Abstract
Background
Antibiotic resistance is currently the most serious global threat to the effective treatment of bacterial infections. Antibiotic resistance has been established to adversely affect both clinical and therapeutic outcomes, with consequences ranging from treatment failures and the need for expensive and safer alternative drugs to the cost of higher rates of morbidity and mortality, longer hospitalization, and high‐healthcare costs. The search for new antibiotics and other antimicrobials continues to be a pressing need in humanity's battle against bacterial infections. Antibiotic resistance appears inevitable, and there is a continuous lack of interest in investing in new antibiotic research by pharmaceutical industries. This review summarized some new strategies for tackling antibiotic resistance in bacteria.
Methods
To provide an overview of the recent research, we look at some new strategies for preventing resistance and/or reviving bacteria's susceptibility to already existing antibiotics.
Results
Substantial pieces of evidence suggest that antimicrobials interact with host immunity, leading to potent indirect effects that improve antibacterial activities and may result in more swift and complete bactericidal effects. A new class of antibiotics referred to as immuno‐antibiotics and the targeting of some biochemical resistance pathway components including inhibition of SOS response and hydrogen sulfide as biochemical underlying networks of bacteria can be considered as new emerging strategies to combat antibiotic resistance in bacteria.
Conclusion
This review highlighted and discussed immuno‐antibiotics and inhibition of SOS response and hydrogen sulfide as biochemical underlying networks of bacteria as new weapons against antibiotic resistance in bacteria.
Keywords: antibiotic resistance, hydrogen sulfide, immuno‐antibiotics, SOS response
Immuno‐antibiotics inhibit the non‐mevalonate or methyl‐D‐erythritol phosphate (MEP) pathway of isoprenoid biosynthesis and riboflavin biosynthesis pathway in bacteria. This image was created with BioRender (https://biorender.com/).
![]()
1. INTRODUCTION
Antibiotics are medications that are used to prevent and treat bacterial infections. Antibiotic resistance, on the contrary, occurs when bacteria change as a result of antibiotic use. A scenario in which bacteria evolve and cease to respond to drugs, making infections more difficult to cure and raising the risk of disease spread, serious sickness, and death. 1 Bacterial resistance refers to a bacterium's ability to withstand or tolerate the effects of antibiotics. The ability of a microorganism to withstand an antimicrobial's growth inhibitory or killing activity at clinically achievable concentrations. 2 Antibiotic resistance is a growing global problem in which antibiotics are no longer effective in the treatment of infectious diseases for which they were specifically designed. The World Health Organization (WHO) has now issued a warning that the world is “running out of antibiotics,” escalating fears about global antibiotic resistance reaching new heights. Lately, the emergence of drug‐resistant bacteria has posed a critical challenge to the treatment of clinical infectious diseases, resulting in a gradual increase in the frequency of nosocomial infections. 3 Antibiotic resistance is on the rise in all parts of the world as bacterial infections are one of the leading causes of illness and mortality. New resistance mechanisms emerge regularly and spread internationally, putting the capacity to treat prevalent infectious diseases in jeopardy. Because of its potential to spread internationally and the resulting restricted treatment options, antibiotic resistance is a major concern in healthcare. 4 As antibiotics become less effective, infections such as pneumonia, tuberculosis, blood poisoning, gonorrhea, and food‐borne diseases become more difficult, if not impossible, to treat. 1 Decades after the first patients received antibiotic treatment, bacterial infections have become a threat once again. We are rapidly approaching a post‐antibiotic era in which common infections and minor injuries can kill again unless immediate and proactive action is taken. 1
In 1929, Sir Alexander Fleming discovered Penicillin, also known as a “wonder drug” with incredible abilities to treat bacterial infections, particularly those caused by Staphylococcus and Streptococcus species. 5 Shortly after penicillin gained extensive usage in 1940, antibiotic resistance became a challenge, so currently more than 95% of Staphylococcus aureus isolates worldwide are penicillin‐resistant. 6 , 7 In response to penicillin resistance, penicillinase‐resistant semisynthetic penicillin, methicillin, was developed. 8 , 9 Unfortunately, 2 years after the introduction of methicillin in the United Kingdom, methicillin‐resistant S. aureus (MRSA) was discovered. 10 The discovery and introduction of broad‐spectrum antibiotics such as streptomycin, chloramphenicol, and tetracycline in the late 1940s and early 1950s ushered in the era of antibiotic chemotherapy. 5 These antibiotics were extremely effective against the bacterial pathogens that they were intended to combat.
Recently, the WHO named antimicrobial resistance among the top 10 global public health threats facing humanity. 11 Antibiotic‐resistant infections are estimated to claim up to 10 million lives per year by 2050, costing the global economy about $100 trillion. 12 The number of resistant bacteria and new ones that are becoming resistant to treatment with all known antibiotics is rising, and few new agents are in the pipeline, necessitating the urgent development of new classes of antibiotics to avoid major global health tragedies. 13 Most importantly, antibiotic resistance jeopardizes contemporary medicine's efforts, development, and successes. Without effective antibiotics for the prevention and treatment of infections that may arise at surgical sites, organ transplantation, chemotherapy, and surgeries such as cesarean sections become much more dangerous. More than 70% of all pathogenic bacteria are thought to be resistant to at least one commercially available antibiotic. 14 , 15 Antibiotic resistance develops at both the hospital and community levels. Although the acquisition and dissemination of resistance genes take time, the evolution of bacterial resistance is significantly accelerated by the unnecessary use and misuse of antibiotics. 1 Furthermore, the increase of multidrug‐resistant (MDR) bacteria has been attributed to the spread of resistance genes between and among bacterial species. 16 The abuse of antibiotics has been severally reported as an important factor leading to the emergence of bacterial resistance, which seriously threatens clinical treatment and calls for more attention. 9 , 16 The higher the frequency of antibiotics used, the greater the possibility of the emergence of resistant bacteria. The emergence of coronavirus disease 2019 (COVID‐19) has not helped, as COVID‐19 is reported to exacerbate antibiotic resistance. According to statistics from five nations, 6.9% of COVID‐19 diagnoses are associated with bacterial infections, with a higher prevalence in patients requiring severe critical care. 17 Furthermore, a multicenter study in the United States found that 72% of COVID‐19 patients were given antibiotics even when they were not clinically indicated, 17 which can further enhance antibiotic resistance. Antibiotic resistance may have worsened under COVID‐19 as a result of overuse of antibiotics in humans, continued misuse in agriculture, and a lack of antimicrobials in the development pipeline. 12
It is pertinent to state that the problem of antimicrobial resistance is aggravated by the lack of interest by pharmaceutical industries in new antimicrobial investment, as they view research for new antimicrobials as “low profit” and believe that resistance will develop for new antimicrobials sooner or later. As a result, they prefer to invest in the development of other drugs for chronic diseases in addition to those used to improve lifestyles. 18 As antimicrobial resistance increases in many parts of the world, it becomes increasingly important to not just search for new antimicrobial agents but to come up with strategies to further reduce the emergence and rate of resistance or possibly counter the speedy development of antibiotic resistance by pathogenic microorganisms. It is mentioned that unsuccessful treatment of bacterial infections associated with antibiotic resistance claims at least 700,000 every year globally, and is projected to be associated with 10 million deaths per year by 2050. 12 Given this very serious projection, it becomes indisputably critical for innovations in antibiotic drug technology and the valuation of new treatments to curb the antibiotic resistance menace. This is critical because failure to address antibiotic resistance jeopardizes the achievement of several Sustainable Development Goals (SDGs) (such as poverty reduction, inequality reduction, clean water, and sanitation) and undermines previous progress. 19 In this review, immune‐antibiotics, the inhibition of SOS response, and hydrogen sulfide as biochemical underlying networks leading to universal antibiotic resistance were explored and discussed as positive strategies.
2. ANTIBIOTIC RESISTANCE AND THE INCREASE OF MULTIDRUG RESISTANCE
In his Nobel Prize acceptance speech in 1945, Sir Alexander Fleming foresaw the dangers of inappropriate penicillin use and the emergence of resistance. 20 Many microorganisms have inherent resistance mechanisms that existed before antimicrobial agents were discovered. The extensive usage of antibiotics in humans and animals has created selective pressure that has encouraged the emergence of resistant isolates. 16 The use of antibiotics places a selective pressure on the microbial population; the more antibiotics used, the greater this pressure. For over 70 years, antibiotics have been used successfully for the treatment of bacterial infections. Many infectious organisms, on the contrary, have evolved resistance to the drugs designed to kill them over time, rendering the agents less effective. A growing number of pathogens have developed resistance to one or more of the antimicrobial agents used to treat them. Bacterial antibiotic resistance has been steadily increasing at an alarming rate over the last few years in healthcare settings and livestock systems. 2 , 3 , 21
Globally, there is a high rate of resistance to antibiotics commonly used to treat common bacterial infections such as hospital‐acquired infections, urinary tract infections, sepsis, sexually transmitted infections, and diarrhea, indicating that effective antibiotics are running out. 2 According to the WHO facts sheet on antimicrobial resistance, resistance to ciprofloxacin, an antibiotic often used to treat urinary tract infections, ranged from 8.4% to 92.9% and 4.1% to 79.4% for Escherichia coli and Klebsiella pneumoniae, respectively. 1 K. pneumoniae can cause life‐threatening infections and resistance to last‐resort treatment of carbapenem antibiotics is encountered globally. Similarly, bacteria resistance to colistin, the only sole resort agent for life‐threatening diseases caused by carbapenem‐resistant Enterobacteriaceae, has been found in several countries and regions. 1 , 22 This has resulted in infections for which there is currently no effective antibiotic treatment. The bacterium S. aureus can be found as normal flora of the skin and is also responsible for infections both in the community and in healthcare facilities. 9 , 23 About 64% of people infected with MRSA infections are more likely to die than people infected with drug‐sensitive species. 1 On the contrary, mortality because of drug‐resistant strains of Pseudomonas aeruginosa infections is ever‐increasing, accounting for about 11% of hospital‐acquired bacterial infections. 24
The management and control of gonorrhea have been hampered by widespread resistance in extremely diverse strains of Neisseria gonorrhoeae. Resistance to sulphonamides, penicillin, tetracyclines, macrolides, fluoroquinolones, and early‐generation cephalosporins has emerged rapidly. 25 Currently, ceftriaxone is considered the only empiric monotherapy for gonorrhea in most countries. 1
Antimicrobial resistance is a complicated and multifaceted issue that is influenced by a variety of variables as follows 14 , 26 :
Increasing density of the bacterial population in health care centers, which allows the transfer of bacteria into the community and the emergence of resistance.
Poor adherence to hygiene measures and protocols in hospitals to help keep the spaces clean, which lead to an increase in antimicrobial resistance (AMR) in bacteria
Antibiotic overuse in agriculture
International travel and trade, which can result in the spread of resistant bacteria and resistance genes.
In some areas, there is a lack of sanitation, which can contaminate water systems and spread resistant bacteria in sewage.
Excessive use of antibiotics in human medicine (e.g., for viral infections). Overuse of broad‐spectrum antibiotics can cause selective pressure on commensal bacteria and expose them to secondary infections.
Among other things, there is a lack of quick diagnostics to aid in the proper use of antibiotics.
MDR organisms are of particular concern. MDR bacteria have developed resistance to one or more of the antibiotics used to treat them. Multi‐drug resistance in an organism may develop when antibiotics are not used properly. 1 Taking antibiotics for the incorrect duration or using antibiotics when they are not needed, such as for viral infections, can both contribute to the development of multidrug‐resistance in an organism. MDR strains can also arise as a result of a biological mechanism conferring resistance to multiple drugs, due to multiple genes conferring resistance to multiple antibiotics being genetically linked together on a chromosome or plasmid, or as a result of multiple mutations conferring resistance to multiple antibiotics evolving in a host. 27
3. BACTERIAL RESISTANCE STRATEGIES AGAINST ANTIBIOTICS
To survive in the presence of an antibiotic, bacterial strains must be able to disrupt one or more of the critical stages required for the antimicrobial agent's effective action. 28 Bacterial species follow one of four basic survival strategies:
Preventing the antibiotic from reaching its target in the bacteria by decreasing its ability to penetrate the microbial cell.
Antibacterial agents are expelled from the cell through the efflux pump mechanism.
Antibiotic inactivation via modification or degradation.
Modification or changes to the antimicrobial target within the bacteria.
4. CONSEQUENCES OF ANTIBIOTIC RESISTANCE
Antibiotic resistance is a major cause of death and economic burden around the world. Since 2013, the US Centers for Disease Control and Prevention have reported an 18% decrease in AMR‐related deaths, but an increase in several severe MDR bacterial infections, including a 315% increase in erythromycin‐resistant group A Streptococcus, a 124% increase in drug‐resistant N. gonorrhoeae, and a 50% increase in extended‐spectrum β‐lactamase‐producing Enterobacteriaceae. 12 Similarly, the prevalence of vancomycin‐resistant S. aureus has shown a 3.5% increase between the years 2006 and 2020, with the highest (16%) recorded in Africa. 7 Multidrug resistant‐tuberculosis (MDR–TB) infected an estimated 3.4% of new tuberculosis (TB) cases in 2018 and 18% of previously treated cases, according to a WHO report. 1 The emergence of resistance to new ‘last resort’ TB drugs used to treat drug‐resistant TB is a major threat.
Because of widespread antibiotic misuse, agricultural antibiotic use, poor‐drug quality, insufficient surveillance, and other factors associated with poor‐healthcare standards, malnutrition, chronic and recurring infection, and the inability to afford more effective and costly drugs, low‐ and middle‐income countries are affected more. 29 There are several consequences associated with antibiotic resistance by microbes. When infectious microbial agents develop resistance to a variety of antibiotics, the following outcomes may occur 1 :
Failure to respond to treatment leads to a long illness and a higher chance of death.
Longer hospital stays and illnesses increase the chance for more people to be affected in the community.
When a first‐line antibiotic is no longer effective, therapy must be transferred to second‐ or third‐line antibiotics, which are always more expensive and occasionally more hazardous.
In low‐income countries, many second‐ and third‐line medicines for drug‐resistant illnesses are unavailable, increasing the risk of resistance to first‐line antibiotics.
Medications are becoming insufficient in these nations to treat microbial infections, and important antibiotics to treat infections caused by resistant microorganisms are missing from the essential drug list.
Antibiotic resistance is jeopardizing contemporary medicine's gains. Without appropriate antibiotics, organ transplants, chemotherapy, and operations become riskier.
5. PROBLEMS MILITATING NEW ANTIBIOTICS DEVELOPMENT
The continuous and rapid decrease in the effectiveness of available antibiotics in the treatment of common bacterial diseases as well as a simultaneous decline in the rate of new drug development is a global healthcare concern. 29 To sustain the use of antibiotics in the treatment of infectious diseases, there is a need for constant replenishment of new drugs and drug classes as existing ones become less effective. There is a worldwide consensus that the need for novel anti‐infective drugs in healthcare is enormous and we are fast running out of time. In 2021, only six of the thirty‐two antibiotics in clinical development met the WHO list for priority infections and were categorized as being novel. 15 Many modern medical advances, particularly in the treatment of infectious diseases, are dependent on the availability of effective antibiotics. However, the antibacterial development pipeline is drying up, and the number of new antibiotics reaching the market is frighteningly low. This is further worsened by the unhurried pace of clinical testing and regulatory approval.
Despite the ongoing need for new antimicrobial drugs, major pharmaceutical companies have abandoned this field. Companies have been driven out of antibacterial research and development due to the increased cost of clinical trials, new regulatory uncertainties over approval requirements, and a low‐economic return. 30 , 31 The ever‐widening gap between the critical public health demand for new antibiotics and the declining potential for new antibacterial medication development has created a worrisome situation. The diminishing number of antibiotics approved for usage by the Food and Drug Administration (FDA) reflects the reluctance of pharmaceutical companies to engage in antibiotic development. 30 Antibacterial drugs' low return on investment when compared with other therapeutics, the difficulty of identifying new compounds through traditional discovery methods, speculation that resistance will certainly develop for new antimicrobials, and regulatory requirements that necessitate large and complex clinical trials for antibiotic approval are all contributing to this decline. 30 , 32 This indicates that, rather than relying solely on the discovery of new antibacterial agents, efforts should be directed toward strategies or treatment alternatives to avoid the emergence or spread of resistance in microbes.
The vast majority of research in the field of antibiotics is centered in academia. The development of new and emerging technologies has aided in the search for new agents and potent strategies. For example, opportunities to investigate biological systems (i.e., metabolic pathways, signaling, immunologic, regulatory pathways) beyond their components are now available. These comprehensive techniques provide novel research strategies for understanding the functional molecular networks created by host‐pathogen interactions in response to treatments. To combat drug resistance, these strategies and technologies must be considered.
6. WAY FORWARD: EMERGING STRATEGIES FOR TACKLING ANTIBIOTIC RESISTANCE
The world urgently needs solutions to the global recalcitrant antibiotic resistance menace, together with the mortality, morbidity, dangers, and economic losses associated with it. According to a report published by WHO, while several new antibiotics are presently being developed, none of them are projected to be effective against the most severe forms of antibiotic‐resistant bacteria. 1 This implies that tackling antibiotic resistance does not only require the discovery and development of new antibiotics nor the establishment of local and international interventions, which could be relatively easily implemented. Rather, it would also require the development of strategies that would limit or completely circumvent the development and rise of resistance to available antibiotics.
6.1. Immuno‐antibiotics as an alternative
Historically, the biological activities of antibiotics have been viewed solely in terms of their direct inhibitory and killing properties in isolation. Antimicrobials, on the contrary, are increasingly being found to network with the host's innate immunity to offer significant indirect effects that improve bacterial clearance, 33 which may lead to more swift and ample effects, thereby decreasing the chance of resistance emergence among residual bacteria. There are reports that innate endogenous host defense peptides have antimicrobial modes of action comparable to peptide antimicrobials given to patients such as daptomycin and colistin, and have evolved to protect the host by preventing pathogenic organisms from establishing infection. 34 Agreeably, there is scientific research on the interactions of antibiotics such as synergy, additivity, indifference, and antagonism, but not much is known about the interactions of antibiotics with the innate immune components. 35 , 36
Although the direct in vitro mechanism of action of an antibiotic is thought to be the driving force behind its antimicrobial efficacy, additional antibiotic effects on bacteria have been linked to better host immunological activities and immune optimization. 37 This includes the ability of antibiotics to affect virulence factors and other immune mechanisms that can modulate host response. 33 In a study by Volk et al., 38 it was discovered that the influence of antibiotic responses on the host immune system resulted in increased IL‐1 and lower IL‐10 production in patients with MRSA bacteremia treated with β‐lactam adjunctive therapy combined with standard antibiotics. Despite the failure of prior attempts to develop the S. aureus vaccine, emerging immunologic‐based therapies that combine virulence factor antibodies with standard therapeutics appear promising. 39 The exclusion of host immunology from antimicrobial pharmacology has undoubtedly resulted in an out‐of‐date understanding of antimicrobial therapy. Henceforward, the cooperation of antibiotics and the immune system should be objectively considered in the future, as this will hopefully evolve this field of science through the application of alternative media, host cytokine responses, and computer modeling. 33 Furthermore, there is a need to reconnect the understanding of innate immunity‐antibiotic relationships to improve treatments with antibiotics, slow bacterial resistance development, and uncover novel therapeutic approaches.
Antibiotics have been shown to target vital bacterial activities such as nucleic acid and protein production, cell membrane, cell wall construction, and essential metabolic pathways. 28 , 40 Bacteria, on the contrary, can develop drug resistance by mutating the bacterial targets that these antibiotics are aimed at, inactivating or pumping out the drugs, or even acquiring resistant genes. In recent years, new classes of antimicrobials known as dual‐acting immuno‐antibiotics (DAIAs) were introduced that target the non‐mevalonate or methyl‐D‐erythritol phosphate (MEP) pathway of isoprenoid biosynthesis and riboflavin biosynthesis pathway in bacteria (Figure 1). 41 , 42 They showed a two‐pronged approach to developing new molecules that can kill MDR microorganisms while also boosting the natural immune response of the host. These new DAIAs, which combine the direct killing capabilities of antibiotics with the inherent capability of the immune system to generate synergy, is regarded to be a potential watershed point in the global fight against AMR. This was based on the theory that uniting the immune system to fight bacteria on two fronts at the same time makes resistance development more difficult. The researchers focused on the MEP metabolic pathway, which is required by almost all bacteria but not found in humans, thereby making it an attractive target for designing antibiotics. 41 , 42 , 43 Singh et al. concentrated on the MEP, also known as the non‐mevalonate pathway, which is involved in the synthesis of isoprenoids, an essential molecule required by most pathogenic bacteria for survival. 41 The true target was the killing of bacteria by the inhibition of the IspH enzyme required for isoprenoid biosynthesis. Given the widespread occurrence of IspH in bacteria, this approach has the potential to target a wide spectrum of bacteria. 41 , 42 , 43
FIGURE 1.
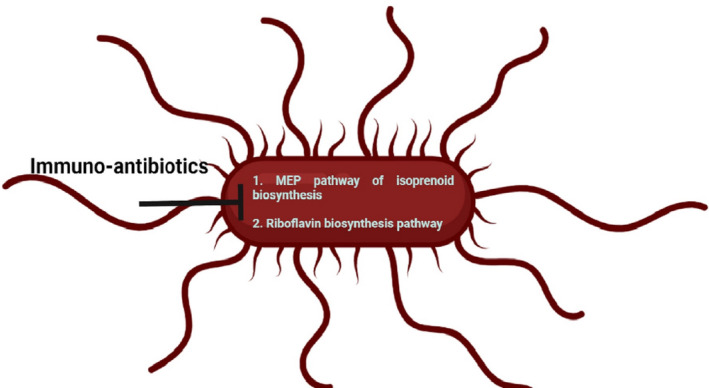
Immuno‐antibiotics inhibit the non‐mevalonate or methyl‐D‐erythritol phosphate (MEP) pathway of isoprenoid biosynthesis and riboflavin biosynthesis pathway in bacteria. This image was created with BioRender (https://biorender.com/)
When tested in vitro on the clinical isolates of antibiotic‐resistant bacteria, the IspH inhibitors were observed to stimulate the immune system with better bacterial clearance and specificity than currently best‐used antibiotics. The bactericidal effects of IspH inhibitors exceeded those of standard pan antibiotics in preclinical models of Gram‐negative bacterial infection, and all compounds examined were found to be safe for humans. 41
The riboflavin biosynthesis pathway is another target for immune‐antibiotics. 42 The riboflavin biosynthesis pathway is present in almost all bacteria and fungi, but it is absent in humans and other animals, which makes it an attractive drug target. 42 Immuno‐antibiotics target the final two steps of the riboflavin synthesis catalyzed by lumazine synthase (RibE or RibH) and riboflavin synthase (RibC). 42
6.2. Biochemical approaches to reducing resistance and increasing susceptibility to available antibiotics
In recent times, scientists have begun to focus research on possible ways to kill antibiotic‐resistant bacteria without the need to necessarily develop new antibiotics. This is an exciting approach considering the cost and challenges linked with the discovery and development of new antibiotics. The main idea of this approach is to neutralize the natural resistance defense mechanisms of microorganisms, thereby making already available antibiotics more effective and lethal. This appears very interesting as we want to achieve a situation where previously existing antibiotics with solid safety profiles become more potent, leading to easy access to more effective antibiotics for the treatment of infectious diseases at a possible cheaper cost. To develop effective alternative therapies, the proper understanding of the mode of action of antibiotics and the mechanisms of resistance in bacteria is very crucial.
Every class of bactericidal antibiotic has a unique mechanism of action that targets a different part of the bacterial cell, leading to the death of the microorganism. It is also widely assumed that these various classes of antibiotics work and kill bacteria in different ways depending on the antibiotics' physiological impacts, such as membrane permeability loss, altered cell morphology, or molecular events, such as essential cellular pathway inhibition. 28 , 40 Still, the impacts of microbial molecular networks generated by exposure to antibiotics directly leading to bacterial cell death remain unclear.
Antibiotic‐mediated bacterial killing is a complex process that starts with physical contact between the drug and its specific target in the bacteria, leading to biochemical, molecular, and ultrastructural changes in the affected bacterium. 44 Drug‐resistant bacteria are constantly evolving and spreading, necessitating a better understanding of the complex mechanisms by which currently existing antibiotics kill bacteria to find new antibacterial therapies. Calhoun et al. 45 and Hong et al. 46 reported that all the antibiotic drugs have a common secondary effect after they have hit their primary targets. According to the report, they force the target bacterium to produce “reactive oxygen species (ROS)” also known as free radicals, which can severely damage the bacteria's DNA and proteins if not quickly defused. This implies that, regardless of their mechanisms of action, all bactericidal antibiotics have the same secondary effect on the bacterial cell, resulting in the organism's death. Research and findings by Kohanski et al. 47 had earlier bolstered this theory by demonstrating that bactericidal antibiotics with distinct cellular targets induced the formation of reactive oxygen species when tested against Gram‐negative and Gram‐positive bacteria. However, this was not the case with bacteriostatic antibiotics, which did not cause hydroxyl radical production. 47
Further investigations have demonstrated that hydroxyl radicals are formed by a Fenton‐like reaction in which ferrous iron is oxidized to ferric iron by peroxide, resulting in hydroxyl radicals. The primary mechanism of peroxide‐induced bacterial death is the establishment of double‐strand DNA breaks (DSBs) 46 , 48 that occur as a result of the Fenton reaction, which can also be induced by antibiotics. 49 , 50 There is also a shred of evidence that when bacteria are exposed to antibiotics, an inducible DNA repair pathway known as the SOS response, which reacts to oxidative stress and DNA impairment, is activated, and that bacterial species unable to form iron–sulfur clusters (a source of iron) are less susceptible to bactericidal drugs. 47 Treatment with high dosages of bactericidal antibiotics produces damaging hydroxyl radicals through a common cellular death pathway involving alterations in the central tricarboxylic acid (TCA) cycle and iron metabolisms. 47 , 49 Furthermore, after exposure to bactericidal antibiotics, there was an observable reduction of nicotinamide adenine dinucleotide (NAD) + hydrogen (H) (NADH) to generate ferrous iron. On the other hand, an impairment of the TCA cycle lowers the concentration of NADH, making bacteria less susceptible to bactericidal drugs. 50 This increase in the production of hydroxyl radicals damages DNA and proteins. This was observed as a common side effect of all tested antibiotics in the study, as well as a common thread in bacterial death. To effectively tackle the increasing threat of antibiotic resistance, we must apply our upward understanding of antibiotic mechanisms to new clinical treatments and approaches. As additional targets in the development of alternative antibiotic therapy, two major uniting targets will be considered: the SOS response and the role of hydrogen sulfide in generalized antibiotic resistance in bacteria.
6.2.1. SOS response: A key step in the development of antibiotic resistance
The SOS response is referred to as an inducible DNA repair process in response to DNA injury and oxidative stress. 51 , 52 The SOS pathway is important in bacterial adaptation, pathogenesis, and diversification, and thus, also important in the development of persister cells, extended tolerance, and stress resistance (including antibiotic resistance) (Figure 2). 53 According to a study conducted by Kohanski et al., it was reported that antibiotic exposure triggered the SOS response, and bacterial mutants that are unable to form iron–sulfur clusters became less susceptible to bactericidal antibiotics. 47 Furthermore, this study strengthens previous evidence that antibiotics such as ciprofloxacin can trigger the SOS response and that the SOS response could be a significant step in the development of drug resistance. 53 Proteins involved in DNA damage repair, such as RecA, an inducer; LexA, a repressor; and chaperones, are produced as part of the SOS response. 53 Repairing damaged DNA typically involves tolerance for minor genetic mutations, which can contribute to the development of antibiotic resistance and persistence.
FIGURE 2.
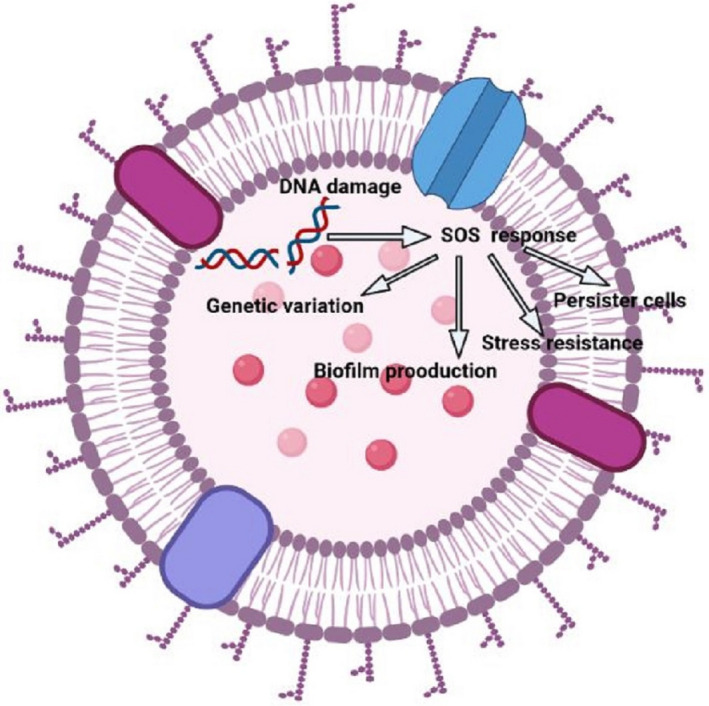
Bacterial SOS response: a schematic view. This image was created with BioRender (https://biorender.com/).
Antibiotics, for example, can cause an increase in ROS levels within the cell, causing DNA, protein, and lipid damage and inducing the SOS response. When more ROS are synthesized than are removed in the cell, oxidative stress ensues. 53 Based on considerable knowledge, bacterial killing, and antibiotic resistance may thus be connected to the formation of hydroxyl radicals. The RecA protein has also been generally identified as the first actor in initiating the SOS response by binding to single‐stranded DNA. 51 , 53 This emerging evidence linking antibiotic action and, as a result, cellular response to hydroxyl radical‐induced macromolecular damage can now be leveraged to develop new antibacterial agents. Inhibitors of the SOS response can not only prevent the development of antibiotic resistance, particularly, when the drug is present in sub‐lethal concentrations, but they can also enhance the activities of bactericidal antibiotics. 47 There are reports on RecA inhibitors that are currently being developed for clinical applications. 54 The SOS response was initially recognized as regulating DNA damage repair. However, it has been observed to play a much broader role. The SOS response triggers a higher rate of mutation, resulting in genetic diversification and microbial adaptation, including antibiotic persistence and resistance. 51 , 53 This method could be applied to any other critical proteins involved in the hydroxyl radical response. They may have been ignored in past efforts to uncover new antibiotic targets since they are not essential for cell growth.
6.2.2. The role of hydrogen sulfide (H2S) in generalized antibiotic resistance in bacteria
Several reports suggest that the production of endogenous hydrogen sulfide by bacteria confers widespread protection against diverse antibiotics in hydrogen sulfide‐synthesizing bacteria, leading to antibiotic tolerance or resistance in virtually all bacteria studied thus far. 55 , 56 Shatalin et al. first reported a new resistance pattern mediated by H2S in several clinical isolates, including S. aureus, Bacillus anthracis, Pseudomonas aeruginosa, and Escherichia coli. 57 Endogenous synthesis of H2S is thought to inhibit the cellular generation of ROS by interfering with the Fenton reaction and boosting ROS‐scavenging enzymes, thereby aiding the development of antibiotic tolerance. On the other hand, genetic and pharmacological disruption of the H2S biosynthesis pathways was found to result in increased antibiotic sensitivity, suggesting that the H2S biosynthetic pathway may be targeted to enhance antibiotic activities or regress resistance. 56 According to several other reports, H2S is often observed to be protective of microbial cells. 55 , 56 , 58
The antibiotic‐induced stress‐triggered Fenton reaction, which results in double‐strand DNA breaks, is recognized as the primary cause of bacterial death due to peroxide formation. 50 , 55 The potential of endogenous H2S to enhance the activities of catalase and superoxide dismutase (SOD) may also contribute to its antioxidant impact. By suppressing the DNA‐damaging Fenton reaction through Fe2+ sequestration and stimulating the key antioxidant enzymes (catalase and SOD), H2S improves bacterial resilience to oxidative stress and antibiotics. 50 Shatalin et al. 57 demonstrated that H2S is endogenously synthesized in S. aureus, B. anthracis, P. aeruginosa, and E. coli via orthologs of cystathionine γ‐lyase (CSE), cystathionine β‐synthase (CBS), or mercaptopyruvate sulfurtransferase (MST). In these bacteria, the H2S‐mediated protective mechanism was doubled, involving the suppression of oxidants produced by the Fenton reaction and the activation of antioxidant enzymes. 57 However, Weikum et al. 59 demonstrated that H2S protection in S. aureus was confined to aminoglycosides. Since it has been established that endogenous H2S reduces the efficacy of several therapeutically used antibiotics by increasing pathogenic bacteria's tolerance, there is a need to consider the inhibition of this “cell protector” as an augmentation therapy against a wide spectrum of pathogens.
There are other reports that exogenous H2S might not have the same protective effects on bacteria as endogenous H2S. This is evidenced by reports that exogenous H2S is cytotoxic to various bacteria, including E. coli and Acinetobacter baumannii. 58 , 60 Podlesek and Bertok 53 made a similar observation while studying the effect of exogenous H2S on A. baumannii, a crucial antimicrobial‐resistant bacterium that lacks the genes coding for H2S biosynthesis. Exogenous H2S was found to be ineffective in protecting A. baumannii against antibiotics. H2S was observed to improve the killing effects of antibiotics such as gentamycin, colistin, rifampicin, and clarithromycin, which are unrelated. 53 Nevertheless, it was further observed that when antibiotic‐sensitive A. baumannii were treated with H2S‐releasing compounds (such as NaHS) together with antibiotics, the drugs' bactericidal activity was substantially higher than when the antibiotics were used alone. In recent years, some H2S‐releasing compounds have been designed for a variety of clinical applications, 61 , 62 and their safety profiles have been reported. 63 It becomes very important to consider H2S‐releasing compounds as a means of improving antibiotic efficacies and reversing resistance in bacteria that do not produce H2S.
7. CAUSE FOR CONCERN
The beneficial and harmful impacts of H2S on animals and plants have been well documented. 64 , 65 Surprisingly, both effects are linked with either protection from or worsening oxidative damage and energy metabolism. 64 Along with nitric oxide and carbon monoxide, hydrogen sulfide has been identified as the third gasotransmitter in mammals and has been connected to a range of metabolic processes. 66 , 67 H2S is produced by cysteine degradation in mammals and bacteria via cystathionine‐lyase (CSE), cystathionine‐synthase (CBS), and 3‐mercaptopyruvate sulfurtransferase (3MST). 68 , 69 This implies that mammalian cells also synthesize H2S, and human cells rely on it. H2S works as a signaling chemical in humans, interacting with a variety of organs ranging from the brain to the smooth muscle. 50 , 68 Nonetheless, the key determinant of the effect of H2S in an organism is noted as the concentration range of H2S. 50 For the most part, lower concentrations of H2S are generally considered cytoprotective, but high doses (millimolar) are considered cytotoxic. 55
The CSE pathway is used by both human and bacterial cells to produce hydrogen sulfide. However, the flavors of human and bacterial CSE differ slightly. 70 , 71 Bacterial CBS, CSE, and 3MST have been found to differ significantly from their mammalian counterparts, 70 suggesting that specific inhibitors targeting these enzymes could be designed. The goal is to find compounds that have a significant affinity for the bacterial CSE, ensuring that both of them are selectively efficient against bacteria and do not have any unexpected side effects on mammalian cells.
8. CONCLUSION
Antibiotic resistance remains an internationally worrisome problem that requires urgent intervention. The idea of antibiotic potentiation by molecules or approaches that block key metabolic pathways is a viable alternative to the “one compound, one target” model that has dominated antibiotic drug development. Although employing this combinatorial technique to build and improve antibiotics presents obstacles in terms of clinical trials and regulatory hurdles. The development of agents with the dual activity of inhibiting bacteria while also enhancing the immune system appears to be a promising strategy. Furthermore, antibiotic‐induced suppression of bacteria's downstream repair processes could be the ultimate haymaker for tackling bacterial pathogens.
CONFLICT OF INTEREST
Authors declared that they have no competing interests.
Nwobodo DC, Ugwu MC , Anie OC, et al. Antibiotic resistance: The challenges and some emerging strategies for tackling a global menace. J Clin Lab Anal. 2022;36:e24655. doi: 10.1002/jcla.24655
Contributor Information
David Chinemerem Nwobodo, Email: nwobododave@gmail.com.
Morteza Saki, Email: mortezasaki1981@gmail.com.
DATA AVAILABILITY STATEMENT
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
REFERENCES
- 1. WHO . Antibiotics resistance. WHO; 2022. Accessed April 21, 2022 https://www.who.int/news‐room/fact‐sheets/detail/antibiotic‐resistance [Google Scholar]
- 2. Antimicrobial Resistance Collaborators (ARC) . Global burden of bacterial antimicrobial resistance in 2019: a systematic analysis. Lancet. 2022;399(10325):629‐655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Zhu Y, Huang WE, Yang Q. Clinical perspective of antimicrobial resistance in bacteria. The opinion on recent clinic antibiotics abuse. Infect Drug Resist. 2022;15:735‐746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Ugwu MC, Shariff M, Nnajide CM, et al. Phenotypic and molecular characterization of β‐lactamases among enterobacterial uropathogens in southeastern Nigeria. Can J Infect Dis Med Microbiol. 2020;2020:5843904‐5843909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Letek M. Alexander Fleming, the discoverer of the antibiotic effects of penicillin. Front Young Minds. 2020;8:159. [Google Scholar]
- 6. Gajdács MJ. The continuing threat of methicillin‐resistant Staphylococcus aureus . Antibiotics. 2019;8(2):52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Wu Q, Sabokroo N, Wang Y, Hashemian M, Karamollahi S, Kouhsari E. Systematic review and meta‐analysis of the epidemiology of vancomycin‐resistance Staphylococcus aureus isolates. Antimicrob Resist Infect Control. 2021;10:101. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 8. Guo Y, Song G, Sun M, Wang J, Wang Y. Prevalence and therapies of antibiotic‐resistance in Staphylococcus aureus . Front Cell Infect Microbiol. 2020;10:107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Faheem SM, Gohar UF, Riaz S. Recent developments in methicillin‐resistant Staphylococcus aureus (MRSA) management and potential antimicrobial alternatives to combat the antibiotic resistance challenge: a review. Pure Appl Biol. 2022;11(2):353‐382. [Google Scholar]
- 10. Ike B, Ugwu MC, Ikegbunam MN, et al. Prevalence, antibiogram and molecular characterization of comunity‐acquired methicillin‐resistant staphylococcus aureus in Awka. Anambra Nigeria Open Microbiol J. 2016;10:211‐221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. World Health Organisation . Antimicrobial resistance; Fact sheet. WHO; 2021. Accessed June 20, 2022 https://www.who.int/news‐room/fact‐sheets/detail/antimicrobial‐resistance [Google Scholar]
- 12. Strathdee SA, Davies SC, Marcelin JR. Confronting antimicrobial resistance beyond the COVID‐19 pandemic and the 2020 US election. Lancet. 2020;396(10257):1050‐1053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Nwobodo DC, Eze PM, Okezie UM, Okafoanyali JO, Okoye FBC, Esimone CO. Bioactive compounds characterization and antimicrobial potentials of crude extract of Curvularia lunata, a fungal endophyte from Elaeis guineensis . Trop J Nat Prod Res. 2022;6(3):395‐402. [Google Scholar]
- 14. Uddin TM, Chakraborty AJ, Khusro A, et al. Antibiotic resistance in microbes: history, mechanisms, therapeutic strategies and future prospects. J Infect Public Health. 2021;14(12):1750‐1766. [DOI] [PubMed] [Google Scholar]
- 15. WHO . Antimicrobial resistance. WHO. 2020. Accessed April 21, 2022. https://www.who.int/news‐room/fact‐sheets/detail/antimicrobial‐resistance [Google Scholar]
- 16. Aworh MK, Kwaga JKP, Hendriksen RS, Okolocha EC, Thakur S. Genetic relatedness of multidrug resistant Escherichia coli isolated from humans, chickens and poultry environments. Antimicrob Resist Infect Control. 2021;10:58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Langford BJ, So M, Raybardhan S, et al. Bacterial co‐infection and secondary infection in patients with COVID‐19: a living rapid review and meta‐analysis. Clin Microbiol Infect. 2020;26(12):1622‐1629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Congressional Budget Office . Research and development in the pharmaceutical industry. CBO; 2021. Accessed April 21, 2022 https://www.cbo.gov/publication/57126 [Google Scholar]
- 19. World Bank . Drug‐resistant infections: a threat to our economic future (discussion draft). World Bank; 2016. Accessed February 1, 2022 http://pubdocs.worldbank.org/en/527731474225046104/AMR‐Discussion‐Draft‐Sept18updated.pdf [Google Scholar]
- 20. Fleming A. NobelPrize.org. Nobel Prize Outreach AB 2022. 2022. Accessed August 8, 2022. https://www.nobelprize.org/prizes/medicine/1945/fleming/facts/ [Google Scholar]
- 21. Tartor YH, Gharieb R, El‐Aziz A, et al. Virulence determinants and plasmid‐mediated colistin resistance mcr genes in gram‐negative bacteria isolated from bovine milk. Front Cell Infect Microbiol. 2021;11:761417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Tartor YH, Abd El‐Aziz NK, Gharieb RMA, et al. Whole‐genome sequencing of gram‐negative bacteria isolated from bovine mastitis and raw milk: the first emergence of colistin mcr‐10 and fosfomycin fosA5 resistance genes in Klebsiella pneumoniae in Middle East. Front Microbiol. 2021;12:770813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Ugwu MC, Anie CO, Ibezim EC, Esimone CO. Antimicrobial evaluation of methicillin‐resistant Staphylococcus aureus nasal carriage amongst healthy students in Agbor, Delta state, Nigeria. Arch Clin Microbiol. 2016;7(2):13. [Google Scholar]
- 24. Nwobodo DC, Ihekwereme CP, Ikem CJ, Okoye FBC. The anti‐pseudomonal potentials of metabolites from some endophytic fungi isolated from Grcinia kola leaves. Novel Res Microbiol J. 2020;4(3):845‐855. [Google Scholar]
- 25. Derbie A, Mekonnen D, Woldeamanuel Y, Abebe T. Azithromycin resistant gonococci: a literature review. Antimicrob Resist Infect Control. 2020;9:138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Reygaert WC. An overview of the antimicrobial resistance mechanisms of bacteria. AIMS Microbiol. 2018;4(3):482‐501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Jacopin E, Lehtinen S, Débarre F, Blanquart F. Factors favouring the evolution of multidrug resistance in bacteria. J R Soc Interface. 2020;17:20200105. [Google Scholar]
- 28. Peterson E, Kaur P. Antibiotic resistance mechanisms in bacteria: relationships between resistance determinants of antibiotic producers, environmental bacteria, and clinical pathogens. Front Microbiol. 2018;9:2928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Haroon RM, Rahman MM, Sultana H, et al. Antibacterial resistance patterns of bacteria isolated from clinical specimens at Uttara IbnSina diagnostic Centre. Dhaka Afr J Microbiol Res. 2020;14(5):175‐181. [Google Scholar]
- 30. Dutescu IA, Hillier SA. Encouraging the development of new antibiotics: are financial incentives the right way forward? A systematic review and case study. Infect Drug Resist. 2021;14:415‐434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Tacconelli E, Carrara E, Savoldi A, et al. Discovery, research, and development of new antibiotics: the WHO priority list of antibiotic‐resistant bacteria and tuberculosis. Lancet Infect Dis. 2017;18(3):318‐327. [DOI] [PubMed] [Google Scholar]
- 32. Ayukekbong JA, Ntemgwa M, Atabe AN. The threat of antimicrobial resistance in developing countries: causes and control strategies. Antimicrob Resist Infect Control. 2017;6:47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Berti A, Rose W, Nizet V, Sakoulas G. Antibiotics and innate immunity: a cooperative effort toward the successful treatment of infections. Open Forum Infect Dis. 2020;7(8):ofaa302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Rima M, Fajloun Z, Sabatier JM, Bechinger B, Naas T. Antimicrobial peptides: a potent alternative to antibiotics. Antibiotics. 2021;10(9):1095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Yeh PJ, Hegreness MJ, Aiden AP, Kishony R. Drug interactions and the evolution of antibiotic resistance. Nat Rev Microbiol. 2009;7(6):460‐466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Tartor YH, Elmowalid GA, Hassan MN, Shaker A, Ashour DF, Saber T. Promising anti‐biofilm agents and phagocytes enhancers for the treatment of Candida albicans biofilm–associated infections. Front Cell Infect Microbiol. 2022;12:807218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Watson K, Russell CD, Baillie JK, et al. Developing novel host‐based therapies targeting microbicidal responses in macrophages and neutrophils to combat bacterial antimicrobial resistance. Front Immunol. 2020;11:786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Volk CF, Burgdorf S, Edwardson G, Nizet V, Sakoulas G, Rose WE. Interleukin (IL)‐1β and IL‐10 host responses in patients with Staphylococcus aureus bacteremia determined by antimicrobial therapy. Clin Infect Dis. 2020;70(12):2634‐2640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Miller LS, Fowler VG, Shukla SK, Rose WE, Proctor RA. Development of a vaccine against Staphylococcus aureus invasive infections: evidence based on human immunity, genetics and bacterial evasion mechanisms. FEMS Microbiol Rev. 2020;44(1):123‐153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Lade H, Kim JS. Bacterial targets of antibiotics in methicillin‐resistant Staphylococcus aureus . Antibiotics. 2021;10(4):398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Singh KS, Sharma R, Reddy PAN, et al. IspH inhibitors kill gram‐negative bacteria and mobilize immune clearance. Nature. 2020;589(7843):597‐602. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 42. Eberl M, Oldfield E, Herrmann T. Immuno‐antibiotics: targeting microbial metabolic pathways sensed by unconventional T cells. Immunother Adv. 2021;1(1):l‐12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Sanapalli BKR, Yele V. Dual acting immuno‐antibiotics: computational investigation on antibacterial efficacy of immune boosters against isoprenoid H enzyme. Assay Drug Dev Technol. 2022;20:225‐236. doi: 10.1089/adt.2022.038 [DOI] [PubMed] [Google Scholar]
- 44. Wong F, Stokes JM, Cervantes B, et al. Cytoplasmic condensation induced by membrane damage is associated with antibiotic lethality. Nat Commun. 2021;12(2321):2321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Calhoun C, Wermuth HR, Hall GA. Antibiotics. StatPearls. 2021. Accessed April 22, 2022; https://www.ncbi.nlm.nih.gov/books/NBK535443/ [Google Scholar]
- 46. Hong Y, Zeng J, Wang X. Post‐stress bacterial cell death mediated by reactive oxygen species. Biological Sciences. 2019;116(20):10064‐10071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Kohanski MA, Dwyer DJ, Hayete B, Lawrence CA, Collins JJ. A common mechanism of cellular death induced by bactericidal antibiotics. Cell. 2007;130(5):797‐810. [DOI] [PubMed] [Google Scholar]
- 48. Chatgilialoglu C, Krokidis MG, Masi A, et al. New insights into the reaction paths of hydroxyl radicals with purine moieties in DNA and double‐stranded oligodeoxynucleotides. Molecules. 2019;24(21):3860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Mironov A, Seregina T, Nagornykh M, et al. Mechanism of H2S‐mediated protection against oxidative stress in Escherichia coli . Proc Natl Acad Sci. 2017;114(23):6022‐6027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Ng SY, Ong KX, Surendran ST, et al. Hydrogen sulfide sensitizes Acinetobacter baumannii to killing by antibiotics. Front Microbiol. 2020;11:1875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Blanchard L, de Groot A. Coexistence of SOS‐dependent and SOS‐independent regulation of DNA repair genes in radiation‐resistant Deinococcus bacteria. Cell. 2021;10:924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Podlesek Z, Bertok D. The Escherichia coli SOS response: Much more than DNA damage repair. IntechOpen. 2021. Accessed April 21, 2022; https://www.intechopen.com/online‐first/78722 [Google Scholar]
- 53. Podlesek Z, Bertok DZ. The DNA damage inducible SOS response is a key player in the generation of bacterial persister cells and population wide tolerance. Front Microbiol. 2020;1:1785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Zhou Z, Pan Q, Lv X, et al. Structural insights into the inhibition of bacterial RecA by naphthalene polysulfonated compounds. iScience. 2021;24(1):101952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Mendes SS, Miranda V, Saraiva LM. Hydrogen sulfide and carbon monoxide tolerance in bacteria. Antioxidants. 2021;10:729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Walsh BJC, Giedroc DP. H2S and reactive sulfur signaling at the host‐bacterial pathogen interface. J Biol Chem. 2020;295(38):13150‐13168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Shatalin K, Shatalina E, Mironov A, Nudler E. H2S: a universal defense against antibiotics in bacteria. Science. 2011;18(334):986‐990. [DOI] [PubMed] [Google Scholar]
- 58. Fu LH, Wei ZZ, Hu K, et al. Hydrogen sulfide inhibits the growth of Escherichia coli through oxidative damage. J Microbiol. 2018;56:238‐245. [DOI] [PubMed] [Google Scholar]
- 59. Weikum J, Ritzmann N, Jelden N, et al. Sulfide protects Staphylococcus aureus from aminoglycoside antibiotics but cannot be regarded as a general defense mechanism against antibiotics. Antimicrob Agents Chemother. 2018;62:e602‐e618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Singapore‐MIT Alliance for Research and Technology (SMART) . New way to make bacteria more sensitive to antibiotics discovered. ScienceDaily. 2020. Accessed April 21, 2022; www.sciencedaily.com/releases/2020/08/200812115250.htm [Google Scholar]
- 61. Zaorska E, Tomasova L, Koszelewski D, Ostaszewski R, Ufnal M. Hydrogen sulfide in pharmacotherapy, beyond the hydrogen sulfide‐donors. Biomolecules. 2020;10(2):323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Wen YD, Wang H, Zhu YZ. The drug developments of hydrogen sulfide on cardiovascular disease. Oxid Med Cell Longev. 2018;2018:4010395‐4010321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Wallace JL, Nagy P, Feener TD, et al. A proof‐of‐concept, phase 2 clinical trial of the gastrointestinal safety of a hydrogen sulfide‐releasing anti‐inflammatory drug. Br J Pharmacol. 2020;177:769‐777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Aroca A, Gotor C, Bassham DC, Romero LC. Hydrogen sulfide: from a toxic molecule to a key molecule of cell life. Antioxidants. 2020;9(7):621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Rubright SLM, Pearce LL, Peterson J. Environmental toxicology of hydrogen sulfide. Nitric Oxide. 2017;71:1‐13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Zhu Z, Chambers S, Zeng Y, Bhatia M. Gases in sepsis: novel mediators and therapeutic targets. Int J Mol Sci. 2022;23:3669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Giuffrè A, Vicente JB. Hydrogen sulfide biochemistry and interplay with other gaseous mediators in mammalian physiology. Oxid Med Cell Longev. 2018;27:6290931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Cao X, Ding L, Xie Z, et al. A review of hydrogen sulfide synthesis, metabolism, and measurement: is modulation of hydrogen sulfide a novel therapeutic for cancer? Antioxid Redox Signal. 2019;31(1):1‐38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Pedre B, Dick T. 3‐Mercaptopyruvate sulfurtransferase: an enzyme at the crossroads of sulfane sulfur trafficking. Biol Chem. 2021;402(3):223‐237. [DOI] [PubMed] [Google Scholar]
- 70. Rahman MA, Glasgow JN, Nadeem S. The role of host‐generated H2S in microbial pathogenesis: new perspectives on tuberculosis. Front Cell Infect Microbiol. 2020;10:586923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Pal VK, Bandyopadhyay P, Singhi A. Hydrogen sulfide in physiology and pathogenesis of bacteria and viruses. IUBMB life. 2018;70(5):393‐410. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.


