Abstract
Background
Colorectal cancer (CRC) has few or no symptoms and is often diagnosed at its end stage. Boeravinone B (BB) is a natural rotenoid which induces an antioxidative effect and has been used in cancer prevention. In this study, we scrutinized the chemoprotective effect of BB against 1,2dimethyl hydrazine (DMH) induced CRC in rats.
Methods
Subcutaneous administration of DMH (40 mg/kg) was used for the induction of CRC in rats, followed by oral administration of BB. The body weight, tumor volume, tumor incidence, and total number of tumors were estimated in all rat groups rats except the normal group. Antioxidant parameters, phase I and II enzymes, and inflammatory cytokines and parameters were estimated at the completion of the study.
Results
DMH induced group rats exhibited a tumor incidence of 100% along with several tumors/polyps per tumor‑bearing rat, while BB treatment remarkably suppressed the incidence of tumors and suppressed polyps per tumor bearing rat. BB treatment significantly (P<0.001) altered the level of antioxidant parameters, phase I and phase II enzymes, and cytokines such as TNF-α, IL-1β, IL-4, IL-6, and IL-10, and treatment significantly (P<0.001) suppressed the level of inflammatory cytokines, including cyclooxygenase-2 (COX-2), prostaglandin E2 (PGE2), and inducible nitric oxide synthase (iNOS).
Conclusions
BB treatment considerably suppresses colon cancer via its antioxidant and anti-inflammatory mechanism.
Keywords: Colorectal cancer (CRC), boeravinone B (BB), oxidative stress, inflammation
Introduction
Cancer is a major cause of mortality and morbidity globally, and colorectal cancer (CRC) is the 3rd most common type in men and women (1). Reports suggest dietary habits and genetic susceptibility can increase the risk of the disease (2), and that a controlled diet may suppress this risk.
DMH is a potent carcinogen commonly used for the induction of colorectal tumors in the rodent model. DMH induces CRC in multiple steps involving discrete microscopic lesions (aberrant cryptic foci) (3,4). Previous reports suggest an active metabolite of DMH excreted via bile is mainly accountable for its carcinogenic effect on colorectal tissue while passing through the digestive system (4). DMH specifically targets DNA and finally induces a generation of methyl adducts with bases of DNA, micronuclei, and mutation, yielding macroscopically visible neoplasms (5). During the metabolism of DMH, methylazoxymethanol and azoxymethane are generated, which also move to colorectal tissue through blood or bile to produce their ultimate carcinogenic metabolite (diazonium ion). This further induces oxidative stress via methylating the biomolecules of colonic epithelial cells leading to promutagenic events as a result of tumor promotion and inflammation (1,6,7).
High reactive oxygen species (ROS) exhibit alteration in various signalling pathways related to the drug resistance, proliferation, invasion, metastasis and survival of tumors (1,8). However, the human body has a unique system for adjusting the level of ROS, as endogenous antioxidant enzymes such as glutathione peroxidase (GPx), superoxide dismutase (SOD), paraoxonase1 (PON1), and catalase (CAT) play crucial roles in suppression it (1,9). Other markers such as phase II detoxification enzymes offer protection from drug toxicity and carcinogenesis effects. Glutathione S transferase (GST), with the help of glutathione (GSH), catalyzes the conjugation electrophilic compound, and quinone reductase (QR) catalyzes the two-electron reduction of nitrogen and quinone oxides (7,10).
The inflammatory reaction plays a crucial role in the expansion of tumors, and previous reports suggest inflammation and oxidative stress facilitate the progression of CRC (10,11). Cancer also increases the level of inflammatory mediators including cyclooxygenase-2 (COX-2). Various pathological and physiological conditions also play a crucial role in the expansion of tumors and the inflammatory reaction. Inflammatory cytokines such as TNF-α participate in the initiation and progression of CRC (4), while the increased level of inflammatory cytokines enhances the level of nuclear factor kappa B (NF-κB).
GDI2 is a GDP dissociation inhibitor 2 which belongs to the small family of chaperone proteins and shows a significant signal transduction molecule which regulates the expansion of various types of cancers including that of the prostate, breast, and thyroid, as well as CRC (12). Various research suggests GDI2 is heavily involved in the regulation of several biological functions including apoptosis, tumor cell proliferation, and cell metabolism and migration (13-15). Therefore, while the function of GDI2 has been previously explored in various types of the cancer, its role has not been explored in the colorectal cancer.
Boeravinone B (BB) is a rotenoid phytoconstituent belonging to the flavonoid family isolated from the Boerhaavia diffusa (16), and previous reports have suggested it has an anticancer effect (17,18). BB also showed a protective effect against acute myoskeletal dysfunction, spondylosis, osteoarthritis, systemic lupus erythematosus, and rheumatoid arthritis (17-21), and an immunomodulator effect against various autoimmune diseases by exhibiting both free radical scavenging activity and anti-inflammatory effects (16,17). In this study, we scrutinized the anticancer effect of BB against DMH induced CRC in rats. We present the following article in accordance with the ARRIVE reporting checklist (available at https://jgo.amegroups.com/article/view/10.21037/jgo-22-733/rc).
Methods
Experimental animals
Swiss Albino Wistar rats (aged: 10–12 weeks; 200±20 g; sex-male) were procured from the animal house of Jinan University and kept in standard laboratory conditions at a temperature of 20±5 ℃; humidity 60%±20% and a cycle of 12 h dark and 12 h light. Animal experiments were performed under a project license (No. GDY2102322) granted by ethics committee of The Affiliated Hospital of Guangdong Medical University, in compliance with The Affiliated Hospital of Guangdong Medical University guidelines for the care and use of animals. A protocol was prepared before the study without registration.
CRC induction
A previously reported method with minor modification was used to induce CRC in the rats.
A subcutaneous administration of DMH (40 mg/kg) was used. Briefly, the toxicant (DMH) was prepared via dissolving into the saline solution and maintained the pH (6.5) using the NaHCO3.
Tested groups
BB was administered to animals in the form of a suspension which was prepared via dissolving it in 1% of carboxymethyl cellulose (CMC).
Experimental design
After successful induction of CRC, rats were divided into groups as described below:
❖ Group I: this normal control group received the vehicle throughout the experimental study;
❖ Group II: this DMH control group received the subcutaneous administration of DMH (40 mg/kg) for 16 weeks;
❖ Group III: this DMH control group received the subcutaneous administration of DMH (40 mg/kg) for 16 weeks and oral administration of BB (5 mg/kg);
❖ Group IV: this DMH control group received the subcutaneous administration of DMH (40 mg/kg) for 16 weeks and oral administration of BB (10 mg/kg);
❖ Group V: this DMH control group received the subcutaneous administration of DMH (40 mg/kg) for 16 weeks and oral administration of BB (15 mg/kg).
Body weight and food and water intake were estimated at regular time intervals. At the termination of the experimental protocol, rats were anesthetized using diethyl ether, and blood was collected via puncturing the cardiac tissue and centrifuged at 15,000 rpm for 5 min to separate the serum. The serum sample was then separated out and kept at −20 ℃ for further analysis.
Antioxidant parameters
Phase I enzymes, including cytochrome C, cytochrome P450, and cytochrome B5, and phase II enzymes such as glutathione S transferase (GST) and UDP-glucuronyltransferase were estimated using a previously reported method with minor modification (3).
Previously reported method was used for the estimation of MDA via using the formation of thiobarbituric acid reactive substances (TBARS) (3,4). Tissue homogenate (0.2), sodlium dodecylsulphate (0.2 mL of 8.1%), acetic acid (1.5 mL), TBA (1.5 mL) were mixed together and finally made up the volume upto 4 mL using the distilled water and heated on water bath for 60 min at 95 ℃. After cooling the sample added the n-butanol/pyridine (5.0 mL) and shaken vigorously and centrifuged for 10 min at 600 ×g. The pink coloured chromogen generates after adding the 2-thiobarbituric acid and estimation the absorbance at 535 nm.
For the estimation of SOD level, the reaction mixture was prepared using the Tris HCl buffer (2.875 mL), PMS (100 µL) and pyrogallol (10 mM HCl) and finally make up volume 3 ml and estimation the absorbance at 420 nm.
For the estimation of GPx activity, briefly, prepared the reaction mixture which contain the 0.1 M phosphate buffer (2 mL), hydrogen peroxide (0.95 mL) and PMS (0.05 mL) and finally make up to the volume 3 mL and determine the absorbance at 240 nm (3,4).
For the estimation of GPx activity, prepare the reaction mixture, which contain the EDTA (0.1 mL), sodium azide (0.1 mL), phosphate buffer saline (1.44 mL) glutathione reductase (0.05 mL), reduced glutathione (0.05 mL), NADPH (0.01 mL), H2O2 (0.01 mL) and PMS (0.1 mL) and estimation the absorbance at 340 nm (3,4).
The previous reported method was used for the determination of GR activity (3,4). Briefly, prepared the reaction mixture contains the PBS (1.65 mL), EDTA (0.1 mL), glutathione (0.05 mL), NADPH (0.1 mL) and PMS (0.1 mL) and make up the volume upto 2 mL and determined the absorbance at 340 nm.
Apoptosis
The level of apoptosis markers was estimated using available kits and following the manufacture’s protocol.
Inflammatory cytokines and mediators
Inflammatory cytokines including interleukin-1β, tumor necrosis factor-α (TNF-α), and interleukin-6 (IL-6), and inflammatory mediators including prostaglandin E2 (PGE2), inducible nitric oxide synthase (iNOS), and COX-2 were estimated using enzyme-linked immunosorbent assay (ELISA) kits (MyBioSource Inc., San Diego, California, USA) as per the manufacture’s instructions.
Reverse transcription‑quantitative PCR (RT‑qPCR)
A Trizol kit was used for the isolation of total RNA from cells (Invitogen, Thermo Fisher Scientific Inc.). For conducting the RT, the following conditions were maintained: 52 min incubation at 42 ℃, followed by 5 min incubation at 85 ℃ to stop the reaction. Following this, RT-qPCR was carried out using SYBR Premix for mRNA PCR kits, while GAPDH was used as the internal standard. Thermocycling conditions used for performing the qPCR were as follows: initial denaturation for 30 s (95 ℃), 5 s for 40 cycles (95 ℃), and 30 s for (60 ℃). The relative mRNA expression was accessed using the 2−ΔΔCq analysis method. The primer sequence was as follows: GDI2 reverse primer 5'-TTTCGTCTCGAGGCTGTTAGTCTTCCCCATAG-3' and reverse primer 5'-CAACGGATACATTGGGGGTAGG-3' forwarded primer 5'-TTTCGTAAGCTTATGGACGAGGAATACGATGT-3'.
Statistical analysis
The statistical significance of the results was estimated using one-way variance of analysis (ANOVA), and the significant difference between groups was scrutinized via Dunnett’s multiple test. P<0.05 was considered as significant.
Results
Tumors
Table 1 shows the number of rats bearing tumors. No tumors were observed in the normal control group rats, while the DMH induced group rats exhibited a 100% tumor incidence with 2.5 tumors/polyps per tumor bearing rat. BB treatment considerably suppressed the tumor incidence along with the no. of tumors/polyps per tumor bearing rat. BB (15 mg/kg) treated rats exhibited the maximum suppression of tumor incidence.
Table 1. The incidence of colorectal tumor, tumor incidence and tumors/polyps per tumor bearing rats.
| No. | Group | No. of rats | Tumor bearing rats | Tumor incidence | Total number of tumor/polyps | No. of tumors/polyps per tumor‑bearing rat |
|---|---|---|---|---|---|---|
| 1 | Normal | 10 | 0 | 0 | 0 | 0 |
| 2 | DMH | 10 | 10 | 100 | 25 | 2.5 |
| 3 | DMH + BB (5 mg/kg) | 10 | 8 | 80 | 18 | 2.25 |
| 4 | DMH + BB (10 mg/kg) | 10 | 5 | 50 | 10 | 2 |
| 5 | DMH + BB (15 mg/kg) | 10 | 2 | 20 | 2 | 1 |
DMH, 1,2-dimethylhydrazine; BB, boeravinone B.
Antioxidants
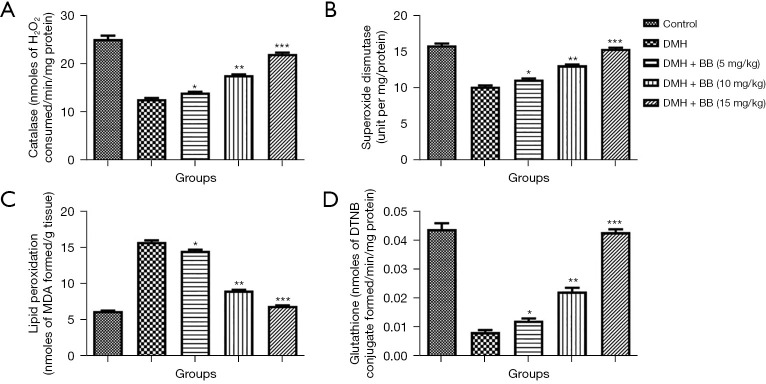
Antioxidant parameters protect organs from oxidative injury. During the expansion and progression of disease, the level of antioxidant parameters suppresses and increases the production of free radicals, which further induce oxidative stress. DMH group rats demonstrated a boosted level of LPO and suppressed level of CAT, GSH, and SOD, while in DMH rats treated with BB, a significantly decreased LPO level and improved level of CAT, SOD, and GSH was seen (Figure 1).
Figure 1.
Effect of BB on the antioxidant parameters of DMH induced CRC in rats. (A) Catalase; (B) superoxide dismutase; (C) lipid peroxidation; and (D) glutathione. All data denote mean ± SEM of six animals per group. Data obtained are significantly different from the DMH treated group (*P<0.05, **P<0.01 and ***P<0001). DMH, 1,2-dimethylhydrazine; CRC, colorectal cancer; BB, boeravinone B.
Phase I and II enzymes
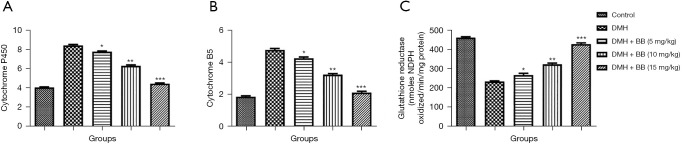
To explore a possible mechanism of action, we estimated the activity of phase I and II enzymes and found DMH induced group rats exhibited boosted levels of cytochrome P450 and cytochrome B5, while reducing glutathione reductase levels. BB treated group rats also significantly (P<0.001) suppressed the level of cytochrome P450 and cytochrome B5, and boosted glutathione reductase levels (Figure 2).
Figure 2.
Effect of BB on the phase I enzymes of DMH induced CRC in rats. (A) Cytochrome P450; (B) cytochrome B5; and (C) glutathione reductase. All data denote mean ± SEM of six animals per group. Data obtained are significantly different from the DMH treated group (*P<0.05, **P<0.01 and ***P<0001). DMH, 1,2-dimethylhydrazine; CRC, colorectal cancer; BB, boeravinone B.
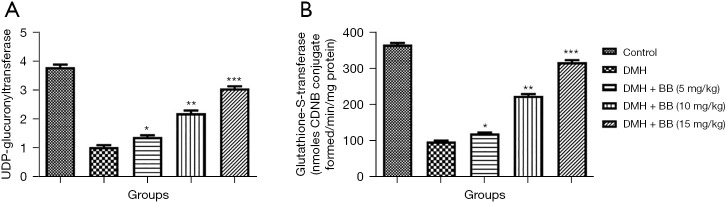
The level of phase II like UDP-glucuronyltransferase and glutathione-S-transferase was estimated in the different group of rats. DMH group rats exhibited suppressed levels of UDP-glucuronyltransferase and glutathione-S-transferase, while BB treatment significantly (P<0.001) enhanced these (Figure 3).
Figure 3.
Effect of BB on the phase II enzymes of DMH induced CRC in rats. (A) UDP-glucuronyltransferase and (B) glutathione-S-transferase. All data denote mean ± SEM of six animals per group. Data obtained are significantly different from the DMH treated group (*P<0.05, **P<0.01 and ***P<0001). DMH, 1,2-dimethylhydrazine; CRC, colorectal cancer; BB, boeravinone B.
Cytokines
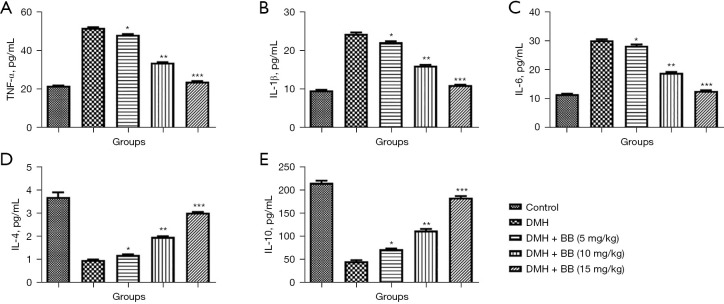
Cytokines play an important role in the expansion of cancer, and in this study DMH induced group rats exhibited boosted levels of TNF-α, IL-1β, and IL-6, and reduced levels of IL-4 and IL-10. BB treated rats had significantly (P<0.001) altered levels of cytokines, and BB (15 mg/kg) almost restored these normal (Figure 4).
Figure 4.
Effect of BB on the inflammatory cytokines of DMH induced CRC in rats. (A) TNF-α; (B) IL-1β; (C) IL-6; (D) IL-4; and (E) IL-10. All data denote mean ± SEM of six animals per group. Data obtained are significantly different from the DMH treated group (*P<0.05, **P<0.01 and ***P<0001). DMH, 1,2-dimethylhydrazine; CRC, colorectal cancer; BB, boeravinone B; TNF-α, tumor necrosis factor-α; IL-1β, interleukin-β; IL-4, interleukin-4; IL-6, interleukin-6; IL-10, interleukin-10.
MPO
MPO levels are usually boosted during cancer, and this was observed in DMH group rats. BB treated group rats showed significantly (P<0.001) reduced levels of MPO, with BB (15 mg/kg) suppressing the level to near normal (Figure 5).
Figure 5.
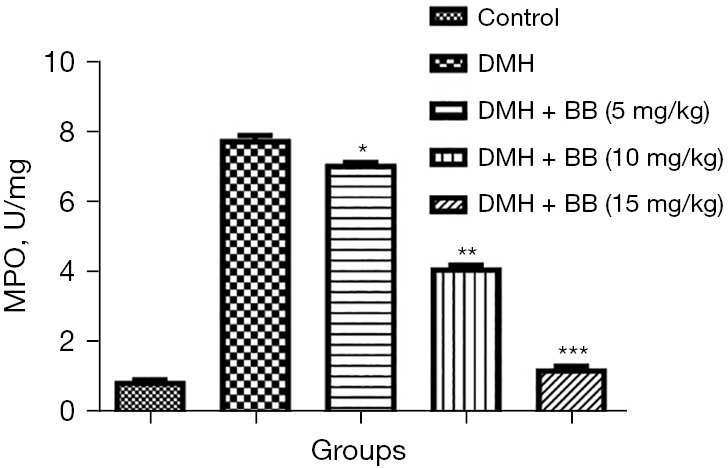
Effect of BB on the MPO activity of DMH induced CRC in rats. All data denote mean ± SEM of six animals per group. Data obtained are significantly different from the DMH treated group (*P<0.05, **P<0.01 and ***P<0001). DMH, 1,2-dimethylhydrazine; CRC, colorectal cancer; BB, boeravinone B; MPO, myeloperoxidase.
Inflammatory mediators
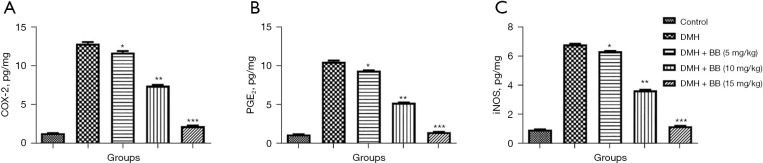
The inflammatory reaction plays a crucial role in the expansion of cancer. In this study, DMH induced CRC rats exhibited boosted levels of COX-2 (Figure 6A), PGE2 (Figure 6B), and iNOS (Figure 6C), while BB treated rats showed a significant (P<0.001) suppression of inflammatory parameter levels. BB (15 mg/kg) treated rats exhibited the maximum reduction in the level of inflammatory parameters.
Figure 6.
Effect of BB on the inflammatory parameters of DMH induced CRC in rats. (A) COX-2, (B) PGE2, and (C) iNOS. All data denote mean ± SEM of six animals per group. Data obtained are significantly different from the DMH treated group (*P<0.05, **P<0.01 and ***P<0001). DMH, 1,2-dimethylhydrazine; CRC, colorectal cancer; BB, boeravinone B; COX-2, cyclooxygenase-2; PGE2, prostaglandin E2; iNOS, inducible nitric oxide synthase.
mRNA expression
DMH induced CRC rats exhibited boosted mRNA expression of GDI2, and BB treated rats significantly (P<0.001) suppressed mRNA expression (Figure 7).
Figure 7.
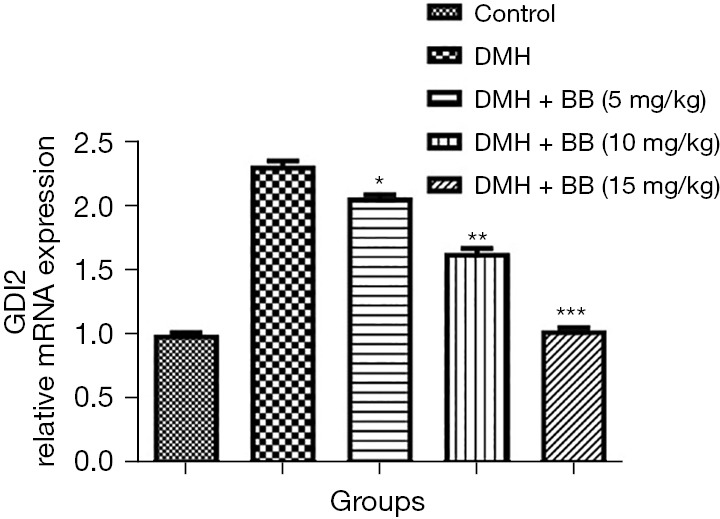
Effect of BB on the mRNA expression of DMH induced CRC in rats. All data denote mean ± SEM of six animals per group. Data obtained are significantly different from the DMH treated group (*P<0.05, **P<0.01 and ***P<0001). DMH, 1,2-dimethylhydrazine; CRC, colorectal cancer; BB, boeravinone B.
Discussion
Previous reports suggest the inflammatory reaction plays a crucial role in the expansion of CRC (22,23). Patients suffering from ulcerative colitis have a higher risk of progression of CRC, and immune cells infiltration generates different types of inflammatory cytokines and chemical factors, which boost the inflammatory reaction and enhance cell differentiation and proliferation (22,23). Chemotherapeutic agents are commonly used in the treatment of all types of the cancers, while plant-based drugs and their phytoconstituents exhibit protective effects with less side effects (4). Herbal drugs and their constituents are less toxic and more effective, and are commonly observed in various fruits, vegetables, and dietary agents (24,25). Phytoconstituents exhibit anti-inflammatory and antioxidant effect against various types of cancers such as renal, breast, hepatic, colorectal, and brain (22,26). Due to numerous effects of plant based phytoconstituents, in this experimental study, we scrutinized the chemoprotective effect of BB against DMH induced CRC in rats.
DMH is a potent carcinogen commonly used for the induction of CRC in rodent models. Previous research suggests the methyldiazonium ion boosts oxidative stress via methylation in the epithelial cells of colon tissue (2,27). Colon cancer follows exposure to carcinogens, and cells may then progress by a series of pre-cancerous lesions, premalignant, and malignant stages (1).
Oxidative stress plays a crucial role in the expansion and progression of gastrointestinal (GI) disease, ranging from chronic enteritis to CRC (1,9). Previous literature suggests oxidative induces inflammation, which further increases the disease load in rodents and humans (1,27). In the rodent model, carcinogens cause the production of free radicals and induce oxidative stress, and the production of ROS can cause the peroxidation of lipids, which further induces cellular injury. As first line antioxidant enzymes, SOD and CAT play a crucial role in scavenging free radicals (28,29) and are involved in endogenous antioxidant mechanisms and protection of cells and tissues against free radicals like hydroxyl (OH) and superoxides (O2). Both are also directly involved in the removal of ROS and act as the primary antioxidant enzymes (30). SOD and CAT are more sensitive to oxidative injury induced via the carcinogen (DMH) and are suppressed by its carcinogenic effect. The increase in free radical production exceeds the scavenging capacity of the endogenous antioxidant system during cancer (31,32). The production of LPO or MDA is one of the significant and appropriate markers of oxidative injury, and an increased level of LPO is found after DMH administration. Consistent with previous literature, our data exhibited a remarkable enhancement in LPO level after DMH administration, while BB treatment considerably suppressed it, suggesting an anticancer effect against CRC.
Enzymatic and non-enzymatic antioxidants are an important part of the body’s defensive system against free radicals (3). GSH is a tripeptide (low molecular weight) cellular antioxidant which protects the lipid membrane from peroxidation via conjugating with the electrophile like 4-hydroxyl-3-nonenal (HNE), which is generated during lipid peroxidation and accordingly reduced during the conjugation reaction (33). GSH and its oxidized products are a major redox system of cells which interact with the -SH group in ROS and can be implicated in the enzymatic detoxification reaction for ROS as a cofactor or coenzyme (3,33). The level of GSH is supressed in CRC, and BB treatment can improve this, demonstrating its antioxidant effect.
DMH is a well-known alkylating agent that induces cellular DNA injury via forming an adduct. While some cells are repaired via DNA repair enzymes, some are not and undergo apoptotic removal (33). Apoptosis is a finely controlled process of cell death, in which caspase plays a crucial role (34,35). Previous research suggests caspases are cysteine dependent enzymes and are activated via oxidative stress (35,36). Caspase-3 is the main executioner caspase and is activated via extrinsic and intrinsic pathways. An increased level of caspase-3 induces the DNA fragmentation and cleavage of specific cellular protein such as lamins, PARP, and caspase during apoptosis (33). DMH induced CRC rats exhibited enhanced levels of caspase-3 and BB treatment considerably suppressed this.
The inflammatory reaction and oxidative stress play a crucial role in the progression and expansion of cancer, and inflammatory mediators such as COX-2 are significant markers, in colon cancer (3,33). In addition, enhanced COX-2 levels further induce colon injury and cause polyp formation, and research has targeted its chemoprotective effect. iNOS (a potent inflammatory marker) is also commonly boosted during cancer (3) and a similar result was observed in this study. DMH induced CRC group rats exhibited boosted levels of COX-2, iNOS, and PGE2, and BB treatment considerably suppressed the level of COX-2.
Inflammatory cytokines, including TNF-α, play a crucial role in the initiation and progression of cancer (11,33). TNF-α is dependent on NF-κB activation, which further boosts inflammation in tissue. In this study, DMH induced CRC group rats exhibited modulated levels of inflammatory cytokines, and BB treatment considerably altered these.
Previous reports show the expression of GDI2 is boosted in esophageal squamous cell carcinoma (37) and pancreatic cancer (38), suggesting it should be considered as a biomarker or potential molecular target for these cancers (15). A reduced expression of GDI2 is also observed in CRC, and it should be considered as a potential target for the treatment of this disease.
Conclusions
Our results suggest the oral administration of BB effectively diminishes the incidence of CRC and suppresses the progression of DMH induced CRC in rats. BB treatment considerably suppressed oxidative stress via altering the level of endogenous antioxidant parameters. The underlying mechanism proposed suggests the chemoprotective effect of BB reduces oxidative stress and the inflammatory reaction, both of which play a crucial role in the expansion of CRC tumors in rodents. BB showed the protective effect against the DMH induced CRC in rats via suppression of inflammatory pathway.
Supplementary
The article’s supplementary files as
Acknowledgments
Funding: None.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. Animal experiments were performed under a project license (Approval Number: GDY2102322) granted by ethics committee of The Affiliated Hospital of Guangdong Medical University, in compliance with The Affiliated Hospital of Guangdong Medical University guidelines for the care and use of animals.
Reporting Checklist: The authors have completed the ARRIVE reporting checklist. Available at https://jgo.amegroups.com/article/view/10.21037/jgo-22-733/rc
Data Sharing Statement: Available at https://jgo.amegroups.com/article/view/10.21037/jgo-22-733/dss
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://jgo.amegroups.com/article/view/10.21037/jgo-22-733/coif). The authors have no conflicts of interest to declare.
(English Language Editor: B. Draper)
References
- 1.López-Mejía A, Ortega-Pérez LG, Magaña-Rodríguez OR, et al. Protective effect of Callistemon citrinus on oxidative stress in rats with 1,2-dimethylhydrazine-induced colon cancer. Biomed Pharmacother 2021;142:112070. 10.1016/j.biopha.2021.112070 [DOI] [PubMed] [Google Scholar]
- 2.Hassan HFH, Mansour AM, Salama SA, et al. The chemopreventive effect of thymol against dimethylhydrazine and/or high fat diet-induced colon cancer in rats: Relevance to NF-κB. Life Sci 2021;274:119335. 10.1016/j.lfs.2021.119335 [DOI] [PubMed] [Google Scholar]
- 3.Hamiza OO, Rehman MU, Tahir M, et al. Amelioration of 1,2 Dimethylhydrazine (DMH) induced colon oxidative stress, inflammation and tumor promotion response by tannic acid in Wistar rats. Asian Pac J Cancer Prev 2012;13:4393-402. 10.7314/APJCP.2012.13.9.4393 [DOI] [PubMed] [Google Scholar]
- 4.Shao P, Li X, Guan X. Chemoprotective effect of crocetin against 1,2 dimethyl hydrazine induced colorectal cancer in albino wistar rats through antioxidant pathway. Pharmacogn Mag 2021;17:360-6. 10.4103/pm.pm_311_20 [DOI] [Google Scholar]
- 5.Jackson PE, O'Connor PJ, Cooper DP, et al. Associations between tissue-specific DNA alkylation, DNA repair and cell proliferation in the colon and colon tumour yield in mice treated with 1,2-dimethylhydrazine. Carcinogenesis 2003;24:527-33. 10.1093/carcin/24.3.527 [DOI] [PubMed] [Google Scholar]
- 6.Bordini HP, Kremer JL, Fagundes TR, et al. Protective effect of metformin in an aberrant crypt foci model induced by 1,2-dimethylhydrazine: Modulation of oxidative stress and inflammatory process. Mol Carcinog 2017;56:913-22. 10.1002/mc.22545 [DOI] [PubMed] [Google Scholar]
- 7.Al Hassan M, Al Battal M, Usta J, et al. Protective effect of sorafenib against 1,2-dimethylhydrazine-induced colorectal cancer in balb/c mice. Oncology and Radiotherapy 2021;15:18-24. [Google Scholar]
- 8.Guéraud F. 4-Hydroxynonenal metabolites and adducts in pre-carcinogenic conditions and cancer. Free Radic Biol Med 2017;111:196-208. 10.1016/j.freeradbiomed.2016.12.025 [DOI] [PubMed] [Google Scholar]
- 9.Hafez EE, Badr E, Mabrouk Y, et al. Molecular genetic evaluation of Cichorium endivia L. as an anticancer agent against colorectal cancer. Int J Phytomedicine 2016;8:551-7. 10.5138/09750185.1916 [DOI] [Google Scholar]
- 10.El Joumaa MM, Taleb RI, Rizk S, et al. Protective effect of Matricaria chamomilla extract against 1,2-dimethylhydrazine-induced colorectal cancer in mice. J Complement Integr Med 2020. 10.1515/jcim-2019-0143 [DOI] [PubMed] [Google Scholar]
- 11.Ghazizadeh Darband S, Saboory E, Sadighparvar S, et al. The modulatory effects of exercise on the inflammatory and apoptotic markers in rats with 1,2-dimethylhydrazine-induced colorectal cancer. Can J Physiol Pharmacol 2020;98:147-55. 10.1139/cjpp-2019-0329 [DOI] [PubMed] [Google Scholar]
- 12.Liu C, Wang W, Lin P, et al. GDI2 is a target of paclitaxel that affects tumorigenesis of prostate cancer via the p75NTR signaling pathway. Biochem Biophys Res Commun 2021;562:119-26. 10.1016/j.bbrc.2021.05.015 [DOI] [PubMed] [Google Scholar]
- 13.Zhang W, Liu Z, Xia S, et al. GDI2 is a novel diagnostic and prognostic biomarker in hepatocellular carcinoma. Aging (Albany NY) 2021;13:25304-24. 10.18632/aging.203748 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ahn K, Felser C, Seshadri R, et al. Giant negative magnetoresistance in GdI2. J Alloys Compd 2000;303-304:252-6. 10.1016/S0925-8388(00)00668-X [DOI] [Google Scholar]
- 15.Zou J, Qian J, Fu H, et al. MicroRNA.15b.5p exerts its tumor repressive role via targeting GDI2: A novel insight into the pathogenesis of thyroid carcinoma. Mol Med Rep 2020;22:2723-32. 10.3892/mmr.2020.11343 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chen Y, Peng L, Shi S, et al. Boeravinone B alleviates gut dysbiosis during myocardial infarction-induced cardiotoxicity in rats. J Cell Mol Med 2021;25:6403-16. Erratum in: J Cell Mol Med 2022;26:3307-8. 10.1111/jcmm.16620 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Yuan S, Zhang T., Boeravinone B. Protects Brain against Cerebral Ichemia Reperfusion Injury in Rats: Possible Role of Anti-inflammatory and Antioxidant. J Oleo Sci 2021;70:927-36. 10.5650/jos.ess21037 [DOI] [PubMed] [Google Scholar]
- 18.Wang Z, Xiao S, Huang J, et al. Chemoprotective Effect of Boeravinone B against DMBA/Croton Oil Induced Skin Cancer via Reduction of Inflammation. J Oleo Sci 2021;70:955-64. 10.5650/jos.ess21055 [DOI] [PubMed] [Google Scholar]
- 19.Posa Krishnamoorthy P, Muthukumaran S. In vitro studies to determine the effect of boeravinone B on human dendritic cells. Pharmacogn Mag 2018;14:465-70. 10.4103/pm.pm_625_17 [DOI] [Google Scholar]
- 20.Biradar SP, Tamboli AS, Khandare RV, et al. Chebulinic acid and Boeravinone B act as anti-aging and anti-apoptosis phyto-molecules during oxidative stress. Mitochondrion 2019;46:236-46. 10.1016/j.mito.2018.07.003 [DOI] [PubMed] [Google Scholar]
- 21.Huang Y, Sun Y, Wang WW, et al. Boeravinone B a natural rotenoid exerts anticancer activity via inducing internalization and degradation of inactivated EGFR and ErbB2 in human colon cancer cells. Am J Transl Res 2018;10:4183-92. [PMC free article] [PubMed] [Google Scholar]
- 22.Sartini M, Bragazzi NL, Spagnolo AM, et al. Coffee Consumption and Risk of Colorectal Cancer: A Systematic Review and Meta-Analysis of Prospective Studies. Nutrients 2019;11:694. 10.3390/nu11030694 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.He X, Sun LM. Dietary intake of flavonoid subclasses and risk of colorectal cancer: evidence from population studies. Oncotarget 2016;7:26617-27. 10.18632/oncotarget.8562 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Borzì AM, Biondi A, Basile F, et al. Olive Oil Effects on Colorectal Cancer. Nutrients 2018;11:32. 10.3390/nu11010032 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Dove-Edwin I, Thomas HJ. Review article: the prevention of colorectal cancer. Aliment Pharmacol Ther 2001;15:323-36. 10.1046/j.1365-2036.2001.00934.x [DOI] [PubMed] [Google Scholar]
- 26.Wang X, Saud SM, Zhang X, et al. Protective effect of Shaoyao Decoction against colorectal cancer via the Keap1-Nrf2-ARE signaling pathway. J Ethnopharmacol 2019;241:111981. Erratum in: J Ethnopharmacol 2021;266:113532. 10.1016/j.jep.2019.111981 [DOI] [PubMed] [Google Scholar]
- 27.Qamar TR, Iqbal S, Syed F, et al. Impact of Novel Prebiotic Galacto-Oligosaccharides on Various Biomarkers of Colorectal Cancer in Wister Rats. Int J Mol Sci 2017;18:1785. 10.3390/ijms18091785 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hamiza OO, Rehman MU, Khan R, et al. Chemopreventive effects of aloin against 1,2-dimethylhydrazine-induced preneoplastic lesions in the colon of Wistar rats. Hum Exp Toxicol 2014;33:148-63. 10.1177/0960327113493307 [DOI] [PubMed] [Google Scholar]
- 29.Senedese JM, Alves JM, Lima IM, et al. Chemopreventive effect of Copaifera langsdorffii leaves hydroalcoholic extract on 1,2-dimethylhydrazine-induced DNA damage and preneoplastic lesions in rat colon. BMC Complement Altern Med 2013;13:3. 10.1186/1472-6882-13-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Bantal V, Ghanta P, Tejasvi P. Piperine Presents Chemo-preventive Property Against 1, 2-Dimethyl Hydrazine Induced Colon Cancer in Mice: Biochemical and Physiological Evidences. Pharmacologia 2018;9:30-8. [Google Scholar]
- 31.Punvittayagul C, Chariyakornkul A, Jarukamjorn K, et al. Protective Role of Vanillic Acid against Diethylnitrosamine- and 1,2-Dimethylhydrazine-Induced Hepatocarcinogenesis in Rats. Molecules 2021;26:2718. 10.3390/molecules26092718 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Baskar AA, Al Numair KS, Gabriel Paulraj M, et al. β-sitosterol prevents lipid peroxidation and improves antioxidant status and histoarchitecture in rats with 1,2-dimethylhydrazine-induced colon cancer. J Med Food 2012;15:335-43. 10.1089/jmf.2011.1780 [DOI] [PubMed] [Google Scholar]
- 33.Khan R, Sultana S. Farnesol attenuates 1,2-dimethylhydrazine induced oxidative stress, inflammation and apoptotic responses in the colon of Wistar rats. Chem Biol Interact 2011;192:193-200. 10.1016/j.cbi.2011.03.009 [DOI] [PubMed] [Google Scholar]
- 34.Caetano BFR, Tablas MB, Pereira NEF, et al. Capsaicin reduces genotoxicity, colonic cell proliferation and preneoplastic lesions induced by 1,2-dimethylhydrazine in rats. Toxicol Appl Pharmacol 2018;338:93-102. 10.1016/j.taap.2017.11.008 [DOI] [PubMed] [Google Scholar]
- 35.Khan R, Rehman MU, Khan AQ, et al. Glycyrrhizic acid suppresses 1,2-dimethylhydrazine-induced colon tumorigenesis in Wistar rats: Alleviation of inflammatory, proliferation, angiogenic, and apoptotic markers. Environ Toxicol 2018;33:1272-83. 10.1002/tox.22635 [DOI] [PubMed] [Google Scholar]
- 36.Rajendran SS, Geetha G, Venkatanarayanan R, et al. Amelioration of 1, 2 dimethylhydrazine induced tumor promotion response by novel benzimidazole derivative nanoparticle in wistar rats. J Young Pharm 2018;10:292-8. 10.5530/jyp.2018.10.65 [DOI] [Google Scholar]
- 37.Kashyap MK, Harsha HC, Renuse S, et al. SILAC-based quantitative proteomic approach to identify potential biomarkers from the esophageal squamous cell carcinoma secretome. Cancer Biol Ther 2010;10:796-810. 10.4161/cbt.10.8.12914 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Sun ZL, Zhu Y, Wang FQ, et al. Serum proteomic-based analysis of pancreatic carcinoma for the identification of potential cancer biomarkers. Biochim Biophys Acta 2007;1774:764-71. 10.1016/j.bbapap.2007.04.001 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
The article’s supplementary files as