Abstract
Suspected changes in the epidemiology of schistosomiasis due to several hybridization reports between human and livestock Schistosoma species in Africa calls for epidemiological investigations among potential high-risk groups and sites. Although the use of wetlands for pastoralism has been linked to schistosomiasis, there is limited information on the epidemiology of the disease among pastoralists in Nigeria. In this study, urine samples from 355 participants from pastoral communities settled around three Ramsar wetlands (Wetlands of International Importance) in Nigeria, (Dagona Sanctuary, Maladumba, and Pandam-Wase) were screened for the eggs of Schistosoma haematobium. Only participants in the Dagona Sanctuary were infected with 34.2% prevalence. Macrohematuria was however observed in some individuals at the Dagona Sanctuary wetland (2.5%) and Maladumba (2.8%). Regular praziquantel administration, functional health care facilities and awareness about schistosomiasis were contributory factors to the contrasting epidemiology of the disease among the study population. Schistosomiasis control requires the inclusion of pastoral and nomadic communities in mass drug administration of praziquantel based on a community-directed intervention strategy.
Keywords: Schistosomiasis, Ramsar, Wetlands, Pastoralists, Nigeria
Introduction
Schistosomiasis is one of the neglected tropical diseases (NTD) which impact the health of over 200 million people mostly in Africa (WHO 2021). Parasitic trematodes in the genus Schistosoma are responsible for schistosomiasis and in Nigeria, the two forms of the disease (urogenital, caused by S. haematobium) and (intestinal by S. mansoni) are endemic (Nduka et al. 2019). The acute and chronic morbidity impacts of schistosomiasis include; anemia, diarrhea, abdominal pain, portal hypertension, hepatic fibrosis, bladder cancer, and hepatomegaly. The current control strategy advocated for the disease focuses on chemotherapy based on the periodic administration of Praziquantel (WHO 2021).
Application of molecular techniques is increasingly confirming the occurrence of hybridization between different species of Schistosoma. Schistosomes are no longer assumed to be host specific, with increasing evidences that human and animal schistosomes can pair to produce hybrids capable of infecting both humans and animals. In West Africa, hybrids of S. haematobium and livestock schistosomes, S. bovis and/or S. curassoni have been reported as evidence of possible zoonotic transmission of the parasite (Webster et al. 2013; Leger et al. 2016, Soentjens et al. 2016; Boon et al. 2018 and Tian-Bi et al. 2019).
In Nigeria, most epidemiological investigation on schistosomiasis focuses on school-aged children who are usually the most impacted by the disease and are therefore the mainly targeted population for mass administration of praziquantel (Faust et al. 2020). However, one of the often-overlooked populations for schistosomiasis epidemiological studies especially on hybridization are pastoral or nomad communities. A link exists between pastoralism and schistosomiasis (Bruun and Aagaard-Hansen 2008). The lifestyle of nomads and pastoral communities in Nigeria poses several challenges to effective schistosomiasis control. Their frequent migration, location in hard-to-reach terrains, and recent highlevels of insecurity associated with the group hinder successful epidemiological mapping of schistosomiasis.
Important locations for studying Schistosoma hybridization are among nomads and pastoral communities residing around wetlands. Wetlands have been identified to play a crucial role in infectious disease epidemiology including the provision of habitats for disease vectors and acting as transmission foci (Finlayson et al. 2015). Human and animal population density around wetland is often high especially during the dry seasons in tropical areas and for pastoralists, it provides an ideal interface for parasites, human and livestock interaction (Berthe and Kone 2008; Rebelo et al. 2010). We present here our findings on the epidemiology of schistosomiasis among pastoral communities surrounding three Ramsar-listed wetlands (Wetlands of International Importance) in Nigeria during collection of samples for molecular investigation on the occurrence and transmission dynamics of hybrid Schistosoma species.
Methodology
Study location
Nigeria presently has 11 Ramsar categorized wetlands among which are; Dagona Sanctuary wetland (Yobe State), Maladumba wetland (Bauchi State), Pandam-Wase wetland (Nasarawa/Plateau State) where sampling for this study was conducted (Fig. 1). The sites were selected based on their potentials as risk areas for Schistosoma species hybridization. The wetlands are known sites for irrigation farming, pastoralism, and fishing. During the dry season, the floodplains and wetlands are frequented by nomadic pastoral groups who settle around the lakes for grazing their animals (Idris 2008; Olalekan et al. 2014).
Fig. 1.
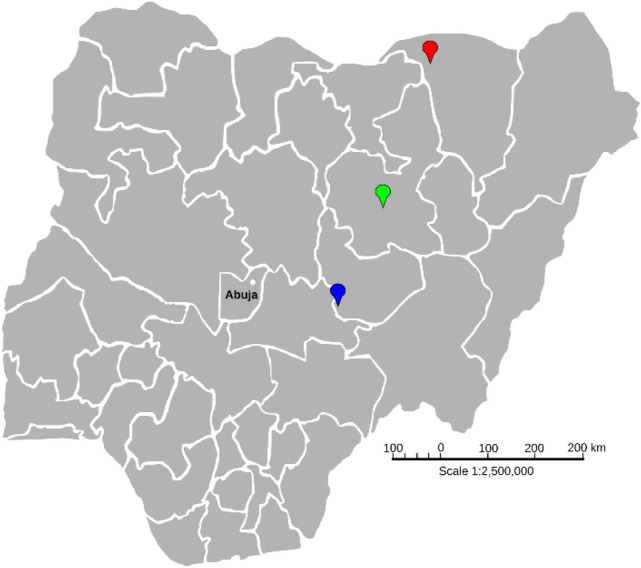
Ramsar listed wetlands in Nigeria sampled for urogenital schistosomiasis. Location pointer: Red, Dagona Sanctuary wetland (12°49′ N, 010°44′ E); Green, Maladumba wetland (10°24′ N, 009°51′ E); Blue, Pandam-Wase wetland (08°42′ N, 008°59′ E)
Sample collection and processing
Sample size (n = 120) for this study was determined based on 95% confidence level of Z score = 1.96 and 5% absolute precision of expected prevalence of 9.5% reference for human infections (Nduka et al. 2019). Ethical approval was obtained from the National Health Research Ethics Committee of Nigeria (NHREC) (protocol number: NHREC/01/01/2007-30/10/2020 and approval number: NHREC/01/01/2007-29/03/2021). Participants were recruited by visiting pastoral settlements around the wetlands from November to December, 2020. Informed consent was obtained from the head of each settlement and individual families after translating the objectives of the study to the participants in a local dialect. Verbal consent was also obtained from the parents/guardians of children. After informed consent by pastoralists, urine samples were collected from voluntary participants.
Each participant was given a clean 50 cm3 wide-mouthed, screw-capped specimen bottle to supply terminal urine between 10:00 am and 2:00 pm. Each bottle was labeled to correspond to the number of the person on a pre-designed epidemiological form. The sex, age, and locality of the subjects were obtained and entered against the appropriate number on the pre-designed epidemiological form on submission of the urine samples. Urine samples from each patient were microscopically examined for the characteristic terminal spine S. haematobium eggs in the laboratory (Montresor et al. 1998). Freshwater snails were collected at each visited wetland site using a scooping net and collected snails were identified and sorted according to species using morphological keys (Brown 1994). Collected snails were screened for cercariae by placing individual snails in petri dishes containing tap water and exposed to artificial light.
Results
A total of 355 participants comprising 212 males and 143 females aged between 2 and 71 years were sampled. The overall prevalence of infection was 11.5% (Table 1). Infection with S. haematobium was recorded only in pastoralists at Dagona Sanctuary wetland (34.2%). Macrohaematuria in urine was observed in 2.8% and 2.5% of pastoralists at Maladumba and Dagona Sanctuary wetland respectively however no Schistosoma egg was recovered in pastoralists from Maladumba wetland. There was no indication of schistosomiasis infection in samples from Pandam-Wase wetland. At the Dagona Sanctuary wetland, males were more infected than females as shown in Table 2. A higher prevalence (46.7%) was observed in the 10–19 years age group followed by the 0–9 years age group (28.9%) while no infection was observed in the age group above 20 years (Table 2). There was no statistically significant variation in differences in age and gender-based infection rates (p < 0.05).
Table 1.
Prevalence of urogenital schistosomiasis in Dagona Sanctuary, Maladumba, and Pandam-Wase wetlands in Nigeria
| Wetland | Total | % Prevalence (N) | % Macrohaematuria (N) |
|---|---|---|---|
| Maladumba | 143 | 0.0 (0) | 2.8 (4) |
| Pandam-Wase | 92 | 0.0 (0) | 0.0 (0) |
| Dagona Sanctuary | 120 | 34.2% (41) | 2.5 (3) |
| Total | 355 | 11.5% (41) | 2.0% (7) |
Table 2.
Prevalence of urogenital schistosomiasisinfection in Dagona Sanctuary wetland settlements according to gender and age
| % Negative (N) | % Positive (N) | % Total (N) | P value | |
|---|---|---|---|---|
| Gender | P < 0.05 | |||
| Male | 59.7 (43) | 40.3 (29) | 60.0 (72) | |
| Female | 75.0 (36) | 25.0 (12) | 40.0 (48) | |
| Age Groups | P < 0.05 | |||
| ≤ 10 | 71.0 (49) | 28.9 (20) | 57.5 (69) | |
| 10–19 | 53.3 (24) | 46.7 (21) | 37.5 (45) | |
| ≥ 20 | 100.0 (6) | 0.0 (0) | 5.0 (6) | |
A total of 130 snails belonging to four genera (Bellamya, Bulinus, Gabiella, and Melanoides) were collected from the three wetlands (Table 3). Most of the collected snails Bellamya spp. (2), Bulinus globusus (76), and Gabiella spp. (27) were from the Dagona Sanctuary wetland. Melanoides tuberculata (21) was recorded only in Maladumba wetland while three B. globusus were the only snails collected in Pandam-Wase wetland. None of the species of snails collected at the Dagona Sanctuary and Maladumba wetland shed ocellate furcocercaria after two days while one out of the three B. globusus from the Pandam-Wase wetland shed ocellate furcocercaria.
Table 3.
Snail species collected in Dagona Sanctuary, Maladumba, and Pandam-Wase wetlands in Nigeria
| Number of snails across wetlands | ||||
|---|---|---|---|---|
| Snail species | Maladumba | Dagona | Pandam-Wase | Total |
| Melanoides tuberculata | 21 | – | – | 21 |
| Bellamya spp. | 1 | 2 | – | 3 |
| Bulinus globusus | – | 76 | 3 | 79 |
| Gabiella spp. | – | 27 | – | 27 |
| Total | 23 | 105 | 3 | 130 |
Discussion
Recent reports of hybridization between human and livestock Schistosoma species have renewed interest in the inquiry of the epidemiology of schistosomiasis among pastoral communities and the contributions of pastoralism to Schistosoma hybridization (Soentjens et al. 2016; Tian-Bi et al. 2019). Nomadic and pastoral communities are usually down the priority list of targeted groups for schistosomiasis intervention. However, these often mobile communities are potential sources of schistosomiasis transmission as they migrate.
Though there have been epidemiological reports on schistosomiasis from the states where the wetlands in our study are located, to the best of our knowledge, the pastoral communities surrounding the wetlands have been poorly investigated. We recorded urogenital schistosomiasis in only one of the three communities investigated in this study (Table 1). A prevalence of 34.2% was recorded in the Dagona wetland communities. A previous investigation by Enabulele et al. (2021) reported 8% prevalence although fewer participants were screened for urogenital schistosomiasis among the same population in the Dagona Sanctuary wetland communities. Infection appears to have peaked at the lower age groups as commonly observed in schistosomiasis infection and males were more infected than females. The high prevalence of infection among the pastoralists settled around the Dagona Sanctuary wetland is not surprising due to the consistent water contact activities by both children and adults despite the presence of schistosomiasis snail vector host, Bulinus globosus in the water. The ox-bow lake is the only source of water for pastoral and farming communities surrounding the area. During the dry season, there is usually migration of pastoralists and their livestock from other communities to the lake, a factor that may have contributed to the high prevalence observed during the study. Also, we could not find a functional health center in the locality and there was no report of recent mass administration of praziquantel in the community.
At the pastoral settlements around Maladumba wetland and Pandam-Wase wetland, no schistosome egg was recovered from the participants. Our finding in the Maladumba wetland is consistent with the report by Nduka et al. (2019) during the national mapping of schistosomiasis in Nigeria in 2015. Although no infection was recovered at the Maladumba wetland, water contact activities such as irrigation and fishing were observed but no vector snails of schistosomiasis were recorded in the water. Interestingly, several studies elsewhere in Bauchi State have consistently reported a low prevalence of urogenital schistosomiasis in Bauchi state (Belonwu 2007; Usman and Babeker 2017). In the Pandam-Wase wetland, we did not record any infection among the population examined though there were constant water contact activities including rice farming, pastoralism, and fishing by the local community members. We however noted the existence of a functional health centre in the locality and yearly administration of praziquantel by the local health authorities through the support of the Carter Centre (Gutman et al. 2008, 2009).
The occurrence of snail species (Bellamya, Bulinus, and Gabiella) encountered in the Dagona sanctuary wetland during the study have been previously documented in Nigeria (Brown 1994; Koudenoukpo et al. 2020). Out of the three species of snails encountered, Bulinus globosus, the snail vector host of S. haematobium was the most abundant. This agrees with the finding of Abe et al. (2017) who reported a very high dominance of B. globosus over other snails in a study on population abundance and bionomics of snail intermediate hosts of trematode parasites in Nasarawa State, Nigeria. The infection status of the snails recovered could not be ascertained as the snails did not shed ocellate furcocercaria after screening for two days which could be due to stress as a result of temperature changes. Subsequent molecular screening of snails is expected to determine the schistosome infection status of the snails (Akinwale et al. 2011).
We did not record any Bulinus species in the Maladumba wetland during this study, however, the occurrence of Bulinus species has been documented by Istifanus et al.(2018) in Bauchi state. The two snail species Melanoides tuberculata and Bellamya sp. found in the wetland are not known to harbour schistosomes although Melanoides tuberculata is a known intermediate host for other trematode parasites (Pinto and Melo 2011). Snail population was almost nonexistent at the Pandam-Wase wetland. Three Bulinus species were the only snail encountered, out of which one shed ocellate furcocercaria (subsequent molecular analysis will be required to confirm the schistosome species). This report is similar to that observed by Ismail et al. (2021) in Sudan who reported complete absence of snails due to presence of and interactions between ecological characteristic such as high turbidity, deep water, low vegetation coverage (near absence of vegetation), high water temperature, and high current speed. Apart from ecological factors, we suspect that the use of agrochemicals in rice farming by the farming communities settled around the lake could be responsible for the reduced diversity and population of snails as these agrochemicals are washed into the water during rainfall. Increased human activities such as dredging and deforestation were also observed around the wetland and could have contributed to the disruption and changes in the ecology of the natural habitat for the snails.
Neglected populations such as pastoral and nomad communities in hard-to-reach areas or conflict zones serve as reservoirs of infections sustaining the continuous transmission of schistosomiasis across a large geographical area. The recent insurgencies in areas where these groups are concentrated further compound the problem as the provision of basic amenities, accessibility to health care, mass chemotherapy programs, and other disease control programs are hampered (Oyeyemi et al. 2020). The goal of achieving the elimination of schistosomiasis cannot become a reality without the inclusion of pastoral communities in schistosomiasis control efforts hence the need for epidemiological data among such risk group. The control of schistosomiasis among pastoral/nomadic populations can be improved by a community-directed distribution of praziquantel. A community-directed approach will be more suited in Nigeria because of the conservative nature and patriarchal structure of most pastoral and nomad communities. This approach will reduce the frequency of visits and risks posed to health workers due to insecurity in the regions. The provision of basic amenities such as good roads for accessibility and potable water supply coupled with mass drug administration is necessary for effective control of schistosomiasis among nomad/pastoral communities.
Acknowledgments
We appreciate all the participants, field assistants and laboratory staffs. We also appreciate the support of Mrs. Bola Akinlosotu who served as the community liaison officer and interpreter.
Author contributions
OGA: Conceptualization, Methodology, Data curation, Investigation, Validation, Writing original draft preparation. AGD, AGH, MPL-Methodology, Investigation. AO-Methodology, Investigation, Validation. EEE: Conceptualization, Methodology, Validation, Writing- Reviewing and Editing, Supervision.
Funding
This work was supported by the International Foundation of Science (IFS) grant I3-B-6522-1 to the first author.
Declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- Abe EM, Ombugadu A, Oluwole AS, Njila HL, Mogaji HO. Population abundance and bionomics of snail intermediate hosts of trematode parasites in Nasarawa State, Nigeria. Res J Parasitol. 2017 doi: 10.3923/jp.2017.8.18. [DOI] [Google Scholar]
- Akinwale OP, Kane RA, Rollinson D, Stothard JR, Ajayi MB, Akande DO, et al. Molecular approaches to the identification of Bulinus species in south-west Nigeria and observations on natural snail infections with schistosomes. J Helminthol. 2011;85:283–293. doi: 10.1017/S0022149X10000568. [DOI] [PubMed] [Google Scholar]
- Belonwu RO. Prevalence and intensity of schistosoma haematobium infection among primary school children in Katagun town, Bauchi state Nigeria. Sahel Med J. 2007;10(1):11–14. doi: 10.4314/smj2.v10i1.12923. [DOI] [Google Scholar]
- Berthe A, Kone B (2008) Wetlands and Sanitation - a View from Africa. In: Ounstedt M, Madgwick J (eds.), Healthy Wetlands, Healthy People. Report of the Shaoxing City Symposium, pp 42–56
- Boon NAM, Broeck FVD, Faye D, Volckaert FAM, Mboup S, Polman K, Huyse T. Barcoding hybrids: heterogeneous distribution of Schistosoma haematobium × Schistosoma bovis hybrids across the Senegal River Basin. Parasitology. 2018;145(5):634–645. doi: 10.1017/S0031182018000525. [DOI] [PubMed] [Google Scholar]
- Brown DS. Freshwater snails of Africa and their medical importance. 2. London: Taylor & Francis; 1994. [Google Scholar]
- Bruun B, Aagaard-Hansen J (2008) The social context of schistosomiasis and its control: an introduction and annotated bibliography. World Health Organization Publication, p 213
- Enabulele EE, Platt RN, Adeyemi E, Agbosua E, Aisien MSO, Ajakaye OG, et al. Urogenital schistosomiasis in Nigeria post receipt of the largest single praziquantel donation in Africa. Acta Trop. 2021 doi: 10.1016/j.actatropica.2021.105916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Faust CL, Osakunor DNM, Downs JA, Kayuni S, Stothard JR, Lamberton PHL, et al. Schistosomiasis control: leave no age group behind. Trends Parasitol. 2020;36(7):582–591. doi: 10.1016/j.pt.2020.04.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finlayson CM, Horwitz P, Weinstein P, editors. Wetlands and human health. Dordrecht: Springer; 2015. p. 275. [Google Scholar]
- Gutman J, Fagbemi A, Alphonsus K, Eigege A, Miri ES, Richards FO., Jr Missed treatment opportunities for schistosomiasis mansoni, in an active programme for the treatment of urinary schistosomiasis in Plateau and Nasarawa states, Nigeria. Ann Trop Med Parasitol. 2008;102(4):335–346. doi: 10.1179/136485908X278810. [DOI] [PubMed] [Google Scholar]
- Gutman J, Richards FO, Jr, Eigege A, Umaru J, Alphonsus K, Miri ES. The presumptive treatment of all school-aged children is the least costly strategy for schistosomiasis control in Plateau and Nasarawa states, Nigeria. Ann Trop Med Parasitol. 2009;103(6):501–511. doi: 10.1179/136485909X451843. [DOI] [PubMed] [Google Scholar]
- Idris M (2008) Damming Nigeria’s wetlands people: communities work together to restore lives and livelihoods. International Rivers
- Ismail H, Ahmed A, Lee YH, Elhag MS, Kim Y, Cha S, Jin Y. Population dynamics of intermediate-host snails in the white Nile river, Sudan: a year-round observational descriptive study. Korean J Parasitol. 2021;59(2):121–129. doi: 10.3347/kjp.2021.59.2.121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Istifanus WA, Panda SM, Sunday ID. Transmission patterns among freshwater snail hosts of schistosomiasis in Bauchi area of Nigeria. GSC Biol Pharm Sci. 2018;2(2):18–24. doi: 10.30574/gscbps.2018.2.2.0006. [DOI] [Google Scholar]
- Koudenoukpo ZC, Odountan OH, Bocxlaer BV, Sablon R, Chikou A, Backeljau T. Checklist of the fresh and brackish water snails (Mollusca, Gastropoda) of Bénin and adjacent West African ecoregions. Zookeys. 2020;942:21–64. doi: 10.3897/zookeys.942.52722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Léger E, Garba A, Hamidou AA, et al. Introgressed animal Schistosomes Schistosoma curassoni and S. bovis naturally infecting humans. Emerg Infect Dis. 2016;22:2212–4. doi: 10.3201/eid2212.160644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Montresor A, Crompton DWT, Hall A, Bundy DAP, Savioli L (1998) Guidelines for the evaluation of soil-transmitted helminthiasis and schistosomiasis at community level: a guide for managers of control programmes, 1988. WHO. https://apps.who.int/iris/handle/10665/63821 (accessed 20 November 2020)
- Nduka F, Nebe OJ, Njepuome N, Dakul DA, Anagbogu IA, Ngege E, et al. Epidemiological mapping of schistosomiasis and soil-transmitted helminthiasis for intervention strategies in Nigeria. Nigerian J Parasitol. 2019;40(2):218–225. doi: 10.4314/njpar.v40i2.18. [DOI] [Google Scholar]
- Olalekan EI, Abimbola LM, Saheed M, Damilola OA. Wetland Resources of Nigeria: Case Study of the Hadejia-Nguru Wetlands. J Poult Fishe Wildl Sci. 2014;2:123. doi: 10.4172/2375-446X.1000123. [DOI] [Google Scholar]
- Oyeyemi OT, de Jesus JW, Grenfell RFQ. Schistosomiasis in Nigeria: Gleaning from the past to improve current efforts towards control. One Health. 2020;11:100183. doi: 10.1016/j.onehlt.2020.100183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pinto HA, De Melo AL. A checklist of trematodes (Platyhelminthes) transmitted by Melanoides tuberculata (Mollusca: Thiaridae) Zootaxa. 2011;2799:15–28. doi: 10.11646/zootaxa.2799.1.2. [DOI] [Google Scholar]
- Rebelo ML, McCartney MP, Finlayson CM. Wetlands of Sub-Saharan Africa: distribution and contribution of agriculture to livelihood. Wetlands Ecol Manage. 2010;18(5):557–572. doi: 10.1007/s11273-009-9142-x. [DOI] [Google Scholar]
- Soentjens P, Cnops L, Huyse T, Yansouni C, De Vos D, Bottieau E, et al. Diagnosis and clinical management of Schistosoma haematobium-Schistosoma bovis hybrid infection in a cluster of travelers returning from Mali. Clin Infect Dis. 2016;63:1626–1629. doi: 10.1093/cid/ciw493. [DOI] [PubMed] [Google Scholar]
- Tian-Bi YT, Webster B, Konan CK, Allan F, Diakité NR, Ouattara M, Salia D, Koné A, Kakou AK, Rabone M, Coulibaly JT, Knopp S, Meïté A, Utzinger J, N’Goran EK, Rollinson D. Molecular characterization and distribution of Schistosoma cercariae collected from naturally infected bulinid snails in northern and central Côte d’Ivoire. Parasit Vectors. 2019;12:117. doi: 10.1186/s13071-019-3381-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Usman AM, Babeker EA (2017) A study on the aspects of epidemiology of urinary and intestinal Schistosomiasis in Bauchi State, Nigeria. Sci World J 12, 4. https://www.ajol.info/index.php/swj/article/view/166103
- Webster BL, Diaw OT, Seye MM, Webster JP, Rollinson D. Introgressive Hybridization of Schistosoma haematobium Group Species in Senegal: Species Barrier Break Down between Ruminant and Human Schistosomes. PLoS Negl Trop Dis. 2013;7(4):e2110. doi: 10.1371/journal.pntd.0002110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- World Health Organization (2021) Schistosomiasis: fact sheet, WHO. https://www.who.int/news-room/fact-sheets/detail/schistosomiasis


