Abstract
Background
Korean Red Ginseng (KRG) was reported to play an anti-inflammatory role, however, previous studies largely focused on the effects of KRG on priming step, the inflammation-preparing step, and the anti-inflammatory effect of KRG on triggering, the inflammation-activating step has been poorly understood. This study demonstrated anti-inflammatory role of KRG in caspase-11 non-canonical inflammasome activation in macrophages during triggering of inflammatory responses.
Methods
Caspase-11 non-canonical inflammasome-activated J774A.1 macrophages were established by priming with Pam3CSK4 and triggering with lipopolysaccharide (LPS). Cell viability and pyroptosis were examined by MTT and lactate dehydrogenase (LDH) assays. Nitric oxide (NO)-inhibitory effect of KRG was assessed using a NO production assay. Expression and proteolytic cleavage of proteins were examined by Western blotting analysis. In vivo anti-inflammatory action of KRG was evaluated with the LPS-injected sepsis model in mice.
Results
KRG reduced LPS-stimulated NO production in J774A.1 cells and suppressed pyroptosis and IL-1β secretion in caspase-11 non-canonical inflammasome-activated J774A.1 cells. Mechanistic studies demonstrated that KRG suppressed the direct interaction between LPS and caspase-11 and inhibited proteolytic processing of both caspase-11 and gasdermin D in caspase-11 non-canonical inflammasome-activated J774A.1 cells. Furthermore, KRG significantly ameliorated LPS-mediated lethal septic shock in mice.
Conclusion
The results demonstrate a novel mechanism of KRG-mediated anti-inflammatory action that operates through targeting the caspase-11 non-canonical inflammasome at triggering step of macrophage-mediated inflammatory response.
Keywords: KRG, Caspase-11, Non-canonical inflammasome, Inflammation, Macrophage
Graphical abstract
1. Introduction
Inflammation is an innate immunity that protects self from microbes and cellular dangers [1]. The inflammatory response is initiated by the interaction of pattern recognition receptors (PRRs) with pathogen-associated molecular patterns (PAMPs) and danger-associated molecular patterns (DAMPs) of invading pathogens [[1], [2], [3]]. The inflammatory response is a two-step process: 1) priming step, which prepares the inflammatory response by increasing the expression of inflammatory molecules, and 2) triggering step, which activates and augments the inflammatory responses via activation of inflammasomes [[4], [5], [6], [7]].
Inflammasomes, assembled in response to PAMPs and DAMPs, are intracellular multiprotein complexes comprising a PRR and inflammatory molecules, which activate the innate immune response [[7], [8], [9]]. Inflammasomes can be categorized into two main groups. The earliest discovered canonical inflammasomes include NLR family inflammasomes, such as NLRP1, NLRP3, NLRC4, NLRP6, and NLPR9 inflammasomes, and an absent in melanoma 2 (AIM2) inflammasome [[7], [8], [9]]. More recently, a group of inflammasomes distinguishable from the canonical inflammasomes has been identified and termed non-canonical inflammasomes [[10], [11], [12]]. Non-canonical inflammasomes include mouse caspase-11 and human caspase-4 and caspase-5 inflammasomes [[10], [11], [12]]. Interestingly, despite the variety of PAMPs and DAMPs that activate canonical inflammasomes, only lipopolysaccharide (LPS) was identified as a direct ligand of non-canonical inflammasomes [[10], [11], [12]]. The canonical and non-canonical inflammasomes differ in their activating ligands, PRR types, and assembled structures but share common downstream signaling pathways. The activation of inflammasomes induces proteolytic processing of gasdermin D (GSDMD) that produces GSDMD N-terminal fragments (GSDMD-NT). The GSDMD-NT are subsequently recruited to cell membranes where they produce GSDMD pores, resulting in pyroptosis, inflammation-induced cell death. Inflammasome activation also induces caspase-1 activation and the caspase-1-induced maturation and secretion of pro-inflammatory cytokines, IL-1β and IL-18 [[13], [14], [15], [16], [17], [18], [19]].
Korean ginseng (Panax ginseng) has long been cultivated as a medicinal plant in far-eastern Asian and North American countries [20,21]. Fresh ginseng rapidly decomposes; therefore, a stable product of ginseng is processed by cycles of repeated steaming and drying to produce a dehydrated and dark red colored ginseng, named Korean Red Ginseng (KRG). Numerous pharmacological compounds in KRG, such as saponins, glycosides, polysaccharides, and essential oils were identified, and KRG was demonstrated to play pharmacological roles in multiple diseases [[22], [23], [24], [25], [26], [27], [28], [29], [30]]. Additionally, KRG was also demonstrated to play an anti-inflammatory role in inflammatory responses and diseases [26,[31], [32], [33], [34], [35]]; but, KRG-mediated anti-inflammatory action in these studies was accomplished by inhibiting the priming step. Recent studies have reported that KRG also has an anti-inflammatory effect by targeting inflammasome activation to inhibit the triggering step of inflammatory responses [[36], [37], [38], [39], [40]]; importantly, these studies focused only on the canonical inflammasomes, and the anti-inflammatory role of KRG through modulating non-canonical inflammasomes has not yet been studied. Therefore, the present study explored in vitro and in vivo anti-inflammatory action of KRG via targeting caspase-11 non-canonical inflammasome as well as the associated underlying mechanisms.
2. Materials and methods
2.1. Materials
KRG water extract and the information of ginsenoside composition (Table 1) was provided by Korea Ginseng Cooperation (Daejon, Korea). J774A.1 mouse macrophages (40067) and HEK293 cells (21573) were purchased at the Korean Cell Line Bank (Seoul, Korea). RPMI 1640 medium (SH30027.01), Dulbecco's modified Eagle's medium (DMEM; SH30243.01), fetal bovine serum (FBS; 16000044), penicillin-streptomycin (15140–22), 4-(2-Hydroxyethyl)piperazine-1-ethanesulfonic acid (HEPES; 15630080), polyethylenimine (PEI; 23966–100), mouse IL-1β uncoated enzyme-linked immunosorbent assay (ELISA) kit (88-7013-88), and streptavidin agarose resin (20347) were purchased at Thermo Fisher Scientific (Waltham, MA, USA). LPS (Escherichia coli O111:B4; tlrl-eblps), Pam3CSK4 (tlrl-pms), and biotin-conjugated LPS (tlrl-lpsbiot) were purchased at InvivoGen (San Diego, CA, USA). FuGENE® HD (E2311) was purchased at Promega (Madison, WI, USA). The Quanti-LDH™ PLUS Cytotoxicity Assay Kit (BCT-LDHP500) was purchased at BIOMAX (Seoul, Korea). Bovine serum albumin (BSA; A0100-010) and Xpert protease inhibitor cocktail solution (P3100-010) were purchased at GenDEPOT (Katy, TX, USA). 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT; HC0793), sodium deoxycholate (194–08311), and sodium dodecyl sulfate (SDS; 81065S1204) were purchased at HanLab (Seoul, Korea). The PCMV-flag-caspase-11 plasmid (21145) was purchased at Addgene (Watertown, MA, USA). Antibodies specific for caspase-11 (ab180673), GSDMD (ab209845), Flag (sc-166355), β-actin (sc-47778) were purchased at abcam (Cambridge, UK) and Santa Cruz Biotechnology (Santa Cruz, CA, USA). An enhanced chemiluminescence (EBP-1073) was purchased at ELPIS-Biotech (Daejeon, Korea).
Table 1.
The ginsenoside composition present in KRG used in this study.
| Ginsenosides | Amounts (mg/g) | Amounts (%) |
|---|---|---|
| Ginsenoside Rb1 | 5.43 | 24.5 |
| Ginsenoside Rg3s | 4.21 | 19.0 |
| Ginsenoside Rc | 1.99 | 9.0 |
| Ginsenoside Rb2 | 1.83 | 8.3 |
| Ginsenoside Rg2s | 1.77 | 8.0 |
| Ginsenoside Rg3r | 1.72 | 7.8 |
| Ginsenoside Rh1 | 1.50 | 6.8 |
| Ginsenoside Rf | 1.48 | 6.7 |
| Ginsenoside Rd | 0.89 | 4.0 |
| Ginsenoside Re | 0.68 | 3.1 |
| Ginsenoside Rg1 | 0.66 | 3.0 |
| Total | 22.16 | 100 |
2.2. Mice
C57BL/6J mice (female, 6-wk-old) were purchased at ORIENT BIO (Seongnam, Korea). Mice were kept in plastic cages with a cycle of 12 h light and 12 h dark and fed pelleted diet and tap water ad libitum. The animal study was conducted in accordance with the Institutional Animal Care and Use Committee of Kyonggi University (#2021–009).
2.3. Cell culture
J774A.1 and HEK293 cells were cultured in RPMI 1640 medium and DMEM, respectively, with 10% heat-inactivated FBS, penicillin, streptomycin, and HEPES (25 mM) at 37 °C in a 5% CO2 incubator.
2.4. Cell viability assay
J774A.1 cells were treated with KRG (1–1000 μg/mL) for 24 h J774A.1 cells pre-treated with KRG (1–1000 μg/mL) for 30 min were treated with LPS (0.5 μg/mL) for 24 h. The cell viability was determined by the MTT assay [41].
2.5. NO production assay
J774A.1 cells pre-treated with KRG (1–1000 μg/mL) for 30 min were then treated with LPS (0.5 μg/mL) for 24 h. NO levels in culture medium was determined by the Griess assay [42].
2.6. Pyroptosis assay
J774A.1 cells pre-incubated with KRG (100 and 150 μg/mL) for 1 h were treated with Pam3CSK4 (1 μg/mL) for 4 h and transfected with LPS (2.5 μg/mL plus 0.25% v/v FuGENE® HD) for 18 h. Pyroptosis of J774A.1 cells was determined by the lactate dehydrogenase (LDH) release in culture medium using a Quanti-LDH™ PLUS Cytotoxicity assay Kit.
2.7. IL-1β production assay
Soluble IL-1β secreted in culture medium was determined by ELISA using a mouse IL-1β ELISA Kit.
2.8. Western blotting analysis
J774A.1 cells pre-incubated with KRG (100 and 150 μg/mL) for 1 h were treated with Pam3CSK4 (1 μg/mL) for 4 h and transfected with LPS (2.5 μg/mL plus 0.25% v/v FuGENE® HD) for 18 h. Whole cell lysates were prepared by lysing the cells in RIPA buffer (25 mM Tris-HCl, pH 7.4, 150 mM NaCl, 1% Nonidet P-40, 0.5% sodium deoxycholate, 0.1% SDS, protease inhibitor cocktail) on ice for 1 h, and the supernatants were obtained by centrifugation as whole cell lysates. The proteins in the whole cell lysates were separated by SDS-PAGE transferred onto polyvinylidene fluoride membranes. The membranes were blocked in TBST containing 5% BSA and incubated with the primary and horseradish peroxidase-linked secondary antibodies. Thereafter, the target proteins were detected using the ECL reagent.
2.9. Caspase-11–LPS binding inhibition assay
HEK293 cells transfected with PCMV-flag-caspase-11 plasmids were incubated in lysis buffer (50 mM Tris-HCl, pH 7.6, 150 mM NaCl, 2 mM EDTA, 1% Triton X-100, protease inhibitor cocktail) on ice for 1 h and incubated with KRG (50 and 100 μg) for 1 h at 4 °C with constant rotation. Biotin-LPS (1 μg) was conjugated with 8 μL of streptavidin agarose resin, and unconjugated ligand was removed by washing the resin three times with lysis buffer. The LPS-conjugated resins were then incubated with the cell lysates for 1 h at 4 °C and washed three times with lysis buffer. The precipitates were dissolved in 1X SDS sample buffer, and analyzed through western blotting.
2.10. In vivo septic shock study
C57BL/6J mice (n = 7 mice per group) were orally administered KRG (200 and 300 mg/kg) every 12 h for a total of 5 times. At 12 h after the final dose, the C57BL/6J mice were challenged via intraperitoneal injection with LPS (54 mg/kg) [12,43,44]. The survival rate and body weight of the mice were monitored for 25 h.
2.11. Statistical analysis
The data are presented as the mean ± standard deviation of at least 3 experiments conducted independently. Statistical significance between the groups was determined by either analysis of variance or the Mann–Whitney test, and P < 0.05 was considered statistically significant (∗P < 0.05, ∗∗P < 0.01).
3. Results
3.1. Inhibitory effect of KRG on NO production in J774A.1 cells
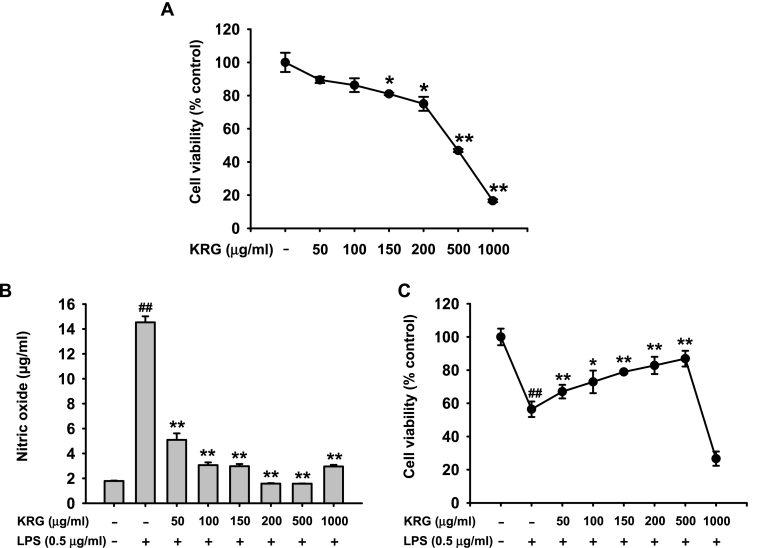
The cytotoxicity of KRG was first evaluated in J774A.1 macrophages. It was found that KRG exerted slight cytotoxicity at concentrations up to 200 μg/mL but severe cytotoxicity at 500 μg/mL (Fig. 1A). Thereafter, the in vitro anti-inflammatory effect of KRG on J774A.1 cells was explored. The results showed that KRG dose-dependently suppressed NO production in LPS-exposed J774A.1 cells (Fig. 1B). Thus, KRG at concentrations up to 500 μg/mL protected J774A.1 cells from LPS stimulation and increased the viability of LPS-exposed J774A.1 cells; however, the viability was dramatically decreased with KRG at a concentration of 1000 μg/mL (Fig. 1C).
Fig. 1.
Inhibitory effect of KRG on NO production in J774A.1 cells. (A) J774A.1 cells were treated with KRG (0–1000 μg/mL) for 24 h, and the cell viability was determined by a MTT assay. J774A.1 cells pre-treated with KRG (0–1000 μg/mL) for 30 min were then treated with LPS (0.5 μg/mL) for 24 h. (B) The NO levels in culture medium were determined by a Griess assay, and (C) the cell viability was determined by a MTT assay. n = 3 independent experiments. ∗P < 0.05, ∗∗P < 0.01 compared to the vehicle-treated control in (A). ##P < 0.01 compared to the vehicle-treated control, and ∗P < 0.05, ∗∗P < 0.01 compared to the stimulator-treated controls (B–C).
3.2. Inhibitory effect of KRG on caspase-11 non-canonical inflammasome activation in J774A.1 cells
An in vitro macrophage model in which the caspase-11 non-canonical inflammasome was activated was established in J774A.1 cells and confirmed by evaluating the cell shape, LDH release, and IL-1β secretion (Fig. S1).
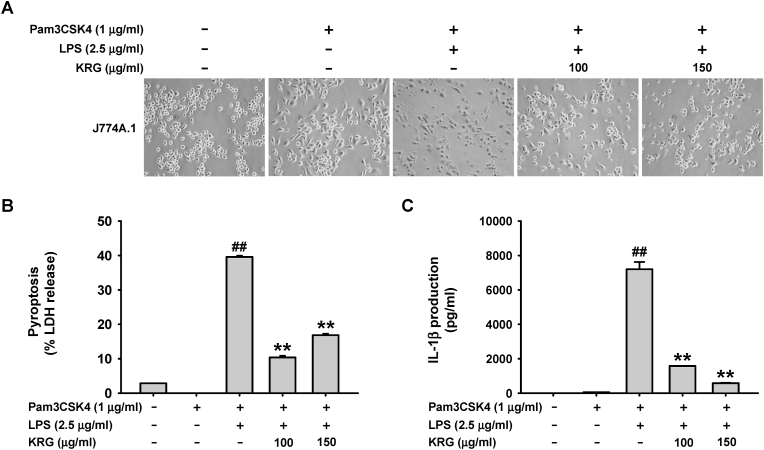
Thereafter, KRG-mediated inhibitory effect on caspase-11 non-canonical inflammasome was investigated in the in vitro J774A.1 cell model. It was found that KRG significantly inhibited pyroptotic death of LPS-triggered J774A.1 cells (Fig. 2A) and suppressed LDH release from LPS-triggered J774A.1 cells (Fig. 2B). Additionally, KRG markedly inhibited IL-1β secretion from LPS-triggered J774A.1 cells (Fig. 2C).
Fig. 2.
Inhibitory effect of KRG on caspase-11 non-canonical inflammasome activation in J774A.1 cells. J774A.1 cells pre-treated with KRG (100 and 150 μg/mL) for 30 min were treated with Pam3CSK4 (1 μg/mL) for 4 h and transfected with LPS (2.5 μg/mL) for 18 h. (A) Cell shapes were observed by a light microscope (magnification: 100 × ) and photographed with a digital camera. (B) Pyroptotic cell death was determined based on LDH release in culture medium. (C) IL-1β levels in culture medium were determined by an ELISA. n = 3 independent experiments. ##P < 0.01 compared to the vehicle-treated control, and ∗∗P < 0.01 compared to the stimulator-treated controls.
3.3. KRG-mediated inhibitory mechanism of caspase-11 non-canonical inflammasome activation in J774A.1 cells
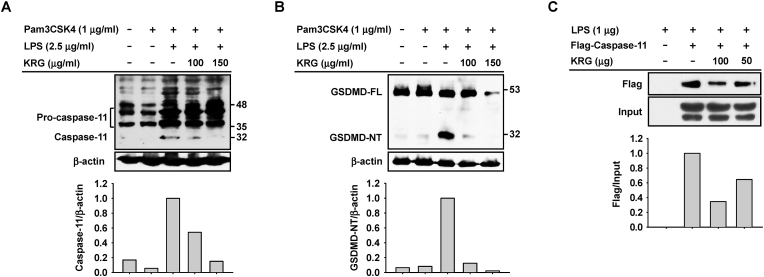
Next, the molecular mechanism by which KRG inhibits caspase-11 non-canonical inflammasome activation during inflammatory responses was investigated in J774A.1 cells. KRG dose-dependently inhibited proteolytic processing of pro-caspase-11 (p43 and p38) to caspase-11 (p32) (Fig. 3A). KRG also inhibited proteolytic processing of full-length GSDMD (GSDMD-FL; p50) to GSDMD-NT (p30) (Fig. 3B). Interestingly, KRG dose-dependently inhibited the direct interaction of caspase-11 with LPS (Fig. 3C).
Fig. 3.
Mechanism of KRG-mediated inhibition of caspase-11 non-canonical inflammasome activation in J774A.1 cells. J774A.1 cells pre-treated with KRG (100 and 150 μg/mL) for 30 min were treated with Pam3CSK4 (1 μg/mL) for 4 h and transfected with LPS (2.5 μg/mL) for 18 h. (A) Pro-caspase-11 and caspase-11 and (B) GSDMD-FL and GSDMD-NT in whole cell lysates were detected by western blotting. (C) The whole lysates of the HEK293 cells transfected with empty or PCMV-flag-caspase-11 plasmids were incubated with LPS (1 μg) in the presence or absence of KRG (50 and 100 μg), and LPS-bound caspase-11 was detected through western blotting.
3.4. In vivo protective effect of KRG on lethal sepsis in mice
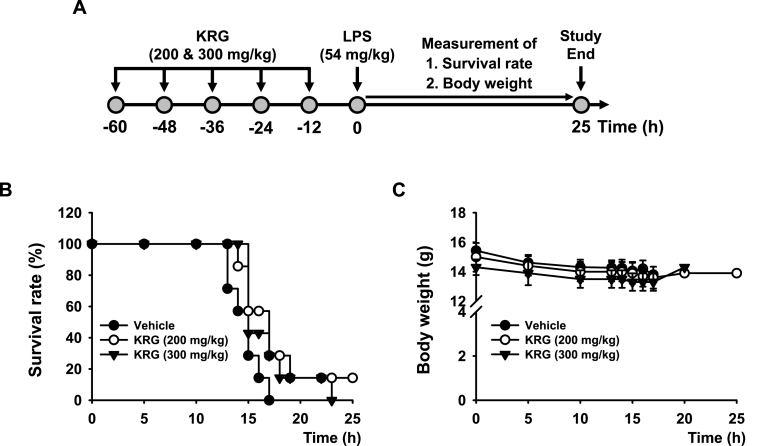
Finally, the in vivo protective effect of KRG against lethal sepsis was investigated using an LPS-challenged mouse model of acute septic shock. Acute septic shock was induced in mice that were previously administered multiple doses of KRG (200 and 300 mg/kg), and the survival rate and body weight of the mice were evaluated for 25 h (Fig. 4A). The results revealed that KRG protected the mice from LPS-triggered lethal sepsis by increasing the survival rate of the septic mice at 200 mg/kg, but interestingly, a higher dose of KRG (300 mg/kg) did not show the protective effect on lethal septic in mice (Fig. 4B). Furthermore, KRG treatment appeared to be well tolerated, with no signs of acute toxicity, such as significant loss of body weight, and none of the surviving mice showed significant changes in body weight during the experiments (Fig. 4C). The P values of the survival rate between groups analyzed by Kaplan-Meier plot are summarized in Table 2.
Fig. 4.
In vivo protective effect of KRG on lethal sepsis in mice. (A) Schematic summary of the experimental schedule. (B) Kaplan–Meier survival plots for the LPS (54 mg/kg)-challenged lethal sepsis mice administered KRG (200 and 300 mg/kg). (C) Body weight of the LPS (54 mg/kg)-challenged lethal sepsis mice administered KRG (200 and 300 mg/kg). n = 7 mice per group.
Table 2.
The P values of the survival rate between groups analyzed by Kaplan-Meier plot.
| Group | Group | P value |
|---|---|---|
| Vehicle | KRG (200 mg/kg) | 0.0418 |
| Vehicle | KRG (300 mg/kg) | 0.1206 |
| KRG (200 mg/kg) | KRG (300 mg/kg) | 0.2707 |
4. Discussion
Although numerous studies demonstrated the anti-inflammatory role of KRG [25,26,32,33,[45], [46], [47], [48]], these studies mostly investigated the priming step of inflammatory responses. Recently, a multiple studies demonstrated the anti-inflammatory action of KRG in the triggering step of inflammatory responses via suppressing the activation of various inflammasomes; however, all these studies investigated only canonical inflammasomes, particularly the NLRP3 inflammasome [31,36,[38], [39], [40],45,49,50]. Therefore, the present study investigated the anti-inflammatory role of KRG by targeting caspase-11 non-canonical inflammasome activated in the triggering step of inflammatory responses in macrophages. First, the pharmacologically effective but safe doses for KRG-mediated inhibition of caspase-11 non-canonical inflammasome were determined. At a concentration of 500 μg/mL, KRG exhibited severe cytotoxicity. Additionally, KRG showed a dose-dependent anti-inflammatory action by suppressing NO production in LPS-stimulated J774A.1 cells and played a protective role in LPS-induced inflammatory responses at doses up to 500 μg/kg. However, KRG concentrations of 100 and 150 μg/mL were selected for further studies given that significant KRG-induced cytotoxicity of J774A.1 cells was detected at 200 μg/mL (Fig. 1A).
To investigate the in vitro inhibitory effect of KRG on the activation of caspase-11 non-canonical inflammasome in macrophages, an in vitro cell model of caspase-11 non-canonical inflammasome activation was first established in J774A.1 cells by priming with Pam3CSK4 and triggering with LPS, which is a selective ligand that activates only the caspase-11 non-canonical inflammasome [6,[16], [17], [18], [19],51,52]. The establishment of the in vitro cell model was confirmed by evaluating pyroptosis and IL-1β secretion in J774A.1 cells (Fig. S1). We next used this model to examine the inhibitory effect of KRG on caspase-11 non-canonical inflammasome activation during inflammatory responses in macrophages. The results demonstrated that KRG dramatically inhibited caspase-11 non-canonical inflammasome activation in J774A.1 cells by suppressing pyroptosis and IL-1β secretion (Fig. 2). Given that LPS triggering activates only the caspase-11 non-canonical inflammasome in macrophages, as previously described, KRG-inhibited pyroptosis and IL-1β secretion in this model is mediated by targeting caspase-11 non-canonical inflammasome.
Using the same in vitro model, we next investigated the potential molecular mechanisms by which KRG inhibits caspase-11 non-canonical inflammasome-induced inflammatory responses in macrophages. Macrophages initially express inactive pro-caspase-11, which is proteolytically cleaved under LPS stimulation, which generates active caspase-11 [6,[16], [17], [18], [19],51,52]. There are multiple proteolytic cleavage sites in pro-caspase-11, and the cleavage patterns differ between types of macrophages [43,[53], [54], [55]]. Previous studies have reported that J774A.1 macrophages express two main forms of pro-caspase-11, p43 and p38, and that LPS-induced proteolytic processing produces two different forms of caspase-11, p32 and p26 [56,57]. The generation of active form of caspase-11, p32, which is proteolytically processed by LPS stimulation in J774A.1 cells, was suppressed by KRG (Fig. 3A). Activation of caspase-11 non-canonical inflammasome subsequently generates GSDMD-NT by proteolytic processing in the 276 aspartic acid residue, resulting in the formation of GSDMD pores in cell membranes and pyroptosis [6,[16], [17], [18], [19],51,52]. Therefore, we next evaluated the effect of KRG on the proteolytic cleavage of GSDMD in LPS-triggered J774A.1 cells. As anticipated, KRG substantially inhibited GSDMD proteolytic cleavage in J774A.1 cells (Fig. 3B). The initiation of caspase-11 non-canonical inflammasome activation is accomplished by direct interaction of pro-caspase-11 with LPS [6,[16], [17], [18], [19],51,52]. Therefore, we evaluated whether KRG competitively inhibits the direct molecular interaction of pro-caspase-11 with LPS and found that KRG inhibited the direct interaction of these two molecules (Fig. 3C). These mechanism studies strongly suggest that KRG significantly prevents caspase-11 non-canonical inflammasome-activated inflammatory responses in macrophages by inhibiting direct interaction of pro-caspase-11 with LPS, thereby inhibiting the activation of caspase-11 and GSDMD by blocking their proteolytic cleavage.
Finally, we investigated the in vivo inhibitory effect of KRG on caspase-11 non-canonical inflammasome activation using a lethal sepsis mouse model. Several in vivo animal models of LPS-induced acute septic shock and lethal sepsis have been reported, and mice intraperitoneally injected with a lethal dose of LPS (54 mg/kg) are the most well-established models [12,43,44]. We investigated the in vivo inhibitory effect of KRG on lethal sepsis induced by caspase-11 non-canonical inflammasome activation using this animal model and found that KRG prevented caspase-11 non-canonical inflammasome-induced lethal sepsis without causing severe toxicity (loss of body weight) in mice (Fig. 4).
In conclusion, we report for the first time a new role for KRG-mediated anti-inflammatory action by targeting caspase-11 non-canonical inflammasome in macrophages using in vitro macrophage and in vivo lethal sepsis mouse models. Moreover, we provide a novel mechanism of KRG-mediated anti-inflammatory action, which involves inhibition of the signaling pathways activated by caspase-11 non-canonical inflammasome via blocking molecular interaction between caspase-11 and its activating ligand, LPS, in macrophages, as illustrated in Fig. 5. Given the evidence of the previous and this study, KRG plays an anti-inflammatory role via targeting not only priming step, but also triggering step of inflammatory responses by inhibiting both canonical and non-canonical inflammasomes in macrophage-mediated inflammatory responses, strongly suggesting that KRG could alleviate inflammatory responses in multiple ways by targeting a wide range of molecules. However, despite the inhibitory role of KRG in the activation of caspase-11 non-canonical inflammasome, the possible ginsenosides or other components in KRG that may be responsible for targeting caspase-11 non-canonical inflammasome are still unclear, which needs to be further investigated. Taken together, this study provide strong scientific basis that KRG, functioning via this novel mechanism, could be an effective and safe complementary and alternative therapeutics to prevent and treat infectious and inflammatory diseases.
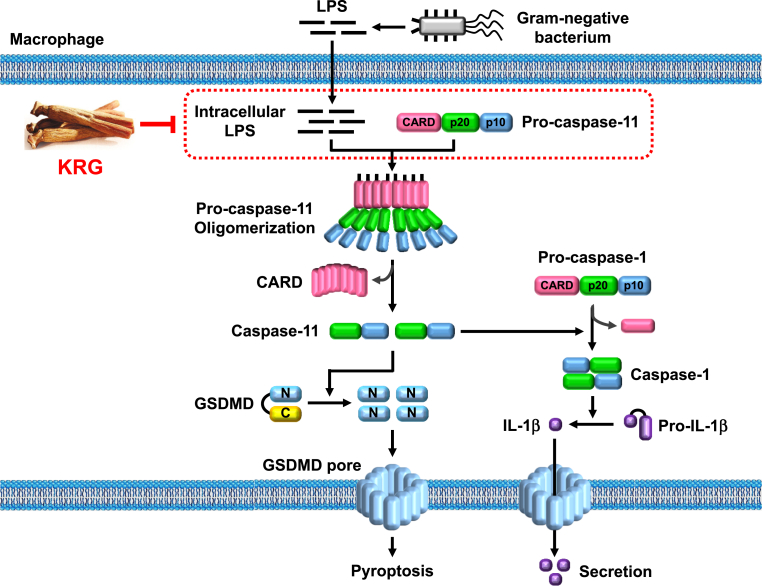
Fig. 5.
Schematic summary of the novel KRG-mediated anti-inflammatory action via targeting caspase-11 non-canonical inflammasome in macrophages.
Declaration of competing interest
The author declares no conflict of interest.
Acknowledgments
This work was supported by Kyonggi University's Graduate Research Assistantship 2021 and a grant from the Korean Society of Ginseng (2020).
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.jgr.2021.12.009.
Appendix A. Supplementary data
The following are the Supplementary data to this article:
References
- 1.Janeway C.A., Jr., Medzhitov R. Innate immune recognition. Annu Rev Immunol. 2002;20:197–216. doi: 10.1146/annurev.immunol.20.083001.084359. [DOI] [PubMed] [Google Scholar]
- 2.Li D., Wu M. Pattern recognition receptors in health and diseases. Signal Transduct Target Ther. 2021;6:291. doi: 10.1038/s41392-021-00687-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.McKernan D.P. Pattern recognition receptors as potential drug targets in inflammatory disorders. Adv Protein Chem Struct Biol. 2020;119:65–109. doi: 10.1016/bs.apcsb.2019.09.001. [DOI] [PubMed] [Google Scholar]
- 4.Yi Young-Su. Syk-MyD88 Axis Is a Critical Determinant of Inflammatory-Response in Activated Macrophages. 2021 doi: 10.3389/fimmu.2021.767366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kelley N., Jeltema D., Duan Y., He Y. The NLRP3 inflammasome: an overview of mechanisms of activation and regulation. Int J Mol Sci. 2019;20 doi: 10.3390/ijms20133328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Yi Y.S. Functional crosstalk between non-canonical caspase-11 and canonical NLRP3 inflammasomes during infection-mediated inflammation. Immunology. 2020;159:142–155. doi: 10.1111/imm.13134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zheng D., Liwinski T., Elinav E. Inflammasome activation and regulation: toward a better understanding of complex mechanisms. Cell Discov. 2020;6:36. doi: 10.1038/s41421-020-0167-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chauhan D., Vande Walle L., Lamkanfi M. Therapeutic modulation of inflammasome pathways. Immunol Rev. 2020;297:123–138. doi: 10.1111/imr.12908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Christgen S., Kanneganti T.D. Inflammasomes and the fine line between defense and disease. Curr Opin Immunol. 2020;62:39–44. doi: 10.1016/j.coi.2019.11.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kayagaki N., Warming S., Lamkanfi M., Vande Walle L., Louie S., Dong J., Newton K., Qu Y., Liu J., Heldens S., Zhang J., Lee W.P., Roose-Girma M., Dixit V.M. Non-canonical inflammasome activation targets caspase-11. Nature. 2011;479:117–121. doi: 10.1038/nature10558. [DOI] [PubMed] [Google Scholar]
- 11.Hagar J.A., Powell D.A., Aachoui Y., Ernst R.K., Miao E.A. Cytoplasmic LPS activates caspase-11: implications in TLR4-independent endotoxic shock. Science. 2013;341:1250–1253. doi: 10.1126/science.1240988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kayagaki N., Wong M.T., Stowe I.B., Ramani S.R., Gonzalez L.C., Akashi-Takamura S., Miyake K., Zhang J., Lee W.P., Muszynski A., Forsberg L.S., Carlson R.W., Dixit V.M. Noncanonical inflammasome activation by intracellular LPS independent of TLR4. Science. 2013;341:1246–1249. doi: 10.1126/science.1240248. [DOI] [PubMed] [Google Scholar]
- 13.Christgen S., Place D.E., Kanneganti T.D. Toward targeting inflammasomes: insights into their regulation and activation. Cell Res. 2020;30:315–327. doi: 10.1038/s41422-020-0295-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Xue Y., Enosi Tuipulotu D., Tan W.H., Kay C., Man S.M. Emerging activators and regulators of inflammasomes and pyroptosis. Trends Immunol. 2019;40:1035–1052. doi: 10.1016/j.it.2019.09.005. [DOI] [PubMed] [Google Scholar]
- 15.Mathur A., Hayward J.A., Man S.M. Molecular mechanisms of inflammasome signaling. J Leukoc Biol. 2018;103:233–257. doi: 10.1189/jlb.3MR0617-250R. [DOI] [PubMed] [Google Scholar]
- 16.Ding J., SnapShot Shao F. The noncanonical inflammasome. Cell. 2017;168:544–544 e541. doi: 10.1016/j.cell.2017.01.008. [DOI] [PubMed] [Google Scholar]
- 17.Yi Y.S. Caspase-11 non-canonical inflammasome: a critical sensor of intracellular lipopolysaccharide in macrophage-mediated inflammatory responses. Immunology. 2017;152:207–217. doi: 10.1111/imm.12787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Yi Y.S. Regulatory roles of the caspase-11 non-canonical inflammasome in inflammatory diseases. Immune Netw. 2018;18:e41. doi: 10.4110/in.2018.18.e41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Yi Y.S. Caspase-11 non-canonical inflammasome: emerging activator and regulator of infection-mediated inflammatory responses. Int J Mol Sci. 2020;21 doi: 10.3390/ijms21082736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lu J.M., Yao Q., Chen C. Ginseng compounds: an update on their molecular mechanisms and medical applications. Curr Vasc Pharmacol. 2009;7:293–302. doi: 10.2174/157016109788340767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zhao B., Lv C., Lu J. Natural occurring polysaccharides from Panax ginseng C. A. Meyer: a review of isolation, structures, and bioactivities. Int J Biol Macromol. 2019;133:324–336. doi: 10.1016/j.ijbiomac.2019.03.229. [DOI] [PubMed] [Google Scholar]
- 22.Mohanan P., Subramaniyam S., Mathiyalagan R., Yang D.C. Molecular signaling of ginsenosides Rb1, Rg1, and Rg3 and their mode of actions. J Ginseng Res. 2018;42:123–132. doi: 10.1016/j.jgr.2017.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hyun S.H., Ahn H.Y., Kim H.J., Kim S.W., So S.H., In G., Park C.K., Han C.K. Immuno-enhancement effects of Korean Red Ginseng in healthy adults: a randomized, double-blind, placebo-controlled trial. J Ginseng Res. 2021;45:191–198. doi: 10.1016/j.jgr.2020.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Nam Y.H., Jeong S.Y., Kim Y.H., Rodriguez I., Nuankaew W., Bhawal U.K., Hong B.N., Kang T.H. Anti-aging effects of Korean Red Ginseng (KRG) in differentiated embryo chondrocyte (DEC) knockout mice. J Ginseng Res. 2021;45:183–190. doi: 10.1016/j.jgr.2020.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Yoon S.J., Kim S.K., Lee N.Y., Choi Y.R., Kim H.S., Gupta H., Youn G.S., Sung H., Shin M.J., Suk K.T. Effect of Korean red ginseng on metabolic syndrome. J Ginseng Res. 2021;45:380–389. doi: 10.1016/j.jgr.2020.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Park S.K., Hyun S.H., In G., Park C.K., Kwak Y.S., Jang Y.J., Kim B., Kim J.H., Han C.K. The antioxidant activities of Korean Red Ginseng (Panax ginseng) and ginsenosides: a systemic review through in vivo and clinical trials. J Ginseng Res. 2021;45:41–47. doi: 10.1016/j.jgr.2020.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hossain M.A., Lee D., Kim B., Kang C.W., Kim N.S., Kim J.H. Korean Red Ginseng attenuates type 2 diabetic cardiovascular dysfunction in Otsuka Long-Evans Tokushima Fatty rats. J Ginseng Res. 2020;44:308–311. doi: 10.1016/j.jgr.2018.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Shin S.J., Nam Y., Park Y.H., Kim M.J., Lee E., Jeon S.G., Bae B.S., Seo J., Shim S.L., Kim J.S., Han C.K., Kim S., Lee Y.Y., Moon M. Therapeutic effects of non-saponin fraction with rich polysaccharide from Korean red ginseng on aging and Alzheimer's disease. Free Radic Biol Med. 2021;164:233–248. doi: 10.1016/j.freeradbiomed.2020.12.454. [DOI] [PubMed] [Google Scholar]
- 29.Hong J.T., Lee M.J., Yoon S.J., Shin S.P., Bang C.S., Baik G.H., Kim D.J., Youn G.S., Shin M.J., Ham Y.L., Suk K.T., Kim B.S. Effect of Korea red ginseng on nonalcoholic fatty liver disease: an association of gut microbiota with liver function. J Ginseng Res. 2021;45:316–324. doi: 10.1016/j.jgr.2020.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Park S.J., Nam J., Ahn C.W., Kim Y. Anti-diabetic properties of different fractions of Korean red ginseng. J Ethnopharmacol. 2019;236:220–230. doi: 10.1016/j.jep.2019.01.044. [DOI] [PubMed] [Google Scholar]
- 31.Yi Y.S. New mechanisms of ginseng saponin-mediated anti-inflammatory action via targeting canonical inflammasome signaling pathways. J Ethnopharmacol. 2021;278:114292. doi: 10.1016/j.jep.2021.114292. [DOI] [PubMed] [Google Scholar]
- 32.Baek K.S., Yi Y.S., Son Y.J., Yoo S., Sung N.Y., Kim Y., Hong S., Aravinthan A., Kim J.H., Cho J.Y. In vitro and in vivo anti-inflammatory activities of Korean Red Ginseng-derived components. J Ginseng Res. 2016;40:437–444. doi: 10.1016/j.jgr.2016.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lee S.Y., Kim M.H., Kim S.H., Ahn T., Kim S.W., Kwak Y.S., Cho I.H., Nah S.Y., Cho S.S., Park K.M., Park D.H., Bae C.S. Korean Red Ginseng affects ovalbumin-induced asthma by modulating IL-12, IL-4, and IL-6 levels and the NF-kappaB/COX-2 and PGE2 pathways. J Ginseng Res. 2021;45:482–489. doi: 10.1016/j.jgr.2020.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lee J.H., Min D.S., Lee C.W., Song K.H., Kim Y.S., Kim H.P. Ginsenosides from Korean Red Ginseng ameliorate lung inflammatory responses: inhibition of the MAPKs/NF-kappaB/c-Fos pathways. J Ginseng Res. 2018;42:476–484. doi: 10.1016/j.jgr.2017.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Saba E., Jeon B.R., Jeong D.H., Lee K., Goo Y.K., Kwak D., Kim S., Roh S.S., Kim S.D., Nah S.Y., Rhee M.H. vol. 2015. Evid Based Complement Alternat Med; 2015. p. 624132. (A novel Korean red ginseng compound gintonin inhibited inflammation by MAPK and NF-kappaB pathways and recovered the levels of mir-34a and mir-93 in RAW 264.7 cells). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ahn H., Han B.C., Hong E.J., An B.S., Lee E., Lee S.H., Lee G.S. Korean Red Ginseng attenuates ultraviolet-mediated inflammasome activation in keratinocytes. J Ginseng Res. 2021;45:456–463. doi: 10.1016/j.jgr.2021.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Wang F., Park J.S., Ma Y., Ma H., Lee Y.J., Lee G.R., Yoo H.S., Hong J.T., Roh Y.S. Ginseng saponin enriched in Rh1 and Rg2 ameliorates nonalcoholic fatty liver disease by inhibiting inflammasome activation. Nutrients. 2021;13 doi: 10.3390/nu13030856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ahn H., Han B.C., Lee S.H., Lee G.S. Fructose-arginine, a non-saponin molecule of Korean Red Ginseng, attenuates AIM2 inflammasome activation. J Ginseng Res. 2020;44:808–814. doi: 10.1016/j.jgr.2020.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kim J., Ahn H., Han B.C., Lee S.H., Cho Y.W., Kim C.H., Hong E.J., An B.S., Jeung E.B., Lee G.S. Korean red ginseng extracts inhibit NLRP3 and AIM2 inflammasome activation. Immunol Lett. 2014;158:143–150. doi: 10.1016/j.imlet.2013.12.017. [DOI] [PubMed] [Google Scholar]
- 40.Chei S., Oh H.J., Jang H., Lee K., Jin H., Choi Y., Lee B.Y. Korean red ginseng suppresses the expression of oxidative stress response and NLRP3 inflammasome genes in aged C57BL/6 mouse ovaries. Foods. 2020;9 doi: 10.3390/foods9040526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Mosmann T. Rapid colorimetric assay for cellular growth and survival: application to proliferation and cytotoxicity assays. J Immunol Methods. 1983;65:55–63. doi: 10.1016/0022-1759(83)90303-4. [DOI] [PubMed] [Google Scholar]
- 42.Guevara I., Iwanejko J., Dembinska-Kiec A., Pankiewicz J., Wanat A., Anna P., Golabek I., Bartus S., Malczewska-Malec M., Szczudlik A. Determination of nitrite/nitrate in human biological material by the simple Griess reaction. Clin Chim Acta. 1998;274:177–188. doi: 10.1016/s0009-8981(98)00060-6. [DOI] [PubMed] [Google Scholar]
- 43.Lee B.L., Stowe I.B., Gupta A., Kornfeld O.S., Roose-Girma M., Anderson K., Warming S., Zhang J., Lee W.P., Kayagaki N. Caspase-11 auto-proteolysis is crucial for noncanonical inflammasome activation. J Exp Med. 2018;215:2279–2288. doi: 10.1084/jem.20180589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kayagaki N., Stowe I.B., Lee B.L., O'Rourke K., Anderson K., Warming S., Cuellar T., Haley B., Roose-Girma M., Phung Q.T., Liu P.S., Lill J.R., Li H., Wu J., Kummerfeld S., Zhang J., Lee W.P., Snipas S.J., Salvesen G.S., Morris L.X., Fitzgerald L., Zhang Y., Bertram E.M., Goodnow C.C., Dixit V.M. Caspase-11 cleaves gasdermin D for non-canonical inflammasome signalling. Nature. 2015;526:666–671. doi: 10.1038/nature15541. [DOI] [PubMed] [Google Scholar]
- 45.Yun M., Yi Y.S. Regulatory roles of ginseng on inflammatory caspases, executioners of inflammasome activation. J Ginseng Res. 2020;44:373–385. doi: 10.1016/j.jgr.2019.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Ratan Z.A., Haidere M.F., Hong Y.H., Park S.H., Lee J.O., Lee J., Cho J.Y. Pharmacological potential of ginseng and its major component ginsenosides. J Ginseng Res. 2021;45:199–210. doi: 10.1016/j.jgr.2020.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Lorz L.R., Kim D., Kim M.Y., Cho J.Y. Panax ginseng-derived fraction BIOGF1K reduces atopic dermatitis responses via suppression of mitogen-activated protein kinase signaling pathway. J Ginseng Res. 2020;44:453–460. doi: 10.1016/j.jgr.2019.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Han S.Y., Kim J., Kim E., Kim S.H., Seo D.B., Kim J.H., Shin S.S., Cho J.Y. AKT-targeted anti-inflammatory activity of Panax ginseng calyx ethanolic extract. J Ginseng Res. 2018;42:496–503. doi: 10.1016/j.jgr.2017.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Yi Y.S. Roles of ginsenosides in inflammasome activation. J Ginseng Res. 2019;43:172–178. doi: 10.1016/j.jgr.2017.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Han B.C., Ahn H., Lee J., Jeon E., Seo S., Jang K.H., Lee S.H., Kim C.H., Lee G.S. Nonsaponin fractions of Korean Red Ginseng extracts prime activation of NLRP3 inflammasome. J Ginseng Res. 2017;41:513–523. doi: 10.1016/j.jgr.2016.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Yi Y.S. Functional interplay between methyltransferases and inflammasomes in inflammatory responses and diseases. Int J Mol Sci. 2021:22. doi: 10.3390/ijms22147580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Yi Y.S. Caspase-11 noncanonical inflammasome: a novel key player in murine models of neuroinflammation and multiple sclerosis. Neuroimmunomodulation. 2021;28:195–203. doi: 10.1159/000516064. [DOI] [PubMed] [Google Scholar]
- 53.Liu M., Zhou K., Xu Z., Ma H., Cao X., Yin X., Zeng W., Zahid A., Fu S., Ni K., Ye X., Zhou Y., Bai L., Zhou R., Jin T. Crystal structure of caspase-11 CARD provides insights into caspase-11 activation. Cell Discov. 2020;6:70. doi: 10.1038/s41421-020-00201-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Abu Khweek A., Amer A.O. Pyroptotic and non-pyroptotic effector functions of caspase-11. Immunol Rev. 2020;297:39–52. doi: 10.1111/imr.12910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Agnew A., Nulty C., Creagh E.M. Regulation, activation and function of caspase-11 during health and disease. Int J Mol Sci. 2021;22 doi: 10.3390/ijms22041506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Ye J., Zeng B., Zhong M., Li H., Xu L., Shu J., Wang Y., Yang F., Zhong C., Ye X., He X., Ouyang D. Scutellarin inhibits caspase-11 activation and pyroptosis in macrophages via regulating PKA signaling. Acta Pharm Sin B. 2021;11:112–126. doi: 10.1016/j.apsb.2020.07.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Lo T.H., Chen H.L., Yao C.I., Weng I.C., Li C.S., Huang C.C., Chen N.J., Lin C.H., Liu F.T. Galectin-3 promotes noncanonical inflammasome activation through intracellular binding to lipopolysaccharide glycans. Proc Natl Acad Sci U S A. 2021:118. doi: 10.1073/pnas.2026246118. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.