Version Changes
Revised. Amendments from Version 2
Addition to the introduction to briefly describe the Delphi method when first introduced in the text and additional text to clarify why macaque welfare assessment information was used as a basis for marmoset welfare.
Abstract
Background: Accurate assessment of the welfare of non-human primates (NHPs) used and bred for scientific purposes is essential for effective implementation of obligations to optimise their well-being, for validation of refinement techniques and novel welfare indicators, and for ensuring the highest quality data is obtained from these animals. Despite the importance of welfare assessment in NHP research, there is little consensus on what should be measured. Greater harmonisation of welfare indicators between facilities would enable greater collaboration and data sharing to address welfare-related questions in the management and use of NHPs.
Methods: A Delphi consultation was used to survey attendees of the 2019 NC3Rs Primate Welfare Meeting (73 respondents) to build consensus on which welfare indicators for macaques and marmosets are reliable, valid, and practicable, and how these can be measured.
Results: Self-harm behaviour, social enrichment, cage dimensions, body weight, a health monitoring programme, appetite, staff training, and positive reinforcement training were considered valid, reliable, and practicable indicators for macaques (≥70% consensus) within a hypothetical scenario context involving 500 animals. Indicators ranked important for assessing marmoset welfare were body weight, NHP induced and environmentally induced injuries, cage furniture, huddled posture, mortality, blood in excreta, and physical enrichment. Participants working with macaques in infectious disease and breeding identified a greater range of indicators as valid and reliable than did those working in neuroscience and toxicology, where animal-based indicators were considered the most important. The findings for macaques were compared with a previous Delphi consultation, and the expert-defined consensus from the two surveys used to develop a prototype protocol for assessing macaque welfare in research settings.
Conclusions: Together the Delphi results and proto-protocol enable those working with research NHPs to more effectively assess the welfare of the animals in their care and to collaborate to advance refinement of NHP management and use.
Keywords: behaviour, Callithrix, Macaca, non-human primate, refinement, welfare assessment, well-being, 3Rs
Research highlights.
Scientific benefit(s)
-
•
Harmonises welfare indicators for macaques, enabling inter-lab comparative studies and also greater data sharing to boost sample sizes for welfare-focused research.
-
•
Ranks welfare indicators for macaques and marmosets and narrows the field for further investigation of those considered most important by experts.
3Rs benefit(s)
-
•
Identifies context appropriate welfare indicators, that are valid, reliable and practicable, allowing better assessment of welfare, minimisation of harm and evaluation of the impact of refinement techniques.
-
•
Potentially benefits the welfare of an estimated 100,000 non-human primates (NHPs) used globally per year in biomedical research.
Practical benefit(s)
-
•
Presents a practical and generalised welfare assessment protocol to support laboratory staff in assessing, monitoring and maximising the health and wellbeing of macaques.
Current applications
-
•
Welfare assessment of macaques bred for and used in research, including in toxicology, neuroscience, infectious disease and other disciplines.
Potential applications
-
•
Welfare assessment of marmosets and other NHP species.
-
•
Benchmarking of welfare standards/quality of life between facilities.
Introduction
Globally, an estimated 100,000 non-human primates (NHPs) are used annually in biomedical research and testing, with a far larger number housed in breeding facilities ( Carlsson et al., 2004; Lankau et al., 2014; Zhang et al., 2014; Vermeire et al., 2017; Grimm, 2018). Accurate assessment of the welfare of these animals is essential for fulfilling ethical and legal obligations to minimise any harm caused by scientific or veterinary procedures, and for the effective implementation of refinement techniques such as analgesia and humane endpoints ( Rennie & Buchanan-Smith, 2006; Jennings & Prescott, 2009; Hawkins et al., 2011; Descovich et al., 2019). It is also important for evaluating enhancements to animal management aimed at promoting positive welfare states and good psychological well-being, such as environmental enrichment and training for cooperation with husbandry ( Chamove, 1989; Segal, 1989; Bassett et al., 2003; Lutz & Novak, 2005; Buchanan-Smith et al., 2005; Buchanan-Smith, 2010a; Coleman & Maier, 2010; Coleman & Novak, 2017). In some countries, there is a requirement for in vivo researchers to report to regulators the ‘actual severity’ experienced by the animals used in their experiments ( European Union, 2010; Home Office, 2014; USDA APHIS, 2018), which is predicated on the ability to recognise and accurately measure pain and distress. Welfare assessment is also a component of the scientific method, because physiological and psychological responses to suffering can significantly affect data quality ( Poole, 1997; Institute for Laboratory Animal Research, 2008). Minimising avoidable suffering is therefore necessary to ensure the validity of the scientific research performed ( Novak & Petto, 1991; Graham & Prescott, 2015; Hannibal et al., 2017; Prescott et al., 2021).
Most NHP facilities have dedicated and highly trained animal care staff who go to great efforts to optimise the well-being of the NHPs in their care ( Coleman, 2011), and effective welfare assessment tools will enable them to better accomplish this. It is recognised that welfare assessment should encompass both physical health and psychological well-being ( National Research Council, 1998; Wolfensohn & Honess, 2005; Jennings & Prescott, 2009). However, working evaluations of laboratory NHP welfare are often based on measurements of various indicators presumed to be related to the extent of failure to cope, or difficulty in coping, with the environment ( Lutz et al., 1991; European Commission, 2002). Modern welfare assessments should also aim to evaluate positive as well as negative states of individuals ( Hawkins et al. 2011; Wolfensohn et al., 2018). Social play, allogrooming, food sharing, exploration, and relaxed gait have been suggested as behavioural indicators of positive NHP welfare in the laboratory, though relatively few have been validated ( University of Stirling, 2011; Blois-Heulin et al., 2015; NC3Rs, 2015; Ahloy-Dallaire et al., 2018; Miller et al., 2020).
Most facilities that house or breed NHPs for research (i.e. laboratories, breeding centres, etc.) utilise a combination of animal-based indices, as this gives the best estimate of an individual NHP’s welfare state ( Novak & Suomi, 1988; National Research Council, 1998; Jennings & Prescott, 2009). These include physical or somatic observations (e.g. susceptibility to disease; growth rate; coat and body condition), physiological measurements (e.g. heart rate; body temperature; plasma cortisol), and structured behavioural assessments (e.g. behavioural repertoire; activity budgets; presence of quantitative or qualitative behavioural abnormalities) ( Poole, 1988; European Commission, 2002; Wolfensohn & Honess, 2005; Gottlieb & Pomerantz, 2021; Novak & Meyer, 2021). Some animal-based indices used in practice, such as stereotyped behaviour (e.g. pacing), have been criticised for their lack of validity or validation ( Poirier & Bateson, 2017; Polanco et al., 2021) and specificity ( Descovich et al., 2019). Regardless, animal-based indices can be used to assess the outcome of providing resources for animal care, such as cage space and a varied diet.
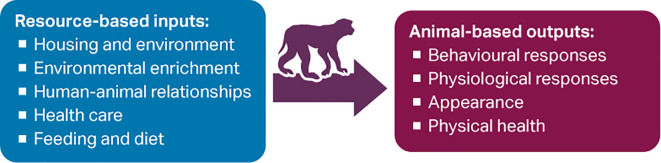
A variety of resource-based indicators, which are variables measured not in the animals but in the environment, are also used to assess welfare (e.g. size and design of enclosures; provision of environmental enrichment; health monitoring programmes). These input-based, engineering criteria are attractive because they are objective, less time intensive, and easy to measure (e.g. during site inspection) ( Johnsen et al., 2001; Mench 2003); however, they are often indirect measures of welfare and can be experienced differently by individuals (e.g. Izzo et al., 2011; Velarde & Dalmau, 2012). Used alone, they do not effectively evaluate the welfare state of individual animals; but used alongside animal-based outcome indices, resource-based input indices can usefully contribute to welfare assessments, and are important for standardising within and between facilities, especially if founded on validated welfare needs ( Beaver & Bayne, 2014; Bennett et al., 2018) ( Figure 1).
Figure 1. Some resource-based inputs and animal-based outputs that can be used to assess non-human primate (NHP) welfare.
Despite the importance of welfare assessment in NHP research, there are few established welfare assessment tools, and little is known about the level of consensus within the research community on whether the available indices are considered valid (i.e. genuinely measuring an aspect of an animal's welfare state), reliable (i.e. can be measured consistently across and between users), and practicable (i.e. can be measured with limited time, resources, and within facility constraints). Truelove et al. (2020) conducted a Delphi consultation, an iterative, multi-stage survey technique, to identify laboratory macaque welfare measures and their relative importance. A list of 115 potential indicators for use in welfare assessment of macaques (54 animal-based and 61 environment-based items) was provided to a panel of macaque experts, predominantly from North America. Experts indicated which indicators were valid, reliable, and practicable to measure using the provided on-site scenario ( Table 1a) and a composite percentage agreement score was assigned to each indicator, allowing subsequent ranking. Among the 39 experts who completed the two rounds of the survey, resource/environment-based measures were considered better suited than animal-based ones for on-site welfare assessment, with the presence of self-harm behaviours and provision of social enrichment considered the most important indicators for assessing macaque welfare; a total of 56 indicators were selected as being valid, reliable, and practicable. The ten indicators with the highest composite respondent percentage agreement score following two rounds of ratings included only one animal-based indicator (self-harm behaviour). These 56 indicators were presented as part of the current study, in part to gauge validity of the measures found in Truelove et al. (2020), as well as to uncover any indices that a different group of experts might accept or reject as useful in assessing macaque welfare and whether any of the indices can be applied to a different primate species used in research (marmosets).
Table 1a. Hypothetical welfare audit scenario.
| You are participating in a welfare audit in an institution housing approximately 500 macaques. Individuals are housed indoors in 25 animal rooms which each hold 5 racks; each rack holds 4 cages and each cage houses 1 animal. Animals are either singly housed with access to one cage or are socially housed in pairs or groups with access to multiple adjacent cages (1 per animal) with a single rack; some individuals are participating in active research studies. |
If there was a broader consensus on appropriate indicators of suffering and well-being in NHPs used for research, and widely applicable welfare assessment tools, then this would help researchers, veterinarians, and other animal care staff better fulfill their obligations to optimise the welfare of the animals in their care. Importantly, it could also facilitate greater collaboration and data sharing between research facilities to address welfare-related research questions, such as the impact of common procedures and putative refinements. Not only would this boost sample sizes for welfare-focused studies, especially those which must piggy-back onto ongoing scientific procedures conducted primarily for another research purpose, but it would also enable inter-laboratory comparative studies to identify how variation in management practices influence animal welfare ( Bliss-Moreau et al., 2021); doing so across an international audience might also identify practices diverging due to differences in culture and research specific to a region (e.g. McMillan et al., 2017; Baker & Prescott, unpublished work). In 2017, the United Kingdom’s national 3Rs centre (NC3Rs) led an international data crowdsourcing project to establish the prevalence and potential triggers for aggression-related injury in group-housed male laboratory mice ( Lidster et al., 2019) – a significant problem affecting the murine research community. In total, 143 animal technicians from 44 facilities collected aggression and husbandry data on over 137,000 mice using a common data collection framework. By comparing the prevalence of aggression and husbandry variables between facilities, the key factors that influence levels of aggression in male mice were identified, leading to recommendations for practical changes to husbandry to minimise aggressive behaviour and improve mouse welfare. This work illustrates the potential for welfare improvements when tapping into the expertise of a large group, regardless of the approach taken (e.g. crowdsourcing, Delphi).
To achieve broad consensus for NHP welfare indicators, and to develop a practical protocol for assessing macaque welfare, advantage was taken of the assembly of a group of NHP experts at the 2019 NC3Rs Primate Welfare Meeting. This international event supports laboratory and breeding centre staff working directly with NHPs to develop, share, and implement evidence-based refinements in NHP use and care. At the 2019 meeting, a hybridised Delphi consultation was undertaken to help harmonise NHP welfare assessment by gaining agreement amongst the experts on a list of macaque welfare indices that are valid, reliable, and practicable. Macaca experts either rejected or accepted the indices as measures of welfare for macaques as did Callithrix experts for marmosets, revealing whether welfare indicators identified for once species are applicable to other NHP species used or bred for research. Additionally, participants were surveyed about the methods used to measure each of these indices.
A classical Delphi consultation is an iterative, multi-stage survey technique that involves controlled feedback to a panel of anonymous subject experts; the consultation results in statistical group consensus on a selected topic as indicated by response stability between rounds ( Van Zolingen & Klaassen, 2003). This is in contrast to the group Delphi/expert workshop approach, in which a panel of experts work together, rather than independently, on a topic to arrive at consensus ( Webler et al., 1991) – all other elements are identical. We integrated both approaches for this study, using a classical Delphi in one round and a group Delphi in another round. Achieving consensus between experts increases the validity of the welfare assessment protocol and ensures that it incorporates a wide range of expert opinions, so that it is not perceived as an imposition from a single group of people ( Boulkedid et al., 2011).
Methods
Online survey software from Qualtrics was used to survey the delegates of the NC3Rs Primate Welfare Meeting (8 November 2019, London) about their views on welfare indices for macaques and marmosets, as part of a hybridised Delphi consultation process. The inclusion criteria were being a delegate of the meeting and being directly involved in the care, use or breeding of NHPs for research, which all participants met. The survey was constructed and administered by the authors. Participation was voluntary and responses were submitted using personal mobile devices. The link to the survey was emailed on the day of the meeting and also displayed at the event. Participants completed a consent statement online at the start of the survey. Additionally, if any participant wished to withdraw consent at any time, they were asked to contact the NC3Rs team who would then remove the data they had supplied. All delegates provided consent and no delegates subsequently retracted consent. Quasi-anonymity was maintained: responses remained unknown to other participants but were known to the authors and response data were coded by username after receipt so that individuals’ responses could not be readily linked. Data collection procedures were approved by the Ethics Committee of the Faculty of Science, Agriculture and Engineering at Newcastle University. All data were managed according to a data management plan for NC3Rs office-led data sharing projects.
Participants were researchers, veterinarians, and animal technologists working directly with NHPs in nine countries (United Kingdom [UK], France, Germany, Hungary, Italy, Netherlands, Sweden, Switzerland, and the United States of America [USA]), with three-quarters based within the UK. Respondents were asked to identify their species of focus (macaque or marmoset) and area of specialty (neuroscience, infectious disease, toxicology, breeding, or other). In this way, we were able to actively control for species as a potential source of bias in the study. The survey method (hybridized Delphi) also addressed two potential biases (dominance effect and Von Restorff effect) through the use of multiple rounds and anonymity ( Hallowell, 2009).
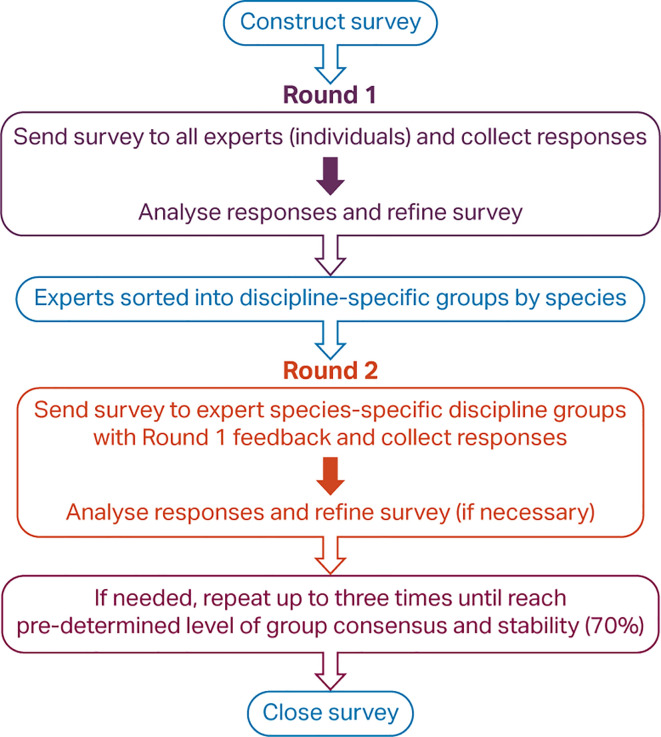
Multiple steps were required to complete the hybridised Delphi consultation process ( Figure 2). First, participants were presented with the scenario in Table 1a. They were then presented with the top ten welfare indices identified as valid, reliable, and practicable (≥70% consensus) for assessing macaque welfare in the Delphi consultation of Truelove et al., 2020 ( Table 1b) and asked:
Figure 2. Steps in a hybridised Delphi process.
Table 1b. Top ten indicators from Truelove et al. (2020).
| Human euthanasia programme |
| Health monitoring programme |
| Food enrichment |
| Physical enrichment |
| Social enrichment |
| Self-harm behaviour |
| Ventilation |
| Behavioural management programme |
| Hear other NHPs |
| Cage furniture |
Q1 “ Which of the following indices do you think are the most valid and reliable for assessing NHP welfare? (select as many indices as you feel are appropriate)”
Q2 “ How practical are the indices you selected for assessing NHP welfare from the top ten?” (with the options: “ Very impractical; Impractical; Neither; Practical; Very practical”)
Next, the participants were presented with a more extensive list of 56 welfare indices from the aforementioned Delphi consultation and asked:
Q3 “ Of the 56 indices, which do you think are the most valid and reliable for assessing NHP welfare? (select as many indices as you feel are appropriate)”
Q4 “ How practical are the indices you selected for assessing NHP welfare from the 56 indices?” (with the options: “ Very impractical; Impractical; Neither; Practical; Very practical”)
Finally, participants had the opportunity to suggest additional indices that they considered to be valid, reliable, and practicable for assessing welfare (Q5; free text responses; 5% threshold).
In a second round of the consultation, the participants were split into pre-assigned groups according to the scientific disciplines they worked in and whether their work involved marmosets or macaques. Working as a group and bearing in mind the same scenario ( Table 1a), participants were provided feedback from the first round as to which welfare indicators were considered valid and reliable, and which were considered practicable. They were asked to discuss and then define how they would measure each of the indices identified as being valid, reliable, and practicable in round 1 (i.e. at or exceeding 70% consensus, as per Truelove et al., 2020 and Leach et al., 2008). Specifically, they were asked to consider the following and then respond as a group:
Q6 “ Are you recording this measure at an individual, group/cage, room or unit level?” (with the option: “ Other [please specify]”)
Q7 “ How would you record this measure? i.e. what method and equipment (if any) would you use?” (free text responses)
Q8 “ How long would you spend recording this measure? i.e. would you measure this intermittently, and how frequently or constantly and over what period of time etc.?” (free text responses)
Q9 “ What proportion of animals/groups/rooms/units would you assess in order to get a meaningful assessment?” (selected choice in 10% intervals from <10% to 100%; with the option: “ Other [please specify]”)
The top ranked welfare indicators for macaques identified during the two Delphi consultations and the information obtained regarding their measurement was then used, along with the expertise of the authors, to construct a prototype protocol for assessing macaque welfare in research settings. There were too few marmoset data to generate a welfare assessment protocol for this species.
Statistical analysis
Many Delphi studies have used percentage measures as their primary indication of consensus, despite disagreement as to whether this is adequate ( Hsu & Sanford, 2007). We set an a priori agreement level of 70% or greater for consensus, as has been done in other animal welfare and healthcare studies (e.g. Leach et al., 2008; Keeney et al., 2011).
Descriptive statistics were used to summarize the participants’ responses per round. For all completed surveys, there were no missing data. For those surveys started but without any collected data (i.e., no answers provided but an identifier was issued), these were removed so as to not inflate the number of participants. Data were imported into Microsoft Excel for Microsoft 365 (2021) and summarised for analysis; participant identifiers were removed to maintain anonymity. Free text comments were analysed qualitatively and were grouped by similar idea by one coder (MAT).
To complete the Delphi process, group stability (i.e. consistency of response between rounds) must be demonstrated ( von der Gracht, 2012); this was achieved by Krippendorff’s alpha coefficient (α) test ( Hayes & Krippendorff, 2007 ). For interpretation, a value of 0 indicates perfect disagreement whereas 1 indicates perfect agreement; a value of 0.667 or more permits (tentative) conclusions to be made ( Krippendorff, 2004).
Generalised macaque welfare assessment protocol (GEN-MAC)
Our generalised macaque welfare assessment protocol aims to offer a practical and context appropriate tool for laboratory staff caring for macaques ( Table 2). It provides a quantitative set of criteria to support staff in monitoring and maximising macaque health and well-being, based on expert consensus. The tool encompasses all four domains of potential welfare compromise (i.e. nutritional, environmental, health, behavioural) identified by the Welfare Quality® project ( Blokhuis et al., 2013) and Mellor et al. (2009). Taken together, the chosen indicators should provide an assessment of an individual animal’s welfare, and hence, when repeatedly measured over time, provide an assessment of its quality of life ( Fraser, 2008). We acknowledge that good animal welfare is more than the mere absence of negative experiences and recognise that the tool incorporates few indicators of positive welfare state currently; however, validation of these is proving difficult (e.g. see Ahloy-Dallaire et al., 2018 for a discussion of the relationship between play and positive affective states). As new indices of positive state are validated, they can be incorporated into this tool.
Table 2. Generalised welfare assessment protocol for laboratory-housed macaques.
| Assessor: ___________ Animal ID: ___________ Date: ___________ | |||
|---|---|---|---|
| Indicator | Score | Scoring criteria | References |
| Animal-based – score for each animal | |||
|
1. Self-harm behaviour
E.g. on inspection or recorded in daily logs. Where seen, more frequent and detailed follow-up observations can be made to assess incidence, severity, and impact. |
0 | Self-harm behaviour not observed; individual not known to self-harm | Reinhardt & Rossell, 2001; Novak 2003, 2021; Polanco et al., 2021 |
| 1 | Self-harm behaviour observed, without physical injury; individual known to self-harm occasionally
(e.g. self-biting, self-hitting, eye poking, head-banging, hair plucking/pulling) |
||
| 2 | Physical injuries present, consistent with self-harm behaviour (e.g. abrasions, lacerations, eye trauma) | ||
|
2. NHP induced injuries
Use 2a and 2b. 2a. Injuries E.g. on inspection or recorded in daily logs. |
0 | No injuries present | Beisner et al., 2019; Crast et al., 2021 |
| 1 | Minor injuries present, consistent with fighting; may or may not require veterinary intervention/treatment (e.g. abrasion/blunt trauma, puncture wound, skin laceration) | ||
| 2 | Severe injuries present, consistent with fighting; veterinary intervention/treatment necessary (e.g. deep or multiple laceration/s, skin puncture + muscle involvement, inflamed or infected wound, bone exposure, degloving; signs of pain such as grimacing, hunched posture, guarding of limb) | ||
| 2b. Lameness
E.g. on inspection or recorded in daily logs. |
0 | No signs of lameness or imbalance when moving; unimpaired/normal locomotion | Lewis & Colgin, 2005; Wren et al., 2013 |
| 1 | Signs of lameness or imbalance when moving (e.g. limping or favouring limb, slow locomotion or uneven rhythm, unable to keep up with the group, guarding of limb); interfering with normal locomotion, may or may not require veterinary intervention/treatment | ||
| 2 | Persistent or long-term signs of lameness or imbalance when moving; inability to locomote normally; requires veterinary intervention/treatment | ||
|
3. Appetite
Choose the most appropriate method for your context: 3a, 3b or 3c for eating, plus 3d or 3e for drinking. 3a. Hand feeding For animals that are typically comfortable taking food from the hand. |
0 | Takes preferred food | Keeling & Wolf, 1975; Wolfensohn & Honess, 2005; Smith et al., 2006; Prescott et al., 2010; Association of Primate Veterinarians, 2019 |
| 1 | Takes only small amounts of preferred food | ||
| 2 | Refuses to take preferred food; reluctant to come forward | ||
| 3b. Eating habits E.g. on inspection at feeding time; or recorded in daily logs. | 0 | Observed to eat normally; evidence of food consumption (e.g. crumbs/scraps) | |
| 1 | Observed to not eat; no evidence of food consumption (e.g. no crumbs/scraps or food intact) | ||
| 2 | Repeatedly observed to not eat (i.e. for more than one day); protracted lack of evidence of food consumption (e.g. no crumbs/scraps or food intact) | ||
| 3c. Food consumption E.g. in the last 24 hours; weigh food or count biscuits/residues); can be recorded at the group level. | 0 | Normal amount of food consumed | |
| 1 | Reduced food consumption (e.g. 25-75% relative to baseline; or 1 SD below the mean) | ||
| 2 | No (or very little) food consumed; reduced faecal output | ||
| 3d. Drinking habits E.g. on inspection at feeding time; or recorded in daily logs. | 0 | Observed to drink normally; evidence of water consumption (e.g. approach water source) | |
| 1 | Observed to not drink; no evidence of water consumption (e.g. does not approach water source) | ||
| 2 | Repeatedly observed to not drink (i.e. for more than one day); protracted lack of evidence of water consumption (e.g. does not approach water source) | ||
| 3e. Fluid consumption E.g. in the last 24 hours; weigh water bottle; can be recorded at the group level. | 0 | Normal amount of fluid consumed | |
| 1 | Reduced fluid consumption (e.g. 25-75% relative to baseline; or 1 SD below the mean) | ||
| 2 | No (or very little) fluid consumed; dry faeces | ||
|
4. Body weight
Use 4a or 4b (age dependent), plus 4c.
Note weight loss is expected for some research protocols. 4a. Body weight change (for adult animals) E.g. relative to previous day or week. |
0 | No change in body weight | Wolfensohn & Honess 2005; Smith et al., 2006 |
| 1 | <10% change in body weight (or 1SD from expected for individual of that age) (Weight loss over a certain period may not be a concern in overweight animals, so check body condition score, 4c) | ||
| 2 | >10% change in body weight (or 2SD from expected for individual of that age) | ||
| 4b. Growth rate (for young, growing animals) E.g. using colony-specific growth curves, or those in the published literature. | 0 | Appears to be growing normally (e.g. body weight within normal range for age and sex, growth is following the relative centile) | Van Wagenen & Catchpole, 1956; Prescott et al., 2010 |
| 1 | Deviation from expected growth rate (e.g. body weight outside normal range for age and sex, deviation from the individuals’ normal growth trajectory, crossing centiles) | ||
| 2 | Ceasing to grow normally (e.g. body weight far outside normal range for age and sex) | ||
| 4c. Body condition score
Condition scoring may be performed non-invasively through observation, or during clinical examination by palpating the thoracic and lumbar vertebrae. |
0 | Body condition score of 3 (normal/optimal) | Wolfensohn & Honess, 2005; Clingerman & Summers, 2012 |
| 1 | Body condition score of 2 (underweight/thin) or 4 (overweight/heavy) | ||
| 2 | Body condition score of 1 (severely underweight/emaciated) or 5 (obese/grossly obese) | ||
| Sub-total: _ /14 | |||
| Resource-based – score for the cage | |||
|
5. Social enrichment
Use 5a and 5b. 5a. Social condition Regardless of whether an exemption from social housing is approved by the IACUC/AWERB. In very rare cases, individuals may thrive without a social partner. |
0 | Continuously socially housed with one or more compatible conspecifics in the same cage/enclosure | Schapiro et al., 1996; Lutz & Novak, 2005; Gilbert & Baker, 2011; Baker et al., 2012, 2014; DiVincenti & Wyatt, 2011; Hannibal et al., 2017; Cassidy et al., 2020 |
| 1 | Intermittent social housing (during part of the day/week) | ||
| 2 | Single housed (no physical contact but visual and olfactory contact provided); OR protected contact (separation of individuals via a barrier that permits social contact but not entry into each other’s cage/enclosure) | ||
| 3 | Social isolation (no sensory contact with conspecifics). | ||
| 5b. Social behaviour E.g. on inspection or recorded in daily logs. | 0 | Frequent prosocial/affiliative interactions observed between social partners; pair/group appears stable | |
| 1 | Both affiliative and agonistic interactions observed between social partners; may be some signs of pair/group instability | ||
| 2 | Frequent agonistic interactions and few affiliative interactions observed between social partners; pair/group appears unstable; OR animal singly housed | ||
|
6. Caging environment
Use 6a and 6b. 6a. Cage dimensions Assessed against, e.g. ILAR Guide; ETS 123; Directive 2010/63/EU. |
0 | Provided with more than the regulatory/accreditation-related minimum space allowance (e.g. via large indoor enclosure, outdoor enclosure, access to additional exercise enclosure/play pen) | Reinhardt et al., 1996; Buchanan-Smith et al., 2004; Griffis et al., 2013 |
| 1 | Provided with the regulatory/accreditation-related minimum space allowance | ||
| 2 | Provided with less than the regulatory/accreditation-related minimum space allowance (e.g. metabolism cage) | ||
| 6b. Vertical space | 0 | Housed in cage floor to ceiling high, with adequate high perching, verandas, etc. to allow all occupants to move to heights about human eye level | Reinhardt, 1992; Nakamichi & Asanuma, 1998; Reinhardt, 2003; Clarence et al., 2006; Maclean et al., 2009; Gottlieb et al., 2014; Lutz & Brown, 2018 |
| 1 | Housed in cage floor to ceiling high, with limited high perches, verandas, etc., meaning not all animals have access | ||
| 2 | Housed in double-tiered (1-over-1) caging that lacks perching at or above human eye level | ||
|
7. Physical enrichment (including cage furniture)
Use 7a and 7b. 7a. Provision of physical enrichment |
0 | Complex environment, with ample physical enrichment provided including structural enhancements (e.g. swings, ladders, shelves, tyres, hammocks, perches) that allow for species-typical locomotion (climbing, leaping, running, etc.), visual barriers, and manipulanda (e.g. toys, mirrors, wood blocks); structural complexity allows for as much of the housing to be used as possible | Bryant et al., 1988; Turner & Grantham, 2002; Honess & Marin, 2006; Waitt et al., 2008, 2010; Griffis et al., 2013; Descovich et al., 2019 |
| 1 | Limited physical enrichment provided (e.g. perches with toys) | ||
| 2 | No physical enrichment provided | ||
| 7b. Use of physical enrichment
E.g. on inspection or recorded in daily logs. Note use of physical enrichment can vary with age. |
0 | Observed to frequently interact with physical enrichment in species-typical, positive way | |
| 1 | Observed to occasionally interact with physical enrichment in species-typical, positive way | ||
| 2 | No interaction (or abnormal interaction) with physical enrichment | ||
| 8. Food enrichment | 0 | Food presentation encourages daily and extended bouts of species-typical foraging behaviour (e.g. scatter feeding of fine forage mix into floor substrate; feeding fresh browse and edible plants from approved sources; utilising puzzle feeders, which require considerable time, manipulation, and fine motor skills for retrieval of food) | Chamove et al., 1982; Boccia 1989a,b; Byrne & Suomi, 1991; Reinhardt, 1994; Doane et al., 2013 |
| 1 | Food presentation encourages species-typical foraging behaviour, but such opportunities are not daily, nor extended (e.g. feeding whole fresh produce) | ||
| 2 | Food presentation does not allow species-typical foraging behaviour (e.g. cafeteria-style presentation in bowls) | ||
| Sub-total: _ /15 | |||
| Staff-based – score for the individual or facility, as appropriate | |||
|
9. Positive reinforcement training (PRT)
Use 9a and 9b. 9a. Individual training performance E.g. on inspection or recorded in daily logs. |
0 | Individual is trained, using positive reinforcement, to voluntarily cooperate with the scientific, veterinary, and husbandry procedures it is exposed to; it reliably cooperates, showing a high degree of compliance and few signs of distress | Prescott et al., 2005; Prescott & Buchanan-Smith, 2007; Perlman et al., 2012; Baker, 2016; McMillan et al., 2017 |
| 1 | Individual is trained, using positive reinforcement, to voluntarily cooperate with the scientific, veterinary and husbandry procedures it is exposed to; however, it does not reliably cooperate and shows signs of distress | ||
| 2 | Individual is not trained to voluntary cooperate with procedures | ||
| 9b. PRT programme | Characteristics of a high-quality programme:
|
||
| 0 | Programme includes more than four of the above characteristics | ||
| 1 | Programme includes two to four of the above characteristics | ||
| 2 | Programme includes less than two of the above characteristics | ||
| 10. Behavioural management programme | Characteristics of a high-quality programme:
|
Bloomsmith, 2017; Bloomsmith et al., 2018; Schapiro, 2021 | |
| 0 | Programme includes more than four of the above characteristics | ||
| 1 | Programme includes two to four of the above characteristics | ||
| 2 | Programme includes less than two of the above characteristics | ||
|
11. Humane euthanasia programme
Use 11a and 11b. 11a. Euthanasia programme |
0 | Personnel responsible for carrying out euthanasia are knowledgeable and competent to perform the procedure in a compassionate, professional, and appropriate manner that avoids distress to the animals; AVMA-approved methods are used | Canadian Council on Animal Care, 2019; Lambeth et al., 2013; American Veterinary Medical Association, 2020 |
| 2 | Personnel responsible for carrying out euthanasia are not suitably trained and competent; euthanasia methods are not AVMA-approved | ||
| 11b. Humane endpoints | Characteristics of a high-quality programme:
|
Association of Primate Veterinarians, 2020; Prescott et al., 2021 | |
| 0 | Programme includes more than four of the above characteristics | ||
| 1 | Programme includes two to four of the above characteristics | ||
| 2 | Programme includes less than two of the above characteristics | ||
| 12. Health monitoring programme | 0 | Comprehensive health monitoring programme is in place, (e.g. as specified by FELASA), to track colony health and prevent disease outbreaks | Balansard et al., 2019 |
| 2 | No comprehensive health monitoring programme | ||
|
13. Staff training
Use 13a and 13b. 13a. Staff training progamme |
0 | All relevant staff (research, veterinary, and animal care) undergo a structured training programme before working with macaques; individual training records are kept and regularly reviewed (e.g. annually) | Wolfensohn & Honess, 2008; Jennings & Prescott, 2009 |
| 1 | A structure programme is in place, but records are not kept or regularly reviewed | ||
| 2 | No structured programme in place | ||
| 13b. Continuing professional development | 0 | All relevant staff (research, veterinary, and animal care) have the opportunity to attend internal and/or external presentations, conferences, and/or workshops on macaque welfare, care, and behaviour (e.g. NC3Rs Primate Welfare Meeting) | |
| 2 | Staff do not have access to continuing professional development opportunities | ||
| Sub-total: _ /16 | |||
| Grand total: _ /45 | |||
|
Judgement:
0-15: Normal; assume good welfare state. 16-30: Welfare compromised; improvements required; monitor carefully. 31-45: Welfare severely compromised; suffering is likely; immediate action required; provide appropriate examination, treatment, and relief (e.g., analgesia, environmental adjustments). | |||
This tool is not intended to replace welfare assessment protocols tailored to specific scientific disciplines, projects, procedures, and adverse effects. Rather it presents an appropriate number of valid, reliable, and practicable indicators for a generalised assessment of “wellness” that can inform and augment existing specific tools. This generalised tool is particularly suited for high level assessments of the outcome/quality of institutional behavioural management programmes and comparisons between laboratories. Where appropriate, facilities working in specific disciplines may wish to supplement this core set of indicators with additional ones listed in Figure 4 or the literature (e.g. Smith et al., 2006; Wolfensohn & Honess, 2005; Honess & Wolfensohn, 2010; Kirchner & Bakker, 2015; Descovich et al., 2019 for neurophysiology). Where physiological measurements are required, the least invasive and most refined method that will provide the necessary data should be used (e.g. Davenport et al., 2006; Rennie & Buchanan-Smith, 2006; Smiley Evans et al., 2015). Awareness of the context for the assessment is important; for example, food intake can be reduced following administration of anaesthetic and analgesic medication, as well as due to pain or illness.
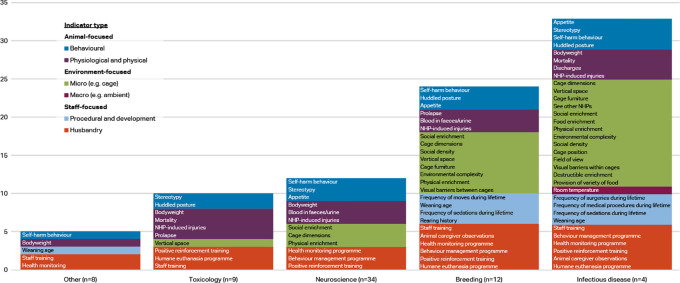
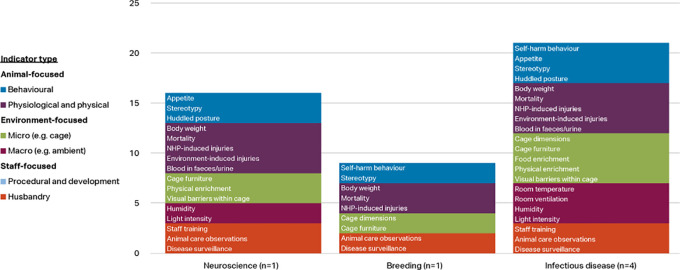
Figure 4. Indicators chosen as reliable and valid by two-thirds of respondents, macaques by discipline and indicator type (n=67 respondents).
Like other welfare assessment tools, this one combines animal-based measures of welfare with indirect resource- and staff-based ones, which are more amenable for assessing the welfare of large numbers or groups of animals when under time constraints. There is evidence that the resource- and staff-based measures included are closely associated with outcomes indicative of good animal welfare in macaques, even if they do not guarantee that any one animal is experiencing a good quality of life ( Jennings & Prescott, 2009; Schapiro et al., 2014). For example, it can be time consuming to measure affiliative social interactions, but an acceptable alternative is to check the macaques are at least socially housed with the opportunity for normal social behaviour and there is no evidence of NHP induced injury. Our approach to scoring of staff-based indicators allows a degree of flexibility and rewards programmes which incorporate elements of good practice, though users can choose to focus on the other indicator types if they wish. Most of the animal-based indicators can be directly and objectively measured after only a short-period of staff training, and they do not overly disturb the animal. The information can be gathered during site inspections, daily observations, physical exams, and other activities, such as handling for scheduled scientific procedures. Where there is the option to conduct more detailed, extended behavioural observations (e.g. analysis of closed-circuit television [CCTV] recordings), we would encourage this as it will provide greater insight into an animal’s welfare state, especially if compared against a baseline measurement of normal behaviour for individual animals during their active phase and prior to any study ( Council on Animal Care, 2019). A pilot study is underway to assess the time commitment required for completion of assessments using the tool, for a variety of group and colony sizes. It is possible that emerging approaches for automated recording and analysis of NHP behaviour will help to reduce the time required in the future ( Rushen et al., 2012; Witham, 2018; Bain et al., 2021).
The multi-dimensional assessment should be performed by experienced staff, ideally with a knowledge of the individual animals, so that changes in welfare status can be more readily identified. The indicators included in the protocol do not require veterinary diagnostic expertise or specialist animal behaviour knowledge to be accurately recorded, but the involvement of such experts in implementing the welfare assessment tool is encouraged, particularly in the interpretation of the findings of assessments using this tool. A team approach, with good communication among those involved and periodic testing of inter-observer reliability, will help to ensure reliable assessments and consistent use of the tool ( Clingerman & Summers, 2012; Lambeth et al., 2013). Individual animal records can be combined to give an overview of the colony, which can be reviewed periodically or compared with data from other colonies. If the tool is used as part of daily health checks, then scores can be compared over time and a greater severity score assigned where there is repeated evidence of impaired welfare.
To facilitate use of the GEN-MAC protocol in practice, an Excel version is available to download in our Extended data ( Leach et al., 2022). The file incorporates the formulae for calculating the welfare scores. We encourage users of the protocol to provide us with feedback, so that the tool can be enhanced; please email mark.prescott@nc3rs.org.uk. When reporting use or adaptation of GEN-MAC in the literature, please use the following citation:
Generalised macaque welfare assessment protocol (GEN-MAC) https://doi.org/10.25405/data.ncl.19106960. From: Prescott MJ, Leach MC, Truelove MA. Harmonisation of welfare indicators for macaques and marmosets used or bred for research. F1000Research 2022: X-X ( https://doi.org/10.12688/f1000research.109380.1).
Results
Demographics
A total of 73 participants took part in this survey ( Leach et al., 2022). Of these 73 respondents, 67 (92%) worked with macaque species (rhesus macaques, Macaca mulatta, and cynomolgus/long-tailed/crab-eating macaques, M. fascicularis): 34 (47%) in neuroscience, 12 (16%) in breeding, nine (12%) in toxicology, four (5%) in infectious disease research, and eight (11%) in other disciplines. Six respondents (8%) worked with common marmosets, Callithrix jacchus: four in infectious research, one in neuroscience, and one in breeding.
Macaque respondents
Round 1, Phase 1 – rating of top ten indices
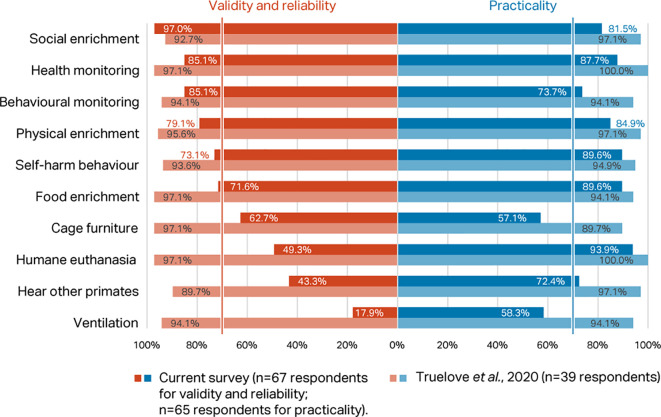
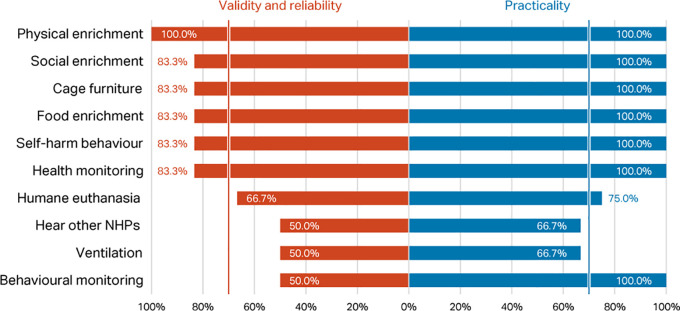
Percentage scores for validity and reliability, and practicability, of the top ten macaque welfare indicators in the Truelove et al. (2020) Delphi were compared with the scores for the same indices in the current Delphi ( Figure 3). Considering respondents working with macaques only, there was agreement between the two consultations that presence of self-harm behaviour, provision of social, food, and physical enrichment, and health and behaviour monitoring are valid, reliable, and practicable welfare indicators for NHPs (>70% consensus). However, whilst included in the top ten indicators in Truelove et al. (2020), cage furniture, humane euthanasia, hear other NHPs, and room ventilation failed to reach the consensus criterion in the current survey.
Figure 3. Comparison of validity and reliability and practicability scores (percentage of respondents) between the two Delphi consultations for macaques, showing the top ten indicators identified in Truelove et al. (2020).
Round 1, Phase 2 – rating of 56 indices
The percentage agreement scores for the more extensive list of macaque welfare indicators presented in Round 1, Phase 2 are given in Table 3. For Table 3 (macaques) and Table 5 (marmosets), “Practical” reflects two practicability categories that have been collapsed (practical + very practical). Those indices reaching less than 70% agreement for practicability have been shaded grey. The 56 indices have also been categorised into the following symbol-coded indicator types:
-
▪
Animal-based: behavioural #, physiological/physical ##
-
▪
Environment-based: micro ^ (i.e. cage), macro ^^ (i.e. ambient)
-
▪
Staff-based: procedural and development +, husbandry ++
Table 3. Ranking of validity and reliability and practicability scores for macaques, 56 indices (n=67 respondents *).
| 56 macaque indicators from Truelove et al. (2020) | Q3. Of the 56 indices, which do you think are the most valid and reliable for assessing NHP welfare? | Q4. How practical are the indices you selected for assessing NHP welfare? | ||
|---|---|---|---|---|
| Potential indicator | Count (n) | Valid, reliable (%) | Count (n) | Practical (%) |
| Self-harm behaviour # | 54 | 80.6 | 44 | 81.5 |
| Social enrichment ^ | 52 | 77.6 | 45 | 86.5 |
| Cage dimensions ^ | 51 | 76.1 | 43 | 84.3 |
| Body weight ## | 49 | 73.1 | 40 | 81.6 |
| Health monitoring programme ++ | 49 | 73.1 | 38 | 79.2 |
| Appetite # | 47 | 70.1 | 35 | 74.5 |
| Staff training ++ | 47 | 70.1 | 38 | 80.9 |
| Positive reinforcement training ++ | 47 | 70.1 | 36 | 76.6 |
| NHP induced injuries ## | 46 | 68.7 | 38 | 84.4 |
| Behavioural management programme ++ | 46 | 68.7 | 34 | 73.9 |
| Vertical space ^ | 45 | 67.2 | 41 | 91.1 |
| Cage furniture ^ | 43 | 64.2 | 40 | 93.0 |
| Blood in faeces/urine ## | 43 | 64.2 | 34 | 79.1 |
| Huddled posture # | 43 | 64.2 | 38 | 88.4 |
| Physical enrichment ^ | 43 | 64.2 | 40 | 93.0 |
| Stereotypy # | 43 | 64.2 | 31 | 72.1 |
| Social density ^ | 42 | 62.7 | 32 | 76.2 |
| Food enrichment ^ | 41 | 61.2 | 38 | 92.7 |
| See other NHPs ^ | 41 | 61.2 | 40 | 97.6 |
| Mortality ## | 40 | 59.7 | 31 | 79.5 |
| Animal caregiver observations ++ | 40 | 59.7 | 35 | 87.5 |
| Weaning age + | 40 | 59.7 | 29 | 72.5 |
| Environmental complexity ^ | 39 | 58.2 | 29 | 74.4 |
| Prolapse ## | 38 | 56.7 | 30 | 78.9 |
| Frequency of surgeries during lifetime + | 37 | 55.2 | 26 | 70.3 |
| Humane euthanasia programme ++ | 36 | 53.7 | 31 | 86.1 |
| Frequency of sedations during lifetime + | 36 | 53.7 | 26 | 72.2 |
| Frequency of medical procedures during lifetime + | 36 | 53.7 | 26 | 72.2 |
| Visual barriers between cages ^ | 34 | 50.7 | 31 | 91.2 |
| Frequency of moves during lifetime + | 33 | 49.3 | 22 | 66.7 |
| Visual barriers within cages ^ | 32 | 47.8 | 29 | 90.6 |
| Discharges ## | 32 | 47.8 | 28 | 90.3 |
| Environmental-induced injuries ## | 31 | 46.3 | 19 | 61.3 |
| Rearing history + | 31 | 46.3 | 22 | 71.0 |
| Provision of variety of food ^ | 30 | 44.8 | 25 | 83.3 |
| Room temperature ^ ^ | 28 | 41.8 | 20 | 71.4 |
| Field of view ^ | 28 | 41.8 | 15 | 55.6 |
| Dyspnoea # | 26 | 38.8 | 19 | 73.1 |
| Sensory enrichment ^ | 26 | 38.8 | 19 | 73.1 |
| Fear of other NHPs # | 25 | 37.3 | 14 | 56.0 |
| Disease surveillance ++ | 24 | 35.8 | 21 | 87.5 |
| Destructible enrichment ^ | 24 | 35.8 | 22 | 91.7 |
| Frequency of chair restraint + | 23 | 34.3 | 12 | 52.2 |
| Light intensity ^ ^ | 22 | 32.8 | 21 | 95.5 |
| Hear other NHPs ^ ^ | 22 | 32.8 | 19 | 86.4 |
| Cage position ^ | 22 | 32.8 | 12 | 57.1 |
| Room cleaning frequency ++ | 22 | 32.8 | 15 | 68.2 |
| Manipulanda ^ | 21 | 31.3 | 15 | 71.4 |
| Humidity ^ ^ | 20 | 29.9 | 15 | 78.9 |
| Room ventilation ^^ | 18 | 26.9 | 14 | 77.8 |
| Daily number of meals ++ | 18 | 26.9 | 14 | 77.8 |
| Intentional exposure to novelty ++ | 18 | 26.9 | 9 | 50.0 |
| See humans ^ | 17 | 25.4 | 15 | 88.2 |
| Daily timing of meals ++ | 15 | 22.4 | 12 | 80.0 |
| Frequency of inoculations during lifetime + | 12 | 17.9 | 9 | 75.0 |
| Provision of browse ^ | 12 | 17.9 | 10 | 83.3 |
NHP=non-human primates.
Fewer respondents replied to Q4.
Table 5. Ranking of validity and reliability and practicability scores for marmosets, 56 indies (n=6 respondents).
| 56 macaque indicators from Truelove et al. (2020) | Q3. Of the 56 indices, which do you think are the most valid and reliable for assessing NHP welfare? | Q4. How practical are the indices you selected for assessing NHP welfare? | ||
|---|---|---|---|---|
| Potential indicator | Count (n) | Valid, reliable (%) | Count (n) | Practical (%) |
| Body weight ## | 6 | 100 | 6 | 100.0 |
| NHP induced injuries ## | 6 | 100 | 5 | 83.3 |
| Cage furniture ^ | 6 | 100 | 6 | 100.0 |
| Huddled posture # | 5 | 83.3 | 5 | 100.0 |
| Mortality ## | 5 | 83.3 | 5 | 100.0 |
| Blood in faeces/urine ## | 5 | 83.3 | 5 | 100.0 |
| Environmentally induced injuries ## | 5 | 83.3 | 4 | 80.0 |
| Physical enrichment ^ | 5 | 83.3 | 5 | 100.0 |
| Self-harm behaviour # | 4 | 66.7 | 4 | 100.0 |
| Stereotypy # | 4 | 66.7 | 4 | 100.0 |
| Appetite # | 4 | 66.7 | 4 | 100.0 |
| Food enrichment ^ | 4 | 66.7 | 4 | 100.0 |
| Cage dimensions ^ | 4 | 66.7 | 3 | 75.0 |
| Visual barriers within cages ^ | 4 | 66.7 | 4 | 100.0 |
| Humidity ^^ | 4 | 66.7 | 3 | 75.0 |
| Room temperature ^^ | 4 | 66.7 | 3 | 75.0 |
| Room ventilation ^^ | 4 | 66.7 | 3 | 75.0 |
| Light intensity ^^ | 4 | 66.7 | 3 | 75.0 |
| Staff training ++ | 4 | 66.7 | 4 | 100.0 |
| Animal caregiver observations ++ | 4 | 66.7 | 4 | 100.0 |
| Disease surveillance ++ | 4 | 66.7 | 4 | 100.0 |
| Discharges ## | 3 | 50.0 | 3 | 100.0 |
| Dyspnoea ## | 3 | 50.0 | 3 | 100.0 |
| Social enrichment ^ | 3 | 50.0 | 3 | 100.0 |
| See other NHPs ^ | 3 | 50.0 | 3 | 100.0 |
| See humans ^ | 3 | 50.0 | 3 | 100.0 |
| Vertical space ^ | 3 | 50.0 | 3 | 100.0 |
| Visual barriers between cages ^ | 3 | 50.0 | 3 | 100.0 |
| Provision of variety of food ^ | 3 | 50.0 | 2 | 66.7 |
| Hear other NHPs ^^ | 3 | 50.0 | 3 | 100.0 |
| Health monitoring programme ++ | 3 | 50.0 | 2 | 66.7 |
| Humane euthanasia programme ++ | 3 | 50.0 | 2 | 66.7 |
| Daily number of meals ++ | 3 | 50.0 | 3 | 100.0 |
| Daily timing of meals ++ | 3 | 50.0 | 3 | 100.0 |
| Rearing history + | 3 | 50.0 | 2 | 66.7 |
| Weaning age + | 3 | 50.0 | 2 | 66.7 |
| Frequency of moves during lifetime + | 3 | 50.0 | 1 | 33.3 |
| Frequency of sedations during lifetime + | 3 | 50.0 | 1 | 33.3 |
| Frequency of surgeries during lifetime + | 3 | 50.0 | 2 | 66.7 |
| Fear of other NHPs # | 2 | 33.3 | 2 | 100.0 |
| Behavioural management programme ++ | 2 | 33.3 | 2 | 100.0 |
| Positive reinforcement training ++ | 2 | 33.3 | 2 | 100.0 |
| Frequency of inoculations during lifetime + | 2 | 33.3 | 1 | 50.0 |
| Frequency of medical procedures during lifetime + | 2 | 33.3 | 1 | 50.0 |
| Cage position ^ | 2 | 33.3 | 1 | 50.0 |
| Field of view ^ | 2 | 33.3 | 2 | 100.0 |
| Social density ^ | 2 | 33.3 | 2 | 100.0 |
| Environmental complexity ^ | 2 | 33.3 | 2 | 100.0 |
| Prolapse ## | 1 | 16.7 | 1 | 100.0 |
| Sensory enrichment ^ | 1 | 16.7 | 1 | 100.0 |
| Destructible enrichment ^ | 1 | 16.7 | 1 | 100.0 |
| Provision of browse ^ | 1 | 16.7 | 1 | 100.0 |
| Manipulanda ^ | 1 | 16.7 | 1 | 100.0 |
| Intentional exposure to novelty ++ | 1 | 16.7 | 1 | 100.0 |
| Room cleaning frequency ++ | 1 | 16.7 | 1 | 100.0 |
| Frequency of chair restraint + | 0 | 0.0 | 0 | 0.0 |
NHP=non-human primates.
Across the 56 welfare indicators presented to the macaque respondents (n=67), only eight (14.3%) met the a priori agreement level of ≥ 70% for validity and reliability; these were also considered practicable measures. Three were animal-based (self-harm behaviour, body weight, appetite), whilst the other five were environment- or staff-based (social enrichment, cage dimensions, health monitoring, staff training, positive reinforcement training). Three of these eight indices selected by respondents are in the top ten of Truelove et al. (2020) (self-harm behaviour, social enrichment, and health monitoring) (see Extended data, Supplementary Table 1 ( Leach et al., 2022)). Consensus for three additional indictors was nearly reached, with agreement levels between 65-69.99%; one was animal-based (NHP induced injuries) and the remainder were staff- and environment-based (behavioural management programme, vertical space).
Items were deemed more practicable to measure than they were valid and reliable, with 48 indicators (85.7%) meeting the threshold for consensus for practicability and two additional indicators approaching consensus (room cleaning frequency, frequency of moves during lifetime). Six indicators did not meet the consensus threshold for practicability when considering the hypothetical scenario; four of these were environment- or staff-based (field of view, intentional exposure to novelty, frequency of chair restraint, cage position) and two were animal-based (fear of other NHPs, environmentally induced injuries).
Round 2 – measurement of selected welfare indicators
Respondents working with macaques were classified into five categories: neuroscience (n=34), toxicology (n=9), infectious disease (n=4), breeding (n=12), and other (n=8). The ‘other’ category included the disciplines of reproduction, surgery, metabolic disease, and ethology. Figure 4 shows the indicators chosen as reliable and valid by two-thirds of macaque respondents (i.e. at or approaching consensus) by discipline and indicator type. Items approaching consensus (65-69.99%) are included in these results as some of the groups had small sample sizes and items would perhaps reach consensus with additional participants. Refer to Extended data Supplementary Table 2 for a complete list of the respondent agreement scores for validity and reliability of the indices by discipline category ( Leach et al., 2022).
More of the 56 welfare indices were considered valid and reliable by at least two-thirds of respondents in the infectious disease category (33 indices) and breeding category (24 indices), than in the other three categories. Whilst the number of animal-focused indices is relatively similar across the main disciplines (6-8 indices), the infectious disease and breeding categories also include procedural and developmental indices, as well as more micro-environment level indicators. Self-harm behaviour is one of the top four indices in all discipline categories but toxicology, and body weight appears as one of the top four as well in all except breeding. Provision of social enrichment appears in each discipline except toxicology and other. Potential explanations for the variation between the disciplines are given in the Discussion section.
Discipline groups discussed and reported how they would measure the potential welfare indicators deemed valid, reliable, and practicable. The top ten indices from the current survey and how they might be measured are given in Table 4. For each indicator, participants recommended that each should be measured at 91-100% of the population being assessed, whether the unit of measurement be at the individual or the facility level, to get a meaningful assessment. All indicators except staff training could be measured at the individual level, and there was a mix of methods for recording the indices, with observation, records, or both being recommended.
Table 4. Method of assessment, recording level, frequency, and duration for top ten indicators for macaques (n=8-10 groups across five disciplines).
| Macaques | Recording level | Method | Notes | Duration and frequency | ||||
|---|---|---|---|---|---|---|---|---|
| Indicator | Individual | Group/Cage | Room | Facility | Observation | Records | ||
| Self-harm behaviour | * | O | R | Observation of behaviour and injury; record incidence and severity | One to multiple daily checks (up to 5 mins.) | |||
| Social enrichment | * | * | O | R | Presence/absence of programme; review of records | Daily or as needed (e.g. pair/group formation) | ||
| Cage dimensions | * | * | O | Per location | As required (i.e. caging change) | |||
| Body weight | * | O | Sedation may be needed | Daily to weekly | ||||
| Health monitoring programme | * | * | R | Inspection; presence/absence programme | Semi-annually or annually; multiple daily checks | |||
| Appetite | * | O | Food consumed and feeding behaviour | Daily checks (1-10 mins. per cage) | ||||
| Staff training | * | R | At personnel level, including competence | Ongoing or annually | ||||
| Positive reinforcement training | * | R | Training logs (behaviour, frequency, duration, training stage, shaping plan) | At each session (daily to weekly) | ||||
| NHP induced injuries | * | O | Visual inspection of the animal | One to multiple daily checks (as long as needed) | ||||
| Behavioural management programme | * | * | O | R | Presence/absence of programme | Semi-annually or annually; multiple daily checks (2-5 mins. per animal) | ||
NHP=non-human primates.
Marmoset respondents
Round 1, Phase 1 – rating of top ten indices
As was the case for macaque respondents, when presented with the top ten indices from Truelove et al. (2020), most respondents working with marmosets considered social, physical, and food enrichment to be indices that can be used to assess welfare, along with self-harm behaviours and the presence of a health monitoring programme; cage dimensions was also considered important. Hearing other NHPs and ventilation were not rated highly (selected by only 50% of respondents) ( Figure 5). A smaller proportion of respondents working with marmosets considered a behavioural management programme to be useful for assessing welfare (50%), than did those working with macaques (82.6%). Of these top ten indices, eight were considered practicable by all respondents, and all ten by at least two-thirds of respondents (potentially a consequence of the small sample size; n=6).
Figure 5. Validity and reliability and practicability scores (% of respondents) for marmosets, top ten indicators from Truelove et al. (2020) (n=6 respondents).
Round 1, Phase 2 – rating of 56 indices
Overall, 21 of the more extensive list of 56 indicators were considered valid and reliable measures for assessing marmoset welfare ( Table 5); eight met consensus, while the other 13 approached consensus. Of these 21, nine are animal-based, with the remainder comprised of environment-based (9) or staff-based (3) indicators. Of the 56 indicators, 50 (89.3%) were rated as practicable by at least two-thirds of the respondents working with marmosets.
The few respondents working with marmosets were classified into three categories: infectious disease (n=4), neuroscience (1) and breeding (n=1). Indicators chosen as reliable and valid by at least two-thirds of these respondents are shown by discipline and indicator type in Figure 6. Items approaching consensus (65-69.99%) are included in these results as the groups had very small sample sizes and items would perhaps reach consensus with additional participants. More of the 56 welfare indices were considered valid and reliable by at least 70% of respondents in the infectious disease category (21 indices) and neuroscience category (16 indices), than in breeding (9). Seven indices were selected in all three disciplines (stereotypy, body weight, mortality, NHP induced injuries, cage furniture, animal care observations, and disease surveillance) and all 16 indices in the neuroscience category were also selected by the infectious disease group.
Figure 6. Indicators chosen as reliable and valid by two-thirds of respondents, marmosets by discipline and indicator type (n=6 respondents).
Round 2 – measurement of selected welfare indicators
The marmoset experts also discussed and reported how they would measure the potential welfare indices deemed valid, reliable, and practicable. The top eight indices from the current survey and how they might be measured for this species are given in Table 6. For each indicator, participants recommended that each should be measured at 91-100% of the population being assessed, whether the unit of measurement be at the individual or the facility level, to get a meaningful assessment. All indicators could be measured at either the individual or cage level, as appropriate, with NHP induced injuries also being assessed at the room level. There was a mix of methods for recording these indices, with observation, records, or both being recommended.
Table 6. Method of assessment, recording level, frequency, and duration for top eight indicators for marmosets (n=3 discipline groups).
| Marmosets | Recording level | Method | Notes | Duration and frequency | ||||
|---|---|---|---|---|---|---|---|---|
| Indicator | Individual | Group/Cage | Room | Facility | Observation | Records | ||
| Body weight | * | O | R | Weight and body condition score | Regular health checks, or when handling animal for experiment or management needs | |||
| NHP induced injuries | * | * | O | As needed (up to 10 minutes) over a year period (for comparison) | ||||
| Cage furniture | * | R | Housing records | |||||
| Huddled posture | * | O | At each physical check (60s) | |||||
| Mortality | * | R | Intermittently as needed | |||||
| Blood in faeces/urine | * | O | Daily checks (up to 1 minute) | |||||
| Environmentally induced injuries | * | O | As needed (up to 5 minutes) over a year period (for comparison) | |||||
| Physical enrichment | * | O | R | Observation of use and records of what is provided. | As needed if injuries occur or items not used. | |||
NHP=non-human primates.
Macaque and marmoset respondents
Considering both macaque and marmoset respondents (N=73), the whole group’s level of disagreement about the validity and reliability of the top ten indicators identified in Truelove et al. (2020) was high in both phases (Phase 1, α=0.1993; Phase 2, α=0.0915); however, levels remained relatively consistent between phases (Δ 0.1078), indicating group stability. The movement that occurred between phases was in the direction of disagreement (signifying divergence). Likely, this was a result of the increased options provided to the respondents between phases (i.e., more potential indices in Phase 2) and not requiring ranking of the same ten items across each phase; respondent fatigue is also a possibility. When asked to indicate which of 56 potential macaque welfare indicators are valid and reliable (Q3) for assessing NHP welfare, those ten initially presented (Q1) shifted in importance as evidenced by the proportion of respondents who selected an item as important for assessing welfare ( Table 7). Across the two phases, the respondents’ average inter-rater agreement was 78%; however, there were 12 respondents who had scores below 70%.
Table 7. Respondent percentage agreement score shift between Phases 1 and 2 of Round 1 for Truelove et al. (2020) top ten indicators.
| Humane euthanasia | Health Monitoring | Food enrichment | Physical enrichment | Social enrichment | Self-harm behaviour | Ventilation | Behavioural management | Hear other NHPs | Cage furniture | |
|---|---|---|---|---|---|---|---|---|---|---|
| Percent of respondents selecting indicator, Q1 (Phase 1) | 50.7 | 84.9 | 72.6 | 80.8 | 95.9 | 74.0 | 20.5 | 82.2 | 43.8 | 64.4 |
| Percent of respondents selecting indicator, Q3 (Phase 2) | 53.4 | 71.2 | 61.6 | 65.8 | 75.3 | 79.5 | 30.1 | 65.8 | 34.2 | 67.1 |
| Percent change between Phases 1 and 2 in Round 1 | 2.7 | -13.7 | -11.0 | -15.1 | -20.5 | 5.5 | 9.6 | -16.4 | -9.6 | 2.7 |
Across the two Delphi consultations, four indicators were identified as important for both marmosets and macaques: body weight, NHP induced injuries, physical enrichment, and cage furniture ( Table 8).
Table 8. Summary of top indicators, comparing species and Delphi consultations.
| Delphi consultation | Self-harm behaviour | Social enrichment | Cage dimensions | Body weight | Health monitoring | Appetite | Staff training | Positive reinforcement training | NHP induced injuries | Behavioural management | Humane euthanasia | Food enrichment | Physical enrichment | Ventilation | Hear other primates | Cage furniture | Huddled posture | Mortality | Environmentally induced injuries | Blood in faeces/urine |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Macaques, top ten, Truelove et al. (2020) (n=39) |
|
|
|
|
|
|
|
|
|
|
||||||||||
| Macaques, top ten, Current survey (n=67) * |
|
|
|
|
|
|
|
|
|
|
||||||||||
| Marmosets, top eight, Current survey (n=6) |
|
|
|
|
|
|
|
|
NHP=non-human primates.
Top eight are valid, reliable, and practicable; NHP induced injuries and behavioural management are valid and reliable.
Discussion
This study aimed to achieve consensus on effective indices of welfare for macaques and marmosets bred and used for research through expert consultation about the validity, reliability and practicability of a range of potential indicators. It builds upon the previous Delphi consultation of Truelove et al. (2020) by surveying a larger population of macaque experts working within a broader range of countries (predominantly within the EU) and collecting information on how top-ranking indices should be measured. The larger population also enabled us to explore differences between disciplines (though sample sizes were small for some discipline categories). By combining data from the two consultations, we were able to develop a generalised protocol for welfare assessment of macaque species.
We chose a Delphi process owing to the ability to survey a large number of experts anonymously and independently, without the opinions of any one respondent/group dominating the discussion, and to provide controlled feedback, helping to reduce noise and converge upon quality indicators. The systematic Delphi approach is more rigorous than other group consensus approaches, like case studies or focus groups ( Boulkedid et al., 2011). However, it does have limitations which impact on our interpretation of the results (outlined below).
Considering first respondents working with common marmosets, of the 56 indicators presented in Round 1 Phase 2, only 37.5% (21/56) were considered valid and reliable for assessing marmoset welfare, and 89.3% (50/56) were rated as practicable by at least two-thirds of the respondents. This is not surprising given these indicators were those furnished from the macaque literature and experts in Truelove et al. (2020). Of the six indicators rated as not practicable, five were staff-based and one was a micro-environment indicator. Of note in terms of species differences is the observation that ambient environment indicators such as humidity, room temperature, room ventilation, and light intensity were considered valid and reliable by two-thirds of marmoset respondents but not so of macaque respondents, probably reflecting the physical needs of these tropical New World monkeys, which are different to those of macaques and temperate living humans ( Buchanan-Smith, 2010b).
Indicators ranked important for assessing the welfare of common marmosets were body weight, NHP and environmentally induced injuries, cage furniture, huddled posture, mortality, blood in excreta, and physical enrichment. These findings should be viewed as preliminary given only six of our 73 respondents (8%) worked with this species and the indices were from Truelove et al. (2020). Nonetheless, whilst they cannot be said to be indicative of the marmoset research community, these findings have value in identifying potential effective indicators that can be further explored in a subsequent Delphi process involving a larger population of subject experts. We consider it important to conduct this exercise, given the resurgence in the use of this species in biomedical research ( Colman et al., 2021). Some of the chosen indicators may reflect signs typically associated with marmoset wasting syndrome, a disease which causes morbidity and mortality in captive colonies ( Ludlage & Masfield, 2003). We note also there was some inter-species application, as indicated by the percentage agreement scores of marmoset experts for seven of the top ten macaque indices in Truelove et al. (2020). It is valuable to have identified welfare assessment indicators that could be applied to multiple NHP species, particularly when conducting a limited-time, on-site assessment.
When presented with 115 potential indicators for macaques, participants in the Truelove et al. (2020) Delphi selected 56 of these as valid, reliable, and practicable within the context of a hypothetical scenario involving 500 animals; environment-based and staff-based indicators (44) were selected more than three times animal-based indicators (12). In the current study and with the same scenario, of the 56 indices, only eight were found to be valid, reliable, and practicable by at least 70% agreement of the macaque respondents. Three of the eight were animal-based (self-harm behaviour, body weight, appetite); the remainder were either environment-based (social enrichment, cage dimensions) or staff-based (health monitoring programme, staff training, positive reinforcement training). In addition, NHP induced injuries (animal-based) and presence of a behavioural management programme (staff-based) approached consensus at 68.7%.
It is notable that no physiological indicators and only one behavioural indicator (self-harm behaviour) are included in the top ten of Truelove et al. (2020), probably reflecting the greater effort required in collecting animal-based data to assess welfare (though 12 animal-based indicators, including body weight, appetite, and NHP induced injuries did reach consensus in Truelove et al., 2020). We speculate that the predominantly European participants of the current Delphi were more open to animal-based indicators than the predominantly North American participants in Truelove et al. (2020) because European macaque colonies tend to be smaller and there is perhaps more staff resource available to obtain information requiring direct measurement. Environment- and staff-based indicators can generally be assessed with more immediacy and ease, and without specialist equipment or judgement (e.g. whether cage furniture is present in the enclosure). However, it should be noted that the mere presence of something does not give a full picture of its contribution to NHP welfare; the quality of the item, and how much it is used, and by which animals, are also important factors.
Of the eight indicators selected in Phase 2, three of these also appeared in the top ten of Truelove et al. (2020) presented in Phase 1 (self-harm behaviour, social enrichment, health monitoring programme), strongly suggesting that these indicators are considered critical for the assessment of macaque welfare. Also exceeding or approaching the 70% threshold in Phase 1 were a behavioural management programme, physical enrichment, and food enrichment, suggesting their consideration as well. Social enrichment was rated the top indicator in Phase 1 (>94% of respondents), reflecting the importance of companionship for psychological well-being in these animals. Social enrichment and self-harm behaviour are well known as important indicators of good and poor welfare, respectively, in NHPs, and there is a large literature on their incidence and relevance to macaque well-being, so it is not surprising that there was consensus agreement on their importance in both Delphi consultations. Fewer than two-thirds of respondents felt a humane euthanasia programme, hearing other NHPs, cage furniture, and ventilation could be used to assess welfare. Opinion was split on how practical cage furniture and ventilation are as welfare indices for macaques. Agreement scores for practicability were generally higher in Truelove et al. (2020) than in the current study, possibly reflecting differences in expert demographics, sample size, and methodology.
A greater number of the 56 indicators were considered valid and reliable by respondents working with macaques in infectious disease research and breeding, than in neuroscience, toxicology and other disciplines, perhaps reflecting a greater awareness of the impact of surgical and husbandry procedures, and the cage environment, on the welfare of these animals ( Figure 4). Macaques in neuroscience also undergo frequent sedations, surgeries, and medical procedures, so it is curious these did not approach consensus in this category but did so in infectious disease. Within neuroscience, body weight and appetite are included in the top four indices, reflecting that many macaques used in neuroscience undergo food or fluid control to motivate them to work on behavioural or cognitive tasks whilst brain activity is measured. Self-harm behaviour and stereotypies are within the top seven, reflecting the practice of monitoring between experimental manipulations behavioural changes which could compromise the validity of the NHP model. Although they did not meet the set threshold for inclusion as items to rate by all participants, additional indicators suggested by respondents within this discipline included activity level (including the presence of depressive-like behaviour or non-alert inactive behaviour), engagement with and performance on experimental tasks, and water intake (again reflecting the scientific procedures involved).
Within infectious disease, more than half of the indicators (33/56) were considered valid and reliable by three-quarters of respondents, with over 40% (14/33) of these being cage-based. Appetite, body weight, and mortality are in the top five, reflecting that many of the macaques used in such studies will experience disease ( Prescott et al., 2021). Additionally, to round out the top five are stereotypy and self-harm behaviour. Similar to neuroscience, these indices reflect the practice of monitoring behavioural changes between experimental manipulations over prolonged periods of time.
Ten indicators were selected by more than two-thirds of respondents in toxicology, drawn from a range of categories – behavioural, physiological, husbandry-based, and cage-based. Body weight and mortality checks are routinely performed as part of regulatory toxicology studies. Most animals are euthanised for pathology when assigned to toxicology studies, which might account for the appearance of a humane euthanasia programme in the top four. Huddled posture may reflect sickness due to test drug administration.
Within breeding, 24 of the 56 indices (42.9%) were selected by more than two-thirds of respondents. Of the top ten, five are cage-based and four husbandry-based, reflecting the large group sizes and number of animals to be monitored in breeding units, as well as the relative lack of need for scientific procedures and for welfare data for a scientific purpose. The inclusion of indicators such as social density, vertical space, environmental complexity, cage furniture, and visual barriers probably reflects the more spacious environments often afforded to breeding animals.
That some indices are contextualized differently across specializations is not surprising. For example, those who require chair restraint for handling of monkeys to do their research might find frequency of such restraint to be a more useful indicator than those who do not. This brings to light the difficulty in assessing animal welfare and the complexities of using indicators – they must be well defined, validated for the species for which they are applied, and need be not only practical to measure but also reliable across time and raters. Agreement on which of these is most important is coloured by culture and work experience as well as discipline perspective ( Duijvesteijn et al., 2014), and when working as a group, the results of the process will also be subject to the composition of the panel. In the current study, the majority of respondents working with macaques did so in neuroscience, and most respondents were primarily from the UK and EU, whereas those in the Truelove et al. (2020) study were primarily from the USA. Differences exist between these regions in how laboratory NHPs are housed and managed, partly due to the oversight regulations. For example, minimum cage space requirements for NHPs are considerably smaller in the USA than in the UK and EU member countries. Under Directive 2010/63/EU ( European Union, 2010), the minimum volume for macaques from three years of age is 1.8m 3 /64ft 3 per animal, reflecting the value placed on providing housing that allows for exercise and the expression of ethologically relevant behaviours, such as running, climbing, leaping and hiding from companions ( NC3Rs, 2017). Under the ILAR Guide ( National Research Council, 2011), the minimum volume per macaque up to 10kg is 0.25m 3/9ft 3, and this space allocation was not increased in the 2011 revision. One possible reason for this disparity in minimum cage space is that UK and EU NHP facilities tend to house considerably fewer animals than those in the USA. Irrespective of these regional differences, our two Delphi consultations have been able to identify critically important indices for macaque welfare.
Conclusions
We have identified context appropriate indicators that are valid, reliable, and practicable for assessing the welfare of macaques and marmosets bred and used for research, including in toxicology, neuroscience, and infectious disease, potentially benefiting far in excess of 100,000 NHPs used globally per year by improving welfare assessment, minimisation of harm and evaluation of the impact of refinement techniques. In ranking potential welfare indicators, we have identified those indicators considered the most important by experts and narrowed the field for further investigation and validation of both species-specific and general indicators. We have used the top-ranking indicators for macaques identified by experts in our two Delphi consultations, and agreement on how these should be measured, to develop a practical and generalised welfare assessment protocol to support laboratory staff in monitoring and optimising macaque health and well-being (GEN-MAC). There were too few marmoset data to generate a welfare assessment protocol for this species, and we recommend further data are collected. Our work to harmonise welfare indicators and assessment should facilitate inter-lab comparative studies, data-sharing to boost sample sizes in research asking welfare-focused questions, and benchmarking of welfare standards between facilities. It would be good to build upon this momentum and achieve further consensus and harmonisation globally, involving Pacific Rim countries in addition to North America and Europe. Further validation of the proto-protocol, and of the top-ranking welfare indicators, would also be welcome. Funding opportunities are available from the NC3Rs, other bioscience organisations, and animal welfare charities.
Data availability
Underlying data
Newcastle University research repository: Survey Data and Supplementary Tables. https://doi.org/10.25405/data.ncl.19106960 ( Leach et al., 2022).
This project contains the following underlying data:
-
•
Survey 1 Data anonymised.xlsx (Anonymised data set generated during Survey 1)
-
•
Survey 2 Data anonymised.xlsx (Anonymised data set generated during Survey 2)
Extended data
Newcastle University research repository: Survey Data and Supplementary Tables. https://doi.org/10.25405/data.ncl.19106960 ( Leach et al., 2022).
This project contains the following extended data:
-
•
Supplementary Table 1.doc (Comparison of validity and reliability and practicability respondent scores between two Delphi exercises, macaques, 56 welfare indices)
-
•
Supplementary Table 2.doc (Macaque respondent agreement scores for validity and reliability by discipline, 56 welfare indices, n=67 respondents)
-
•
GEN-MAC protocol.xlsx (Generalised macaque welfare assessment protocol aims to offer a practical and context appropriate tool for laboratory staff caring for macaques. It provides a quantitative set of criteria to support staff in monitoring and maximising macaque health and well-being, based on expert consensus.)
Data are available under the terms of the Creative Commons Zero “No rights reserved” data waiver (CC0 1.0 Public domain dedication).
Acknowledgements
We are grateful to all participants of the 2019 Primate Welfare Meeting for sharing their views and practices.
Funding Statement
The author(s) declared that no grants were involved in supporting this work.
[version 3; peer review: 3 approved]
References
- Ahloy-Dallaire J, Espinosa J, Mason G: Play and optimal welfare: does play indicate the presence of positive affective states?. Behav. Processes. 2018;156:3–15. 10.1016/j.beproc.2017.11.011 [DOI] [PubMed] [Google Scholar]
- American Veterinary Medical Association: AVMA Guidelines for the Euthanasia of Animals. Schaumburg, Illinois: AVMA; 2020 ed 2020. Reference Source [Google Scholar]
- Anderson JR, Chamove AS: Allowing captive primates to forage. Standards in Laboratory Animal Management. Potters Bar, UK: Universities Federation for Animal Welfare;1984;253–256. [Google Scholar]
- Association of Primate Veterinarians: Guidelines for Assessment of Acute Pain in Nonhuman Primates. J. Am. Assoc. Lab. Anim. Sci. 2019;58(6):748–749. [PMC free article] [PubMed] [Google Scholar]
- Association of Primate Veterinarians: Association of Primate Veterinarians’ Humane Endpoint Guidelines for Nonhuman Primates in Biomedical Research. J. Am. Assoc. Lab. Anim. Sci. 2020;59(1):6–8. [PMC free article] [PubMed] [Google Scholar]
- Bain M, Nagrani A, Schofield D, et al. : Automated audiovisual behavior recognition in wild primates. Sci. Adv. 2021;7(46):eabi4883. 10.1126/sciadv.abi4883 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baker KC: Survey of 2014 behavioral management programs for laboratory primates in the United States. Am. J. Primatol. 2016;78:780–796. 10.1002/ajp.22543 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baker KC, Crockett CM, Lee GH, et al. : Pair housing for female longtailed and rhesus macaques in the laboratory: behavior in protected contact versus full contact. J. Appl. Anim. Welf. Sci. 2012;15:126–143. 10.1080/10888705.2012.658330 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baker KC, Bloomsmith MA, Oettinger B, et al. : Comparing options for pair housing rhesus macaques using behavioral welfare measures. Am. J. Primatol. 2014;76:30–42. 10.1002/ajp.22190 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balansard I, Cleverley L, Culter KC, et al. : Revised recommendations for health monitoring of non-human primate colonies (2018): FELASA Working Group Report. Lab. Animals. 2019 Oct;53(5):429–446. 10.1177/0023677219844541 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bassett L, Buchanan Smith HM, McKinley J, et al. : Effects of training on stress-related behavior of the common marmoset ( Callithrix jacchus) in relation to coping with routine husbandry procedures. J. Appl. Anim. Welf. Sci. 2003;6(3):221–233. 10.1207/S15327604JAWS0603_07 [DOI] [PubMed] [Google Scholar]
- Beaver V, Bayne K: Animal welfare assessment considerations. Laboratory Animal Welfare. Bayne K, Turner PV, editors. London: Academic Press, Elsevier;2014;29–38. 10.1016/B978-0-12-385103-1.00004-X [DOI] [Google Scholar]
- Beisner BA, Wooddell LJ, Hannibal DL, et al. : High rates of aggression do not predict rates of trauma in captive groups of macaques. Appl. Anim. Behav. Sci. 2019;212:82–89. 10.1016/j.applanim.2019.01.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bennett AJ, Bailoo JD, Dutton M, et al. : Psychological science applied to improve captive animal care: a model for development of a systematic, evidence-based assessment of environ mental enrichment for nonhuman primates. PsyArXiv. 2018. 10.17605/OSF.IO/79XKY [DOI] [Google Scholar]
- Bliss-Moreau E, Amara RR, Buffalo EA, et al. : National Primate Research Center Consortium Rigor and Reproducibility Working Group. Improving rigor and reproducibility in nonhuman primate research. Am. J. Primatol. 2021 Sep 20;83:e23331. 10.1002/ajp.23331 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blois-Heulin C, Rochais C, Camus S, et al. : Animal welfare: could adult play be a false friend?. Anim. Behav. Cogn. 2015;2(2):156–185. 10.12966/abc.05.04.2015 Reference Source [DOI] [Google Scholar]
- Blokhuis M, Miele M, Veissier I, et al. : Improving Farm Animal Welfare, Science and Society Working Together: The Welfare Quality Approach. Wageningen, The Netherlands: Wageningen Academic Publishers;2013. 10.3920/978-90-8686-770-7 Reference Source [DOI] [Google Scholar]
- Bloomsmith MA: Behavioral management of laboratory primates: principles and projections. Handbook of Primate Behavioral Management. Schapiro SJ, editor. New York: CRC Press;2017;497–513. 10.1201/9781315120652-29 [DOI] [Google Scholar]
- Bloomsmith MA, Perlman JE, Hutchinson E, et al. : Behavioral management programs to promote laboratory animal welfare. Management of Animal Care and Use Programs in Research, Education, and Testing. 2nd ed. Weichbrod RH, Thompson GA, Norton JN, editors. Boca Raton, FL: CRC Press/Taylor & Francis;2018, Chapter 5. 10.1201/9781315152189-5 [DOI] [PubMed] [Google Scholar]
- Boccia ML: Preliminary report on the use of a natural foraging task to reduce aggression and stereotypies in socially housed pigtail macaques. Lab. Prim. Newsl. 1989a;28(1):3–4. Reference Source [Google Scholar]
- Boccia ML: Long-term effects of a natural foraging task on aggression and stereotypies in socially housed pigtail macaques. Lab. Prim. Newsl. 1989b;28:18–19. Reference Source [Google Scholar]
- Boulkedid R, Abdoul H, Loustau M, et al. : Using and reporting the Delphi method for selecting healthcare quality indicators: a systematic review. PLoS ONE. 2011;6(6):e20476. 10.1371/journal.pone.0020476 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bryant CE, Rupniak NM, Iversen SD: Effects of different environmental enrichment devices on cage stereotypies and autoaggression in captive cynomolgus monkeys. J Med Primatol. 1988;17(5):257–269. 10.1111/j.1600-0684.1988.tb00388.x [DOI] [PubMed] [Google Scholar]
- Buchanan-Smith HM: Environmental enrichment for primates in laboratories. Adv. Sci. Res. 2010a;5:41–56. 10.5194/asr-5-41-2010 Reference Source [DOI] [Google Scholar]
- Buchanan-Smith HM: Marmosets and tamarins. The UFAW Handbook on the Care and Management of Laboratory and Other Research Animals. Kirkwood J, Hubrecht RC, editors. Chichester: Wiley Blackwell;2010b;543–563. 10.1002/9781444318777.ch36 [DOI] [Google Scholar]
- Buchanan-Smith HM, Prescott MJ, Cross NJ: What factors should determine cage size for primates in the laboratory?. Anim. Welfare. 2004;13:S197–S201. [Google Scholar]
- Buchanan-Smith HM, Rennie AE, Vitale A, et al. : Harmonising the definition of refinement. Anim. Welfare. 2005;14(4):379–384. Reference Source [Google Scholar]
- Byrne GD, Suomi SJ: Effects of woodchips and buried food on behavior patterns and psychological well-being of captive rhesus monkeys. Am. J. Primatol. 1991;23(3):141–151. 10.1002/ajp.1350230302 [DOI] [PubMed] [Google Scholar]
- Canadian Council on Animal Care: CCAC Guidelines: Nonhuman Primates. Ottawa, Ontario: CCAC;2019. Reference Source [Google Scholar]
- Carlsson HE, Schapiro SJ, Farah I, et al. : Use of primates in research: a global overview. Am. J. Primatol. 2004;63(4):225–237. 10.1002/ajp.20054 [DOI] [PubMed] [Google Scholar]
- Cassidy LC, Hannibal DL, Semple S, et al. : Improved behavioral indices of welfare in continuous compared to intermittent pair-housing in adult female rhesus macaques ( Macaca mulatta). Am. J. Primatol. 2020;82:e23189. 10.1002/ajp.23189 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chamove AS: Environmental enrichment: a review. Animal Technology. 1989;40(3):155–178. Reference Source [Google Scholar]
- Chamove AS, Anderson JR, Morgan-Jones SC, et al. : Deep woodchip litter: hygiene, feeding, and behavioral enhancement in eight primate species. Int. J. Study Anim. Problems. 1982;3(4):308–318. Reference Source [Google Scholar]
- Clarence WM, Scott JP, Dorris MC, et al. : Use of enclosures with functional vertical space by captive rhesus monkeys ( Macaca mulatta) involved in biomedical research. J. Am. Assoc. Lab. Anim Sci. 2006;45(5):31–34. [PubMed] [Google Scholar]
- Clingerman KJ, Summers L: Validation of a body condition scoring system in rhesus macaques ( Macaca mulatta): inter- and intra-rater variability. J. Am. Assoc. Lab. Anim Sci. 2012;51(1):31–36. [PMC free article] [PubMed] [Google Scholar]
- Coleman K: Caring for nonhuman primates in biomedical research facilities: scientific, moral and emotional considerations. Am. J. Primatol. 2011;73(3):220–225. 10.1002/ajp.20855 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coleman K, Maier A: The use of positive reinforcement training to reduce stereotypic behavior in rhesus macaques. Appl. Anim. Behav. Sci. 2010;124(3-4):142–148. 10.1016/j.applanim.2010.02.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coleman K, Novak MA: Environmental enrichment in the 21st century. ILAR J. 2017;58(2):295–307. 10.1093/ilar/ilx008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colman RJ, Capuano S, Bakker J, et al. : Marmosets: welfare, ethical use, and IACUC/regulatory considerations. ILAR J. 2021;ilab003. 10.1093/ilar/ilab003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crast J, Bloomsmith MA, Remillard CM, et al. : Contribution of adult sex ratio to trauma and reproductive output in large breeding groups of rhesus macaques ( Macaca mulatta). Anim. Welfare. 2021;30(4):479–492. 10.7120/09627286.30.4.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davenport MD, Tiefenbacher S, Lutz CK, et al. : Analysis of endogenous cortisol concentrations in the hair of rhesus macaques. Gen. Comp. Endocrinol. 2006;147(3):255–261. 10.1016/j.ygcen.2006.01.005 [DOI] [PubMed] [Google Scholar]
- Descovich K, Richmond S, Leach M, et al. : Opportunities for refinement in neuroscience: indicators of wellness and post-operative pain in laboratory macaques. ALTEX. 2019;36(4):535–554. 10.14573/altex.1811061 [DOI] [PubMed] [Google Scholar]
- DiVincenti L, Jr, Wyatt JD: Pair housing of macaques in research facilities: a science-based review of benefits and risks. J. Am. Assoc. Lab. Anim. Sci. 2011;50(6):856–863. [PMC free article] [PubMed] [Google Scholar]
- Doane CJ, Andrews K, Schaefer LJ, et al. : Dry bedding provides cost-effective enrichment for group-housed rhesus macaques ( Macaca mulatta). J. Am. Assoc. Lab. Anim. Sci. 2013;52(3):247–252. [PMC free article] [PubMed] [Google Scholar]
- Duijvesteijn N, Benard M, Reimert I, et al. : Same pig, different conclusions: stakeholders differ in qualitative behaviour assessment. J. Agric. Environ. Ethics. 2014;27(6):1019–1047. 10.1007/s10806-014-9513-z [DOI] [Google Scholar]
- European Commission: The welfare of non-human primates used in research, Report of the Scientific Committee of Animal Health and Animal Welfare, Adopted on 17 December 2002. Brussels: European Commission;2002. Reference Source [Google Scholar]
- European Union: Directive 2010/63/EU of the European Parliament and of the Council of 22 September 2010 on the protection of animals used for scientific purposes. Brussels: Official Journal of the European Union L 276/33;2010. Reference Source [Google Scholar]
- Fraser D: Understanding Animal Welfare: The Science in its Cultural Context. Ames, Iowa, USA: Wiley-Blackwell;2008. 978-1-405-13695-2. [Google Scholar]
- Gilbert MH, Baker KC: Social buffering in adult male rhesus macaques ( Macaca mulatta): Effects of stressful events in single vs. pair housing. J. Med. Primatol. 2011;40:71–78. 10.1111/j.1600-0684.2010.00447.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gottlieb D, Pomerantz O: Utilizing behavior to assess welfare. Behavioral Biology of Laboratory Animals. Coleman K, Schapiro SJ, editors. Boca Raton, FL: CRC Press;2021;51–64. [Google Scholar]
- Gottlieb DH, O'Connor JR, Coleman K: Using porches to decrease feces painting in rhesus macaques ( Macaca mulatta). J. Am. Assoc. Lab. Anim. Sci. 2014;53(6):653–656. [PMC free article] [PubMed] [Google Scholar]
- Graham ML, Prescott MJ: The multifactorial role of the 3Rs in shifting the harm-benefit analysis in animal models of disease. Eur. J. Pharmacol. 2015;759:19–29. 10.1016/j.ejphar.2015.03.040 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Griffis CM, Martin AL, Perlman JE, et al. : Play caging benefits the behavior of singly housed laboratory rhesus macaques ( Macaca mulatta). J. Am. Assoc. Lab. Anim. Sci. 2013;52(5):534–540. [PMC free article] [PubMed] [Google Scholar]
- Grimm D: Record number of monkeys being used in U.S. research. Science. 2018 2 Nov 2018. 10.1126/science.aav9290 Reference Source [DOI] [Google Scholar]
- Hallowell MR: Techniques to minimize bias when using the Delphi method to quantify construction safety and health risks . Construction Research Congress 2009: Building a Sustainable Future. Reston, VA: ASCE;2009;1489–1498. 10.1061/41020(339)151 [DOI] [Google Scholar]
- Hannibal DL, Bliss-Moreau E, Vandeleest J, et al. : Laboratory rhesus macaque social housing and social changes: Implications for research. Am. J. Primatol. 2017;79:1–14. 10.1002/ajp.22528 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hawkins P, Morton DB, Burman O, et al. : A guide to defining and implementing protocols for the welfare assessment of laboratory animals: Eleventh report of the BVAAWF/FRAME/RSPCA/UFAW Joint Working Group on Refinement. Lab. Animals. 2011;45(1):1–13. 10.1258/la.2010.010031 [DOI] [PubMed] [Google Scholar]
- Hayes AF, Krippendorff K: Answering the call for a standard reliability measure for coding data. Commun. Methods Meas. 2007;1(1):77–89. 10.1080/19312450709336664 [DOI] [Google Scholar]
- Home Office: Advisory notes on recording and reporting the actual severity of regulated procedures. London: UK Government;2014. Reference Source [Google Scholar]
- Honess PE, Marin CM: Enrichment and aggression in primates. Neurosci. Biobehav. Rev. 2006;30(3):413–436. 10.1016/j.neubiorev.2005.05.002 [DOI] [PubMed] [Google Scholar]
- Honess P, Wolfensohn S: The extended welfare assessment grid: a matrix for the assessment of welfare and cumulative suffering in experimental animals. ATLA. 2010;38(3):205–212. 10.1177/026119291003800304 [DOI] [PubMed] [Google Scholar]
- Hsu CC, Sandford BA: The Delphi technique: making sense of consensus. Pract. Assess. Res. Eval. 2007;12(10):1–8. 10.7275/pdz9-th90 [DOI] [Google Scholar]
- Institute for Laboratory Animal Research: Recognition and Alleviation of Distress in Laboratory Animals. Washington, D.C.: National Academy Press;2008. 10.17226/11931 [DOI] [Google Scholar]
- Izzo GN, Bashaw MJ, Campbell JB: Enrichment and individual differences affect welfare indicators in squirrel monkeys ( Saimiri sciureus). J. Comp. Psychol. 2011;125(3):347–352. 10.1037/a0024294 [DOI] [PubMed] [Google Scholar]
- Jennings M, Prescott MJ: Refinements in husbandry, care and common procedures for non-human primates: Ninth report of the BVAAWF/FRAME/RSPCA/UFAW Joint Working Group on Refinement. Lab. Animals. 2009;43(S1):1–47. 10.1258/la.2008.007143 [DOI] [PubMed] [Google Scholar]
- Johnsen PF, Johannesson T, Sandøe P: Assessment of farm animal welfare at herd level: many goals, many methods. Acta Agric. Scand., Section A - Animal Science. 2001;51(S30):26–33. 10.1080/090647001316923027 [DOI] [Google Scholar]
- Keeling ME, Wolf RH: Medical management of the rhesus monkey. The Rhesus Monkey: Management, Reproduction, and Pathology. Bourne GH, editor. New York, NY: Academic Press;1975;11–96. [Google Scholar]
- Keeney S, Hasson F, McKenna H: Conducting the research using the Delphi technique. The Delphi Technique in Nursing and Health Research. West Sussex, UK: Wiley-Blackwell;2011;69–83. 10.1002/9781444392029.ch5 [DOI] [Google Scholar]
- Kirchner MK, Bakker J: Construction of an integrated welfare assessment system (MacWel) for macques ( Macaca spp.) in human husbandry. Int. Conf. Dis. Zoo Wild Anim. Szentiks CA, Schumann A, editors. Leibniz Institute for Zoo and Wildlife Research;2015. Reference Source [Google Scholar]
- Krippendorff K: Reliability in content analysis. Hum. Commun. Res. 2004;30(3):411–433. 10.1111/j.1468-2958.2004.tb00738.x [DOI] [Google Scholar]
- Lambeth SP, Schapiro SJ, Bernacky BJ, et al. : Establishing ‘quality of life’ parameters using behavioural guidelines for humane euthanasia of captive non-human primates. Anim. Welfare. 2013;22(4):429–435. 10.7120/09627286.22.4.429 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lankau EW, Turner PV, Mullan RJ, et al. : Use of nonhuman primates in research in North America. J. Am. Assoc. Lab. Anim. Sci. 2014;53(3):278–282. Reference Source [PMC free article] [PubMed] [Google Scholar]
- Leach MC, Thornton PD, Main DCJ: Identification of appropriate measures for the assessment of laboratory mouse welfare. Anim. Welf. 2008;17(2):161–170. Reference Source [Google Scholar]
- Leach M, Prescott M, Truelove M: Survey Data and Supplementary Tables. Newcastle University;2022. Dataset. 10.25405/data.ncl.19106960.v1 [DOI] [Google Scholar]
- Lewis AD, Colgin LMA: Pathology of noninfectious diseases of the laboratory primate. The Laboratory Primate. London, UK: Elsevier Ltd.;2005;47–74. 10.1016/B978-012080261-6/50004-0 [DOI] [Google Scholar]
- Lidster K, Owen K, Browne B, et al. : Cage aggression in group-housed laboratory male mice: an international data crowdsourcing project. Sci. Rep. 2019;9:15211. 10.1038/s41598-019-51674-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ludlage E, Mansfield K: Clinical care and diseases of the common marmoset ( Callithrix jacchus). Comp. Med. 2003;53(4):369–382. [PubMed] [Google Scholar]
- Lutz CK, Brown TA: Porches as enrichment for singly housed cynomolgus macaques ( Macaca fascicularis). J. Am. Assoc. Lab. Anim. Sci. 2018;57(2):134–137. [PMC free article] [PubMed] [Google Scholar]
- Lutz CK, Novak MA: Environmental enrichment for nonhuman primates: theory and application. ILAR J. 2005;46(2):178–191. 10.1093/ilar.46.2.178 [DOI] [PubMed] [Google Scholar]
- Lutz C, Well A, Novak M: Stereotypic and self-injurious behaviour in rhesus macaques: a survey and retrospective analysis of environment and early experience. Anim. Behav. 1991;60:1–15. 10.1002/ajp.10075 [DOI] [PubMed] [Google Scholar]
- McMillan J, Bloomsmith MA, Prescott MJ: An international survey of approaches to chair restraint of nonhuman primates. Comp. Med. 2017;67(5):1–10. [PMC free article] [PubMed] [Google Scholar]
- MacLean EL, Prior SR, Platt ML, et al. : Primate location preference in a double-tier cage: the effects of illumination and cage height. J. Appl. Anim. Welf. Sci. 2009;12(1):73–81. 10.1080/10888700802536822 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mellor DJ, Patterson-Kane E, Stafford KJ: The Sciences of Animal Welfare. UFAW Animal Welfare Series. Oxford, UK: Wiley-Blackwell;2009. [Google Scholar]
- Mench JA: Assessing animal welfare at the farm and group level: a United States perspective. Anim. Welfare. 2003;12(4):493–503. Reference Source [Google Scholar]
- Miller LJ, Vicino GA, Sheftel J, et al. : Behavioral diversity as a potential indicator of positive animal welfare. Animals. 2020;10(7):1211. 10.3390/ani10071211 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakamichi M, Asanuma K: Behavioral effects of perches on group-housed adult female Japanese monkeys. Percept. Mot. Skills. 1998;87(2):707–714. 10.2466/pms.1998.87.2.707 [DOI] [PubMed] [Google Scholar]
- National Research Council. Committee on Well-Being of Nonhuman Primates: The Psychological Well-Being of Nonhuman Primates. Washington, D.C.: National Academies Press;1998. 10.17226/4909 [DOI] [Google Scholar]
- National Research Council: Guide for the Care and Use of Laboratory Animals. Eighth ed. Washington D.C.: National Academies Press;2011. 10.17226/12910 [DOI] [Google Scholar]
- NC3Rs: The Macaque Website. London: NC3Rs;2015. Reference Source [Google Scholar]
- NC3Rs: Non-human Primate Accommodation, Care and Use. London: NC3Rs;2017. Reference Source [Google Scholar]
- Novak MA: Self-injurious behavior in rhesus monkeys: new insights into its etiology, physiology, and treatment. Am. J. Primatol. 2003;59(1):3–19. 10.1002/ajp.10063 [DOI] [PubMed] [Google Scholar]
- Novak MA: Self-Injurious behavior in rhesus macaques: issues and challenges. Am. J. Primatol. 2021;83:e23222. 10.1002/ajp.23222 [DOI] [PubMed] [Google Scholar]
- Novak MA, Meyer JS: Abnormal behavior in animals in research settings. Behavioral Biology of Laboratory Animals. Coleman K, Schapiro SJ: editors. Boca Raton, FL: CRC Press;2021;27–50. [Google Scholar]
- Novak MA, Petto AJ: Through the Looking Glass: Issues of Psychological Well-being in Captive Nonhuman Primates. Washington, DC: American Psychological Association;1991. [Google Scholar]
- Novak MA, Suomi SJ: Psychological well-being of primates in captivity. Am. Psychol. 1988;43(10):765–773. 10.1037/0003-066X.43.10.765 [DOI] [PubMed] [Google Scholar]
- Perlman JE, Bloomsmith MA, Whittaker MA, et al. : Implementing positive reinforcement animal training programs at primate laboratories. Appl. Anim. Behav. Sci. 2012;137(3-4):114–126. 10.1016/j.applanim.2011.11.003 [DOI] [Google Scholar]
- Poirier C, Bateson M: Pacing stereotypies in laboratory rhesus macaques: implications for animal welfare and the validity of neuroscientific findings. Neurosci. BioBehav. Reviews. 2017;83:508–515. 10.1016/j.neubiorev.2017.09.010 [DOI] [PubMed] [Google Scholar]
- Polanco A, McCowan B, Niel L, et al. : Recommendations for abnormal behaviour ethograms in monkey research. Animals. 2021;11(5):1461. 10.3390/ani11051461 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poole TB: Behaviour, housing and welfare of non-human primates. New Developments in Biosciences: Their Implications for Laboratory Animal Science. Beynen AC, Solleveld HA, editors. Dordrecht: Martinus Nijhoff Publishers;1988;231–237. 978-94-010-7973-0. [Google Scholar]
- Poole T: Happy animals make good science. Lab. Animals. 1997;31(2):116–124. 10.1258/002367797780600198 [DOI] [PubMed] [Google Scholar]
- Prescott MJ, Buchanan-Smith HM: Training laboratory-housed non-human primates, Part 1: A UK survey. Anim. Welfare. 2007;16:21–26. [Google Scholar]
- Prescott MJ, Bowell VA, Buchanan-Smith HM: Training laboratory-housed non-human primates, Part 2: Resources for developing and implementing training programmes. Anim. Technol. Welfare. 2005;4:133–148. Reference Source [Google Scholar]
- Prescott MJ, Brown VJ, Flecknell PA, et al. : Refinement of the use of food and fluid control as motivational tools for macaques used in behavioural neuroscience research: report of a working group of the NC3Rs. J. Neurosci. Methods. 2010;193:167–188. 10.1016/j.jneumeth.2010.09.003 [DOI] [PubMed] [Google Scholar]
- Prescott MJ, Clark C, Dowling WE, et al. : Opportunities for refinement of non-human primate vaccine studies. Vaccines. 2021;9:284. 10.3390/vaccines9030284 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reinhardt V: Space utilization by captive rhesus macaques. Anim. Technol. 1992;11:61–66. 10.1002/zoo.1430110108 Reference Source [DOI] [Google Scholar]
- Reinhardt V: Caged rhesus macaques voluntary work for ordinary food. Primates. 1994;35:95–98. 10.1007/BF02381490 Reference Source [DOI] [Google Scholar]
- Reinhardt V: Legal loophole for subminimal floor area for caged macaques. J. Appl. Anim. Welf. Sci. 2003;6(1):53–56. 10.1207/S15327604JAWS0601_05 [DOI] [PubMed] [Google Scholar]
- Reinhardt V, Rossell M: Self-biting in caged macaques: cause, effect, and treatment. J. Appl. Anim. Welf. Sci. 2001;4:285–294. 10.1207/S15327604JAWS0404_05 [DOI] [Google Scholar]
- Reinhardt V, Liss C, Stevens C: Space requirement stipulations for caged non-human primates in the United States: a critical review. Anim. Welfare. 1996;5(4):361–372. Reference Source [Google Scholar]
- Rennie AE, Buchanan-Smith HM: Refinement of the use of non-human primates in scientific research. Part III: refinement of procedures. Anim. Welf. 2006;15:239–261. Reference Source [Google Scholar]
- Rushen J, Chapinal N, Passille AM: Automated monitoring of behavioural-based animal welfare indicators. Anim. Welfare. 2012;21:339–350. 10.7120/09627286.21.3.339 [DOI] [Google Scholar]
- Schapiro SJ: An overview of behavioral management for laboratory animals. Behavioral Biology of Laboratory Animals. Coleman K, Schapiro SJ, editors. Boca Raton, FL: CRC Press;2021;65–86. [Google Scholar]
- Schapiro SJ, Bloomsmith MA, Suarez SA, et al. : Effects of social and inanimate enrichment on the behavior of yearling rhesus monkeys. Am. J. Primatol. 1996;40(3):247–260. [DOI] [PubMed] [Google Scholar]
- Schapiro SJ, Coleman K, Akinyi M, et al. : Chapter 13 - Nonhuman Primate welfare in the research environment. American College of Laboratory Animal Medicine - Laboratory Animal Welfare. Bayne K, Turner PV, editors. London: Academic Press, Elsevier;2014;197–212. 10.1016/B978-0-12-385103-1.00013-0 [DOI] [Google Scholar]
- Segal EF: Housing, Care and Psychological Well-being of Captive and Laboratory Primates. Park Ridge, NJ: Noyes Publications;1989. 9780815512011. [Google Scholar]
- Smiley Evans T, Barry PA, Gilardi KV, et al. : Optimization of a novel non-invasive oral sampling technique for zoonotic pathogen surveillance in nonhuman primates. PLoS. Negl. Trop. Dis. 2015;9(6):e0003813. 10.1371/journal.pntd.0003813 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith JJ, Hadzic V, Li XB, et al. : Objective measures of health and well-being in laboratory rhesus monkeys ( Macaca mulatta). J. Med. Primatol. 2006;35:388–396. 10.1111/j.1600-0684.2006.00188.x [DOI] [PubMed] [Google Scholar]
- Truelove MA, Martin JE, Langford FM, et al. : The identification of effective welfare indicators for laboratory-housed macaques using a Delphi consultation process. Sci. Rep. 2020;10:20402. 10.1038/s41598-020-77437-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turner PV, Grantham LE: Short-term effects of an environmental enrichment program for adult cynomolgus monkeys. Contemp. Top. Lab. Anim. Sci. 2002;41(5):13–17. [PubMed] [Google Scholar]
- University of Stirling: Common Marmoset Care. Stirling: University of Stirling;2011. Reference Source [Google Scholar]
- USDA APHIS: United States Department of Agriculture, Animal and Plant Health Inspection Service. Code of Federal Regulations Title 9, Volume 1, Part 2, § 2.36 (As of 1 January 2018). 2018. Reference Source
- Van Wagenen G, Catchpole HR: Physical growth of the rhesus monkey ( Macaca mulatta). American J. Phys. Anthropol. 1956;14:245–273. 10.1002/ajpa.1330140219 [DOI] [PubMed] [Google Scholar]
- Van Zolingen SJ, Klaassen CA: Selection processes in a Delphi study about key qualifications in senior secondary vocational education. Technol. Forecast. Soc. Change. 2003;70(4):317–340. 10.1016/S0040-1625(02)00202-0 [DOI] [Google Scholar]
- Velarde A, Dalmau A: Animal welfare assessment at slaughter in Europe: moving from inputs to outputs. Meat Science. 2012;92(3):244–251. 10.1016/j.meatsci.2012.04.009 [DOI] [PubMed] [Google Scholar]
- Vermeire T, Epstein M, Badin RA, et al. : Final opinion on the need for non-human primates in biomedical research, production and testing of products and devices (update 2017). Brussels: Scientific Committee on Health, Environmental and Emerging Risks (SCHEER), European Commission;2017. Reference Source [Google Scholar]
- Von der Gracht HA: Consensus measurement in Delphi studies. Technol. Forecast. Soc. Change. 2012;79(8):1525–1536. 10.1016/j.techfore.2012.04.013 [DOI] [Google Scholar]
- Waitt CD, Honess PE, Bushmitz M: Creating housing to meet the behavioural needs of long-tailed macaques. Lab. Prim. Newsl. 2008;47(4):1–5. Reference Source [Google Scholar]
- Waitt CD, Bushmitz M, Honess PE: Designing environments for aged primates. Lab. Prim. Newsl. 2010;49(3):5–9. Reference Source [Google Scholar]
- Witham CL: Automated face recognition of rhesus macaques. J. Neurosci. Methods. 2018;300:157–165. 10.1016/j.jneumeth.2017.07.020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Webler T, Levine D, Rakel H, et al. : A novel approach to reducing uncertainty: the group Delphi. Technol. Forecast. Soc. Change. 1991;39(3):253–263. 10.1016/0040-1625(91)90040-M [DOI] [Google Scholar]
- Wolfensohn S, Honess P: Handbook of Primate Husbandry and Welfare. Oxford: John Wiley & Sons;2005. 10.1002/9780470752951 [DOI] [Google Scholar]
- Wolfensohn S, Sharpe S, Hall I, et al. : Refinement of welfare through development of a quantitative system for assessment of lifetime experience. Anim. Welfare. 2015;24(2):139–149. 10.7120/09627286.24.2.139 Reference Source [DOI] [Google Scholar]
- Wolfensohn S, Shotton J, Bowley H, et al. : Assessment of welfare in zoo animals: towards optimum quality of life. Animals. 2018;8(7):110. 10.3390/ani8070110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wren MA, Caskey JR, Liu DX, et al. : Septic arthritis due to moraxella osloensis in a rhesus macaque ( Macaca mulatta). Comp. Med. 2013;63(6):521–527. [PMC free article] [PubMed] [Google Scholar]
- Zhang XL, Pang W, Hu XT, et al. : Experimental primates and non-human primate (NHP) models of human diseases in China: current status and progress. Dongwuxue Yanjiu. 2014;35(6):447–464. 10.13918/j.issn.2095-8137.2014.6.447 [DOI] [PMC free article] [PubMed] [Google Scholar]