Abstract
Pseudomonas aeruginosa is a gram-negative bacterium that secretes many proteins into the extracellular medium via the Xcp machinery. This pathway, conserved in gram-negative bacteria, is called the type II pathway. The exoproteins contain information in their amino acid sequence to allow targeting to their secretion machinery. This information may be present within a conformational motif. The nature of this signal has been examined for P. aeruginosa exotoxin A (PE). Previous studies failed to identify a common minimal motif required for Xcp-dependent recognition and secretion of PE. One study identified a motif at the N terminus of the protein, whereas another one found additional information at the C terminus. In this study, we assess the role of the central PE domain II composed of six α-helices (A to F). The secretion behavior of PE derivatives, individually deleted for each helix, was analyzed. Helix E deletion has a drastic effect on secretion of PE, which accumulates within the periplasm. The conformational rearrangement induced in this variant is predicted from the three-dimensional PE structure, and the molecular modification is confirmed by gel filtration experiments. Helix E is in the core of the molecule and creates close contact with other domains (I and III). Deletion of the surface-exposed helix F has no effect on secretion, indicating that no secretion information is contained in this helix. Finally, we concluded that disruption of a structured domain II yields an extended form of the molecule and prevents formation of the conformational secretion motif.
Pseudomonas aeruginosa is a gram-negative bacterium and opportunistic human pathogen that produces several extracellular proteins that contribute to its virulence (10). Those exoproteins are involved in alteration of the host tissues and in the disorganization of cellular functions. The secreted proteins include degradative enzymes such as elastase and toxins such as exotoxin A (PE), an ADP-ribosyltransferase (45). The secretion into the extracellular medium of the majority of these virulence factors, including elastase and PE, requires the so-called general secretory pathway (32). This pathway is widely conserved in gram-negative bacteria, i.e., Klebsiella oxytoca (Pul) (33), Erwinia species (Out) (24, 36), Vibrio cholerae (Eps) (39), Aeromonas species (Exe) (19, 21), Xanthomonas campestris (Xps) (5), Legionella pneumophila (Lsp) (14, 23), Escherichia coli (Gsp) (11), and Pseudomonas species (Xcp) (4, 9, 13). Briefly, the exoproteins using this pathway are synthesized as precursors with an N-terminal signal peptide which is cleaved off during translocation across the cytoplasmic membrane via the Sec machinery (6). The proteins are subsequently released into the periplasm, where they fold into what appears to be their final conformation. Several studies have demonstrated that disulfide bond formation and chaperone-assisted folding are crucial steps in the generation of a protein competent for the final step in the secretion process (2, 3, 7, 31). During this step, the folded mature proteins are translocated across the outer membrane via a specialized machinery called Xcp in P. aeruginosa, i.e., the main terminal branch of the general secretory pathway, or type II pathway (10). This machinery is composed of 12 xcp gene products (XcpP to XcpZ and XcpA) distributed within the bacterial cell envelope. The unravelling of protein-protein interactions within this macromolecular complex is one of the major issues addressed by researchers in the field.
In addition to the interactions required for the assembly and functioning of the machinery, the mechanism allowing specific recognition of secreted substrates as distinct from periplasmic resident proteins appears to be a key event. Indeed, translocation across the outer membrane is a highly specific process that requires targeting features for the unambiguous recognition of the secreted protein by the secretion machinery. The features permitting specific exoprotein recognition by the Xcp and “Xcp-like” machineries in other bacteria are poorly understood. In P. aeruginosa, exoproteins are structurally highly diverse (e.g., PE, elastase, and lipase) and do not contain obvious sequence similarities that might constitute a common secretion signal. The absence of obvious linear motifs among various substrates sorted by a common unique machinery and the ability of the Xcp machinery to transport folded polypeptides suggest the existence of a conformational motif, a specific structure found only within the mature, folded protein. Consequently, this secretion signal might be identified in proteins that have known three-dimensional structures.
P. aeruginosa mature PE is a 613-amino-acid protein with a molecular size of 66,600 Da. The toxin enters eukaryotic cells by receptor-mediated endocytosis and is then translocated into the cytosol (22), where it catalyzes ADP-ribosylation of elongation factor 2, resulting in protein synthesis inhibition and cell death. The three-dimensional structure of PE shows the existence of three distinct domains (1), the functions of which have been elucidated (20). Domain I, which encompasses amino acids 1 to 252 (Ia) and 365 to 404 (Ib), is responsible for cell recognition; domain II (residues 253 to 364) is involved in translocation of the toxin across the membrane of intracellular compartments; and domain III (residues 405 to 613) forms the catalytic domain. Previous studies using hybrid and truncated proteins have reported the existence of discrete recognition signals in PE. Those signals map either to domain Ia or to the 305 C-terminal residues of PE (15, 26, 28). More particularly, domain I was shown to allow secretion of β-lactamase by the Xcp system (26). Whether those signals constitute independent recognition motifs or function synergetically remains to be elucidated. In order to determine whether other regions of the protein contain secretion information essential in the recognition process, we have focused our investigations on central domain II. This domain of PE is composed of six consecutive α-helices named A to F. We previously deleted each of these helices independently (43). In this study we analyzed the effect of these deletions on secretion by P. aeruginosa and showed that features within domain II are important for efficient recognition of PE by the Xcp secretion machinery.
MATERIALS AND METHODS
Bacterial strains and culture conditions.
Escherichia coli strain TG1, used for propagating recombinant plasmids, was grown at 37°C in L broth for isolation of plasmids. P. aeruginosa strain PAK-NT was used as the standard strain for assaying for protein secretion. PAK-NT is an exotoxin A (toxA) mutant obtained by insertion of a gentamicin resistance gene cassette (42). Recombinant broad-host-range plasmids were introduced into PAK-NT by triparental mating with pRK2013 as a helper plasmid (8). Protein secretion was assayed in plasmid-bearing PAK-NT strains grown at 30°C in L broth. Ampicillin (50 μg/ml for E. coli) and carbenicillin (300 μg/ml for P. aeruginosa) were used where appropriate.
Recombinant DNA techniques, plasmids, and subclones.
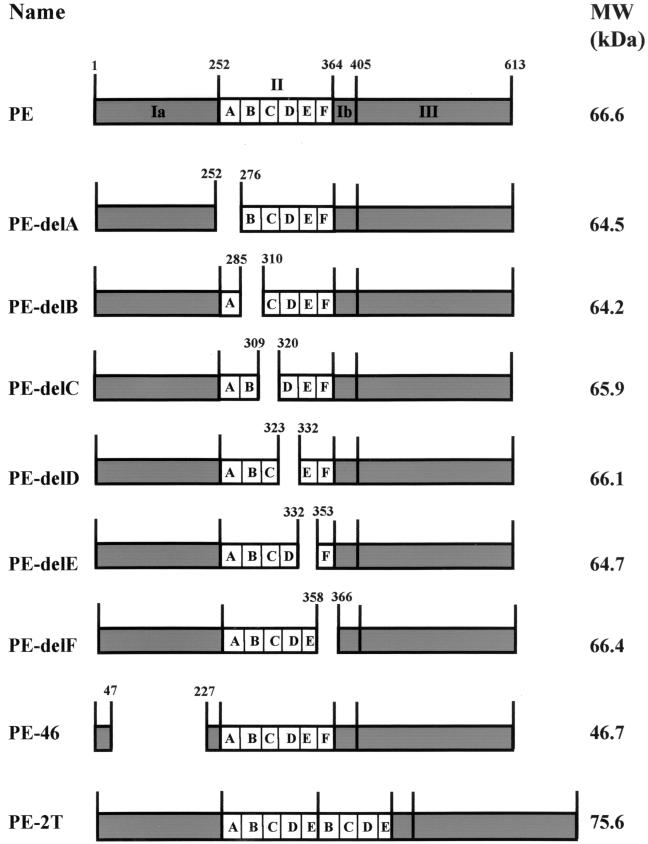
General procedures for the isolation, analysis, and manipulation of DNA were as described by Sambrook et al. (38). Plasmids encoding PE, PE-2T, and PE lacking helix A, B, C, D, E, or F from PE domain II have been previously described (43) and are schematically represented in Fig. 1. XbaI-EcoRI inserts carrying the genes encoding those PE derivatives were subcloned into the broad-host-range plasmid pMMB67HE (12) digested with XbaI and EcoRI, yielding pMRV1PE (PE wild type), pMRV2T (PE-2T), pMRV1A (PE-delA), pMRV1B (PE-delB), pMRV1C (PE-delC), pMRV1D (PE-delD), pMRV1E (PE-delE), and pMRV1F (PE-delF). PE-46 has a deletion of residues 48 to 226 within domain I and was recloned into pMMB67HE, yielding pMRV46.
FIG. 1.
Schematic representation of the PE derivatives used in this study. The representation is not drawn to scale, and helices A to F of domain II are indicated. Numbers represent residues. The names of the PE derivatives are indicated on the left. The predicted molecular sizes are indicated on the right.
Subcellular fractionation of PE and its derivatives.
PAK-NT carrying recombinant plasmids was grown to an optical density at 600 nm (OD600) of 3 to 4 (stationary phase). An equivalent of 3 OD600 units of culture was separated into cell and supernatant by centrifugation. The proteins were precipitated from the supernatants with 15% trichloroacetic acid (TCA) for 30 min at 4°C, resuspended in sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) sample buffer and heated for 7 min at 95°C. Cell pellets were directly resuspended in SDS-PAGE sample buffer and heated. The periplasmic fraction was prepared as described previously (18). Cell pellets were first washed in 50 mM Tris-HCl (pH 7.4), resuspended in 50 mM Tris-HCl (pH 7.4)–200 mM CaCl2 and gently shaken for 30 min at 30°C. Samples were then incubated for 5 min on ice and then for 15 min at room temperature. These incubation steps were repeated once, and finally the cells were pelleted by centrifugation. The supernatants, corresponding to the periplasmic fluid, were precipitated with 15% TCA. Cell pellets and TCA-precipitated supernatants were then resuspended and heated in SDS-PAGE sample buffer as above.
SDS-PAGE and immunoblotting.
Samples were solubilized in SDS-PAGE sample buffer and separated using 11% polyacrylamide gels containing SDS. Proteins were then blotted onto nitrocellulose membranes which were developed using goat or rabbit antiserum directed against PE, followed by a peroxidase-conjugated rabbit anti-goat or goat anti-rabbit immunoglobulin G detected by chemiluminescence (kit from Pierce). To quantify nonspecific leakage, periplasmic β-lactamase was also probed using specific antibodies. Quantitative estimation of the relative amounts of PE detected was carried out using the Image Quant program (Molecular Dynamics) after scanning the chemiluminescence films.
Gel filtration.
PE and derivatives were purified as described previously (43). Purified toxins (at ∼0.5 mg/ml) were applied (200-μl sample volume) to a Superdex 200 column (HR10/30; Pharmacia). The column was calibrated using yeast alcohol dehydrogenase (150 kDa), human transferrin (80 kDa), bovine serum albumin (66 kDa), and bovine carbonic anhydrase (29 kDa). Proteins were eluted in 750-μl fractions using phosphate-buffered saline at 250 μl/min and monitored at 280 nm.
Graphic computer analysis of PE three-dimensional structure.
The atomic coordinates were kindly provided by David McKay, since the Protein Data Bank entry only contains the Cα positions of domain III. The graphic inspection and analysis were performed using Turbo-Frodo (37).
RESULTS
Influence of domain II deletions on PE secretion.
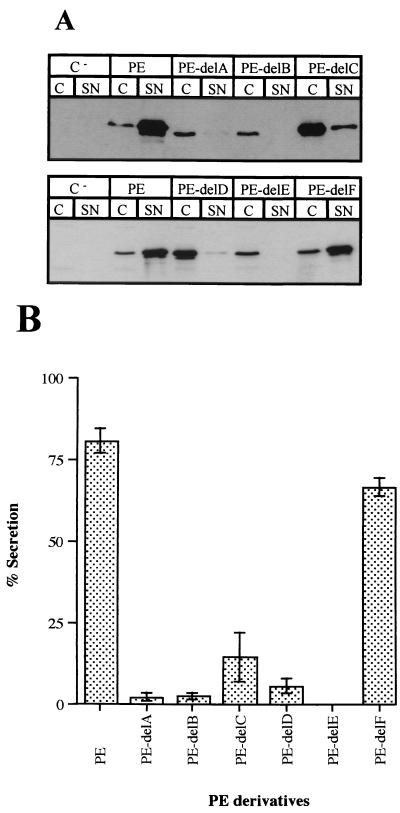
Six PE variants, each lacking one of the helices from PE domain II, were constructed previously (43) (Fig. 1). The genes encoding these truncated forms of PE were cloned in the broad-host-range plasmid pMMB67HE under control of the tac promoter and introduced into a P. aeruginosa strain, PAK-NT. This strain is an exotoxin A (toxA) mutant obtained by insertion of a gentamicin resistance gene cassette (42). PAK-NT cells carrying each of the truncated PEs were grown to an OD600 of 3 to 4. No addition of isopropyl-β-d-thiogalactoside (IPTG) was required to obtain sufficient expression of PE and its derivatives in P. aeruginosa. After immunoblotting using an antibody directed against PE, the secretion level of the truncated PE forms could be evaluated by comparing signals obtained with cell extracts and precipitated culture supernatants (e.g., Fig. 2A). The only truncated PE form that was efficiently secreted was the variant lacking helix F (PE-delF). Inspection of data from three independent experiments indicated that the truncated PEs fall into distinct categories with respect to their average secretion level (Fig. 2B). PE-delF is secreted almost as efficiently as wild-type PE, indicating that this helix has a marginal role, if any, in the secretion of PE. The PE with a deletion of helix C (PE-delC) exhibited a greatly reduced level of secretion (10 to 20%) but was still clearly detectable at levels higher than could be explained by nonspecific leakage in the supernatant fraction (Fig. 2A). The PE forms with deletions of helices A, B, and D (PE-delA, PE-delB, and PE-delD, respectively) were extremely poorly secreted (<5%), but this amount is significant since no quantifiable amount of the normally periplasmically located β-lactamase was ever detected in the supernatant fraction (data not shown). Finally, deletion of helix E (PE-delE) had the most drastic effect on secretion, since no PE-delE was ever detected in the supernatant fraction in any of the experiments performed.
FIG. 2.
(A) Secretion by P. aeruginosa PAK-NT of PE and PE derivatives deleted within domain II. PAK-NT cells containing the pMMB67HE vector (C−) or pMMB67HE derivatives carrying genes encoding wild-type PE and various deletions of domain II helices A to F (PE-delA to PE-delF) were separated into cellular (C) and extracellular (SN) fractions. Samples were loaded on SDS–11% PAGE gels followed by immunoblotting using a rabbit antiserum directed against PE. Only that part of the gel where whole-length PE and PE derivatives migrated is shown. (B) Quantitative analysis of PE secretion levels. The Western blots presented in panel A were scanned, and bands were quantified using Image Quant (Molecular Dynamics). The values presented are means of three independent experiments.
Nonsecreted PE forms are periplasmically located.
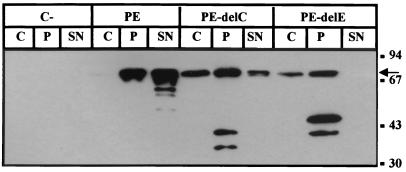
We next examined whether the lack of secretion was due to inefficient outer membrane translocation of the PE forms via the Xcp machinery or to a block in the first step of translocation across the inner membrane via the Sec machinery. Cells of PAK-NT containing the different PE derivatives were grown as previously described, and periplasmic extracts were prepared (see Materials and Methods). Samples were loaded on SDS-PAGE gels followed by immunoblotting with antibodies directed against PE. The results obtained indicated that in all cases, the nonsecreted PE forms were mostly located in the periplasmic fraction (Fig. 3 and data not shown). It should be noted that the amount of proteins detected in the cytoplasmic fraction is due to inefficient release of the periplasmic fraction, as seen for β-lactamase (data not shown). The limiting step in the secretion process thus appeared to be the Xcp-dependent outer membrane translocation. This observation confirmed the previously reported data which showed that the PE derivatives were periplasmic when produced by E. coli (43). However, the truncated forms of PE seemed to be more sensitive to proteolytic degradation than the wild-type PE form (Fig. 3), which suggests a difference in folding state and structure (see the Discussion section). Interestingly, full-length PE-delE was clearly detectable in the periplasm but was not found in the supernatant fraction, indicating that lack of secretion is not due to proteolytic degradation in the periplasm. Indeed, in the case of PE-delC, the intact protein was recovered from the supernatant in spite of the proteolytic degradation (Fig. 3).
FIG. 3.
Subcellular localization of PE derivatives in PAK-NT cells containing the pMMB67HE vector (C−) or pMMB67HE carrying genes encoding wild-type PE, PE-delC, or PE-delE. Samples were loaded on SDS–11% PAGE followed by immunoblotting using a goat antiserum directed against PE. C, cell fraction (cytoplasm plus membrane); P, periplasmic fraction; SN, extracellular fraction. Molecular size markers are indicated on the right (in kilodaltons). The position of PE and derivatives is indicated by an arrow.
Permissivity of insertion at helix F.
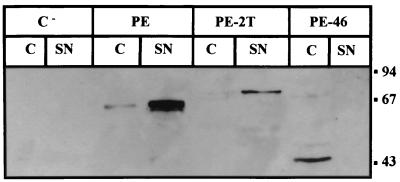
We have shown that deletion of helix F did not affect secretion of PE. This observation suggests that no secretion information is contained within this helix and that this deletion does not disturb the protein's conformation and subsequently its recognition by the Xcp machinery. The position occupied by helix F might be a permissive site for the insertion of protein domains without disturbing the efficient secretion of the hybrid proteins by the Xcp machinery. We examined the secretion of a previously engineered PE protein (43) in which a large part of PE domain II has been duplicated by the insertion of the B, C, D, and E helices at the position of the F helix (PE-2T) (Fig. 1). The gene was recloned in the broad-host-range vector pMMB67HE and introduced into PAK-NT, and secretion was analyzed by immunoblotting as above. The results (Fig. 4) revealed that PE-2T, which as expected was larger than wild-type PE, was efficiently secreted into the extracellular medium even though weakly produced, indicating that the increased molecular size of the PE derivative PE-2T (75.6 kDa instead of 66.6 kDa) does not affect secretion.
FIG. 4.
Secretion by P. aeruginosa PAK-NT of PE and the PE derivatives PE-2T and PE-46. PAK-NT cells containing the pMMB67HE vector (C−) or pMMB67HE carrying genes encoding wild-type PE or PE-2T and PE-46 derivatives were separated into cellular (C) and extracellular (SN) fractions. Samples were loaded on SDS–11% PAGE followed by immunoblotting using a rabbit antiserum directed against PE. Molecular size markers are indicated on the right (in kilodaltons).
Influence of domain II deletions on PE molecular size.
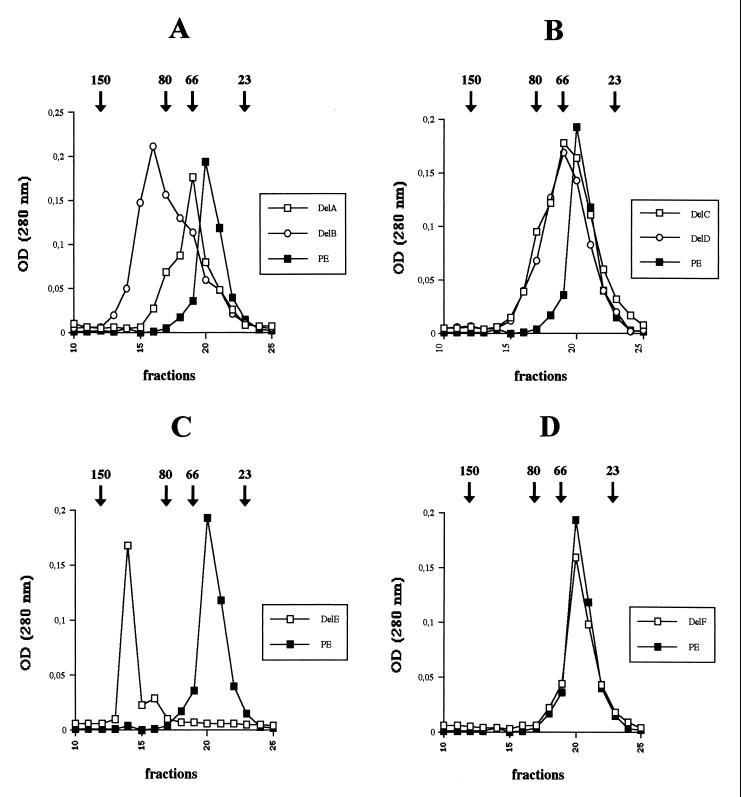
It was previously reported that some of the truncated PE forms migrated more slowly than PE on nonreducing gels (43), indicating a slightly less compact tertiary structure. This was particularly marked in the case of PE-delE, which is totally secretion defective. We decided to investigate further the potential relationship between compaction and secretion by analyzing the different variants using gel filtration on a Superdex 200 column (Fig. 5). The results obtained showed that PE-delE is the most affected variant form, since it eluted in fraction 14, whereas PE eluted with fraction 20 (Fig. 5C). The shift in position of the PE-delE elution peak seems to be due to relaxation of the molecule rather than to the formation of dimers, since this peak is very sharp, reflecting a homogenous population of molecules. Moreover, dimers were not observed on nonreducing SDS-PAGE (43). PE-delF coeluted with PE in fraction 20 (Fig. 5D), indicating that no major structural changes have occurred in the molecule and in line with the fact that this variant form conserved all PE biological activities, including the ability to be secreted by the bacterium. PE-delC and PE-delD, which were both secreted at significant levels, had a high elution peak in fraction 20 as well (Fig. 5B). In contrast, PE-delA and PE-delB had more clearly retarded elution peaks (Fig. 5A), which may indicate why they are poorly, or not, secreted.
FIG. 5.
Molecular size determination of PE derivatives by gel filtration chromatography. Purified toxins were analyzed on Superdex 200. Elution profiles of PE, PE-delA, and PE-delB (A), PE, PE-delC, and PE-delD (B), PE and PE-delE (C), and PE and PE-delF (D). The positions of the different molecular size markers are noted at the top (in kilodaltons).
DISCUSSION
The type II secretory pathway is widely conserved in gram-negative bacteria. Yet species specificity appears to be highly stringent, since heterologous secretion hardly ever occurred, at least efficiently. One striking example concerns the very similar Cel5 (formerly EGZ) (41) and CelV cellulases, which cannot be heterologously secreted by the very closely related producer strains, Erwinia chrysanthemi and Erwinia carotovora, respectively (34). There is thus little doubt that specific targeting signals must be present within the sequence of the substrates secreted by the type II machinery and that this signal is only efficiently recognized by the secretion machinery of the organism that produces this substrate. Though several attempts have been made to define these targeting signals in different systems, no common features have emerged. The use of gene fusions with β-lactamase or alkaline phosphatase allowed the identification of specific regions implicated in the secretion process. The results obtained with Klebsiella oxytoca pullulanase hybrids identified two distal regions within the primary sequence, between residues 1 and 78 and 735 and 814, which are both necessary to promote secretion of β-lactamase across the outer membrane (40). In the case of P. aeruginosa PE, residues 60 to 120 appeared to be sufficient for directing β-lactamase secretion (26). However, in the case of E. carotovora polygalacturonase (PehA), no more than the last two amino acids of the PehA could be excluded from a PehA-Bla hybrid without blocking secretion (29, 30). Passenger proteins might contain structures incompatible with efficient secretion by the type II machineries.
Other approaches using simple deletions have also been carried out. In this way, it could be shown that deletions of either of the two previously identified pullulanase secretion motifs reduces but does not abolish secretion (40). In addition, a truncated form of PE containing the N-terminal 30 residues connected to the C-terminal 370 residues, thus lacking the previously mentioned residues 60 to 120, is also secreted (28). These results indicate that more than a single region within the primary sequence might function as a targeting motif. Whether these regions function independently or synergetically remains to be elucidated. Studies done with cellulase Cel5 from E. chrysanthemi previously established that secretability is probably not controlled by a single discrete region but requires information present on the whole length of the protein. Indeed, in this case it appears that information for outer membrane translocation is present in both the catalytic and cellulose-binding domains (35).
Failure to identify discrete elements within the primary sequence of type II-transported substrates led to the hypothesis that the targeting signal might be a conformational motif. This speculation is also strongly supported by the fact that type II-secreted proteins acquire a high level of folding within the periplasm, even before they are translocated across the outer membrane. In particular, it has been shown that disulfide bond formation is a prerequisite for secretion of E. chrysanthemi Cel5 and K. oxytoca pullulanase (2, 31), and aerolysin and cholera toxin oligomerize within the periplasm prior to secretion by the type II machinery (16, 17). The conformational motif could be located in one area of the folded molecule, such an area being generated by residues coming from diverse regions of the primary sequence. This hypothesis may fit with the identification of the two distal motifs within the K. oxytoca pullulanase (40). Alternatively, the motif could be a defined region of the primary sequence but highly dependent on the folding of the molecule for its proper exposition to the secretion machinery.
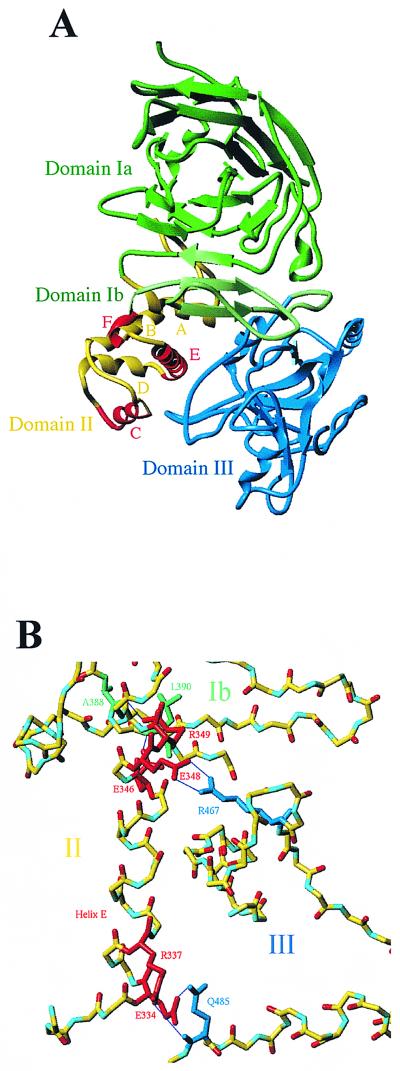
PE from P. aeruginosa is a single-chain cytotoxin, arranged into three major structural domains (1) (Fig. 6A). It belongs to the group of exoproteins secreted by the type II secretion machinery (Xcp) (10), and it was shown that a periplasmic intermediate is part of the normal route for its extracellular secretion (27). We examined the role of domain II, which previous studies indicated did not contain information needed for secretion. Domain II is composed of six α-helices (A to F) that form the translocation domain. We showed that deletion of any of these helices except F reduces or abolishes secretion of PE. Importantly, we thus first concluded that no secretion information was contained within helix F.
FIG. 6.
(A) Ribbon representation of the three-dimensional structure of PE, indicating the organization into domains. Domains Ia and Ib are shown in green and light green, respectively. Domain II is in yellow with the exception of helices C, E, and F, which are red, and domain III is blue. Helix E both plays a central role in the structuration of domain II and ensures the interconnection of domain II with domains I and III. Helices C and F are rather short and highly surface exposed, with few contacts to other structural elements and no contacts to domain I or domain III. The figure was produced with the program Turbo-Frodo (38). (B) Close-up view of helix E, highlighting the zones of interactions with other domains. All residues are represented as backbone except those in domain II (residues in red) that are involved in hydrogen bonds formed with residues of domain III (residues in blue) and with residues from domain Ib (residues in light green).
In order to seek an explanation for the observed differences in secretion level between the truncated PE forms, the three-dimensional structure of PE (1) was inspected by graphic means. We have shown that PE-delE is totally incompetent for secretion to the extracellular medium. The structural evidence that the mutational deletion of helix E in domain II must lead to dramatic changes in the structure of the protein is illustrated in Fig. 6A and B. Besides the fact that helix E spans the entire domain II (21 residues), it forms tight links to the neighboring domains Ib and III (Fig. 6A). Residues E334 and R337, at the N-terminal end of helix E, are hydrogen bonded to Q485 of domain III (Fig. 6B). A double-bridged salt link is formed by E348, at the C-terminal end of helix E, and R467 in domain III (Fig. 6B). Residues E346 and R349 form hydrogen bonds with the main chain N (L390) and CO (A388) groups of residues, respectively, situated in the β-strand of domain Ib (Fig. 6B). Helix E is also implicated in numerous hydrophobic contacts, not only with the surrounding helices of the same domain but also with hydrophobic residues in domains Ib and domain III. The breakdown of all of these stabilizing contacts and links most probably leads to incorrect folding of domain II and could also disturb proper domain organization, with domain I and III possibly falling apart. Helix F, the deletion of which did not interfere with the secretion properties of the protein, is a very short helix (9.2 Å) of two complete helical turns. The helix is located at the tip of a surface loop, is highly surface exposed, and does not make any major hydrogen bonds with neighboring helices (Fig. 6A). Presumably, the distance of 9.2 Å, corresponding to the length of helix F, can easily be bridged by the surrounding residues that are not involved in secondary structures without perturbing the overall fold. PE-delC is another form of PE whose secretion is drastically reduced. Helix C is of an intermediate length (14.8 Å) and exposed at the surface of the folded PE (Fig. 6A). Within domain II, it is a rather isolated helix with few close contacts and no particular hydrogen bonds to the surrounding helices. The residues preceding (301 to 309) and residues following (318 to 323) helix C (310 to 317) are unstructured loop regions, and therefore, one can imagine that, with small conformational rearrangements, these stretches could replace helix C without drastically influencing the overall fold of domain II. The presence of three glycine residues in the loop between helices C and D supports this hypothesis.
The predicted changes in the structure of domain II caused by all deletions except that of PE-delF are confirmed by the previous observation that none of the truncated PE forms has a functional domain II; i.e., none of these variants was transported into the cytosol after internalization by eukaryotic cells (43). Interestingly, it was also previously shown that none of these deletions within PE domain II interfered with cell binding or internalization or enzymatic activity, indicating that domains I and III are correctly folded (43). Thus, if there is a secretion signal in domain I or III and it is sufficient for secretion, the truncated proteins studied here should be efficiently secreted. This was not the case, however, even though they were correctly exported to the periplasm. The lack of translocation across the outer membrane might be due either to the absence of a secretion motif, contained in domain II and essential for recognition by the Xcp machinery, or to a default in the presentation of a secretion signal(s) contained in other domains because of the incorrect folding of the molecule. Since domain I alone may trigger β-lactamase secretion in an Xcp-dependent fashion (26), the first alternative appears unlikely. Moreover, when we analyzed the fate of a protein from which most of domain Ia has been deleted (residues 48 to 226), all of the molecules were retained within the cell (Fig. 4). This confirms that information critical for secretion is contained in this particular domain. The hypothesis that altering domain II may alter the overall conformation of the molecule and exposure of the secretion signal is restricted by the observation that the structures of domains I and III are not altered in these variants truncated within domain II. Consequently we postulated that the lack of secretion in this case is due to a separation of the two folded domains and a change in the molecular dimension due to the formation of a hinge by the destructured domain II. The fact that truncated PE proteins such as PE-delE and PE-delC displayed increased susceptibility to periplasmic proteases favors this hypothesis. Moreover, gel filtration experiments showed that indeed the Stockes radius of these molecules is increased. This is obvious for PE-delE, which eluted in a totally different fraction from wild-type PE (Fig. 5C). Interestingly, PE-delE is the most affected of the domain II helix deletion mutants with respect to translocation across the outer membrane. On the other hand, PE-delF, which is the only variant whose secretion is unaffected, has an elution profile identical to that of PE (Fig. 5D). The weakly secreted PE-delC and PE-delD also have a high peak of elution in the fraction corresponding to PE (Fig. 5B), in contrast to the nonsecreted PE-delA and PE-delB forms (Fig. 5A). Thus, the gel filtration data correlate well with the secretion behavior.
Several lines of evidence presented here suggest that PE domain II is important for secretion of the molecule. Alterations within this domain, although not affecting the folding and function of the other domains of the molecule, yield nonsecretory PE derivatives. However, we suggest that this domain does not contain the targeting signal per se but might be important for the proper positioning of the signal within the molecule and subsequently for its presentation to the secretion machinery. The role of a properly folded domain II might be to keep in close contact domains I and III, both of which have been proposed to contain targeting informations. Thus, modifications in domain II do not influence the structure of the adjacent domains but might disturb the level of compaction of these domains in the overall molecule in which they might be loosely packed, as suggested above for PE-delE. This hypothesis is supported by the analysis of the three-dimensional structure of PE and the structural description of PE-delE and is in line with recently reported data on E. chrysanthemi PelC exoprotein (25). In this study, the authors suggest that an exposed region at the C terminus of the protein contains the targeting information but proper positioning of this signal is dependent on two other regions present within the core of the molecule. The fact that even minor sequence changes, as described for the channel-forming toxin aerolysin (44), can alter presentation of the secretion signal makes our task of identifying the common features of the secretory information among type II-dependent secreted proteins very difficult. One approach which may give fruitful information will be to systematically and randomly mutagenize structural genes for model proteins such as PE, for which the three-dimensional structure is available, in order to identify those critical regions of the molecule. We are currently addressing this issue using P. aeruginosa PE as a model system.
ACKNOWLEDGMENTS
We thank Steve Lory for providing PAK-NT. We are indebted to David McKay for PE atomic coordinates. We thank all members of Andrée Lazdunski's laboratory for helpful discussion and Laurent Roux for his interest in this work. We are grateful to Claude Lazdunski, Tony Pugsley, and Frederic Barras for critical reading of the manuscript.
Romé Voulhoux is supported by the Ministry of Research and Technology and the Fondation pour le Recherche Médicale (FRM). This work was partly supported by the French Cystic Fibrosis Foundation (AFLM), by Biotech Framework grant BIO4-CT960119 from the European Union as part of the Cell Factories Network to A.F., and by a grant from the Association pour la recherche contre le cancer to B.B.
REFERENCES
- 1.Allured V S, Collier R J, Carroll S F, McKay D B. Structure of exotoxin A of Pseudomonas aeruginosa at 3.0-Angstrom resolution. Proc Natl Acad Sci USA. 1986;83:1320–1324. doi: 10.1073/pnas.83.5.1320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bortoli-German I, Brun E, Py B, Chippaux M, Barras F. Periplasmic disulphide bond formation is essential for cellulase secretion by the plant pathogen Erwinia chrysanthemi. Mol Microbiol. 1994;11:545–553. doi: 10.1111/j.1365-2958.1994.tb00335.x. [DOI] [PubMed] [Google Scholar]
- 3.Braun P, Tommassen J, Filloux A. Role of the propeptide in folding and secretion of elastase of Pseudomonas aeruginosa. Mol Microbiol. 1996;19:297–306. doi: 10.1046/j.1365-2958.1996.381908.x. [DOI] [PubMed] [Google Scholar]
- 4.de Groot A, Gerritse G, Tommassen J, Lazdunski A, Filloux A. Molecular organization of the xcp gene cluster in Pseudomonas putida: absence of an xcpX (gspK) homologue. Gene. 1999;226:35–40. doi: 10.1016/s0378-1119(98)00570-8. [DOI] [PubMed] [Google Scholar]
- 5.Dums F, Dow J M, Daniels M J. Structural characterization of protein secretion genes of the bacterial phytopathogen Xanthomonas campestris pathovar campestris: relatedness to secretion systems of other gram-negative bacteria. Mol Gen Genet. 1991;229:357–364. doi: 10.1007/BF00267456. [DOI] [PubMed] [Google Scholar]
- 6.Economou A. Following the leader: bacterial protein export through the Sec pathway. Trends Microbiol. 1999;7:315–320. doi: 10.1016/s0966-842x(99)01555-3. [DOI] [PubMed] [Google Scholar]
- 7.El Khattabi M, Ockhuijsen C, Bitter W, Jaeger K E, Tommassen J. Specificity of the lipase-specific foldases of gram-negative bacteria and the role of the membrane anchor. Mol Gen Genet. 1999;261:770–776. doi: 10.1007/s004380050020. [DOI] [PubMed] [Google Scholar]
- 8.Figurski D H, Helinski D R. Replication of an origin-containing derivative of plasmid RK2 dependent on a plasmid function provided in trans. Proc Natl Acad Sci USA. 1979;76:1648–1652. doi: 10.1073/pnas.76.4.1648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Filloux A, Bally M, Ball G, Akrim M, Tommassen J, Lazdunski A. Protein secretion in gram-negative bacteria: transport across the outer membrane involves common mechanisms in different bacteria. EMBO J. 1990;9:4323–4329. doi: 10.1002/j.1460-2075.1990.tb07881.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Filloux A, Michel G, Bally M. GSP-dependent protein secretion in Gram-negative bacteria: the Xcp system of Pseudomonas aeruginosa. FEMS Microbiol Rev. 1998;22:177–198. doi: 10.1111/j.1574-6976.1998.tb00366.x. [DOI] [PubMed] [Google Scholar]
- 11.Francetic O, Pugsley A P. The cryptic general secretory pathway (gsp) operon of Escherichia coli K-12 encodes functional proteins. J Bacteriol. 1996;178:3544–3549. doi: 10.1128/jb.178.12.3544-3549.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Furste J P, Pansegrau W, Frank R, Blocker H, Scholz P, Bagdasarian M, Lanka E. Molecular cloning of the plasmid RP4 primase region in a multi-host-range tacP expression vector. Gene. 1986;48:119–131. doi: 10.1016/0378-1119(86)90358-6. [DOI] [PubMed] [Google Scholar]
- 13.Gerritse G, Ure R, Bizoullier F, Quax W J. The phenotype enhancement method identifies the Xcp outer membrane secretion machinery from Pseudomonas alcaligenes as a bottleneck for lipase production. J Biotechnol. 1998;64:23–38. doi: 10.1016/s0168-1656(98)00101-1. [DOI] [PubMed] [Google Scholar]
- 14.Hales L M, Shuman H A. Legionella pneumophila contains a type II general secretion pathway required for growth in amoebae as well as for secretion of the Msp protease. Infect Immun. 1999;67:3662–3666. doi: 10.1128/iai.67.7.3662-3666.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hamood A N, Olson J C, Vincent T S, Iglewski B H. Regions of toxin A involved in toxin A excretion in Pseudomonas aeruginosa. J Bacteriol. 1989;171:1817–1824. doi: 10.1128/jb.171.4.1817-1824.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hardie K R, Schulze A, Parker M W, Buckley J T. Vibrio spp. secrete proaerolysin as a folded dimer without the need for disulphide bond formation. Mol Microbiol. 1995;17:1035–1044. doi: 10.1111/j.1365-2958.1995.mmi_17061035.x. [DOI] [PubMed] [Google Scholar]
- 17.Hirst T R, Holmgren J. Conformation of protein secreted across bacterial outer membranes: a study of enterotoxin translocation from Vibrio cholerae. Proc Natl Acad Sci USA. 1987;84:7418–7422. doi: 10.1073/pnas.84.21.7418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hoshino T, Kageyama M. Purification and properties of a binding protein for branched-chain amino acids in Pseudomonas aeruginosa. J Bacteriol. 1980;141:1055–1063. doi: 10.1128/jb.141.3.1055-1063.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Howard S P, Critch J, Bedi A. Isolation and analysis of eight exe genes and their involvement in extracellular protein secretion and outer membrane assembly in Aeromonas hydrophila. J Bacteriol. 1993;175:6695–6703. doi: 10.1128/jb.175.20.6695-6703.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hwang J, Fitzgerald D J, Adhya S, Pastan I. Functional domains of Pseudomonas exotoxin identified by deletion analysis of the gene expressed in E. coli. Cell. 1987;48:129–136. doi: 10.1016/0092-8674(87)90363-1. [DOI] [PubMed] [Google Scholar]
- 21.Karlyshev A V, MacIntyre S. Cloning and study of the genetic organization of the exe gene cluster of Aeromonas salmonicida. Gene. 1995;158:77–82. doi: 10.1016/0378-1119(95)00139-w. [DOI] [PubMed] [Google Scholar]
- 22.Kounnas M Z, Morris R E, Thompson M R, FitzGerald D J, Strickland K, Saelinger C B. The alpha 2-macroglobulin receptor/low density lipoprotein receptor-related protein binds and internalizes Pseudomonas exotoxin A. J Biol Chem. 1992;267:12420–12423. [PubMed] [Google Scholar]
- 23.Liles M R, Edelstein P H, Cianciotto N P. The prepilin peptidase is required for protein secretion by and the virulence of the intracellular pathogen Legionella pneumophila. Mol Microbiol. 1999;31:959–970. doi: 10.1046/j.1365-2958.1999.01239.x. [DOI] [PubMed] [Google Scholar]
- 24.Lindeberg M, Collmer A. Analysis of eight out genes in a cluster required for pectic enzyme secretion by Erwinia chrysanthemi: sequence comparison with secretion genes from other gram-negative bacteria. J Bacteriol. 1992;174:7385–7397. doi: 10.1128/jb.174.22.7385-7397.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lindeberg M, Boyd C M, Keen N T, Collmer A. External loops at the C terminus of Erwinia chrysanthemi pectate lyase C are required for species-specific secretion through the Out type II pathway. J Bacteriol. 1998;180:1431–1437. doi: 10.1128/jb.180.6.1431-1437.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lu H M, Lory S. A specific targeting domain in mature exotoxin A is required for its extracellular secretion from Pseudomonas aeruginosa. EMBO J. 1996;15:429–436. [PMC free article] [PubMed] [Google Scholar]
- 27.Lu H M, Mizushima S, Lory S. A periplasmic intermediate in the extracellular secretion pathway of Pseudomonas aeruginosa exotoxin A. J Bacteriol. 1993;175:7463–7467. doi: 10.1128/jb.175.22.7463-7467.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.McVay C S, Hamood A N. Toxin A secretion in Pseudomonas aeruginosa: the role of the first 30 amino acids of the mature toxin. Mol Gen Genet. 1995;249:515–525. doi: 10.1007/BF00290577. [DOI] [PubMed] [Google Scholar]
- 29.Palomaki T, Saarilahti H T. The extreme C-terminus is required for secretion of both the native polygalacturonase (PehA) and PehA-Bla hybrid proteins in Erwinia carotovora subsp. carotovora. Mol Microbiol. 1995;17:449–459. doi: 10.1111/j.1365-2958.1995.mmi_17030449.x. [DOI] [PubMed] [Google Scholar]
- 30.Palomaki T, Saarilahti H T. Isolation and characterization of new C-terminal substitution mutations affecting secretion of polygalacturonase in Erwinia carotovora ssp. carotovora. FEBS Lett. 1997;400:122–126. doi: 10.1016/s0014-5793(96)01369-5. [DOI] [PubMed] [Google Scholar]
- 31.Pugsley A P. Translocation of a folded protein across the outer membrane in Escherichia coli. Proc Natl Acad Sci USA. 1992;89:12058–12062. doi: 10.1073/pnas.89.24.12058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Pugsley A P. The complete general secretory pathway in gram-negative bacteria. Microbiol Rev. 1993;57:50–108. doi: 10.1128/mr.57.1.50-108.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Pugsley A P, Francetic O, Hardie K, Possot O M, Sauvonnet N, Seydel A. Pullulanase: model protein substrate for the general secretory pathway of gram-negative bacteria. Folia Microbiol. 1997;42:184–192. doi: 10.1007/BF02818976. [DOI] [PubMed] [Google Scholar]
- 34.Py B, Salmond G P C, Chippaux M, Barras F. Secretion of cellulases in Erwinia chrysanthemi and Erwinia carotovora is species-specific. FEMS Microbiol Lett. 1991;79:315–322. [Google Scholar]
- 35.Py B, Chippaux M, Barras F. Mutagenesis of cellulase EGZ for studying the general protein secretory pathway in Erwinia chrysanthemi. Mol Microbiol. 1993;7:785–793. doi: 10.1111/j.1365-2958.1993.tb01169.x. [DOI] [PubMed] [Google Scholar]
- 36.Reeves P J, Whitcombe D, Wharam S, Gibson M, Allison G, Bunce N, Barallon R, Douglas P, Mulholland V, Stevens S, Walker D, Salmond G P C. Molecular cloning and characterization of 13 out genes from Erwinia carotovora subspecies carotovora: genes encoding members of a general secretion pathway (GSP) widespread in gram-negative bacteria. Mol Microbiol. 1993;8:443–456. doi: 10.1111/j.1365-2958.1993.tb01589.x. [DOI] [PubMed] [Google Scholar]
- 37.Roussel A, Cambillau C. Silicon Graphics geometry partners directory. Mountain View, Calif: Silicon Graphics; 1991. Turbo-Frodo program; p. 86. [Google Scholar]
- 38.Sambrook J, Fritsch E F, Maniatis T. Molecular cloning: a laboratory manual. Cold Spring Harbor, N.Y: Cold Spring Harbor Laboratory Press; 1989. [Google Scholar]
- 39.Sandkvist M, Michel L O, Hough L P, Morales V M, Bagdasarian M, Koomey M, DiRita V J. General secretion pathway (eps) genes required for toxin secretion and outer membrane biogenesis in Vibrio cholerae. J Bacteriol. 1997;179:6994–7003. doi: 10.1128/jb.179.22.6994-7003.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Sauvonnet N, Pugsley A P. Identification of two regions of Klebsiella oxytoca pullulanase that together are capable of promoting beta-lactamase secretion by the general secretory pathway. Mol Microbiol. 1996;22:1–7. doi: 10.1111/j.1365-2958.1996.tb02650.x. [DOI] [PubMed] [Google Scholar]
- 41.Simpson H, Barras F. Functional analysis of the carbohydrate-binding domains of Erwinia chrysanthemi Cel5 (endoglucanase Z) and an Escherichia coli putative chitinase. J Bacteriol. 1999;181:4611–4616. doi: 10.1128/jb.181.15.4611-4616.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Starnbach M N, Lory S. The fliA (rpoF) gene of Pseudomonas aeruginosa encodes an alternative sigma factor required for flagellin synthesis. Mol Microbiol. 1992;6:459–469. doi: 10.1111/j.1365-2958.1992.tb01490.x. [DOI] [PubMed] [Google Scholar]
- 43.Taupiac M P, Bebien M, Alami M, Beaumelle B. A deletion within the translocation domain of Pseudomonas exotoxin A enhances translocation efficiency and cytotoxicity concomitantly. Mol Microbiol. 1999;31:1385–1393. doi: 10.1046/j.1365-2958.1999.01280.x. [DOI] [PubMed] [Google Scholar]
- 44.Wong K R, Buckley J T. Site-directed mutagenesis of a single tryptophan near the middle of the channel-forming toxin aerolysin inhibits its transfer across the outer membrane of Aeromonas salmonicida. J Biol Chem. 1991;266:14451–14456. [PubMed] [Google Scholar]
- 45.Wretlind B, Bjorklind A, Pavlovskis O R. Role of exotoxin A and elastase in the pathogenicity of Pseudomonas aeruginosa strain PAO experimental mouse burn infection. Microb Pathog. 1987;2:397–404. doi: 10.1016/0882-4010(87)90046-5. [DOI] [PubMed] [Google Scholar]