Abstract
Background and purpose
Diagnostics and treatment of developmental dysplasia of the hip (DDH) are highly variable in clinical practice. To obtain more uniform and evidence-based treatment pathways, we developed the ‘Dutch guideline for DDH in children < 1 year’. This study describes recommendations for unstable and decentered hips.
Materials and methods
The Appraisal of Guidelines for Research and Evaluation criteria (AGREE II) were applied. A systematic literature review was performed for six predefined guideline questions. Recommendations were developed, based on literature findings, as well as harms/benefits, patient/parent preferences, and costs (GRADE).
Results
The systematic literature search resulted in 843 articles and 11 were included. Final guideline recommendations are (i) Pavlik harness is the preferred first step in the treatment of (sub) luxated hips; (ii) follow-up with ultrasound at 3–4 and 6–8 weeks; (iii) if no centered and stable hip after 6–8 weeks is present, closed reduction is indicated; (iv) if reduction is restricted by limited hip abduction, adductor tenotomy is indicated; (v) in case of open reduction, the anterior, anterolateral, or medial approach is advised, with the choice based on surgical preference and experience; (vi) after reduction (closed/open), a spica cast is advised for 12 weeks, followed by an abduction device in case of residual dysplasia.
Interpretation
This study presents recommendations on the treatment of decentered DDH, based on the available literature and expert consensus, as Part 2 of the first official and national evidence-based ‘Guideline for DDH in children < 1 year’. Part 1 describes the guideline sections on centered DDH in a separate article.
Keywords: DDH, hip dysplasia, guideline, review, treatment
Introduction
Developmental dysplasia of the hip (DDH) has a reported incidence of 3–4% in children under the age of 6 months. Actual dislocations are reported in 0.1–0.2% (1). If undiagnosed or untreated, consequences can be severe, including pain and severe disabilities in the activities of daily living due to early onset osteoarthritis or a dislocated hip (2). Nevertheless, diagnostic and treatment methods are highly variable in clinical practice and often depend on local agreements or protocols. To obtain more uniform and evidence-based treatment pathways for DDH, the Dutch Orthopaedic Society (NOV) developed the ‘Guideline for DDH in children under the age of 1 year’ in cooperation with the Dutch Knowledge Institute of Medical Specialists (KiMS) and delegates of several related medical specialties and the Dutch hip patient association. Part 1 of the guideline describes the diagnostics and treatment of stable and centered DDH (3). Part 2, as described in the current article, focuses on decentered hips.
In the current clinical practice in the Netherlands, there is selective DDH screening (i.e. ultrasound of the hips in case of risk factors or clinical abnormalities). Generally, the first step of treatment is an abduction device for all types of DDH, including centered and decentered hips. In case of a persisting decentered hip, closed or open reduction and a spica cast are applied. However, for example, type and duration of abduction device and spica cast treatment are variable, and many questions on clinical decision-making remain unanswered.
For this Part 2 of the guideline, we investigated key issues in the treatment of patients diagnosed with DDH (Graf D/III/IV) under the age of 1 year, with regard to the type of abduction device, traction and/or adductor tenotomy, surgical approach for open reduction, spica cast treatment, and follow-up after reduction.
Materials and methods
Guideline development
The Pediatric Orthopedic Society of the NOV initiated the process of guideline development in December 2018, in cooperation with the Knowledge Institute of the Federation of Medical Specialists (KiMS). A Guideline Committee was composed, including a board member of the Dutch hip patient association, seven pediatric orthopedic surgeons (including an epidemiologist), two methodologists of the KiMS, a radiologist, and a youth health care physician.
The guideline was developed for all providers of treatment for children with DDH under the age of 1 year in the Netherlands. The first aim was to improve and unify the care for these children with evidence-based medicine. Secondly, the guideline provides uniform and comprehensive information on patients, patient groups, parents, and caregivers. Thirdly, the guideline identifies knowledge gaps that are relevant subjects for future research projects.
Methodology and workflow
The guideline was developed based on the international Appraisal of Guidelines for Research & Evaluation II (AGREE II) instrument (4). The approach and methodology of guideline development are similar to previously published Dutch guidelines (5, 6, 7, 8), including another pediatric guideline, reporting on clubfeet (9). This approach is described in short in the following paragraphs. For a more elaborate description, we refer to the clubfoot paper.
The guideline development process had the following phases: a preparative phase, a development phase, a commentary phase, and an authorization phase. During all phases, the Guideline Committee had meetings on a regular basis. Decisions and final recommendations were made by consensus, and all members of the committee agreed on the final contents of the guideline.
Guideline questions
During preparative phase, key issues were discussed and prioritized in cooperation with relevant stakeholders, including patient societies. Using these key issues, guideline questions were formulated in the patient, intervention, comparison, and outcomes format (PICO) (10), with regard to patients with Graf D/III/IV DDH under the age of 1 year:
What are the outcomes of a Pavlik harness compared to other abduction devices with regard to successful reduction, residual dysplasia, and complications?
In case of unsuccessful treatment with an abduction device: If closed reduction in DDH is restricted by limited hip abduction, is traction or adductor tenotomy preferable to facilitate reduction?
In case of unsuccessful closed reduction: What are the (un)favorable effects of a surgical reduction through a medial or anterior approach, compared to other surgical approaches of the hip?
After successful reduction (closed or open): What are the (un)favorable effects of a short period of spica cast treatment compared to a longer period?
After successful reduction (closed or open): What is the preferable method of diagnostic assessment during follow-up in spica cast?
After successful reduction (closed or open): What are the (un)favorable effects of spica cast treatment followed by an abduction device, compared to spica cast treatment without a subsequent abduction device?
Relevant outcome measures
The Guideline Committee considered the following outcome as critical for decision-making: successful and maintained reduction.
Important outcomes for decision-making were residual dysplasia in follow-up as defined in the included studies (expressed in, e.g., acetabular index (AI) (11) or Severin classification) (12), complications (including avascular necrosis (AVN) and neuropathy), and secondary procedures. Additionally, with regards to surgery (PICO research question 3), blood loss and operative time were considered important outcomes.
Search strategy and study selection
In the development phase, a systematic literature search was performed for each PICO guideline question, using the databases MEDLINE (Ovid) and Embase (Elsevier). Detailed search strategies are depicted in Supplementary Appendix 1 (see section on supplementary materials given at the end of this article).
For each PICO, relevant literature was selected by two members of the Guideline Committee after screening of titles, abstracts, and full texts, based on the following inclusion criteria: (i) comparative study; (ii) patients younger than 1 year with Graf D/III/IV DDH; (iii) at least one of the selected critical or important outcome measures reported. Language was limited to English and Dutch. Reference lists of included articles were cross-checked for additional relevant articles.
Formulation of literature conclusions and guideline recommendations
The available scientific evidence after study selection was recapitulated in literature conclusions. For each conclusion, the level of evidence was assigned using the grading of recommendations assessment, development, and evaluation (GRADE) method (13). Guideline recommendations were formulated using the literature conclusions, as well as considerations with regard to for example, patients’ and parents’ values and preferences, organizational issues, and costs and potential harm of treatments.
Commentary phase and authorization
After formulation of the recommendations, the guideline was edited and finalized by all members of the Guideline Committee. For the commentary phase, this concept guideline was presented to 19 relevant Dutch societies involved in the care of children with DDH. The Guideline Committee discussed submitted comments and altered the guideline where relevant. The final version was presented to the involved societies and was formally authorized on January 11, 2021 (https://richtlijnendatabase.nl/richtlijn/ddh_dysplastische_heupontwikkeling_bij_kinderen_onder_n_jaar/startpagina_-_ddh.html#tab-content-general).
Results
For each PICO, results and literature conclusions are described below. Results are summarized in Table 1 and literature conclusions and recommendations in Table 2.
Table 1.
Summary of included studies.
| Reference/intervention | Study type | Number of | Follow-up | Outcomes | |||||
|---|---|---|---|---|---|---|---|---|---|
| Patients | Hips | Graf D/II/IV) | Duration | Loss to follow-up | Primary | Secondary | Complications | ||
| PICO 1: Pavlik harness vs other abduction devices | |||||||||
| Zidka et al. (14) | RCCS | ||||||||
| Pavlik harness | 137 | 48 | 119 days | 16 patients (14%) | Graf I ultrasound: 100% | ||||
| Frejka pillow | 145 | 26 | 95 days | 7 patients (5%) | Graf I ultrasound: 100% | ||||
| Wilkinson et al. (15) | RCCS | 6–12 months | 0% | None | |||||
| (i) Craig splint | 28 | Spica cast: 3 hips (10.7%); Operation: 1 hip (3.6%) |
|||||||
| (ii) Pavlik harness | 43 | Spica cast: 10 hips (23.3%); Operation: 3 hips (7%) |
|||||||
| (iii) Von Rosen splint | 26 | Spica cast: 0; Operation: 0 |
|||||||
| No splint | 37 | Spica cast: 8 hips (21.6%); Operation: 2 hips (5.4%) |
|||||||
| Atar et al. (16) | RCCS | ||||||||
| Pavlik harness | 40 | 48 | 1.8 years (1–5) | 0% | Successful reduction: 42/48 (88%) | AVN 3/48 (6%) | |||
| Frejka splint | 70 | 84 | 1.5 (1–4) 0% | 0% | Successful reduction: 76/84 (90%) | AVN 6/84 (7%) | |||
| PICO 2: Closed reduction restricted by limited hip abduction – traction vs adductor tenotomy | |||||||||
| Carney et al. (17) | RCRS | ||||||||
| Traction | 2 | 91 months (24–163) | N/A | Successful reduction: 100% | Residual dysplasia 2/2 (100%) |
AVN 1/2 (50%) | |||
| Adductor longus tenotomy | 8 | Successful reduction: 100% | Residual dysplasia 6/8 (75%) |
AVN 2/8 (25%) | |||||
| Closed reduction without traction or tenotomy | 5 | Successful reduction: 100% | Residual dysplasia 5/5 (100%) |
AVN 3/9 (33%) | |||||
| Both traction and tenotomy | 5 | Successful reduction: 100% | Residual dysplasia 2/5 (40%) |
AVN 2/5 (40%) | |||||
| PICO 3: Unsuccessful closed reduction – surgical reduction through a medial or anterior approach vs other surgical approaches | |||||||||
| Duman et al. (18) | RCCS | AVN and femoral neuropathy | |||||||
| Arthroscopic-assisted | 26 | 26 | 24 months (24–30) | 4 | Successful reduction: 26/26; Successful functional outcome (MacKay score): 18 (81.8%); AI: 27° (19–36); | Blood loss: 9 mL (5–15); Operative time: 32 min (30–40) | 0 | ||
| Medial approach (Ludloff) | 28 | 28 | Successful reduction: 27/28; Successful functional outcome (MacKay score): 17 (80.9%); AI: 26° (11–39); | Blood loss: 35 mL (15–55); Operative time: 34 min (30–40) | 0 | ||||
| Yorgancigil et al. (19) | RCCS | Successful functional outcome (MacKay)(P = 0.23); AI postoperative P = 0.226 |
Revision surgery (P = 0.170) | AVN and femoral neuropathy (P= 0.933) | |||||
| Anterior approach | 17 | 22 | 84.0 ± 29.5 months | NR | 18 hips (81.8%); AI postoperative: 21.23° ± 3.70 | 4 hips (18.1%) | 5 hips (22.7%) | ||
| Medial approach | 19 | 21 | 75.2 ± 19.6 months | NR | 17 hips (80.9%); AI postoperative: 21.86° ± 3.93 | 3 hips (14.3%) | 5 hips (23.8%) | ||
| Hoellwarth et al. (20) | RCCS | AI, mean (P = 0.23) | Revision surgery (P = 0.48) | AVN and femoral neuropathy), P= 0.32 |
|||||
| Anterior approach | 18 | 19 | 6.2 ± 3.2 months | NR; Incomplete data: 22 | 17° (6–25) | 7 hips (37%) | 10 hips (53%) | ||
| Medially based approach | 14 | 19 | 6.1 ± 2.8 months | NR; Incomplete data: 2 | 19° (11–33) | 4 hips (21%) | 6 hips (32%) | ||
| Holman et al. (21) | RCCS | Successful reduction | AVN, femoral neuropathy and osteonecrosis | ||||||
| Anterior approach | 141 for surgery; 48 in follow-up study | 27 years (13–54) | 96 hips | 9 (19%) re-dislocations | 20 hips (42%) | 9 hips (18.8%) | |||
| Medial approach Ludloff | 38 for surgery; 18 in follow-up study | 20 hips | 2 (11%) re-dislocations | 9 hips (50 %) | 1 hip (6 %) | ||||
| Tarasolli et al. (22) | PCS | Successful reduction | Acetabular index (P= 0.18) | AVN, femoral neuropathy, and osteonecrosis (P = 0.52) | |||||
| Anterior approach | 21 | 22 | 61 months (28–100) | 1 | 0 re-dislocations | Absolute mean: 24° (15°–34°)Mean decrease: 7.9° (6°–10°) | 4 (18%) | ||
| Medial approach | 22 | 26 | 77 months (26–228) | 0 | 1 (4%) re-dislocations | Absolute mean: 25° (16°–35°)Mean decrease: 8.8° (4°–12°) | 3 (12%) | ||
| Matsushita et al. (23) | RCCS | 11–14 months | NR | Successful functional outcome (MacKay), | Due to (sub)luxation | AVN, femoral neuropathy, and osteonecrosis | |||
| Wide exposure method | 27 | 32 | 24 (77.4%); Residual dysplasia: 4 hips (12.9%) class III, 1 hip (3.2%) class IV |
0 | 1 hip (3.2%) | ||||
| Medial approach | 24 | 31 | 24 (75%); Residual dysplasia: 11 hips (34.4%) class III, 3 hips (9.4%) class IV; P<0.05 |
10 hips (31.3%) | 7 hips (21.9%) | ||||
| PICO 4: After successful surgical reduction (closed or open) – short period of spica cast treatment compared vs longer period | |||||||||
| Emara et al. (26) | RCCS | ±12 years | NR | Clinical assessments according to McKay criteria (P = 0.612) | Radiographic assessment according to Severin classification (P = 0.449) | ||||
| Spica removed after 4 weeks followed by abduction brace | 32 | 38 | E = 81.6%, G= 18.4% | Ia= 81.6%, Ib =15.8% | AVN: 15.8%; (P = 0.015); Other: 0 hips | ||||
| Spica removed after 12 weeks, then started ambulation without brace | 24 | 29 | E = 86.2%, G= 13.8% | Ia = 69%, Ib = 24.1%, II = 6.9% | AVN: 48.3%; Other: 5 hips; (P = 0.029) | ||||
AI, acetabular index; PCS, prospective cohort study; RCCS, retrospective comparative cohort study; RCRS, retrospective chart review study.
Table 2.
Conclusions and recommendations.
| Guideline question | Conclusions | Recommendations1 | GRADE |
|---|---|---|---|
| (i) Reduction with Pavlik harness vs other abduction devices | There were no significant differences reported comparing the Pavlik harness to the Frejka pillow, Craig splint, and Von Rosen splint, with regard to successful reduction (around 90%), complication rates, secondary procedures, and residual dysplasia. (Zidka et al. 2019, Wilkinson et al. 2002, Atar et al. 1993). The great majority of literature on abduction devices is on the Pavlik harness, and it is by far the most applied device in the Netherlands. It can be applied for all grades of DDH. |
Use the Pavlik Harness as the first step in treatment for (sub)luxated DDH hips in babies under the age of 1 year. Follow-up with ultrasound is recommended at 3–4 and 6–8 weeks. |
Very low1 |
| (ii) Unsuccessful Pavlik treatment → closed reduction restricted by limited hip abduction – traction vs adductor tenotomy | In 1 comparative study, no significant differences were reported for successful reduction, residual dysplasia, secondary procedures, AVN, or other complications. (Carney et al. 2005). In 4 non-comparative studies, 39–79% of patients still had an indication for adductor tenotomy after traction (Brougham et al. 1990, Burgos-Flores et al. 1993, Forlin et al. 1992, Gogus et al. 1997). |
Perform adductor tenotomy, and not traction, if closed reduction is restricted by limited hip abduction. | Very low1 |
| (iii) Unsuccessful closed reduction → surgical reduction through a medial or anterior approach vs other surgical approaches | No significant differences were reported for successful reduction, functional outcome, secondary procedures, AVN or other complications, blood loss, and operative time between approaches. (Duman et al. 2019, Yorgancigil et al. 2016, Hoelwarth et al. 2015, Holman et al. 2012, Tarasolli et al. 2014, Matsushita et al. 1999). The scientific evidence and general clinical experience for arthroscopic procedures or the wide-exposure method are highly limited, in contrast to the anterior and medial approaches. |
Use either the anterior, anterolateral, or medial approach, based on surgical preference and experience. | Very low1
|
| (iv) After successful surgical reduction (closed or open): short period of spica cast treatment compared vs longer period |
No significant differences were found with regard to to successful reduction and residual dysplasia (Emara et al. 2019) comparing 4 weeks of spica cast and a 11–13 months weaning regime with an abduction splint, compared to a spica cast for 12 weeks. | The recommended duration of spica cast treatment after closed or open reduction is 12 weeks. | Very low1 |
| (v) Preferable method of diagnostic assessment during follow-up in spica cast | No comparative studies were found. | Use transinguinal ultrasound for the evaluation of the hip after reduction and during follow-up in spica cast. When not available, an arthrogram is advisable after reduction, followed by standard radiographs during follow-up. When in doubt, MRI or low-dose CT can be applied. |
Not applicable |
| (vi) Subsequent abduction device after spica cast treatment – yes or no | No comparative studies were found. | (Additional) Treatment with an abduction device after spica cast treatment is advised under the age of 1 year old, in cases with severe residual dysplasia. | Not applicable |
Recommendations are based on the literature conclusions, as well as the clinical considerations as described in the text.
1GRADE Level of evidence was downgraded by one level because of study limitations, including bias by indication, no adjustment for confounding, or low numbers of patients (imprecision).
DDH, developmental dysplasia of the hip; GRADE, grading recommendations assessment, development, and evaluation (Guyatt et al. 2008).
Guideline question 1: Pavlik harness vs other abduction devices
A systematic literature search on April 25, 2019, resulted in 132 hits and 20 studies were initially selected by title and abstract screening. After reading the full texts, 3 were included.
Zidka et al. published a retrospective study, comparing outcomes in 286 children with DDH, treated with either a Frejka pillow (n = 145; 26 Graf D/III/IV) or a Pavlik harness (n = 137; 48 Graf D/III/IV), with a follow-up of 4–34 weeks (14). The Frejka pillow was used as the preferred device in milder dysplastic hips, while unstable and decentered hips were treated more frequently with the Pavlik harness.
Wilkinson et al. retrospectively compared the effectiveness of several abduction devices in 96 babies (134 hips) with Graf III or IV DDH, with a mean follow-up of 6–12 months (15). Twenty-eight hips were treated with the Craig splint (28 Graf III), 43 with the Pavlik harness (40 Graf III and 3 Graf IV), and 26 with the Von Rosen splint (24 Graf III and 2 Graf IV). In 28 children (37 hips), no abduction device was applied.
In a retrospective study by Atar et al., the Frejka splint and Pavlik harness were compared in patients with dislocated hips (16). Atar included 110 babies (73 girls, 132 hips). Eighty-four hips were treated with the Frejka splint and 48 with the Pavlik harness. Average duration of treatment was 3.8 months (range, 3–9) with the Frejka splint and 4.5 months (range, 3–8) with the Pavlik. Mean follow-up was 1.5–1.8 years (range, 1–5) in both groups.
None of these studies accounted for confounding variables, despite, for example, differences in age and/or DDH classification between treatment groups.
Successful reduction was achieved in 88–100% of children in these studies. None of the studies found a significant difference for successful reduction between the studied abduction devices.
Residual dysplasiaand AI were not investigated in the included studies.
Secondary procedureswere not reported in the studies by Zídka and Atar. Wilkonson et al. reported 1% secondary procedures in the Craig splint group, 3% in the Pavlik group, 0% in the Von Rosen splint group, and 2% in the group without abduction device. No statistical analysis was performed.
Complicationswere not reported in the study by Zídka. In the study by Wilkinson, no complications were observed. Atar et al. reported AVN in 6 of 84 (7%) hips of children treated with a Frejka splint and 3 of 48 (6%) hips in the Pavlik group. No statistical analysis was performed.
Guideline question 2: closed reduction restricted by limited hip abduction – traction vs adductor tenotomy
A systematic literature search, on April 25, 2019, on comparisons of the two treatments, resulted in 41 hits and 10 studies were initially selected based on title and abstract. With full-text evaluation, only one study met the inclusion criteria.
Carney et al. performed a retrospective study to investigate the association between age and the presence of the ossific nucleus of the femoral head at the time of closed reduction and the incidence of acetabular dysplasia and AVN in follow-up (17). In total, 24 patients were younger than 1 year at the time of closed reduction: 9 had a closed reduction without traction or tenotomy, 2 had only traction, 8 only tenotomy, and 5 children had both interventions. Follow-up ranged from 28 to 163 months.
Successful reductionwas achieved in all 24 children under the age of 1 year
Residual dysplasia was reported in both children who had traction (100%), in 6 after adductor tenotomy (75%), in 2 who had both traction and tenotomy (40%), and in all children who had undergone no adductor intervention (100%).
Secondary procedureswere reported for persisting residual dysplasia in the follow-up of 6 patients. However, there was no description of whether this concerned children < 1 year at the time of closed reduction or whether they had undergone tenotomy and/or traction.
Avascular necrosis (AVN) occurred in one of two children who had traction (50%), in two of eight after tenotomy (25%), in two of five after both traction and adductor tenotomy (40%) and in three of nine children who had no adductor intervention (33%).
Other complicationsbesides AVN were not reported.
Guideline question 3: unsuccessful closed reduction – surgical reduction through a medial or anterior approach vs other surgical approaches
A systematic literature search, on July 22, 2019, on the medial approach vs other approaches and the anterior/anterolateral approach vs other approaches, resulted in 347 hits. After screening of titles and abstracts, 19 studies were selected, of which 6 met the inclusion criteria after full-text evaluation.
Duman performed a retrospective comparative cohort study in patients < 18 months of age with DDH, comparing arthroscopic-assisted vs medial approach (Ludloff) open reduction (18). In total, 26 patients (26 hips; median age: 12 months (range: 7–17)) were included in the arthroscopic group and 28 (28 hips; median age: 11 months (range: 6–17)) in the medial approach group. Minimum follow-up was 24 months.
Yorgancigil performed a retrospective comparative cohort study in children undergoing open reduction via anterior (Smith–Petersen) or medial approach (Ferguson), after failed closed reduction (19). Seventeen patients (22 hips) had an anterior and 19 (21 hips) a medial approach. Mean age in both groups was 14.6 (s.d. = 2.6) and 13.0 months (s.d. = 2.9), respectively. Minimal follow-up was until the age of 5 years.
Hoellwarth performed a retrospective comparative study comparing aged-matched DDH cohorts undergoing either medial approach (interval not specified) or anterior approach open reduction (Smith–Petersen) (20). Eighteen patients (19 hips) had an anterior and 14 (19 hips) a medial approach. Mean age in the anterior group was 6.1 months (s.d. = 3.2) and 5.9 (s.d. = 2.7) in the medial group. Minimal follow-up was 2 years. A multivariable Cox regression analysis was performed to compare the outcomes in both groups while accounting for possible confounding factors.
Holman (2012) performed a retrospective cohort study on children with DDH and open reduction (21). The anterior (surgical interval not reported) was compared with the medial approach (Ludloff) open reduction. Fifty-three patients (66 hips) participated in the study (anterior: 48 hips; medial: 18 hips). Mean age in the anterior and medial groups at time of surgery was 2.9 years (range: 0.3–8.1) and 1.4 years (range: 0.4–3.5), respectively. Mean follow-up was 25 years (range: 14–35).
Two additional studies were included that not completely satisfied our inclusion criteria because a substantial part of the study population was >1 year. The guideline development group nevertheless decided to include these studies because of their clinical relevance and lack of alternative available literature.
Tarasolli (2014) performed a prospective cohort study on children with DDH in whom closed reduction had failed (22). The medial approach (Ludloff) and the anterior approach (Smith-Petersen) were compared. Totally 21 children (22 hips) were included in the anterior group and 22 (26 hips) in the medial group. Mean age in the anterior and medial group was 18 (range: 12–24) and 11 months (range: 3–24), respectively. Mean follow-up was 70 months (range: 26–228).
Matsushita (1999) performed a retrospective cohort study in children with congenital hip dislocation (23), to compare the wide exposure method (circumferential capsulotomy, including the release of the gluteus medius and minimus, and short external rotators), with the medial approach (Ludloff). Twenty-seven patients (32 hips) had the wide exposure method and 24 (31 hips) had the medial approach. Mean age at surgery was 18 months (range, 12–31) and 12 months (range, 5–30), respectively. Mean follow-up was 16 years.
No variables were taken into consideration to adjust for possible confounding factors in the studies of Duman, Yorgancigil, Holman, Tarasolli, and Matsushita.
Successful reductionwas assessed in three studies comparing the anterior, medial, and/or arthroscopic approach (18, 21, 22). Re-dislocations were reported in various treatment groups, ranging between 0 and 19%, without reported statistical differences.
Functional outcome was described in three studies comparing the anterior, medial, wide, and/or arthroscopic approach (18, 19, 23). All used the Mac-Kay criteria, which take into account hip stability, pain, stiffness, and walking pattern (24). Excellent scores were reported in 62–82%, without statistical differences between groups.
Residual dysplasiawas analyzed in five papers (18, 19, 20, 22, 23), comparing all types of the mentioned approaches, with various durations of follow-up. In four studies, dysplasia was quantified with AI and in one study, with the Severin Classification (25). With regard to AI, no differences were found between groups. For the Severin classification (III or IV), Matshushita reported 21% residual dysplasia in the wide exposure group, vs 52% in the medial approach group (P < 0.05).
Secondary procedures for residual dysplasia were reported in two studies (21, 23). Re-intervention rates varied between 0 and 50%. No statistically significant differences were described between the medial, anterior, and wide exposure approaches.
Secondary procedures for other indicationswere described in 14–37% of patients in two studies (19, 20), without information on the indications for these surgeries. No statistically significant differences were described between the medial and anterior approaches in these studies.
Blood loss was assessed in the study by Duman et al. (18), reporting higher blood loss in patients with a medial approach, compared to arthroscopic assisted reduction: a median of 35 (range: 15–55) vs 9 (range: 5–15) mL. No statistic investigations were performed.
Duman et al. also assessed operative time (18). They reported no differences between operative time for medial approach vs arthroscopic-assisted reduction: a median of 34 (range, 30–40) vs 32 (range, 30–40) min. There was no statistic evaluation.
Complications (including AVN)were reported in 3–53% in five studies comparing all types of included approaches (18, 19, 20, 21, 22). No statistically significant differences were described between approaches.
Guideline question 4: after successful surgical reduction (closed or open) – short period of spica cast treatment vs longer period
A systematic literature search, on May 13, 2019, on the duration of spica cast treatment after reduction, resulted in 191 hits and 28 studies were initially selected based on title and abstract screening. After reading the full texts, only one comparative study could be included. In this study, a significant portion of patients was >1-year-old. However, the Guideline Committee decided to include this study as the best available evidence.
Emara et al. performed a retrospective cohort study, comparing two groups of DDH patients with different durations of hip spica cast immobilization after open reduction (anterior approach) (26). The intervention consisted of removing the spica cast after 4 weeks, followed by an abduction device for 18 h/day for 1 month, 14 h/day for 1 month, 10 h/day for 1 month, and lastly 8 h/day for 8–10 months. In the control group, the spica cast was removed after 12 weeks, that is without weaning. A total of 32 patients (38 hips) were included in the intervention group and 24 (29 hips) in the control group. Mean age of both groups was 16 months. Follow-up was around 12 years in both groups, respectively. No variables were taken into consideration to adjust for possible confounding factors.
Successful reduction (no re-dislocation) was 100% in both groups. No re-dislocations were reported.
Residual dysplasia was classified using the Severin classification (25). In the intervention group, 82% were regarded as class Ia (normal) and 18% class Ib (normal). In the control group, 69% had class Ia, 24% class Ib, and 7% class II (moderate deformity of femoral head). These differences were not statistically significant (P = 0.449). There were no children classified with residual dysplasia (Severin classification > III).
Complicationsandsecondary procedures were not reported.
Guideline question 5: method of diagnostics during follow-up in spica cast
On October 15, 2019, we performed a systematic literature search on diagnostics during spica cast treatment, resulting in 132 hits and 11 studies were initially selected with title and abstract screening. After full text evaluation, no studies could be included.
Guideline question 6: subsequent abduction device after spica cast treatment
The systematic literature search on May 13, 2019, resulted in no comparative studies assessing an abduction device after spica cast treatment. However, the above-mentioned study by Emara et al. did compare abduction device treatment after spica cast in two groups, but, in this study, the duration of spica cast treatment varied between groups as well (26). And as reported, no significant differences were found in results between both groups, with regard to re-dislocations, residual dysplasia, and other predefined relevant outcomes.
Discussion
We present a summary of the Dutch national guideline on the diagnosis and treatment of DDH, with the focus on decentered hips (Graf D/III/IV) in this specific article (Part 2). For Part 1, on centered hips, we refer to the article of Van Bergen et al. (3). To the best of our knowledge, this is the first official and national evidence-based guideline on the treatment of DDH. It was developed by members of the pediatric society of the NOV, with the support of the Knowledge Institute of the Federation of Medical Specialists and in co-operation with specialists from several relevant backgrounds, including orthopedic surgery, youth health care, radiology, and the Dutch hip patient association. The goal of Part 2 of this guideline is to use evidence-based medicine to improve and unify the care for children with DDH (Graf D/III/IV) under the age of 1 year, with regard to the type of abduction device, traction and/or adductor tenotomy, approach for surgical reductions, follow-up, and duration of spica cast treatment after surgical reduction.
For guideline question 1, we evaluated the results of several abduction devices, which is generally the first step in the treatment of decentered hips in babies. With the legs in ‘the human position’ (90–100° of hip flexion and comfortable abduction), reduction and centralization of the hip can be obtained (27). Based on the three included studies, no clear recommendation can be given on which type of abduction device to apply. Successful reduction rates for all studied devices (Pavlik harness, Frejka pillow, Craig splint, and Von Rosen splint) are reported around 90%, without differences in complication rates, secondary procedures, and residual dysplasia. However, the great majority of literature on abduction devices is on the Pavlik harness, and it is by far the most applied device in the Netherlands. Additionally, it is suited for all types of DDH, specifically in the first months of life: for reductions of dislocations and for maintaining the centered hip, as well as for the treatment of DDH Graf 2 B/C. Consequently, the Guideline Committee recommends the Pavlik harness as the first step of treatment for decentered DDH in babies. Follow-up with ultrasound is recommended at 3–4 and 6–8 weeks, based on current clinical practice in the Netherlands, expert opinion, patient’s/parent’s burden, and to prevent Pavlik disease by prolonged Pavlik treatment of (sub)luxated hips. In case of no stable and centered hip within 6–8 weeks of Pavlik treatment, closed reduction is indicated.
Closed reduction can be restricted by limited abduction due to adductor tightness. For guideline question 2, the outcomes of traction and adductor tenotomy were investigated. Only one comparative study was found: Carney et al. found no differences for successful reduction, residual dysplasia, secondary procedures, AVN, or other complications. In four (non-comparative) studies that were additionally identified by the literature search, 91–100% were treated with traction (28, 29, 30, 31). In 39–79% of these patients, there was still an indication for adductor tenotomy after traction. Furthermore, traction requires days or even weeks of treatment, with additional burden on patient, caretakers, and the health care system, while adductor tenotomy can be performed in the operating theater in the same session as closed reduction and spica casting. Therefore, the Guideline Committee recommends adductor tenotomy, if closed reduction is restricted by limited hip abduction.
When closed reduction is unsuccessful, open reduction is indicated. For guideline question 3, the (un)favorable effects of available surgical approaches were compared. The medial approach according to Ludloff (surgical interval: adductor brevis and pectineus) was first described (1913) (32). Several variants of the medial approach exist, including the Ferguson approach (interval: adductor brevis and gracillis) (33) and the Weinstein and Ponseti approach (interval: pectineus and neurovascular bundle) (34). In the more recent literature, the anterior approach is upcoming. Generally, the anterior approach refers to the Smith–Petersen technique (interval: sartorius and tensor fascia lata) (35) or the Watson–Jones approach (anterolateral, surgical interval: gluteus medius and tensor fascia lata) (36). It is advocated that these anterior approaches offer better possibilities for capsule tightening and for preserving medial circumflex vessels, which in turn would lead to less (re)dislocations and AVN. Additionally, anterior approaches can be easily combined with peri-acetabular procedures in older children, when indicated. Lastly, arthroscopic approaches are emerging in the literature and clinical practice, possibly due to the increasing implementation in adult hip surgery. Overall, six studies were found, comparing the anterior/anterolateral and/or medial approach with other surgical approaches. No significant or clinically relevant differences were found for successful reduction, functional outcome, secondary procedures, AVN or other complications, blood loss, and operative time, except for residual dysplasia in the study by Matshushita (23). In this latter study, there was less residual dysplasia in the ‘wide exposure’ group, as compared to the medial approach group. However, the level of evidence in this study was low, as for the five other studies. Based on this review of comparative literature, no clear recommendation can be given on the type of surgical approach to use in these children. In practice, 73% of Dutch pediatric orthopedic surgeons use the anterior approach and 27% the medial approach (37). Furthermore, there is no evidence for the superiority of alternative approaches, including the wide exposure method and arthroscopic assisted procedures. Lastly, all reported approaches have more or less similar peri- and post-operative care regimes. The recommendation of the Guideline Committee is therefore to use the anterior, anterolateral, or medial approach, based on surgical preference and experience.
After closed or open reduction, a spica cast is indicated to maintain the reduction of the hip. In the Netherlands, the duration of spica cast treatment is generally 12 weeks in clinical practice. However, variations between institutions range from 6 to 16 weeks. For guideline question 4, we investigated the optimal duration of spica cast treatment and only one study was found. In a retrospective cohort study by Emara et al., two spica cast treatment regimes after reduction were compared: (i) spica cast for 4 weeks, followed by a weaning regime with an abduction device for 11–13 months and (ii) spica cast for 12 weeks. As the group with the short spica cast period had additional treatment with an abduction device for 8–10 months, it is complicated to draw conclusions on short vs longer duration of spica cast treatment. Also, no significant differences were found between both groups, with regard to successful reduction and residual dysplasia. Additionally, the level of evidence of the concerning study was low. Hence, based on the available literature on the duration of cast treatment and/or subsequent abduction device, no clear recommendation can be made. The Guideline Committee recommends to maintain 12 weeks as the standard duration of spica cast treatment after closed or open reduction. A shorter duration might increase the risk of, for example, re-dislocation or residual dysplasia, and a longer duration increases the burden for patient and parents.
During spica cast treatment, it is important to evaluate the position of the femoral head on a regular basis. For guideline question 5, we investigated the preferable method of imaging. Diagnostic methods vary in clinical practice, including (transinguinal) ultrasound, radiographs, low-dose CT, and arthrography. No comparative literature on these diagnostic follow-up methods was found. The preference of the Guideline Committee is to prevent unnecessary irradiation, costs, and burden on the child. The recommendation is to use transinguinal ultrasound for the evaluation of the hip directly after reduction and during follow-up in spica cast (33). When not available, use fluoroscopy directly after reduction, followed by standard radiographs during follow-up. When in doubt, use an arthrogram directly after reduction, or MRI or low-dose CT in follow-up, to assess the position of the femoral head in the hip joint.
Lastly, we studied the (un)favorable effects of spica cast treatment followed by an abduction device compared to spica cast treatment without a subsequent abduction device for guideline question 6. Only one study was found, assessing the use of an abduction device after spica cast treatment (26). As mentioned previously, this study by Emara et al. compares two groups, with variations in both abduction device application, as well as duration of spica cast treatment. No significant differences were found. The consensus of the Guideline Committee is that (additional) treatment with an abduction device is feasible (and possibly of added value) until the age of 1-year-old, in cases with severe residual dysplasia after spica cast treatment.
There are some strengths and weaknesses to take into account when interpreting our results. This is the first evidence-based national guideline on the diagnostics and treatment of DDH under the age of 1 year. The Guideline Committee had a multidisciplinary background, and the guideline was reviewed by 19 relevant Dutch societies involved in the care of children with DDH, including the patient association. Furthermore, the Guideline Committee followed the GRADE and AGREE methodologies and was supported by the NOV and the Dutch Knowledge Institute of Medical Specialists (KiMS). Additionally, recommendations that not only based on literature but also on non-scientific considerations were taken into account for each recommendation, including patients’ and parents’ values and preferences, costs, implementation, and organizational factors (see https://richtlijnendatabase.nl/richtlijn/ddh_dysplastische_heupontwikkeling_bij_kinderen_onder_n_jaar/startpagina_-_ddh.html). However, although we were able to make some clear recommendations for the treatment of children with DDH under the age of 1 year, there appears to be a lack of high-level evidence on DDH management. Furthermore, only comparative studies published in English or Dutch were included, which is the standard method for Dutch guideline development. As a consequence, possibly relevant case series were excluded. Also, the guideline is based on a (subjective) selected set of key issues. Consequently, there are still many knowledge gaps on DDH to investigate. And in the end, guideline recommendations, although based on the highest available level of evidence, are formulated in a consensus process among the committee members. This may leave room for bias. Lastly, the guideline is as strong as its supporting literature.
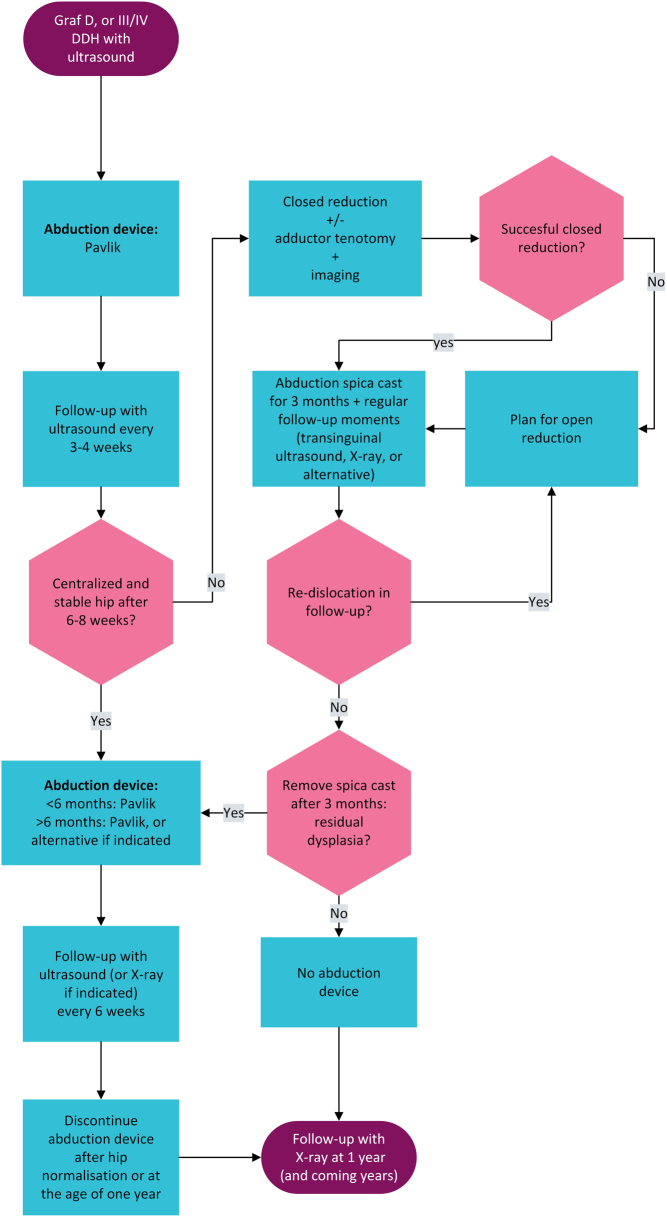
The recommendations as formulated in this clinical practice guideline resulted in the treatment flowchart presented in Fig. 1. The reported results and recommendations have been published and implemented in the Netherlands in 2021 and can not only be used in clinical decision-making but also for patient/parents/caregiver information. However, the availability of literature on DDH is very limited, specifically with regard to comparative studies. Additional large prospective and comparative studies are required to obtain more evidence on the treatment of DDH.
Figure 1.
Flowchart guiding treatment of unstable DDH.
Supplementary Material
ICMJE Conflict of Interest Statement
The authors declare that there is no conflict of interest that could be perceived as prejudicing the impartiality of the research reported.
Funding Statement
This work did not receive any specific grant from any funding agency in the public, commercial, or not-for-profit sector.
Author contribution statement
All authors were members of the guideline committee and approved the final version of the paper. In addition, P d W selected the studies, drafted the guideline, and wrote the paper, C v B commented on the drafts, F W and B d G analyzed the included studies, M F selected the studies, and M W supervised the guideline development.
References
- 1.Boere-Boonekamp MM.Screening for Developmental Dysplasia of the Hip: a Cohort Study to Evaluate the Screening Protocol for Early Detection of Developmental Dysplasia of the Hip in the Dutch Infant Health Care Programme. Enschede: Twente University, 1996. [Google Scholar]
- 2.Murphy SB, Ganz R, Muller ME. The prognosis in untreated dysplasia of the hip. A study of radiographic factors that predict the outcome. Journal of Bone and Joint Surgery: American Volume 199577985–989. ( 10.2106/00004623-199507000-00002) [DOI] [PubMed] [Google Scholar]
- 3.van Bergen CJA, de Witte PB, Willeboordse F, de Geest BL, Foreman-van Dongelen MMHP, Burger BJ, den Hartog YM, van Linge JH, Pereboom RM, Robben SGF, et al. Treatment of centered developmental dysplasia of the hip under the age of 1 year: an evidence-based clinical practice guideline - Part 1. EFORT Open Reviews 20227498 ––505.. ( 10.1530/EOR-21-0125) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Brouwers MC, Kho ME, Browman GP, Burgers JS, Cluzeau F, Feder G, Fervers B, Graham ID, Grimshaw J, Hanna SE, et al. AGREE II: advancing guideline development, reporting and evaluation in health care. Canadian Medical Association Journal 2010182E839–E842. ( 10.1503/cmaj.090449) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Diercks R, Bron C, Dorrestijn O, Meskers C, Naber R, de Ruiter T, Willems J, Winters J, van der Woude HJ. & Dutch Orthopaedic Association. Guideline for diagnosis and treatment of subacromial pain syndrome A multidisciplinary review by the Dutch Orthopaedic Association. Acta Orthopaedica 201485314–322. ( 10.3109/17453674.2014.920991) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Koeter S, Van Tienen TG, Rijk PC, Vincken PWJ, Segers MJM, Van Essen B, Van Melick N, Stegeman BH, Van Arkel ERA. Dutch Guideline on Knee arthroscopy Part 2: Non-meniscus intra-articular knee injury: a multidisciplinary review by the Dutch Orthopaedic Association. Acta Orthopaedica 20219281–84. ( 10.1080/17453674.2020.1850081) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Van Arkel ERA, Koeter S, Rijk PC, Van Tienen TG, Vincken PWJ, Segers MJM, Van Essen B, Van Melick N, Stegeman BH. Dutch guideline on knee arthroscopy Part 1, the meniscus: a multidisciplinary review by the Dutch Orthopaedic Association. Acta Orthopaedica 20219274–80. ( 10.1080/17453674.2020.1850086) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Itz CJ, Willems PC, Zeilstra DJ, Huygen FJDutch Society of Anesthesiologists,, Dutch Orthopedic Association & Dutch Neurosurgical Society. Dutch multidisciplinary guideline for invasive treatment of pain syndromes of the lumbosacral spine. Pain Practice 20161690–110. ( 10.1111/papr.12318) [DOI] [PubMed] [Google Scholar]
- 9.Besselaar AT, Sakkers RJB, Schuppers HA, Witbreuk MMEH, Zeegers EVCM, Visser JD, Boekestijn RA, Margés SD, Van der Steen MCM, Burger KNJ. Guideline on the diagnosis and treatment of primary idiopathic clubfoot. Acta Orthopaedica 201788305–309. ( 10.1080/17453674.2017.1294416) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Armstrong EC.The well-built clinical question: the key to finding the best evidence efficiently. WMJ 19999825–28. [PubMed] [Google Scholar]
- 11.KLEINBERG SL.The acetabular index in infants in relation to congenital dislocation of the hip. Archives of Surgery 1936321049–1054. ( 10.1001/archsurg.1936.01180240137007) [DOI] [Google Scholar]
- 12.Severin E.Contribution to the knowledge of congenital dislocation of the hip joint. Acta Chirurgica Scandinavica 1941. [Google Scholar]
- 13.Schunemann HJ, Oxman AD, Brozek J, Glasziou P, Bossuyt P, Chang S, Muti P, Jaeschke R, Guyatt GH. GRADE: assessing the quality of evidence for diagnostic recommendations. Evidence-Based Medicine 200813162–163. ( 10.1136/ebm.13.6.162-a) [DOI] [PubMed] [Google Scholar]
- 14.Zidka M, Dzupa V. Pavlik harness and Frejka pillow: compliance affects results of outpatient treatment. Archives of Orthopaedic and Trauma Surgery 20191391519–1524. ( 10.1007/s00402-019-03179-7) [DOI] [PubMed] [Google Scholar]
- 15.Wilkinson AG, Sherlock DA, Murray GD. The efficacy of the Pavlik harness, the Craig splint and the von Rosen splint in the management of neonatal dysplasia of the hip. A comparative study. Journal of Bone and Joint Surgery: British Volume 200284716–719. ( 10.1302/0301-620x.84b5.12571) [DOI] [PubMed] [Google Scholar]
- 16.Atar D, Lehman WB, Tenenbaum Y, Grant AD. Pavlik harness versus Frejka splint in treatment of developmental dysplasia of the hip: bicenter study. Journal of Pediatric Orthopedics 199313311–313. ( 10.1097/01241398-199305000-00006) [DOI] [PubMed] [Google Scholar]
- 17.Carney BT, Rogers M, Minter CL. Reliability of acetabular measures in developmental dysplasia of the hip. Journal of Surgical Orthopaedic Advances 20051473–76. [PubMed] [Google Scholar]
- 18.Duman S, Camurcu Y, Sofu H, Ucpunar H, Akbulut D, Yildirim T. Arthroscopic versus open, medial approach, surgical reduction for developmental dysplasia of the hip in patients under 18 months of age. Acta Orthopaedica 201990292–296. ( 10.1080/17453674.2019.1599775) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Yorgancigil H, Aslan A. Comparison of the clinical and radiological outcomes of open reduction via medial and anterior approach in devleopmental dysplasia of the hip. Eklem Hastalik Cerrahisi 20162774–80. ( 10.5606/ehc.2016.17) [DOI] [PubMed] [Google Scholar]
- 20.Hoellwarth JS, Kim YJ, Millis MB, Kasser JR, Zurakowski D, Matheney TH. Medial Versus anterior open reduction for developmental hip dislocation in age-matched patients. Journal of Pediatric Orthopedics 20153550–56. ( 10.1097/BPO.0000000000000338) [DOI] [PubMed] [Google Scholar]
- 21.Holman J, Carroll KL, Murray KA, MacLeod LM, Roach JW. Long-term follow-up of open reduction surgery for developmental dislocation of the hip. Journal of Pediatric Orthopedics 201232121–124. ( 10.1097/BPO.0b013e3182471aad) [DOI] [PubMed] [Google Scholar]
- 22.Tarassoli P, Gargan MF, Atherton WG, Thomas SR. The medial approach for the treatment of children with developmental dysplasia of the hip. Bone and Joint Journal 201496–B406–413. ( 10.1302/0301-620X.96B3.32616) [DOI] [PubMed] [Google Scholar]
- 23.Matsushita T, Miyake Y, Akazawa H, Eguchi S, Takahashi Y. Open reduction for congenital dislocation of the hip: comparison of the long-term results of the wide exposure method and Ludloff's method. Journal of Orthopaedic Science 19994333–341. ( 10.1007/s007760050113) [DOI] [PubMed] [Google Scholar]
- 24.McKay DW.A comparison of the innominate and the pericapsular osteotomy in the treatment of congenital dislocation of the hip. Clinical Orthopaedics and Related Research 197498124–132. ( 10.1097/00003086-197401000-00013) [DOI] [PubMed] [Google Scholar]
- 25.Severin E.Contribution to the knowledge of congenital dislocation of the hip joint: late results of closed reduction and arthrographic studies of recent cases. JAMA 1942118565–566. ( 10.1001/jama.1942.02830070071035) [DOI] [Google Scholar]
- 26.Emara K, Kersh MAA, Hayyawi FA. Duration of immobilization after developmental dysplasia of the hip and open reduction surgery. International Orthopaedics 201943405–409. ( 10.1007/s00264-018-3962-3) [DOI] [PubMed] [Google Scholar]
- 27.Pavlik A.[A harness for treatment of congenital hip dislocation in infants]. Acta Chirurgiae Orthopaedicae et Traumatologiae Cechoslovaca 19532093–100. [PubMed] [Google Scholar]
- 28.Brougham DI, Broughton NS, Cole WG, Menelaus MB. Avascular necrosis following closed reduction of congenital dislocation of the hip. Review of influencing factors and long-term follow-up. Journal of Bone and Joint Surgery. British Volume 199072557–562. ( 10.1302/0301-620X.72B4.2380203) [DOI] [PubMed] [Google Scholar]
- 29.Burgos-Flores J, Ocete-Guzman G, Gonzalez-Herranz P, Hevia-Sierra E, Amaya-Alarcon S. Factors responsible for the development of avascular necrosis secondary to the treatment of congenital dislocation of the hip. International Orthopaedics 199317305–307. ( 10.1007/BF00181705) [DOI] [PubMed] [Google Scholar]
- 30.Forlin E, Choi IH, Guille JT, Bowen JR, Glutting J. Prognostic factors in congenital dislocation of the hip treated with closed reduction. The importance of arthrographic evaluation. Journal of Bone and Joint Surgery: American Volume 1992741140–1152. ( 10.2106/00004623-199274080-00003) [DOI] [PubMed] [Google Scholar]
- 31.Gogus MT, Aksoy MC, Atay OA, Acaroglu RE, Surat A. Treatment of congenital dislocation of the hip. Results of closed reduction and immobilization in the hip spica cast. Turkish Journal of Pediatrics 199739499–503. [PubMed] [Google Scholar]
- 32.Ludloff K.Zur blutigen Einrenkung der angeborenen Hüftluxation. Z Orthopaedics 190822272–276. [Google Scholar]
- 33.Ferguson Jr. AB.Primary open reduction of congenital dislocation of the hip using a median adductor approach. Journal of Bone and Joint Surgery: American Volume 197355671–689. ( 10.2106/00004623-197355040-00001) [DOI] [PubMed] [Google Scholar]
- 34.Weinstein SL, Ponseti IV. Congenital dislocation of the hip. Journal of Bone and Joint Surgery: American Volume 197961119–124. ( 10.2106/00004623-197961010-00021) [DOI] [PubMed] [Google Scholar]
- 35.Smit-Petersen MN.A new supra-articular subperiosteal approach to the hip joint. Journal of Bone and Joint Surgery: American Volume 1917s2-15592–5. [Google Scholar]
- 36.Watson-Jones R.Fractures of the neck of the femur. British Journal of Surgery 200523787–808. ( 10.1002/bjs.1800239213) [DOI] [Google Scholar]
- 37.Heeres RH, Witbreuk MM, van der Sluijs JA. Diagnosis and treatment of developmental dysplasia of the hip in the Netherlands: national questionnaire of paediatric orthopaedic surgeons on current practice in children less than 1 year old. Journal of Children’s Orthopaedics 20115267–271. ( 10.1007/s11832-011-0355-1) [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.



 This work is licensed under a
This work is licensed under a