Abstract
Introduction
Kidney Disease: Improving Global Outcomes (KDIGO) 2012 guidelines classify chronic kidney disease (CKD) risk or prognosis using estimated glomerular filtration rate (eGFR) and urinary albumin-to-creatinine ratio (UACR). We assessed patient characteristics and outcomes according to the KDIGO classification, using data from DISCOVER CKD (NCT04034992).
Methods
Data were extracted from the US integrated Limited Claims and Electronic Health Record Dataset and TriNetX databases, and the UK Clinical Practice Research Datalink linked to Hospital Episode Statistics and Office for National Statistics databases. Eligible patients were aged ≥18 years with CKD, and identified by 2 consecutive eGFR measures (5 to <75 ml/min/1.73 m2; ≥90 days apart [maximum 730]) from January 2008. Index date was the second eGFR measurement; patients were categorized using the UACR measure closest to the index. Outcomes included patient characteristics, eGFR or UACR measurement frequency, and clinical outcomes per baseline KDIGO classification.
Results
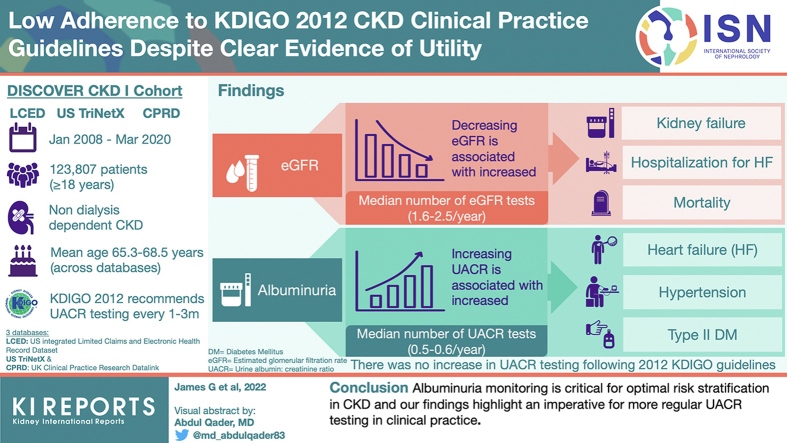
Across databases, only 8.6% of patients with 2 eGFR measures had ≥1 UACR measures. Among 123,807 eligible patients, prevalence of heart failure, hypertension, and type 2 diabetes increased with increasing albuminuria. Incidence rates of mortality and adverse cardiovascular and renal outcomes increased with declining baseline eGFR, and particularly with increasing albuminuria. Median number of eGFR and UACR tests per year post-index ranged from 1.6 to 2.5 and 0.5 to 0.6, respectively, across databases; there was no clear increase in UACR testing frequency following the KDIGO 2012 guidelines.
Conclusion
Albuminuria monitoring is critical for optimal risk stratification in CKD, and our findings highlight an imperative for more regular UACR testing in clinical practice.
Keywords: chronic kidney disease, DISCOVER CKD, estimated glomerular filtration rate, Kidney Disease: Improving Global Outcomes, retrospective, urinary albumin-to-creatinine ratio
Graphical abstract
Chronic kidney disease (CKD), a common and often undiagnosed and asymptomatic condition in its early stages, is defined by abnormalities of kidney structure or function present for >3 months.1 Abnormalities include markers of kidney damage, the most important being elevated urinary albumin (albuminuria; defined as an urinary albumin-to-creatine ratio [UACR] of ≥30 mg/g) and a decreased glomerular filtration rate (GFR). Since 1990, the prevalence of CKD and associated mortality have increased by 29.3% and 41.5%, respectively.2 Worldwide, the number of individuals with CKD in 2017 was estimated at approximately 840 million.3
Accurate screening, diagnosis, and risk stratification are important in CKD management, and are critical for slowing disease progression, managing complications, reducing risks, and optimizing patient outcomes.4,5 While GFR assessment allows estimation of remaining renal function, this measure does not provide fully comprehensive prognostic information when used in isolation.6,7 In recent decades, the value of albuminuria as an important predictor of renal disease progression, as well as cardiovascular and mortality risk, independent of GFR, has become apparent; studies have demonstrated how assessment of albuminuria provides improved risk stratification in patients staged according to estimated GFR(eGFR).8, 9, 10, 11 In light of these findings, the 2012 Kidney Disease: Improving Global Outcomes (KDIGO) group developed a classification system to diagnose and stratify risk or prognosis in patients with CKD, based on combined assessment of serum creatinine-based eGFR and UACR.1
Despite these recommendations and the strong epidemiological evidence linking increased albuminuria with CKD progression, kidney failure, cardiovascular events, and premature mortality,8, 9, 10, 11, 12 recent data on rates of UACR testing in patients with CKD in routine clinical practice are lacking, and available evidence suggest that testing rates are low.13, 14, 15, 16, 17 Moreover, few contemporary studies describe the outcomes of patients with CKD categorized according to the KDIGO 2012 classification. A recent review of studies employing this classification system indicated that, although the proportion of patients with CKD who fall into the high-risk and very high-risk KDIGO categories is low, these patients have the highest disease burden in terms of comorbidities, particularly diabetes, hypertension, and cardiovascular disease, which are risk factors for adverse cardiovascular and renal outcomes.6 Another review reported an increasing risk of cardiovascular morbidity or mortality and all-cause mortality, as well as increased costs and resource utilization, as eGFR declined and albuminuria increased.18 Additionally, in a recent systematic literature review of clinical trials in CKD since 2012, few trials reported on changes in UACR from baseline.19
Application of the KDIGO 2012 classification system to large CKD cohorts from multiple countries will facilitate a better understanding of the risks faced by patients with CKD according to their eGFR or UACR status, with the ultimate goal of improving the prognosis and management of patients with CKD. DISCOVER CKD (ClinicalTrials.gov Identifier: NCT04034992) is a hybrid and multinational observational cohort study in patients with CKD.20 DISCOVER CKD aims to provide contemporary real-world insight to inform clinical practice, and improve understanding of the epidemiology, clinical, and economic burden of CKD.20 The primary aim of the present analysis was to use real-world data from the DISCOVER CKD retrospective cohort to provide contemporary data on how patient characteristics, medication use, and outcomes change as CKD progresses, according to the KDIGO 2012 classification system of eGFR and albuminuria. The secondary aim was to assess measurement frequency of eGFR and UACR in routine clinical practice to determine adherence to guideline recommendations for testing.
Methods
The DISCOVER CKD retrospective cohort captured patients with CKD beginning January 1, 2008, through to March 2020.
Patient Population
Data were extracted from 3 databases: US integrated Limited Claims and Electronic Health Record Dataset (LCED), US TriNetX, and UK Clinical Practice Research Datalink (CPRD; ISAC protocol number, 19_226A4) linked to Hospital Episode Statistics and Office for National Statistics databases.
LCED, collated by IBM Watson Health (Armonk, NY), is a static dataset comprising approximately 4.4 million patients and covering the period from January 2012 through to June 2018 and September 2018 for claims and electronic medical record data, respectively. TriNetX (Cambridge, MA) is a global federated health research network dataset, integrating data from multiple healthcare organizations, including hospitals, primary care, and specialty treatment providers.21, 22, 23 This study utilized a subset of data from the analytics data subset,21 which included electronic health records from approximately 38 million patients in 35 healthcare organizations in the US between January 2008 and March 2020. CPRD is a primary care database comprising anonymized medical records collected as part of routine care from general practitioners, with coverage of 60 million UK patients.24,25 The present analysis utilized data from the CPRD GOLD database (between January 2008 and January 2020), linked to Hospital Episode Statistics and Office for National Statistics death registration data where available.
As reported previously,20 adults (aged ≥18 years at index date) with nondialysis-dependent CKD, identified by 2 consecutive eGFR measures (5 to <75 ml/min/1.73 m2; recorded ≥90 days apart [maximum 730 days]) from January 1, 2008, were eligible for inclusion. Patients were required to have ≥1 UACR measurement within 1 year before or up to 5 years after the index date (the measure closest to index was used to categorize patients). The index date was the date of second eGFR measurement. Exclusion criteria included having <1 year of medical history available before index, death within 30 days of index (where available in data source), history of type 1 diabetes mellitus, or a history of renal transplant or renal replacement therapy at index.
Patient Characteristic Measures
Available covariates for the DISCOVER CKD cohort have been described elsewhere.20 Characteristics pertinent to the present analyses include age, sex, eGFR, UACR, medication use, comorbidities, and laboratory findings.
Clinical Outcome Measures
Incidence of adverse clinical outcomes of interest was ascertained from diagnostic or procedural codes and eGFR measures (for kidney outcomes); further details are provided in Supplementary Table S1. Outcomes assessed included kidney, cardiovascular, and mortality outcomes as follows: ≥50% sustained reduction in eGFR, development of kidney failure, hospitalization for heart failure (hHF), stroke, myocardial infarction, all-cause mortality (CPRD and TriNetX), and cardiovascular mortality (CPRD).
Two sensitivity analyses were conducted as follows: (i) all-event rates for hHF were computed to capture recurrent events, and (ii) to account for adverse clinical events occurring prior to a UACR measure, incidence rates of all outcomes of interest were computed using the classifying UACR (value closest to index) as the index date in patients for whom this measure occurred after the second eGFR. In patients for whom this measure occurred before the second eGFR, the eGFR remained as the index.
eGFR and UACR Testing
To assess adherence to guideline recommendations for ongoing patient monitoring, we calculated the number of eGFR and UACR measures recorded per patient during each year of follow-up. To investigate the implementation of UACR recording following the KDIGO 2012 guidelines, we also compared the number of UACR measures before and during or after 2012.
Statistical Analysis
Baseline or index characteristics were summarized descriptively for each database or cohort and further stratified using KDIGO category.1 Frequency of days with an eGFR or UACR measure during follow-up (index measures excluded) are reported; annualized rates of UACR measurements pre-2012 and during or after-2012 were also calculated. Adverse clinical outcomes are reported as incidence rates per 100 patient-years, including estimated 95% confidence intervals.
Results
Patient Disposition and Overall Characteristics
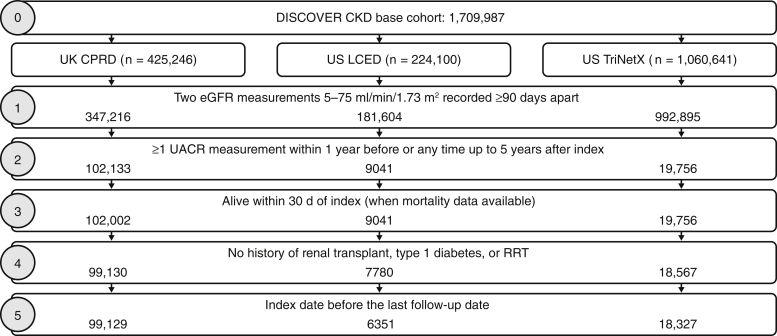
A total of 123,807 patients with CKD from the United Kingdom and United States were included in the study cohort (Figure 1). Mean age across databases ranged from 65.3 to 68.5 years, and 45.0% to 49.8% of the patients were male. Comorbidities, particularly type 2 diabetes (37.3%–68.4%), coronary heart disease (17.4%–22.4%), and hypertension (58.7%–85.7%), were prevalent (Table 1). The median (interquartile range) length of follow-up was 4.7 (2.6–6.9), 3.4 (2.0–4.7), and 2.8 (1.4–4.4) years for CPRD, LCED, and TriNetX, respectively.
Figure 1.
Patient flow diagram. CKD, chronic kidney disease; CPRD, Clinical Practice Research Datalink; eGFR, estimated glomerular filtration rate; LCED, Limited Claims and Electronic Health Record Dataset; RRT, renal replacement therapy; UACR, urinary albumin-to-creatinine ratio.
Table 1.
Patient demographics at baseline
| Characteristics | CPRD (n = 99,129) | LCED (n = 6351) | TriNetX (n = 18,327) |
|---|---|---|---|
| Age at index, yr, mean (SD) | 68.5 (11.3) | 65.3 (10.5) | 65.7 (11.7) |
| Male sex, n (%) | 48,820 (49.2) | 3165 (49.8) | 8252 (45.0) |
| BMI, kg/m2, mean (SD) | 30.1 (6.2) | 32.3 (6.9) | 30.6 (5.1) |
| Laboratory values | |||
| Albumin, g/dl, mean (SD) | 4.2 (0.4) | 4.3 (0.3) | 4.0 (0.5) |
| Missinga, % | 18.4 | 50.6 | 31.6 |
| eGFR, ml/min/1.73 m2, median (IQR) | 64.0 (55.1, 70.2) | 65.0 (56.3, 70.5) | 61.7 (50.1, 69.2) |
| Missinga, % | 0 | 0 | 0 |
| Ferritin, ng/ml, mean (SD) | 132.3 (164.6) | 151.8 (186.7) | 182.2 (251.0) |
| Missinga, % | 86.9 | 91.6 | 90.7 |
| HbA1c, %, mean (SD) | 7.0 (1.6) | 6.9 (1.4) | 7.3 (1.6) |
| Missinga, % | 55.3 | 34.1 | 29.6 |
| Missinga (T2D and HbA1c), % | 4.0 | 23.2 | 13.4 |
| Missinga (no T2D and HbA1c), % | 85.8 | 57.8 | 59.7 |
| Potassium, mmol/l, mean (SD) | 4.5 (0.5) | 4.3 (0.5) | 4.3 (0.5) |
| Missinga, % | 5.7 | 3.6 | 2.5 |
| Serum bicarbonate, mmol/l, mean (SD) | 26.5 (3.0) | 26.2 (3.0) | 26.2 (3.3) |
| Missinga, % | 81.9 | 7.9 | 1.9 |
| UACR, mg/g, mean (SD) | 64.2 (263.7) | 71.8 (323.9) | 143.6 (437.5) |
| Missinga, % | 57.8 | 40.3 | 46.4 |
| Uric acid, μmol/l, mean (SD) | 382.5 (94.6) | 371.1 (94.5) | 373.9 (94.7) |
| Missinga, % | 95.6 | 89.6 | 92.2 |
| Comorbidities/complications, n (%) | |||
| Acute kidney injury | 1701 (1.7) | 339 (5.3) | 1372 (7.5) |
| Coronary heart disease | 17,294 (17.4) | 1422 (22.4) | 3657 (20.0) |
| T2D | 36,960 (37.3) | 4345 (68.4) | 11,892 (64.9) |
| HF | 5033 (5.1) | 533 (8.4) | 1735 (9.5) |
| Hyperkalemia | 449 (0.5) | 166 (2.6) | 504 (2.8) |
| Hypertension | 58,217 (58.7) | 5440 (85.7) | 13,898 (75.8) |
| Myocardial infarction | 7380 (7.4) | 368 (5.8) | 998 (5.4) |
| Stroke | 5046 (5.1) | 983 (15.5) | 1669 (9.1) |
| Prescription medications, n (%) | |||
| RAASib | 51,032 (51.5) | 4248 (66.9) | 8953 (48.9) |
| Diureticsc | 34,161 (34.5) | 3039 (47.9) | 7097 (38.7) |
| Statins | 52,473 (52.9) | 4159 (65.5) | 8828 (48.2) |
| DPP4i | 3237 (3.3) | 676 (10.6) | 1611 (8.8) |
| Insulin | 4478 (4.5) | 923 (14.5) | 4371 (23.9) |
| Metformin | 24,037 (24.2) | 2832 (44.6) | 6283 (34.3) |
ACE, angiotensin-converting enzyme; ARB, angiotensin receptor blocker; BMI, body mass index; CPRD, Clinical Practice Research Datalink; DPP4i, dipeptidyl peptidase-4; eGFR, estimated glomerular filtration rate; HbA1c, glycated hemoglobin; HF, heart failure; IQR, interquartile range; LCED, Limited Claims and Electronic Health Record Dataset; MRA, mineralocorticoid receptor antagonist; RAASi, renin-angiotensin-aldosterone system inhibitor; SD, standard deviation; T2D, type 2 diabetes; UACR, urinary albumin-to-creatinine ratio.
Data were either missing and/or not recorded in clinical practice.
Includes ACE inhibitors and ARBs.
Includes MRA, loop diuretics, and thiazide.
Frequency of UACR and eGFR Testing
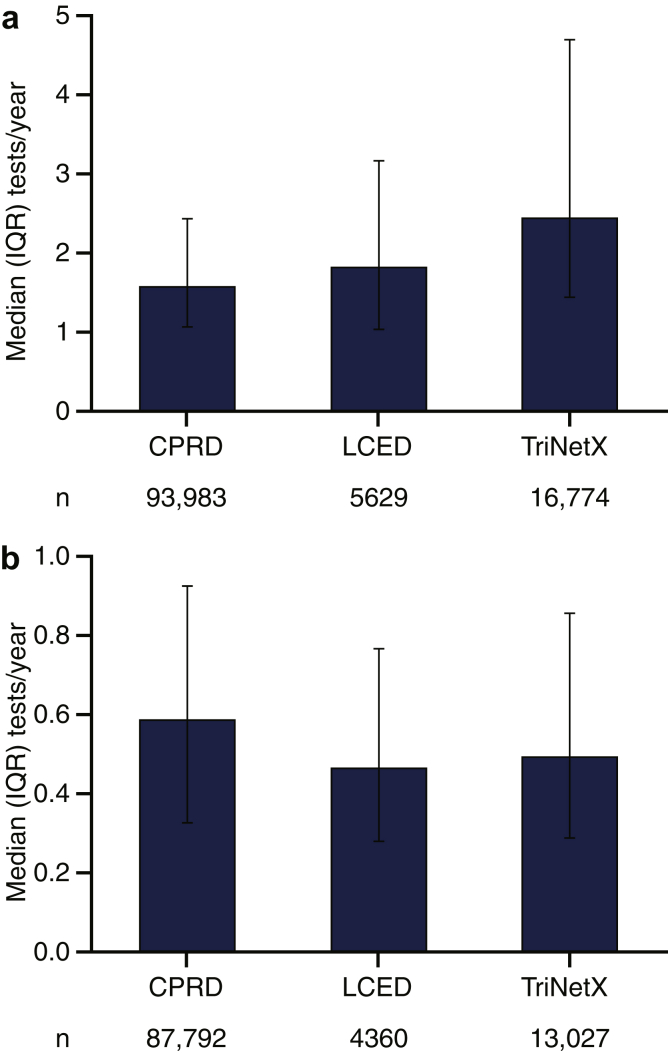
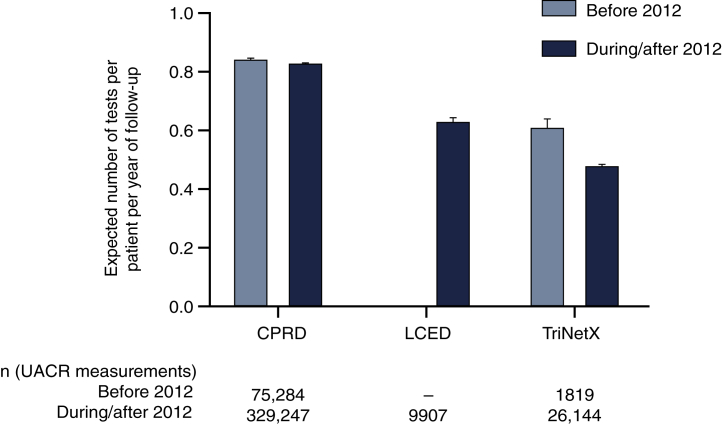
Among patients in the DISCOVER CKD base cohort, only 8.6% of patients with 2 eligible eGFR values had ≥1 UACR measurement and could be classified according to KDIGO 2012 guideline recommendations (Figure 1). After index, the median number of eGFR tests per year ranged from 1.6 to 2.5 across databases (Figure 2a), with increased testing frequency as eGFR decreased and UACR increased (Supplementary Figure S1). Frequency of UACR testing during follow-up was similar between databases (median number of tests per year during follow-up: 0.5–0.6) (Figure 2b) and there was no clear increase in testing frequency following the introduction of KDIGO guidelines in 2012 (Figure 326). Testing frequency did not appear to vary with eGFR or UACR levels (Supplementary Figure S2).
Figure 2.
Frequency of (a) eGFR and (b) UACR testing during follow-up. Numbers of tests inferred from the number of days with a test result during follow-up post-index. CPRD, Clinical Practice Research Datalink; eGFR, estimated glomerular filtration rate; IQR, interquartile range; LCED, Limited Claims and Electronic Health Record Dataset; UACR, urinary albumin-to-creatinine ratio.
Figure 3.
Impact of KDIGO 2012 guidelines on frequency of UACR testing during follow-up. Numbers of tests inferred from the number of days with a test result during follow-up post-index. UACR data were not available from before 2012 in LCED. Data are rates with 95% confidence intervals, calculated according to the method reported by Ulm, K.26 CPRD, Clinical Practice Research Datalink; KDIGO, Kidney Disease: Improving Global Outcomes; LCED, Limited Claims and Electronic Health Record Dataset; UACR, urinary albumin-to-creatinine ratio.
Patient Characteristics by KDIGO 2012 Classification
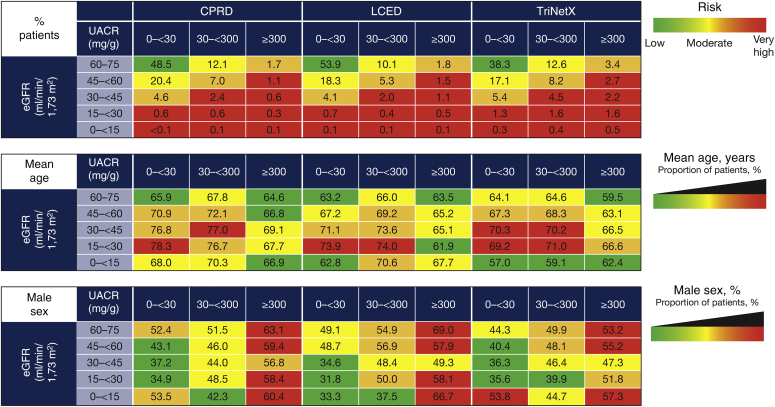
Across databases, over half of patients had CKD consistent with the low or moderately increased risk KDIGO categories (68.0%–82.3%; Figure 4). The proportions of patients in the high-risk and very high-risk categories were 11.2% to 17.0% and 5.9% to 15.1%, respectively (Figure 4). There were trends toward increased age both with declining eGFR (for eGFR >15 ml/min/1.73 m2) and a higher proportion of males with increasing albuminuria severity (Figure 4).
Figure 4.
Patient categorization and baseline characteristics by KDIGO category at index. Color coding is based on odds ratio quartile (Q) for each outcome within each database: green = Q1 and below; yellow = Q1 to Q2; orange = Q2 to Q3; red = Q3 and above. CPRD, Clinical Practice Research Datalink; eGFR, estimated glomerular filtration rate; KDIGO, Kidney Disease: Improving Global Outcomes; LCED, Limited Claims and Electronic Health Record Dataset; UACR, urinary albumin-to-creatinine ratio.
The prevalence of several comorbidities and history of complications tended to be higher with declining eGFR and increasing albuminuria (Supplementary Figure S3). This was particularly evident for history of acute kidney injury, hHF, and hyperkalemia. There were also trends toward higher hypertension and type 2 diabetes prevalence with increasing albuminuria. Prescriptions for renin-angiotensin-aldosterone system inhibitors (RAASis, including angiotensin-converting enzyme inhibitors and angiotensin receptor blockers), diuretics, and insulin generally increased with declining eGFR and increasing albuminuria. The proportion of patients prescribed RAASi among those with UACR >300 mg/g was 63.8%, 79.7%, and 56.4% in the CPRD, LCED, and TriNetX databases, respectively. Conversely, metformin prescriptions decreased with declining eGFR and increasing albuminuria (Supplementary Figure S4). Mean serum levels of ferritin, phosphate, parathyroid hormone, potassium, and uric acid were generally higher with declining eGFR and increasing albuminuria, whereas levels of bicarbonate, hemoglobin, and iron were lower (Supplementary Figure S5).
Adverse Clinical Outcomes
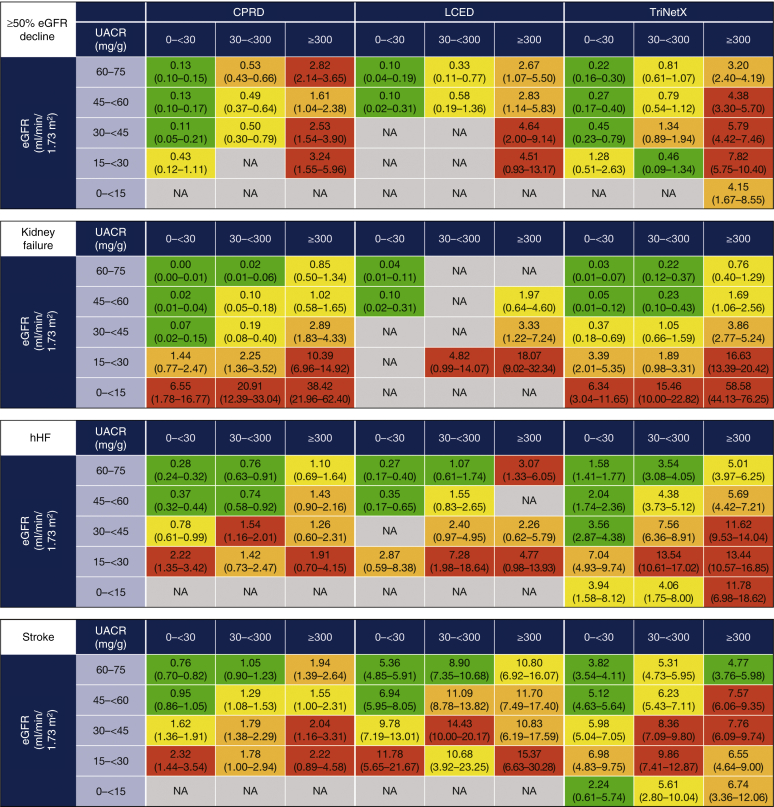
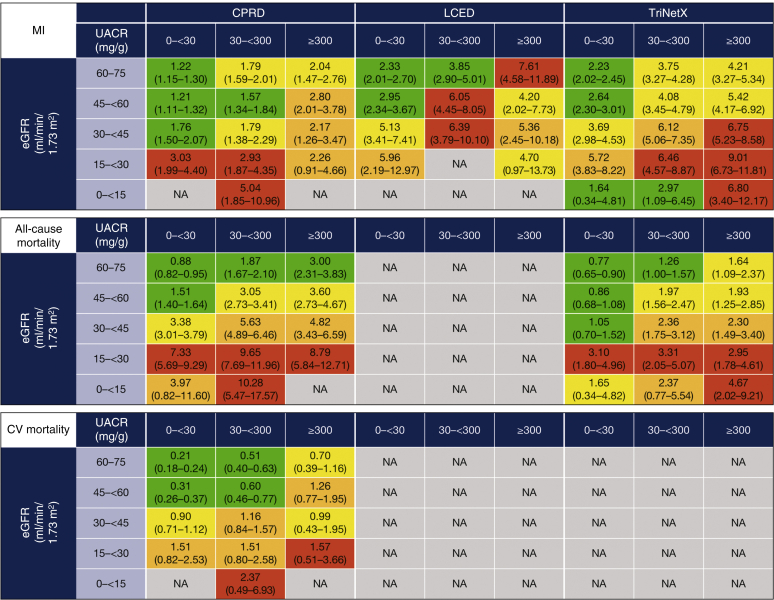
Across databases, there was a considerable burden of CKD in terms of adverse outcomes, even in low-risk KDIGO categories, and increased incidence rates of kidney failure with declining eGFR (Figure 5). Within each eGFR category, rates of both kidney outcomes (≥50% eGFR decline and kidney failure) increased with increasing albuminuria. There were also increased incidence rates of cardiovascular outcomes (hHF, stroke, and myocardial infarction) per 100 patient-years with declining eGFR (Figure 5). Within each eGFR category, the incidence rates of cardiovascular outcomes increased with increasing albuminuria, particularly in patients with less severely reduced eGFR. Results from the sensitivity analysis capturing all-event rates of hHF are shown in Supplementary Figure S6. There were increased incidence rates of all-cause mortality (in CPRD and TriNetX) and cardiovascular mortality (in CPRD) with both declining eGFR and increasing albuminuria within each eGFR category (Figure 5). Patterns in incidence rates of clinical outcomes using the classifying UACR measurement as the index date in patients where this occurred after the eGFR index date were consistent with findings from the main analysis (Supplementary Figure S7).
Figure 5.
Incidence rates per 100 PY of clinical outcomes during follow-up by KDIGO category. Data are incidence rate (95% CI), calculated as the number of specific events that occur during patient follow-up time at risk (time in the study until first event or loss to follow-up). Color coding is based on odds ratio quartile (Q) for each outcome within each database: green = Q1 and below; yellow = Q1 to Q2; orange = Q2 to Q3; red = Q3 and above. Kidney failure was defined as progression to CKD stage 5 (sustained eGFR ≤15 ml/min/1.73 m2) or initiation of chronic RRT for >30 days (2 dialysis codes 30–365 days apart) or kidney transplant. Mortality data were not available for US LCED, US TriNetX reports only in-hospital deaths, and incidence rates for some outcomes were not available where there were low patient or event numbers (e.g., LCED eGRF <15 ml/min/1.73 m2). CI, confidence interval; CPRD, Clinical Practice Research Datalink; CV, cardiovascular; CKD, chronic kidney disease; eGFR, estimated glomerular filtration rate; hHF, hospitalization for heart failure; KDIGO, Kidney Disease: Improving Global Outcomes; LCED, Limited Claims and Electronic Health Record Dataset; MI, myocardial infarction; NA, not available (owing to low patient/event numbers); PY, patient-years; RRT, renal replacement therapy; UACR, urinary albumin-to-creatinine ratio.
Discussion
This is one of the largest studies since the launch of the KDIGO 2012 guidelines, assessing patient characteristics and outcomes according to the KDIGO 2012 classification system using comprehensive real-world data from DISCOVER CKD, a multinational observational cohort study that includes data from the UK and US. Key findings include the following: (i) an increased incidence of adverse clinical outcomes with declining baseline eGFR and increasing albuminuria, and (ii) a low frequency of UACR testing in patients with CKD, with no evidence of change following the launch of the KDIGO 2012 guidelines. Among patients in the DISCOVER CKD base cohort, <10% of those with 2 eGFR measurements had an available UACR measurement, which is low considering the baseline characteristics of the cohort and KDIGO recommendations for UACR testing.
Most patients included in the study cohort had CKD consistent with the low and moderately increased risk KDIGO categories, as defined by eGFR and UACR ranges. Across databases, 18% to 32% of patients were classified as high-risk or very high-risk, and approximately 10% of these patients had eGFR of 60 to 75 ml/min/1.73 m2. Therefore, they may not have received a CKD diagnosis or not have been perceived as high-risk based on eGFR alone. As CKD risk (in terms of KDIGO classification1) increased, there were trends toward increased age, comorbidity burden, and laboratory abnormalities. Patients in the highest-risk KDIGO categories had the highest CKD disease burden in terms of comorbidities, consistent with previous findings.6 Trends in prescription of key medications, including metformin and RAASi, were generally consistent with guideline recommendations,1,27,28 although the absolute proportion of patients prescribed RAASi (angiotensin-converting enzyme inhibitors and angiotensin receptor blockers) still remained suboptimal given the evidence supporting their efficacy in patients with CKD and proteinuria.1,29
Incidence rates of adverse clinical outcomes reflected the significant risk of adverse cardiovascular and kidney outcomes, and mortality among patients with impaired kidney function, even among those in low-risk KDIGO categories, as observed previously.30, 31, 32 The associations between declining eGFR and/or increasing albuminuria with increased incidence of adverse clinical outcomes concur with previous findings.8, 9, 10, 11,16,33, 34, 35 Nevertheless, our findings add important detail to understanding of contemporary kidney, cardiovascular, and mortality risk across the KDIGO 2012 classification by applying the classification to a large real-world CKD cohort. Importantly, the findings support the premise that albuminuria is an independent risk predictor of adverse outcomes in CKD and that including albuminuria status in risk stratification provides valuable prognostic information. In general, incidence of adverse clinical outcomes was higher in US databases than in the CPRD, which is consistent with the primary care setting of CPRD with secondary Hospital Episode Statistics linkage. In particular, the increased hHF incidence but lower mortality in TriNetX reflects the high proportion of hospital-based data and capture of only in-hospital deaths in this database.
Across databases, eGFR testing frequency increased with declining kidney function, in line with KDIGO 2012 and UK National Institute for Health and Care Excellence recommendations, which recommend assessing eGFR and albuminuria at least annually in most patients, and more frequently in individuals at increased risk of progression.1,36 Nevertheless, testing frequency was still less than recommended for patients in the highest-risk KDIGO categories.1,36 Furthermore, the low rates of UACR testing across KDIGO categories and lack of improvement since 2012 suggest there has been insufficient implementation of new clinical guidelines in practice. KDIGO 2012 guidelines recommend monitoring albuminuria at least every 1 to 3 months in patients within the highest-risk categories,1 and implementation of this recommendation was not evident from our findings. These findings are particularly concerning in the context of the increased risks of adverse clinical outcomes seen with increasing albuminuria within the present study cohort. Moreover, albuminuria status can serve as a key element in treatment-related decision making, such as RAASi initiation.27
Our findings regarding UACR testing concur with those from other studies, which observed suboptimal rates of UACR testing or recording in patients at high-risk of developing CKD14,15 or with a confirmed CKD diagnosis.16,17 Although some studies have reported improvements in rates of albuminuria measurement over time,37,38 data from the present analysis suggests that absolute testing rates remain low and require improvement, despite release of the KDIGO 2012 guidelines and the low cost of conducting these tests in routine care.
Potential explanations for the low rates of UACR testing in real-world practice include the existence of country-specific practices and interpretation of guidelines, including screening for UACR based on indications that are independent of eGFR. For example, some studies have reported increased UACR testing rates in patients with CKD and concomitant diabetes,17 which may reflect policy-based incentivization and diabetes treatment guidelines recommending routine UACR monitoring, as well as perception of a higher clinical risk in this CKD subpopulation.28
Other reasons could include physician reluctance to conduct urine tests owing to perceived inconvenience or burden, or a preference to collect only morning samples. Some countries might also use alternative measures such as urinary dipstick tests to assess proteinuria or timed urine collections to measure albuminuria, despite UACR measurement being the preferred “gold standard” and the most accurate means of evaluating albuminuria.1,39 Results from dipstick tests may also be less likely to be recorded in patient medical records than UACR tests. Other factors may include the relatively high eGFR values in the study cohort and high representation of patients treated in primary care settings, where there may be less visibility of the KDIGO guidelines than in specialist nephrology practices.
To our knowledge, this analysis is the first to assess patient characteristics and outcomes by the application of the KDIGO 2012 classification system to a large (>100,000 patients) real-world cohort of patients with CKD. Additional strengths of this study include the longitudinal assessment of clinical outcomes, large range of covariates assessed, granularity of data, and use of databases from primary care and hospital-based care that are generalizable to populations from which they derive. Limitations of this study include those inherent to retrospective observational data, in that conclusions about causality cannot be made. Additionally, the findings are only generalizable to patients meeting the study eligibility criteria within the geographies represented in the study cohorts.
The study databases also have several limitations, including the potential for coding errors as electronic health record and claims data are not collected for research purposes. Mortality data were not available from LCED, and only the year of in-hospital deaths are recorded in TriNetX, which may have led to mortality being underestimated. LCED and TriNetX also capture data from secondary care, meaning included patients are likely to have a higher comorbidity burden or more advanced disease compared with primary care; additional limitations of TriNetX have been described previously.40 In the UK CPRD, prescriptions and laboratory parameters are only available from primary care, and there is potential for under-reporting or misclassification of outcomes of interest and of events occurring in emergency care being missed. Across all 3 study databases, it is possible some patients with late-stage CKD were lost to other databases (e.g., Medicare in the United States and the UK Renal Registry in the United Kingdom). Additionally, not all patients included in the study had a diagnostic code for CKD, and eGFR measures were used to infer the presence of CKD in these patients. There was also a high proportion of missing data for some laboratory parameters (e.g., ferritin and uric acid), which may reflect the relatively mild CKD in many patients, many of whom would have been treated in primary care settings rather than in specialist nephrology practice settings.
As a selection criterion, UACR measurement may have enriched the study population with a higher burden of comorbidities, due to the increased likelihood of UACR monitoring at baseline in patients with CKD of increased severity. In addition, a considerable proportion of the source population did not have a UACR measurement recorded, an exclusion criterion that restricted the size of the final study population, and UACR was not systematically recorded in all patients during the study period. While some patients may have undergone urinary protein assessments (e.g., using urinary dipsticks), and have recorded urinary protein-to-creatinine values rather than UACR, these data were not available in the study datasets. We chose to include only patients with UACR measurements because this is the “gold standard” method, as noted previously. If alternative means of measuring albuminuria are routinely being used in place of UACR in clinical practice, the reasons for this practice occurring is of significant interest, given the well communicated advantages of UACR versus other methods, its endorsement by clinical guidelines,1,39 and low cost.
Taken together, these findings highlight the opportunity for improvements in the care of patients with CKD (Table 2), including appropriate risk stratification by measuring UACR more frequently in clinical practice, and implementation of evidence-based strategies to reduce kidney failure and adverse cardiovascular outcomes.
Table 2.
Practice points: potential strategies for improving guideline implementation in clinical practice
| Strategy | Description |
|---|---|
| 1 | Patient engagement to increase knowledge of the rationale behind eGFR and UACR testing |
| 2 | Referral of requesting doctors to clinical guidelines by pathologists |
| 3 | Implementation of HCP education strategies to encourage both initial screening and periodic follow-up monitoring of eGFR and UACR |
| 4 | Financial incentives for implementation of best practice in healthcare settings |
eGFR, estimated glomerular filtration rate; HCP, healthcare professional; UACR, urinary albumin-to-creatinine ratio.
Conclusion
This large CKD cohort study provides contemporary and valuable insight into real-world clinical characteristics and outcomes of patients with CKD stratified according to both eGFR and albuminuria status. The findings emphasize the increased clinical burden, particularly in terms of comorbidities and adverse clinical outcomes, associated with declining eGFR and increasing albuminuria per KDIGO 2012 classification, and emphasize the additional prognostic accuracy afforded by stratifying patients according to albuminuria status. The data also highlight an imperative for more regular or routine and mandated UACR testing in all patients with CKD in routine clinical practice for prognostic and monitoring purposes.
Disclosure
GJ, HC, SK, JJGS, MA, and SN are employees of, and hold or may hold stock in AstraZeneca. JJC reports institutional grants from Astellas, AstraZeneca, and Vifor Pharma; speaker fees from AstraZeneca, Abbott, and Nutricia; and consultancy for AstraZeneca and Bayer. RPF is an employee of Arbor Research Collaborative for Health, which receives global support for the ongoing DOPPS Programs (provided without restriction on publications by a variety of funders; for details see https://www.dopps.org/AboutUs/Support.aspx). RPF also reports research grants from Fresenius Medical Care, nonfinancial support from Akebia, AstraZeneca, Bayer, Boehringer, Novo Nordisk, and FibroGen; as well as personal fees from Travere Therapeutics and consulting fees from George Clinical outside the submitted work. HJLH reports grants and other fees from AbbVie, AstraZeneca, Bayer, Boehringer Ingelheim, Chinook, CSL Pharma, Dimerix, Gilead, Janssen, Merck, Mitsubishi Tanabe, Mundipharma, Novo Nordisk, and Travere Therapeutics. CSPL is supported by a Clinician Scientist Award from the National Medical Research Council of Singapore; has received research support from AstraZeneca, Bayer, Boston Scientific, Medtronic, Roche Diagnostics, and Vifor Pharma; has served as consultant or on the Advisory Board/Steering Committee/Executive Committee for Abbott Diagnostics, Amgen, Applied Therapeutics, AstraZeneca, Bayer, Biofourmis, Boehringer Ingelheim, Boston Scientific, Corvia Medical, Cytokinetics, Darma Inc., Eko.ai Pte Ltd., Jana Care, Janssen Research & Development LLC, Medtronic, Menarini Group, Merck, MyoKardia, Novartis, Novo Nordisk, Radcliffe Group Ltd., Roche Diagnostics, Sanofi, Stealth BioTherapeutics, The Corpus, Vifor Pharma, and WebMD Global LLC; and serves as co-founder and non-executive director of Us2.ai Pte Ltd. EK is a consultant for AstraZeneca. NK is a consultant for AstraZeneca, Boehringer Ingelheim, and Kyowa Hakko Kirin, and receives honoraria from Kyowa Hakko Kirin and Daiichi Sankyo. MNK reports research grants from AstraZeneca and Boehringer Ingelheim; has served as consultant or on the advisory board for Alnylam Pharmaceuticals, Amgen, AstraZeneca, Bayer, Boehringer Ingelheim, Eli Lilly, Esperion, Janssen, Merck (diabetes and cardiovascular), Novo Nordisk, Pharmacosmos, Sanofi, and Vifor Pharma; receives honoraria from AstraZeneca, Boehringer Ingelheim, and Novo Nordisk; and other research support from AstraZeneca. ML is supported by the Slovenian Research Agency; has received research support from Roche Diagnostics; has served as consultant or on the advisory board/steering committee for AstraZeneca, Boehringer Ingelheim, Novartis, and Vifor Pharma; has received personal fees from Abbott, Amgen, AstraZeneca, Boehringer Ingelheim, Novartis, Sanofi, Servier, and Vifor Pharma. CP reports advisory board membership for AstraZeneca, Boehringer Ingelheim, Eli Lilly, and Vifor Pharma; as well as speaker fees for AstraZeneca, Janssen Cilag, Novartis, Otsuka, and Vifor Pharma. DCW reports personal fees and nonfinancial support from AstraZeneca, as well as personal fees from Astellas, Bayer, Boehringer Ingelheim, GlaxoSmithKline, Janssen, Mundipharma, Napp, Reata, Tricida, and Vifor Fresenius.
Acknowledgments
The authors acknowledge the contributions of Alyshah Abdul Sultan for support with the study design and data interpretation, and for providing comments on an initial manuscript outline. Medical writing support was provided by Lucy Ambrose, DPhil; and editorial support was provided by Rachael Cazaly, both of Core, Knutsford, UK, supported by AstraZeneca according to Good Publication Practice guidelines (Link). The sponsor, AstraZeneca, was involved in the study design, and collection, analysis, and interpretation of data. Nevertheless, ultimate responsibility for opinions, conclusions, and data interpretation lies with the authors. This analysis was funded by AstraZeneca.
Data Availability
The datasets generated during and/or analyzed during the current study are available upon reasonable request in accordance with AstraZeneca’s data sharing policy described at https://astrazenecagroup-dt.pharmacm.com/DT/Home.
Author Contributions
All authors contributed to the development of the manuscript, including reviewing, providing feedback, and approving the final version of the submitted manuscript.
Footnotes
Figure S1. Frequency of eGFR testing per KDIGO category.
Figure S2. Frequency of UACR testing per KDIGO category.
Figure S3. Patient comorbidities/complications by KDIGO category at index.
Figure S4. Prescription medications by KDIGO category at Index.
Figure S5. Laboratory parameters (in serum/whole blood) by KDIGO category at index.
Figure S6. Incidence rates and all-event rates per 100 PY of hospitalization for heart failure: overall (a) and by KDIGO (b, c, d) category.
Figure S7. Incidence rates per 100 PY of clinical outcomes during follow-up by KDIGO category (sensitivity analysis).
Table S1. Ascertainment of clinical outcomes.
Plain Language Summary Infographic.
Supplementary Material
Figure S1. Frequency of eGFR testing per KDIGO category.
Figure S2. Frequency of UACR testing per KDIGO category.
Figure S3. Patient comorbidities/complications by KDIGO category at index.
Figure S4. Prescription medications by KDIGO category at Index.
Figure S5. Laboratory parameters (in serum/whole blood) by KDIGO category at index.
Figure S6. Incidence rates and all-event rates per 100 PY of hospitalization for heart failure: overall (a) and by KDIGO (b, c, d) category.
Figure S7. Incidence rates per 100 PY of clinical outcomes during follow-up by KDIGO category (sensitivity analysis).
Table S1. Ascertainment of clinical outcomes.
Plain Language Summary Infographic.
References
- 1.KDIGO KDIGO 2012 Clinical practice guideline for the evaluation and management of chronic kidney disease. Kidney Int Suppl. 2012;3:1–150. doi: 10.1038/ki.2013.243. [DOI] [PubMed] [Google Scholar]
- 2.Bikbov B., Purcell C.A., Levey A.S. Global, regional, and national burden of chronic kidney disease, 1990-2017: a systematic analysis for the Global Burden of Disease Study 2017. Lancet. 2020;395:709–733. doi: 10.1016/S0140-6736(20)30045-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Jager K.J., Kovesdy C., Langham R., et al. A single number for advocacy and communication-worldwide more than 850 million individuals have kidney diseases. Nephrol Dial Transplant. 2019;34:1803–1805. doi: 10.1093/ndt/gfz174. [DOI] [PubMed] [Google Scholar]
- 4.Chen T.K., Knicely D.H., Grams M.E. Chronic kidney disease diagnosis and management: a review. JAMA. 2019;322:1294–1304. doi: 10.1001/jama.2019.14745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Rysz J., Gluba-Brzozka A., Franczyk B., et al. Novel biomarkers in the diagnosis of chronic kidney disease and the prediction of its outcome. Int J Mol Sci. 2017;18 doi: 10.3390/ijms18081702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Murton M., Goff-Leggett D., Bobrowska A., et al. Burden of chronic kidney disease by KDIGO categories of glomerular filtration rate and albuminuria: a systematic review. Adv Ther. 2021;38:180–200. doi: 10.1007/s12325-020-01568-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Polkinghorne K.R. Estimated glomerular filtration rate versus albuminuria in the assessment of kidney function: what’s more important? Clin Biochem Rev. 2014;35:67–73. [PMC free article] [PubMed] [Google Scholar]
- 8.Astor B.C., Matsushita K., Gansevoort R.T., et al. Lower estimated glomerular filtration rate and higher albuminuria are associated with mortality and end-stage renal disease. A collaborative meta-analysis of kidney disease population cohorts. Kidney Int. 2011;79:1331–1340. doi: 10.1038/ki.2010.550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Matsushita K., van der Velde M., Astor B.C., et al. Association of estimated glomerular filtration rate and albuminuria with all-cause and cardiovascular mortality in general population cohorts: a collaborative meta-analysis. Lancet. 2010;375:2073–2081. doi: 10.1016/S0140-6736(10)60674-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.van der Velde M., Matsushita K., Coresh J., et al. Lower estimated glomerular filtration rate and higher albuminuria are associated with all-cause and cardiovascular mortality. A collaborative meta-analysis of high-risk population cohorts. Kidney Int. 2011;79:1341–1352. doi: 10.1038/ki.2010.536. [DOI] [PubMed] [Google Scholar]
- 11.Gansevoort R.T., Matsushita K., van der Velde M., et al. Lower estimated GFR and higher albuminuria are associated with adverse kidney outcomes. A collaborative meta-analysis of general and high-risk population cohorts. Kidney Int. 2011;80:93–104. doi: 10.1038/ki.2010.531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mace-Brickman T., Eddeen A.B., Carrero J.J., et al. The risk of stroke and stroke type in patients with atrial fibrillation and chronic kidney disease. Can J Kidney Health Dis. 2019;6 doi: 10.1177/2054358119892372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Tuttle K.R., Alicic R.Z., Duru O.K., et al. Clinical characteristics of and risk factors for chronic kidney disease among adults and children: an analysis of the CURE-CKD Registry. JAMA Netw Open. 2019;2 doi: 10.1001/jamanetworkopen.2019.18169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Saran R., Robinson B., Abbott K.C., et al. US Renal Data System 2019 annual data report: epidemiology of kidney disease in the United States. Am J Kidney Dis. 2020;75:A6–A7. doi: 10.1053/j.ajkd.2019.09.003. [DOI] [PubMed] [Google Scholar]
- 15.Szczech L.A., Stewart R.C., Su H.L., et al. Primary care detection of chronic kidney disease in adults with type-2 diabetes: the ADD-CKD Study (awareness, detection and drug therapy in type 2 diabetes and chronic kidney disease) PLoS One. 2014;9 doi: 10.1371/journal.pone.0110535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Olufade T., Lamerato L., Sanchez J.J.G., et al. Clinical outcomes and healthcare resource utilization in a real-world population reflecting the DAPA-CKD Trial participants. Adv Ther. 2021;38:1352–1363. doi: 10.1007/s12325-020-01609-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Fraser S.D., Parkes J., Culliford D., et al. Timeliness in chronic kidney disease and albuminuria identification: a retrospective cohort study. BMC Fam Pract. 2015;16:18. doi: 10.1186/s12875-015-0235-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Darlington O., Dickerson C., Evans M., et al. Costs and healthcare resource use associated with risk of cardiovascular morbidity in patients with chronic kidney disease: evidence from a systematic literature review. Adv Ther. 2021;38:994–1010. doi: 10.1007/s12325-020-01607-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Garcia Sanchez J.J., Thompson J., Scott D.A., et al. Treatments for chronic kidney disease: A systematic literature review of randomized controlled trials. Adv Ther. 2021;39:193–220. doi: 10.1007/s12325-021-02006-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Pecoits-Filho R., James G., Carrero J.J., et al. Methods and rationale of the DISCover CKD global observational study. Clin Kidney J. 2021;14:1570–1578. doi: 10.1093/ckj/sfab046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Harrison P.J., Luciano S., Colbourne L. Rates of delirium associated with calcium channel blockers compared to diuretics, renin-angiotensin system agents and beta-blockers: an electronic health records network study. J Psychopharmacol. 2020;34:848–855. doi: 10.1177/0269881120936501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.The TriNetX global health research network . 2022. TriNetX.https://trinetx.com/our-network [Google Scholar]
- 23.Topaloglu U., Palchuk M.B. Using a federated network of real-world data to optimize clinical trials operations. JCO Clin Cancer Inform. 2018;2:1–10. doi: 10.1200/CCI.17.00067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Herrett E., Gallagher A.M., Bhaskaran K., et al. Data resource profile: Clinical Practice Research Datalink (CPRD) Int J Epidemiol. 2015;44:827–836. doi: 10.1093/ije/dyv098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Clinical Practice Research Datalink . 2022. CPRD.https://www.cprd.com/ [Google Scholar]
- 26.Ulm K. A simple method to calculate the confidence interval of a standardized mortality ratio (SMR) Am J Epidemiol. 1990;131:373–375. doi: 10.1093/oxfordjournals.aje.a115507. [DOI] [PubMed] [Google Scholar]
- 27.Kidney Disease: Improving Global Outcomes (KDIGO) Blood Pressure Work Group KDIGO 2021 Clinical Practice Guideline for the Management of Blood Pressure in Chronic Kidney Disease. Kidney Int. 2021;99:S1–S87. doi: 10.1016/j.kint.2020.11.003. [DOI] [PubMed] [Google Scholar]
- 28.American Diabetes Association 11. Microvascular complications and foot care: standards of medical care in diabetes-2021. Diabetes Care. 2021;44(suppl 1):S151–S167. doi: 10.2337/dc21-S011. [DOI] [PubMed] [Google Scholar]
- 29.Staruschenko A., Bhalla V., Rangaswami J. SGLT2 inhibitors: diabetic kidney disease and beyond. Am J Physiol Ren Physiol. 2020;319:F780–F781. doi: 10.1152/ajprenal.00518.2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Heerspink H.J.L., Stefansson B.V., Correa-Rotter R., et al. Dapagliflozin in patients with chronic kidney disease. N Engl J Med. 2020;383:1436–1446. doi: 10.1056/NEJMoa2024816. [DOI] [PubMed] [Google Scholar]
- 31.Foley R.N., Murray A.M., Li S., et al. Chronic kidney disease and the risk for cardiovascular disease, renal replacement, and death in the United States Medicare population, 1998 to 1999. J Am Soc Nephrol. 2005;16:489–495. doi: 10.1681/ASN.2004030203. [DOI] [PubMed] [Google Scholar]
- 32.Tonelli M., Wiebe N., Culleton B., et al. Chronic kidney disease and mortality risk: a systematic review. J Am Soc Nephrol. 2006;17:2034–2047. doi: 10.1681/ASN.2005101085. [DOI] [PubMed] [Google Scholar]
- 33.Hallan S.I., Matsushita K., Sang Y., et al. Age and association of kidney measures with mortality and end-stage renal disease. JAMA. 2012;308:2349–2360. doi: 10.1001/jama.2012.16817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Carrero J.J., Grams M.E., Sang Y., et al. Albuminuria changes are associated with subsequent risk of end-stage renal disease and mortality. Kidney Int. 2017;91:244–251. doi: 10.1016/j.kint.2016.09.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lees J.S., Welsh C.E., Celis-Morales C.A., et al. Glomerular filtration rate by differing measures, albuminuria and prediction of cardiovascular disease, mortality and end-stage kidney disease. Nat Med. 2019;25:1753–1760. doi: 10.1038/s41591-019-0627-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Chronic kidney disease in adults: assessment and management. NICE; 2014. https://www.nice.org.uk/guidance/cg182/resources/chronic-kidney-disease-in-adults-assessment-and-management-pdf-35109809343205 [PubMed] [Google Scholar]
- 37.Perkins R.M., Chang A.R., Wood K.E., et al. Incident chronic kidney disease: trends in management and outcomes. Clin Kidney J. 2016;9:432–437. doi: 10.1093/ckj/sfw044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Feakins B., Oke J., McFadden E., et al. Trends in kidney function testing in UK primary care since the introduction of the quality and outcomes framework: a retrospective cohort study using CPRD. BMJ Open. 2019;9 doi: 10.1136/bmjopen-2018-028062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Inker L.A., Astor B.C., Fox C.H., et al. KDOQI US commentary on the 2012 KDIGO clinical practice guideline for the evaluation and management of CKD. Am J Kidney Dis. 2014;63:713–735. doi: 10.1053/j.ajkd.2014.01.416. [DOI] [PubMed] [Google Scholar]
- 40.Sultan A.A., James G., Wang X., et al. Incidence of uncommon clinical events in USA patients with dialysis-dependent and nondialysis-dependent chronic kidney disease: analysis of electronic health records from TriNetX. Nephron. 2021;145:462–473. doi: 10.1159/000516280. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The datasets generated during and/or analyzed during the current study are available upon reasonable request in accordance with AstraZeneca’s data sharing policy described at https://astrazenecagroup-dt.pharmacm.com/DT/Home.