Abstract
Introduction
Data collected through ongoing, state-based, cross-sectional health surveys could be used to better understand the contribution of respiratory symptoms to impaired health among the US adult population.
Methods
We used the 2015 Behavioral Risk Factor Surveillance System telephone health survey in four states (Kentucky, Florida, South Carolina, Texas) to describe the relationship between symptoms, associated factors such as tobacco smoking, and health impairments. Self-reported productive cough, shortness of breath (SOB), and dyspnea on exertion (DOE) were categorized as minimal, moderate, or severe. Data were analyzed using multiple logistic regression models with age as a covariate to assess relationships of symptoms with other factors.
Results
Among adults ≥ 18 years, respiratory impairment [current asthma, chronic obstructive pulmonary disease (COPD), or a current moderate or severe symptom] occurred in 39.1% of the population. More than half of adults reporting moderate or severe symptoms had not been diagnosed with asthma or COPD, particularly with DOE and productive cough. Subjects were at greater risk of moderate and severe SOB or productive cough with increasing age, prolonged smoking duration (≥ 20 years), being an ever-smoker, or if reporting COPD, current asthma, or any other comorbidity except cancer. Morbid obesity [body mass index (BMI) > 35 kg/m2] was associated with severe DOE at a rate similar to current asthma or COPD (25.6%, 95% CI 20.9–30.3%; 20.8%, 95% CI 16.4–25.1%; 21.3%, 95% CI 17.5–25.1%, respectively); it was the most common cause of DOE. SOB was associated with worse general health impairment and limited ambulation compared with other symptoms. Tobacco smoking prevalence and race varied among states, affecting symptom prevalence.
Conclusion
In the largest US survey in decades, we provide a current perspective of respiratory symptoms among adults of all ages. While known risk factors were apparent, low-risk persons also frequently reported symptoms and impairments.
Supplementary Information
The online version contains supplementary material available at 10.1007/s41030-022-00194-9.
Keywords: Cross-sectional health survey, Behavioral Risk Factor Surveillance System, Productive cough, Shortness of breath, Dyspnea on exertion, Quality of life, Smoking duration, Asthma, COPD, Obesity
Key Summary Points
| Characterizing respiratory symptoms in the general population through health surveys helps define the burden of disease. Current, cross-sectional data are lacking in the USA, particularly in young adults and across different races/ethnicities. |
| In a four-US-state survey, representing ~ 17% of the US adult population, dyspnea on exertion was the most prevalent respiratory symptom, followed by productive cough and shortness of breath. |
| Shortness of breath was the respiratory symptom most associated with health impairments. |
| While asthma, chronic obstructive pulmonary disease (COPD), and coronary heart disease were common causes of dyspnea on exertion, morbid obesity was the most prevalent comorbidity in subjects reporting this symptom. |
| Young persons continue to smoke at the highest rates among adults, with cough and dyspnea on exertion more common than shortness of breath in this age group. |
Introduction
Characterizing respiratory health is important to the understanding of overall health in the population. Respiratory symptoms are common in the population as a number of health conditions can cause breathlessness or cough, including obstructive lung diseases, heart diseases, anxiety/depression, obesity, gastrointestinal reflux, and deconditioning. These symptoms are important because dyspnea and chronic mucus hypersecretion are associated with increased morbidity and mortality, particularly in obstructive lung diseases, but even in the absence of airflow obstruction [1–5]. There is increasing interest in better understanding lung health at the population level, a topic to be evaluated in the upcoming Lung Health Cohort Millennials study in the USA [6].
Cross-sectional and longitudinal surveys of respiratory symptoms in adults have been conducted in Europe [2, 7–10], internationally [3, 11], and in the USA [12–16]. Current data about respiratory symptoms in young and older adults in the USA are sparse [15, 16], particularly the former. The US Centers for Disease Control and Prevention’s (CDC) Behavioral Risk Factor Surveillance System (BRFSS) is an annual, nationally based, state-administered telephone health survey conducted in > 400,000 adults 18 years and older, and is the world’s largest ongoing population health survey. This type of health survey is also used in some other countries, including South Korea and Ecuador. It has been a primary epidemiologic source for the prevalence and burden of COPD and asthma among and within US states. In 2012, a module containing four questions was added to one state’s BRFSS to serve as proof of concept to describe respiratory symptoms in a general adult population [16].
For the current study, we expanded the use of this module [16] in four states’ BRFSS in 2015 to assess respiratory symptoms in a larger, more diverse population. Our aims were to further examine the BRFSS as a survey tool to describe lung health in the USA, provide a recent characterization of sociodemographics and health behaviors of persons with respiratory symptoms among all ages of adults, and report on symptoms from a state-based perspective. Some of the results have been reported as a meeting abstract [17].
Methods
Study Population
We incorporated an adult respiratory health module (Table 1) into the 2015 BRFSS for four states—Florida (FL), Kentucky (KY), South Carolina (SC), and Texas (TX), in which an estimated 17.7% of the US population [18] and 18.7% of patients with COPD [19] reside. The BRFSS has been approved as exempt research by the CDC’s institutional review board. The study was a secondary data analysis of deidentified, publicly available data. It was also approved by the Duke University institutional review board.
Table 1.
Respiratory module and health impairment questions with possible responses using the 2015 Behavioral Risk Factor Surveillance System telephone health survey in four states (Florida, Kentucky, South Carolina, Texas)
| Measure | Respiratory module questions | Definitions |
|---|---|---|
| Ever-smoking status and duration of smoking |
Have you smoked more than 100 cigarettes in your lifetime? If yes, subjects were asked How many years have you smoked tobacco products? ___ years |
Years of tobacco smoking (categorized as 0, 1–9, 19–19, 29–29, ≥ 30 years) |
| Shortness of breath | During the past 30 days, how often have you felt short of breath?—Would you say “all of the time,” “most of the time,” “some of the time,” “a little of the time,” or “none of the time”? |
Severe—all or most of the time Moderate—little or some of the time Minimal—none of the time |
| Productive cough | During the past 30 days, how often did you cough up mucus or phlegm? Would you say “every day,” “most days,” “a few days,” “only with colds,” or “never”? |
Severe—every day or most days Moderate—a few days Minimal—only with colds or never |
| Dyspnea on exertion | How much do you agree or disagree with the following statement? In the past year, I am not as physically active as I once was because of my shortness of breath: agree strongly, agree slightly, neither agree or disagree, disagree slightly, disagree strongly |
Severe—agree strongly or slightly Moderate—neither agree nor disagree Minimal—disagree slightly or strongly |
Telephone Survey
The BRFSS is a random, digit-dialed cellphone and landline survey of non-institutionalized adults ≥ 18 years, composed of a core section of > 90 questions about sociodemographics, health behaviors, and self-report of healthcare provider diagnosed chronic diseases. In addition to core BRFSS questions, the respiratory module (Table 1) was asked of all states’ respondents over the 12-month period except FL where this was asked over 6 months. State-specific modules added to the core survey are sometimes only undertaken over a 6 month period, as chosen by FL in this instance. Comorbities were self-reported and obesity was defined by World Health Organization classifications [20].
Respiratory Symptoms
The respiratory module contained three questions to characterize the type and frequency of self-reported respiratory symptoms and a fourth question to quantitate tobacco exposure on the basis of years smoked (duration). The previously published module questions [16] inquiring about shortness of breath (SOB), productive cough, and dyspnea on exertion (DOE) were derived by consensus of investigators using respiratory-symptom-based questionnaires [21–25]. We considered DOE as breathlessness due to physical activity [26]. Each of the three symptoms was categorized as minimal, moderate, or severe.
Respiratory and Health Impairment
We defined impairment of respiratory health by the report of any moderate and severe respiratory symptom, current asthma, or COPD. Questions used to define and analyze general health-related quality of life (HRQoL) and ambulation are presented in Supplementary Materials.
Analysis
Respondents were excluded from analysis if data were missing for smoking status, smoking duration, or one or more respiratory symptoms. All analyses was conducted using SAS 9.4 to account for the complex BRFSS sampling. BRFSS method applies an iterative proportional fitting, or raking to address the nonresponsive bias. This method allows for education level, marital status, and home ownership in addition to other traditional demographic characteristics used in post-stratification to increase the representativeness of estimates. Analysis was done collectively for all states and for selected measures by individual state. First, we calculated the percentage and 95% confidence intervals (CI) of sociodemographics, health behaviors, health impairment, and chronic diseases. For comparisons of prevalence between subgroups, statistical significance was determined by t-tests. Prevalence was compared by risk status using pairwise t-tests. A P-value < 0.05 was considered statistically significant for all tests. Other than an age-specific category, all prevalence estimates were age-adjusted to the 2000 US standard population. A trend analysis of each symptom with age and tobacco duration was performed using models regressing moderate or severe SOB, cough, and DOE with each measure using the Wald test of linearity and odds ratio for age increment (SAS Institute, SURVEYLOGISTIC).
Results
A total of 44,849 respondents were asked the core questions; the number of respondents was 9739 (FL), 8806 (KY), 11,607 (SC), and 14,697 (TX). The response rates for landline, cellphone, and combined respectively for each state were as follows: FL 37.0%, 37.1%, and 37.0%; KY 62.1%, 51.6%, and 59.0%; SC 52.1%, 51.5%, and 50.4%; and TX 32.9%, 41.0%, and 34.4%. After exclusion of respondents with incomplete data, 31,875 subjects’ data were analyzed. (Supplemental Materials Table 1). More than half of the subjects were ≥ 45 years old, female, white, and with at least some college education. Racial/ethnic minorities (Blacks, Hispanics) and young adults (18–34 years old) were well represented (> 2500 each). The number of Hispanics participating in the survey in each state was as follows: 13.0% (FL), 1.0% (KY), 1.9% (SC), and 29.8% (TX).
Respiratory Symptoms
We found the frequency of respiratory symptoms was impacted by increasing age, race/ethnicity, nearly all comorbidities, years of tobacco smoking, and state of residence. In contrast, sex had little effect on symptom prevalence. DOE was the most commonly reported symptom, followed by productive cough, then SOB (Supplementary Materials Table 1). Figure 1 shows age-related frequencies of symptoms, COPD, current asthma, and current smoking. There was a significant linear relationship between increasing age and each symptom. In the youngest age groups (18–24 and 25–34 years old), productive cough was the most common moderate and severe symptom, and with increasing age, DOE became the most frequent symptom. Among the elderly, productive cough became as prevalent as DOE. When comparing solely severe symptoms in 18–24-year-olds, differences became statistically significant for DOE, SOB, and productive cough compared with 45–54-year-olds, 55–64-year-olds, and 65–74-year-olds, respectively. While the prevalence of asthma was somewhat stable across age groups, the increase in COPD prevalence became evident in the 55–64-year-olds.
Fig. 1.
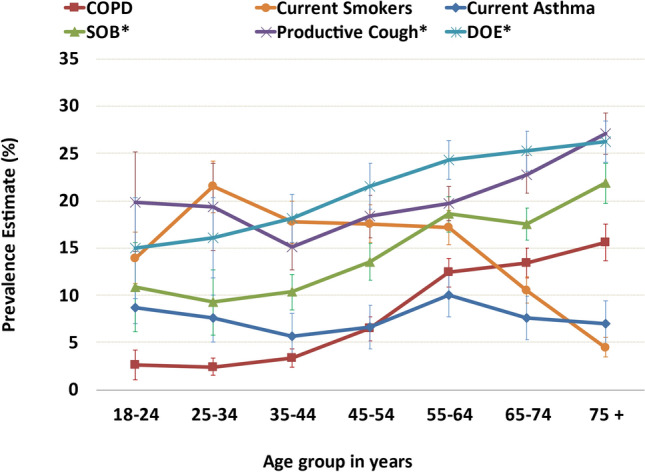
Age-based frequencies of moderate or severe respiratory symptoms, current asthma, COPD, and current smoking in adults ≥ 18 years old using the 2015 BRFSS health survey in four US states (Florida, Kentucky, South Carolina, and Texas). COPD chronic obstructive pulmonary disease, DOE dyspnea on exertion, SOB shortness of breath. Test of linearity between age and each symptom was significant (P < 0.001) for SOB (odds ratio [OR] 1.017, 95% CI 1.013–1.022), productive cough (OR 1.008, 95% CI 1.004–1.012), and DOE (OR 1.012, 95% CI 1.009–1.016). *Moderate to severe productive cough: cough up mucus or phlegm either every day, most days, or a few days each month. *Moderate to severe shortness of breath: short of breath either all of the time, most of the time, or some of the time. *Moderate to severe dyspnea on exertion: not as physically active as I once was because of my shortness of breath, either agreeing strongly or slightly, or neither agree nor disagree
State-based differences were evident for symptoms where the frequency of moderate or severe symptoms was highest in KY, with the exception of moderate productive cough, which had a higher frequency in FL and TX. At least one in five KY adults reported severe symptoms (Table 2) While past smoking rates were not different among states, current smoking rates were highest in KY and, thus, likely an important contributor to this state having the highest rates for all three symptoms.
Table 2.
State-specific age-adjusted prevalence of respiratory symptoms in adults ≥ 18 years old using the 2015 Behavioral Risk Factor Surveillance System health survey (Florida, Kentucky, South Carolina, and Texas)
| Smoking status | SOB | Productive cough | DOE | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Current smoker | Former smoker | Never smoker | Severea | Moderateb | Minimalc | Severe d | Moderatee | Minimalf | Severeg | Moderateh | Minimali | |
| % (95% CI) | % (95% CI) | % (95% CI) | % (95% CI) | % (95% CI) | % (95% CI) | % (95% CI) | % (95% CI) | % (95% CI) | % (95% CI) | % (95% CI) | % (95% CI) | |
| FL | 15.0 (13.1–17.0) | 25.8 (23.8–27.8) | 59.2 (56.8–61.7) | 3.1 (2.3–4.1) | 7.1 (5.9–8.3) | 73.9 (71.7–76.2) | 6.7 (5.5–7.9) | 8.5 (7.1–9.9) | 68.8 (66.5–71.2) | 10.8 (9.2–12.3) | 4.8 (3.7–5.8) | 66.2 (63.8–68.6) |
| KY | 26.4 (24.4–28.3) | 23.4 (21.8–25.0) | 50.3 (48.2–52.3) | 6.9 (5.8–8.0) | 11.4 (10.2–12.6) | 75.6 (73.9–77.4) | 12.2 (10.9–13.5) | 10.7 (9.5–11.8) | 70.3 (68.5–72.1) | 19.3 (17.7–20.9) | 5.1 (4.2–6.1) | 65.9 (64.0–67.8) |
| SC | 20.2 (18.9–21.5) | 23.4 (22.2–24.5) | 56.4 (54.9–57.9) | 3.6 (3.1–4.1) | 8.1 (7.3–9.0) | 68.3 (67.0–70.0) | 7.8 (7.1–8.6) | 9.6 (8.7–10.5) | 62.3 60.9–63.8) | 10.7 (9.9–11.6) | 4.6 (4.0–5.2) | 63.0 (61.6–64.4) |
| TX | 15.2 (14.1–16.5) | 20.8 (19.5–22.0) | 64.0 (62.4.-65.6) | 2.2 (1.8–2.5) | 5.0 (4.4–5.6) | 40.7 (39.4–42.0) | 4.1 (36.1–46.3) | 5.1 (4.5–5.7) | 38.6 (37.3–39.8) | 9.1 (8.3–9.9) | 2.0 (1.6–2.4) | 34.9 (33.6–36.2) |
SOB shortness of breath, DOE dyspnea on exertion, FL Florida, KY Kentucky, SC South Carolina, TX Texas
aSevere shortness of breath (SOB): all of the time or most of the time in the last 30 days
bModerate SOB: some of the time in the last 30 days
cMinimal SOB a little of the time or none of the time over the last 30 days
dSevere productive cough: cough up phlegm or mucus every day or most days in the last 30 days
eModerate productive cough: cough up phlegm or mucus a few days in the last 30 days
fMinimal productive cough: cough up phlegm or mucus only with colds or never in the last 30 days
gSevere dyspnea on exertion (DOE): agree slightly or strongly that physical activity is affected by shortness of breath over the past year
hModerate DOE: neither agree nor disagree that physical activity is affected by shortness of breath over the past year
iMinimal DOE: disagree slightly or strongly that physical activity is affected by shortness of breath over the past year
Comorbidities, except cancers, were more prevalent in persons with moderate or severe respiratory symptoms than in those with minimal symptoms (Supplementary Materials Table 2). Arthritis and depression were the health conditions most commonly reported in persons with moderate or severe respiratory symptoms. While some of the highest frequencies occurred in persons with COPD and current asthma, symptoms were also common in CHD, stroke, diabetes mellitus, kidney disease, and morbid obesity.
Figure 2 is a proportional Venn diagram showing symptoms, COPD, and asthma, where 39.1% of subjects reported either a moderate symptom, severe symptom, current asthma, or COPD. There was substantial overlap for all symptoms with asthma and COPD, yet there were many symptomatic persons reporting neither condition.
Fig. 2.
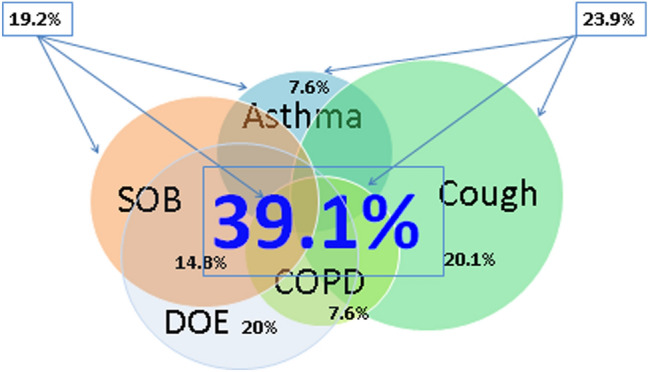
Proportional Venn diagram of moderate and severe symptoms, asthma, and chronic obstructive pulmonary disease (COPD) in adults ≥ 18 years old using the 2015 BRFSS health survey in four US states (Florida, Kentucky, South Carolina, and Texas). *Values in legend reported as mean (95% CI). DOE dyspnea on exertion, SOB shortness of breath. Asthma: 7.6% (7.0–8.1%) of adults reported currently having asthma. Chronic obstructive pulmonary disease (COPD): 7.6% (7.1–8.2%) of adults reported having been told they have chronic obstructive pulmonary disease, emphysema, or chronic bronchitis. Productive cough: 20.1% (18.6–21.6%) of adults reported moderate or severe productive cough within last 30 days, and 24.2% of these reported overlap with current asthma, 22.7% with COPD, 32.4% with moderate or severe DOE, and 27.1% with moderate or severe SOB. Dyspnea on exertion (DOE): 20% (18.7–21.3%) or adults reported moderate or severe DOE in the last year, and 24.4% of these reported overlap with current asthma, 41.0% with COPD, 29.6% with severe or moderate cough, and 77.9% with moderate to severe SOB. Shortness of breath (SOB): 14.8% (13.6–16.2%) of adults reported moderate or severe SOB within last 30 days, and 17.9% of these reported overlap with current asthma, 27.1% overlap with COPD, 27.1% with moderate and severe productive cough, and 25.3% overlap with moderate and severe SOB. Asthma–COPD–SOB: 19.2% (18.3–20.1%) of adults were found to have either asthma, COPD, or shortness of breath within last 30 days. Asthma–COPD–cough: 23.9% (22.9–24.9%) of adults were found to have either asthma, COPD, or productive cough. Asthma–COPD–cough–SOB–DOE: 39.1% (37.5–40.8%) of adults were found to have either asthma, COPD, productive cough, or SOB within last 30 days or DOE in 12 months
Severe DOE was more common in people who were morbidly obese (N = 2523) than with COPD (N = 1994), but fewer obese subjects reported severe SOB (N = 852) than those with COPD (N = 1036). A J-shaped relationship was apparent in persons with morbid obesity (WHO classes II and II) for severe SOB or severe DOE, while a U-shaped relationship was seen with BMI and severe productive cough (Supplementary Materials Table 2). A nonsignificant trend indicating severe SOB and productive cough are less frequent in overweight, non-obese groups (BMI 25–29.9 kg/m2) compared with other BMI groups.
Racial/ethnic differences were evident as whites were more likely than Blacks and Hispanics to report moderate and severe productive cough; Hispanics were the least likely to report moderate or severe SOB (Fig. 3, Supplementary Materials Table 1). There was no statistically significant difference in moderate or severe DOE based on race/ethnicity.
Fig. 3.
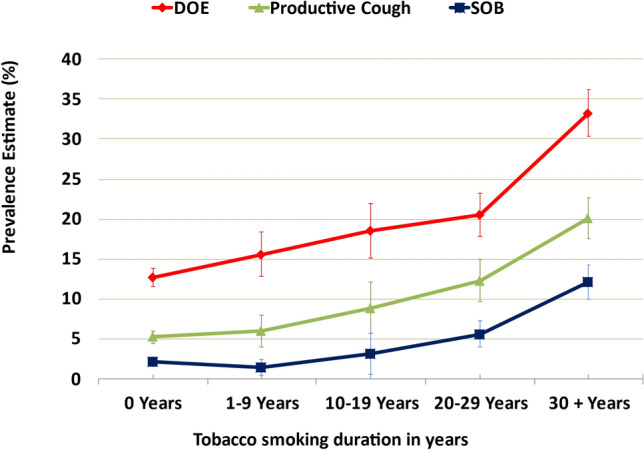
Moderate and severe respiratory symptoms* and tobacco smoking duration in adults ≥ 18 years old using the 2015 BRFSS health survey in four US states (Florida, Kentucky, South Carolina, and Texas). DOE dyspnea on exertion, SOB shortness of breath. Test of linearity between years smoked; each symptom was significant (P < 0.001), for SOB (OR 1.017, 95% CI 1.013–1.022), productive cough (OR 1.008, 95% CI 1.004–1.012), and DOE (OR 1.012, 95% CI 1.009–1.016). *Moderate to severe productive cough: cough up mucus or phlegm either every day or most days each month. *Moderate to severe shortness of breath: short of breath either all of the time or most of the time. *Moderate to severe dyspnea on exertion: not as physically active as I once was because of my shortness of breath, agreeing strongly or slightly
Tobacco Smoking
Tobacco use in the four states differed, where KY had the highest rates of ever-smokers and TX the lowest. The impact of smoking status on the frequency of respiratory symptoms was evident (current > former > never-smokers), especially in those with severe symptoms (Fig. 4, Supplementary Materials Table 1). There was a statistically significant linear relationship for each respiratory symptom with years of tobacco smoking.
Fig. 4.
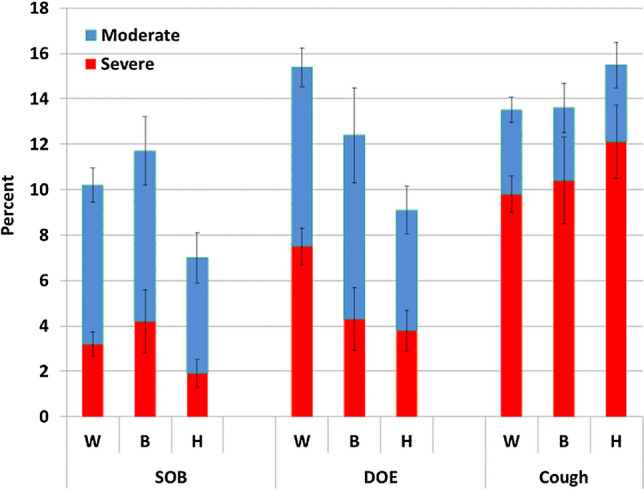
Moderate and severe respiratory symptoms and race/ethnicity in adults ≥ 18 years old using the 2015 BRFSS health survey in four US states (Florida, Kentucky, South Carolina, and Texas)*. DOE dyspnea on exertion, SOB shortness of breath (SOB), W white, non-Hispanic, B Black, non-Hispanic, H Hispanic. *Moderate to severe productive cough: cough up mucus or phlegm either every day, most days or a few days each month. *Moderate to severe shortness of breath: short of breath either all of the time, most of the time, or some of the time. *Moderate to severe dyspnea on exertion: not as physically active as I once was because of my shortness of breath, either agreeing. strongly or slightly, or neither agree nor disagree
Respiratory and Health Impairments
Fair or poor overall health, worse mental distress, and activity limitation were more common with moderate or severe symptoms than in those with minimal symptoms (Supplementary Materials Table 3) While productive cough and DOE affecting physical and mental health were common, severe SOB was the symptom with the highest prevalence of health impairments and activity limitation (Supplementary Materials Table 3).
Discussion
In the largest cross-sectional study conducted in the USA in decades, we report the frequency and burden of respiratory symptoms among adults of all ages and many of the factors associated with a higher risk. Nearly 40% of adults reported at least one moderate or severe respiratory symptom, current asthma, or COPD and an even greater percentage of these reported mental and physical impairments. Morbid obesity was a major factor contributing to SOB and DOE among the population. Young adults (< 35 years old) are a group for which US data are lacking. There was a surprisingly high frequency (> 15%) of moderate or severe productive cough in the last 30 days and DOE in the last year in this group. With increasing age, DOE became the most common symptom until the oldest group, when productive cough occurred at a similar frequency. We provide evidence that tobacco smoking duration was strongly associated with symptoms. Prior studies have principally relied on tobacco intensity (e.g., pack-years) or smoking status as the tobacco exposure metric.
Our findings align with others in which the risks of breathlessness and cough are affected by age [8, 11–15], sociodemographics [12, 16], race [16], current tobacco use [3, 11, 27], and comorbidities such as obesity [28–30], COPD [16], asthma [27], heart disease [27, 29, 31], and depression [32]. DOE was expected to be the most common symptom in this study, as it occurs in nonpulmonary conditions, including obesity and deconditioning, that are frequent among the US population [33], is often the first symptom associated with chronic lung diseases, and worsens with progression of chronic lung disease [34–36]. Although it is unknown what portion of subjects reporting moderate or severe respiratory symptoms had airflow obstruction by spirometry in the current study, the presence of symptoms is invariably important, as evidenced by current recommendations [37]. Symptoms affect overall well-being and long-term outcomes [1, 2, 4, 5, 15, 38]. In fact, chronic respiratory symptoms are a better predictor of mortality than forced expiratory volume in 1 s (FEV1) [4, 5].
Many nonpulmonary diseases can cause or worsen respiratory-related symptoms. This study highlights the association of obesity [28–30], heart disease [29, 31], and depression [32] with the cardinal symptoms of lung disease. Increased symptoms are well known to occur in patients with COPD who have low BMI [39], but we did not find this to be true with DOE or SOB in the general adult population. Morbid obesity significantly impacted symptoms. In fact, severe DOE and serious difficulty walking or climbing stairs in morbid obesity approached that seen with COPD and asthma. Cardiac disease is also common in persons with frequent dyspnea [29, 31], and we found coronary heart disease two to six times more likely in persons with moderate or severe respiratory symptoms, especially SOB.
Prior population-based studies have measured lung function but not symptoms [38], enrolled a specific ethnicity or race [40, 41], only enrolled older adults [12, 42], or were reported decades ago [13, 14]. Studies from other countries have reported on respiratory symptoms in young adults, but race and ethnicity differ from the USA [5, 7, 8, 10, 42, 43]. A US longitudinal study (CARDIA) recently reported on respiratory symptoms in 2749 adults aged 18–30 years old where 12.7% of subjects reported persistent (“usually”) cough and phlegm [15]. We found productive cough on most or all days in the last month in 6.7% of participants aged 18–24 and 9.5% of participants 25–34 years old. The prevalence of SOB walking up an incline or hurrying on the level was 8.1% in CARDIA, whereas we found that physical activity in nearly 10% of 18–34 year olds was strongly or slightly affected by breathing problems (severe DOE). With the frequency of these impairments, many more targeted longitudinal studies on the respiratory health of adolescents and young adults should be conducted.
Chronic cough and phlegm production early in adult life is considered a marker for development of COPD and is associated with lung function decline [43], even in nonsmokers [44, 45]. In a longitudinal study of a British-birth cohort, chronic mucus hypersecretion and cough in 20-year-olds were associated with airflow obstruction later in life [43]. Since the first Surgeon General’s Report on tobacco smoking in 1964, current smoking rates in the USA have declined substantially [46]. This is evident in the current study where, among 25–34-year-olds, ever-smoking rates are about 50% lower than those reported for the same age group from the 1970s [47]. However, while current smoking in the USA has decreased, the age group with the highest smoking rates remains the 25–34-year-olds [47], as found in this study.
The significant differences in respiratory symptoms among states was apparent when comparing KY with TX and FL and are likely related to the higher smoking rates and racial differences. In the USA, smoking [47] and COPD [19] rates are highest in KY and perhaps the highest in the world. In the BOLD study that included 29 countries, the symptom scores and COPD prevalence were highest from the site in KY [11, 42]. The effects of race and ethnicity on the prevalence of respiratory symptoms in this study is consistent with others where whites report the highest rates and Hispanics the lowest. Hispanics have different smoking behaviors with lower rates of ever-smoking and cumulative smoking compared with whites [40].
We chose to rely on years of tobacco smoking (duration) as it is practical for a large epidemiologic survey and in fact is a better predictor of health risks than pack-years. A recent study found years of tobacco smoking among a group of patients with COPD was more strongly associated with reduced spirometry, emphysema, 6-min walk, and impaired respiratory health than pack-years or cigarettes per day [48]. Smoking duration has also been shown to be a stronger predictor of risk of lung cancer and cardiac disease than pack-years [49, 50]. We found the frequency of respiratory symptoms increased significantly between 10 and 20 years of smoking, and especially at ≥ 30 years.
A surprising extent of respiratory symptoms was evident in never-smoking respondents and those without COPD or current asthma. While tobacco smoking is a leading cause of chronic productive cough, about 10% of never-smokers in our study reported moderate or severe productive cough and accounted for one-third of all persons reporting productive cough. Since up to 30% of COPD occurs in nonsmokers [51, 52], our population-based estimates of productive cough in young adulthood give us pause that the current successes in the war on smoking may not appreciably diminish the burden of COPD in our lifetime.
A strength of this cross-sectional, population-based study is that it can be used to understand the sociodemographic nature of respiratory health in the USA. We also provide data in persons < 40 years old, which is quite sparse currently for the USA. While some health measures are available in databases and medical records, data on respiratory symptoms and patient-reported health impairments are usually unavailable. Longitudinal research studies such as CARDIA [15] and MESA [53] among others are vitally important to understanding lung health; however, they are often not a true representation of the current population as they are not cross-sectional and do not adapt to dynamic census changes and associated social, racial, and cultural differences. Although BRFSS is cross-sectional, the survey is weighted to account for population differences and is administered annually; therefore, it is a better reflection of the general population.
We also provided insights into the relationships among respiratory symptoms and health impairments. While DOE was the most common symptom, SOB had the biggest impact on quality-of-life measures. All health impairments were much less likely in persons reporting minimal or no respiratory symptoms, highlighting the burden of respiratory symptoms on well-being.
This study has several limitations, including those inherent to BRFSS. As responses were obtained by telephone survey, we relied on self-report of medical conditions and smoking history. Diagnoses were not confirmed through review of medical records, which could result in misclassification of subjects. Spirometry was not available for study subjects. We did not collect data regarding nontobacco environmental causes of respiratory symptoms such as biomass fuels, local air quality, or occupation. Response rates reported for this study are consistent with prior BRFSS surveys at 40–50%; weighting, callbacks, and use of cellphones are employed to minimize bias in respondent participation. We did not identify patients with less common lung diseases such as cystic fibrosis or the nearly 2% of adults with heart failure [54]. While the respiratory module employed for this study was derived from multiple questionnaires [21–25], it characterizes the most common symptoms associated with obstructive lung diseases—mucus production and dyspnea. However, we did not use standardized questionnaires for dyspnea or chronic bronchitis. The St George Respiratory Questionnaire uses 30-day recall for mucus production and cough, similar to the current study. Using COPDGene subject data, this question showed good sensitivity (0.87) and specificity (0.77) compared with the traditional definition of chronic bronchitis that requires 2-year recall [55]. Lastly, we were unable to characterize all causes of physical impairment leading to SOB or DOE, such as deconditioning.
Conclusion
We provide up-to-date insight into the prevalence, associated comorbidities, and burden of respiratory symptoms among adults in four US states that represent 17.7% of the US population. We found a substantial portion of adults reported significant respiratory symptoms and associated impairment—influenced by aging, race/ethnicity, BMI, pulmonary and nonpulmonary diseases, and long-term use of tobacco. Considered low risk, young adults and never-smokers also reported significant symptom frequencies. There were many more adults with symptoms than those who report having COPD and current asthma, particularly with productive cough and DOE and in morbid obesity. The currently poor state of respiratory health among adults in the USA should be targeted to better understand causes among the population including greater utilization of spirometry and a better understanding of the impact of non-obstruction in persons with significant respiratory symptoms. Cross-sectional surveys such as the BRFSS are a useful tool to describe respiratory health in a population and, if applied repeatedly, might serve to monitor respiratory health from geographic and societal perspectives.
Supplementary Information
Below is the link to the electronic supplementary material.
Acknowledgements
In memoriam to our colleague, Janet Reaves, RN, who encouraged us to use the BRFSS survey to better understand lung diseases in the USA.
Funding
This study was funded through a grant from GlaxoSmithKline, which had no role in the collection, analysis and interpretation of data; in the writing of the report; or in the decision to submit the article for publication. No funding or sponsorship was received for the publication of this article.
Author Contributions
All authors (RP, KH, JO, JD, NL, SK, WL, DM, and CS) contributed to the conceptualization, study design, interpretation of data analysis, and preparation/completion of the manuscript. SK also contributed to data curation, KH also performed the formal analysis. RP also conceptualized the study and acquired funding.
Disclosures
Roy Pleasants—Grants: Boheringer Ingelheim, Astra Zeneca, Teva; Njira L. Lugogo—Grant and personal fees: GlaxoSmithKline; Monica Kraft- Grants: NIH, Sanofi, Am Lung Assn, Chiesi, Astra Zeneca; Consulting: Astra Zeneca, Sanofi; Royalties: Elsevier; Co founder and Chief Medical Officer of RaeSedo, Inc,; David M. Mannino—Consulting: Astra Zeneca, GlaxoSmithKline. Personal Fees: UpToDate. Charlie Strange—Consulting: Bronchus, GlaxoSmithKline, Morair, Pulmangae, UpToDate, Takeda, Vertex. Research: Adverum, AstraZeneca, CSA Medical, Grifols, Nuvaira, Takeda, and Vertex. Medical Director: AlphaNet. Khosrow Heidari, Jim Donohue, Jill Ohar and Sarojini Kanotra have nothing to disclose.
Compliance with Ethics Guidelines
The BRFSS has been approved as exempt research by the CDC’s institutional review board. The study was a secondary data analysis of deidentified, publicly-available data. It was also approved by the Duke University Institutional Review Board.
Data Availability
The data presented in this study are now available by contacting each state’s agency responsible for the Behavioral Risk Factor Surveillance System.
References
- 1.Meek PM, Petersen H, Washko GP, et al. Chronic bronchitis is associated with worse symptoms and quality of life than chronic airflow obstruction. Chest. 2015;148(2):408–416. doi: 10.1378/chest.14-2240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Vestbo J, Prescott E, Lange P. Association of chronic mucus hypersecretion with FEV1 decline and chronic obstructive pulmonary disease morbidity. Am J Respir Crit Care Med. 1996;153(5):150–155. doi: 10.1164/ajrccm.153.5.8630597. [DOI] [PubMed] [Google Scholar]
- 3.De Marco R, Accordini S, Cerveri I, et al. An international survey of chronic obstructive pulmonary disease in young adults according to GOLD stages. Thorax. 2004;59(2):120–125. doi: 10.1136/thorax.2003.011163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Nishimura K, Izumi T, Tsukino M, Oga T. Dyspnea is a better predictor of 5-year survival than airway obstruction in patients with COPD. Chest. 2002;121(5):1434–1440. doi: 10.1378/chest.121.5.1434. [DOI] [PubMed] [Google Scholar]
- 5.Figarska SM, Boezen HM, Vonk JM. Dyspnea severity, changes in dyspnea status and mortality in the general population: the Vlagtwedde/Vlaardingen study. Eur J Epidemiol. 2012;27(11):867–876. doi: 10.1007/s10654-012-9736-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lung Health Cohort Millennials study. https://www.lung.org/research/about-our-research/videos/lung-health-cohort-focuses-on. Accessed 31 Mar 2022.
- 7.Cerveri I, Accordini S, Verlato A, Corsico G, Zoia MC, Casoli L, Burney P, De Marco R. Variations in the prevalence across countries of chronic bronchitis and smoking habits in young adults. Eur Respir J. 2001;18(1):85–92. doi: 10.1183/09031936.01.00087101. [DOI] [PubMed] [Google Scholar]
- 8.Maio S, Baldacci S, Carrozzi L, et al. Respiratory symptoms/diseases prevalence is still increasing: a 25-yr population study. Respir Med. 2016;110(1):58–65. doi: 10.1016/j.rmed.2015.11.006. [DOI] [PubMed] [Google Scholar]
- 9.Pelkonen M, Notkola IL, Nissinen A, et al. Thirty-year cumulative incidence of chronic bronchitis and COPD in relation to 30-year pulmonary function and 40-year mortality: a followup in middle-aged rural men. Chest. 2006;130(4):1129–1137. doi: 10.1378/chest.130.4.1129. [DOI] [PubMed] [Google Scholar]
- 10.Voll-Aanerud M, Eagana TML, Wentzel-Larsenb T, et al. Respiratory symptoms, COPD severity, and health related quality of life in a general population sample. Respir Med. 2008;102(3):399–406. doi: 10.1016/j.rmed.2007.10.012. [DOI] [PubMed] [Google Scholar]
- 11.Mejza F, Gnatiuc L, Buist AS, et al. Prevalence and burden of chronic bronchitis symptoms: results from the BOLD study. Eur Respir J. 2017;50(5):1700621. doi: 10.1183/13993003.00621-2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wheaton AG, Ford ES, Thompson WW, et al. Pulmonary function, chronic respiratory symptoms, and health-related quality of life among adults in the United States-National Health and Nutrition Examination Survey 2007–2010. BMC Public Health. 2013;13:854. doi: 10.1186/1471-2458-13-854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hammond ECH. Some preliminary findings on physical complaints from a prospective study of 1,064,004 men and women. Am J Pub Health. 1964;54(1):11–23. doi: 10.2105/AJPH.54.1.11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Miller A, Thornton JC, Anderson HA, Selikoff IJ. Clinical respiratory abnormalities in Michigan. Chest. 1988;94(6):1187–1194. doi: 10.1378/chest.94.6.1187. [DOI] [PubMed] [Google Scholar]
- 15.Kalhan R, Dransfield MT, Colangelo LA, et al. Symptoms in young adults and future lung disease: the CARDIA Lung Study. Am J Respir Crit Care Med. 2018;197(12):1616–1624. doi: 10.1164/rccm.201710-2108OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Pleasants R, Heidari K, Wheaton A, et al. Targeting chronic obstructive pulmonary disease by state-based surveillance. J COPD. 2015;12(6):680–689. doi: 10.3109/15412555.2015.1043424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Pleasants R, Heidari K, Ohar J, et al. Prevalence of COPD and high risk for COPD in a four state health survey. Am J Respir Crit Care Med. 2017;195:A3668. [Google Scholar]
- 18.United States Census. Bureau. https://factfinder.census.gov/faces/tableservices. Accessed 31 Mar 2022.
- 19.Ford ES, Croft JB, Mannino DM. COPD Surveillance—United States, 1999–2011. Chest. 2013;144(1):284–305. doi: 10.1378/chest.13-0809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.WHO. Obesity: preventing and managing the global epidemic. 2000. Geneva, World Health Organization. [PubMed]
- 21.Price DB, Tinkelman DG, Nordyke RJ, Isonaka S, Halbert RJ. Scoring system and clinical application of COPD diagnostic questionnaires. Chest. 2006;129(6):1531–1539. doi: 10.1378/chest.129.6.1531. [DOI] [PubMed] [Google Scholar]
- 22.Calverley PM, Nordyke RJ, Halbert RJ, Isonaka S, Nonikov D. Development of a population-based screening questionnaire for COPD. COPD. 2005;2(2):225–232. doi: 10.1081/COPD-57594. [DOI] [PubMed] [Google Scholar]
- 23.Martinez FJ, Raczek AE, Seifer FD, COPD-PS clinician working group et al. Development and initial validation of a self-scored COPD Population Screener Questionnaire (COPD-PS) COPD. 2008;2(5):85–95. doi: 10.1080/15412550801940721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Yawn BP, Mapel DW, Mannino DM, et al. Development of the lung function questionnaire (LFQ) to identify airflow obstruction. Int J COPD. 2010;5(2):1–10. [PMC free article] [PubMed] [Google Scholar]
- 25.Jones PW, Quirk FH, Baveystock CM, Littlejohns P. A self-completed measure of health status for chronic airflow limitation. Am Rev Respir Dis. 1992;145(6):1321–1327. doi: 10.1164/ajrccm/145.6.1321. [DOI] [PubMed] [Google Scholar]
- 26.https://medical-dictionary.thefreedictionary.com/exertional+dyspnea. Accessed 31 Mar 2022.
- 27.Enright PL, Kronmal PA, Higgins MW, Schenker EM, Haponik EF. Prevalence and correlates of respiratory symptoms and disease in the elderly. Chest. 1994;106(3):827–834. doi: 10.1378/chest.106.3.827. [DOI] [PubMed] [Google Scholar]
- 28.Liu Y, Pleasants R, Ohar J, et al. Body mass index, respiratory symptoms, and respiratory conditions among South Carolina adults, 2012. Respir Med. 2015;109(7):851–859. doi: 10.1016/j.rmed.2015.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Van Mourik Y, Rutten FH, Moons KGM, Bertens LCM, Hoes AW, Reitsma JB. Prevalence and underlying causes of dyspnea in older people: a systematic review. Age Ageing. 2014;43(3):319–326. doi: 10.1093/ageing/afu001. [DOI] [PubMed] [Google Scholar]
- 30.Peters U, Dixon AE, Thl S, Nordestgaard BG, et al. Effect of obesity on lung function. Expert Rev Respir Med. 2018;12(9):755–767. doi: 10.1080/17476348.2018.1506331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Güder G, Brenner S, Störk S, Hoes A, Rutten FH. Chronic obstructive pulmonary disease in heart failure: accurate diagnosis and treatment. Eur J Heart Failure. 2014;16(12):1273–1282. doi: 10.1002/ejhf.183. [DOI] [PubMed] [Google Scholar]
- 32.Neuman A, Gunnbjornsdottir M, Tunsater A, et al. Dyspnea in relation to symptoms of anxiety and depression: a prospective population study. Respir Med. 2006;100(10):1843–1849. doi: 10.1016/j.rmed.2006.01.016. [DOI] [PubMed] [Google Scholar]
- 33.https://www.cdc.gov/obesity/data/prevalence-maps.html. Accessed 31 Mar 2022.
- 34.Colak Y, Afza S, Nordestgaard BG, Vesto J, Lange P. Prevalence, characteristics, and prognosis of early chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2020;201(6):671–680. doi: 10.1164/rccm.201908-1644OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Martinez FJ, Han MK, Allinson JP, et al. At the root: defining and halting progression of early chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2018;197(12):1540–1551. doi: 10.1164/rccm.201710-2028PP. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Sherman CB, Xu X, Speizer FE, et al. Longitudinal lung function decline in subjects with respiratory symptoms. Am J Respir Crit Care Med. 1992;146(4):855–859. doi: 10.1164/ajrccm/146.4.855. [DOI] [PubMed] [Google Scholar]
- 37.https://goldcopd.org/gold-reports Accessed 20 Apr 2020.
- 38.Feinleib M, Kannel WB, Garrison RJ, et al. The Framingham Offspring Study. Design and preliminary data Prev Med. 1975;4(4):518–525. doi: 10.1016/0091-7435(75)90037-7. [DOI] [PubMed] [Google Scholar]
- 39.Decramer M, De Benedetto F, Del Ponte A, Marinari S. Systemic effects of COPD. Respir Med. 2005;99(Suppl B):S3–S10. doi: 10.1016/j.rmed.2005.09.010. [DOI] [PubMed] [Google Scholar]
- 40.Samet JM, Schrag SD, Howard CA, Key CR, Pathak DR. Respiratory disease in a New Mexico population sample of Hispanic and non-Hispanic whites. Am Rev Respir Dis. 1982;125(2):157–161. doi: 10.1164/arrd.1982.125.2.152. [DOI] [PubMed] [Google Scholar]
- 41.Taylor HA, Wilson JG, Jones DW, et al. Toward resolution of cardiovascular health disparities in African Americans: design and methods of the Jackson Heart Study. Ethn Dis. 2005;15(4 suppl 6):S6-4–S6-17. [PubMed] [Google Scholar]
- 42.Gronseth R, Vollmer WM, Hardie JA, et al. Predictors of dyspnea prevalence: results from the BOLD study. Eur Respir J. 2014;43(6):1610–1620. doi: 10.1183/09031936.00036813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Allinson JP, Hardy R, Donaldson GC, et al. The presence of chronic mucus hypersecretion across adult life in relation to chronic obstructive pulmonary disease development. Am J Respir Crit Care Med. 2016;193(6):662–672. doi: 10.1164/rccm.201511-2210OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Harmsen L, Thomsen SF, Ingebrigtsen T, et al. Chronic mucus hypersecretion: prevalence and risk factors in younger individuals. Int J Tuberc Lung Dis. 2010;14(8):1052–1058. [PubMed] [Google Scholar]
- 45.DeMarco R, Accordini S, Cerveri I, et al. Incidence of chronic obstructive lung disease in a young cohort according to the presence of cough or phlegm. Am J Respir Crit Care Med. 2007;175(1):32–39. doi: 10.1164/rccm.200603-381OC. [DOI] [PubMed] [Google Scholar]
- 46.Surgeon General Report - U.S. Department of Health and Human Services. The health consequences of smoking - 50 years of progress. A report of the Surgeon General. Atlanta, GA : U.S. Department of Health and Human Services, Centers for Disease Control and Prevention, National Center for Chronic Disease Prevention and Health Promotion, Office on Smoking and Health, 2014. Printed with corrections, January 2014.
- 47.Phillips E, Wang TW, Husten CG, et al. Tobacco product use among adults—United States, 2015. MMWR Morb Mortal Wkly Rep. 2017;66:1209–1215. doi: 10.15585/mmwr.mm6644a2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Bhatt SP, Kim YI, Harrington KF, et al. Smoking duration alone provides stronger risk estimates of chronic obstructive pulmonary disease than pack-years. Thorax. 2018;73:414–421. doi: 10.1136/thoraxjnl-2017-210722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Remen T, Pintos J, Abrahamowicz M, Siemiatycki J. Risk of lung cancer in relation to various metrics of smoking history: a case–control study in Montreal. BMC Cancer. 2018;18:1275. doi: 10.1186/s12885-018-5144-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Lubin JH, Couper D, Lutsey PL, Woodward M, Yatsuya H, Huxley RR. Risk of cardiovascular disease from cumulative cigarette use and the impact of smoking intensity. Epidemiology. 2016;27:395–404. doi: 10.1097/EDE.0000000000000437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Behrendt CE. Mild and moderate-to-severe COPD in nonsmokers: distinct demographic profiles. Chest. 2005;128(3):1239–1244. doi: 10.1378/chest.128.3.1239. [DOI] [PubMed] [Google Scholar]
- 52.Lamprecht B, Schirnhofer L, Kaiser B, Buist S, Studnicka M. Non-reversible airway obstruction in never smokers: results from the Austrian BOLD study. Respir Med. 2008;102(12):1833–1838. doi: 10.1016/j.rmed.2008.07.007. [DOI] [PubMed] [Google Scholar]
- 53.Bild DE, Bluemke DA, Burke GL, et al. Multi-ethnic study of atherosclerosis: objectives and design. Am J Epidemiol. 2002;156(9):871–881. doi: 10.1093/aje/kwf113. [DOI] [PubMed] [Google Scholar]
- 54.Benjamin EJ, Muntner P, Alonso A, et al. Heart disease and stroke statistics—2019 update: a report from the American Heart Association. Circ. 2019;139:e56–e528. doi: 10.1161/CIR.0000000000000659. [DOI] [PubMed] [Google Scholar]
- 55.Kim V, Crapo J, Zhou H, Jones PW, Silverman EK, Cornellus A, Make BJ, Criner GJ, and the COPDGene Investigators Comparison between an alternative and the classic definition of chronic bronchitis in COPDGene. Ann Am Thorac Soc. 2015;12:332–339. doi: 10.1513/AnnalsATS.201411-518OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data presented in this study are now available by contacting each state’s agency responsible for the Behavioral Risk Factor Surveillance System.


