(2):215–276. [Google Scholar]
- Tadesse Z, Hailemariam, Kolaczinski JH. Potential for integrated control of neglected tropical diseases in Ethiopia. Trop Mede Hygiene. 2008;102:213–221. doi: 10.1016/j.trstmh.2007.09.005. [DOI] [PubMed] [Google Scholar]
- Tedla S. Intestinal helminthiasis in Ethiopia. Helminthologia. 1981;23:43–48. [Google Scholar]
- Tedla S, Jemaneh L. Distribution of Ancylosto maduodenale and Necator americanus in Ethiopia. Eth Med J. 1985;23:149–158. [PubMed] [Google Scholar]
- Tefera E, Belay T, Mekonnen SK, Zeynudin A, Belachew T. Prevalence and intensity of soil-transmitted helminths among school children of elementary school, Jimma, South West Ethiopia. Pan Afric Med J. 2017;27:88. doi: 10.11604/pamj.2017.27.88.8817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WHO (2003) Preventative Chemotherapy in Human Helminthiasis: Coordinated use of Antihelminthic Drugs in Control Interventions: A manual for Health Professionals and Programme Managers. Issue 1, Geneva, Switzerland: 29
- WHO (2006) Preventive chemotherapy in human helminthiasis: coordinated use of anthelminthic drugs in control interventions: a manual for health professionals and programme managers
- WHO (2012) Soil-transmitted helminthiases: eliminating soil-transmitted helminthiases as a public health problem in children: progress report 2001–2010 and strategic plan 2011–2020. Switzerland
- WHO (2019) Intestinal parasites: Burden and trends of soil transmitted helminths infection. Fact sheet
- Worku L, Damte D, Endris M, Tesfa H, Aemero M (2014) Schistosoma mansoni Infection and Associated Determinant Factors among School Children in Sanja Town,Northwest Eth J Parasit Res792536 [DOI] [PMC free article] [PubMed]
- Yoseph A, Beyene H. The high prevalence of intestinal parasitic infections is associated with stunting among children aged 6–59 months in BorichaWoreda, Southern Ethiopia: a cross-sectional study. BMC Pub Health. 2020;20:1270. doi: 10.1186/s12889-020-09377-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Raw data can be obtained from the corresponding author upon reasonable request.


