Abstract
Background
Urinary tract infections (UTIs) and bacterial resistance to antibiotics is global health problem and a threat to public health in many countries.
Aims
The study aimed to determine the prevalence of MDR Escherichia coli and Klebsiella pneumoniae in UTI patients.
Materials & methods
The midstream urine samples of 120 patients were collected and cultured as described by the protocols at the respective sample collection sites on MacConkey Blood agar. Samples were tested by using the fully automated VITEK 2 Compact system for Gram‐negative identification and detection of antimicrobial susceptibility of microorganisms.
Results
The most prevalent pathogen was E. coli, which was found in 82 (68.3%) urine samples, followed by K. pneumonia, found in 38 (31.7%) urine samples. As far as antibiotic resistance is concerned, E. coli isolates were found to be highly resistant for ceftriaxone (89.0% of the isolates), ampicillin (86.6%), levofloxacin (82.9%), cefotaxime (79.3%), aztreonam (74.4%), ceftazidime (68.3%) and gentamicin, piperacillin, and trimethoprim‐sulfamethoxazole, 54.9 and 53.7%, respectively. The E. coli isolates were found to be relatively less resistant to imipenem (2.4%), cefepime (34.1%), and ciprofloxacin (35.4%). For K. pneumonia isolates, high resistance rates were observed for piperacillin (81.6%), levofloxacin (78.9%), ampicillin (76.3%), cefotaxime (73.7%), trimethoprim‐sulfamethoxazole (71.1%), ceftazidime (65.8%), gentamicin (63.2%), cefepime (50.0%), and aztreonam (44.7%). However, moderate resistance rates were detected for these were found to be less resistant for imipenem (13.2%), ceftriaxone (31.6%), and ciprofloxacin (36.8%).
Conclusion
E. coli and K. pneumoniae from the clinical isolates displayed high resistance to many antibiotics in UTI patients.
Keywords: antibiotic resistance, E. coli, Klebsiella, MDR, UTIs
E. coli isolates were found to be highly resistant for ceftriaxone (89.0% of the isolates), ampicillin (86.6%), levofloxacin (82.9%), cefotaxime (79.3%), aztreonam (74.4%), ceftazidime (68.3%) and gentamicin, piperacillin, and trimethoprim‐sulfamethoxazole, 54.9 and 53.7% respectively. The E. coli isolates were found to be relatively less resistant to imipenem (2.4%), cefepime (34.1%), and ciprofloxacin (35.4%). For K. pneumonia isolates, high resistance rates were observed for piperacillin (81.6%), levofloxacin (78.9%), ampicillin (76.3%), cefotaxime (73.7%), trimethoprim‐sulfamethoxazole (71.1%), ceftazidime (65.8%), gentamicin (63.2%), cefepime (50.0%), and aztreonam (44.7%). However, moderate resistance rates were detected for these were found to be less resistant for imipenem (13.2%), ceftriaxone (31.6%), and ciprofloxacin (36.8%).
1. INTRODUCTION
Urinary tract infections (UTIs) are one of the most common bacterial infections found in humans after respiratory tract infections because used broad‐spectrum antibiotics and the indiscriminate use of antibiotics. 1 These account for nearly 150–200 million cases worldwide every year with approximately 40% females and 12% males experiencing at least one symptomatic episode of UTI in their lifetime. 2 These along with constituting 40%–50% of all the bacterial infections acquired in hospitals leading to increased morbidity and prolonged hospitalization also lead to substantial economic burden. 3 Patients with UTIs may be divided into two groups; complicated groups that occur without a causative agent, while uncomplicated infections occur in immunosuppression patients, diabetes mellitus, and anatomically or functionally abnormal urinary tracts. 4 The primary cause of UTI is Gram‐negative bacteria with the most common causative pathogen is Escherichia coli followed by other species such as Klebsiella pneumonia, and Proteus mirabilis. 5 Some Gram‐positive bacteria such as Enterococcus faecalis and Staphylococcus saprophyticus also cause UTIs. 6 , 7 Various antibiotics have been recommended by international guidelines for the treatment of UTI, which include nitrofurantoin monohydrate, trimethoprim‐sulfamethoxazole, fosfomycin trometamol, pivmecillinam, fluoroquinolones, and beta‐lactams. 8 However, indiscriminate and widespread use of antibiotics has led to the problem of antibiotic resistance in pathogens causing UTI such as extended‐spectrum beta‐lactamases (ESBL)‐producing Gram‐negative bacteria, that are mainly resistant to most of the available antibiotics except carbapenem group and these bacteria are increasing widely in the population. 9 This increased antibiotic resistance and appearance of multidrug resistant (MDR) pathogens due to inadequate use of antibiotics without testing for susceptibility have led to the situation of an ineffective UTI treatment. 9 Recently, several studies demonstrated that the probability of MDR in pathogens causing UTI in index infection increases when the use of antibiotics has been initiated four weeks to one year before the index infection. Resistance was found to be more strongly associated with the use of antimicrobials such as fluoroquinolones and antipseudomonal penicillin as well as prolonged use of any antimicrobial before the presentation of UTI. 10 In term of the drug resistance, authors found that the prevalent antibiotic resistance among bacteria is due to the production of many Gram‐negative bacteria toxins that especially belonging to Enterobacteriaceae. 11
As per the information provided by European Antimicrobial Resistance Surveillance Network (EARS‐Net), the main European Union (EU) surveillance system for MDR pathogens isolated from the infection in the bloodstream from many countries, the E. coli and K. pneumonia were found to be the most common pathogens, showing resistance to the most common classes of antibacterial used in clinics; and therefore, these drugs could not be used for treatments of UTIs. Which is leading to the development of MDR bacterial strains resistance to antibiotics with a rise in mortality due to complications associated with UTIs. 11 , 12 Data from Italy showed high resistance rates against aminopenicillin, aminoglycosides and fluoroquinolones in E. coli isolates and against aminoglycosides and fluoroquinolones in K. pneumonia isolates. 13
Using enzyme production has been a well‐established mechanism for resistance to antibiotics by bacteria. The synthesis of β‐lactamase or Extended‐spectrum β‐lactamases (ESBLs) by these Gram‐negative bacteria has been considered as the main mechanism for resistance to β‐lactam drugs. 14 These pathogens carry genes that encode for resistance to other classes of antibiotics as well and are, therefore, classified as MDR organisms. 15 Excessive use of antibiotics has been a major factor for resistance of these ESBL producing isolates to the third‐generation cephalosporins. 14 As discussed above, these enzymes could even break fourth‐generation cephalosporins at the β‐lactam ring except carbapenems and cephamycin. 11 As per a report by Padmini et al., the E. coli and K. pneumoniae that produce ESBL are resistant to a broad range of β‐lactam drugs, also including third‐generation cephalosporins. 15 Along with β‐lactamases, bacteria also produce various other enzymes, which could metabolize the antibiotics such as aminoglycoside‐modifying enzymes and chloramphenicol acetyltransferases, which act by inactivating the antibiotics before they could confer their effect. Modifications in the target site are also a mechanism in which the efficacy of antibiotics is lost due to their inability to bind to their target. 16 , 17
The above literature suggests that understanding the concerned bacterial pathogens causing UTIs and the analysis of their susceptibility to antibiotics is very important for a successful empirical antibiotic‐treatment regimen. Moreover, the regional differences in the pattern of antibiotic—resistance should also be studied to choose antimicrobials for the treatment of UTI based on the local resistance profile of these uropathogens. Published data suggested a high prevalence of MDR in most common species of bacteria causing UTI such as E. coli and K. pneumoniae.
Keeping all these points in consideration, the present study was designed with an intent to determine the prevalence of MDR E. coli and K. pneumoniae in UTI patients and to determine antibiotic resistance patterns for risk assessment for recurrent UTIs, and help to facilitate the appropriate antibiotic selection for UTIs.
2. METHODS
2.1. Collection of samples
The samples were collected from May 2020 to June 2021 from private clinics. Written consent was obtained from all the patients participating in this study. This study included a total of 120 patients (46 males and 74 females) suffering from recurrent urinary tract infections at different ages. Patients with well‐known risk factors for UTI and abnormalities of the urinary system and vesicoureteral reflux were excluded from the study. All the patients enrolled in the study had not taken any antibiotic at least three days before the sampling.
2.2. Urine culture and Antibiotic susceptibility test
The midstream urine samples (120) were collected and cultured according to the protocols at the respective sample collection sites on MacConkey blood agar and incubated at 37°C overnight to detect the presence of bacterial colonies for samples. MacConkey agar used because it is a selective and differential medium that helps us diagnose Gram‐negative rods and Enterobacteriaceae based on lactose fermentation and is used to culture many samples such as urine and other samples. The presence of bacterial colonies ≥105 CFU/ml was considered an infection with UTI. After incubation, the morphological characteristics for colonies were determined using gram stain and some biochemical tests. The antibiotic susceptibility was tested by using the fully automated VITEK 2 Compact system for Gram‐negative identification to determine the minimum inhibitory concentration (MIC) and antimicrobial susceptibility test to ceftriaxone (CRO; 30 μg), ampicillin (AM; 10 μ), levofloxacin (10 μg), cefotaxime (CTX; 30 μ), aztreonam (ATM; 30 μg), ceftazidime (CAZ; 30 μg), gentamicin (CN; 10 μg), piperacillin (TPZ; 30 μg), trimethoprim‐sulfamethoxazole (STX; 1.25/23.75 μg), imipenem (IPM; 10 μg), cefepime (FEP; 30 μg), and ciprofloxacin (CIP; 5 μg). Five to ten colonies were selected from each sample. All susceptibility results are interpreted according to the Clinical Laboratory Standards Institute (CLSI) guidelines. Furthermore, quality control by American type culture collection (ATCC) strains in hospital laboratory was done.
Some of isolated samples contain both E. coli and K. pneumonia, approximately, two isolates (of all isolated samples).
In addition, in the culture of these samples we found Proteus mirabilis, Enterobacter, Enterococcus, Enterobacter cloacae, Enterococcus faecalis, besides, Gram‐negative organisms (E. coli, and K. pneumonia); however, Gram‐positive organisms (ex; S. aureus) also isolated.
If bacterial isolates are able to develop resistance to three or more of the antibiotic are called multiple drugs resistance (MDR). 18
2.3. Statistical analysis
All the data were analyzed using SPSS software. Calculations of mean values and standard deviation (SD) were made for the characterization of the study population. We tested the resistance of MDR E. coli and K. pneumoniae to many antibiotics. The statistical significance of the difference of data was assessed by a Chi‐square test. Unpaired t‐test was used to compered between groups of two isolates. p values <0.05 were considered statistically significant.
3. RESULTS
From all the urine culture samples tested, 82 (68.3%) were E. coli, and 38 (31.7%) were K. pneumonia. Among relation to gender, E. coli infection found in 37 (30.8%) male and 45 (37.5%) female, whereas K. pneumonia infection documented in 9 (7.5%) male, and 29 (24.2%) female, with a statistical significant difference (Chi‐square = 4.1822; p = 0.04; Table 1).
TABLE 1.
Distribution of the isolates according to gender
| Gender | E. coli | K. pneumoniae | Total | |||
|---|---|---|---|---|---|---|
| No. | % | No. | % | No. | % | |
| Male | 37 | 30.8 | 9 | 7.5 | 46 | 38.3 |
| Female | 45 | 37.5 | 29 | 24.2 | 74 | 61.7 |
| Total | 82 | 68.3 | 38 | 31.7 | 120 | 100 |
| Chi‐square = 4.1822; p = 0.04 | ||||||
In relation to the age, the overall mean age of patients infected with E. coli was (32.16 ± 14.39 years), which was significantly younger than overall mean age of patients infected with K. pneumonia (37.24 ± 14.37 years), (t‐test = 4.361; 95% CI = 5.791–15.73; p = 0.024). In addition, female in this study were infected with E. coli very early in their life than other. In the other words, the mean age of female samples isolated with E. coli was (26.48 ± 14.57 years), while age of female with K. pneumonia was (31.33 ± 14.15 years) with a highly significant difference (t‐test = 12.233; 95% CI = 11.565–25.432; p = 0.0001). Furthermore, mean age of male samples isolated with E. coli was (30.0 ± 14.36 years), while age of male with K. pneumonia was (36.5 ± 14.12 years) with a highly significant difference (t‐test = 10.761; 95% CI = 10.884–30.782; p = 0.0001; Table 2).
TABLE 2.
Distribution of the isolates according to age
| E. coli | K. pneumonia | Unpaired t‐test | 95% CI | p‐value | |
|---|---|---|---|---|---|
|
Age (years) Mean ± SD |
32.16 ± 14.39 | 37.24 ± 14.37 | 4.361 | 5.791–15.73 | 0.024 |
|
Age of male (years) Mean ± SD |
30.0 ± 14.36 | 36.5 ± 14.12 | 10.761 | 10.884–30.782 | 0.0001 |
|
Age of female (years) Mean ± SD |
26.48 ± 14.57 | 31.33 ± 14.15 | 12.233 | 11.565–25.432 | 0.0001 |
The E. coli isolates were found to be highly resistant to ceftriaxone (n = 73; 89.0%), ampicillin (n = 71; 86.6%), levofloxacin (n = 68; 82.9%), cefotaxime (n = 65; 79.3%), aztreonam (n = 61; 74.4%), ceftazidime (n = 56; 68.3%), gentamicin (n = 45; 54.9%), piperacillin (n = 44; 53.7%), and trimethoprim‐sulfamethoxazole (n = 44; 53.7%), respectively. The E. coli isolates were found to be relatively less resistant to imipenem (n = 2; 2.4%), cefepime (n = 28; 34.1%), and ciprofloxacin (n = 29; 35.4%), respectively. For K. pneumonia isolates, high resistance rates were observed for piperacillin (n = 31; 81.6%), levofloxacin (n = 30; 78.9%), ampicillin (n = 29; 76.3%), cefotaxime (n = 27; 73.7%), trimethoprim‐sulfamethoxazole (n = 28; 71.1%), ceftazidime (n = 25; 65.8%), gentamicin (n = 24; 63.2%), cefepime (n = 19; 50.0%), and aztreonam (n = 17; 44.7%), respectively.
However, moderate resistance rates were detected for these were found to be less resistant for imipenem (n = 5; 13.2%), ceftriaxone (n = 12; 31.6%), and ciprofloxacin (n = 14; 36.8%), respectively (Figure 1). The bacterial isolates were resistant to more than three antibiotics, therefore, classified as MDR.
FIGURE 1.
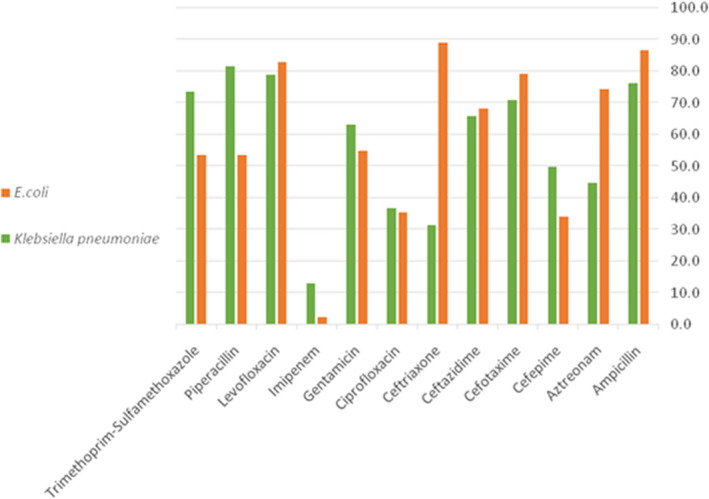
Rates of antimicrobial resistance of the collected E. coli and K. pneumoniae
4. DISCUSSION
Urinary tract infection is one of the most common infectious diseases in the world and is caused by both Gram‐positive and Gram‐negative bacteria. 19 The study of bacterial resistance to multiple antibiotics is crucial in deciding on the appropriate treatment for the infection resulting from it as the spread of these MDR bacterial strains poses a great risk to the health of individuals of all ages. The majority of individuals are exposed to urinary tract infections due to changes in various anatomical and physiological characteristics.
In this study, we observed that the infection rates in females (61.7%) were higher than in males (38.3%). In this study, the overall mean age of patients infected with E. coli was (32.16 ± 14.39 years) significantly younger than overall mean age of patients with K. pneumonia (37.24 ± 14.37 years), (t‐test = 4.361; 95% CI = 5.791–15.73; p = 0.024). In other words, the mean age of female samples isolated with E. coli is (26.48 ± 14.57 years), while age of female with K. pneumonia is (31.33 ± 14.15 years) with a highly significant difference (p = 0.0001). Furthermore, mean age of male samples isolated with E. coli is (30.0 ± 14.36 years), while mean age of male with K. pneumonia is (36.5 ± 14.12 years) with a highly significant difference (p = 0.0001). The results of this study were in congruence with another study by Regmi et al, where they observed growth culture (23.75%) in the younger age group 21–30 years and the age group 41–50 years (13.66%) from urine samples. 20 In Saudi Arabia, it was observed that the incidence of urinary tract infection in females was more than that of males at 82.5% with the mean (SD) age of 36.5 (12.2) years. 21 In Isfahan, Iran, a study showed that the number of females with UTIs was 263 (89%), which was much more than the males, which were 32 (11%). 22 The high incidence of UTIs in women is due to multiple reasons including according to female anatomy, female urethra is much shorter in length in women than men, more sensitive skin of the external urethral meatus in women is mostly mucosa (moist tissue lining the inside of the vagina, and this skin is thinner and more sensitive than most of the skin on the body), female urethra is located closer to the rectum, sexual contact can allow bacteria near the vagina to get into the urethra, vaginal irritation due to spermicide or a diaphragm for birth control, menopause, and pregnancy, which make women more susceptible to infection. 23 The most common prevalent pathogens in the urine isolate were E. coli (n = 82; 68.3%) followed by K. pneumonia (n = 38; 31.7%). Previous studies differed from ours in recording prevalence rates of MDR bacteria isolated from urine samples. The results of the present study are roughly consistent with a study in Saudi Arabia, showing that the most prevalent bacteria were E. coli (66%) and K. pneumoniae (11.4%), respectively. 24 Similarly, in another study, Regmi et al, observed that most of the isolates were also E. coli (65.84%) and K. pneumoniae (12.42%). 20 On the other hand, in Isfahan, Iran, the prevalence rates of E. coli and K. pneumoniae were 50.1% and 23.3%, respectively, 22 whereas in Nepal, Kattel et al., reported the prevalence of E. coli (59.59%) and K. pneumoniae (10.78%). 25 A contrary report showed that the majority of isolates were K. pneumoniae (n = 116; 76.3%) followed by E. coli (n = 26; 17.1%). The study from Saudi Arabia showed, predominance of E. coli (n = 157; 37.6%), followed by K. pneumoniae (n = 70; 16.7%). 21 Most of the studies agreed on the ordering of the uropathogens from most common to least, as they included E. coli, Klebsiella, Pseudomonas, Enterobacter, and possible Candida sp. 26 , 27
Overall, our findings suggest that there is a large percentage of resistance shown by bacteria isolated from the urine samples. E. coli isolates showed high resistance to ceftriaxone (89%), ampicillin (86.6%), levofloxacin (82.9%), cefotaxime (79.3%), aztreonam (74.4%), ceftazidime (68.3%), gentamicin (54.9%), piperacillin (53.7%), and trimethoprim‐sulfamethoxazole (53.7%). Though they appeared less resistant for imipenem (2.4%), cefepime (34.1%), and ciprofloxacin (35.4%), respectively. K. pneumonia isolates were highly resistant for piperacillin (81.6%), levofloxacin (78.9%), ampicillin (76.3%), cefotaxime (73.7%), trimethoprim‐sulfamethoxazole (71.1%), ceftazidime (65.8%), gentamicin (63.2%,), cefepime (50.0%), and aztreonam (44.7%), respectively. However, they found to be less resistant for imipenem (13.2%), ceftriaxone (31.6%,), and ciprofloxacin (36.8%), respectively. A study conducted in the USA showed an increase in the rates of ampicillin (97.8%), trimethoprim‐sulfamethoxazole (92.8%), and ciprofloxacin (38.8%) resistance, respectively. 28 Another study from the UK showed higher rates of ampicillin resistance (55%) in E. coli isolates. 29 In India, the resistance rates to trimethoprim‐sulfamethoxazole, gentamicin, and ciprofloxacin were 83.3%, 48.8%, and 46%, respectively. 30 Leski et al., 2016 showed high gentamycin (72.9%) and ciprofloxacin resistance (47.1%) resistance. 31 Alanazi et al, reported high ciprofloxacin (72.7%) and ampicillin (42.85%) resistance in their study. 32 All the studies differed in the resistance rates of bacteria that cause UTIs, which could be attributed to many factors, like the study population and differences in the geographical location. One of the reasons for the development of bacterial resistance to antibiotics is the indiscriminate use of antibiotics by the patient without any medical advice. This leads to the emergence of mutant strains, thus enhancing their ability to prevent drugs from reaching them. Numerous studies have shown that most urinary tract pathogens have altered their resistance to change over time in many countries. 7 , 16 , 33 , 34
E. coli and K. pneumoniae isolates from urinary tract infections have been discussed in several empirical kinds of literature and conducted in many countries. 35 , 36 , 37 , 38 However, the paucity of investigations into E. coli and K. pneumoniae isolated from patients with urinary tract infections in the Iraqi context remained rare. This study contributes to bridging the literature gap by investigating in‐depth such an issue in Basra.
This study is not free from limitations. Among the gaps in this study was whether the patient repeated the use of the same antibiotics when they were reinfected again with UTI without a prescription from medical advice or not. The current study focused on specific pathogens. It is an important to conduct other studies to study the resistance of other pathogens while evaluating the patterns of resistance spread in hospitals.
5. RECOMMENDATIONS
The spread of antibiotic resistance is a major health problem that must be taken to consideration because it has a significant impact on the health of the individual in society. This study recommended the need to reduce the indiscriminate use of antibiotics and the spread of resistant bacteria that cause UTIs and to develop a policy to be followed in hospitals and private clinics after the establishment of other studies. A requirement to detect bacterial strains resistant to antibiotics and evaluate the genetic basis of the resistance patterns spread inside Iraq and compare it with other countries.
6. CONCLUSION
In this study, our results demonstrated that urinary tract infections were higher in females than in males, (61.7% vs. 38.3%). The most prevalent pathogens found in the urine isolates were E. coli (82; 68.3%) followed by K. pneumonia (38; 31.7%). E. coli isolates exhibited high resistance rates for ceftriaxone, ampicillin, levofloxacin, cefotaxime, aztreonam, ceftazidime, gentamicin, piperacillin, and trimethoprim‐sulfamethoxazole, while it appeared less resistant for imipenem, cefepime, and ciprofloxacin. K. pneumonia isolates were also highly resistant to piperacillin, levofloxacin, ampicillin, cefotaxime, trimethoprim‐sulfamethoxazole, cefotaxime, ceftazidime, gentamicin, cefepime, and aztreonam, whereas, relatively less resistant to imipenem, ceftriaxone, and ciprofloxacin. Molecular investigation of genes encoding these antimicrobial resistance markers are highly recommended in order to better understand the molecular epidemiology of the collected isolates.
CONFLICT OF INTEREST
None.
Jalil MB, Al Atbee MYN. The prevalence of multiple drug resistance Escherichia coli and Klebsiella pneumoniae isolated from patients with urinary tract infections. J Clin Lab Anal. 2022;36:e24619. doi: 10.1002/jcla.24619
Contributor Information
Mays B. Jalil, Email: mays.basil@kunoozu.edu.iq.
Mohammed Younus Naji Al Atbee, Email: Mohammed.naji@uobasrah.edu.iq.
DATA AVAILABILITY STATEMENT
Mays Jalil. (2022). The prevalence of multiple drug resistance Escherichia coli and Klebsiella pneumoniae isolated from patients with urinary tract infections. https://doi.org/10.5281/zenodo.5822380
REFERENCES
- 1. Bischoff S, Walter T, Gerigk M, Ebert M, Vogelmann R. Empiric antibiotic therapy in urinary tract infection in patients with risk factors for antibiotic resistance in a German emergency department. BMC Infect Dis. 2018;18(1):56. doi: 10.1186/s12879-018-2960-9 PMID: 29373965; PMCID: PMC5787273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Micali S, Isgro G, Bianchi G, Miceli N, Calapai G, Navarra M. Cranberry and recurrent cystitis: more than marketing? Crit Rev Food Sci Nutr. 2014;54(8):1063‐1075. doi: 10.1080/10408398.2011.625574 PMID: 24499122. [DOI] [PubMed] [Google Scholar]
- 3. Asadi Karam MR, Habibi M, Bouzari S. Urinary tract infection: pathogenicity, antibiotic resistance, and development of effective vaccines against Uropathogenic Escherichia coli. Mol Immunol. 2019;108:56‐67. doi: 10.1016/j.molimm.2019.02.007 Epub 2019 Feb 18. PMID: 30784763. [DOI] [PubMed] [Google Scholar]
- 4. Nitzan O, Elias M, Chazan B, Saliba W. Urinary tract infections in patients with type 2 diabetes mellitus: a review of prevalence, diagnosis, and management. Diabetes Metab Syndr Obes. 2015;8:129‐136. doi: 10.2147/DMSO.S51792 PMID: 25759592; PMCID: PMC4346284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Flores‐Mireles AL, Walker JN, Caparon M, Hultgren SJ. Urinary tract infections: epidemiology, mechanisms of infection and treatment options. Nat Rev Microbiol. 2015;13(5):269‐284. doi: 10.1038/nrmicro3432 Epub 2015 Apr 8. PMID: 25853778; PMCID: PMC4457377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Säemann M, Hörl WH. Urinary tract infection in renal transplant recipients. Eur J Clin Invest. 2008;38(Suppl 2):58‐65. doi: 10.1111/j.1365-2362.2008.02014.x PMID: 18826483. [DOI] [PubMed] [Google Scholar]
- 7. Gupta K, Hooton TM, Naber KG, et al. International clinical practice guidelines for the treatment of acute uncomplicated cystitis and pyelonephritis in women: a 2010 update by the Infectious Diseases Society of America and the European Society for Microbiology and Infectious Diseases. Clin Infect Dis. 2011;52(5):e103‐e120. doi: 10.1093/cid/ciq257 PMID: 21292654. [DOI] [PubMed] [Google Scholar]
- 8. Adamus‐Białek W, Baraniak A, Wawszczak M, et al. The genetic background of antibiotic resistance among clinical uropathogenic Escherichia coli strains. Mol Biol Rep. 2018;45(5):1055‐1065. doi: 10.1007/s11033-018-4254-0 Epub 2018 Jul 14. PMID: 30008141; PMCID: PMC6156760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Khawcharoenporn T, Vasoo S, Singh K. Urinary tract infections due to multidrug‐resistant enterobacteriaceae: prevalence and risk factors in a Chicago Emergency Department. Emerg Med Int. 2013;2013:258517. doi: 10.1155/2013/258517 Epub 2013 Oct 31. PMID: 24307946; PMCID: PMC3844142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Saravanan M, Ramachandran B, Barabadi H. The prevalence and drug resistance pattern of extended‐spectrum β–lactamases (ESBLs) producing Enterobacteriaceae in Africa. Microb Pathog. 2018;114:180‐192. doi: 10.1016/j.micpath.2017.11.061 [DOI] [PubMed] [Google Scholar]
- 11. European Centre for Disease Prevention and Control . Antimicrobial resistance surveillance in Europe 2014. Annual Report of the European Antimicrobial Resistance Surveillance Network (EARS‐Net). ECDC; 2015. [Google Scholar]
- 12. Spellberg B, Bartlett JG, Gilbert DN. The future of antibiotics and resistance. N Engl J Med. 2013;368(4):299‐302. doi: 10.1056/NEJMp1215093 PMID: 23343059; PMCID: PMC3617123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Chaudhary U, Aggarwal R. Extended‐spectrum β‐lactamases (ESBL)—an emerging threat to clinical therapeutics. Indian J Med Microbiol. 2004;22(2):75‐80. doi: 10.1016/s0255-0857(21)02884-x [DOI] [PubMed] [Google Scholar]
- 14. Mazzariol A, Bazaj A, Cornaglia G. Multi‐drug‐resistant gram‐negative bacteria causing urinary tract infections: a review. J Chemother. 2017;29(sup1):2‐9. doi: 10.1080/1120009X.2017.1380395 PMID: 29271736. [DOI] [PubMed] [Google Scholar]
- 15. Padmini N, Ajilda AAK, Sivakumar N, Selvakumar G. Extended‐spectrum β‐lactamase producing Escherichia coli and Klebsiella pneumoniae: critical tools for antibiotic resistance pattern. J Basic Microbiol. 2017;57(6):460‐470. doi: 10.1002/jobm.201700008 Epub 2017 Apr 11. PMID: 28397262. [DOI] [PubMed] [Google Scholar]
- 16. Livermore DM, Pearson A. Antibiotic resistance: location, location, location. Clin Microbiol Infect. 2007;13(Suppl 2):7‐16. doi: 10.1111/j.1469-0691.2007.01724.x PMID: 17488371. [DOI] [PubMed] [Google Scholar]
- 17. Foxman B. The epidemiology of urinary tract infection. Nat Rev Urol 2010;7(12):653–60. 10.1038/nrurol.2010.190. PMID: 21139641. [DOI] [PubMed] [Google Scholar]
- 18. Gad GF, El‐Domany RA, Zaki S, Ashour HM. Characterization of Pseudomonas aeruginosa isolated from clinical and environmental samples in Minia, Egypt: prevalence, antibiogram and resistance mechanisms. J Antimicrob Chemother. 2007;60(5):1010‐1017. doi: 10.1093/jac/dkm348 Epub 2007 Sep 29. PMID: 17906321. [DOI] [PubMed] [Google Scholar]
- 19. Cohen‐Nahum K, Saidel‐Odes L, Riesenberg K, Schlaeffer F, Borer A. Urinary tract infections caused by multi‐drug resistant Proteus mirabilis: risk factors and clinical outcomes. Infection. 2010;38(1):41‐46. doi: 10.1007/s15010-009-8460-5 Epub 2009 Dec 7. PMID: 19998053. [DOI] [PubMed] [Google Scholar]
- 20. Regmi N, Kafle S, Paudyal R. Multi‐drug resistance uropathogens isolated from mid urine samples. J Inst Sci Technol. 2018;23(1):39‐42. [Google Scholar]
- 21. Al Wutayd O, Al Nafeesah A, Adam I, Babikir IH. The antibiotic susceptibility patterns of uropathogens isolated in Qassim. Saudi Arabia J Infect Dev Countries. 2018;12(11):946‐952. doi: 10.3855/jidc.10553 [DOI] [PubMed] [Google Scholar]
- 22. Shariatpanahi SS. Prevalence of Enterobacteriaceae involved in urinary tract infection and antimicrobial susceptibility pattern in adult outpatients in Isfahan. Third Iran Congr Med Bacteriol Milad Hosp Tehran; 2015. https://www.researchgate.net/publication/283794334_Prevalence_of_Enterobacteriaceae_involved_in_urinary_tract_infection_and_antimicrobial_susceptibility_pattern_in_adult_outpatients_in_Isfahan
- 23. August SL, De Rosa MJ. Evaluation of the prevalence of urinary tract infection in rural Panamanian women. PLoS One. 2012;7(10):e47752. doi: 10.1371/journal.pone.0047752 Epub 2012 Oct 19. PMID: 23094080; PMCID: PMC3477127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Ahmad S, Al‐Juaid NF, Alenzi FQ, Mattar EH, Bakheet OE‐S. Prevalence, antibiotic susceptibility pattern, and production of extended‐spectrum beta‐lactamases amongst clinical isolates of Klebsiella pneumoniae at armed forces Hospital in Saudi Arabia. J Coll Physicians Surg Pak. 2009;19:264‐265. 04.2009/JCPSP.264265. [PubMed] [Google Scholar]
- 25. Kattel HP, Acharya J, Mishra SK, Bp R, Pokhrel BM. Bacteriology of urinary tract infection among patient attending TU teaching hospital, Kathmandu, Nepal. J Nepal Assoc Med Lab Sci. 2008;9(1):25‐29. [Google Scholar]
- 26. Chopra I, Schofield C, Everett M, et al. Treatment of health‐care‐associated infections caused by gram‐negative bacteria: a consensus statement. Lancet Infect Dis. 2008;8(2):133‐139. doi: 10.1016/S1473-3099(08)70018-5 PMID: 18222164. [DOI] [PubMed] [Google Scholar]
- 27. Jaggi N, Sissodia P, Sharma L. Control of multidrug‐resistant bacteria in a tertiary care hospital in India. Antimicrob Resist Infect Control. 2012;1:23. doi: 10.1186/2047-2994-1-23 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Karlowsky JA, Kelly LJ, Thornsberry C, Jones ME, Sahm DF. Trends in antimicrobial resistance among urinary tract infection isolates of Escherichia coli from female outpatients in the United States. Antimicrob Agents Chemother. 2002;46(8):2540‐2545. doi: 10.1128/AAC.46.8.2540-2545.2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Bean DC, Krahe D, Wareham DW. Antimicrobial resistance in community and nosocomial Escherichia coli urinary tract isolates, London 2005‐2006. Ann Clin Microbiol Antimicrob. 2008;7:13. doi: 10.1186/1476-0711-7-13 PMID: 18564430; PMCID: PMC2440378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Manikandan S, Ganesapandian S, Singh M, Kumaraguru AK. Antimicrobial susceptibility pattern of urinary tract infection causing human pathogenic bacteria. Asian J Med Sci. 2011;3(2):56‐60. [Google Scholar]
- 31. Leski TA, Taitt CR, Bangura U, et al. High prevalence of multidrug‐resistant Enterobacteriaceae isolated from outpatient urine samples but not the hospital environment in Bo, Sierra Leone. BMC Infect Dis. 2016;16:167. doi: 10.1186/s12879-016-1495-1 PMID: 27090787; PMCID: PMC4836052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Alanazi MQ, Alqahtani FY, Aleanizy FS. An evaluation of E. coli in urinary tract infection in emergency department at KAMC in Riyadh, Saudi Arabia: retrospective study. Ann Clin Microbiol Antimicrob. 2018;7(1):3. doi: 10.1186/s12941-018-0255-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Lomazzi M, Moore M, Johnson A, Balasegaram M, Borisch B. Antimicrobial resistance ‐ moving forward? BMC Public Health. 2019;19:858. doi: 10.1186/s12889-019-7173-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Magliano E, Grazioli V, Deflorio L, et al. Gender and age‐dependent etiology of community‐acquired urinary tract infections. Sci World J. 2012;2012:349597. doi: 10.1100/2012/349597 Epub 2012 Apr 26. PMID: 22629135; PMCID: PMC3351074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Al Yousef SA, Younis S, Farrag E, Moussa HS, Bayoumi FS, Ali AM. Clinical and laboratory profile of urinary tract infections associated with extended Spectrum β‐lactamase producing Escherichia coli and Klebsiella pneumoniae. Ann Clin Lab Sci. 2016;46(4):393‐400. PMID: 27466299. [PubMed] [Google Scholar]
- 36. Arana DM, Rubio M, Alós JI. Evolution of antibiotic multiresistance in Escherichia coli and Klebsiella pneumoniae isolates from urinary tract infections: a 12‐year analysis (2003–2014). Enferm Infecc Microbiol Clin. 2017;35(5):293–298. English, Spanish. 10.1016/j.eimc.2016.02.018. Epub 2016 Apr 5. PMID: 27056582. [DOI] [PubMed] [Google Scholar]
- 37. Iqbal Z, Mumtaz MZ, Malik A. Extensive drug‐resistance in strains of Escherichia coli and Klebsiella pneumoniae isolated from paediatric urinary tract infections. J Taibah Univ Med Sci. 2021;16(4):565‐574. doi: 10.1016/j.jtumed.2021.03.004 PMID: 34408614; PMCID: PMC8348552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Bader MS, Loeb M, Leto D, Brooks AA. Treatment of urinary tract infections in the era of antimicrobial resistance and new antimicrobial agents. Postgrad Med. 2020;132(3):234‐250. doi: 10.1080/00325481.2019.1680052 Epub 2019 Oct 24. PMID: 31608743. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Mays Jalil. (2022). The prevalence of multiple drug resistance Escherichia coli and Klebsiella pneumoniae isolated from patients with urinary tract infections. https://doi.org/10.5281/zenodo.5822380


