Abstract
Background
C‐Jun N‐terminal kinase pathway‐associated phosphatase (JKAP) modulates the T cell receptor and mitogen‐activated protein kinase pathway‐mediated autoimmunity, thus participating in the pathogenesis of autoimmune diseases. This study aimed to explore the clinical implication of JKAP in inflammatory bowel disease (IBD) children.
Methods
C‐Jun N‐terminal kinase pathway‐associated phosphatase, tumor necrosis factor‐α (TNF‐α), interleukin‐23, interferon‐γ (T‐helper 1 secreted cytokine), and interleukin‐17A (T‐helper 17 secreted cytokine) in serum samples from 140 IBD children (including 60 Crohn's disease (CD) children and 80 ulcerative colitis (UC) children) were detected by ELISA. Meanwhile, JKAP from serum samples of 10 healthy controls (HCs) was also detected by ELISA.
Results
C‐Jun N‐terminal kinase pathway‐associated phosphatase was reduced in CD children (median (interquartile range (IQR)): 51.6 (36.8–69.5) pg/ml) and UC children (median (IQR): 57.5 (43.4–78.5) pg/ml) compared with HCs (median (IQR): 101.8 (70.0–143.2) pg/ml) (both p < 0.05). In CD children, JKAP was negatively correlated with C‐reactive protein (CRP) (p = 0.016) and erythrocyte sedimentation rate (ESR) (p = 0.029); while in UC children, JKAP was also negatively correlated with CRP (p = 0.006) and ESR (p = 0.022). Regarding the correlation of JKAP with disease activity, it presented negative correlations with PCDAI (p = 0.001) and PUCAI (p = 0.002). Besides, JKAP was negatively related to TNF‐α (both p < 0.05) but not interleukin‐23 (both p>0.05) in CD and UC children. Additionally, JKAP was not correlated with interferon‐γ in CD or UC children (both p>0.05), while negatively correlated with interleukin‐17A in CD and UC children (both p < 0.05).
Conclusion
C‐Jun N‐terminal kinase pathway‐associated phosphatase shows low expression and negative correlations with inflammation, disease activity, and T‐helper 17 cells in IBD children.
Keywords: C‐Jun N‐terminal kinase pathway‐associated phosphatase, disease activity, inflammation, inflammatory bowel disease children, T‐helper 17
C‐Jun N‐terminal kinase pathway‐associated phosphatase (JKAP) modulates the T cell receptor and mitogen‐activated protein kinase pathway‐mediated autoimmunity, thus participating in the pathogenesis of autoimmune diseases, while its clinical implication in inflammatory bowel disease (IBD) children remains unclear. The current study reveals that JKAP shows low expression and negative correlations with inflammation, disease activity, and T‐helper 17 cells in IBD children.

1. INTRODUCTION
Inflammatory bowel disease (IBD) is an autoimmune disease that mainly includes Crohn's disease (CD) and ulcerative colitis (UC), which impacts the gastrointestinal tract in both adults and children. 1 , 2 In children, IBD may also affect their growth and even lead to reduced quality of life and psychological health. 3 , 4 In recent decades, the incidence of IBD in children is steadily increasing in Europe, North American, and Asia, which renders IBD in children a critical health issue internationally. 5 , 6 Although nutritional management, 5‐aminosalicylic acid (5‐ASA), glucocorticoids, immunosuppressive agents, and biologics may be useful in the treatment of IBD in children, there still lacks biomarkers to reflect the risk and characteristics of IBD in children, which could hinder the in‐time management of IBD in children. 4 , 7 , 8 , 9 , 10
C‐Jun N‐terminal kinase (JNK) pathway‐associated phosphatase (JKAP) is a dual‐specificity phosphatase to activate the JNK pathway and thus participates in various cellular functions, including malignant behavior of cancer cells, cell motility, and notably, T cell receptor (TRC) signaling‐mediated autoimmunity. 11 , 12 , 13 Clinically, a bunch of studies has reported the potential of JKAP as a biomarker in autoimmune diseases. For instance, it is suggested that JKAP is associated with inflammation and disease activity in patients with rheumatoid arthritis, ankylosing spondylitis, and psoriasis. 14 , 15 , 16 Another interesting study discloses that JKAP is insufficiently expressed and negatively correlates with disease activity in IBD patients 17 ; however, this study only enrolled adult IBD patients, and it is restricted by the limited sample size (N = 81). Therefore, the role of JKAP as a biomarker in IBD should be verified in a larger cohort, and whether JKAP could also act as a biomarker in IBD in children remained unclear.
The current study enrolled 140 IBD children (including 60 CD children and 80 UC children) and detected JKAP level in their serum, which aimed to explore the correlation of JKAP with T‐helper (Th) 1, Th17 cells inflammation, and disease activity in children with IBD.
2. METHODS
2.1. Participants
Between July 2019 and January 2022, this study serially enrolled a total of 140 IBD children (including 60 CD children and 80 UC children) who were admitted to the hospital. The inclusion criteria were: (i) confirmed as IBD (CD or UC) according to conventional radiological, endoscopic, and histological examinations; (ii) less than 16 years old; (iii) willing to provide peripheral blood (PB) samples. The exclusion criteria were: (i) complicated with other immune system diseases; (ii) had a prior history of hematologic malignancies or solid tumors. In addition, during the same period, a total of 10 healthy children who underwent health examinations in the hospital were also recruited in the study as health controls (HCs). The HCs who had a history of IBD or met the exclusion criteria for IBD children were ineligible for the study. The study was permitted by Ethics Committee of The Affiliated Hospital of Qingdao University. The statutory guardians of all participants signed the informed consents.
2.2. Data document
Clinical characteristics were collected from CD children and UC children at admission, as well as from HCs after enrollment, which included age, gender, height, weight, C‐reactive protein (CRP), and erythrocyte sedimentation rate (ESR). Additionally, pediatric Crohn's disease activity index (PCDAI) was assessed among CD children, based on which the disease activity was evaluated, then classified as quiescent, mild, moderate, and severe. 18 Besides, pediatric ulcerative colitis activity index (PUCAI) was assessed among UC children, then the disease severity was evaluated and classified as remission, mild, moderate, and severe. 19 Treatments for all patients were also recorded, including 5‐ASA, glucocorticoid treatment, immunosuppressant treatment, biologics treatment.
2.3. Sample collection and assessment
Peripheral blood samples were obtained from IBD children at admission and HCs after enrollment, then serum samples were separated to assess the levels of JKAP, proinflammatory cytokines TNF‐α and interleukin‐23 (IL‐23)), Th1 cell‐secreted cytokine (interferon‐gamma (IFN‐γ)), and Th17 cell‐secreted cytokine (interleukin‐17A (IL‐17A)) by enzyme‐linked immunosorbent assay (ELISA) using commercial Human ELISA Kits. All kits used in the study analysis were purchased from Shanghai Enzyme‐linked Biotechnology Co., Ltd, and the experimentations were performed in strict accordance with the protocols from manufacturers.
2.4. Statistics
Statistical analysis and graphs construction were carried out using SPSS V.24.0 (IBM Corp.) and GraphPad Prism V.6.01 (GraphPad Software Inc.), respectively. Differences of clinical characteristics among three groups were analyzed using one‐way analysis of variance (ANOVA) test, Chi‐square test, and Kruskal–Wallis H rank‐sum test. Comparison of JKAP level among three groups was determined using Kruskal–Wallis H rank‐sum test, and comparison between groups was adjusted using Bonferroni test. The ability of JKAP level in differentiating participants was assessed using receiver‐operating characteristic (ROC) analysis and area under the curve (AUC). The association of two variables was evaluated using Spearman's rank correlation test. The association between treatment and JKAP level was assessed by Wilcoxon rank‐sum test. A p value < 0.05 indicated statistical significance.
3. RESULTS
3.1. Clinical characteristics
The UC children had a mean age of 8.5 ± 2.6 years with 34 (42.5%) males and 46 (57.5%) females; meanwhile, the CD children showed a mean age of 8.6 ± 2.8 years with 29 (48.3%) males and 31 (51.7%) females; besides, the HCs presented a mean age of 8.8 ± 2.3 years, consisting of 4 (40.0%) males and 6 (60.0%) females. The comparison analysis revealed that no difference was found in age, gender, height, or weight among UC children, CD children, and HCs (all p > 0.05), whereas CRP (median (interquartile range (IQR)): 31.1 (18.0–45.5) versus 25.2 (16.9–43.2) versus 2.6 (1.4–3.3) mg/L) and ESR (median (IQR): 29.9 (19.2–40.9) versus 30.5 (20.6–45.1) versus 10.1 (8.8–13.7) mm/h) were different among the three groups (both p < 0.001). Moreover, TNF‐α, IL‐23, and IL‐17A were similar between UC children and CD children (all p > 0.05), but IFN‐γ showed an increase in CD children compared with UC children (p < 0.001). Regarding disease activity of UC children, the median (IQR) value of PUCAI score was 25.0 (15.0–40.0); meanwhile, there were 12 (15.0%) children with remission disease, 39 (48.8%) children with mild UC, and 29 (36.2%) children with moderate or severe UC. In terms of disease activity in CD children, the median (IQR) value of PCDAI score was 25.0 (17.5–40.0); besides, the numbers of children with quiescent, mild, and moderate/severe CD were 9 (15.0%), 28 (46.7%), and 23 (38.3%), respectively (Table 1).
TABLE 1.
Characteristics of IBD children and HCs
| Items |
HCs (N = 10) |
UC children (N = 80) |
CD children (N = 60) |
Statistic (F/χ 2/H/Z) | p Value |
|---|---|---|---|---|---|
| Age (years), mean ± SD | 8.8 ± 2.3 | 8.5 ± 2.6 | 8.6 ± 2.8 | 0.058 | 0.944 |
| Gender, No. (%) | 0.566 | 0.753 | |||
| Male | 4 (40.0) | 34 (42.5) | 29 (48.3) | ||
| Female | 6 (60.0) | 46 (57.5) | 31 (51.7) | ||
| Height (cm), mean ± SD | 134.8 ± 13.1 | 128.7 ± 18.0 | 133.1 ± 20.2 | 1.206 | 0.302 |
| Weight (kg), mean ± SD | 29.9 ± 8.6 | 27.9 ± 8.7 | 28.9 ± 10.0 | 0.314 | 0.731 |
| CRP (mg/L), median (IQR) | 2.6 (1.4–3.3) | 31.1 (18.0–45.5) | 25.2 (16.9–43.2) | 27.682 | <0.001 |
| ESR (mm/h), median (IQR) | 10.1 (8.8–13.7) | 29.9 (19.2–40.9) | 30.5 (20.6–45.1) | 22.440 | <0.001 |
| PUCAI, median (IQR) | ‐ | 25.0 (15.0–40.0) | ‐ | ‐ | ‐ |
| Severity of UC, No. (%) | ‐ | ‐ | |||
| Remission | ‐ | 12 (15.0) | ‐ | ||
| Mild | ‐ | 39 (48.8) | ‐ | ||
| Moderate or severe | ‐ | 29 (36.2) | ‐ | ||
| PCDAI, median (IQR) | ‐ | ‐ | 25.0 (17.5–40.0) | ‐ | ‐ |
| Severity of CD, No. (%) | ‐ | ‐ | |||
| Quiescent | ‐ | ‐ | 9 (15.0) | ||
| Mild | ‐ | ‐ | 28 (46.7) | ||
| Moderate or severe | ‐ | ‐ | 23 (38.3) | ||
| TNF‐α (pg/ml), median (IQR) | ‐ | 67.4 (52.8–107.0) | 83.2 (57.4–147.2) | −1.756 | 0.079 |
| IL−23 (pg/ml), median (IQR) | ‐ | 107.0(85.2–160.0) | 124.1 (91.2–172.9) | −1.392 | 0.164 |
| IFN‐γ (pg/ml), median (IQR) | ‐ | 9.8 (8.0–12.5) | 12.9 (9.6–17.2) | −3.622 | <0.001 |
| IL−17A (pg/ml), median (IQR) | ‐ | 75.7 (59.2–90.8) | 70.8 (55.7–101.4) | −0.101 | 0.920 |
| 5‐ASA treatment, No. (%) | ‐ | 72 (90.0) | 10 (16.7) | 75.985 | <0.001 |
| Glucocorticoid treatment, No. (%) | ‐ | 26 (32.5) | 23 (38.3) | 0.513 | 0.474 |
| Immunosuppressant treatment, No. (%) | ‐ | 11 (13.8) | 32 (53.3) | 25.244 | <0.001 |
| Biologics treatment, No. (%) | ‐ | 10 (12.5) | 20 (33.3) | 8.838 | 0.003 |
Abbreviations: 5‐ASA, 5‐aminosalicylic acid; CD, Crohn's disease; CRP, C‐reactive protein; ESR, erythrocyte sedimentation rate; HCs, health controls; IBD, inflammatory bowel disease; IFN‐γ, interferon‐gamma; IL‐17A, interleukin 17A; IL‐23, interleukin 23; IQR, interquartile range; PCDAI, pediatric Crohn's disease activity index; PUCAI, pediatric ulcerative colitis activity index; SD, standard deviation; TNF‐α, tumor necrosis factor alpha; UC, ulcerative colitis.
3.2. JKAP level
C‐Jun N‐terminal kinase pathway‐associated phosphatase presented a distinctive expression among CD children (median (IQR): 51.6 (36.8–69.5) pg/ml), UC children (median (IQR): 57.5 (43.4–78.5) pg/ml), and HCs (median (IQR): 101.8 (70.0–143.2) pg/ml) (p < 0.001); meanwhile, it was reduced in CD children (p < 0.001) and UC children (p = 0.005) compared with HCs; in addition, JKAP was similar between CD children and UC children (p = 0.281) (Figure 1A). Further ROC curve analysis showed that JKAP illustrated good potential in discriminating CD children (AUC: 0.868, 95% confidence interval (CI): 0.759–0.978) and UC children (AUC: 0.824, 95% CI: 0.687–0.961) from HCs; JKAP at the best cut‐off point was 73.25 pg/ml for discriminating CD children versus HCs and 83.65 pg/ml for discriminating UC children versus HCs, accordingly (Figure 1B).
FIGURE 1.

C‐Jun N‐terminal kinase pathway‐associated phosphatase expression in IBD children and HCs. Comparison of JKAP expression among UC children, CD children, and HCs (A); Ability of JKAP in discriminating UC and CD children from HCs (B)
3.3. Correlation of JKAP with inflammation markers
In CD children, JKAP showed a negative correlation with CRP (rs = −0.310, p = 0.016) (Figure 2A) and ESR (rs = −0.281, p = 0.029) (Figure 2B). In UC children, JKAP also displayed negative correlations with both CRP (rs = −0.307, p = 0.006) (Figure 2C) and ESR (rs = −0.257, p = 0.022) (Figure 2D).
FIGURE 2.
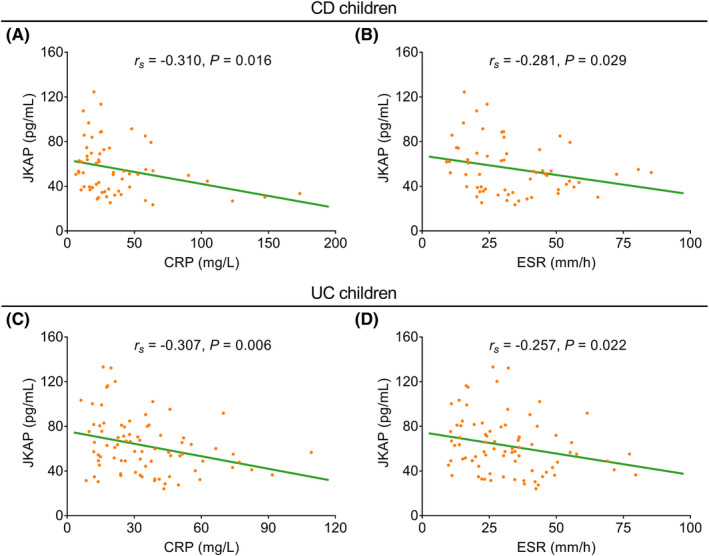
Correlation of JKAP with inflammatory markers in IBD children. Correlation of JKAP with CRP (A) and ESR (B) in CD children; correlation of JKAP with CRP (C) and ESR (D) in UC children
3.4. Correlation of JKAP with disease activity
C‐Jun N‐terminal kinase pathway‐associated phosphatase was negatively correlated with PCDAI score in CD children (rs = −0.421, p = 0.001) (Figure 3A); after categorizing CD children into children with quiescent, mild, and moderate/severe CD, it was revealed that JKAP was negatively correlated with disease severity in CD children (rs = −0.392, p = 0.002) (Figure 3B). Simultaneously, JKAP showed a reverse correlation with PUCAI score in UC children (rs = −0.342, p = 0.002) (Figure 3C); UC children were also divided into children with remission, mild, or moderate/severe UC; correlation analysis presented that JKAP was also negatively correlated with disease severity in UC children (rs = −0.241, p = 0.031) (Figure 3D).
FIGURE 3.

Correlation of JKAP with disease activity in IBD children. Correlation of JKAP with PCDAI (A) and comparison of JKAP among patients with different severity (B) in CD children; correlation of JKAP with PUCAI (C) and comparison of JKAP among patients with different severity (D) in UC children
3.5. Correlation of JKAP with proinflammatory cytokines
C‐Jun N‐terminal kinase pathway‐associated phosphatase was negatively correlated with TNF‐α in both CD children (rs = −0.437, p < 0.001) and UC children (rs = −0.238, p = 0.033). However, it presented no correlation with IL‐23 in neither CD children (rs = −0.240, p = 0.065) nor UC children (rs = −0.205, p = 0.068) (Figure 4A–D).
FIGURE 4.

Correlation of JKAP with proinflammatory cytokines in IBD children. Correlation of JKAP with TNF‐α (A) and IL‐23 (B) in CD children; correlation of JKAP with TNF‐α (C) and IL‐23 (D) in UC children
3.6. Correlation of JKAP with Th1‐ and Th17‐secreted cytokines
C‐Jun N‐terminal kinase pathway‐associated phosphatase was not correlated with IFN‐γ (rs = −0.186, p = 0.156) (Figure 5A), while it illustrated a negative correlation with IL‐17A (rs = −0.342, p = 0.008) (Figure 5B); however, it was not correlated with IFN‐γ/IL‐17A ratio (rs = −0.149, p = 0.256) (Figure 5C) in CD children. Meanwhile in UC children, JKAP showed no correlation with IFN‐γ (rs = −0.101, p = 0.373) (Figure 5D), but it was negatively correlated with IL‐17A (rs = −0.255, p = 0.022) (Figure 5E); besides, it was not correlated with IFN‐γ/IL‐17A ratio (rs = −0.035, p = 0.756) (Figure 5F) in UC children. In addition, JKAP did not vary in IBD children with or without 5‐ASA, glucocorticoid, immunosuppressant, or biologics treatment (all p > 0.05) (Figure 6A–H).
FIGURE 5.

Correlation of JKAP with cytokines secreted by Th1 and Th17 in IBD children. Correlation of JKAP with IFN‐γ (A), IL‐17A (B), and IFN‐γ/IL‐17A ratio (C) in CD children; correlation of JKAP with IFN‐γ (D), IL‐17A (E), and IFN‐γ/IL‐17A ratio (F) in UC children
FIGURE 6.

Correlation of JKAP with treatment in IBD children. Comparison of JKAP in CD children with or without 5‐ASA (A), glucocorticoid (B), immunosuppressant (C), or biologics (D) treatment. Comparison of JKAP in UC children with or without 5‐ASA (E), glucocorticoid (F), immunosuppressant (G), or biologics (H) treatment
4. DISCUSSION
C‐Jun N‐terminal kinase pathway‐associated phosphatase is reported to suppress TCR signaling and autoimmunity through inactivating lymphocyte‐specific protein‐tyrosine kinase (Lck). 13 Based on this finding, several studies have inquired about the clinical role of JKAP in autoimmune diseases, which illustrate that JKAP is lower in patients with rheumatoid arthritis or IBD. 14 , 17 However, whether JKAP also presented aberrant expression in IBD children remained unclear. In the current study, it was observed that JKAP was reduced in CD children and UC children compared with HCs. A possible explanation was that: a low level of JKAP might activate the TCR signaling through activating Lck, while activated TCR signaling was a critical inducer of IBD. 13 , 20 Therefore, JKAP was lower in IBD children compared with HCs. Currently, the diagnosis of IBD is mainly based on a complex combination of clinical symptoms, laboratory findings, endoscopy, histopathological examination, and imageology examination; meanwhile, there lacks a gold standard for the diagnosis of IBD, which may cause a delay in the treatment of IBD. 21 Our data revealed that JKAP showed good potential in discriminating IBD children from HCs, which implied that JKAP might be helpful for the diagnosis of IBD in children. However, since the main objective of this study was not to explore the effect of JKAP on diagnosing IBD in children, this hypothesis could be explored in further studies.
It is worth noting that JKAP is able to reflect the disease activity in patients with autoimmune diseases. For instance, JKAP is negatively correlated with psoriasis area severity index score in patients with psoriasis 15 ; meanwhile, JKAP is associated with lower CD activity index in adult patients with CD 22 ; besides, JKAP presents a negative correlation with 28‐joints disease activity score based on ESR in rheumatoid arthritis patients. 23 These studies suggest that JKAP could serve as a potential biomarker to timely monitor disease activity in autoimmune diseases. However, whether this correlation also existed in IBD children should be investigated. In the present study, it was observed that JKAP was negatively correlated with inflammation markers, proinflammatory cytokine, and disease activity scores in IBD children. Possible explanations might be that: (1) as a phosphatase, JKAP might downregulate inflammation through several pathways, such as the JNK pathway and the TCR pathway. 12 , 13 Therefore, JKAP was negatively correlated with inflammation in IBD children; (2) through downregulating inflammation, JKAP connected to reduced disease activity in IBD patients.
CD4+ T cells are critically involved in the pathophysiology of IBD. For instance, it is reported that Th1 and Th17 are dysregulated in patients with IBD. 24 Meanwhile, Th1 and Th17 also modulate the progression of IBD. 25 Apart from these, previous studies also present that JKAP not only correlates with Th1 and Th17 in several comprehensive diseases such as chronic obstructive pulmonary disease, Alzheimer's disease, and Parkinson's disease, but also modulates the differentiation of Th1 and Th17 in IBD. 17 , 26 , 27 , 28 Therefore, it was reasonable to deduce that JKAP was also correlated with Th1 and Th17 in IBD children. In the current study, it was observed that JKAP presented a negative correlation with Th17 in IBD children, which could be a result from that JKAP might suppress the differentiation of Th17 in IBD children. 17 Meanwhile, since Th17 critically regulates the pathogenesis and progression of IBD, these above‐mentioned data could also explain the correlation of JKAP with IBD risk and activity.
Although several interesting results were found, there existed some limitations in this study. Firstly, this study had a relatively low sample size, which might cause low statistical power. Secondly, the clinical role of JKAP in unclassified IBD children could be further explored. Thirdly, the molecular mechanism of JKAP in regulating the differentiation of CD4+ T cells into Th17 cells in IBD could be investigated in the future. Fourthly, the monitoring of longitudinal change of JKAP in IBD children should be conducted in the future. Fifthly, the correlation of JKAP with treatment response to biologics in IBD children could be investigated in further studies. Sixthly, the comparison of JKAP as a biomarker between pediatric and adult IBD children could be explored in the future.
Conclusively, JKAP is insufficiently expressed and negatively correlates with inflammation, disease activity, and Th17 cells in IBD children.
CONFLICT OF INTEREST
The authors have no relevant financial or non‐financial interests to disclose.
ACKNOWLEDGEMENT
None.
Wang C, Bai C, Mao C, et al. JNK pathway‐associated phosphatase illustrates low expression and negative correlations with inflammation, disease activity, and T‐helper 17 cells in inflammatory bowel disease children. J Clin Lab Anal. 2022;36:e24488. doi: 10.1002/jcla.24488
DATA AVAILABILITY STATEMENT
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
REFERENCES
- 1. Rubalcava NS, Gadepalli SK. Inflammatory bowel disease in children and adolescents. Adv Pediatr. 2021;68:121‐142. [DOI] [PubMed] [Google Scholar]
- 2. Flynn S, Eisenstein S. Inflammatory bowel disease presentation and diagnosis. Surg Clin North Am. 2019;99(6):1051‐1062. [DOI] [PubMed] [Google Scholar]
- 3. Fuller MK. Pediatric inflammatory bowel disease: special considerations. Surg Clin North Am. 2019;99(6):1177‐1183. [DOI] [PubMed] [Google Scholar]
- 4. Keethy D, Mrakotsky C, Szigethy E. Pediatric inflammatory bowel disease and depression: treatment implications. Curr Opin Pediatr. 2014;26(5):561‐567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Park S, Kang Y, Koh H, Kim S. Increasing incidence of inflammatory bowel disease in children and adolescents: significance of environmental factors. Clin Exp Pediatr. 2020;63(9):337‐344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Huang JG, Aw MM. Pediatric inflammatory bowel disease in Asia: epidemiology and natural history. Pediatr Neonatol. 2020;61(3):263‐271. [DOI] [PubMed] [Google Scholar]
- 7. Hart L, Verburgt CM, Wine E, et al. Nutritional therapies and their influence on the intestinal microbiome in pediatric inflammatory bowel disease. Nutrients. 2022;14(1):4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Salvador‐Martín S, Melgarejo‐Ortuño A, López‐Fernández LA. Biomarkers for optimization and personalization of anti‐TNFs in pediatric inflammatory bowel disease. Pharmaceutics. 2021;13(11):1786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Wlazło M, Kierkuś J. Dual biologic therapy for the treatment of pediatric inflammatory bowel disease: a review of the literature. J Clin Med. 2022;11(7):2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Lu Q, Yang MF, Liang YJ, et al. Immunology of inflammatory bowel disease: molecular mechanisms and therapeutics. J Inflamm Res. 2022;15:1825‐1844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Sekine Y, Ikeda O, Hayakawa Y, et al. DUSP22/LMW‐DSP2 regulates estrogen receptor‐alpha‐mediated signaling through dephosphorylation of Ser‐118. Oncogene. 2007;26(41):6038‐6049. [DOI] [PubMed] [Google Scholar]
- 12. Li JP, Fu YN, Chen YR, Tan TH. JNK pathway‐associated phosphatase dephosphorylates focal adhesion kinase and suppresses cell migration. J Biol Chem. 2010;285(8):5472‐5478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Li JP, Yang CY, Chuang HC, et al. The phosphatase JKAP/DUSP22 inhibits T‐cell receptor signalling and autoimmunity by inactivating Lck. Nat Commun. 2014;5:3618. [DOI] [PubMed] [Google Scholar]
- 14. Sun L, Tu J, Chen X, et al. JNK pathway‐associated phosphatase associates with rheumatoid arthritis risk, disease activity, and its longitudinal elevation relates to etanercept treatment response. J Clin Lab Anal. 2021;35(4):e23709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Cailing E, Fang Y, Wu S, Meng Z, Qin G, Yang J. Dual specificity phosphatase 22 relates to skin lesion degree and biologics history, while its longitudinal elevation during treatment reflects better outcome in psoriasis patients. J Clin Lab Anal. 2022;36(2):e24199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Zhou X, Li M. JKAP serves as a potential biomarker for the evaluation of inflammatory condition, disease activity, and treatment response to TNF inhibitor in ankylosing spondylitis patients. Mod Rheumatol. 2022;32(3):613‐618. [DOI] [PubMed] [Google Scholar]
- 17. Zhou R, Chang Y, Liu J, et al. JNK pathway‐associated phosphatase/DUSP22 suppresses CD4(+) T‐cell activation and Th1/Th17‐cell differentiation and negatively correlates with clinical activity in inflammatory bowel disease. Front Immunol. 2017;8:781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Otley A, Loonen H, Parekh N, Corey M, Sherman PM, Griffiths AM. Assessing activity of pediatric Crohn's disease: which index to use? Gastroenterology. 1999;116(3):527‐531. [DOI] [PubMed] [Google Scholar]
- 19. Kerur B, Litman HJ, Stern JB, et al. Correlation of endoscopic disease severity with pediatric ulcerative colitis activity index score in children and young adults with ulcerative colitis. World J Gastroenterol. 2017;23(18):3322‐3329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Fujii H, Shinzaki S, Iijima H, et al. Core fucosylation on T cells, required for activation of T‐cell receptor signaling and induction of colitis in mice, is increased in patients with inflammatory bowel disease. Gastroenterology. 2016;150(7):1620‐1632. [DOI] [PubMed] [Google Scholar]
- 21. Maaser C, Sturm A, Vavricka SR, et al. ECCO‐ESGAR guideline for diagnostic assessment in IBD Part 1: initial diagnosis, monitoring of known IBD, detection of complications. J Crohns Colitis. 2019;13(2):144‐164. [DOI] [PubMed] [Google Scholar]
- 22. Shi X, Yang W, Wang N, Zhu J. Circulating JNK pathway‐associated phosphatase level correlates with decreased risk, activity, inflammation level and reduced clinical response to tumor necrosis factor‐alpha inhibitor in Crohn disease patients. Medicine (Baltimore). 2019;98(33):e16622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Song D, Zhu X, Wang F, Sun J. Longitudinal monitor of Jun N‐terminal kinase pathway associated phosphatase reflects clinical efficacy to triple conventional disease‐modifying anti‐rheumatic drugs treatment in rheumatoid arthritis patients. Inflammopharmacology. 2021;29(4):1131‐1138. [DOI] [PubMed] [Google Scholar]
- 24. Zhu Q, Zheng P, Zhou J, et al. Andrographolide affects Th1/Th2/Th17 responses of peripheral blood mononuclear cells from ulcerative colitis patients. Mol Med Rep. 2018;18(1):622‐626. [DOI] [PubMed] [Google Scholar]
- 25. Saez A, Gomez‐Bris R, Herrero‐Fernandez B, Mingorance C, Rius C, Gonzalez‐Granado JM. Innate lymphoid cells in intestinal homeostasis and inflammatory bowel disease. Int J Mol Sci. 2021;22(14):7618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Gao W, Gao L, Yang F, Li Z. Circulating JNK pathway‐associated phosphatase: a novel biomarker correlates with Th17 cells, acute exacerbation risk, and severity in chronic obstructive pulmonary disease patients. J Clin Lab Anal. 2022;36(1):e24153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Zeng J, Liu J, Qu Q, Zhao X, Zhang J. JKAP, Th1 cells, and Th17 cells are dysregulated and inter‐correlated, among them JKAP and Th17 cells relate to cognitive impairment progression in Alzheimer's disease patients. Ir J Med Sci. 2021. doi: 10.1007/s11845-021-02749-2. Online ahead of print. [DOI] [PubMed] [Google Scholar]
- 28. Yang Q, Zhuang J, Cai P, Li L, Wang R, Chen Z. JKAP relates to disease risk, severity, and Th1 and Th17 differentiation in Parkinson's disease. Ann Clin Transl Neurol. 2021;8(9):1786‐1795. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.


