Abstract
Bacteroides conjugative transposons (CTns) are thought to transfer by first excising themselves from the chromosome to form a nonreplicating circle, which is then transferred by conjugation to a recipient. Earlier studies showed that transfer of most Bacteroides CTns is stimulated by tetracycline, but it was not known which step in transfer is regulated. We have cloned and sequenced both ends of the Bacteroides CTn, CTnDOT, and have used this information to examine excision and integration events. A segment of DNA that contains the joined ends of CTnDOT and an adjacent open reading frame (ORF), intDOT, was necessary and sufficient for integration into the Bacteroides chromosome. Integration of this miniature form of the CTn was not regulated by tetracycline. Excision of CTnDOT and formation of the circular intermediate were detected by PCR, using primers designed from the end sequences. Sequence analysis of the PCR products revealed that excision and integration involve a 5-bp coupling sequence-type mechanism possibly similar to that used by CTn Tn916, a CTn found originally in enterococci. PCR analysis also demonstrated that excision is a tetracycline-regulated step in transfer. The integrated minielement containing intDOT and the ends of CTnDOT did not excise, nor did a larger minielement that also contained an ORF located immediately downstream of intDOT designated orf2. Thus, excision involves other genes besides intDOT and orf2. Both intDOT and orf2 were disrupted by single-crossover insertions. Analysis of the disruption mutants showed that intDOT was essential for excision but orf2 was not. Despite its proximity to the integrase gene, orf2 appears not to be essential for excision.
Many strains of Bacteroides species carry large self-transmissible elements called conjugative transposons (CTns). Many of these CTns are related to an element called CTnDOT. CTnDOT carries a tetracycline resistance gene, tetQ, and an erythromycin resistance gene, ermF (2, 23, 25). A schematic view of CTnDOT is shown in Fig. 1. Intercellular transposition of CTns is thought to occur by the following steps. The CTn initiates conjugal transfer by excising from the chromosome to form a circular intermediate. This intermediate is nicked at the transfer origin (oriT), and a single-stranded copy is transferred to the recipient cell, recircularized, and integrated into the recipient's genome (15, 17, 21). The region of CTnDOT that mediates the nicking and transfer reactions has been located and sequenced (11; G. Bonheyo, D. Graham, N. B. Shoemaker, and A. A. Salyers, submitted for publication), but virtually nothing is known about the excision and integration steps. In particular, there is only indirect evidence for the existence of the hypothetical circular intermediate that presumably forms during the excision reaction and later integrates in the recipient. In this paper, we present direct evidence that the circular intermediate is formed during the excision process and is the form that integrates into the chromosome.
FIG. 1.
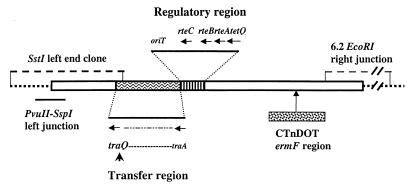
Schematic map of the integrated forms of CTnERL and CTnDOT. The chromosomal DNA flanking the integrated element(s) is shown as dotted lines. The 13-kbp region containing ermF that is contained on CTnDOT but is not on CTnERL is indicated by the patterned rectangle and arrow below the line. The regulatory region of the elements contains the tetQ-rteA-rteB-rteC gene cluster that encodes proteins that are induced by tetracycline and that regulate activities of CTnDOT and CTnERL. Adjacent to this region is the oriT-mob and the transfer region that contains the transfer genes (traA to traQ). A sequence of traQ cloned on an insertional vector was used to clone the CTnDOT-chromosome left end junction from BT4107N1 on an SstI fragment. A PvuII-SspI fragment was subcloned and sequenced. The CTnDOT right end-chromosome junction was cloned from the same strain on a 6.2-kbp EcoRI fragment.
Previously, integration and excision of CTns have been studied in detail only in the case of Tn916, a CTn first found in enterococci (5, 7). Analysis of integration and excision events mediated by Tn916 led to the proposal of a novel model for integration and excision (3). During excision, staggered cuts are made 6 bp from either end. Then, each single-stranded 6-bp overhang is joined to the other end of the CTn to create a circular form in which six bases of double-stranded but unpaired DNA separate the ends of the CTn (5, 21). The 6-bp segments are called coupling sequences. At some point after excision, the 6-bp region is resolved in favor of one coupling sequence or the other (12). In natural hosts of Tn916, this resolution seems to occur very quickly in the donor cell, but it could also occur during the conjugal transfer step. After conjugal transfer of a single-stranded copy of the CTn, integration occurs in the recipient. The integration process does not duplicate the target site. Recently, the integrase of Tn916 and an Xis protein have been purified and shown to bind DNA adjacent to the ends of the CTn (10, 15).
The CTnDOT-type elements differ in a number of ways from Tn916. First, Tn916 integrates relatively randomly, whereas CTnDOT appears to integrate site selectively into about seven sites on the Bacteroides chromosome (2). Second, there is no sequence similarity between CTnDOT and Tn916 in any of the regions so far sequenced. Thus, it was not clear whether CTnDOT would excise and integrate similarly to Tn916 or by a different mechanism. A third difference between CTnDOT and Tn916 is that transfer of CTnDOT is stimulated 1,000- to 10,000-fold by tetracycline (19), a fold increase much higher than the tetracycline stimulation reported for Tn916 transfer (5). The tetracycline-dependent stimulation of CTnDOT transfer is mediated by proteins encoded by three regulatory genes: rteA, rteB, and rteC (18, 29). No regulatory genes analogous to the rte genes of CTnDOT have been found on Tn916. Previous studies of the transfer region of the Bacteroides CTns showed that transfer of a plasmid that contained the CTn transfer region was constitutive (11; Bonheyo et al., submitted). In this paper, we show that excision is a tetracycline-regulated step in transfer.
Also found in Bacteroides species are integrated elements that are much smaller than the CTns. These elements, exemplified by NBU1, Tn5520, and Tn4555, have been called mobilizable transposons because they rely on the CTns for transfer functions. The integrases of NBU1, Tn5520, and Tn4555 have been sequenced, and all are members of the lambda family of integrases (27, 32, 33). NBU1 excision more closely resembles excision of phage lambda in that there is an att site formed by the joined ends that integrates into an identical site in the chromosome. Excision of Tn4555 is more similar to that of Tn916 (31). Although the mobilizable transposons rely absolutely on the CTns for transfer, there is so far no evidence for any significant sequence similarity between them and the CTns. In this paper, we show that the integrases of two Bacteroides CTns are not closely related to those of the mobilizable transposons, although they are also members of the lambda integrase family.
MATERIALS AND METHODS
Bacterial strains, conjugations, and DNA manipulations.
Bacterial strains used in this study are listed in Table 1. Methods of DNA extraction, cloning and Southern blotting analysis, and conjugation protocols have been described previously (9, 16, 20, 24).
TABLE 1.
Bacterial strains and plasmids
| Strain or plasmid | Relevant phenotypea | Source and/or description |
|---|---|---|
| E. coli | ||
| DH5αMCR | RecA | Gibco BRL |
| EM24NR | RecA Strr Nalr Rifr | RecA derivative of LE392 with spontaneous mutations to rifampin and nalidixic acid resistance (20) |
| S17-1 | RecA Tpr TraRP4 | Contains the transfer functions of RP4 integrated in the chromosome (28) |
| BW19851 | RecA Tpr TraRP4 Pir+ | S17-1 with pirR6K in uidA; supports the replication of Pir-dependent vectors (13) |
| Bacteroides thetaiotaomicron 5482 | ||
| BT4001 | (Rifr) | Spontaneous rifampin-resistant mutant of BT5482 |
| BT4107N1 | (Thy− Tcr Emr) | Spontaneous thymidine-requiring mutant of BT5482 (BT4100) containing CTnDOT (1) inserted at attDOT-JS 2 site |
| BT4107-1 | (Thy− Tcr Emr) | BT4100 with CTnDOT inserted in attDOT-JS 1 |
| BT4107-3 | (Thy− Tcr Emr) | BT4100 with CTnDOT inserted in attDOT-JS 3 |
| BT4107-4 | (Thy− Tcr Emr) | BT4100 with CTnDOT inserted in attDOT-JS 4 |
| BT4107-5 | (Thy− Tcr Emr) | BT4100 with CTnDOT inserted in attDOT-JS 5 |
| BT4104N1-3 | (Thy− Tcr) | BT4100 with ΩCTnERL |
| BT4004-5 | (Rifr Tcr) | BT4001 with ΩCTnERL |
| BT4004-Ωint | (Rifr Tcr Emr Exc−) | CTnERL with insertion of pCQW1-int; excision minus (this study) |
| BT4004-Ωorf2 | (Rifr Tcr Emr Exc+) | CTnERL with insertion of pCQW1-orf2; excision plus (this study) |
| Plasmids | ||
| pGEM-T | Apr | E. coli PCR cloning vector (Promega) |
| pGEM-T:DOT-JS2 | Apr | PCR product of CTnDOT target site DOT-JS 2 cloned onto pGEM-T (this study) |
| pNLY3 | (Cmr) Cmr Apr | Bacteroides suicide vector contains IS4351-cat and the oriTRK2 cloned into pUC19. It is Cmr in both E. coli and Bacteroides recipients and can be mobilized by IncPα plasmid RP1 or the RP4 transfer functions in E. coli S17-1 |
| pNLY3:DOT-LE | (Cmr Rep−) Apr | 15-kbp CTnDOT left junction on pNLY3 (this study) |
| pDOT5B/SstI | Apr | 5.5-kbp PvuII-SspI fragment subcloned from pNLY3:DOT-LE into SmaI site of pUC19 (this study) |
| pXBU1 | Apr | 1.5-kbp CTnXBU4422 right junction on pUC19 (26) |
| pUC:DRJ | Apr | 6.2-kbp CTnDOT right junction cloned on EcoRI site of pUC19 (this study) |
| pGERM | (Emr Rep−) Apr | Bacteroides pUC19-based suicide vector containing ermG and oriTRK2 that mobilized by RP4 transfer functions in E. coli S17-1 chromosome (35) |
| pDJE1.1 | (Emr Int−) Apr | 1.1-kbp DJE PCR product subcloned from pGEM-T into pGERM (this study) |
| pDJE2.3 | (Emr Int−) Apr | 2.3-kbp DJE PCR product subcloned from pGEM-T into SstI-SphI sites of pGERM; contains intDOT and integrates efficiently into Bacteroides target sites (this study) |
| pDJE2.6 | (Emr Int+) Apr | 2.6-kbp DJE PCR product subcloned from pGEM-T into pGERM; contains intDOT and orf2; integrates but cannot excise (this study) |
Phenotypes in parentheses are expressed only in Bacteroides; phenotypes outside the parentheses are expressed in E. coli. Abbreviations: Apr, ampicillin resistant; Rifr, rifampin resistant; Emr, erythromycin resistant; Tcr, tetracycline resistant; Cmr, chloramphenicol resistant; Int+ or Int−, ability or inability to integrate; Rep−, cannot replicate.
Cloning of the CTnDOT right end.
Previously, we fortuitously trapped a CTn from Bacteroides uniformis, CTnXBU4422, on a plasmid (25). The ends of CTnXBU4422 hybridize with the ends of CTnDOT, and so we used the CTnXBU4422 right end as a hybridization probe to assist in cloning of the CTnDOT right end. A 2.2-kbp DNA fragment containing the CTnXBU4422 right end from plasmid pXBU1 (25) was used as a probe in Southern blots to identify the DOT right-end fragment. Chromosomal DNA from BT4107N1, which contains CTnDOT, was digested with EcoRI, and a 6.2-kbp fragment hybridized to the probe. Restriction fragments around 6.2 kbp were recovered from a low-melting-point agarose gel, cloned into the EcoRI site of pUC19, and transformed into Escherichia coli. Colony hybridization was used to detect positive colonies using the CTnXBU4422 right-end fragment probe. The 6.2-kbp EcoRI fragment on pUC19:DRJ that hybridized to the probe was sequenced partially to locate the right end. The end was located initially by comparing the sequence of the clone with the sequence of the XBU4422 right end (2) and later to the sequences of the CTnDOT joined ends (DJE) and the BT4107N1 integration site. A 2.5-kbp region of the CTnDOT right end was double-strand sequenced. Sequencing was performed by the University of Illinois Biotechnology Genetic Engineering Facility with an Applied Biosystems model 373A version 2.0.1S dye terminator automated sequencer.
Cloning of the CTnDOT left end.
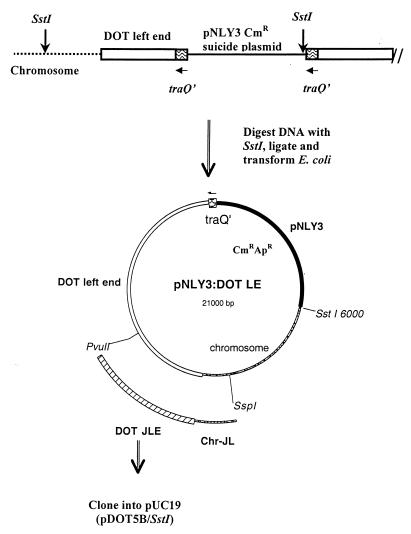
Initially, we tried to clone the CTnDOT left end by using the XBU4422 left-end fragment as the probe but were not successful. Therefore, we decided to clone by using a Bacteroides suicide plasmid to integrate into a sequenced region that was next to the left end of CTnDOT. We would then clone out the plasmid with as much of the adjacent left-end region CTnDOT as possible into E. coli. The vector used was pNLY3, a pUC19-based vector containing the oriT of RK2 for mobilization out of E. coli and IS4351-cat, which is expressed in both E. coli and Bacteroides hosts (Table 1). The region of homology used for the integration was the internal region of traQ (Bonheyo et al.) which by Southern blots appeared to be >15 kbp from the CTnDOT left end (Fig. 1). A 368-bp fragment of traQ was amplified by PCR and cloned into pGEM-T (Promega, Madison, Wis.). The fragment was sequenced to determine orientation and subcloned into pNLY3.
The resulting suicide plasmid was mobilized from E. coli S17-1 to BT4107N1 with selection for chloramphenicol resistance (Cmr). Several Cmr transconjugants were obtained, and integration of the suicide plasmid into the traQ region was verified by Southern blot analyses. Chromosomal DNA isolated from these transconjugants was digested with SstI that cut once at the right end of the integrated form of the plasmid and in the chromosomal region beyond the left end of CTnDOT (Fig. 2). The SstI-digested DNA was cleaned, ligated, and used to transform E. coli selecting for ampicillin resistance (Apr) and Cmr on pNLY3 (Fig. 2). One of the clones obtained, designated pNLY3:DOT-LE, had a 15-kbp fragment containing the CTnDOT left end and some chromosomal sequences. Because this clone was extremely unstable in E. coli, a 5.5-kbp PvuII-SspI fragment that hybridized to the CTnXBU4422 left-end probe was subcloned into the SmaI site of pUC19 for sequencing (pDOT5B/SstI). To make sure we had wild-type sequence in this unstable region, we designed two primers (DLJ/R451F and DLJ/U487F [Table 2]), based on the sequences obtained from pDOT5B/SstI, to PCR amplify the DOT left junction region directly from BT4107N1. The sequence of the amplicon was identical to that of the PvuII-SspI subclone.
FIG. 2.
Cloning of the CTnDOT left junction. The integrated insertional shuttle vector pNLY3 containing 368 bp of traQ, pNLY3:traQ′, is shown integrated into the traQ region of ΩCTnDOT in BT4107N1. The Bacteroides transconjugant was selected as Cmr. Southern blots verified the insertion into traQ. The DNA from the transconjugant was digested with SstI, which was determined to cut one time in the distal end of the vector and in the chromosome beyond the end of CTnDOT, using the left-end probe from CTnXBU4422. The digested DNA was first ligated and then used to transform E. coli. A large unstable plasmid, pNLY3:DOT-LE, that contained about 15 kbp of addition CTnDOT left end (LE) and chromosomal DNA at the left junction (JL) was isolated from a Cmr Apr transformant. A 5.5-kbp PvuII-SspI fragment was a CTnDOT-chromosome junction fragment that contained the CTnDOT left end (DOT JLE) and the chromosomal left end junction (Chr-JL) and was subcloned onto pUC19 (pDOT5B/SstI) for sequencing.
TABLE 2.
PCR primers used in this study
| Primer | Sequence (5′-3′) |
|---|---|
| DLJ/U487F | CTA TGG GCA GAA GAG ACT AAG |
| DLJ/U430F | GCG GAA AGT GAA TGC GAG GTG |
| DLJ/INW-1/R127R | GCT ACT TTT GCA TAC GAA GAG |
| DLJ/INW-1/R241R | GAA AAC CCG TAA AAG CAC CTC |
| DLJ/R451F | TTG TAC TCG CTC GTG ATA ACT |
| DRJ/R343F | CTC GGA CAT ATT TCG GAC AG |
| DRJ/R593F | ATG CCT GCT GTT TCT CTT TGG |
| DRJ/R1729F | CTT TCA TGC CAG CCT CCA CCT |
| DRJ/R2242F | GCC ATC CAT ACC CGT TTG TC |
| DRJ/R2700R | AGG CAC TGT CAA GTC ATA GC |
| ERL-F1 | AGA ATA TGC GCA AAG GCT CG |
Detection of the joined ends of the excised CTnDOT.
The sequences obtained from the ends of the integrated CTnDOT in BT4107N1 were used to design primers DRJ/R1729F and DLJ/INW-1/R241R (Table 2). These primers are directed out of the ends of the integrated element and cannot form a PCR product unless the element excises and forms a circular intermediate. Ten independent cultures of BT4107N1 were grown overnight with and without tetracycline and tested for the production of PCR-amplified excision product; 10-μl aliquots of different overnight cultures were used directly in PCR. The cells were spun down and resuspended in the volume of water needed for the PCR. The cycling conditions were as follows: (i) 5 min at 95°C; (ii) 25 to 35 cycles of 1 min at 94°C, 1 min at 51°C, and 2 min at 72°C; (iii) final extension of 5 min at 72°C. The PCR products were cloned onto pGEM-T and sequenced.
Comparison of the left and right ends of CTnDOT and CTnERL.
Two primers (ERL-F1 and DLJ/INW-1/R241R [Table 2]) were used to PCR amplify the CTnERL joined ends. DNA from tetracycline-induced cells of strain BT4104, which contain a CTnERL insertion in the chromosome, was used as a source of template in a PCR. The PCR product was cloned onto pGEM-T and sequenced.
Disruptions in intERL and orf2.
The conjugative transposon CTnERL was nearly identical to CTnDOT at their right ends, and the joined ends of CTnERL could also be detected by PCR. This similarity allowed us to make disruptions in the putative int gene and orf2 (open reading frame 2) in the right end of CTnERL to test for their effect on CTn excision. We could then use erythromycin resistance (Emr) as the selection marker for the insertions. Insertions were then made in the integrase gene (intERL) and orf2ERL in BT4004. Disruptions in the integrase gene were made by cloning the 423-bp PCR-amplified internal fragment of intERL into pCQW1 (6), a plasmid that replicates in E. coli but not in Bacteroides hosts. Similarly, a 193-bp fragment of orf2 was cloned into pCQW1. The pCQW1 clones, pCQW1-int and pCQW1-orf2, were mobilized into BT4004 and were shown to integrate into the correct region of CTnERL by Southern blots. The resulting BT4004 Emr transconjugants with the insertions in CTnERL were then tested for the ability of CTnERL to excise and form a circular intermediate as detected by PCR amplification of the ERL joined ends (EJE).
Use of inverse PCR to clone the left junctions of CTnDOT inserted in different sites (DOT-JS).
Initially, we cloned and sequenced the DOT left and right junctions from one CTnDOT insertion strain, BT4107N1. The integration site in this strain was designated DOT-JS 2 and was PCR amplified by primers DRJ/R2700R and DLJ/U487F (Table 2). The only obvious sequence similarity between the CTnDOT ends and the integration site was a 10-bp sequence (5 bp away from the left end of the integrated CTnDOT) with high sequence identity to a 10-bp sequence present in the CTnDOT right end. To determine whether a similar sequence was found adjacent to the left ends of CTnDOT inserted in different sites, we examined the left-end junction sequences of four other strains, identified by pulsed-field gel analysis to have CTnDOT in different NotI bands (2). Inverse PCR, with out-directed primers anchored inside the left end of CTnDOT, was used to obtain left junctions from each of the four strains (BT4107-1, BT4107-3, BT4107-4, and BT4107-5). Specifically, chromosomal DNA from the four Bacteroides strains was digested with NlaIII and then ligated at a DNA concentration of about 2 μg/ml to favor monomeric circularization. After ligation, the circularized DNA molecules were used as templates in typical PCRs. The inverse PCR primers were DLJ/INW-1/R127R and DLJ/U430F (Table 2). The resultant PCR products were cloned into the pGEM-T vector and sequenced. The cloned junction sites were designated DOT-JS 1, DOT-JS 3, DOT-JS 4, and DOT-JS 5, respectively.
Construction of miniature versions of CTnDOT.
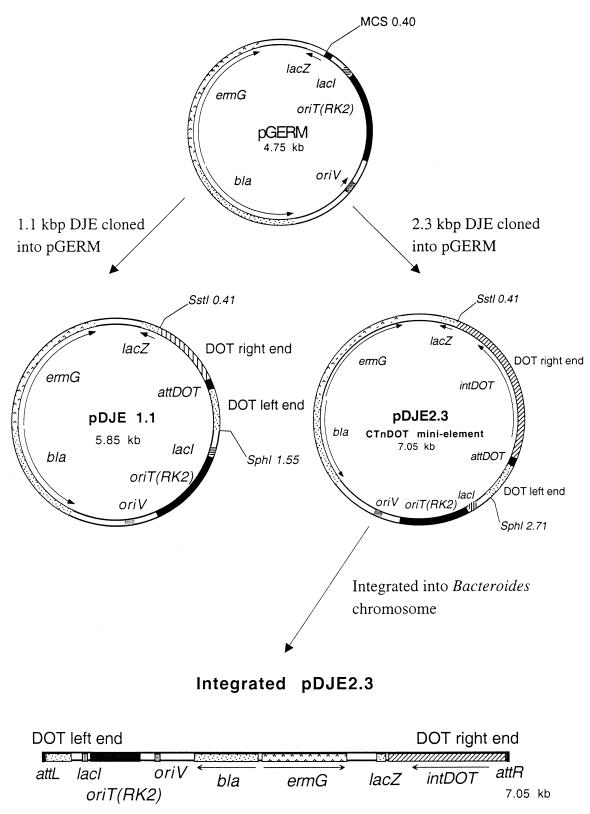
To identify the gene or genes necessary and sufficient to mediate integration of the circular form of CTnDOT into the chromosome, we PCR amplified three different CTnDOT joined ends (DJE) products, using DNA from tetracycline-induced BT4107N1 cells as a template. The sequences of the primers used to produce the various PCR products are shown in Table 2. The smallest DJE PCR product (primers DRJ/R1729F and DLJ/INW-1/R241R) was 1.1 kbp and contained only the N-terminal region of the putative integrase (intDOT). The 2.3-kbp DJE product (primers DRJ/R593F and DLJ/INW-1/R241R) included the entire intDOT and the 2.6-kbp product (primers DRJ/R343F and DLJ/INW-1/R241R) included the intDOT plus a second ORF (orf2) downstream of intDOT (see Results). All three DJE PCR products were first cloned into pGEM-T and then subcloned into the Bacteroides suicide vector pGERM (Fig. 3). These different pGERM:DJE plasmids (Table 1) were transformed into E. coli strain S17-1 and mobilized into BT4001, a strain that does not contain any known conjugative transposon, to test for the ability to integrate into BT4001 chromosome. The vectors that were capable of integrating were tested with and without pAMS9 (29) providing the tetracycline-regulated regulatory genes (rteA, rteB, and rteC) for the ability to excise using PCR amplification to detect the joined ends of the excised circular form.
FIG. 3.
Construction of the CTnDOT minielements pDJE1.1 and pDJE2.3. The Bacteroides shuttle suicide vector pGERM that contains the Bacteroides selection marker ermG and the oriTRK2 cloned on pUC19 was used to construct the CTnDOT minielements to test for integration in Bacteroides hosts. The smallest minielement constructed contained a 1.1-kbp PCR product that included the CTnDOT joined ends (DJE fragment or attDOT) produced from the excised circular form of CTnDOT, subcloned into pGERM from pGEM-T. The smallest PCR product subcloned into pGERM that integrated normally into B. thetaiotaomicron target or attB sites was the 2.3-kbp DJE product that included attDOT and intDOT from the right end of CTnDOT cloned into pGERM. This CTnDOT minielement construct was called pDJE2.3. The map of an integrated pDJE2.3 is shown at the bottom. The element-chromosome junction at the left is labeled attL, and the junction at the right is labeled attR. The locations and orientations of the genes on the integrated plasmid are indicated.
Comparison of DOT minielement junction sequences (DME-JS) with DOT-JS.
To determine if the CTnDOT minielement has the same site selectivity as the wild-type CTnDOT, it was necessary to clone and compare their integration sites (attB). Sixteen Bacteroides Emr transconjugants with insertions of the CTnDOT minielement were isolated from 16 individual filter matings. Chromosomal DNA from these transconjugants was digested with KpnI, which does not cut inside the CTnDOT minielement. The digested DNA fragments were ligated and transformed into E. coli with selection for Apr. Plasmids in the transformants contained the minielement plus DNA adjacent to both ends of the integrated minielement. These DME-JS sites were sequenced using the primers DRJ/R2242F (right junction) and DLJ/INW-1/R127R (left junction).
RESULTS
Sequence features of the ends of CTnDOT.
The sequence of the left end of CTnDOT did not contain any ORFs larger than 600 bp within the first 2 kbp from the end. Of the few smaller ORFs in this region, none had any amino acid sequence similarity to known integrases. The sequence of the right end, however, had an ORF whose first possible start site was 329 bp from the right end. Advanced Blastp (1) analysis of the deduced amino acid sequence of this ORF showed low sequence similarity to known integrases, such as the integrase of phage P2 (26% identity) and the integrases of the Bacteroides mobilizable transposons NBU1 (24% identity [27]), NBU2 (34), Tn5520 (49% identity [33]) and Tn4555 (26% identity [32]). The putative integrase gene was 1,233 bp in length. Previously, we had obtained a small amount of sequence near the ends of a cryptic CTn, CTnXBU4422, but had not identified any potential integrase gene. The integrase gene of CTnDOT is the first such gene from a Bacteroides CTn to be sequenced and characterized.
An alignment of amino acid sequences in essential regions of several members of the site-specific recombinase family (14) such as P2 integrase, YqkM (Bacillus subtilis), and XerC (Lactobacillus leichmannii) is shown in Fig. 4, along with the integrases of four mobilizable integrated Bacteroides elements, NBU1, NBU2, Tn5520 (33), and Tn4555 (32). The Tn5520 integrase was 75% identical to the putative CTnDOT integrase in the box II region shown in Fig. 4, with close to 50% identity throughout the whole length of the protein. The sequence identity to the other integrases was confined to the C-terminal domains shown in Fig. 4. The highly conserved triad HRY amino acid residues in box II of the C-terminal segment, which are characteristic of lambdoid phage integrases, were seen in the IntDOT deduced amino acid sequence and the other Bacteroides integrases. The amino acids are at positions H345, R348, and Y381 in the putative CTnDOT integrase. However, the arginine (R) residue in box I, which is also conserved in the lambda integrase family and in the mobilizable transposons NBU1, NBU2, and Tn4555, was not found at the appropriate position in the CTnDOT integrase. Instead, it had a serine (S) at that position. The R in box I was also replaced in IntERL (S) and Int-Tn5520 (A). The arginine is one of the residues thought to be important for lambda integrase catalysis. Either this arginine can be replaced by the residues shown here or this substitution may indicate slight differences in the catalytic events catalyzed by the CTn and Tn5520 integrases.
FIG. 4.
Sequence comparison of the C-terminal regions IntDOT and IntERL to related integrases. The box I and box II regions in the C-terminal ends of the lambda family of site-specific integrases as defined by Nunes-Düby et al. (14) are shown. The conserved arginine (R) in box I and the HRY triad in box II are in boldface and boxed. The GH doublet in box II that is also highly conserved is shown in boldface. The integrases are grouped: IntDOT, IntERL, IntTn5520 (33), and IntTn4555 (32) are at the top, followed by integrases from two other Bacteroides mobilizable transposons, NBU1 (27) and NBU2 (34). The third group contains members of the lambda family of integrases that come from other organisms: YqkM (Bacillus subtilis), P2 integrase, and XerC (Lactobacillus leichmannii). The shading indicates the regions of identity in the Int proteins relative to the IntDOT sequence shown in the top line. The locations in the integrase amino acid sequences of the amino acids involved in the alignments are shown at the ends of the sequences.
Immediately downstream (12 bp) from the putative stop codon of the CTnDOT integrase, and transcribed in the same direction away from the end, was a second ORF that was 360 bp in length. This ORF was designated orf2. Although this gene had no homologs in the databases, its proximity to the putative int gene suggested that it might play a role in excision, by analogy to lambda xis. Like lambda Xis, the predicted amino acid sequence of the protein encoded by orf2 indicated that the protein would be small (120 amino acids) and basic (pI = 8.5).
An alignment of the CTnDOT left and right end sequences showed 23-bp imperfect inverted repeats (Fig. 5A) similar to that seen for CTnXBU4422 (2). It is possible that the inverted repeats and the flanking sequence at the two ends of CTnDOT are DNA binding sites of proteins which are involved in integration and excision. Future work on the mechanism of CTnDOT transposition will be needed to determine whether this is the case.
FIG. 5.
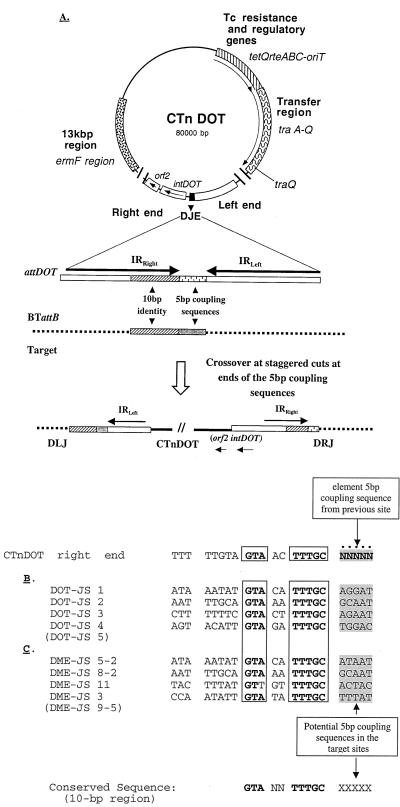
Model for the integration of CTnDOT into Bacteroides target sites. (A) Circular intermediate of CTnDOT after conjugal transfer and prior to integration with the known regions of the element indicated. The attDOT in the DJE is enlarged to show the 23-bp imperfect inverted repeats (IRRight and IRLeft), the 10 bp of high similarity to a Bacteroides target site (BTattB), and a 5-bp coupling sequence derived from its last site of integration. Details of the 10-bp alignment of the attDOT region and some attB sites are shown in panels B and C. Following staggered cuts flanking the coupling sequences in the attDOT and attB, the CTnDOT (or minielement) integrates into the target site. The 10-bp consensus sequence in the CTnDOT right end in the circular form is shown above the attB sequences in panels B and C, with the coupling sequence indicated by N's. The sequences in panel B are from the chromosomal target sites prior to integration of the circular form of the CTnDOT (DOT-JS). The sequences in panel C are from the chromosomal site prior to integration of pDJE2.3 (DME-JS). A conserved sequence GTANNTTTGC (10-bp region) was derived from a comparison of the CTnDOT right end and its target sites. The minielement target sites DME-JS 5-2 and DME-JS 8-2 are the same as the wild-type CTnDOT sites DOT-JS 1 and DOT-JS 2, respectively. After conjugal transfer and integration of pDJE2.3 (not shown), the coupling sequence from pDJE2.3 was found at the left side of the integrated element, adjacent to the 10-bp attB sequence, in DME-JS 3, 5-2, and 9-5 and the right side of the integrated pDJE2.3 in DME-JS 8-2 and 11 (sequence not shown). Parentheses indicate that the site was the same as the site above it.
We also obtained 1 to 2 kbp of sequence from the ends of two other Bacteroides CTns, CTnERL and CTnXBU4422, shown to be related to CTnDOT by Southern blots. There was high sequence identity between the ends of CTnXBU4422 and the ends of CTnDOT as was predicted from previous Southern hybridization results (25). The sequences of the CTnERL ends were virtually identical to those of CTnDOT (95% identity). At the right end of CTnERL are found an intERL and orf2, which were, respectively, 96 and 93% identical in predicted amino acid sequence to the proteins encoded by intDOT and orf2 on CTnDOT.
Sequence features of the integration site.
Since CTnDOT appeared to integrate site selectively, we expected to see some sequence similarity between one or both ends of CTnDOT and the integration sites. Comparisons of the sequences of the ends of CTnDOT with the sequences of the integration sites revealed some sequence similarity. One candidate for a recognition sequence was a 10-bp sequence that was immediately adjacent to the site where CTnDOT entered the chromosome and at the right end of the circular form of the element (Fig. 5A). In the integrated form, this target 10-bp sequence was 5 bp from the left end. Using inverse PCR, we obtained sequences adjacent to the left end of CTnDOT from insertions in four other chromosomal sites. Similar 10-bp sequences were seen in all four cases (Fig. 5B). Comparison of the four different insertion site sequences (DOT-JS 1 to DOT-JS 5) allowed us to define a consensus sequence that may be responsible for the site selectivity of the insertion process. The fact that this region is so small fits with the likelihood that there are multiple sites in the chromosome that could serve as integration sites for CTnDOT (2).
The integrase gene and the joined end sequences are sufficient for integration into the Bacteroides chromosome.
To determine whether the putative integrase gene near the right end of CTnDOT was sufficient for integration in Bacteroides, we constructed a CTnDOT minielement which contained the joined ends of CTnDOT and the putative integrase gene (Fig. 3). The pGERM-based minielement, pDJE2.3, was mobilized into BT4001 with selection for Emr. Approximately 10−3 to 10−4 transconjugants per recipient were obtained in each of several independent matings. Since this is comparable to the frequency of transfer of a plasmid that replicates in Bacteroides species, the integration efficiency of the minielement may be close to 100% once the element enters the recipient. We also constructed a DJE vector that contained the joined ends of CTnDOT but only a truncated integrase gene, pDJE1.1 (Fig. 3). When pDJE1.1 was mobilized into BT4001 under the same conditions, no transconjugants (<10−9 per recipient) were obtained. These results prove that the joined ends alone were not sufficient for integration but that including the intDOT ORF allowed the plasmid to integrate. The fact that integration occurred independently of tetracycline stimulation and independently of the rte genes on CTnDOT or CTnERL, which mediate tetracycline-inducible activities of the CTns, demonstrates that integration is not the tetracycline-regulated step in CTnDOT transfer.
To confirm that the minielement was integrating by the ends of the element and integrating into similar sites to those favored by CTnDOT, we cloned and sequenced the left junction sequences from five minielement insertions (DME-JS). We found two integration events in which the minielement used the same sites used by CTnDOT (DME-JS 5-2 and DME-JS 8-2). In the other three sites, good matches to the 10-bp consensus region were found immediately at the left end, next to the sequences that would become coupling sequences when the element excised (Fig. 5C). Only scattered sequence identity was also found between the CTnDOT left end and the minielement target sites (data not shown). Sequence analysis of the junction region also revealed that the minielement was in fact integrating precisely at the ends used by the intact CTnDOT. Thus, the minielement acts like the parent CTn with respect to integration.
Coupling sequences of CTnDOT in BT4107N1.
Sequence analysis of the right and left ends of the element, as defined by comparison of junction regions with the sequence of the joined ends, revealed that when CTnDOT excises from chromosome, it brings 5 bp of chromosomal sequence with it. We call these sequences “coupling sequences” following the model for the conjugative transposon Tn916 (21, 22). When the excised CTn forms a circular intermediate, the coupling sequence is located between the joined ends (Fig. 5A). According to the model for Tn916 excision, the initial form of the excised element has coupling sequences from the two ends of the element, which form a short region of heterology. In Enterococcus faecalis, as Manganelli et al. (12) have shown, the heterology is rapidly eliminated in the donor in favor of one coupling sequence or the other. Thus, prior to conjugal transfer, either coupling sequence could be found in the excised circular form. To determine if this was the case for CTnDOT, PCRs were performed to amplify the 1.1-kbp DOT joined ends segment from DNA isolated from 10 independent tetracycline-induced BT4107N1 cultures as template. The amplicons were sequenced and compared to detect the coupling sequences. In 4 of the 10 excision events, the excised CTnDOT joined ends contained the GCAAT chromosomal sequence from the left side, and 6 contained AATTC from the right side. This is the outcome expected if excision of CTnDOT occurred similarly to that of Tn916. The PCR approach used here would not distinguish between a region of heterology and a region resolved in favor of one coupling sequence or the other, but the fact that both coupling sequences were seen indicates that excision involved an intermediate that contained both coupling sequences at one time.
When the excised form is transferred by conjugation to a recipient, only one strand is transferred and so only one coupling sequence reaches the recipient. As expected, sequence analysis of pDJE2.3 integration events placed the same coupling sequence on one side or the other. pDJE2.3 was constructed from BT4107N1, and the coupling sequence was AATTC. After pDJE2.3 integrated in the target sites shown in Fig. 5C, AATTC replaced the 5-bp sequence shown in Fig. 5C at the left junction in three of five cases and was found at the right junction in two of five cases. This behavior is similar to that seen in the case of conjugal transfer of Tn916 (21).
To determine if CTnERL coupling sequences were similar in size to those of CTnDOT, we used PCR to amplify the joined ends from 10 independent excision events of CTnERL from a particular site, BT4104N1-3. In this case, coupling sequences were also seen, but in five cases the coupling sequence was 5 bp long (AATAC, from left side) and in the other five cases it was only 4 bp long (GAAA, from right side). To determine whether this was a consistent feature of CTnERL excision, we examined the coupling sequences from a different CTnERL insertion strain, BT4004-5. In this case, the coupling sequences were both 4 bp in length (GTTT or TCCT). Thus, it appears that CTnDOT-type elements can have coupling sequences of either 4 or 5 bp in length.
Importance of the integrase for excision.
Results presented above show that the int gene was needed for integration. To determine whether the integrase was essential for excision, we constructed a single-crossover disruption in the integrase gene of CTnERL, BT4004-Ωint. Excision of wild-type CTnERL was easily detectable by PCR amplification of the joined ends, but no excision was detected in the disruption strain (Table 3). Thus the int gene appears to be essential for excision. Excision of the wild-type CTnERL element occurred only after exposure of cells to tetracycline. This was also true for CTnDOT. These results demonstrate that excision is a tetracycline-regulated step in transposition. To determine whether the downstream orf2 played a role in excision, we made a single-crossover insertion into it. The disruption mutant, BT4004-Ωorf2, was still able to excise. This shows not only that orf2 is not essential for excision but that loss of the ability to excise caused by the disruption in integrase was not due to a polar effect on orf2 or to insertion of a large DNA segment into this region of the CTn.
TABLE 3.
PCR amplification of joined ends to detect excision
| Strain | Tetracycline induction | PCR excision product |
|---|---|---|
| BT4004 (CTnERL) | − | No |
| + | Yes | |
| BT4004-Ωint | − | No |
| + | No | |
| BT4004-Ωorf2 | − | No |
| + | Yes | |
| BT4107 (CTnDOT) | − | No |
| + | Yes | |
| BT4001 (ΩpDJE2.6, pAMS9) | − | No |
| + | No |
Since orf2 was so small, it was possible that the disruption mutant allowed enough of the protein to be made for excision to occur. Accordingly, we also constructed a larger minielement, pDJE2.6, which contained both intDOT and orf2. pDJE2.6 integrated at the same frequency in BT4001 as pDJE2.3. Earlier studies had indicated that there were three regulatory proteins on CTnDOT and CTnERL, RteA, RteB, and RteC, which control tetracycline-dependent CTn activities (19, 27, 30, 31). Accordingly, we provided in trans a plasmid, pAMS9 (29), that carried tetQ, rteA, rteB, and rteC in the strain that had pDJE2.6 integrated into its chromosome. The PCR assay to detect the joined ends was done using DNA from the Bacteroides cells grown with and without tetracycline in the medium. No excision product was seen (Table 3). Excision either did not occur at all or was too inefficient to be detected even by PCR.
DISCUSSION
Although the Bacteroides CTns of the CTnDOT family share no discernible DNA sequence similarity with Tn916 in any of the sequences within 3 kbp of either end of the element, these two types of elements seem to integrate and excise by similar methods. That is, excision brings along a coupling sequence from chromosomal DNA adjacent to the excision site and introduces a coupling sequence into a target site when it integrates after having been transferred by conjugation. In earlier studies of the sequences of the ends of CTnXBU4422, which had been accidentally trapped on a plasmid in B. uniformis, we had concluded that CTnXBU4422 integrated by a blunt-ended integration mechanism. At that time, however, we were unable to detect the joined ends of CTnXBU4422 after excision and did not know if there was a bias for the left-end or right-end coupling sequences to be excised with the element. We have since sequenced the PCR products of the joined ends and found that the excised form of CTnXBU4422 detected in B. uniformis 0061 contained the 4-bp left-end sequence (CCCG) about half the time and the 5-bp right-end sequence (GAAAA) about half the time. However, insertions into the plasmid targets on a plasmid replicating in B. uniformis contained the CCCG coupling sequence at the right end of the integrated element in 9 out of 10 cases tested and GAAAA was at the right end in one (2). The reason for the observed bias is not understood. We were also unable to observe conjugal transfer and integration of CTnXBU4422 into B. thetaiotaomicron from B. uniformis, either from the chromosomal insertion or from a plasmid containing ΩCTnXBU4422 (25).
Whereas Tn916 integrates randomly (21, 22), CTnDOT and CTnERL exhibit some site selectivity. This site selectivity may be due to a 10-bp consensus sequence at the right end of the CTn, which has sequence identity to a 10-bp sequence adjacent to the integration site. IntDOT and IntTn916 are both members of the lambda family of site-specific integrases, but the amino acid sequence identity is below 30% even within the C-terminal region of the proteins. The integrase most closely related to the CTnDOT integrase was that of Tn5520 (49% identity and 69% similarity), a 5.5-kbp Bacteroides fragilis integrated element (33), which is probably mobilized by a CTnDOT-type element. There was also limited amino acid sequence similarity to the mobilizable transposons Tn4555, NBU1, and NBU2. The sequence of the integrase of another integrated mobilizable Bacteroides element, Tn4399 (8), is not yet available.
A major difference between the Bacteroides CTns and Tn916 is that the excision reaction catalyzed by the Bacteroides CTns is dependent on tetracycline stimulation and appears to be more complex than that of Tn916. The excision of CTnDOT and related CTns requires more than just the int gene and an xis-like ORF downstream of int. By contrast, the Tn916 int and xis genes seem to be sufficient for excision (15). In the case of the Bacteroides CTns, the int gene was essential for excision as well as for integration, but the downstream orf2 was neither essential for nor sufficient for excision. The as yet unidentified excision genes may well be regulatory genes, although adding known CTn regulatory genes (rteA, rteB, and rteC) in trans with the integrated pDJE2.6 did not induce pDJE2.6 to excise. Future studies will involve identifying genes and DNA sequences that are required for CTnDOT excision.
ACKNOWLEDGMENTS
We thank Kelly Hayes for assistance in the cloning and sequencing of the left end of CTnDOT.
This work was supported by grant AI22383 (A.A.S.) and grant GM28717 (J.F.G.) from the National Institutes of Health.
REFERENCES
- 1.Altschul S F, Madden T L, Schaffler A A, Zhang J, Zhang Z, Miller W, Lipman D J. Gapped Blast and Psi-Blast: a new generation of protein database search programs. Nucleic Acids Res. 1997;25:3389–3402. doi: 10.1093/nar/25.17.3389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bedzyk L A, Shoemaker N B, Young K E, Salyers A A. Insertion and excision of Bacteroides conjugative chromosomal elements. J Bacteriol. 1992;174:166–172. doi: 10.1128/jb.174.1.166-172.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Caparon M G, Scott J R. Excision and insertion of the conjugal transposon Tn916 involves a novel recombination mechanism. Cell. 1989;59:1027–1034. doi: 10.1016/0092-8674(89)90759-9. [DOI] [PubMed] [Google Scholar]
- 4.Celli J, Trieu-Cuot P. Circularization of Tn916 is required for expression of the transposon-encoded transfer functions: characterization of long tetracycline-inducible transcripts reading through the attachment site. Mol Microbiol. 1998;28:103–117. doi: 10.1046/j.1365-2958.1998.00778.x. [DOI] [PubMed] [Google Scholar]
- 5.Clewell D B, Flannagan S E. The conjugative transposons of gram-positive bacteria. In: Clewell D B, editor. Bacterial conjugation. New York, N.Y: Plenum Press; 1993. pp. 369–393. [Google Scholar]
- 6.Feldhaus M J, Hwa V, Cheng Q, Salyers A A. Use of an Escherichia coli β-glucuronidase gene as a reporter gene for investigation of Bacteroides promoters. J Bacteriol. 1991;173:4540–4543. doi: 10.1128/jb.173.14.4540-4543.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Franke A E, Clewell D B. Evidence for a chromosome-borne resistance transposon (Tn916) in Streptococcus faecalis that is capable of “conjugal” transfer in the absence of a conjugative plasmid. J Bacteriol. 1981;145:494–502. doi: 10.1128/jb.145.1.494-502.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hecht D W, Thompson J S, Malamy M H. Characterization of the termini and transposition products of Tn4399, a conjugal mobilizing transposon of Bacteroides fragilis. Proc Natl Acad Sci USA. 1989;86:5340–5344. doi: 10.1073/pnas.86.14.5340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Holdeman L V, Moore W E C. Anaerobe laboratory manual. 4th ed. Blacksburg, Va: Virginia Polytechnic Institute and State University; 1975. [Google Scholar]
- 10.Jia Y H, Churchward G. Interactions of the integrase protein of the conjugative transposon Tn916 with its specific DNA binding sites. J Bacteriol. 1999;181:6114–6223. doi: 10.1128/jb.181.19.6114-6123.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Li L Y, Shoemaker N B, Salyers A A. Location and characteristics of the transfer region of a Bacteroides conjugative transposon and regulation of transfer genes. J Bacteriol. 1995;177:4992–4999. doi: 10.1128/jb.177.17.4992-4999.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Manganelli R, Ricci S, Pozzi G. The joint of Tn916 circular intermediates is a homoduplex in Enterococcus faecalis. Plasmid. 1997;38:71–78. doi: 10.1006/plas.1997.1300. [DOI] [PubMed] [Google Scholar]
- 13.Metcalf W W, Jiang W, Wanner B L. Use of the rep technique for allele replacement to construct new Escherichia coli hosts for maintenance of R6Kv origin plasmids at different copy numbers. Gene. 1994;138:1–7. doi: 10.1016/0378-1119(94)90776-5. [DOI] [PubMed] [Google Scholar]
- 14.Nunes-Düby S E, Kwon H J, Tirumalai R S, Ellenberger T, Landy A. Similarities and differences among 105 members of the Int family of site-specific recombinases. Nucleic Acids Res. 1998;26:391–406. doi: 10.1093/nar/26.2.391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Rudy C, Taylor K L, Hinerfeld D, Scott J R, Churchward G. Excision of a conjugative transposon in vitro by the Int and Xis proteins of Tn916. Nucleic Acids Res. 1997;25:4061–4066. doi: 10.1093/nar/25.20.4061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Saito H, Miura K I. Preparation of transforming deoxy-ribonucleic acid by phenol treatment. Biochim Biophys Acta. 1963;72:619–629. [PubMed] [Google Scholar]
- 17.Salyers A A, Shoemaker N B. Conjugative transposons: the force behind the spread of antibiotic resistance genes among Bacteroides clinical isolates. Anaerobe. 1995;1:143–150. doi: 10.1006/anae.1995.1011. [DOI] [PubMed] [Google Scholar]
- 18.Salyers A A, Shoemaker N B, Stevens A M. Tetracycline regulation of conjugal transfer genes. In: Hoch J A, Silhavy T J, editors. Two-component signal transduction. Washington, D.C.: American Society for Microbiology; 1995. pp. 393–400. [Google Scholar]
- 19.Salyers A A, Shoemaker N B, Stevens A M, Li L Y. Conjugative transposons: an unusual and diverse set of integrated gene transfer elements. Microbiol Rev. 1995;59:579–590. doi: 10.1128/mr.59.4.579-590.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sambrook J, Fritsch E F, Maniatis T. Molecular cloning: a laboratory manual. 2nd ed. Cold Spring Harbor, N.Y: Cold Spring Harbor Press; 1989. [Google Scholar]
- 21.Scott J R, Bringel F, Marra D, Van Alstine G, Rudy C K. Conjugative transposition of Tn916: preferred targets and evidence for conjugative transfer of a single strand and for a double-stranded circular intermediate. Mol Microbiol. 1994;11:1099–1108. doi: 10.1111/j.1365-2958.1994.tb00386.x. [DOI] [PubMed] [Google Scholar]
- 22.Scott J R, Churchward G G. Conjugative transposition. Annu Rev Microbiol. 1995;49:367–397. doi: 10.1146/annurev.mi.49.100195.002055. [DOI] [PubMed] [Google Scholar]
- 23.Shoemaker N B, Barber R D, Salyers A A. Cloning and characterization of a Bacteroides conjugal tetracycline-erythromycin resistance element by using a shuttle cosmid vector. J Bacteriol. 1989;171:1294–1302. doi: 10.1128/jb.171.3.1294-1302.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Shoemaker N B, Getty C, Guthrie E P, Salyers A A. Regions in Bacteroides plasmids pBFTM10 and pB8-51 that allow Escherichia coli-Bacteroides shuttle vectors to be mobilized by IncP plasmids and by a conjugative Bacteroides tetracycline resistance element. J Bacteriol. 1986;166:959–965. doi: 10.1128/jb.166.3.959-965.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Shoemaker N B, Salyers A A. A cryptic 65-kilobase-pair transposonlike element isolated from Bacteroides uniformis has homology with Bacteroides conjugal tetracycline resistance elements. J Bacteriol. 1990;172:1694–1702. doi: 10.1128/jb.172.4.1694-1702.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Shoemaker N B, Salyers A A. Tetracycline-dependent appearance of plasmidlike forms in Bacteroides uniformis 0061 mediated by conjugal Bacteroides tetracycline resistance elements. J Bacteriol. 1988;170:1651–1657. doi: 10.1128/jb.170.4.1651-1657.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Shoemaker N B, Wang G R, Salyers A A. The Bacteroides mobilizable insertion element, NBU1, integrates into the 3′ end of a Leu-tRNA gene and has an integrase that is a member of the lambda integrase family. J Bacteriol. 1996;178:3594–3600. doi: 10.1128/jb.178.12.3594-3600.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Simon R, Priefer U, Puhler A. A broad host range mobilization system for in vivo genetic engineering: transposon mutagenesis in gram negative bacteria. Bio/Technology. 1983;1:784–791. [Google Scholar]
- 29.Stevens A M, Shoemaker N B, Li L Y, Salyers A A. Tetracycline regulation of genes on Bacteroides conjugative transposons. J Bacteriol. 1993;175:6134–6141. doi: 10.1128/jb.175.19.6134-6141.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Stevens A M, Shoemaker N B, Salyers A A. The region of a Bacteroides conjugal chromosomal tetracycline resistance element which is responsible for production of plasmidlike forms from unlinked chromosomal DNA might also be involved in transfer of the element. J Bacteriol. 1990;172:4271–4279. doi: 10.1128/jb.172.8.4271-4279.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Tribble G D, Parker A C, Smith C J. The Bacteroides mobilizable transposon Tn4555 integrates by a site-specific recombination mechanism similar to that of the gram-positive bacterial element Tn916. J Bacteriol. 1997;179:2731–2739. doi: 10.1128/jb.179.8.2731-2739.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Tribble G D, Parker A C, Smith C J. Genetic structure and transcriptional analysis of a mobilizable, antibiotic resistance transposon from Bacteroides. Plasmid. 1999;42:1–12. doi: 10.1006/plas.1999.1401. [DOI] [PubMed] [Google Scholar]
- 33.Vedantam G, Novicki T J, Hecht D W. Bacteroides fragilis transfer factor Tn5520: the smallest bacterial mobilizable transposon containing single integrase and mobilization genes that function in Escherichia coli. J Bacteriol. 1999;181:2564–2571. doi: 10.1128/jb.181.8.2564-2571.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wang J, Shoemaker N, Wang G-R, Salyers A A. Characterization of a Bacteroides mobilizable transposon, NBU2, which carries a functional lincomycin resistance gene. J Bacteriol. 2000;182:3559–3571. doi: 10.1128/jb.182.12.3559-3571.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]