Abstract
Background
Thyroid nodule prevalence is increasing lately, especially in diabetes, but the mechanism of which is not clear. In this study, we investigated if osteoprotegerin (OPG) is involved in the pathogenesis of thyroid nodules in diabetes.
Methods
A total of 7568 individuals with detailed information and ultrasound examination results were studied for the prevalence of thyroid nodules. Among them, 1883 were with type 2 diabetes and 5685 were non‐diabetic. Then, 1120 individuals were randomly selected for the measurement of OPG. Diabetic rats were made by feeding a high‐fat‐high‐fructose diet for 28 weeks. Rats fed with a normal diet were as controls. Fresh thyroid tissues were obtained and fixed, dehydrated, and embedded in paraffin for hematoxylin‐eosin staining and observing pathological changes. qPCR and western blot were used to detect OPG expression in rat thyroid tissues.
Results
We found that HbA1c is an independent risk factor for thyroid nodules (Exp [β] = 1.158, p < 0.001). The prevalence of thyroid nodules in type 2 diabetes was higher than that in non‐diabetes (53.9% vs. 46.7%, p < 0.001). Serum OPG levels were significantly elevated in the diabetes group than in the non‐diabetes group (3160.17 pg/ml vs. 2819.39 pg/ml, p < 0.01). The expression of OPG increased significantly in the thyroid tissues of diabetic rats.
Conclusion
Osteoprotegerin may be associated with thyroid nodule development in diabetes.
Keywords: diabetes, osteoprotegerin, thyroid nodule
In human, the prevalence of thyroid nodules in type 2 diabetes was much higher than that in non‐diabetes. Serum OPG levels were significantly elevated in the diabetes group thanin the non‐diabetes group. In animals, expression of OPG increased significantly in the thyroid tissues of diabetic mice.
1. INTRODUCTION
Thyroid nodule, which is the most common endocrine nodule, is a severe clinical problem. With the improvement of physical examination technology, thyroid nodules are noted on ultrasound in up to 70% population. 1 , 2
Diabetes mellitus (DM), the most popular metabolic disease, is known to be a major disease that causes a variety of tumors and proliferation. 3 , 4 DM and thyroid disease are two closely related disorders. Previous studies have also shown that patients with type 2 DM (T2DM) had a significantly higher incidence of thyroid dysfunction. 5 , 6 , 7 Some studies pointed out that thyroid dysfunction is linked to increased thyroid nodule and even thyroid cancer incidence rate. 8 , 9 However, the relationship between thyroid nodules and T2DM is still unclear.
Osteoprotegerin (OPG) is from the tumor necrosis factor receptor superfamily, which inhibits osteoclastogenesis by binding to its ligand RANKL, thus preventing bone resorption. 10 The expression of OPG was increased in varieties of tissues, including heart, kidney, liver, stomach, bone, and thyroid gland. 11 , 12 Additionally, OPG has been shown to play an important role not only in regulating bone metabolism but also in tumorigenesis. 13 , 14 Our previous study demonstrated that serum OPG is associated with the formation of thyroid nodules. 15 These findings suggest that OPG is connected with the pathogenesis of thyroid nodules. However, the relationship between OPG and thyroid nodules in T2DM patients is still unclear.
In the present study, we conducted a two‐stage study to investigate whether OPG plays a role in thyroid nodule development in diabetes. The subjects of the human part were from a big survey conducted in Chongming District in shanghai. Since we could not get a thyroid tissue sample in the epidemic survey, to test the relationship between OPG and thyroid hyperplasia in vivo, we made type 2 diabetic rats by feeding a high‐fat‐high‐fructose diet and investigated the expression of OPG in thyroid tissue of diabetic rats. It is worthwhile to study the role of OPG in the pathogenesis of thyroid cell proliferation in diabetes.
2. METHODS
2.1. Subjects
The subjects in this study were from the REACTION study which was conducted in 2011. 16 The data on the human parts are from the baseline survey of subsamples from Chongming District in Shanghai, China. Detailed information was described in the previously study. 15 All participants had signed informed consent, the Institutional Review Board of Xinhua Hospital affiliated to Shanghai Jiaotong University School of Medicine approved this study. A total of 9930 subjects agreed to participate in our survey. After exclusion of individuals who did not have thyroid ultrasonographic examination data, 7568 individuals were studied. Among them, 1883 were diabetic according to WHO 1999 diabetes diagnosis criteria and 5685 were non‐diabetic. Then, 1120 individuals (278 diabetic and 842 non‐diabetic) were randomly selected for the measurement of OPG levels.
2.2. Measurement of OPG
Fasting serum samples were collected and stored at −80°C before determination. The serum OPG levels were measured in duplicate using a Duoset ELISA kit (DY805; R&D Systems) according to the manufacturer's instructions. The ELISA system had an intra‐assay coefficient of variation of 3%–9% and an inter‐assay coefficient of variation of 4%–10%, respectively.
2.3. Thyroid nodule measurement
Thyroid ultrasonographic inspection was completed by two ultrasonic experts using a high‐resolution B‐mode tomographic ultrasound system (Esaote Biomedica SpA) with a 10‐MHz transducer. A thyroid nodule is described as a discrete lesion within the thyroid gland that is radiologically distinct from the adjacent thyroid parenchyma. 17 Solid lesions and mixed cystic–solid lesions were included, we excluded all the cystic lesions.
2.4. Animals
Male Sprague–Dawley rats aged 4‐week‐old were purchased from Shanghai Slac Corporation. The animals were put in an environment with a constant temperature (22 ± 2°C) and a 12‐h light/dark cycle and fed with a regular diet and clean water ad libitum for 1 week. The rats were randomly assigned into two groups, the normal diet group (ND) and high‐fat‐high‐fructose diet group (HFHFD). During the whole experiment process, food intake was recorded, and body weight and fasting blood glucose (FBG) levels were monitored. A glucose tolerance test (GTT) was conducted after the rats were fed for 28 weeks. Oneweek later, the insulin tolerance test (ITT) was performed. At the end of the experiment, all animals were decapitated and the blood was gathered, centrifuged at 1000 g for 15 min at 4°C, and stored at −80°C for later analysis. The thyroid tissues were removed, frozen in liquid nitrogen, and stored at −80°C.
2.5. Blood parameters
Serum FINS levels were determined by ELISA kit (EZRMI‐13K, Merckmilipore). HOMA‐IR was calculated using the formula: FBG (mmol/L) × FINS (μU/L)/22.5. Serum total triglyceride (TG), total cholesterol (TC), low‐density lipoprotein cholesterol (LDL‐C), and high‐density lipoprotein cholesterol (HDL‐C) levels were detected using an automatic biochemical analyzer (ROCHE COBASc702).
2.6. H&E staining
Fresh thyroid tissues were fixed, dehydrated, and embedded in paraffin. Then, the paraffin sections in 4–6 μm were taken for hematoxylin–eosin (H&E) staining and observing pathological changes under a microscope.
2.7. Quantitative real‐time PCR analysis
Total RNA was extracted using Trizol Reagent (Invitrogen). cDNA was synthesized from total RNA using the kit (Takara) in accordance with the manufacturer’s instructions. Quantitative RT‐PCR was performed in triplicate using a real‐time PCR system (Applied Biosystems). The β‐actin gene was treated as the endogenous control. The relative gene expression levels were estimated using the 2−ΔΔCt method.
2.8. Western blot analysis
Thyroid tissues were lysed in RIPA buffer containing a protease inhibitor (Beyotime Institute of Biotechnology). A 30 μg of total protein was separated by a sodium dodecyl sulfate‐polyacrylamide gel electrophoresis and transferred to the polyvinylidene difluoride membrane. Then, the membrane was incubated with the OPG primary antibody overnight at 4°C after blocking with 5% skim milk. OPG primary antibody was purchased from Abcam. Blots were incubated with a proper horseradish conjugated secondary antibody (Beyotime Institute of Biotechnology) for 1 h at room temperature. Antibody‐bound protein was detected by ECL (Merkmillipore) and quantified using scanning densitometry. Relative protein expression levels were corrected with Tubulin expression.
2.9. Statistical analysis
SPSS 25 software (SPSS Inc.) was used for statistical analysis. Data were presented as means ± SD, median (interquartile range), or number (percent). We used Student's t test to compare the differences between groups, the chi‐square test to compare the prevalence rate of thyroid nodules in diabetes and non‐diabetes groups, and logistic regression to estimate risk factors for thyroid nodules. Potential confounders including age, gender, blood pressure, and BMI were all adjusted in the regression model. p value < 0.05 was interpreted as statistically significant.
3. RESULTS
In total, 9930 participants were recruited and agreed to participate in the survey. After excluding individuals who did not have thyroid ultrasonographic examination results, 7568 individuals were studied (2411 men and 5157 women). Among them, 1883 were diabetic (734 men and 1149 women) according to WHO 1999 diabetes diagnosis criteria and 5685 were non‐diabetic (1677 men and 4008 women); 1015 of the 1883 diabetic participants had thyroid nodules (340 men and 675 women) and 2652 of the 5685 non‐diabetic participants had thyroid nodules (678 men and 1974 women). The basic characteristics of the participants in the diabetic and non‐diabetic groups were shown in Table 1.
TABLE 1.
Clinical and laboratory characteristics according to diabetes status
| Characteristics | All | Diabetes | Non‐diabetes |
|---|---|---|---|
| N | 7568 | 1883 | 5685 |
| Age (year) | 56.27 ± 7.70 | 58.93 ± 6.97 | 55.39 ± 7.73 |
| Sex (Male/Female) | 2411/5157 | 734/1149 | 1677/4008 |
| BMI (kg/m2) | 24.71 ± 5.31 | 25.53 ± 3.50 | 24.44 ± 5.77 |
| Thyroid nodule (n) | 3667 | 1015 | 2652 |
| SBP (mm Hg) | 130.27 ± 20.30 | 136.83 ± 20.07 | 128.11 ± 19.91 |
| DBP (mm Hg) | 80.32 ± 10.55 | 81.75 ± 10.36 | 79.85 ± 10.57 |
| FBG (mmol/l) | 6.30 ± 1.69 | 8.10 ± 2.52 | 5.71 ± 0.52 |
| HbA1C (%) | 6.00 ± 1.01 | 6.95 ± 1.55 | 5.68 ± 0.41 |
| HOMA‐IR | 1.79 (1.28, 2.64) | 2.72 (1.79, 3.88) | 1.64 (1.22, 2.31) |
| HDL‐C (mmol/L) | 1.24 ± 0.32 | 1.21 ± 0.31 | 1.25 ± 0.32 |
| LDL‐C (mmol/L) | 2.62 ± 0.77 | 2.70 ± 0.81 | 2.60 ± 0.75 |
| TC (mmol/L) | 4.68 ± 1.02 | 4.84 ± 1.07 | 4.63 ± 1.01 |
| TG (mmol/L) | 1.36 (0.97, 2.01) | 1.65 (1.14, 2.45) | 1.29 (0.93, 1.87) |
Note: Data are means ± SD or median (interquartile range).
3.1. HbA1c is an independent risk factor for thyroid nodules
The logistic regression analysis revealed that variables independently related to prevalent thyroid nodules were HbA1c (Exp [β] = 1.101, p < 0.001), age (Exp [β] = 1.042, p < 0.001), BMI (Exp [β] = 1.035, p < 0.001), and sex (Exp [β] = 1.687, p < 0.001) (see Table 2).
TABLE 2.
Logistic regression analysis showing variables independently associated with thyroid nodules
| Independent variables | β | Exp (β) | 95% CI | p Value |
|---|---|---|---|---|
| HbA1c | 0.147 | 1.158 | (1.106, 1.213) | <0.001 |
| Age | 0.041 | 1.042 | (1.036, 1.049) | <0.001 |
| BMI | 0.035 | 1.035 | (1.020, 1.051) | <0.001 |
| Sex | 0.523 | 1.687 | (1.521, 1.871) | <0.001 |
Note: The variables entered in the analysis also included SBP, DBP, TC, HDL‐C, LDL‐C, and TG which were all excluded from the model.
Increased serum OPG level is associated with increased prevalence of thyroid nodules in diabetes patients.
The prevalence of thyroid nodules was assessed in type 2 diabetes patients and subjects without diabetes. We found that the prevalence of thyroid nodules in type 2 diabetes was increased significantly than that in non‐diabetes (53.9% vs. 46.7%, p < 0.001, Figure 1A).
FIGURE 1.
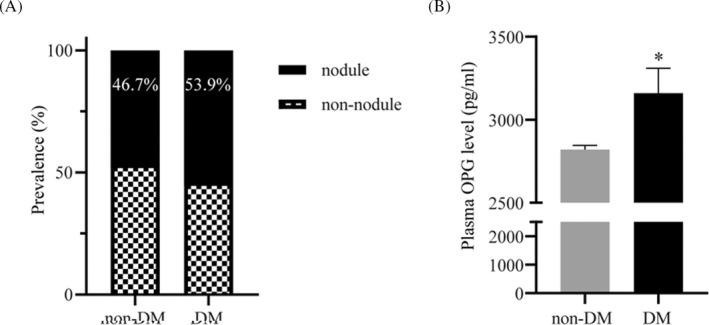
Serum OPG levels were up‐regulated significantly in diabetic patients compared with non‐diabetic patients. (A) Prevalence of thyroid nodules in diabetic patients and non‐diabetic patients. (B) Serum OPG levels in diabetic patients and non‐diabetic patients were analyzed by ELISA. *p < 0.01
Serum levels of OPG were compared between non‐diabetes and diabetes patients by ELISA. We found significantly increased OPG levels in the diabetes group than in the non‐diabetes group (3160.17 pg/ml vs. 2819.39 pg/ml, p < 0.001, Figure 1B). This suggested that OPG may play an important role in thyroid nodule development in diabetes.
3.2. Type 2 diabetes promotes proliferation of thyroid tissue in rats
To explore the correlation between type 2 diabetes and thyroid nodules, we invested the thyroid pathology in rats fed with HFHFD. Insulin resistance and glucose intolerance are present in type 2 DM rats. During 28 weeks of different diet feeding, the body weight every 4 weeks in the HFHFD group was higher than that in the ND group (Figure 2A). At the end of 28 weeks, oral GTT were performed and results showed the presence of glucose intolerance (Figure 2B). Furthermore, the ITT showed profound insulin resistance in HFHFD animals (Figure 2C). Hyperplasia of thyroid follicular was observed in the H&E stained thyroid tissue of HFHFD rats (Figure 2D). Additionally, insulin sensitivity was also assessed using fasting insulin level (FINS) and homeostasis model assessment of insulin resistance (HOMA‐IR). Both FINS and HOMA‐IR were significantly (p < 0.05) increased in HFHFD‐induced type 2 DM animals (Figure 3A,B).
FIGURE 2.
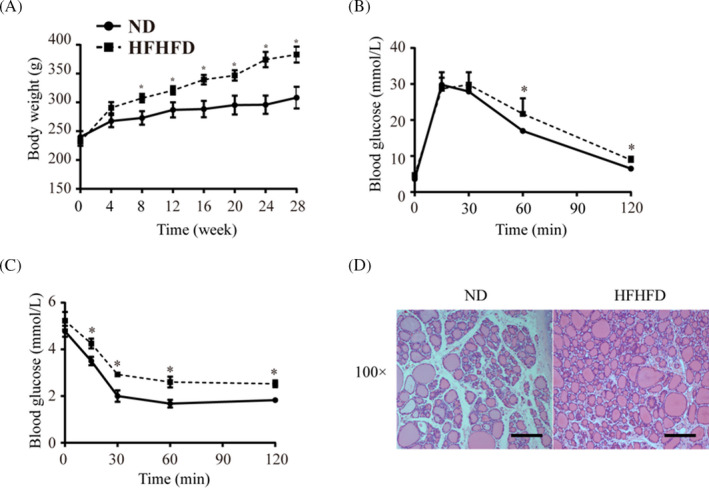
Insulin resistance promotes cell proliferation of the thyroid. (A) Body weight of rats fed with HFHFD and ND. (B) IPGTT of HFHFD‐fed and ND‐fed rats. (C) ITT of HFHFD‐fed and ND‐fed rats. (D) Representative H&E images of thyroid tissue of HFHFD rats and ND rats. H&E, hematoxylin and eosin. Scale bar = 100 μm. *p < 0.01
FIGURE 3.
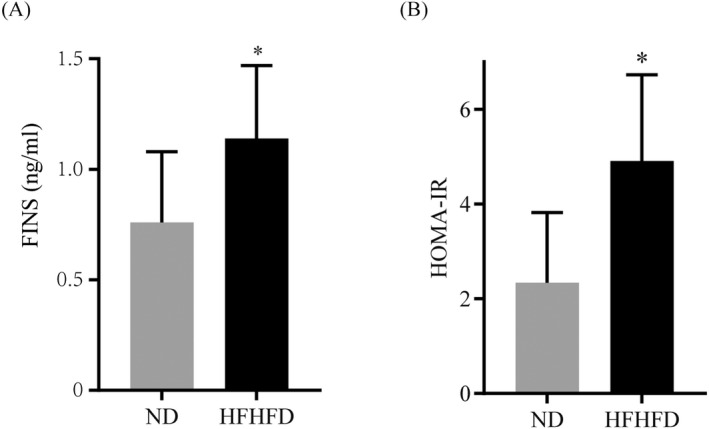
Insulin resistance was assessed after HFHFD feeding. (A) FINS of HFHFD‐fed and ND‐fed rats. (B) IPGTT of HFHFD‐fed and ND‐fed rats. *p < 0.01
3.3. Expression of OPG increases in thyroid glands of diabetes rats
We hypothesized that diabetes promotes the development of thyroid nodules via the OPG signal pathway. The expression of OPG in thyroid tissues of HFHFD and ND rats was examined. qPCR results showed that OPG mRNA expression level was obviously upregulated in the HFHFD group compared with the ND group (Figure 4A). Then, we analyzed the protein levels of OPG in thyroid tissues by Western blot and found that OPG protein levels were higher in thyroid tissues of HFHFD than that of ND group (Figure 4B,C).
FIGURE 4.
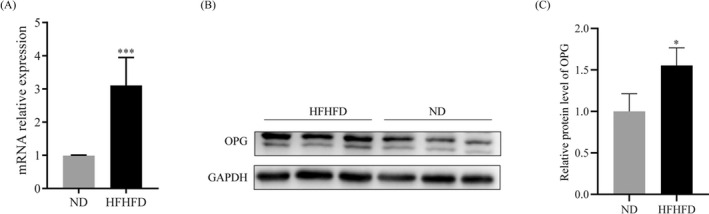
Osteoprotegerin is highly expressed in the thyroid tissue of HFHFD‐fed rats. (A) OPG mRNA level in rat thyroid tissues in the two groups analyzed by Q‐PCR. (B and C) OPG protein level in rat thyroid tissues in the two groups was determined by Western blot. *p < 0.01
4. DISCUSSION
As we all know, diabetes leads to the occurrence and progression of various tumors, and the mechanism of which has been a hotspot in recent years. However, studies focusing on how diabetes promotes thyroid tumors are limited. Previous studies have shown that human thyroid follicular cells can produce OPG. Abundant OPG expression is found in both benign and malignant removed thyroid specimens. 11 , 18 And our previous research results show that increased OPG levels are connected to the onset of thyroid nodules. 15 These studies suggest that OPG participants in the proliferation of thyroid cells.
Insulin resistance, the central step in the onset of type 2 diabetes, is a key reason for promoting thyroid cell proliferation. 19 , 20 , 21 , 22 Clinical studies have shown that insulin resistance in patients with thyroid nodules is much more severe than that in non‐thyroid nodule patients, and there is a significant correlation between insulin resistance and thyroid nodules. 23 In this study, we confirmed the higher incidence of thyroid nodules in diabetic patients than that in non‐diabetic patients, consistent with the results of our previous meta‐analysis. 24 The specific mechanism by which insulin resistance promotes thyroid cell proliferation is not yet clear.
Studies have found that blood OPG levels in patients with metabolic syndrome are significantly increased, 25 , 26 and our research has also found that blood OPG levels in type 2 diabetic patients are significantly higher than that in normal controls. 27 Studies have confirmed that in obese patients, insulin resistance is positively correlated with serum OPG levels. These findings suggest that OPG and HOMA‐IR are significantly positively correlated, 28 OPG may be one of the serum markers of insulin resistance. 29 , 30 We herein observed an obvious up‐regulation of OPG in diabetic serum, which further confirmed that OPG may media the incidence of thyroid nodules in diabetic patients.
Therefore, we hypothesize that OPG is involved in the proliferation of thyroid cells caused by insulin resistance and promotes the occurrence of thyroid nodules. And our in vivo study showed that insulin resistance could promote thyroid cell proliferation, with the increased expression of OPG in the thyroid gland. Therefore, it may be specific for OPG in the correlation between insulin and thyroid nodules.
According to previous reports, hyperinsulinemia itself can promote cell proliferation through the insulin receptor and mitogen‐activated protein kinase pathway. Studies have found that OPG/RANKL/RANK and proteoglycans polymerize in a certain proportion to form a large complex, which can further activate the ERK and p38 signal transduction pathways. 31 After activation, it can further activate the downstream targeting molecule to inhibit cell apoptosis and promote cell proliferation. 32 , 33 Other studies have shown that OPG can promote angiogenesis. 34 These may be the mechanisms by which insulin resistance promotes the occurrence of thyroid nodules through OPG, but the specific molecular mechanism still needs further study.
In summary, we found that the thyroid nodule prevalence rate was higher in diabetes than that in non‐diabetes, OPG levels were significantly increased in diabetic subjects compared to non‐diabetic participants. In our previous study, we found that OPG is related to thyroid nodules. According to all these findings, we think that OPG may be the factor that mediated the elevated thyroid nodule prevalence in diabetes. Consistent with this, we also found thyroid cell proliferation in diabetic rats and increased expression of OPG in thyroid tissues of diabetic rats, which further support our conclusion.
The advance of this study is that we tried to connect OPG with increased thyroid prevalence in diabetes. However, our research also has some limitations. One of the limitations is lacking cytological and/or histopathological data because we could not conduct thyroid biopsy in such a big population. Another limitation is that the response rate of women was higher than men, resulting in more women participants in this study. The third limitation is that we did not explore the detailed pathway through which OPG effects. In future study, we will continue to carry out research on this subject.
AUTHOR CONTRIBUTIONS
Hongmei Zhang Qing Su and Li Qin designed this experiment. Hui Ran and Dazhi Huang wrote the manuscript. Yixin Niu, Weiwei Zhang, and Xiaoyong Li participated in the epidemiological survey, collecting the data. Ning Lin and Zhen Yang analyzed the data. Hongmei Zhang and Hui Ran conducted the animal experiment. Dazhi Huang carried out the western blot experiment. All authors had read the manuscript and approved the final version.
FUNDING INFORMATION
This work was supported by Shanghai Pujiang Program (2019PJD033).
CONFLICT OF INTEREST
The authors declare none competing interests.
INFORMED CONSENT
Written informed consent was obtained from all subjects before recruitment.
Huang D, Niu Y, Zhang W, et al. OPG is associated with thyroid nodule development in type 2 diabetes. J Clin Lab Anal. 2022;36:e24615. doi: 10.1002/jcla.24615
Contributor Information
Hui Ran, Email: ranhui0928@163.com.
Hongmei Zhang, Email: zhanghongmei02@xinhuamed.com.cn.
DATA AVAILABILITY STATEMENT
The datasets used and analyzed during the current study are available from the corresponding author on request.
REFERENCES
- 1. Walsh JP. Managing thyroid disease in general practice. Med J Aust. 2016;205(4):179‐184. [DOI] [PubMed] [Google Scholar]
- 2. Wong R, Farrell SG, Grossmann M. Thyroid nodules: diagnosis and management. Med J Aust. 2018;209(2):92‐98. [DOI] [PubMed] [Google Scholar]
- 3. Tsilidis KK, Kasimis JC, Lopez DS, Ntzani EE, Ioannidis JP. Type 2 diabetes and cancer: umbrella review of meta‐analyses of observational studies. BMJ. 2015;350:g7607. [DOI] [PubMed] [Google Scholar]
- 4. Shlomai G, Neel B, LeRoith D, Gallagher EJ. Type 2 diabetes mellitus and cancer: the role of pharmacotherapy. J Clin Oncol. 2016;34(35):4261‐4269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Brandt F, Thvilum M, Almind D, et al. Morbidity before and after the diagnosis of hyperthyroidism: a nationwide register‐based study. PloS One. 2013;8(6):e66711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Gronich N, Deftereos SN, Lavi I, Persidis AS, Abernethy DR, Rennert G. Hypothyroidism is a risk factor for new‐onset diabetes: a cohort study. Diabetes Care. 2015;38(9):1657‐1664. [DOI] [PubMed] [Google Scholar]
- 7. Thvilum M, Brandt F, Almind D, Christensen K, Brix TH, Hegedüs L. Type and extent of somatic morbidity before and after the diagnosis of hypothyroidism. A nationwide register study. PloS One. 2013;8(9):e75789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Polyzos SA, Kita M, Efstathiadou Z, et al. Serum thyrotropin concentration as a biochemical predictor of thyroid malignancy in patients presenting with thyroid nodules. J Cancer Res Clin Oncol. 2008;134(9):953‐960. [DOI] [PubMed] [Google Scholar]
- 9. Cappelli C, Pirola I, Gandossi E, et al. Could serum TSH levels predict malignancy in Euthyroid patients affected by thyroid nodules with indeterminate cytology? Int J Endocrinol. 2020;2020:7543930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Khosla S. Minireview: the OPG/RANKL/RANK system. Endocrinology. 2001;142(12):5050‐5055. [DOI] [PubMed] [Google Scholar]
- 11. Sood SK, Balasubramanian S, Higham S, Fernando M, Harrison B. Osteoprotegerin (OPG) and related proteins (RANK, RANKL and TRAIL) in thyroid disease. World J Surg. 2011;35(9):1984‐1992. [DOI] [PubMed] [Google Scholar]
- 12. Montagnana M, Lippi G, Danese E, Guidi GC. The role of osteoprotegerin in cardiovascular disease. Ann Med. 2013;45(3):254‐264. [DOI] [PubMed] [Google Scholar]
- 13. Sarink D, Schock H, Johnson T, et al. Circulating RANKL and RANKL/OPG and breast cancer risk by ER and PR subtype: results from the EPIC cohort. Cancer Prev Res (Phila). 2017;10(9):525‐534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Infante M, Fabi A, Cognetti F, Gorini S, Caprio M, Fabbri A. RANKL/RANK/OPG system beyond bone remodeling: involvement in breast cancer and clinical perspectives. J Exp Clin Cancer Res. 2019;38(1):12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Zhang H, Yang Z, Zhang W, et al. Higher serum osteoprotegerin levels in subjects with thyroid nodules. Endocr Pract. 2016;22(4):412‐419. [DOI] [PubMed] [Google Scholar]
- 16. Ning G, Reaction Study G . Risk evaluation of cAncers in Chinese diabeTic individuals: a lONgitudinal (REACTION) study. J Diabetes. 2012;4(2):172‐173. [DOI] [PubMed] [Google Scholar]
- 17. American Thyroid Association Guidelines Taskforce on Thyroid N, Differentiated Thyroid Cancer , Cooper DS, Doherty GM, et al. Revised American Thyroid Association management guidelines for patients with thyroid nodules and differentiated thyroid cancer. Thyroid. 2009;19(11):1167‐1214. [DOI] [PubMed] [Google Scholar]
- 18. Heymann MF, Riet A, Le Goff B, Battaglia S, Paineau J, Heymann D. OPG, RANK and RANK ligand expression in thyroid lesions. Regul Pept. 2008;148(1–3):46‐53. [DOI] [PubMed] [Google Scholar]
- 19. Panagiotou G, Komninou D, Anagnostis P, et al. Association between lifestyle and anthropometric parameters and thyroid nodule features. Endocrine. 2017;56(3):560‐567. [DOI] [PubMed] [Google Scholar]
- 20. Xu W, Chen Z, Li N, et al. Relationship of anthropometric measurements to thyroid nodules in a Chinese population. BMJ Open. 2015;5(12):e008452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Zheng L, Yan W, Kong Y, Liang P, Mu Y. An epidemiological study of risk factors of thyroid nodule and goiter in Chinese women. Int J Clin Exp Med. 2015;8(7):11379‐11387. [PMC free article] [PubMed] [Google Scholar]
- 22. Diez JJ, Iglesias P. An analysis of the relative risk for goitre in euthyroid patients with type 2 diabetes. Clin Endocrinol (Oxf). 2014;80(3):356‐361. [DOI] [PubMed] [Google Scholar]
- 23. Heidari Z, Mashhadi MA, Nosratzehi S. Insulin resistance in patients with benign thyroid nodules. Arch Iran Med. 2015;18(9):572‐576. [PubMed] [Google Scholar]
- 24. Zhang HM, Feng QW, Niu YX, Su Q, Wang X. Thyroid nodules in type 2 diabetes mellitus. Curr Med Sci. 2019;39(4):576‐581. [DOI] [PubMed] [Google Scholar]
- 25. Bernardi S, Fabris B, Thomas M, et al. Osteoprotegerin increases in metabolic syndrome and promotes adipose tissue proinflammatory changes. Mol Cell Endocrinol. 2014;394(1–2):13‐20. [DOI] [PubMed] [Google Scholar]
- 26. Perez de Ciriza C, Moreno M, Restituto P, et al. Circulating osteoprotegerin is increased in the metabolic syndrome and associates with subclinical atherosclerosis and coronary arterial calcification. Clin Biochem. 2014;47(18):272‐278. [DOI] [PubMed] [Google Scholar]
- 27. Niu Y, Yang Z, Li X, et al. Association of osteoprotegerin with impaired glucose regulation and microalbuminuria: the REACTION study. BMC Endocr Disord. 2015;15:75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Suliburska J, Bogdanski P, Gajewska E, Kalmus G, Sobieska M, Samborski W. The association of insulin resistance with serum osteoprotegerin in obese adolescents. J Physiol Biochem. 2013;69(4):847‐853. [DOI] [PubMed] [Google Scholar]
- 29. Ayina Ayina CN, Sobngwi E, Essouma M, et al. Osteoprotegerin in relation to insulin resistance and blood lipids in sub‐Saharan African women with and without abdominal obesity. Diabetol Metab Syndr. 2015;7:47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Duan P, Yang M, Wei M, Liu J, Tu P. Serum Osteoprotegerin is a potential biomarker of insulin resistance in Chinese postmenopausal women with prediabetes and type 2 diabetes. Int J Endocrinol. 2017;2017:8724869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Baud'huin M, Duplomb L, Ruiz Velasco C, Fortun Y, Heymann D, Padrines M. Key roles of the OPG‐RANK‐RANKL system in bone oncology. Expert Rev Anticancer Ther. 2007;7(2):221‐232. [DOI] [PubMed] [Google Scholar]
- 32. Peng XX, Zhang SH, Wang XL, et al. Panax Notoginseng flower saponins (PNFS) inhibit LPS‐stimulated NO overproduction and iNOS gene overexpression via the suppression of TLR4‐mediated MAPK/NF‐kappa B signaling pathways in RAW264.7 macrophages. Chin Med. 2015;10:15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Barroso‐González J, Auclair S, Luan S, et al. PACS‐2 mediates the ATM and NF‐κB‐dependent induction of anti‐apoptotic Bcl‐xL in response to DNA damage. Cell Death Differ. 2016;23(9):1448‐1457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Goswami S, Sharma‐Walia N. Osteoprotegerin secreted by inflammatory and invasive breast cancer cells induces aneuploidy, cell proliferation and angiogenesis. BMC Cancer. 2015;15:935. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets used and analyzed during the current study are available from the corresponding author on request.


