Abstract
The essential cell division protein, FtsZ, from Mycobacterium tuberculosis has been expressed in Escherichia coli and purified. The recombinant protein has GTPase activity typical of tubulin and other FtsZs. FtsZ polymerization was studied using 90° light scattering. The mycobacterial protein reaches maximum polymerization much more slowly (∼10 min) than E. coli FtsZ. Depolymerization also occurs slowly, taking 1 h or longer under most conditions. Polymerization requires both Mg2+ and GTP. The minimum concentration of FtsZ needed for polymerization is 3 μM. Electron microscopy shows that polymerized M. tuberculosis FtsZ consists of strands that associate to form ordered aggregates of parallel protofilaments. Ethyl 6-amino-2,3-dihydro-4-phenyl-1H-pyrido[4,3-b][1,4]diazepin-8-ylcarbamate (SRI 7614), an inhibitor of tubulin polymerization synthesized at Southern Research Institute, inhibits M. tuberculosis FtsZ polymerization, inhibits GTP hydrolysis, and reduces the number and sizes of FtsZ polymers.
Bacteria have a variety of genes that are critical for cell division, among them the fts genes (reviewed in references 20, 24, and 26). Cell division occurs at the site of formation of the contractile Z ring, which is composed of a polymer of FtsZ. FtsZ, a 40-kDa protein, is ubiquitous in eubacteria and archaea. Although it has only weak sequence homology to mammalian tubulin, it does contain the tubulin signature motif GGGTGS/TG (7, 9), which is believed to be necessary for the GTPase activity of tubulin. FtsZ polymerizes to form the Z ring in a GTP-dependent manner, analogous to the polymerization of tubulin to form microtubules (4, 6, 10, 21, 32). The three-dimensional structures of Methanococcus jannaschii FtsZ and α- and β-tubulin are quite similar (16, 17) and reveal that FtsZ and tubulin form a unique family of GTPases (24).
Although the polymerization of tubulin has been studied extensively, understanding FtsZ polymerization has been hampered until recently by the lack of a rapid, easy assay. To date the primary method of examining FtsZ polymerization has relied on centrifugation of the reaction mixture followed by an examination of the pellet or by electron microscopy. Most of the published work has dealt with FtsZ from Escherichia coli (1, 6, 20). Recently, Mukherjee and Lutkenhaus (22) introduced a light-scattering assay that uses a fluorometer to monitor polymer formation and dissolution. We have used this method to extend our understanding of FtsZ by examining the dynamics of Mycobacterium tuberculosis FtsZ polymerization in vitro. M. tuberculosis FtsZ polymerization is similar to that of E. coli FtsZ in many respects. However, there are some significant differences between the two, with M. tuberculosis FtsZ showing some characteristics more reminiscent of its homolog tubulin than the E. coli protein. This work represents the first study of M. tuberculosis FtsZ, a critical cell division protein for a pathogenic organism of worldwide medical importance.
MATERIALS AND METHODS
Purification of FtsZ.
The M. tuberculosis FtsZ coding sequence was subcloned into the NcoI site of pET15b (Novagen). The resulting plasmid, pJD168, was used to transform E. coli BL21(DE3)/pLysS. Cells were incubated at 32°C in Luria-Bertani (LB) media containing 0.4% glucose for 1 h. Five hundred microliters of transformed cells was added to 250 ml of fresh LB medium containing 0.4% glucose, 100 μg of ampicillin/ml, and 34 μg of chloramphenicol/ml and incubated overnight at 32°C. The cells were pelleted by centrifugation at room temperature. They were then resuspended in 4 liters of prewarmed LB medium containing 0.2% glucose, 100 μg of ampicillin/ml, and 34 μg of chloramphenicol/ml, and the culture was shaken with good aeration at 32°C. When the culture reached an A600 of ∼0.4, expression of FtsZ was induced with 1 mM isopropyl-β-d-thiogalactopyranoside. Cells were harvested 3 h later, chilled (8 to 10°C) quickly, centrifuged, washed with ice-cold phosphate-buffered saline, repelleted, and stored at −80°C.
The following procedures were performed at 4°C. The frozen cell pellet from 1 liter of the E. coli culture was resuspended in 30 ml of lysis buffer (20 mM sodium phosphate buffer [pH 7.8], 500 mM NaCl, 2 mM phenylmethylsulfonyl fluoride [PMSF], 4 μg of pepstatin A/ml, 4 μg of leupeptin/ml, 1 mM benzamidine, and 20 μg of soybean trypsin inhibitor/ml) and sonicated briefly to loosen the gum-like pellet. The cell suspension was digested for 30 min with 1 mg of DNase and then extracted by two passes through a French press at 15,000 to 20,000 lb/in2. The solution was clarified by centrifugation at 27,000 × g for 20 min and then applied to a Ni2+-agarose cartridge (Pharmacia) equilibrated with 10 mM imidazole–20 mM sodium phosphate–0.5 M NaCl, pH 7.8. The column was washed with 15 ml of equilibration buffer containing 100 mM imidazole. Recombinant FtsZ was eluted with 5 ml of equilibration buffer containing 250 mM imidazole. The eluate was immediately passed over Sephadex G-25 columns (PD-10; Pharmacia) equilibrated with 25 mM HEPES-NaOH (pH 7.2)–100 mM KCl–0.1 mM EDTA–1 mM dithiothreitol (DTT)–10% glycerol. The N-terminal six-His tag was removed by digestion on ice for 2 h with 0.5 U of thrombin (Sigma)/ml of FtsZ. Thrombin was removed by passing the sample over a benzamidine-agarose column (Sigma; flow rate, ∼1 ml/min) equilibrated with desalting buffer (27). Protease inhibitors (2 mM PMSF, 1 mM benzamidine, 2 mM 1,10-phenanthroline, and 20 μg of soybean trypsin inhibitor, 4 μg of pepstatin A, 10 μg of 4-amidinophenylmethylsulfonyl fluoride 50 μg of aprotinin, 4 μg of leupeptin, and 40 μg of TLCK/ml) were added to the pooled fractions. The sample was applied to a gel filtration column (Pharmacia HiLoad 26/60 Superdex 200 prepgrade) equilibrated with a buffer containing 25 mM HEPES-NaOH (pH 7.2), 1 mM EDTA, 50 mM KCl, 1 mM DTT, and 10% glycerol. Absorbance was monitored at 280 nm. The protease cocktail was added to the pooled fractions. After concentration (Millipore BioMax 15-1000) to 20 mg/ml, the sample was dialyzed against 25 mM HEPES-NaOH (pH 7.2)–1 mM DTT–0.1 mM EDTA–10% glycerol. The protease cocktail was added a third time, and the protein was stored at −80°C.
Light-scattering assay for FtsZ polymerization.
The polymerization and depolymerization of purified FtsZ were monitored by the method described by Mukherjee and Lutkenhaus for E. coli FtsZ (22). Light scattering was measured in a thermostatically (30°C) controlled Aminco-Bowman series 2 luminescence spectrometer using 0.5-ml quartz cuvettes (cell, 2 by 10 mm; Hellma). Excitation and emission wavelengths were 400 nm with a slit width of 2 nm. The gain was typically set at 375 V but was increased if needed to give a maximum response between 0.1 and 0.15. FtsZ (500 μg/ml; 13 μM) was incubated in 50 mM MES (morpholineethanesulfonic acid)-NaOH (pH 6.5)–100 mM KCl–5 mM MgCl2 to establish a baseline. GTP (40 μM) was added (final volume, 300 μl), and data were collected for an additional 50 to 60 min. Changes in concentrations of a component for a particular experiment are indicated in the text or figure legend.
Inhibitor studies.
The effect of different compounds on M. tuberculosis FtsZ polymerization and depolymerization was monitored using the light-scattering assay described above. Compounds were added to the reaction mixture, and a baseline was established. GTP was added to initiate polymerization, and light-scattering data were collected for an additional 50 to 60 min. The maximum light scattering was calculated by subtracting the baseline value from the peak value. The percentage of control activity was calculated by comparison with an assay without the compound. When dimethyl sulfoxide (DMSO) was used as the solvent, the control contained the same amount of DMSO (2 to 4%). A semilog plot of percentage of control activity versus compound concentration was used to calculate the 50% inhibition concentration (IC50). Vinblastine, paclitaxel (Taxol), albendazole, colchicine, and 3-methoxybenzamidine were purchased from Sigma. Ethyl 6-amino-2,3-dihydro-4-phenyl-1H-pyrido[4,3-b][1,4]diazepin-8-ylcarbamate (SRI 7614; molecular weight, 325.37) was synthesized as previously described (30).
Electron microscopy.
FtsZ (500 μg/ml; 13 μM) was incubated in 50 mM MES-NaOH (pH 6.5)–100 mM KCl–MgCl2 at either 0, 5, or 10 mM. GTP (40 μM) was added, and a 5-μl aliquot was withdrawn 25 min later and placed on a carbon-coated copper grid (300 mesh). After blotting, the grids were negatively stained using 1% uranyl acetate and blotted again. The grids were viewed with a Hitachi H-7000 electron microscope (acceleration voltage, 75 kV). Photographs were taken with type 4489 film. Inhibition of M. tuberculosis FtsZ polymerization by SRI 7614 (10 mM) was examined at 5 mM MgCl2.
Mass spectrometry and amino acid sequencing.
Matrix-assisted laser desorption ionization–time of flight (MALDI-TOF) mass spectra were obtained on a Voyager Elite mass spectrometer (positive mode) with delayed-extraction technology (PerSeptive Biosystems). The acceleration voltage was set at 25 kV, and 10 to 50 laser shots were summed. The matrix was sinapinic acid (Aldrich) dissolved in CH3CN–0.1% CF3CO2H (1:1). The spectrometer was calibrated with apomyoglobin or bovine serum albumin. Samples were diluted 1:10 with matrix before pipetting 1 μl onto a smooth plate. N-Terminal sequencing was done by automated Edman degradation on a gas phase microsequencing system (model PI 2090E; Beckman). The amino acid residue released in a given cycle was identified from the difference chromatogram (comparison with the previous cycle).
Miscellaneous.
The GTPase activity of 25 μl of sample containing FtsZ was monitored using the method described by Mukherjee et al. (23). Briefly, FtsZ was incubated at 30°C with 40 μM [γ-32P]GTP (250 to 400 cpm/pmol)–100 mM MES-NaOH (pH 6.5)–100 mM KCl–5 mM MgCl2 for 60 min (final volume, 50 μl). Radioactive inorganic phosphate was extracted with 0.1 M HClO4 containing 1 mM KH2PO4 followed by the addition of sodium molybdate and isopropyl acetate. Aliquots of the organic phase were measured in a liquid scintillation counter. Discontinuous sodium dodecyl sulfate-polyacrylamide gel electrophoresis (15% acrylamide gel) was used to monitor the purification and to determine subunit molecular weight (15). Protein concentrations were determined by the Bradford procedure (3), using bovine gamma globulin as the standard. The amount of nucleotide bound to the protein (50 μM FtsZ) was determined from a 3% perchloric acid extract (29). The absorbance of the supernatant at 257 nm was measured, and the concentration was calculated from a standard curve of GDP (5 to 200 μM). Strong anion-exchange high-pressure liquid chromatography was used to identify the nucleotide.
RESULTS AND DISCUSSION
Purification.
Recombinant M. tuberculosis FtsZ was purified to greater than 95% purity by Ni2+-agarose affinity chromatography, thrombin digestion to remove the N-terminal six-His tag, and gel filtration. Early experiments, in which a higher concentration of thrombin and overnight incubation were used to remove the six-His tag, indicated that FtsZ was unusually sensitive to proteolytic degradation, especially by thrombin. SDS-PAGE, N-terminal sequencing, and MALDI-TOF mass spectrometry results showed that internal proteolytic digestion had occurred in addition to removal of the six-His tag. Consequently, special care was taken to protect the protein from proteases and to eliminate the thrombin used to remove the N-terminal six-His tag. The gel filtration column was monitored by measuring optical density at 280 nm, GTPase activity assays, and SDS-PAGE. The majority of FtsZ was eluted from the column in a single peak corresponding to a molecular mass of 95,500 Da. The fractions from the peak were pooled, concentrated, dialyzed against buffer containing 10% glycerol, and stored in aliquots at −80°C. Under these conditions the protein was stable for several months. A typical yield from a 1-liter E. coli culture was 30 mg of FtsZ.
The molecular mass of FtsZ, determined by SDS–PAGE, was 45,700 Da. It migrated somewhat higher than anticipated from the calculated molecular weight. Mass spectrometric analysis (MALDI-TOF) confirmed, however, that it had the correct mass (observed, 39,064.30 Da; calculated, 39,036.45 Da; masses include an N-terminal Gly-Ser-His that remains after thrombin digestion). N-Terminal sequencing confirmed the expected sequence of GSHMTPPHNY. FtsZ was eluted from a gel filtration column as a series of aggregates of decreasing molecular mass from ∼2,000,000 Da (void volume) to 95,500 Da (major peak). Since the subunit molecular mass is 39,036 Da, the 95,000-Da peak is likely a FtsZ dimer. Under similar conditions (no nucleotide or Mg2+), E. coli FtsZ has been shown by analytical ultracentrifugation and chemical cross-linking to exist as a mixture of ∼70% dimer, 15% trimer, and 15% monomer (29). M. jannaschii FtsZ has also been reported to exist as an oligomer (16).
Purified FtsZ (5 μM) had GTPase activity, converting around 6.9 nmol of GTP to GDP per mg of FtsZ per h. Unlike what was found for E. coli FtsZ (23), heating M. tuberculosis FtsZ did not increase the GTPase activity. GDP was bound at a ratio of 0.35 mol of GDP/mol of FtsZ, about one-half the amount found for E. coli FtsZ (21, 29). Since the GTPase activity is cooperative (29, 31), it is difficult to make direct comparisons between the published specific activity of E. coli FtsZ and our specific activity for M. tuberculosis FtsZ of 6.9 nmol mg−1 h−1. However, it appears that M. tuberculosis FtsZ hydrolyzes GTP at a significantly slower rate than E. coli FtsZ, which has been reported to have a Vmax of 30 μmol mg−1 h−1 (29).
Characterization of M. tuberculosis FtsZ with a light-scattering assay.
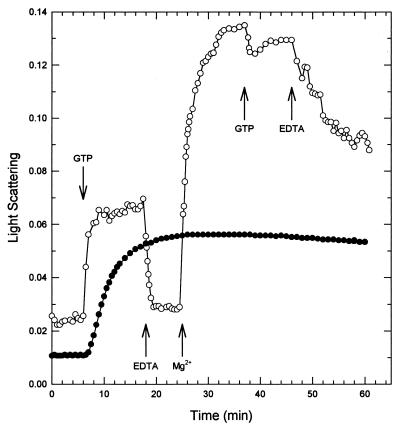
M. tuberculosis FtsZ polymerization was measured using the conditions described for E. coli FtsZ (22). FtsZ (10 μM) was incubated at 30°C in 50 mM MES-NaOH, pH 6.5, containing 10 mM MgCl2 and 25 mM KCl. There was an immediate increase in light scattering upon addition of 1 mM GTP, reaching a plateau in about 10 min (Fig. 1). The light scattering was remarkably stable, dropping by <10% in 5 h. Once polymerization had occurred, neither increasing the temperature to 45°C nor lowering it to 1°C induced depolymerization (data not shown). However, addition of 20 mM EDTA caused the light scattering to immediately return to baseline. FtsZ could be repolymerized, as indicated by an increase in light scattering, by adding 25 mM MgCl2 to the reaction mixture (Fig. 1).
FIG. 1.
Polymerization of M. tuberculosis FtsZ. M. tuberculosis FtsZ (10 μM) was assayed under the same conditions as those used for E. coli FtsZ (22). The protein was incubated in 50 mM MES-NaOH (pH 6.5)–10 mM MgCl2–25 mM KCl in a fluorometer cuvette at 30°C. The 90° angle light scattering was monitored to obtain a baseline. After 6 min, 1 mM GTP was added (●). The dynamic nature of the assay is shown in the other curve (○), in which the addition of 1 mM GTP was followed by 20 mM EDTA, 25 mM MgCl2, 1 mM GTP, and finally 20 mM EDTA.
Polymerization and depolymerization of M. tuberculosis FtsZ are clearly much slower than those of E. coli FtsZ (22). Polymerization occurs very rapidly for E. coli FtsZ (<30 s), with a stable phase lasting about 15 min, followed by complete depolymerization within another 10 min. Under identical conditions, M. tuberculosis FtsZ takes about 10 min to reach maximum polymerization, followed by a stable phase lasting at least 5 h, kinetics that are more similar to those of mammalian tubulin. Unlike tubulin, however, FtsZ could not be depolymerized by a temperature shift to 1°C. It is tempting to speculate that the slower dynamics of M. tuberculosis FtsZ, compared to those of E. coli FtsZ, are related to its lower GTPase activity. Perhaps the rates of polymerization and depolymerization of FtsZ are proportional to the growth rate of the organism. E. coli, with its much faster cell division time, may need a more dynamic cell division protein than the slower-growing M. tuberculosis. Experiments to evaluate these hypotheses are under way.
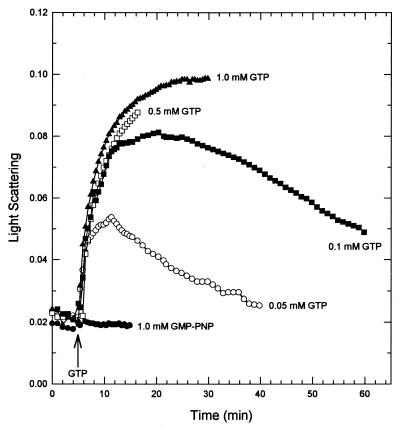
Polymerization and depolymerization were dependent on the concentration of GTP used to initiate the reaction (Fig. 2). With 5 mM MgCl2, 0.2 to 1 mM GTP initiated an increase in light scattering that was still increasing 25 min after addition. (For clarity, the 0.2 and 0.3 mM GTP curves are not plotted since they are identical to the 1 mM GTP curve.) Lower concentrations of GTP (0.05 to 0.1 mM) resulted in both polymerization and depolymerization within 25 min. No increase in light scattering was seen when either 1 mM GDP (data not shown) or 5′-guanylylimidodiphosphate, a nonhydrolyzable GTP analog, replaced GTP.
FIG. 2.
GTP dependence of FtsZ polymerization. FtsZ (10 μM) polymerization was initiated by the addition of various amounts of GTP (arrow) or the nonhydrolyzable GTP analogue 5′-guanylylimidodiphosphate (GMP-PNP).
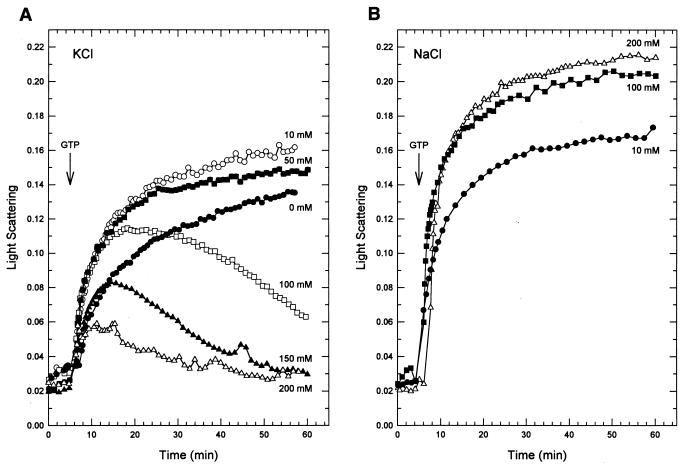
Since the rate of GTP hydrolysis by E. coli FtsZ is affected by the KCl concentration (23), we examined the effect of KCl on polymerization of M. tuberculosis FtsZ (Fig. 3A). With 10 or 50 mM KCl, addition of GTP started an increase in light scattering that continued for 1 h. Without KCl, polymerization was slower than with low KCl but was still continuing at the end of 1 h. Higher concentrations of KCl (100 and 200 mM) led to an increase in light scattering followed by a decrease. The maximum amount of light scattering decreased with increasing KCl concentration. The effect on depolymerization by KCl appears to be specific (Fig. 3B), since in the presence of 100 or 200 mM NaCl polymerization was still increasing at 1 h. E. coli FtsZ GTPase activity can be stimulated by KCl but not NaCl (23), and increasing the KCl concentration is associated with a shortening of the steady-state phase of polymerization (22).
FIG. 3.
Effects of salt on FtsZ polymerization. FtsZ polymerization was initiated by the addition of 0.05 mM GTP (arrow). (A) Effect of various amounts of KCl. (B) Effect of various amounts of NaCl.
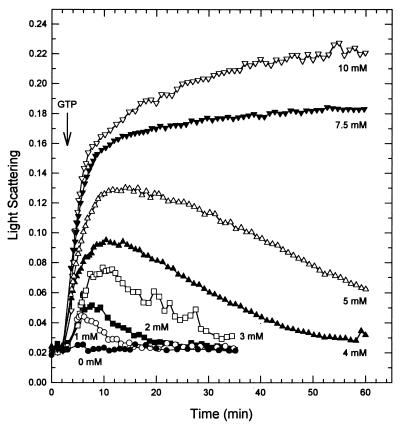
M. tuberculosis FtsZ, like other GTPases, requires Mg2+ for hydrolysis of GTP to GDP (6, 23, 29). As MgCl2 was increased from 1 to 5 mM, there was an augmentation in the maximum polymerization of M. tuberculosis FtsZ followed by a return to baseline (Fig. 4). Above 5 mM MgCl2, only polymerization was observed. FtsZ did not polymerize in the absence of MgCl2. This is in contrast to E. coli FtsZ, for which the addition of GTP in the absence of MgCl2 produces an increase in light scattering approximately one-third the level obtained with 10 mM MgCl2 (22).
FIG. 4.
M. tuberculosis FtsZ polymerization requires Mg2+. FtsZ polymerization was initiated by the addition of 0.04 mM GTP (arrow) in the presence of various amounts of MgCl2.
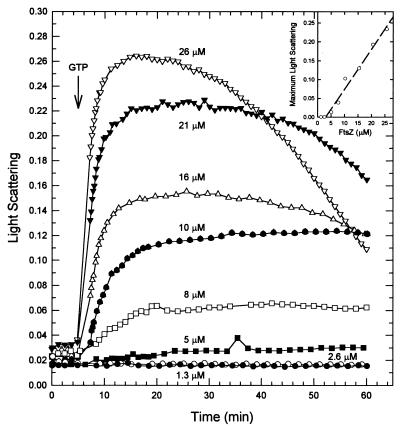
An examination of light scattering as a function of FtsZ concentration allowed a determination of the minimum concentration of protein required for polymerization. FtsZ at different concentrations was incubated in polymerizing buffer containing 100 mM KCl and 5 mM MgCl2. After the addition of 0.05 mM GTP, the maximal amount of light scattering was measured and plotted against the FtsZ concentration (Fig. 5). The value for the critical concentration required for polymerization was 3 μM (120 μg/ml). This was slightly higher than the critical concentration for E. coli FtsZ, determined by sedimentation to be 1.5 μM (21), but was similar to the value of 2.5 μM obtained from a light-scattering assay (22).
FIG. 5.
M. tuberculosis FtsZ polymerizes above a critical concentration. Polymerization of FtsZ at different concentrations was initiated by the addition of 0.05 mM GTP (arrow). (Inset) The net maximum change in light scattering is plotted against the FtsZ concentration. The intercept on the abscissa is the critical concentration of FtsZ required for polymerization to proceed.
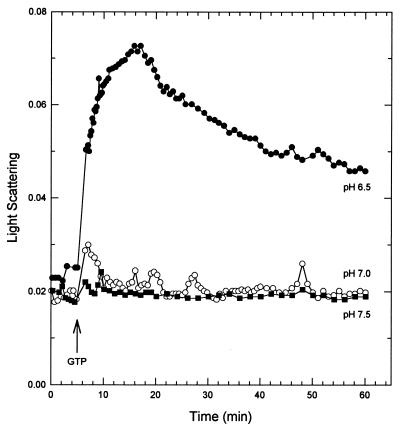
M. tuberculosis FtsZ polymerization was markedly reduced at neutral or alkaline pH (Fig. 6). GTP-dependent polymerization at pH 6.5, 7.0, and 7.5 in a buffer system that maintained a constant ionic strength over this range (50 mM HEPES, 50 mM MES, 100 mM ethanolamine [8]) was monitored. The maximum light scattering at pH 7.0 was less than one-fourth that at pH 6.5. No polymerization was seen at pH 7.5. E. coli FtsZ appears to be more tolerant of pH changes. An increase in light scattering, roughly equivalent to the response seen at pH 6.9, was clearly observable at pH 7.5 and 7.9 (22).
FIG. 6.
Effect of pH on FtsZ polymerization. FtsZ (13 μM) was polymerized at three pH values in a constant-ionic-strength buffer (50 mM HEPES, 50 mM MES, 100 mM ethanolamine) containing 100 mM KCl and 5 mM MgCl2. Polymerization was initiated by the addition of 0.04 mM GTP (arrow).
Inhibitor studies.
One of the major goals of our group is to develop compounds capable of inhibiting M. tuberculosis cell division. FtsZ, as an essential cell division protein, makes an attractive drug target. To this end, we examined the effect of several known inhibitors of tubulin polymerization or depolymerization in the FtsZ light-scattering assay. Tubulin is known to have at least three separate sites, distinct from the GTP binding site, to which inhibitors bind (for a review, see reference 12). Compounds binding to either the colchicine site or the vinca domain site (vinblastine) inhibit polymer assembly. Compounds binding to the third site prevent depolymerization (paclitaxel). The maximum increase in light scattering after GTP addition was used to monitor the reaction. Vinblastine and paclitaxel had no effect on the reaction at concentrations up to 1 mM (data not shown). Colchicine, however, inhibited the reaction with an IC50 of 1.3 mM. Albendazole, which also binds to the tubulin-colchicine site (13), did not interfere with FtsZ polymerization. Although assessment of this compound may have been limited by its solubility, the apparent lack of binding could be due to differences between the FtsZ and tubulin binding sites. 3-Methoxybenzamidine, an ADP-ribosyltransferase inhibitor, has been shown to inhibit Bacillus subtilis cell division by interacting with FtsZ (25). No effect in the light-scattering assay using M. tuberculosis FtsZ was seen (data not shown).
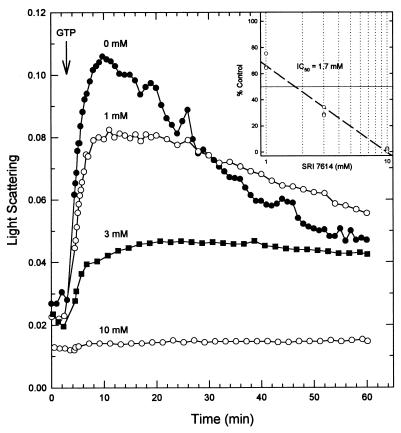
Ethyl 6-amino-2,3-dihydro-4-phenyl-1H-pyrido[4,3-b][1,4] diazepin-8-ylcarbamate (SRI 7614) has good activity (MIC at which 99% of the isolates are inhibited [MIC99], 19 μM; data not shown) against M. tuberculosis in vitro (5). This compound belongs to a series of mitotic inhibitors that compete with colchicine binding to pig brain tubulin (2). We found that SRI 7614 inhibited FtsZ polymerization with an IC50 (1.7 mM) similar to that of colchicine (Fig. 7). The difference of 2 orders of magnitude between the in vitro MIC99 and the polymerization IC50 is comparable to the difference of 1 to 3 orders of magnitude usually seen between analogous assays with tubulin inhibitors (11, 14, 28). Taken together, these results indicate that FtsZ has a binding site similar to the colchicine binding site of tubulin and that compounds directed at this site will be effective inhibitors of M. tuberculosis growth. X-ray crystallography studies of FtsZ complexed with SRI 7614 are clearly critical to further defining the characteristics of this target and are ongoing in our laboratory.
FIG. 7.
Inhibition of M. tuberculosis FtsZ polymerization by SRI 7614 (ethyl 6-amino-2,3-dihydro-4-phenyl-1H-pyrido[4,3-b][1,4]diazepin-8-ylcarbamate). FtsZ (13 μM) was incubated with different concentrations of SRI 7614 in polymerization buffer. Polymerization was initiated by the addition of 0.04 mM GTP (arrow). (Inset) Determination of IC50 value. The net maximum change in light scattering as a percentage of the control is plotted versus the log of the inhibitor concentration. The line is the best fit to the data from three experiments.
GTP hydrolysis and electron microscopy studies.
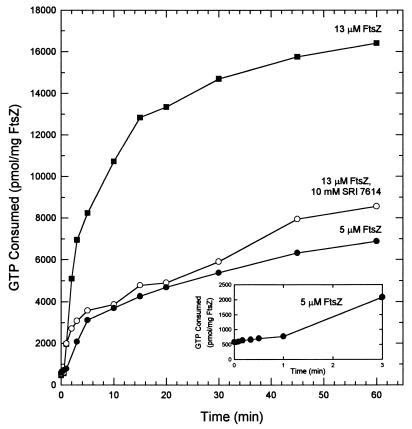
GTP hydrolysis occurs immediately at concentrations of FtsZ (13 μM) that support rapid polymerization (Fig. 8). Rapid GTP hydrolysis continues for about 10 min, roughly paralleling the rapid polymerization. At this point, about 30% of the GTP in the assay mixture has been hydrolyzed. A slower rate of hydrolysis accompanies FtsZ depolymerization. By 60 min, 50% of the GTP remained. At 5 μM FtsZ, just above the critical concentration, hydrolysis proceeds very slowly for 1 min (Fig. 8, inset), followed by a more rapid hydrolysis rate. At 60 min, 10% of the GTP has been used. SRI 7614 inhibited GTP hydrolysis by M. tuberculosis FtsZ. During rapid polymerization (10 min), GTPase activity, compared to that of the control, was inhibited by 70%.
FIG. 8.
Time course for GTP hydrolysis of M. tuberculosis FtsZ. At various times, 25-μl aliquots were withdrawn and assayed for release of 32P. The kinetics of GTP hydrolysis with 100 mM KCl and 5 mM MgCl2 were determined at a concentration slightly above the minimum concentration required for polymerization (5 μM) and at a concentration that gave both polymerization and depolymerization within 60 min (13 μM; Fig. 7). Inhibition by SRI 7614 (10 mM, six times the IC50 value) was determined with 13 μM FtsZ. (Inset) Expansion of the time course for 5 μM FtsZ.
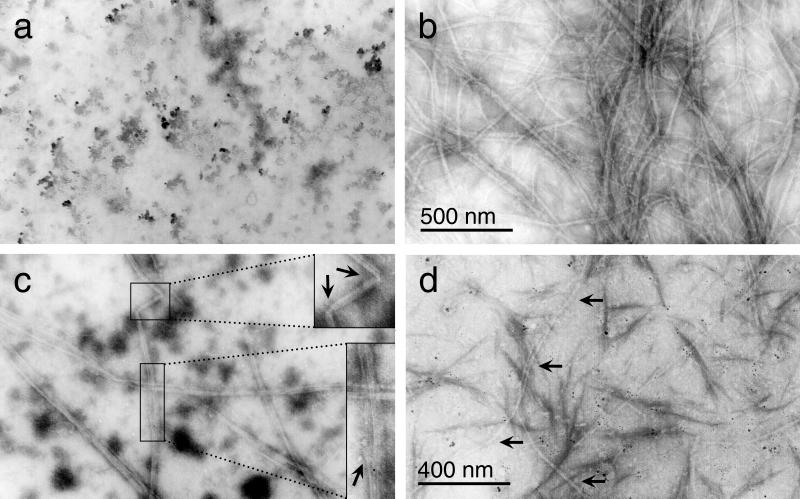
Electron micrographs showed that M. tuberculosis FtsZ polymerized to form strands (3.7-nm diameter) that combined as ordered aggregates of parallel protofilaments (Fig. 9). Polymerization required both Mg2+ (Fig. 9a) and GTP (data not shown). This differs from E. coli FtsZ, where polymers were seen in the absence of Mg2+ (22). The aggregated protofilaments had a diameter of 30 nm in the presence of 10 mM MgCl2 but were narrower (∼17 nm) at 5 mM MgCl2 (Fig. 9b and c). They were composed of several protofilaments (paired strands; see below) 7.5 to 10 nm wide. The polymers often exceeded 4.5 μm in length, and one was measured at 8 μm. They also showed lateral compression, fraying, and bends (Fig. 9c, insets). In the presence of SRI 7614, the aggregates were shorter and narrower than the controls without inhibitor (Fig. 9d). In particular, the ordered aggregates had a diameter of only 7.5 nm and perhaps therefore are actually single protofilaments. SRI 7614 may prevent the lateral association of protofilaments into larger aggregates. In addition, there were fewer of these aggregates than in the controls. The grids had to be searched diligently to find even a few sections containing FtsZ polymers. These differences would account for the inhibition seen in the light-scattering assay (Fig. 7).
FIG. 9.
Electron microscopic analysis of FtsZ polymers. Polymerization was initiated at different MgCl2 concentrations. (a) No MgCl2; (b) ordered aggregates of parallel protofilaments formed at 5 mM MgCl2; (c) 10 mM MgCl2 (insets, blowups showing bending [top] and fraying [bottom]); (d) 10 mM SRI 7614 and 5 mM MgCl2. Magnifications, ×40,000 (a to c) and ×50,000 (d).
With M. jannaschii FtsZ as a model (16, 17), a strand one subunit wide would be about 3.5 nm in diameter. The ordered aggregates formed by M. tuberculosis FtsZ appear to be composed of protofilaments (∼7.5 nm in diameter) two subunits in width, as was seen in an electron microscopic reconstruction of M. jannaschii FtsZ sheets (18). M. tuberculosis FtsZ protofilaments often had a longitudinal striation consistent with their being composed of two strands. The overall diameters of our polymers are similar to those seen for both E. coli and M. jannaschii FtsZ. E. coli FtsZ forms polymers 7 to 20 nm wide (22), and M. jannaschii FtsZ forms tubes 23 nm in diameter (18). E. coli FtsZ-GDP stabilized by DEAE-dextran forms tubes composed of curved protofilaments resulting in a shallow helix, whereas GTP favors the formation of straight protofilaments (19). The ordered parallel aggregates of M. tuberculosis FtsZ, formed in the presence of GTP (Fig. 9), were composed of straight protofilaments.
Tubulin and FtsZ polymerization are clearly complicated phenomena. Over the years, many models for tubulin polymerization have been proposed. One especially complicated issue has been the link between GTP hydrolysis and all of the stages of polymer formation and dissolution. M. tuberculosis FtsZ, with its clearly defined polymerization, steady-state, and depolymerization steps, may be a good model for better understanding the function of and connection between these processes.
ACKNOWLEDGMENTS
This work was supported by an internal research and development grant awarded by Southern Research Institute (SRI 1045). The mass spectrometer was purchased by funds from an NIH Shared Instrumentation Grant (S10RR11329) and from a Howard Hughes Medical Institute infrastructure support grant to UAB.
The M. tuberculosis FtsZ overexpression plasmids were a generous gift from Joseph DeVito, Laboratory of Mycobacteria, Food and Drug Administration, Rockville, Md. Joseph DeVito also provided the expression protocol used in these studies. We thank Kelly Morrison for determining the N-terminal sequences and Lori Coward for measuring the mass spectra, which were carried out at the Peptide Synthesis and Analysis and the Mass Spectrometry Shared Facilities of the University of Alabama at Birmingham Comprehensive Cancer Center (P30 CA13148-27). The electron micrographs were prepared by Edward Phillips at the UAB High Resolution Imaging Facility, Kent Keyser, director.
REFERENCES
- 1.Bi E, Lutkenhaus J. FtsZ ring structure associated with division in Escherichia coli. Nature. 1991;354:161–164. doi: 10.1038/354161a0. [DOI] [PubMed] [Google Scholar]
- 2.Bowdon B J, Waud W R, Wheeler G P, Hain R, Dansby L, Temple C., Jr Comparison of 1,2-dihydropyrido[3,4-b]pyrazines (1-deaza-7,8-dihydropteridines) with several other inhibitors of mitosis. Cancer Res. 1987;47:1621–1626. [PubMed] [Google Scholar]
- 3.Bradford M M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- 4.Bramhill D, Thompson C M. GTP-dependent polymerization of Escherichia coli FtsZ protein to form tubules. Proc Natl Acad Sci USA. 1994;91:5813–5817. doi: 10.1073/pnas.91.13.5813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Collins L, Franzblau S G. Microplate alamar blue assay versus BACTEC 460 system for high-throughput screening of compounds against Mycobacterium tuberculosis and Mycobacterium avium. Antimicrob Agents Chemother. 1997;41:1004–1009. doi: 10.1128/aac.41.5.1004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.de Boer P, Crossley R, Rothfield L. The essential bacterial cell-division protein FtsZ is a GTPase. Nature. 1992;359:254–256. doi: 10.1038/359254a0. [DOI] [PubMed] [Google Scholar]
- 7.de Pereda J M, Leynadier D, Evangelio J A, Chacon P, Andreu J M. Tubulin secondary structure analysis, limited proteolysis sites, and homology to FtsZ. Biochemistry. 1996;35:14203–14215. doi: 10.1021/bi961357b. [DOI] [PubMed] [Google Scholar]
- 8.Ellis K J, Morrison J F. Buffers of constant ionic strength for studying pH-dependent processes. Methods Enzymol. 1982;87:405–426. doi: 10.1016/s0076-6879(82)87025-0. [DOI] [PubMed] [Google Scholar]
- 9.Erickson H P. FtsZ, a prokaryotic homolog of tubulin? Cell. 1995;80:367–370. doi: 10.1016/0092-8674(95)90486-7. [DOI] [PubMed] [Google Scholar]
- 10.Erickson H P, Stoffler D. Protofilaments and rings, two conformations of the tubulin family conserved from bacterial FtsZ to α/β- and γ-tubulin. J Cell Biol. 1996;135:5–8. doi: 10.1083/jcb.135.1.5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hamel E. Potent new antimitotic natural products from marine animals which act in the vinca domain of tubulin. Cell Pharmacol. 1993;1(Suppl. 1):S47–S52. [Google Scholar]
- 12.Hamel E. Antimitotic natural products and their interaction with tubulin. Med Res Rev. 1996;16:207–231. doi: 10.1002/(SICI)1098-1128(199603)16:2<207::AID-MED4>3.0.CO;2-4. [DOI] [PubMed] [Google Scholar]
- 13.Ireland C M, Gull K, Gutteridge W E, Pogson C I. The interaction of benzimidazole carbamates with mammalian microtubule protein. Biochem Pharmacol. 1979;28:2680–2682. doi: 10.1016/0006-2952(79)90049-2. [DOI] [PubMed] [Google Scholar]
- 14.Lacey E, Watson T R. Activity of benzimidazole carbamates against L1210 mouse leukemia cells: correlation with in vitro tubulin polymerization assay. Biochem Pharmacol. 1985;34:3603–3605. doi: 10.1016/0006-2952(85)90742-7. [DOI] [PubMed] [Google Scholar]
- 15.Laemmli U K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970;227:680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- 16.Löwe J. Crystal structure determination of FtsZ from Methanococcus jannaschii. J Struct Biol. 1998;124:235–243. doi: 10.1006/jsbi.1998.4041. [DOI] [PubMed] [Google Scholar]
- 17.Löwe J, Amos L A. Crystal structure of the bacterial cell-division protein FtsZ. Nature. 1998;391:203–206. doi: 10.1038/34472. [DOI] [PubMed] [Google Scholar]
- 18.Löwe J, Amos L A. Tubulin-like protofilaments in Ca2+-induced FtsZ sheets. EMBO J. 1999;18:2364–2371. doi: 10.1093/emboj/18.9.2364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lu C, Reedy M, Erickson H P. Straight and curved conformations of FtsZ are regulated by GTP hydrolysis. J Bacteriol. 2000;182:164–170. doi: 10.1128/jb.182.1.164-170.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lutkenhaus J, Addinall S G. Bacterial cell division and the Z ring. Annu Rev Biochem. 1997;66:93–116. doi: 10.1146/annurev.biochem.66.1.93. [DOI] [PubMed] [Google Scholar]
- 21.Mukherjee A, Lutkenhaus J. Dynamic assembly of FtsZ regulated by GTP hydrolysis. EMBO J. 1998;17:462–469. doi: 10.1093/emboj/17.2.462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mukherjee A, Lutkenhaus J. Analysis of FtsZ assembly by light scattering and determination of the role of divalent metal cations. J Bacteriol. 1999;181:823–832. doi: 10.1128/jb.181.3.823-832.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mukherjee A, Dai K, Lutkenhaus J. Escherichia coli cell division protein FtsZ is a guanine nucleotide binding protein. Proc Natl Acad Sci USA. 1993;90:1053–1057. doi: 10.1073/pnas.90.3.1053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Nogales E, Downing K H, Amos L A, Löwe J. Tubulin and FtsZ form a distinct family of GTPases. Nat Struct Biol. 1998;5:451–458. doi: 10.1038/nsb0698-451. [DOI] [PubMed] [Google Scholar]
- 25.Ohashi Y, Chijiiwa Y, Suzuki K, Takahashi K, Nanamiya H, Sato T, Hosoya Y, Ochi K, Kawamura F. The lethal effect of a benzamide derivative, 3-methoxybenzamide, can be suppressed by mutations within a cell division gene, ftsZ, in Bacillus subtilis. J Bacteriol. 1999;181:1348–1351. doi: 10.1128/jb.181.4.1348-1351.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Rothfield L I, Justice S S. Bacterial cell division: the cycle of the ring. Cell. 1997;88:581–584. doi: 10.1016/s0092-8674(00)81899-1. [DOI] [PubMed] [Google Scholar]
- 27.Schmer G. The purification of bovine thrombin by affinity-chromatography on benzamidine-agarose. Hoppe-Seyler's Z Physiol Chem. 1972;353:810–814. doi: 10.1515/bchm2.1972.353.1.810. [DOI] [PubMed] [Google Scholar]
- 28.Shi Q, Chen K, Morris-Natschke S L, Lee K-H. Recent progress in the development of tubulin inhibitors as antimitotic antitumor agents. Curr Pharm Des. 1998;4:219–248. [PubMed] [Google Scholar]
- 29.Sossong T M, Jr, Brigham-Burke M R, Hensley P, Pearce K H., Jr Self-activation of guanosine triphosphatase activity by oligomerization of the bacterial cell division protein FtsZ. Biochemistry. 1999;38:14843–14850. doi: 10.1021/bi990917e. [DOI] [PubMed] [Google Scholar]
- 30.Temple C, Jr, Rener G A. Antimitotic agents: ring analogues and derivatives of ethyl[(S)-5-amino-1,2-dihydro-2-methyl-3-phenylpyrido[3,4-b]pyrazin-7-yl]carbamate. J Med Chem. 1992;35:4809–4812. doi: 10.1021/jm00104a005. [DOI] [PubMed] [Google Scholar]
- 31.Wang X, Lutkenhaus J. The FtsZ protein of Bacillus subtilis is localized at the division site and has GTPase activity that is dependent upon FtsZ concentration. Mol Microbiol. 1993;9:435–442. doi: 10.1111/j.1365-2958.1993.tb01705.x. [DOI] [PubMed] [Google Scholar]
- 32.Yu X C, Margolin W. Ca2+-mediated GTP-dependent dynamic assembly of bacterial cell division protein FtsZ into asters and polymer networks in vitro. EMBO J. 1997;16:5455–5463. doi: 10.1093/emboj/16.17.5455. [DOI] [PMC free article] [PubMed] [Google Scholar]