Abstract
Cleavage at the F plasmid nic site within the origin of transfer (oriT) requires the F-encoded proteins TraY and TraI and the host-encoded protein integration host factor in vitro. We confirm that F TraY, but not F TraM, is required for cleavage at nic in vivo. Chimeric plasmids were constructed which contained either the entire F or R100-1 oriT regions or various combinations of nic, TraY, and TraM binding sites, in addition to the traM gene. The efficiency of cleavage at nic and the frequency of mobilization were assayed in the presence of F or R100-1 plasmids. The ability of these chimeric plasmids to complement an F traM mutant or affect F transfer via negative dominance was also measured using transfer efficiency assays. In cases where cleavage at nic was detected, R100-1 TraI was not sensitive to the two-base difference in sequence immediately downstream of nic, while F TraI was specific for the F sequence. Plasmid transfer was detected only when TraM was able to bind to its cognate sites within oriT. High-affinity binding of TraY in cis to oriT allowed detection of cleavage at nic but was not required for efficient mobilization. Taken together, our results suggest that stable relaxosomes, consisting of TraI, -M, and -Y bound to oriT are preferentially targeted to the transfer apparatus (transferosome).
Conjugation is the horizontal transfer of DNA from donor to recipient bacteria via plasmid-derived transfer (tra) proteins and other host-encoded factors. F, R1, and R100-1 (a derepressed mutant of R100 [7]) are closely related members of the IncF group of self-transmissible plasmids (19) which exhibit plasmid specificity (53). The plasmids were differentiated at the level of transcriptional control of the major tra operon as well as by properties associated with the conjugative pilus, including antigenicity, phage sensitivity, entry exclusion, and mating-pair stabilization (7). In addition, specificity at the level of DNA processing has also been described (17, 53).
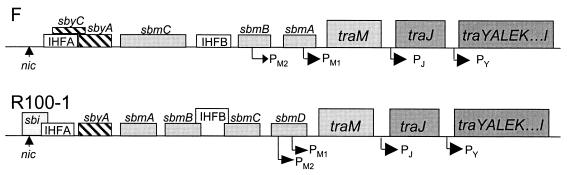
TraY, encoded by the first gene in the traYI operon, binds at two sites in F oriT (sbyA and sbyC [32, 34]) and one site in oriT of R100 (sbyA [27]) (Fig. 1). The relaxase, TraI, cleaves a single strand of DNA in oriT at a site now called nic and covalently binds to the 5′ end (10, 29, 33, 36). In addition to relaxase activity, F TraI also contains an ATP-dependent helicase activity in the large carboxyl-terminal domain of the molecule (12). Integration host factor (IHF) binds two sites in both the F (51) and the R100 (14, 28) oriT regions. Both intrinsic bends and bends induced by IHF (51) are proposed to fulfill the three-dimensional structural requirements at oriT necessary for cleavage at nic.
FIG. 1.
Diagram of the binding sites in oriT of the F and R100-1 plasmids. The traM and traJ genes and the traY-I operon are also shown (not to scale). PM1, PM2, PJ, and PY refer to promoters for the two traM transcripts, traJ and traYI transcripts, respectively. See the text for details.
In the F plasmid, IHF and TraY are required for the nicking reaction in vitro (38), and assembly of the resulting “relaxosome” occurs in a specific order, with TraI binding after IHF and TraY (26). Similar characteristics have been shown for the closely related plasmid R100 (3, 23). The determination of the position of nic was established for F (47, 50) and R100-1 (29), which are equivalent except for a 2-bp difference in the sequence immediately adjacent to nic (19). This difference occurs within the TraI binding site (sbi) for R100-1 (3). TraI has been localized to the cytoplasm (6), but upon overexpression in the presence of TraD it has been shown to be associated with the inner membrane (12). TraD is proposed to be the coupling protein that links the relaxosome to the transferosome, a complex of proteins presumably located at the base of the pilus that forms the transport apparatus (45).
TraM is a cytoplasmic protein of 14.5 kDa which forms tetramers in solution (20, 52). It binds to three sites in F oriT (sbmA, -B, and -C [15]) and four sites in oriT of R100 (sbmA to -D [1]). In F, one of these sites, sbmC, is associated with transfer, while the other two, sbmA and sbmB, are involved in the autoregulation of traM transcription (40). Removal of sbmA and sbmB (Fig. 1) decreases mating efficiency 100-fold, while the additional deletion of sbmC results in a further 100-fold decrease in the efficiency of mobilization of a plasmid containing a cloned version of oriT (22). TraM from the F-like plasmids R100-1 and R1 also autoregulate their transcription (2, 46). The amino-terminal region of TraM is responsible for DNA binding in a plasmid-specific manner (31). The F and R100-1 TraM proteins are 127 amino acids long and are 88.9% identical and 95.3% similar, with 11 of the 14 differences occurring in the first 37 amino acids of the proteins. TraM has also been shown to be associated with the inner membrane (6, 15), possibly via the inner membrane protein TraD (16).
Previous work had shown that TraM, TraY, and TraI from F, R100-1, and R1 plasmids showed plasmid specificity for their homologous oriT regions (17, 53), with TraM and TraY thought to have more specificity than TraI based on sequence variation and the number of alleles (19). Because of the clear differences between the F and R100-1 mating-pair formation systems (7) and the plasmid specificity exhibited by the transfer proteins that bind oriT, chimeric plasmids that are hybrids of the F and R100-1 oriT regions were constructed. These were used to assay whether plasmid specificity is simply a function of DNA recognition by the transfer proteins or whether protein-protein interactions also affected nicking and transfer efficiency.
MATERIALS AND METHODS
Bacterial strains, plasmids, and growth conditions.
Plasmids used in this study are listed in Table 1. The following Escherichia coli strains were used in this study: CS2198 (waaJ19::TnlacZ of CS1999) (43); DH5α [ΔlacU169 (φ80 lacZΔM15) supE44 hsdR17 recA1 endA1 gyrA96 (Nalr) thi-1 relA1] (8, 25); ED24 (F− Spcr Lac−) (54); ED2149 [F− lacΔU124 Δ(nadA aroG gal attL bio)] (13); JE2571-1 (30); and XK1200 [F− Nalr lacΔU124 Δ(nadA aroG gal attL bio gyrA)] (37). Cells were grown in Luria-Bertani (LB) medium (8) or on LB medium with 1.5% agar (Difco Laboratories) supplemented with the appropriate antibiotics at the following final concentrations: ampicillin, 50 μg/ml; kanamycin, 25 μg/ml; and tetracycline, 10 μg/ml.
TABLE 1.
Plasmids used in this study
| Plasmida | Description | Source or reference |
|---|---|---|
| pOX38 | Circularized 55-kb HindIII fragment of F | 24 |
| pOX38-Km | pOX38 with a Kanr cassette inserted into the HindIII site | 11 |
| pOX38-traMK3 | pOX38 with a Kanr cassette inserted into the SalI site within traM | 40 |
| pOX38-traY244 | pOX38 with a Kanr cassette replacing the BclI fragment in traY | 35 |
| pNY300 | 1.08-kbp BglII fragment of F containing oriT, traM, and the beginning of traJ cloned into pUC18 | 18 |
| pRF105 | 1.045-kbp BamHI fragment containing R100-1 oriT, traM, and the beginning of traJ cloned into pUC18 | This work |
| pRF315 | F nic and TraY binding sites linked to R100-1 TraM binding sites and traM cloned into pUC18 (Fig. 2B) | This work |
| pRF206 | F nic linked to R100-1 TraY and TraM binding sites, and traM cloned into pUC18 (Fig. 2B) | This work |
| pKJ4 | 1.98-kbp PCR-amplified fragment of F containing the end of traJ, traY, and traA cloned into pT7.4 | This work |
| pRS31 | 14.5-kbp EcoRI F plasmid fragment containing traI cloned into pSC101 | 48 |
| pLDLF007 | 650-bp DraI fragment containing F TraM binding sites and traM cloned into pT7.4 | 15 |
| R100-1 | A derepressed mutant of the R100 self-transmissible plasmid | 55 |
| pT7.4 | pBR322-based expression vector containing a T7 RNA polymerase promoter | 49 |
Plasmids used in this study are listed with most applicable references.
Recombinant DNA techniques.
Restriction enzymes and T4 DNA ligase were supplied by Boehringer Mannheim and used according to standard procedures (8) except as noted. Plasmids were transformed using CaCl2-competent cells (44) or by electroporation using a Bio-Rad Gene Pulser at 2.5 V, 25 μFD, and 200 Ω. DNA fragments used to create plasmid constructs were isolated from acrylamide by crushing the excised bands containing the fragments and eluting them overnight in 300 μl of 500 mM ammonium acetate and 1 mM EDTA at 37°C, followed by phenol extraction and ethanol precipitation.
Construction of plasmids.
pNY300 (18) was constructed by digesting F with BglII and inserting the 1,080-bp fragment into the BamHI site of pUC18 that contains the F oriT (nic, IHFA, sbyA, and sbmABC) and the F traM gene. pRF105, which is the R100-1 equivalent of pNY300, was constructed using a serendipitous mutation in R100-1 which created a BamHI site 135 bp upstream of nic (19). Digestion with BamHI generated a 1,045-bp fragment which was inserted into the BamHI site of pUC18. Fortuitously situated DraI sites between sbyA and sbmC in F and between nic and sbyA in R100-1 allowed construction of hybrid plasmids in which nic and sbyA, as well as the TraM region, were shuffled. pRF315 was constructed by linking the F nic and TraY binding site (sbyA) to the R100-1 TraM binding sites (sbmABCD) and the traM gene. It was constructed by digesting a PCR product generated from pRF105 using LFR51 (AAATAGAGAGTCGTTGGCGATCC) and reverse (TCACACAGGAAACAGCTATGACCA) primers with EcoRI to yield an 830-bp fragment. This was ligated to the 260-bp DraI and HindIII fragment of pNY300 and inserted into pUC18 digested with EcoRI and HindIII. pRF315 had 1 bp missing from the DraI site and an additional 2 bp near the beginning of sbmC which did not affect its ability to be mobilized by pOX38-Km or pOX38traMK3 compared to the mobilization frequency of pNY300. pRF206 was constructed by linking the F nic to the R100-1 TraY and TraM binding sites (sbyA and sbmABCD) and the traM gene. It was constructed by digesting a PCR product generated from pNY300 using the Universal (GGGTTTTCCCAGTCACGACG) and RFE4 (AAAACGTAAATCAGCAAAAACTTGTT) primers with HindIII to give a 209-bp fragment. This was ligated to an 888-bp fragment of pRF105 digested with EcoRI and DraI and inserted into pUC18 digested with EcoRI and HindIII. One base pair was removed from the DraI site during construction, which did not affect its mobilization frequency by R100-1 compared to pRF105. pKJ4 is an EcoRV-EcoRI fragment containing traY and traA cloned into pT7.4. This construct was created using the EcoRV site in traJ (19) and an EcoRI site engineered by PCR into the 3′ end of traA. All plasmids were sequenced to verify their construction.
Plasmid nicking assays.
Nicking assays for pOX38-Km and its derivatives were done as previously described (21, 41). For the chimeric plasmids, a primer was annealed to a sequence within pUC18 to generate a single-stranded product which terminated at either nic or at a restriction enzyme site downstream from nic. DraI was used to terminate the products for plasmids pNY300 and pRF315 (91 bases from nic), while HinfI was used for pRF206 and pRF105 (98 bases from nic). For chimeric plasmids 3-ml cell cultures containing a chimeric plasmid were grown to an optical density at 600 nm (OD600) of 0.4. Cells were lysed and plasmid DNA was purified using the complete method of Birnboim and Doly (9). DNA was dissolved in 30 μl of Milli-Q water. Then, 2 μl of this DNA was completely digested with the appropriate restriction enzyme. The DNA was ethanol precipitated and dissolved in 10 μl of Milli-Q water. Of this, 0.1 or 0.01 μl was added to the nicking reaction depending on the DNA concentration. The nicking reaction mixture included 11.5 μl of a mixture containing 41.5 μl Milli-Q water, 5 μl of 10× Thermopol Buffer, 1 μl of 10 mM deoxynucleoside triphosphate, 500 pmol of Universal primer, and 2 μl (ca. 20 μCi) of [α-32P]dCTP (Amersham Pharmacia Biotech). Reactions were denatured for 2 min at 94°C before the addition of 2 μl of diluted Vent Polymerase (0.5 μl of polymerase with 8 μl of Milli-Q water) (New England Biolabs). Reactions were amplified by using an MJ Research MiniCycler at 94°C for 30 s, 61°C for 30 s, and 72°C for 1 min for 35 cycles. The reaction mixtures were then removed, rolled on parafilm to remove the remaining mineral oil, and ethanol precipitated. DNA was dissolved in 15 μl of Milli-Q water and 5 μl of Sequencing Stop Solution (USB Biochemicals). Next, 10 μl of each reaction mixture was denatured at 85°C for 10 min and loaded onto a 6% polyacrylamide gel containing 8 M urea. A dideoxy-sequencing reaction of each plasmid using Universal primer was performed with Sequenase (USB Biochemicals) and loaded as a standard.
Quantitation of nicking efficiency.
Gels containing the nicking and sequencing reactions were exposed to a Molecular Dynamics Phosphor Screen overnight and analyzed by a Molecular Dynamics PhosphorImager 445-SI. Band intensities were quantitated using ImageQuant version 4.2a. Bands located at nic were compared to bands located at the DraI or HinfI restriction enzyme sites to determine the percentage of cleavage in each sample. Occasionally, other prominent bands were also found in a single lane, and the values of these bands were added to those of the bands located at the restriction enzyme sites. Background values were also subtracted from both band intensities at nic and the restriction enzyme sites.
Mobilization efficiency assays.
Recipient and donor cells were grown to early log phase (OD600 of 0.4) with appropriate antibiotic selection. Cells were washed twice and resuspended in the same volume of medium. Then, 100 μl each of donor and recipient cells were added to 800 μl of medium and incubated at 37°C for 30 min. Cells were vortexed and placed on ice. Serial dilutions of the mating cultures were made using 1× SSC (0.15 M sodium chloride, 0.015 M sodium citrate; pH 7.0). A 10-μl portion of each dilution was spot-dropped onto selective plates containing combinations of antibiotics to select for transconjugants containing mobilizable plasmids or self-transmissible plasmids, donors, or recipients. Plates were dried and then incubated at 37°C overnight. Mating efficiency is reported as the number of transconjugants per 100 donors. Mobilization assays were done using pOX38-Km or pOX38-traMK3 in E. coli DH5α as donor cells and ED24 as recipient cells. Mobilization assays in the presence of R100-1 used E. coli JE2571-1/R100-1 as donor cells and CS2198 (Kmr) as recipient cells.
RESULTS
Identifying factors required for cleavage in vivo.
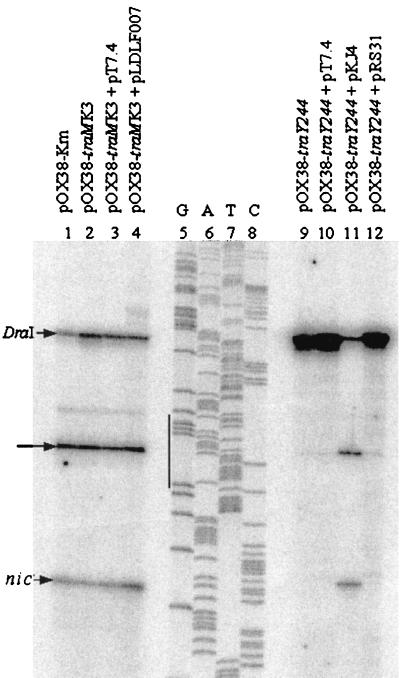
The role of TraM in promoting cleavage at nic was assessed using a nicking assay with pOX38-Km and its derivatives pOX38-traMK3 (traM) and pOX38-traY244 (traY) in E. coli XK1200 (Fig. 2). Removal of TraY by mutation in plasmid pOX38-traY244 abolished cleavage at nic (Fig. 2, lane 9), while supplying TraY in trans (pKJ4) restored cleavage (Fig. 2, lane 11). The addition of the traI gene in trans (pRS31) did not result in cleavage (Fig. 2, lane 12), suggesting that TraI required TraY for both its expression and relaxase activity.
FIG. 2.
Nicking reactions of pOX38-Km and its derivatives. Plasmids present in each experiment are listed above each lane. The DraI site and the cleavage site (nic) are indicated with arrows. The sequencing ladder is used to identify the nic and DraI sites and was performed using the same primer as in the nicking reactions. A nonspecific band is identified with an arrow between nic and the DraI site. The IHF binding site (IHFA) is designated by a vertical line next to the G lane in the sequencing reaction.
The level of cleavage at nic in the traM mutant, pOX38-traMK3, was equivalent to that of the wild-type plasmid pOX38-Km (Fig. 2, lanes 1 and 2). A slight increase in the level of cleavage was seen upon the addition of extra TraM in trans (pLDLF007; traM transcribed from its own promoters) compared to the vector control (pT7.4) (Fig. 2, lanes 3 and 4). A band was routinely found between nic and the DraI site (Fig. 2, middle arrow) which was within the AT-rich region containing IHFA, the first IHF binding site (51). The intensity of this band reflected the level of cleavage at nic and was greatly reduced when TraY was absent (Fig. 2, lane 9), suggesting that termination at this site is dependent on relaxosome formation. In the absence of cleavage at nic, the band located at the DraI site was intensified, as expected (Fig. 2, lane 9), and was approximately equivalent to the sum of the intensities of bands at nic, IHFA, and DraI in other samples.
Mobilization assays of chimeric plasmids.
The chimeric plasmids (Fig. 3) were tested for their ability to be mobilized by R100-1, F (pOX38-Km), and an F traM mutant, pOX38-traMK3. They were also tested for their ability to complement pOX38-traMK3, as well as their effect on the transfer of the pOX38-Km plasmid (Tables 2 and 3). pNY300 was mobilized in the presence of pOX38-Km and pOX38-traMK3 (since pNY300 supplies F TraM) but not in the presence of R100-1 (Table 2). Similarly, pRF105 was mobilized by R100-1 but not by pOX38-Km or pOX38-traMK3, a finding which is consistent with the previously determined plasmid specificity of TraM for its cognate oriT region (53) (Fig. 3).
FIG. 3.
Sequences of the oriT regions of the chimeric plasmids. Sequences are aligned at nic according to the study of Frost et al. (19). Binding sites for F proteins and IHF are shown above the pNY300 sequence, while the equivalent binding sites for R100-1 are represented below the pRF105 sequence. nic is identified by an arrow above the sequences. Sequences were compared by PILEUP in GCG, and 100% homology is represented by black boxes, 75% homology is represented by gray boxes with white lettering, and 50% homology is represented by gray or white boxes with black lettering. The DraI sites used for the cloning of pRF315 and pRF206 are shown as dark gray lines above and below the pNY300 and pRF105 sequences, respectively. Below the sequences is a diagram of the F (clear boxes) and R100-1 (black boxes) sequences for each chimeric oriT region.
TABLE 2.
Mobilization of chimeric plasmids
| Transfer plasmida | No. of transconjugants/100 donors with chimeric plasmid:
|
||||
|---|---|---|---|---|---|
| pNY300 | pRF315 | pRF206 | pRF105 | pUC18 | |
| pOX38-Km | 110 | 51 | 71 | 0 | 0 |
| pOX38-traMK3 | 7.0 | 6.7 | 5.2 | 0.008 | 0 |
| R100-1 | 0.004 | 0.009 | 6.4 | 28 | 0 |
Column contains plasmids that supply transfer functions.
TABLE 3.
Effect of chimeric plasmids on pOX38-Km and pOX38-traMK3 transfer
| Transfer plasmida | No. of transconjugants/100 donors with chimeric plasmid:
|
||||
|---|---|---|---|---|---|
| pNY300 | pRF315 | pRF206 | pRF105 | pUC18 | |
| pOX38-Km | 97 | 83 | 36 | 35 | 133 |
| pOX38-traMK3 | 3.7 | 0.007 | 0 | 0 | 0 |
Column contains plasmids that supply transfer functions.
pRF315 was mobilized efficiently only in the presence of pOX38-Km and pOX38-traMK3 (51 and 6.7 transconjugants per 100 donors, respectively; Table 2). Since TraM is required for transfer, the R100-1 TraM supplied by pRF315 was able to bind to sbmABCD on pRF315 and interact with the F tra proteins supplied by the pOX38 plasmids. The lack of mobilization of pRF315 by R100-1 (<0.01 transconjugants per 100 donors) suggests that the F nic and sbyA sequences were not bound by R100-1 TraI and TraY or, if bound, were unable to function.
pRF206 was mobilized in the presence of pOX38-Km and R100-1 (71 and 6.4 transconjugants per 100 donors, respectively), suggesting that both F and R100-1 are able to mobilize this construct at approximately the same level at which they mobilized pNY300 and pRF105, respectively.
In a set of mating efficiency assays, pNY300 (supplying F TraM), but not pRF315, pRF206, or pRF105 (supplying R100-1 TraM), was able to complement the traM mutation in pOX38-traMK3 (Table 3), suggesting that TraM must bind in cis to nic for transfer to occur. Since pOX38-Km transferred at normal levels in the presence of all four chimeric plasmids, the presence of R100-1 TraM did not appear to exert a dominant-negative effect on F TraM function.
Nicking assays of chimeric plasmids.
The phenotypes of cleavage and transfer have been used to define whether the relaxosome is stable and whether it is able to interact with the transfer machinery (transferosome) to effect DNA transfer (22). To differentiate between these possibilities, nicking assays were performed on the four chimeric plasmids in both F and R100-1 backgrounds (Fig. 4). An extra band (hatched arrow, Fig. 4) immediately above nic and not associated with the band at IHFA was routinely seen in all samples and was considered to be an artifact of sample preparation using the high-copy-number vector pUC18.
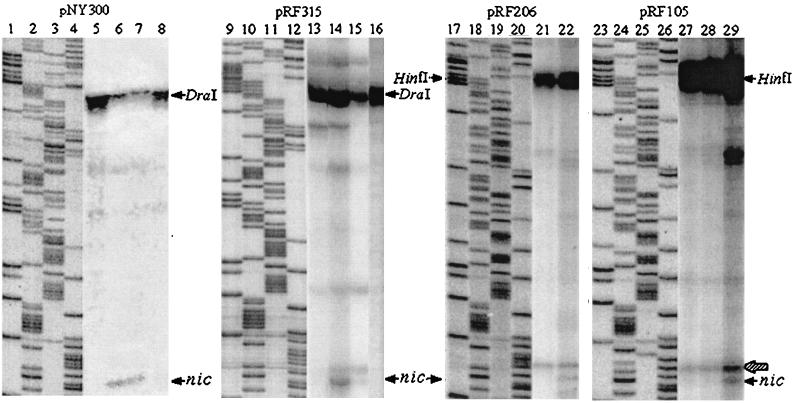
FIG. 4.
Examples of nicking reactions using the chimeric plasmids from Fig. 3. Lanes 1 to 4, 9 to 12, 17 to 20, and 23 to 26 show the sequencing reaction for each plasmid using the universal primer (G, A, T, and C, respectively). Lanes 5 to 8 show the nicking reactions for pNY300, and lanes 13 to 16 show the reactions for pRF315 in DH5α alone and with pOX38-Km, pOX38-traMK3, and R100-1, respectively. Lanes 21 and 22 show the nicking reactions for pRF206 with pOX38-Km and R100-1, respectively. Data for pRF206 in the presence of pOX38-traMK3 are not shown. Lanes 27 to 29 show the nicking reactions of pRF105 with pOX38-Km, pOX38-traMK3, and R100-1, respectively. The nic and restriction enzyme sites are identified with arrows. An example of a nonspecific band is identified with a hatched arrow.
In agreement with the mating efficiency results, pNY300 was cleaved at nic in the presence of pOX38-Km and pOX38-traMK3 (approximately 15% of the plasmids) but not R100-1, while pRF105 was cleaved only in the presence of R100-1 (2%). pRF315 was cleaved by pOX38-Km (12%) and to a lesser extent by pOX38-traMK3 (4%), a finding which is in agreement with the mobilization results for this plasmid (Table 2). Unexpectedly, pRF206 was cleaved efficiently in the presence of R100-1 (3%), but cleavage was not detectable in the presence of pOX38-Km or pOX38-traMK3. Some cleavage of pRF206 was presumed to occur since mobilization of pRF206 was comparable to that of pNY300 and pRF315 in an F (pOX38-Km) background (Table 2).
DISCUSSION
F TraM is not required for efficient cleavage at nic by TraI, a finding which agrees with previous results. Everett and Willetts (17) have shown that cleavage occurs in vivo in the presence of a traM mutation using a lambda nicking assay, while in vitro studies demonstrated that cleavage required TraI, TraY, and IHF (26). Achtman et al. (5) showed that a mutation in traM (JCFL102) affected transfer ability but not phage sensitivity (pilus formation), which was interpreted as a requirement for TraM in DNA metabolism during transfer. Kingsman and Willetts (30) showed that the traM102 mutation affected the initation of DNA synthesis in the donor after mating pair formation had occurred. In the R1 plasmid, TraM has both a regulatory role in the expression of pili (42) and in the level of nicking (31), underscoring the interesting differences between the F and R1 systems. Interestingly, supplying F TraM in trans from a multicopy plasmid increased the amount of cleavage at nic, suggesting that the equilibrium between nicked and un-nicked DNA was shifted toward the relaxed species, as seen in the R1 system (31).
The organization of the oriT region of the R100-1 plasmid closely resembles that of the F plasmid, with plasmid specificity being defined at the level of TraI, -M, and -Y binding at their cognate sites within oriT. Once binding to the DNA has taken place, further specificity could be provided by protein-protein interactions between these proteins within the relaxosome as well as with other proteins involved in the transfer process. Thus, the level of relaxation at nic could reflect the ability of TraY to bind the oriT region independently of TraI (altering the conformation of the DNA near nic, thereby affecting TraI function) or reflect the presence of direct interactions between TraY and TraI. Similarly, the interaction of TraM with these proteins, as well as interactions between TraM within the relaxosome and the transfer apparatus, could also define plasmid specificity.
In the present study, the R100-1 TraM protein of pRF315, pRF206, and pRF105 was not able to complement the traM mutation in the F plasmid derivative pOX38-traMK3. This was not due to decreased cleavage at nic since TraM is not required for this step in F transfer. Since purified F TraM has a low affinity for R100-1 TraM binding sites as measured by electrophoretic mobility shift assay (data not shown), R100-1 TraM might also have a correspondingly low affinity for F TraM binding sites. If this assumption is correct, TraM must be bound to sites in cis to nic for the relaxosome complex for transfer to occur. This is further supported by evidence that pOX38-traMK3 can in turn efficiently mobilize pRF315, where R100-1 TraM is bound to its cognate sites on the chimeric plasmid. Since the level of transfer of pOX38-Km was unaffected by the presence of the chimeric plasmids supplying R100-1 TraM, there appeared to be no dominant-negative effect resulting from having both types of TraM within the same cell. Either mixed oligomers are fully functional or F TraM is preferentially selected to bind to F oriT, a further example of plasmid specificity.
Cleavage of both pRF206 and pRF105 was barely detectable compared to pRF315 or pNY300 in the presence of pOX38-Km. This is in contrast to the mobilization data where pRF206 was efficiently mobilized by pOX38-Km but mobilization was not detectable for pRF105. The only differences between pRF206 and pRF105 are 2 bp adjacent to nic which are within the TraI binding site for the R100-1 plasmid (3). Neither pRF206 nor pRF105 contain the F TraY binding sites which are required for efficient cleavage in an F background. The F TraI relaxase appeared to recognize its cognate cleavage site on pRF206 at a low level and form a small number of stable relaxosomes (TraI covalently bound to the 5′ end of the cleaved nic site) which could be mobilized efficiently.
Comparable results were obtained with the chimeric plasmids in an R100-1 background with one exception. pRF315 was neither cleaved nor transferred by R100-1, suggesting that the R100-1 TraI was not able to form stable relaxosomes in the presence of the F nic and TraY binding sites. Since R100-1 TraI cleaved F nic only if R100-1 TraY was bound in cis to its cognate binding site (pRF206), TraY apparently provides another level of specificity in the R100-1 system.
A specific function for F TraM has not yet been defined. TraM is essential for transfer (4, 40), and its ability to bind DNA near nic and interact with TraD (16) suggests that TraM may anchor the DNA to the membrane. TraM has also been proposed to promote relaxosome formation via formation of a nucleosome-like structure at oriT which adjusts the superhelical density and promotes cleavage and unwinding in preparation for transfer (31, 40). The presence of TraM in the inner membrane in vivo has been demonstrated using multicopy clones of traM (15), as has TraI in the presence of TraD (39). Thus, three steps are required for stable relaxosome formation, which is essential for interaction with the transferosome prior to transfer. TraY binding appears to promote TraI binding but cleavage requires the correct sequence within the TraI binding site near nic. TraM must be bound in cis to nic presumably to allow the complete relaxosome access to the transferosome via TraD. Each of these steps contributes to plasmid specificity.
ACKNOWLEDGMENTS
We thank Jan Manchak for excellent technical assistance.
This research was supported by the Medical Research Council of Canada. R.A.F. is supported by a studentship from the Alberta Heritage Foundation for Medical Research.
REFERENCES
- 1.Abo T, Inamoto S, Ohtsubo E. Specific DNA binding of the TraM protein to the oriT region of plasmid R100. J Bacteriol. 1991;173:6347–6354. doi: 10.1128/jb.173.20.6347-6354.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Abo T, Ohtsubo E. Repression of the traM gene of plasmid R100 by its own product and integration host factor at one of the two promoters. J Bacteriol. 1993;175:4466–4474. doi: 10.1128/jb.175.14.4466-4474.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Abo T, Ohtsubo E. Characterization of the functional sites in the oriT region involved in DNA transfer promoted by sex factor plasmid R100. J Bacteriol. 1995;177:4350–4355. doi: 10.1128/jb.177.15.4350-4355.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Achtman M, Willetts N, Clark A J. Beginning a genetic analysis of conjugational transfer determined by the F factor in Escherichia coli by isolation and characterization of transfer-deficient mutants. J Bacteriol. 1971;106:529–538. doi: 10.1128/jb.106.2.529-538.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Achtman M, Willetts N, Clark A J. Conjugational complementation analysis of transfer-deficient mutants of Flac in Escherichia coli. J Bacteriol. 1972;110:831–842. doi: 10.1128/jb.110.3.831-842.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Achtman M, Manning P A, Edelbluth C, Herrlich P. Export without proteolytic processing of inner and outer membrane proteins encoded by F sex factor tra cistrons in Escherichia coli minicells. Proc Natl Acad Sci USA. 1979;76:4837–4841. doi: 10.1073/pnas.76.10.4837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Anthony A G, Klimke W A, Manchak J, Frost L S. Comparison of proteins involved in pilus synthesis and mating pair stabilization from the related plasmids F and R100-1: insights into the mechanism of conjugation. J Bacteriol. 1999;181:5149–5159. doi: 10.1128/jb.181.17.5149-5159.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ausubel F M, Brent R, Kingston R E, Moore D D, Seidman J G, Smith J A, Struhl J A, editors. Current protocols in molecular biology and supplements. New York, N.Y: John Wiley & Sons, Inc.; 1987. [Google Scholar]
- 9.Birnboim H C, Doly J. A rapid alkaline extraction procedure for screening recombinant plasmid DNA. Nucleic Acids Res. 1979;7:1513–1523. doi: 10.1093/nar/7.6.1513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Byrd D R, Matson S W. Nicking by transesterification: the reaction catalysed by a relaxase. Mol Microbiol. 1997;25:1011–1022. doi: 10.1046/j.1365-2958.1997.5241885.x. [DOI] [PubMed] [Google Scholar]
- 11.Chandler M, Galas D J. Cointegrate formation mediated by Tn9. II. Activity of IS1 is modulated by external DNA sequences. J Mol Biol. 1983;170:61–91. doi: 10.1016/s0022-2836(83)80227-7. [DOI] [PubMed] [Google Scholar]
- 12.Dash P K, Traxler B A, Panicker M M, Hackney D D, Minkley E G., Jr Biochemical characterization of Escherichia coli DNA helicase I. Mol Microbiol. 1992;6:1163–1172. doi: 10.1111/j.1365-2958.1992.tb01555.x. [DOI] [PubMed] [Google Scholar]
- 13.Dempsey W B, Willetts N S. Plasmid cointegrates of prophage lambda and R factor R100. J Bacteriol. 1976;126:166–176. doi: 10.1128/jb.126.1.166-176.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Dempsey W B, Fee B E. Integration host factor affects expression of two genes at the conjugal transfer origin of plasmid R100. Mol Microbiol. 1990;4:1019–1028. doi: 10.1111/j.1365-2958.1990.tb00674.x. [DOI] [PubMed] [Google Scholar]
- 15.Di Laurenzio L, Frost L S, Paranchych W. The TraM protein of the conjugative plasmid F binds to the origin of transfer of the F and ColE1 plasmids. Mol Microbiol. 1992;6:2951–2959. doi: 10.1111/j.1365-2958.1992.tb01754.x. [DOI] [PubMed] [Google Scholar]
- 16.Disque-Kochem C, Dreiseikelmann B. The cytoplasmic DNA-binding protein TraM binds to the inner membrane protein TraD in vitro. J Bacteriol. 1997;179:6133–6137. doi: 10.1128/jb.179.19.6133-6137.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Everett R, Willetts N. Characterisation of an in vivo system for nicking at the origin of conjugal DNA transfer of the sex factor F. J Mol Biol. 1980;136:129–150. doi: 10.1016/0022-2836(80)90309-5. [DOI] [PubMed] [Google Scholar]
- 18.Frost L, Lee S, Yanchar N, Paranchych W. finP and fisO mutations in FinP anti-sense RNA suggest a model for FinOP action in the repression of bacterial conjugation by the Flac plasmid JCFL0. Mol Gen Genet. 1989;218:152–160. doi: 10.1007/BF00330578. [DOI] [PubMed] [Google Scholar]
- 19.Frost L S, Ippen-Ihler K, Skurray R A. Analysis of the sequence and gene products of the transfer region of the F sex factor. Microbiol Rev. 1994;58:162–210. doi: 10.1128/mr.58.2.162-210.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Frost L S, Fekete R A, Penfold S S, Manchak J. Factors that control TraM expression: the level of TraM defines F plasmid transfer proficiency. Plasmid. 1997;37:220–221. [Google Scholar]
- 21.Frost L S, Manchak J. F− phenocopies: characterization of expression of the F transfer region in stationary phase. Microbiology. 1998;144:2579–2587. doi: 10.1099/00221287-144-9-2579. [DOI] [PubMed] [Google Scholar]
- 22.Fu Y H, Tsai M M, Luo Y N, Deonier R C. Deletion analysis of the F plasmid oriT locus. J Bacteriol. 1991;173:1012–1020. doi: 10.1128/jb.173.3.1012-1020.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Fukuda H, Ohtsubo E. Roles of TraI protein with activities of cleaving and rejoining the single-stranded DNA in both initiation and termination of conjugal DNA transfer. Genes Cells. 1997;2:735–751. doi: 10.1046/j.1365-2443.1997.1580356.x. [DOI] [PubMed] [Google Scholar]
- 24.Guyer M S, Reed R R, Steitz J A, Low K B. Identification of a sex-factor-affinity site in E. coli as gamma delta. Cold Spring Harbor Symp Quant Biol. 1980;1:135–140. doi: 10.1101/sqb.1981.045.01.022. [DOI] [PubMed] [Google Scholar]
- 25.Hanahan D. Studies on transformation of Escherichia coli with plasmids. J Mol Biol. 1983;166:557–580. doi: 10.1016/s0022-2836(83)80284-8. [DOI] [PubMed] [Google Scholar]
- 26.Howard M T, Nelson W C, Matson S W. Stepwise assembly of a relaxosome at the F plasmid origin of transfer. J Biol Chem. 1995;270:28381–28386. [PubMed] [Google Scholar]
- 27.Inamoto S, Ohtsubo E. Specific binding of the TraY protein to oriT and the promoter region for the traY gene of plasmid R100. J Biol Chem. 1990;265:6461–6466. [PubMed] [Google Scholar]
- 28.Inamoto S, Abo T, Ohtsubo E. Binding sites of integration host factor in oriT of plasmid R100. J Gen Appl Microbiol. 1990;36:287–293. [Google Scholar]
- 29.Inamoto S, Yoshioka Y, Ohtsubo E. Site- and strand-specific nicking in vitro at oriT by the TraY-TraI endonuclease of plasmid R100. J Biol Chem. 1991;266:10086–10092. [PubMed] [Google Scholar]
- 30.Kingsman A, Willetts N. The requirements for conjugal DNA synthesis in the donor strain during Flac transfer. J Mol Biol. 1978;122:287–300. doi: 10.1016/0022-2836(78)90191-2. [DOI] [PubMed] [Google Scholar]
- 31.Kupelwieser G, Schwab M, Hogenauer G, Koraimann G, Zechner E L. Transfer protein TraM stimulates TraI-catalyzed cleavage of the transfer origin of plasmid R1 in vivo. J Mol Biol. 1998;275:81–94. doi: 10.1006/jmbi.1997.1436. [DOI] [PubMed] [Google Scholar]
- 32.Lahue E E, Matson S W. Purified Escherichia coli F-factor TraY protein binds oriT. J Bacteriol. 1990;172:1385–1391. doi: 10.1128/jb.172.3.1385-1391.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lanka E, Wilkins B M. DNA processing reactions in bacterial conjugation. Annu Rev Biochem. 1995;64:141–169. doi: 10.1146/annurev.bi.64.070195.001041. [DOI] [PubMed] [Google Scholar]
- 34.Luo Y, Gao Q, Deonier R C. Mutational and physical analysis of F plasmid traY protein binding to oriT. Mol Microbiol. 1994;11:459–469. doi: 10.1111/j.1365-2958.1994.tb00327.x. [DOI] [PubMed] [Google Scholar]
- 35.Maneewannakul K, Kathir P, Endley S, Moore D, Manchak J, Frost L, Ippen-Ihler K. Construction of derivatives of the F plasmid pOX-tra715: characterization of traY and traD mutants that can be complemented in trans. Mol Microbiol. 1996;22:197–205. doi: 10.1046/j.1365-2958.1996.00087.x. [DOI] [PubMed] [Google Scholar]
- 36.Matson S W, Morton B S. Escherichia coli DNA helicase I catalyzes a site- and strand-specific nicking reaction at the F plasmid oriT. J Biol Chem. 1991;266:16232–16237. [PubMed] [Google Scholar]
- 37.Moore D, Wu J H, Kathir P, Hamilton C M, Ippen-Ihler K. Analysis of transfer genes and gene products within the traB-traC region of the Escherichia coli fertility factor, F. J Bacteriol. 1987;169:3994–4002. doi: 10.1128/jb.169.9.3994-4002.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Nelson W C, Howard M T, Sherman J A, Matson S W. The traY gene product and integration host factor stimulate Escherichia coli DNA helicase I-catalyzed nicking at the F plasmid oriT. J Biol Chem. 1995;270:28374–28380. [PubMed] [Google Scholar]
- 39.Panicker M M, Minkley E G., Jr Purification and properties of the F sex factor TraD protein, an inner membrane conjugal transfer protein. J Biol Chem. 1992;267:12761–12766. [PubMed] [Google Scholar]
- 40.Penfold S S, Simon J, Frost L S. Regulation of the expression of the traM gene of the F sex factor of Escherichia coli. Mol Microbiol. 1996;20:549–558. doi: 10.1046/j.1365-2958.1996.5361059.x. [DOI] [PubMed] [Google Scholar]
- 41.Perwez T, Meyer R. MobB protein stimulates nicking at the R1162 origin of transfer by increasing the proportion of complexed plasmid DNA. J Bacteriol. 1996;178:5762–5767. doi: 10.1128/jb.178.19.5762-5767.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Polzleitner E, Zechner E L, Renner W, Fratte R, Jauk B, Hogenauer G, Koraimann G. TraM of plasmid R1 controls transfer gene expression as an integrated control element in a complex regulatory network. Mol Microbiol. 1997;25:495–507. doi: 10.1046/j.1365-2958.1997.4831853.x. [DOI] [PubMed] [Google Scholar]
- 43.Pradel E, Parker C T, Schnaitman C A. Structures of the rfaB, rfaI, rfaJ, and rfaS genes of Escherichia coli K-12 and their roles in assembly of the lipopolysaccharide core. J Bacteriol. 1992;174:4736–4745. doi: 10.1128/jb.174.14.4736-4745.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Sambrook J, Fritsch E F, Maniatis T. Molecular cloning: a laboratory manual. 2nd ed. Cold Spring Harbor, N.Y: Cold Spring Harbor Laboratory Press; 1989. [Google Scholar]
- 45.Sastre J I, Cabezon E, de la Cruz F. The carboxyl terminus of protein TraD adds specificity and efficiency to F-plasmid conjugative transfer. J Bacteriol. 1998;180:6039–6042. doi: 10.1128/jb.180.22.6039-6042.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Schwab M, Reisenzein H, Hogenauer G. TraM of plasmid R1 regulates its own expression. Mol Microbiol. 1993;7:795–803. doi: 10.1111/j.1365-2958.1993.tb01170.x. [DOI] [PubMed] [Google Scholar]
- 47.Sherman J A, Matson S W. Escherichia coli DNA helicase I catalyzes a sequence-specific cleavage/ligation reaction at the F plasmid origin of transfer. J Biol Chem. 1994;269:26220–26226. [PubMed] [Google Scholar]
- 48.Skurray R A, Nagaishi H, Clark A J. Molecular cloning of DNA from F sex factor of Escherichia coli K-12. Proc Natl Acad Sci USA. 1976;73:64–68. doi: 10.1073/pnas.73.1.64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Tabor S, Richardson C C. A bacteriophage T7 RNA polymerase/promoter system for controlled exclusive expression of specific genes. Proc Natl Acad Sci USA. 1985;82:1074–1078. doi: 10.1073/pnas.82.4.1074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Thompson T L, Centola M B, Deonier R C. Location of the nick at oriT of the F plasmid. J Mol Biol. 1989;207:505–512. doi: 10.1016/0022-2836(89)90460-9. [DOI] [PubMed] [Google Scholar]
- 51.Tsai M M, Fu Y H, Deonier R C. Intrinsic bends and integration host factor binding at F plasmid oriT. J Bacteriol. 1990;172:4603–4609. doi: 10.1128/jb.172.8.4603-4609.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Verdino P, Keller W, Strohmaier H, Bischof K, Lindner H, Koraimann G. The essential transfer protein TraM binds to DNA as a tetramer. J Mol Biol. 1999;274:37421–37428. doi: 10.1074/jbc.274.52.37421. [DOI] [PubMed] [Google Scholar]
- 53.Willetts N, Maule J. Specificities of IncF plasmid conjugation genes. Genet Res. 1986;47:1–11. doi: 10.1017/s0016672300024447. [DOI] [PubMed] [Google Scholar]
- 54.Willetts N S, Finnegan D J. Characteristics of E. coli K12 strains carrying both an F prime and an R factor. Genet Res. 1970;16:113–122. doi: 10.1017/s0016672300002329. [DOI] [PubMed] [Google Scholar]
- 55.Yoshioka Y, Ohtsubo H, Ohtsubo E. Repressor gene finO in plasmids R100 and F: constitutive transfer of plasmid F is caused by insertion of IS3 into F finO. J Bacteriol. 1987;169:619–623. doi: 10.1128/jb.169.2.619-623.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]