Abstract
In this study, six heavy metals (Pb, Fe, Cu, Ni, Cd, and Mn) have been measured in water, and muscles from mullet (Mugil cephalus), tilapia (Oreochromis niloticus), and African catfish (Clarias gariepinus) collected from Lake Manzal, Egypt. In addition, the existence of different encysted metacercariae in fish muscle with an evaluation of cell-mediated immune response in infected muscles was also investigated. Water samples generally contained less than the permissible level of heavy metals. The metal accumulation levels in muscle were: Pb > Ni > Cd > Cu > Fe > Mn. The levels of Pb and Ni in the muscles exceed the permissible limits, while the concentration of Mn varied significantly (p < 0.05) depending on fish species. Based on the estimated weekly intake in this study, the EWI values of these heavy metals are below the established Provisional Permissible Tolerable Weekly Intake. On the other hand, Prohemistomum vivax encysted metacercaria were found in the muscle of O. niloticus and C. gariepinus with the intensity of 1–10 cyst per 1 cm of muscle. While M. cephalus was found to be infected with Heterophyes heterophyes EMC. TNF- α1 was 10 folds upregulated in O. niloticus than in control fish. IL-1β expression in O. niloticus was upregulated by 15 folds compared with the control one. By examining C. gariepinus, the MHC II gene expression was increased by 15-fold in comparison to the control group.
Keywords: Encysted metacercaria, Gene expression analysis, Heavy metals, Health risk
Introduction
Agricultural and industrial development has led to heavy metal contamination of aquatic ecosystems, a significant health risk to fish and many invertebrates due to their toxic characteristics, non-biodegradability, and bioaccumulation at the base of food chains (Kouamenan et al. 2020; Khalefa et al. 2021). The marine and coastal environment become contaminated by metals such as As, Cr, Cu, Mn, Ni, and Pb due to many anthropogenic sources, including sewage discharges and agricultural runoff, harbor activities, and ship-land litter (Katip et al. 2012). These metals accumulate in various fish species due to differences in uptake and depuration. It was determined by factors such as the season and the water's chemical and physical properties (Rajeshkumar and Li 2018). Additionally, other variables such as wind speed and precipitation, suspended solids, conductivity, salinity, pH, temperature, and organic substance concentrations influence heavy metal concentrations (Katip et al. 2012).
Egypt's Lake Manzala is considered the most valuable source of fish. It is regarded as the most productive lake in Egypt, contributing about 44% (2004–2013) and increasing to about 56% in 2013 from the total annual production of the Northern Delta Lakes (GAFRD 2014). The lake drainage water is collected from five highly polluted drains (Bahr El-Baqar, Hadous, Al-Serw, Maitreya, and Faraskur), known as the significant sources of pollution (Abu Khatita et al. 2017). The Bahr El-Baqar drain is the most polluted drain pumping into Lake Manzala and contributes about 45% of the total discharge (Abdelsalam et al. 2021).
Fish are usually used to determine heavy metal pollution in aquatic ecosystems (Kouamenan et al. 2020). Heavy metals enter the fish bodies indirectly and directly through the permeable membranes in their gills and muscles. Accordingly, heavy metals concentrations in fish reflect those in the water and sediment of aquatic ecosystems (Rajeshkumar and Li 2018). Since fish muscles are the most edible part of the fish, and potential contamination risks are of great concern to humans, researching pollutants in fish muscles is essential, despite their lower accumulation potential than livers and gills (Kouamenan et al. 2020). Egypt's aquatic environments have been contaminated with toxic substances, such as heavy metals, posing a threat to public water supplies, aquatic life, and ecosystems. Contamination with heavy metals increases the susceptibility of fish to pathogens in surrounding waters (Ezzat et al. 2012).
There is a severe economic loss among fishes in open water resources and fish culture caused by metacercariae infections. Many of these infections have public health implications (Mahdy et al. 2021). Fish parasites and heavy metal monitoring help in assessing the aquatic ecosystem health (Mehana et al. 2020). It has been reported that O. niloticus and C. gariepenus are highly susceptible to encysted metacercarial infection in Egypt (Attia et al. 2021a, c). Furthermore, several parasitic diseases reduce wild and cultured fish's growth rates and market value (Abdelsalam et al. 2016, 2020). Parasitic diseases may cause gastrointestinal, skin, and gill abrasions that facilitate the invasion of other opportunistic microbes, including bacteria, viruses, and fungi (Ezzat et al. 2012). The aim of our study was to clarify the relationship between heavy metal concentrations (Pb, Cu, Cd, Ni, Fe) and some parasitic diseases infecting edible fish species in Lake Manzala (tilapia, catfish, and mullet) together with the immunological evaluation of the infected muscles using tumor necrosis factor alpha-1 (TNFα-1) and interluckin1β (IL-1β) in both O. niloticus, and M. cephalus; and major histocompatibility class II (MHC II) in C. Gariepinus.
Materials and methods
The study area
The present study took place at Lake Manzala, Port Said Governorate, where fish farms, drains, villages, and agricultural lands surrounding the lake. There were three sites where samples were collected; site 1: along Lake Manzala, site 2: at the Bahr El-Baqar drain, and site 3: a private fish farm at Shader Azzam that uses water from the drainage system.
Sample collection
Water samples were collected from three different locations at a depth of 30 cm below the water's surface using a PVC vertical water sampler. Each water sample was placed in polyethylene bottles previously washed with acid (0.01 N HNO3) and rinsed with distilled water, after which the bottles were placed in a cooler at 4 °C and sent to the lab for further analysis. For total metals concentration, samples were digested by Nitric Acid Digestion according to APHA (2000).
Fish medium-sized fish of tilapia, mullet, and catfish (15 for each fish species) were caught from three sites with the help of fishers from Manzala Lake-Egypt. After dissecting the fish samples, 25 g of dorsal muscle tissues were collected and cooled to − 20 °C in polyethylene bags. One gram of previously oven-dried muscle tissues was ignited and digested using concentrated HNO3 and HCl according to the procedures recommended by AOAC (2005).
Heavy metals analysis
The selected heavy metals manganese (Mn), iron (Fe), copper (Cu), nickel (Ni), cadmium (Cd), and lead (Pb) were measured using an atomic absorption spectrometer (model Thermo Electron Corporation S Series AA Spectrometer) following the procedure adopted by Fozia et al. (2008). Also, analytical blanks were organized similarly. The results were expressed in μg/g wet weight of fish tissues.
Physical and chemical properties of water
Based on the Standard Methods defined by APHA (2000), temperature, conductivity, and dissolved oxygen (DO) were measured with a digital meter directly in the field, while the total chloride was measured by 50 ml sample titration against 0.0141 N silver nitrate solution using potassium chromate as an indicator.
Parasitological examination
Each fish was scraped from skin mucous and gills. From each scrape, permanent smears were made and fixed using absolute methanol and stained using Giemsa stain. The permanent slides were screened for the presence of any protozoan parasites as well as monogenean trematoda and photographed using an Olympus CX33 microscope (Japan); (Younis et al. 2020). The muscles of each fish (fins muscle; muscle around the head; tail muscle); were examined by compression of 1 cm of muscles between two slides then examined under a stereoscopic microscope; the encysted metacercaria (EMC); which were collected and identified according to Attia et al. (2021a). The collected metacercariae were determined according to the presence of other suckers (oral, ventral, and genital suckers), shape, cysts size (in diameters), and shape of the excretory bladder.
Quantitive real-time PCR protocol
Sampling
One cm of infected muscle with EMC was aseptically dissected. Negative control muscles were collected in the same manner sampled from five non-infected healthy fish collected from fish reared in a pond free from any parasites and bacteria.
RNA isolation
According to the manufacturer's instructions, total mRNA from 100 mg of muscles was extracted using the RNA isolation kit (Ambion, Applied Biosystems). The infected muscle was homogenized with Lysing Matrix D tubes (MP Biomedicals) using the FastPrep-24 homogenizer (Attia et al. 2021a). The produced mRNA was assessed for its purity and quantity using Nanodrop (Thermo Scientific). The produced mRNA was transcribed reversely by the cDNA Archive Kit (Applied Biosystems) with a guide of the manufacturer's protocol (Attia et al. 2021b, c).
qRT-PCR
There were three different fish species; O. niloticus, M. cephalus, and C. gariepinus. For O. niloticus and M. cephalus, tumor necrosis factor alpha-1 (TNFα-1) and interluckin1β (IL-1β) were evaluated, while the major histocompatibility class II (MHC II) was investigated for C. gariepinus. β-actin was used as sample normalization and as a reference gene for each fish species. The primers used were deposited in Table 1; Suprapto et al. (2017), Byadgi et al. (2016), Abdel-Mageid et al. (2020), Heinecke and Buchmann (2013), Praveen et al. (2006). The assay of quantitative PCR was done using Step One™ Real-Time PCR System (Applied Biosystems, USA). The quantitive PCR was performed following Attia et al. (2020, 2021c).
Table 1.
Pair primers were used in the quantitative real-time PCR
| Gene | Gene Sequence | References |
|---|---|---|
|
MHC class II (C. gariepinus) |
F'-ATGTCCAAGCTGCTGAAGATT | Suprapto et al. (2017) |
| R'-TGCCGTCTGACTTCTTCACC | ||
|
IL-1β (M.cephalus) |
F'- GAGGAGCTTGGTGCAGAACA | Byadgi et al. (2016) |
| R'- CTTTGTTCGTCACCTCCTCCA | ||
|
TNFα (M.cephalus) |
F'- GCGCAGTCTGTCATTGGTT | Abdel-Mageid et al. (2020) |
| R'- ACTGGACACGCTCACTGTAGTG | ||
|
IL-1β (O.niloticus) |
F'-TGCACTGTCACTGACAGCCAA | Heinecke, Buchmann (2013) |
| R'- ATGTTCAGGTGCACTATGCGG | ||
|
TNFα-1 (O.niloticus) |
F'-GGTTAGTTGAGAAGAAATCACCTGCA | Praveen et al. (2006) |
| R'- GTCGTCGCTATTCCCGCAGATCA |
Statistical analysis
Data were expressed as mean and standard error (SE). One-way ANOVA and independent-sample t-test were used to compare the variability of the concentrations of each metal. Results were analyzed using PASW Statistics, Version 18.0 software (SPSS Inc., Chicago, IL, USA). p < 0.05 indicates a significant difference.
Results
Parasitological finding
Prohemistomum vivax encysted metacercaria were found in the muscle of O. niloticus and C. gariepinus with the intensity of 1–10 cyst per 1 cm of muscle. The Prohemistomum vivax were oval to round in shape; which had 318–375 (346 ± 4.8) μm in length and 290-315 μm (300 ± 5.7) in width. The cysts were brownish with the fragile double wall in the outer layer while the inner one was hard. There were two lobulated sacs on both sides of the cyst; (Fig. 1a and b). Heterophyes heterophyes EMC was found in the muscles of M. cephalus. The cyst was spherical and had a thick wall of 5.5–12.7 μm (9.5 ± 4.5). The cyst was 160–365 (275 μm ± 3.7) in length × 160–370 (275 μm ± 3.60) in width. The cyst had spines on its tegument, which contain several brown pigments; (Fig. 1c).
Fig. 1.
Fish muscle had Prohemistomum vivax encysted metacercaria (EMC) in O. niloticus (A), C. gariepinus (B) and M. cephalus had Heterophyes heterophyes (C)
Expression of immunological genes in infected muscles with EMC
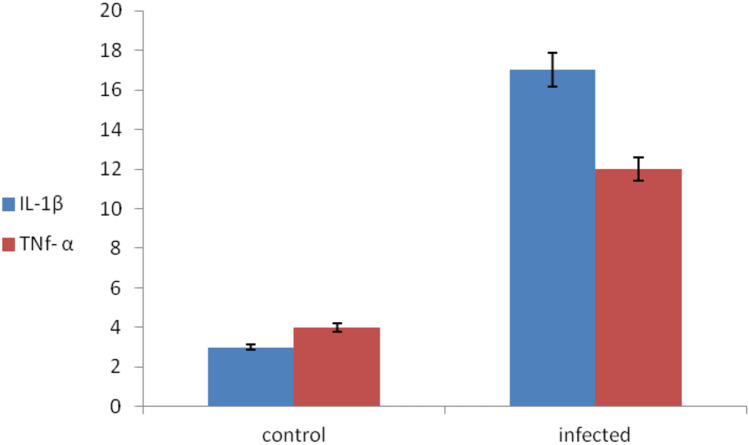
The expression of TNF- α1 was 12 folds upregulated in infected tilapia than in the control one. The expression of IL-1β in infected tilapia was upregulated by 17 folds compared with the control one. On the other hand, TNF- α1 was upregulated by 14 folds in infected mullets than in the control one. While the expression of the IL-1β gene was upregulated by 25 folds in infected mullets when compared with the control fish. The expression of the MHC II gene was upregulated by 17-fold in infected catfish compared with the control group (Figs. 2, 3).
Fig. 2.
Evaluation of immunological cells in O. niloticus (IL-1β and TNf- α)
Fig. 3.
Evaluation of immunological cells in Mugil cephalus (IL-1β and TNf- α)
Water parameters analysis
In Table 2, we presented the results of physical and chemical parameters. There is no significant difference (p > 0.05) between locations along the study area. The values of pH, temperature, DO, EC, and chloride were measured in the samples. All the study sites were on the alkaline side, with pH values between 7.2 ± 0.03 and 7.9 ± 0.05. The temperatures ranged from 17.3 ± 0.25 °C to 19 ± 0.17 °C. In our study, the minimum DO value of (5.9 ± 0.06 mg/l) was recorded at site 2, whereas at site 3, the maximum value of (7.03 ± 0.09 mg/l) was recorded. A site 1, water EC values reached maximum levels of 3.78 ± 0.10 mhos/cm; at the farm, minimum values were found at 1.57 ± 0.3 mhos/cm.
Table 2.
Physicochemical parameters at different sites at Lake Manzala
| Location | Parameters Mean ± SE | ||||
|---|---|---|---|---|---|
| pH | Temp. ( ºc) | DO (mg/l) | EC(mhos/cm) | Chloride (g−1/l) | |
| Site 1 | 7.23 ± 0.03 | 18.00 ± 10.00 | 6.77 ± 0.07 | 3.78 ± 0.01 | 0.81 ± 0.01 |
| Site 2 | 7.93 ± 0.05 | 19.00 ± 0.17 | 5.90 ± 0.06 | 2.33 ± 0.03 | 0.54 ± 0.01 |
| Site 3 | 7.90 ± 0.01 | 17.30 ± 0.25 | 7.03 ± 0.09 | 1.57 ± 0.03 | 0.75 ± 0.00 |
| Minimum | 7.20 | 17.00 | 5.80 | 1.50 | 0.53 |
| Maximum | 8.10 | 19.00 | 7.20 | 3.80 | 0.82 |
| P -value | 0.273 | 0.357 | 0.403 | 0.750 | 0.094 |
SE standard error, DO dissolved oxygen, EC electrical conductivity
p < 0.05 is significance
Heavy metals analysis in water
The concentration of Pb, Fe, Cu, and Ni were observed in site 1 and site 2 and not detected in site 3; while Mn was observed in sites 2 and 3 but not observed at site 1. Cadmium (Cd) was detected in all sites (Table 3). There was a significant difference (p < 0.05) observed between different study areas in the concentration of Pb, Fe, Cu, Ni, and Cd. Lead (Pb) presence in sites 1 and 2 was (0.001, 0.0007 mg/L), respectively, below the permissible limit recommended by USEPA (2006). Iron (Fe) mean concentration was 0.43 mg/L at site 1 and 0.83 mg/L at site 2. The highest concentrations of (Cu) were observed at sites 2 and 1, respectively. Nickel (Ni) revealed a higher significant (p < 0.05) mean at site 1 and site 2 (0.11 and 0.06 mg/L), respectively. The mean concentration of Cd was(0.030, 0.020, and 0.020) at sites 1, 2, and 3, respectively. Mn concentrations observed in sites 2 and 3 (0.010 and 0.52 mg/l), respectively. This finding revealed that the concentrations of Cd and Cu in water were above the permissible limit (USEPA 2006).
Table 3.
Concentration of heavy metals (Mean concentrations ± SE) in water samples collected from the three sites at Lake Manzala
| Location | Heavy metals (mg/L) mean ± SE | |||||
|---|---|---|---|---|---|---|
| Lead (Pb) | Iron (Fe) | Copper (Cu) | Nickel (Ni) | Cadmium (Cd) | Manganese (Mn) | |
| Site 1 | 0.0010 ± 0.00 | 0.43 ± 0.03 | 0.007 ± 0.003 | 0.11 ± 0.003 | 0.030 ± 0.0003 | ND |
| Site 2 | 0.0007 ± 0.0003 | 0.83 ± 0.03 | 0.033 ± 0.012 | 0.06 ± 0.03 | 0.020 ± 0.0003 | 0.010 ± 0.0003 |
| Site 3 | ND | ND | ND | ND | 0.020 ± 0.0003 | 0.52 ± 0.038 |
| P-value | 0.0004* | 0.008* | 0.014* | 0.041* | 0.045* | 0.200 |
| USEPA (2006) | 0.0025 | 1.00 | 0.013 | – | 0.0025 | – |
| WHO (2011) | 0.01 | – | 2.00 | 0.02 | – | 0.40 |
SE standard error, ND not detected
*p < 0.05 is significant
Heavy metals analysis in fish
Mn concentration showed significant (p < 0.05) variations concerning fish species. However, there's no significant (p < 0.05) difference in the rest of the metals (Table 4). The detected metal accumulation levels in muscles tissues were in the following order: Pb > Cd > Ni > Cu > Fe > Mn. The mean concentrations of Pb between the three fish species were (1.15, 1.80, and 1.78) in M. cephalus, O. niloticus, and C. gariepinus, respectively. Among all metals, Fe showed the highest means (19.97 μg/g wet weight) in C. gariepinus muscles. However, Cd was found to be the lowest mean value (0.007 μg/g wet weight) in C. gariepinus muscles.
Table 5.
The mean weekly intake of trace metals (μg/kg body weight/week) through consumption of different fish species by an adult person (70 kg) from Lake Manzala
| Fish samples | Weekly intake of metal per body weight (μg/kg/body weight/w) | ||||||
|---|---|---|---|---|---|---|---|
| Common name | Scientific name | Pb | Fe | Ni | Cu | Cd | Mn |
| Mugil | M. cephalus | 5.77 | 36.39 | 4.82 | 1.25 | 0.055 | 8.54 |
| tilapia | O. niloticus | 9.04 | 41.43 | 1.10 | 1.05 | 0.055 | 25.76 |
| Catfish | C. gariepinus | 8.94 | 100.28 | 10.14 | 1.76 | 0.035 | 6.78 |
| PTWI | 25.00 | 5600 | 35.00* | 3500 | 7.00 | 980.00 | |
PTWI provisional tolerable weekly intake by FAO/WHO (2004)
*PTWI provisional tolerable weekly intake byTürkmen et al. (2009)
Table 4.
The concentrations of heavy metals in muscle of fish collected from fish farms supplied by water from El-Manzala Lake
| Fish samples | Heavy Metal Concentration (μg/g wet weight) (Mean ± SE) | ||||||
|---|---|---|---|---|---|---|---|
| Common name | Scientific name | Pb | Fe | Ni | Cu | Cd | Mn |
| Mullet | M. cephalus | 1.15 ± 0.32 | 11.23 ± 4.44 | 0.96 ± 0.47 | 0.25 ± 0.18 | 0.011 ± 0.003 | 1.71 ± 0.34 b |
| Tilapia | O. niloticus | 1.80 ± 0.97 | 8.25 ± 1.85 | 0.22 ± 0.02 | 0.21 ± 0.02 | 0.011 ± 0.005 | 5.13 ± 0.40 a |
| Catfish | C. gariepinus | 1.78 ± 0.47 | 19.97 ± 5.06 | 2.02 ± 1.16 | 0.35 ± 0.16 | 0.007 ± 0.001 | 1.35 ± 0.34 b |
| P-value | 0.733 | 0.183 | 0.284 | 0.765 | 0.709 | 0.001* | |
| Permissible level | 0.214 | 50.0 | 0.50 mg (WHO 1989) | 30.00 (WHO 1989) | 0.050 | 2–11 mg/day | |
a,b Different superscripts within the same column indicate significant difference at P < 0.05; SE standard error
PL the recommended daily intake for an adult according to WHO (2011)
Most metals showed no significant relationship (p > 0.05) between their concentrations in water and muscle, whereas Fe demonstrated a meaningful positive relationship (p < 0.05) between its concentration in water and muscle (Fig. 4).
Fig. 4.
the correlation of mean values of heavy metal concentration in water and fish tissue
Accumulation factor
The bioaccumulation factor for the lead was highest in the muscles of tilapia and catfish (4.45 and 4.45, respectively) and lowest in mullet samples (2.87). Catfish samples had high iron concentrations during winter (47.55), but mullet and tilapia samples had low concentrations (26.74 and 19.64). A rising nickel bioaccumulation factor of 36.07 was recorded in catfish muscles, and a decreasing level of 3.93 was detected in the tilapia muscle. We determined a maximum and a minimum value of 26.92 and 16.15 of bioaccumulation factors for copper in the muscle of catfish and tilapia, respectively. It is worth mentioning that, Cadmium bioaccumulation factors were less than one. It was (0.25, 0.25, and 0.16) in mullet, tilapia, and catfish, respectively. Accordingly, the manganese bioaccumulation factor for catfish and mullet was 7.58 to 9.6, respectively, while it reached a remarkable increase of level 28.82 in tilapia (Fig. 5).
Fig. 5.
Transfer factor of heavy metals in fish tissues
Discussion
This study evaluated two main stressors (heavy metals and parasitic infection) in (Nile tilapia, mullet and catfish) with their effects on cellular mediated immunity. In our study, two genes TNF-α, IL-1β were evaluated in O. niloticus and M. cephalus muscles infected by different EMC, which revealed its upregulation compared to the standard control negative group. The pro-inflammatory cytokines are secreted by different stimulated immunological cells (as macrophages and eosinophils against other stressors and parasitic infection). In catfish infected with EMC revealed the upregulation of the MHCII. The high levels of MHCII and high levels of antigen presentation were recorded in monogenic trematoda infection in catfish (Attia et al. 2021c); the MHC II gene is immunoglobulins secreted to interact with T cells for beginning and initiation of adaptive immune response and had a significant role in antigen-presenting cells. In contrast to this study, the MHC II β were down-regulated in Gyrodactylus spp. infection (Lindenstrøm et al. 2004).
Parasite-infected tilapia had higher concentrations of Cu, Fe, and Zn in their musculature than non-infected fish. These parasites may impair metal detoxification in the fish. According to Sures (2008) asserted that parasites might amplify the toxic effects of heavy metals by interfering with host protection mechanisms. According to Pietrock and Goater (2005), Cd is a toxic metal to different trematodes cercariae.
According to our results of physical and chemical parameters of water samples in Table 2, there is no significant difference (p > 0.05) between locations along the study area. All the study sites were on the alkaline side, with pH values between 7.2 ± 0.03 and 7.9 ± 0.05. This result agrees with the average pH of the Manzala region recorded by Abu Khatita et al. (2017). The biology of heavy metals and nutrients is influenced by the pH of water (Hacısalihoğlu and Karaer 2016). The temperatures ranged from 17.3 ± 0.25 °C to 19 ± 0.17 °C. The oxygen content is measured to determine the organic load, nutrient input, and biological activity (Abu Khatita et al. 2017). It's not only the direct toxicity of contaminants that kills some fish but also, the absence of oxygen is caused by contaminant degradation. In our study, the minimum DO value of (5.9 ± 0.06 mg/l) was recorded at site 2, whereas at site 3, the maximum value of (7.03 ± 0.09 mg/l) was recorded. Abu Khatita et al. (2017) reported DO = 5.0 mg/l at Manzala lagoon, while Bahr Elbakar lagoon DO = 5.9 mg/l.
In site 1, water EC values reached maximum levels of (3.78 ± 0.10 mhos/cm); at the farm, minimum values were found at (1.57 ± 0.3 mhos/cm). Any aquatic ecosystem's biodiversity and biomass are influenced by salinity. Water discharged from a polluted site has high salinity. Several areas measured the salinity of El-Manzala Lake and determined it was brackish (Hagras et al. 2018). We found that chloride at the studied sites varied depending on the receiving fresh or marine water. It was recorded on sites 1, 2, and 3 (0.81 ± 0.01, 0.54 ± 0.01, and 0.75 ± 0.00 g−1/l), respectively. Waste introduced by industrial activities and domestic wastes at site 1 caused high chloride concentrations (Hagras et al. 2018).
We observed a significant difference in Pb, Fe, Cu, Ni, and Cd concentration between the study areas. The amount of Pb and Fe was found to contain low levels of contamination in the three drains. Based on the findings in the present study, the concentrations of Cd and Cu in the water exceeded the permissible limits. On the other hand, we found that sites 1 and 2 had the highest concentrations of Cu. The Cd contamination was analyzed in the following order: site 1 > site 2 > site 3. According to El-Amier et al. (2018), the high level of Cd in the Bahr El-Baqar drain may be attributed to an increased discharge of untreated industrial waste. We studied the metal accumulation levels in tilapia muscle in the following order: Pb > Cd > Ni > Cu > Fe > Mn, comparable to those obtained in the muscle of Nile tilapia at Shoubra El-Khaema in the southern Nile (Abdel-Khalek 2015). Waterborne contaminants accumulate in fish based on their uptake and elimination rates. We have measured the Pb levels in three species of fish as follows: O. niloticus > C. gariepinus > M. cephalus had values of 1.80 ± 0.97, 1.78 ± 0.47, and 1.15 ± 0.32 μg/g, respectively. This study shows that the Pb concentrations in the muscles are above the permissible limit (0.214 μg/g) set by WHO (2011).
Iron was (19.97 ± 5.06, 11.23 ± 4.44, and 8.25 ± 1.85 μg /g wet weights) in catfish, mullet, and tilapia, respectively, which is still below the permissible limit (50 μg/g) for WHO (2011). Also, this value is lower than that reported by El-Khatib et al. (2020) at Bahr El-Baqar. Moreover, the amount of Cd was found to be the lowest mean value (0.01, 0.01, and 0.007 μg/g wet weight) for Mullet, Tilapia, and Catfish, respectively. It is noted that this value was lower than the values measured by Bahnasawy et al. (2009). They calculated a significant increase (p < 0.05) in Cd concentration in fish muscles at Shadar Azzam farm (0.29 μg/g dry weight). Also, a higher value of 10.84 μg/g dry weight was obtained by Authman et al. (2013). In our study, the value of the measured Ni concentrations was higher than the permissible limits, similar to the data reported by Solgi and Mirmohammadvali, (2021) whose found that the Ni concentrations in muscle and gill were significantly higher than those analyzed in other studies. Nickel is an essential component of crude oil. C. gariepinus was the most affected species due to the food habits because they are benthic and predatory fish (El-Shaer and Alabssawy 2019).
From Fig. 4, metals concentrations in water and muscle tissues did not correlate significantly (except Fe). It was higher in fish tissues than water, and this suggests metal accumulation in organs and biomagnifications. Agricultural pollution can result in contaminant residues that are hundreds or thousands of times higher than water, sediment, and food (Labonne et al. 2001). The obtained results showed that the quantity of heavy elements found in fish was higher than in water. In this regard, it is confirmed by (Abd-El-Khalek et al. 2012), who observed that, in aquatic organisms, fish accumulate metals at concentrations many times greater than those found in water or sediment; the concentrations differ in different body organs.
The trace of metal bioaccumulation in fishes is affected by environmental factors such as exogenous, endogenous, and ambient water (water metal bioavailability, temperature, and alkalinity). The endogenous factors include species, size, physiological state, and feeding behavior (Moiseenko and Kudryavtseva 2001). Transfer factors from water (except for Cd) were all above 1.00, which indicates the fish species accumulate the metals. This result is in agreement with many previous studies (Canpolat et al. 2014). TF values were measured from water, sediment, and plants of Tilapia nilotica fish in Nasser Lake by Rashed (2001), with only the values from water for all metals exceeding 1.00, indicating that the fish accumulate the metals from the water.
Based on the mean PTWI value, humans are thought to consume a contaminant without appreciable risk over a lifetime. Joint Food and Agricultural Organization for the United Nations (FAO)/World Health Organization (WHO) Expert Committee on Food Additives (JECFA) established the PTWI (Alipour et al. 2015). Adult Egyptians were assumed to weigh 70 kg on average. Based on the EWI values in this study, the estimated weekly intake of these heavy metals is below the PTWI. Generally, eating the edible muscles of this species of fish from Manazala Lake is not harmful. Similar results were recorded by Abdel-Mohsien and Mahmoud (2015), where they estimated that the weekly intake of Cd and Pb for adult persons 70 kg consuming fish in Egypt was 7.94 and 15.84 μg/week, which is below PTWI. In literature, many estimates were published by researchers for the intake of fish on a weekly or daily basis (Türkmen et al. 2009). Based on the results of Abdel-Kader and Mourad (2020), the estimated weekly intakes of Cd, As, Pb, and Hg were greater than the PTWI in children, youth, and adults.
In contrast, the estimated weekly intake of Pb by children, youths, and adults, as well as Hg and As by adults, is below the recommended PTWI. Consequently, based on the consumption of fish species from Manzala Lake, the estimated weekly intake of elements for adults eating fish in Egypt is thousands of times less than the recommended PTWI by FAO/WHO. However, these trace element concentrations exceed the maximum permissible limits recommended in some fish samples by Egypt, FAO, WHO, and the EC. The toxic element obtained from fish depends not only on its concentration but also on how much fish is consumed. Therefore, Egyptians are at risk of harmful effects of trace elements only if they consume more fish than is suggested by this study.
Conclusion
We concluded that heavy metal concentrations in water samples were within permissible limits, except that Cd and Cu exceeded the permissible limits set by USEPA (2006), while metal accumulated in muscle tissue with Pb > Ni > Cd > Cu > Fe > Mn. Pb and Ni were above the permissible limits recommended by WHO (2011) and WHO (1989), while Mn concentration showed significant (p < 0.05) variations for fish species. Fe, Pb, Ni, Mn, and Cu concentrations in fish samples were significantly higher than in water. At the same time, the bioaccumulation factor (BAF) varied from 0.16 for Cd to 47.55 for Fe in catfish C gariepinus, which was the most affected species. According to this study, an average weekly intake of 70 kg for someone consuming fish in Egypt is much lower than the Provisional permissible Tolerable Weekly Intake (PTWI). Generally, people who ingest significant amounts of fish from Manzala Lake do not pose a health risk, but caution should be exercised if they eat a big part of edible fish. To clarify the relationship between heavy metals and parasitic infection, we evaluated the infected muscles using the tumor necrosis factor alpha-1 (TNF*-1) and interlockin-1* (IL-1*) for O. niloticus and M. cephalus, as well as the major histocompatibility class II (MHC II) for C. gariepinus.
Acknowledgements
The authors are grateful to Elshaimaa Ismael, Faculty of Veterinary medicine, Cairo University, for her kind help for statistical data analysis.
Authors Contribution
All authors sharing in the aim of works; MAM; MA ZE: collect the samples and identify the clinical sign on the fish; MA; HSK analyze the water quality and toxic substance in fish tissues; MMA examine the parasites inside the fishes and analyze the gene expression; All authors sharing in writing this manuscript and revise it.
Funding
No funding supporting this work.
Declarations
Conflict of interest
All authors declare that they have no conflict of interest.
Ethical approval
This article does not contain any studies with human or animal subjects.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- Abdel-Kader HH, Mourad MH. Trace elements exposure influences proximate body composition and antioxidant enzyme activities of the species tilapia and catfish in Burullus Lake- Egypt: human risk assessment for the consumers. Environ Sci Pollut Res. 2020;27(35):43670–43681. doi: 10.1007/s11356-020-10207-2. [DOI] [PubMed] [Google Scholar]
- Abdel-Khalek AA. Risk assessment, bioaccumulation of metals, and histopathological alterations in Nile tilapia (Oreochromis niloticus) facing degraded aquatic conditions. Bull Environ Contam Toxicol. 2015;94(1):77–83. doi: 10.1007/s00128-014-1400-9. [DOI] [PubMed] [Google Scholar]
- Abd-El-Khalek DE, El-Gohary SE, El-Zokm GM. Assessment of heavy metals pollution in Oreochromis niloticus in EL-Max Fish Farm, Egypt. Egyptian J Exper Biol (zool) 2012;8(2):215–222. [Google Scholar]
- Abdel-Mageid AD, Zaki AG, El Senosi YA, Fahmy HA, El Asely AM, Abo-AlEla HG, El-Kassas S. Modulatory effect of lipopolysaccharide on immune-related gene expression and serum protein fractionation in grey mullet, Mugil Cephalus. Aquac Res. 2020;51(4):1643–1652. doi: 10.1111/are.14510. [DOI] [Google Scholar]
- Abdel-Mohsien HS, Mahmoud MA. Accumulation of some heavy metals in Oreochromis niloticus from the Nile in Egypt: potential hazards to fish and consumers. J Environ Prot. 2015;6(09):1003. doi: 10.4236/jep.2015.69089. [DOI] [Google Scholar]
- Abdelsalam M, Ewiss MZ, Khalefa HS, Mahmoud MA, Elgendy MY, Abdel-Moneam DA. Coinfections of Aeromonas spp., Enterococcus faecalis, and Vibrio alginolyticus isolated from farmed Nile tilapia and African catfish in Egypt, with an emphasis on poor water quality. Microbial Pathogen. 2021;160:105213. doi: 10.1016/j.micpath.2021.105213. [DOI] [PubMed] [Google Scholar]
- Abdelsalam M, Abdel-Gaber R, Mahmoud MA, Mahdy OA, Khafaga NI, Warda M. Morphological, molecular and pathological appraisal of Callitetrarhynchus gracilis plerocerci (Lacistorhynchidae) infecting Atlantic little tunny (Euthynnus alletteratus) in Southeastern Mediterranean. J Adv Res. 2016;7(2):317–326. doi: 10.1016/j.jare.2015.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abdelsalam M, Attia MM, Mahmoud MA (2020) Comparative morphomolecular identification and pathological changes associated with Anisakis simplex larvae (Nematoda: Anisakidae) infecting native and imported chub mackerel (Scomber japonicus) in Egypt. Regional Stud Marine Sci 39, 101469.
- Aboul Ezz AS, Abdel-Razek SE. Heavy metal accumulation in the Tilapia nilotica L. and in the waters of Lake Manzalah. Egyptian J Appl Sci. 1991;6(6):37–52. [Google Scholar]
- Abu Khatita AM, Shaker IM, Shetaia SA. Water quality assessment and potential health risk of Manzala lake-Egypt. Al Azhar Bull Sci. 2017;9:119–136. [Google Scholar]
- Albering HJ, Rila JP, Moonen EJ, Hoogewerff JA, Kleinjans JC. Human health risk assessment in relation to environmental pollution of two artificial freshwater lakes in The Netherlands. Environ Health Perspect. 1999;107(1):27–35. doi: 10.1289/ehp.9910727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alipour H, Pourkhabbaz A, Hassanpour M. Estimation of potential health risks for some metallic elements by consumption of fish. Water Qual Expo Health. 2015;7(2):179–185. doi: 10.1007/s12403-014-0137-3. [DOI] [Google Scholar]
- AOAC (2005) Association of official analytical chemists, Official methods of analysis. 18th edn, Arlington, VA, USA
- APHA (2000) Standard methods for the examination of water and wastewater, 18th ed. American Publich Health Association (APHA), American Water Works Association (AWWA) and Water Pollution Control Federation (WPCF), Washington, DC
- Attia MM, El-Gameel SM, Ismael E. Evaluation of tumor necrosis factor-alpha (TNF-α); gamma interferon (IFN-γ) genes and oxidative stress in sheep: immunological responses induced by Oestrus ovis (Diptera: Oestridae) infestation. J Parasit Dis. 2020;44(2):332–337. doi: 10.1007/s12639-020-01220-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Attia MM, Elgendy MY, Prince A, El-Adawy MM, Abdelsalam M. Morphomolecular identification of two trichodinid coinfections (Ciliophora: Trichodinidae) and their immunological impacts on farmed Nile Tilapia. Aquacult Res. 2021;00:1–9. doi: 10.1111/are.15281. [DOI] [Google Scholar]
- Attia MM, Abdelsalam M, Korany RMS, et al. Characterization of digenetic trematodes infecting African catfish (Clarias gariepinus) based on integrated morphological, molecular, histopathological, and immunological examination. Parasitol Res. 2021 doi: 10.1007/s00436-021-07257-x. [DOI] [PubMed] [Google Scholar]
- Attia MM, Elgendy MY, Abdelsalam M, Hassan A, Prince A, Salaeh NMKL, Younis NA. Morpho-molecular identification of Heterophyes heterophyes encysted metacercariae and its immunological and histopathological effects on farmed Mugil cephalus in Egypt. Aquacult Int. 2021 doi: 10.1007/s10499-021-00708-3. [DOI] [Google Scholar]
- Authman MM, Abbas HH, Abbas WT. Assessment of metal status in drainage canal water and their bioaccumulation in Oreochromis niloticus fish in relation to human health. Environ Monit Assess. 2013;185(1):891–907. doi: 10.1007/s10661-012-2599-8. [DOI] [PubMed] [Google Scholar]
- Bahnasawy M, Khidr AA, Dheina N. Seasonal variations of heavy metals concentrations in mullet, Mugil cephalus and Liza ramada (Mugilidae) from Lake Manzala, Egypt. Egyptian J Aquatic Biol Fish. 2009;13(2):81–100. doi: 10.21608/ejabf.2009.2034. [DOI] [Google Scholar]
- Byadgi O, Chen YC, Barnes AC, Tsai MA, Wang PC, Chen SC. Transcriptome analysis of grey mullet (Mugil cephalus) after challenge with Lactococcus garvieae. Fish Shellfish Immunol. 2016;58:593–603. doi: 10.1016/j.fsi.2016.10.006. [DOI] [PubMed] [Google Scholar]
- Canpolat O, Eroğlu M, Çoban MZ, Düşükcan M. Transfer factors and bioaccumulation of some heavy metals in muscle of a freshwater fish species: a human health concern. Fresenius Environ Bull. 2014;23:418–425. [Google Scholar]
- El-Amier YA, El-Alfy MA, Nofal MM. Macrophytes potential for removal of heavy metals from aquatic ecosystem, Egypt: using metal accumulation index (MAI) Plant Arch. 2018;18(2):2131–2144. [Google Scholar]
- El-Khatib Z, Azab MA, Abo-Taleb AHH, Al-Absawy NMA, Toto MMM. Effect of heavy metals in irrigation water of different fish farms on the quality of cultured fish. Egyptian J Aquatic Biol Fish. 2020;24(5):261–277. doi: 10.21608/ejabf.2020.104648. [DOI] [Google Scholar]
- El-Shaer FM, Alabssawy AN. Assessment of heavy metals concentration in water and edible tissues of Nile tilapia (Oreochromis niloticus) and (Clarias gariepinus) from Burullus Lake, Egypt with liver histopathological as pollution indicator. J Egypt Soc Parasitol. 2019;49(1):183–194. doi: 10.21608/jesp.2019.68301. [DOI] [Google Scholar]
- Ezzat SM, ElKorashey RM, Sherif MM. The economical value of Nile Tilapia Fish "Oreochromis niloticus" in relation to water quality of lake Nasser, Egypt. J Am Sc. 2012;8(9):234–247. [Google Scholar]
- FAO/WHO (2004) Summary of evaluations performed by the joint FAO/WHO expert committee on food additives (JECFA 1956–2003), (first through sixty first meetings). ILSI Press International Life Sciences Institute
- Fozia A, Muhammad AZ, Muhammad A, Zafar MK. Effect of chromium on growth attributes in sunflower (Helianthus annuus L.) J Environ Sci. 2008;20(12):1475–1480. doi: 10.1016/S1001-0742(08)62552-8. [DOI] [PubMed] [Google Scholar]
- GAFRD . The general authority for fishery resources development: summary production statistics. Cairo: Egypt; 2014. [Google Scholar]
- Hacısalihoğlu S, Karaer F (2016) Relationships of heavy metals in water and surface sediment with different chemical fractions in Lake Uluabat, Turkey. Polish J Environ Stud 25(5)
- Hagras AE, Elbaghdady HAM, Gouda AM. Assessment of water quality and heavy metals in water, sediments, and some organs of African catfish (Clarias gariepinus) in El-Serw Drain, Nile Delta, Egypt. Int J Environ. 2018;7(4):124–141. [Google Scholar]
- Katip A, Karaer F, Ileri S, Sarmasik S, Aydogan N, Zenginay S. Analysis and assessment of trace elements pollution in sediments of Lake Uluabat, Turkey. J Environ Biol. 2012;33(5):961. [PubMed] [Google Scholar]
- Khalefa HS, Abdel-Moneam DA, Ismael E, Waziry MMF, Ali MSG, Zaki MM. The effect of alterations in water quality parameters on the occurrence of bacterial diseases in different aquatic environments. Adv Anim Veter Sci. 2021;9(12):2084–2094. doi: 10.17582/journal.aavs/2021/9.12.2084.2094. [DOI] [Google Scholar]
- Kouamenan NM, Coulibaly S, Atse BC, Goore BG. Human health risk assessment and effects some heavy metals in the tissue of two species of Cichlidae (Hemichromis fasciatus and Tilapia zillii× Tilapia guineensis) from the Western Part of the Ebrie Lagoon, Côte D'Ivoire. Int J Sci Res. 2020;9(4):160–167. [Google Scholar]
- Labonne M, Othman DB, Luck JM. Pb isotopes in mussels as tracers of metal sources and water movements in a lagoon (Thau Basin, S France) Chem Geol. 2001;181(1–4):181–191. doi: 10.1016/S0009-2541(01)00281-9. [DOI] [Google Scholar]
- Lindenstrøm T, Secombes CJ, Buchmann K. Expression of immune response genes in rainbow trout skin induced by Gyrodactylus derjavini infections. Vet Immunol Immunopathol. 2004;97:137–148. doi: 10.1016/j.vetimm.2003.08.016. [DOI] [PubMed] [Google Scholar]
- Mahdy OA, Abdel-Maogood SZ, Abdelsalam M, Shaalan M, Abdelrahman HA, Salem MA. Epidemiological study of fish- borne zoonotic trematodes infecting Nile tilapia with first molecular characterization of two heterophyid flukes. Aquac Res. 2021;52:4475–4488. doi: 10.1111/are.15286. [DOI] [Google Scholar]
- Mehana EE, Khafaga AF, Elblehi SS, Abd El-Hack ME, Naiel MA, Bin-Jumah M, Othman SI, Allam AA. Biomonitoring of heavy metal pollution using acanthocephalans parasite in ecosystem: an updated overview. Animals. 2020;10:811. doi: 10.3390/ani10050811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moiseenko TI, Kudryavtseva LP. Trace metal accumulation and fish pathologies in areas affected by mining and metallurgical enterprises in the Kola Region, Russia. Environ Pollut. 2001;114(2):285–297. doi: 10.1016/S0269-7491(00)00197-4. [DOI] [PubMed] [Google Scholar]
- Nasrullah H, Nababan YI, Yanti DH, Hadiantho D, Sri N, Zairin M, Ekasari J, Alimuddin A. Identification and expression analysis of C-type and g-type lysozymes genes after Aeromonas hydrophila infection in African catfish. J Aquacult Indonesia. 2019;18(2):1–10. doi: 10.19027/jai.18.2.1-10. [DOI] [Google Scholar]
- Pietrock M, Goater CP. Infectivity of Ornithodiplostomum ptychocheilus and Posthodiplostomum minimum (Trematoda:Diplostomidae) cercariae following exposure to cadmium. J Parasitol. 2005;91(4):854–856. doi: 10.1645/GE-473R.1. [DOI] [PubMed] [Google Scholar]
- Rajeshkumar S, Li X. Bioaccumulation of heavy metals in fish species from the Meiliang Bay, Taihu Lake, China. Toxicol Rep. 2018;5:288–295. doi: 10.1016/j.toxrep.2018.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rashed MN. Cadmium and lead levels in fish (Tilapia nilotica) tissues as biological indicator for lake water pollution. Environ Monit Assess. 2001;68(1):75–89. doi: 10.1023/A:1010739023662. [DOI] [PubMed] [Google Scholar]
- Solgi E, Mirmohammadvali S. Comparison of the heavy metals, copper, iron, magnesium, nickel, and zinc between muscle and gills of four benthic fish species from Shif Island (Iran) Bull Environ Contam Toxicol. 2021;106(4):658–664. doi: 10.1007/s00128-021-03155-1. [DOI] [PubMed] [Google Scholar]
- Sures B. Host–parasite interactions in polluted environments. J Fish Biol. 2008;73:2133–2142. doi: 10.1111/j.1095-8649.2008.02057.x. [DOI] [Google Scholar]
- Türkmen M, Türkmen A, Tepe Y, Töre Y, Ateş A. Determination of metals in fish species from Aegean and Mediterranean seas. Food Chem. 2009;113(1):233–237. doi: 10.1016/j.foodchem.2008.06.071. [DOI] [Google Scholar]
- USEPA (2006) Resources for information on risk-based concentration table. Available at http://www.epa.gov/reg3hwmd/risk/human/rbc/rbc1006.pdf
- WHO (2011). Guideline for drinking-water quality (fourth ed), World Health Organization, Geneva
- World Health Organization, WHO (1989) Heavy metals-environmental aspects. Guidelines for drinking-water quality, WHO, Geneva, 4th edn, 2011, pp 1, 430–431, 475
- Younis NA, Laban SE, Al-Mokaddem AK, Attia MM. Immunological status and histopathological appraisal of farmed Oreochromis niloticus exposed to parasitic infections and heavy metal toxicity. Aquacult Int. 2020 doi: 10.1007/s10499-020-00589-y. [DOI] [Google Scholar]