Abstract
No standard options existed for human epidermal growth factor receptor 2 (HER2)‐positive advanced breast cancer that progresses after second‐line trastuzumab emtansine therapy before 2020. The purpose of this study was to examine the efficacy of pertuzumab retreatment after disease progression following pertuzumab‐containing therapy for HER2‐positive locally advanced or metastatic breast cancer for the first time. This randomized, open‐label, multicenter phase III trial was undertaken in 93 sites in Japan. Eligible patients with HER2‐positive breast cancer who had received pertuzumab, trastuzumab, and chemotherapy as first‐ and/or second‐line therapy were randomly assigned (1:1) to: (i) pertuzumab, trastuzumab, and physician's choice chemotherapy (PTC), or (ii) trastuzumab and physician's choice chemotherapy (TC). The primary end‐point was investigator‐assessed progression‐free survival (PFS). Between August 1, 2015 and December 31, 2018, 219 patients were randomized to PTC (n = 110) or TC (n = 109). Median follow‐up was 14.2 months (interquartile range, 9.0–22.2), and median PFS was 5.3 months (95% confidence interval [CI], 4.0–6.6) with PTC and 4.2 months (95% CI, 3.2–4.8) with TC (stratified hazard ratio 0.76 [95% CI upper limit 0.967]; p = 0.022). Progression‐free survival was improved by adding pertuzumab in all prespecified subgroups. The PTC arm showed a trend towards better overall survival and duration of response, but similar objective response and health‐related quality of life. The incidence of treatment‐related adverse events was similar between groups except for diarrhea. Pertuzumab retreatment contributes to disease control for HER2‐positive locally advanced or metastatic breast cancer previously treated with pertuzumab‐containing regimens.
Keywords: advanced breast cancer, heavily pretreated, HER2‐positive, pertuzumab, trastuzumab
This randomized, open‐label, multicentre phase 3 trial examined the efficacy of pertuzumab retreatment after disease progression following pertuzumab‐containing therapy for HER2‐positive locally advanced or metastatic breast cancer for the first time. Patients randomly assigned to pertuzumab, trastuzumab and physician #x2019;s choice chemotherapy had improved progression‐free survival compared with those who received trastuzumab and physician #x2019;s choice chemotherapy (5.3 vs 4.2 months; p=0.022). Pertuzumab retreatment also showed a trend towards better overall survival and duration of response, and contributes to disease control for HER2‐positive locally advanced or metastatic breast cancer previously treated with pertuzumab‐containing regimens.

Abbreviations
- AE
adverse event
- B‐TOI
B‐Trial Outcome Index
- CI
confidence interval
- CR
complete response
- DoR
duration of response
- FACT‐B
Functional Assessment of Cancer Therapy – Breast
- FAS
full analysis set
- HER2/3
human epidermal growth factor receptor 2/3
- HR
hazard ratio
- HR‐QoL
health‐related quality of life
- ITT
intention‐to‐treat
- LVEF
left ventricular ejection fraction
- ORR
overall response rate
- OS
overall survival
- PET
positron emission tomography
- PFS
progression‐free survival
- PR
partial response
- PTC
pertuzumab, trastuzumab, and physician's choice of chemotherapy
- TC
trastuzumab and physician's choice of chemotherapy
- T‐DM1
trastuzumab emtansine
- TTD
time to deterioration
1. INTRODUCTION
Current standard of care for HER2‐positive advanced breast cancer involves HER2‐targeted combinations for first‐line treatment, specifically consisting of trastuzumab, pertuzumab, and a taxane. 1 , 2 For HER2‐positive advanced breast cancer that progresses during or after such first‐line therapy, T‐DM1 is strongly recommended, based on two large phase III trials. 3 , 4 Subsequent treatment options exist but there is no single standard of care for third‐line or later treatment. Until approximately 2019, further HER2‐targeted therapy‐based treatment was recommended, including lapatinib plus capecitabine or trastuzumab, trastuzumab and combinations of chemotherapy, or endocrine therapy (if hormone receptor‐positive) for HER2‐positive disease but with insufficient evidence to recommend one particular regimen. However, since 2020, several promising options after T‐DM1 have been reported, including the addition of tucatinib to trastuzumab and capecitabine, 5 trastuzumab deruxtecan, 6 and margetuximab plus chemotherapy. 7 These provide survival benefits and antitumor activity, including in particular clinical or genetic subgroups.
Continual trastuzumab treatment is effective following progression of HER2‐positive advanced breast cancer. 8 , 9 Clinical practice guidelines recommend subsequent anti‐HER2 treatment for progression beyond anti‐HER2 therapy for patients with HER2‐positive advanced breast cancer. 1 , 2 However, the efficacy or safety of sequential dual HER2 blockade with pertuzumab and trastuzumab after progression is underreported. Pertuzumab retreatment is one of the most important issues in treatment sequencing for HER2‐positive advanced breast cancer previously exposed to pertuzumab, especially from the viewpoint of resistance to anti‐HER2 Abs. Also, it is unclear whether retreatment with pertuzumab and trastuzumab in combination with chemotherapy is effective after T‐DM1.
The purpose of this study was to evaluate the efficacy and safety of pertuzumab, trastuzumab, and chemotherapy as pertuzumab retreatment compared with trastuzumab and chemotherapy in locally advanced or metastatic breast cancer patients previously treated with pertuzumab.
2. MATERIALS AND METHODS
2.1. Trial design and patients
This multicenter, open‐label, randomized controlled, phase III trial recruited participants from 93 institutes in Japan (Table S1). Eligible women aged 20 years or older with histologically confirmed invasive breast cancer, HER2‐positive status confirmed by immunohistochemical analysis (3+ indicating positive status) and/or in situ hybridization (amplification ratio ≥2.0 indicating positive status), and history of pertuzumab and trastuzumab‐containing chemotherapy for locally advanced or metastatic breast cancer (although the latest regimen before enrolment must not include pertuzumab) were enrolled. Full inclusion criteria, as well as exclusion criteria, are detailed in Table S2.
This study was carried out in accordance with the Declaration of Helsinki and the Ethical Guidelines for Clinical Research of the Japanese Ministry of Health, Labor and Welfare. An independent ethics committee for each participating site approved the protocol and any modifications. Each participant provided written informed consent before enrolment.
2.2. Randomization and masking
Eligible patients were randomly assigned at baseline (1:1) to receive either PTC or TC. A minimization approach ensured treatment arms were balanced with respect to predefined patient factors as well as patient numbers in each group. Treatment stratification factors were estrogen receptor status (positive/negative), duration of previous pertuzumab therapy (first‐line, <180 days/≥180 days; second‐line, <120 days/≥120 days), previous number of regimens for locally advanced or metastatic breast cancer (2/3), and site of metastases (visceral/nonvisceral).
Uniform random numbers for randomization were generated by a computer program with the Mersenne twister method used for the generation algorithm. The allocation system was tested before study initiation to ensure it met plan requirements. As there was no placebo arm, neither clinicians nor patients were masked to treatment allocation.
2.3. Procedures
Pertuzumab was given intravenously as an 840 mg loading dose followed by 420 mg maintenance doses every 3 weeks. Trastuzumab was given intravenously as an 8 mg/kg loading dose followed by 6 mg/kg maintenance doses every 3 weeks. Physician's choice of chemotherapy agents were chosen by investigators before randomization and options, the safety of which had been confirmed in combination with pertuzumab and trastuzumab, consisted of docetaxel, 10 paclitaxel, 11 nab‐paclitaxel, 12 vinorelbine, 13 eribulin, 14 capecitabine, 15 or gemcitabine. 16 Doses were given every 3 weeks based on results of previous clinical trials for HER2‐positive metastatic breast cancer, as detailed in Table S3. Treatment continued until tumor progression was observed, an intolerable AE occurred, or consent was withdrawn. Subsequent unrestricted treatment after protocol treatment was possible at the discretion of the attending physician.
Tumor assessment for target and nontarget lesions was carried out at screening, every 6 weeks for 6 months after enrolment, every 9 weeks thereafter, and at the end of treatment. Evaluation of treatment effect and disease progression was undertaken with RECIST version 1.1, 17 with all patients undergoing at least chest and abdominal computed tomography or MRI at screening and tumor evaluation throughout the study as at screening. 18 Clinical progression was defined when the investigator determined that exacerbation was detected by methods other than those defined in RECIST version 1.1, and included ultrasonography, bone scintigraphy, PET determination, worsening of subjective symptoms, and elevated tumor markers.
2.4. Outcomes
The primary end‐point was PFS, as assessed by investigators. Progression‐free survival was defined as the period from registration to the date of disease progression or death from any cause. Disease progression is equivalent to tumor progression, as defined in RECIST version 1.1, or clinical progression as described above.
Secondary end‐points were PFS in patients with T‐DM1 as the latest regimen, ORR, DoR, OS, HR‐QoL, and safety‐related end‐points. Objective response rate was defined as the proportion of patients with measurable disease whose best overall response was either CR or PR as assessed by the attending physician. Duration of response was defined as the period from the day of first overall response (CR or PR) to the first day of objective confirmation of recurrence, death, or disease progression by investigators' assessment. Overall survival was defined as the period from registration to the date of death regardless of cause.
Health‐related quality of life was primarily evaluated using the B‐TOI, which is the total score of physical well‐being, functional well‐being, and breast cancer subscale among the domains that make up the FACT‐B, 19 an index for HR‐QoL used in previous clinical trials. 20 The B‐TOI scores in the PTC and TC were analyzed such that the time from enrolment to the time at which a clinically meaningful decrease in B‐TOI score (≥5 points) represented the TTD for each patient. 21
Safety was monitored continuously using the Japan Clinical Oncology Group/NCI's Common Terminology Criteria for Adverse Events version 4.0. 17 Left ventricular ejection fraction measurement was undertaken by the echocardiogram/multigated acquisition scan method at the time of screening, every four cycles, and at the end of treatment. Of the AEs, infusion reaction, neutrophil count reduction, diarrhea, stomatitis, cardiac events, and skin‐related events were reported in terms of all severity grades, and other AEs were reported as grade 3 or higher.
2.5. Statistical analysis
The target sample size was calculated according to the results of the TH3RESA trial, 3 which based the sample size calculation on a median PFS for physician's choice of chemotherapy of 4.0 months. This study hypothesized that PTC will increase the median PFS to 5.5 months, representing a change of 1.5 months over standard therapy, with 325 PFS events providing an 86.5% power to detect an HR of 0.739 for PFS at a one‐sided 5% level of significance. Assuming 333 eligible patients were needed and a drop‐out rate of 10%, a total of 370 patients were to be enrolled
For the primary end‐point analysis, PFS assessed by investigators was based on the ITT population and estimated by the Kaplan–Meier method with between‐group differences compared using the stratified log–rank test. The Cox proportional hazards model was used to calculate the hazard ratio of the PTC arm to the TC arm and the upper limit 95% CI (one‐sided significance level set at 0.05). For the secondary end‐points analysis, including PFS in patients who had immediate prior T‐DM1 treatment, DoR, and OS, the hypothesis test was exploratory, so an unstratified log–rank test was also carried out along with the stratified log–rank test. Sensitivity analysis was based on the FAS and undertaken by the same method used to analyze the ITT population as described above. The FAS consisted of all registered patients who started the study according to the allocation procedure and had at least some data. However, patient data were reviewed to exclude patients whose pre‐enrolment objective data did not meet the selection criteria and those who withdrew consent before postregistration treatment. For the response rate analysis, the point estimate of the difference between groups was calculated along with the upper limit 95% CI using the χ2‐test.
Regarding HR‐QoL, the analysis population comprised all patients undergoing a baseline FACT B‐TOI assessment and more than one postbaseline assessment. Time to deterioration for each patient was calculated using the Kaplan–Meier method, which was then used to calculate the median TTD value for each treatment group with intergroup comparisons undertaken using a stratified log–rank test. In addition, the hazard ratio of TTD and two‐sided 95% CI between the groups were calculated using the Cox proportional hazard model, with treatment as the stratification factor.
The significance level for comparison tests was set at 0.05 (one‐sided) for primary and secondary end‐point analyses and 0.05 (two‐sided) for the HR‐QoL analysis with no adjustments made for multiplicity and no imputation methods applied for missing data.
Statistical analysis was undertaken using SAS version 9.0 (SAS).
This study is registered with ClinicalTrials.gov (NCT02514681), the Japan Registry of Clinical Trials (jRCTs041180153), and the University Hospital Medical Information Network (UMIN000018202).
3. RESULTS
3.1. Patient characteristics
In total, 219 patients were enrolled and randomized (PTC, n = 110; TC, n = 109) between August 1, 2015 and December 31, 2018 (data cut‐off: July 31, 2019). Figure 1 summarizes the patient disposition following randomization. The median (interquartile range) follow‐up time was similar in the PTC (14.4 [9.0, 21.0] months) and TC (14.2 [8.7, 22.5] months) arms (p = 0.926). In general, demographic and other baseline characteristics were similar between treatment arms (Table 1).
FIGURE 1.
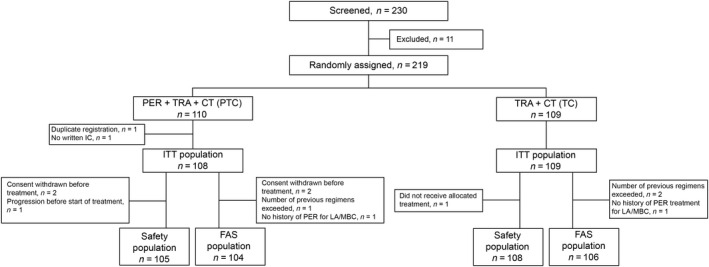
Selection of 219 women with HER2‐positive advanced breast cancer treated with pertuzumab (PER) + trastuzumab (TRA) + chemotherapy (CT) (PTC) or TRA + CT (TC). FAS, full analysis set; IC, informed consent; ITT, intention‐to‐treat; LA/MBC, locally advanced/metastatic breast cancer
TABLE 1.
Demographic and baseline clinical characteristics of 219 women with HER2‐positive advanced breast cancer treated with pertuzumab + trastuzumab + chemotherapy (PTC) or trastuzumab + chemotherapy (TC)
| Characteristic | PTC (N = 108) | TC (N = 109) |
|---|---|---|
| Age, years; median (min, max) | 57 (27, 81) | 60 (32, 83) |
| ECOG performance status, n (%) | ||
| 0 | 78 (72.2) | 73 (67.0) |
| 1, 2 | 30 (27.8) | 36 (33.0) |
| Estrogen receptor, n (%) | ||
| Positive | 58 (53.7) | 64 (58.7) |
| Negative | 50 (46.3) | 45 (41.3) |
| Visceral disease involvement, n (%) | 73 (67.6) | 75 (68.8) |
| Location of metastatic site, n (%) | ||
| Lung | 32 (29.6) | 33 (30.3) |
| Liver | 22 (20.4) | 19 (17.4) |
| Bone | 21 (19.4) | 24 (22.0) |
| Brain | 1 (0.9) | 1 (0.9) |
| Measurable disease, n (%) | 90 (83.3) | 92 (84.4) |
| Number of previous CT regimens, n (%) | ||
| 2 | 61 (56.5) | 65 (59.6) |
| 3 | 46 (42.6) | 42 (38.5) |
| 4 | 1 (0.9) | 1 (0.9) |
| 5 | 0 (0) | 1 (0.9) |
| Duration of previous pertuzumab exposure as first‐line therapy, a days | ||
| <180 | 19 (17.6) | 20 (18.3) |
| ≥180 | 65 (60.2) | 66 (60.6) |
| Duration of previous pertuzumab exposure as second‐line therapy, days | ||
| <120 | 4 (3.7) | 2 (1.8) |
| ≥120 | 19 (17.6) | 20 (18.3) |
| Previous exposure to anti‐HER2 therapy for LA/MBC, a n (%) | ||
| Pertuzumab | 107 (99.1) | 108 (99.1) |
| Trastuzumab | 107 (99.1) | 108 (99.1) |
| T‐DM1 | 104 (96.3) | 108 (99.1) |
| Lapatinib | 15 (13.9) | 15 (13.8) |
| Others | 9 (8.3) | 8 (7.3) |
| T‐DM1 as the latest regimen before randomization | 82 (75.9) | 89 (81.7) |
Abbreviations: CT, chemotherapy; ECOG, Eastern Cooperative Oncology Group; LA/MBC, locally advanced/metastatic breast cancer; max, maximum; min, minimum; T‐DM1, trastuzumab emtansine.
One case in each treatment group did not receive pertuzumab for LA/MBC, but received pertuzumab during the perioperative period.
3.2. Efficacy
After a median follow‐up period of 14.2 months, 90 (83.3%) patients in the PTC arm and 94 (86.2%) patients in the TC arm had PFS events. Fourteen of 90 PFS events (15.6%) in the PTC arm and 16 of 94 PFS events (17.0%) in the TC arm were judged to be clinical progression (Table S4). The PTC regimen was associated with a significant improvement in median PFS (5.3 months [95% CI, 4.0–6.6]) compared with TC (4.2 months [95% CI, 3.2–4.8]; stratified HR 0.76 [one‐sided 95% CI upper limit, 0.967]; p = 0.022; Figure 2A). According to a prespecified subgroup analysis, the benefit of PTC in relation to improvement in PFS was present for all subgroups (Figure 3).
FIGURE 2.
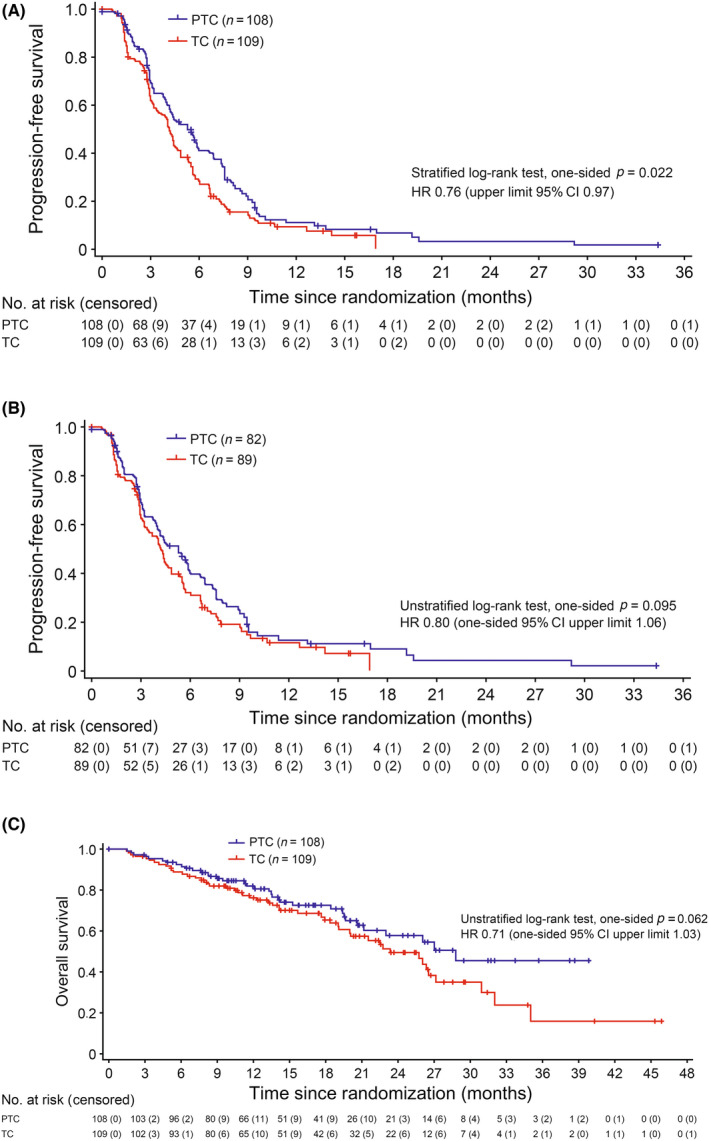
Survival among 219 women with HER2‐positive advanced breast cancer treated with pertuzumab + trastuzumab + chemotherapy (PTC) or trastuzumab + chemotherapy (TC). (A) Progression‐free survival assessed by investigators (intention‐to‐treat [ITT] population). (B) Progression‐free survival in patients treated with trastuzumab emtansine as the latest regimen (ITT population). (C) Overall survival (ITT population). CI, confidence interval; HR, hazard ratio
FIGURE 3.
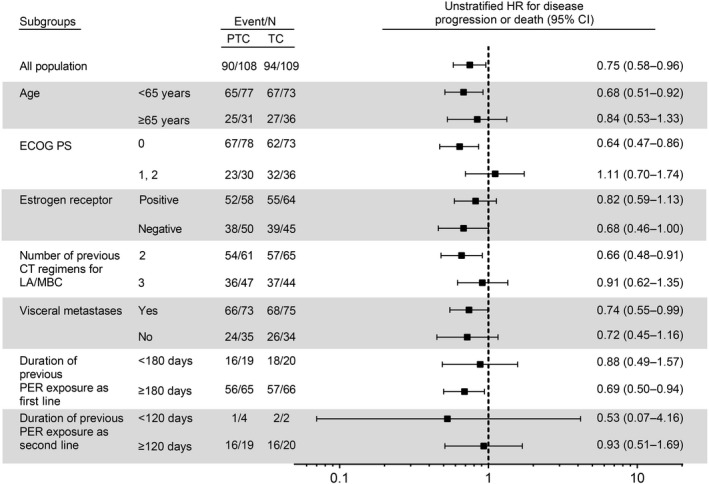
Prespecified progression‐free survival subgroup analysis among 219 women with HER2‐positive advanced breast cancer treated with pertuzumab (PER) + trastuzumab + chemotherapy (PTC) or trastuzumab + chemotherapy (TC). CI, confidence interval; CT, chemotherapy; HR, hazard ratio; LA/MBC, locally advanced/metastatic breast cancer; PS, performance status
Among patients treated with T‐DM1 as the latest regimen, median PFS also showed a similar trend towards improvement with PTC (median PFS 5.3 months [95% CI, 3.7–6.6] for PTC compared with 4.2 months [95% CI, 3.2–5.3] for TC; unstratified HR 0.80 [one‐sided 95% CI upper limit, 1.06]; p = 0.095; Figure 2B).
Thirty‐five (32.4%) patients in the PTC arm died compared with 49 (45.0%) patients in the TC arm. Median OS showed a trend towards improvement with PTC (median PFS 28.8 months [95% CI, 21.2–NR] for PTC compared with 23.4 months [95% CI, 19.1–27.1] for TC; unstratified HR 0.71 [one‐sided 95% CI upper limit, 1.03]; p = 0.062; Figure 2C).
The ORR in the PTC arm (19.5 [95% CI upper limit, 27.9]%) was similar to that in the TC arm (20.7 [95% CI upper limit, 29.1]%; odds ratio 0.957 [95% CI upper limit, 1.778]; Table 2). However, the DoR in the PTC arm (8.3 [95% CI upper limit, 18.2] months) was longer than in the TC arm (4.1 [95% CI upper limit, 13.4] months; stratified HR 0.66 [one‐sided 95% CI upper limit, 1.369]; unstratified HR 0.49 [one‐sided 95% CI upper limit, 0.945]; Table 2).
TABLE 2.
Summary of best overall response, overall response rate, and duration of response by investigator assessment (measurable disease) among 219 women with HER2‐positive advanced breast cancer treated with pertuzumab + trastuzumab + chemotherapy (PTC) or trastuzumab + chemotherapy (TC)
| PTC (N = 90) | TC (N = 92) | |
|---|---|---|
| Best overall response, n (%) | ||
| CR | 0 (0.0) | 0 (0.0) |
| PR | 17 (18.9) | 18 (19.6) |
| SD | 57 (63.3) | 49 (53.3) |
| PD | 13 (14.4) | 20 (21.7) |
| Unknown | 1 (1.1) | 3 (3.3) |
| Missing | 2 (2.2) | 2 (2.2) |
| Overall response rate (CR + PR), n (%) | 17 (19.5) | 18 (20.7) |
| (upper limit 95% CI) | (27.9) | (29.1) |
| Odds ratio for overall response (upper limit 95% CI) | 0.957 (1.778) | |
| Duration of response (CR + PR) | n = 17 | n = 18 |
| Median (upper limit 95% CI), months | 8.3 (18.2) | 4.1 (13.4) |
| Unstratified HR (95% CI upper limit) | 0.490 (0.945) | |
| Stratified HR (95% CI upper limit) | 0.656 (1.369) | |
Abbreviations: CI, confidence interval; CR, complete response; HR, hazard ratio; PD, progressive disease; PR, partial response; SD, stable disease.
3.3. Safety
Duration of treatment exposure for patients who received PTC was longer than for patients who received TC (median [minimum, maximum] number of cycles on study treatment 7.0 (0, 54) for PTC arm vs. 5.0 1 , 25 for TC arm; Table S5). Differences between treatment groups in terms of physician's choice of chemotherapy mainly related to eribulin (more common with PTC) and vinorelbine (more common with TC, Table S5). There were no statistically significant differences between the two groups in the frequency of AEs of grade 3 or higher, serious AEs, death from AEs, treatment discontinuation due to AEs, or chemotherapy dose reduction due to AEs (Table 3). One AE‐related death was recorded each in the PTC arm (drug‐induced lung injury) and TC arm (respiratory failure).
TABLE 3.
Summary of adverse events (AEs) among 219 women with HER2‐positive advanced breast cancer treated with pertuzumab + trastuzumab + chemotherapy (PTC) or trastuzumab + chemotherapy (TC)
| AEs, n (%) | PTC (N = 105) | TC (N = 108) | p value |
|---|---|---|---|
| Grade ≥3 AE | 65 (61.9) | 75 (69.4) | 0.253 |
| Serious AE | 19 (18.1) | 23 (21.3) | 0.608 |
| Death due to AE | 1 (1.0) | 1 (0.9) | 1.000 |
| Treatment discontinuation due to AE | 19 (18.1) | 11 (10.2) | 0.115 |
| Dose reduction of chemotherapeutic agents due to grade ≥3 AE | 26 (24.8) | 27 (25.0) | 1.000 |
| LVEF (<50% or ≥ 15% reduction from baseline) | 3 (2.9) | 0 (0.0) | 0.118 |
| AEs of special interests, n (%) | All grade | Grade ≥3 | All grade | Grade ≥3 |
|---|---|---|---|---|
| Stomatitis | 24 (22.9) | 2 (1.9) | 17 (15.7) | 2 (1.9) |
| Diarrhea | 22 (21.0) | 3 (2.9) | 7 (6.5) | 1 (0.9) |
| Neutropaenia | 82 (78.1) | 48 (45.7) | 79 (73.1) | 52 (48.1) |
| Febrile neutropaenia | 16 (15.2) | 16 (15.2) | 18 (16.7) | 18 (16.7) |
| Infusion reaction | 2 (1.9) | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| Cardiac dysfunction | 1 (1.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| Skin and subcutaneous disorder | 24 (22.9) | 0 (0.0) | 18 (16.7) | 2 (1.9) |
Abbreviations: LVEF, left ventricular ejection fraction.
Regarding AEs of special interest, any‐grade diarrhea was more common in the PTC arm, in which one patient with cardiac dysfunction was also recorded (Table 3). However, there was no statistical difference in the transition of LVEF values between the two groups (Figure S1). Grade 3 or higher AEs, other than those of special interest, occurred at a similar frequency in the PTC and TC arms (Tables S6 and S7).
3.4. Heath‐related quality of life
In terms of HR‐QoL, the analysis population was comprised of 87 (80.6%) patients in the PTC arm and 91 (83.5%) patients in the TC arm. Overall, there were 51 events (58.6%) in the PTC arm and 46 events (50.5%) in the TC arm. The median TTD was 2.8 (95% CI, 1.6–4.3) months with active treatment and 4.3 (95% CI, 2.9–6.0) months with control treatment (stratified HR 1.28 [95% CI, 0.856–1.903]; Figure 4). However, the difference between groups was nonsignificant according to the log–rank test (p = 0.231).
FIGURE 4.
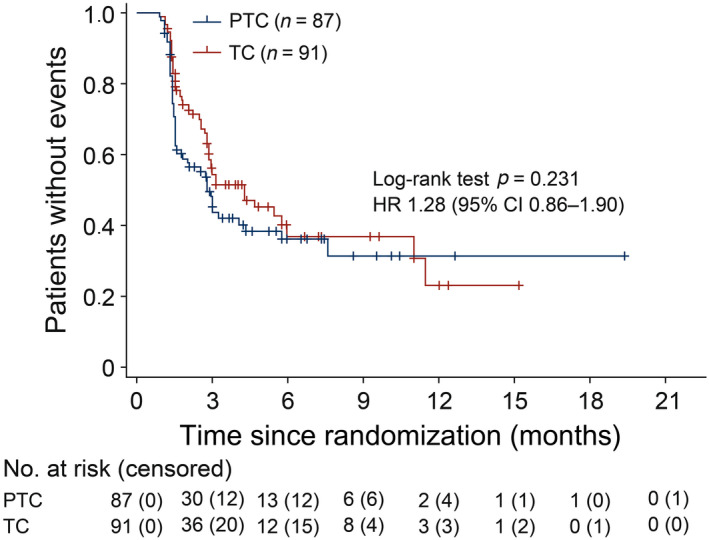
Time to deterioration in Functional Assessment of Cancer Therapy – Breast Trial Outcome Index (intention‐to‐treat population) among women with HER2‐positive advanced breast cancer treated with pertuzumab + trastuzumab + chemotherapy (PTC) or trastuzumab + chemotherapy (TC). CI, confidence interval; HR, hazard ratio
3.5. Sensitivity analysis
According to a sensitivity analysis using the FAS population (PTC arm, n = 104; TC arm, n = 106; Figure 1), patient background characteristics were well balanced between the two treatment groups (Tables S8, S9, S10). As assessed by investigators, PFS in patients with T‐DM1 as the latest regimen, ORR, DoR, and OS in the FAS population were similar to those for the ITT population (Tables S11, S12; Figures S2, S3, S4). Table S12 summarizes the effectiveness results in the ITT and FAS populations.
4. DISCUSSION
This is the first report to assess the efficacy and safety of pertuzumab retreatment in patients previously treated with pertuzumab in combination with TC for HER2‐positive locally advanced or metastatic breast cancer. Pertuzumab added to standard trastuzumab and chemotherapy significantly improved PFS without HR‐QoL deterioration for that setting. Retreatment with pertuzumab did not improve ORR, but stable disease was 10% higher and progressive disease was 7% lower, and the DoR was prolonged by 4 months. This leads to the thought that there is a PFS prolongation effect of pertuzumab retreatment. This study also showed that, among patients who had received T‐DM1, dual HER2 blockade with pertuzumab and trastuzumab tended to prolong PFS more than single HER2 blockade with trastuzumab.
The rationale for dual HER2 blockade by adding pertuzumab to trastuzumab in breast cancer could be related to inhibition of HER3 as a potential therapeutic target. 22 Human epidermal growth factor receptor 2 forms a heterodimer or homodimer with the HER family and activates intracellular signals, such as the PI3K pathway and the MAPK pathway. Among these dimers, the ligand‐dependent HER2–HER3 signal has the strongest proliferative activity. 23 , 24 Trastuzumab can block ligand‐independent HER2–HER3 signals, 25 but cannot block ligand‐dependent HER2–HER3 signals. Pertuzumab, in contrast, can block ligand‐gated HER2–HER3 signals. 26 The combination of both can block a wide range of HER2 signals. 27 , 28 In the CLEOPATRA study undertaken in the first‐line setting for patients with HER2‐positive metastatic breast cancer, the addition of pertuzumab to trastuzumab in combination with docetaxel significantly improved PFS and OS versus addition of placebo. 10 A subgroup study of CLEOPATRA using outcomes similar to those in the present trial also concluded that combining pertuzumab with trastuzumab and docetaxel had no adverse impact on HR‐QoL. 20 Following progression after first‐line trastuzumab‐based dual blockade therapy, T‐DM1 has become the standard second‐line treatment based on high quality evidence from several studies such as TH3RESA 3 and EMILIA. 4 , 29 The Marianne trial, which examined the effect of adding pertuzumab to T‐DM1, failed to show that pertuzumab and T‐DM1 was superior to T‐DM1 in first‐line treatment. 30 Although no comparative studies of T‐DM1 versus pertuzumab and T‐DM1 in second‐ or later‐line treatment have been carried out, T‐DM1 monotherapy is still the most recommended second‐line treatment. In in vitro and in vivo studies, pertuzumab and trastuzumab significantly suppressed tumor growth activity and tumor growth of T‐DM1‐resistant HER2‐positive cell lines compared to single treatment with trastuzumab or pertuzumab. 31 The present open‐label randomized controlled trial provided results that support this basic research.
Few effective options are available for HER2‐positive advanced breast cancer that progresses after second‐ or later‐line therapy. However, recent studies have sought to address this therapeutic need. Tucatinib, an oral, selective inhibitor of the HER2 tyrosine kinase, has been investigated for heavily pretreated HER2‐positive metastatic breast cancer previously treated with trastuzumab, pertuzumab, and T‐DM1 and shown promising PFS and OS results, including in patients with brain metastases. 5 Margetuximab is a chimeric, Fc‐engineered, immune‐activating mAb with enhanced innate and adaptive immunity compared with trastuzumab. A randomized trial (SOPHIA) showed that margetuximab plus chemotherapy improved median PFS by investigator assessment compared with trastuzumab (5.7 vs. 4.4 months; HR 0.70 [95% CI, 0.56–0.87]; p = 0.001). 7 These results are similar to those of the present trial although, in contrast, the ORR in the SOPHIA trial favored margetuximab treatment whereas the DoR was not significantly different between treatment groups. Trastuzumab deruxtecan, an Ab–drug conjugate of trastuzumab, has shown promising results in an open‐label, single‐group, multicenter, phase II study in HER2‐positive metastatic breast cancer previously treated with T‐DM1. 6 However, trastuzumab deruxtecan was associated with interstitial lung disease among approximately 14% of patients, which would require careful monitoring. Also, data from larger, randomized studies of trastuzumab deruxtecan are awaited to fully understand its role in these patients.
Although emerging treatment options after T‐DM1 exist, not all countries have ready or affordable access to these drugs. Therefore, from a practical prescribing perspective, these study results show that retreatment with HER2 dual blockade is effective and an option for third‐ or fourth‐line treatment in countries where the above drugs are not available. Furthermore, as use of this combination is established, side‐effects related to anti‐HER2 therapy are readily predictable. Of particular importance, there is no clear guideline on how to treat recurrence in patients who have received pertuzumab and/or T‐DM1 in the neoadjuvant and adjuvant settings. Recently, based on the results of the Neosphere and TRYPHAENA trials, 32 , 33 which showed the additional effect of pertuzumab as preoperative treatment, and the APHINITY trial, 34 which showed the additional effect of pertuzumab as postoperative treatment, the use of pertuzumab in neoadjuvant and/or adjuvant settings is widely used. Furthermore, the KATHERINE study 35 verified the effect of postoperative T‐DM1 on residual invasive disease after completion of taxane‐based neoadjuvant chemotherapy plus anti‐HER2 therapy using trastuzumab and underlies the increasing use of T‐DM1 in this setting. However, there are few data on anti‐HER2 therapy for recurrent disease in patients who receive pertuzumab perioperatively or T‐DM1 postoperatively and more data are needed to address the efficacy of PTC specifically in this patient population. Based on this background and the findings of this study, dual HER2 blockade with pertuzumab and trastuzumab is expected to be more effective than single HER2 blockade with trastuzumab for postoperative recurrence among patients treated with pertuzumab and trastuzumab before and/or after surgery. Furthermore, PTC is expected to be more effective than trastuzumab and chemotherapy in recurrent patients during or after postoperative T‐DM1 for residual invasive tumor after neoadjuvant anti‐HER2 therapy.
The main limitations of this study are the open‐label study design and the fact that the planned enrolment has not yet been reached. Furthermore, there is a slight bias between the two groups in the chemotherapy selection with, in particular, more eribulin coadministered in the PTC group. This study used available medications and one person assigned to the TC group was actually treated with pertuzumab. Furthermore, cross‐over was allowed and, including the above issues, a total of seven patients with eligibility violations were included in the ITT analysis. However, in the sensitivity analysis using the FAS, the PFS assessed by the attending physician was also significantly prolonged in the pertuzumab‐containing group. However, this limitation is likely to underestimate rather than overestimate the true treatment effect of the dual blockade therapy. The introduction of new drugs has dramatically changed the landscape of postoperative and recurrent treatment for HER2‐positive breast cancer, but this result seems to be extremely important for considering the treatment resistance mechanism of pertuzumab in combination with trastuzumab. Currently, we are undertaking translational research to assist selection of cases that require pertuzumab addition.
In conclusion, these results suggest that retreatment with pertuzumab as third‐ or fourth‐line chemotherapy could be considered for patients with HER2‐positive locally advanced or metastatic breast cancer previously treated with pertuzumab‐containing regimens.
AUTHOR CONTRIBUTIONS
Y.Y. contributed to the literature search. N.M., T.T. (Toyama), H.T., F.H., and S.S. contributed to the conception and design of the study. T.T. (Takano) and H.T. contributed to the study resources. N.M. and T.Y. contributed to data curation. N.T., T.Y., and S.S. contributed to the formal analysis. T.T. (Toyama) and S.S. contributed to study supervision. N.T., N.M., T.Y. (Yoshinami), T.Y. (Yamanaka), H.T., F.H., and S.S. contributed to the investigation. N.T. and T.Y. (Yoshinami) contributed to the methodology. N.M. and T.Y. (Yoshinami) contributed to project administration. N.T. and T.T. (Toyama) were involved in writing the original draft. N.T., N.M., T.Y. (Yoshinami), T.Y. (Yamanaka), T.T. (Takano), F.H., and S.S. were involved in reviewing and editing the manuscript. All authors approved the final manuscript.
FUNDING INFORMATION
Chugai Pharmaceutical Co., Ltd.
DISCLOSURE
Y.Y. receives lecture fees, honoraria, or other fees from AstraZeneca K.K., Chugai Pharmaceutical Co., Ltd., and Pfizer Japan Inc., research funds from AstraZeneca K.K., Chugai Pharmaceutical Co., Ltd., Daiichi‐Sankyo Co., Ltd., and MSD K.K., and scholarship endowments from Chugai Pharmaceutical Co., Ltd. and Kyowa Kirin Co. Ltd. H.I. receives research funds from AstraZeneca K.K., Chugai Pharmaceutical Co., Ltd., Daiichi‐Sankyo Co., Ltd., Eli Lilly and Company, and MSD K.K. N.M. receives lecture fees, honoraria, or other fees from AstraZeneca K.K., Chugai Pharmaceutical Co., Ltd., Eisai Co. Ltd., Eli Lilly and Company, and Pfizer Japan Inc., research funds from AstraZeneca K.K., Chugai Pharmaceutical Co. Ltd., Daiichi‐Sankyo Co. Ltd., Eisai Co., Ltd., Eli Lilly and Company, Kyowa Kirin Co. Ltd., MSD K.K., Novartis Pharma K.K., Pfizer Japan Inc., and Sanofi K.K. (contract with the facility), and is a member of the editorial board of Cancer Science. M.T. (Takahashi) receives lecture fees, honoraria, or other fees from AstraZeneca K.K., Eisai Co. Ltd., Eli Lilly and Company, and Pfizer Japan Inc., and scholarship endowments from Kyowa Kirin Co., Ltd. and Taiho Pharmaceutical Co., Ltd. T.Y. (Yoshinami) receives lecture fees, honoraria, or other fees from Chugai Pharmaceutical Co., Ltd., Kyowa Kirin Co. Ltd., and Pfizer Japan Inc. T.U. receives lecture fees, honoraria, or other fees from AstraZeneca K.K., Chugai Pharmaceutical Co., Ltd., Eisai Co. Ltd., and Novartis Pharma K.K. T.T. (Toyama) receives lecture fees, honoraria, or other fees from Pfizer Japan Inc., and research funds from Chugai Pharmaceutical Co., Ltd. and Eisai Co. Ltd. T.T. (Takano) receives lecture fees, honoraria, or other fees from Chugai Pharmaceutical Co., Ltd., Celltrion Healthcare Japan Co., Daiichi‐Sankyo Co. Ltd., Eisai Co. Ltd., and Eli Lilly and Company. K.T. receives lecture fees, honoraria, or other fees from Chugai Pharmaceutical Co., Ltd. and Daiichi‐Sankyo Co. Ltd., research funds from Daiichi‐Sankyo Co. Ltd., Eli Lilly and Company, and Pfizer Japan Inc. F.H. receives lecture fees, honoraria, or other fees from Chugai Pharmaceutical Co., Ltd., Eisai Co., Ltd., Eli Lilly and Company, Kyowa Kirin Co., Ltd., and Pfizer Japan Inc. N.N. receives lecture fees, honoraria, or other fees from AstraZeneca K.K., Chugai Pharmaceutical Co., Ltd., Daiichi‐Sankyo Co., Ltd., Eisai Co., Ltd., Eli Lilly and Company, and Pfizer Japan Inc., research funds from Chugai Pharmaceutical Co., Ltd., Daiichi‐Sankyo Co., Ltd., Eisai Co., Ltd., Nippon Kayaku Co., Ltd., and Pfizer Japan Inc., and is a member of the editorial board of Cancer Science. S.S. receives lecture fees, honoraria, or other fees from AstraZeneca K.K., Chugai Pharmaceutical Co., Ltd., Daiichi‐Sankyo Co., Ltd., Eisai Co., Ltd., Eli Lilly and Company, Kyowa Kirin Co., Ltd., and Pfizer Japan Inc., research funds from Chugai Pharmaceutical Co., Ltd. and Daiichi‐Sankyo Co., Ltd., scholarship endowments from Chugai Pharmaceutical Co., Ltd. and Taiho Pharmaceutical Co., Ltd., and is a member of the editorial board of Cancer Science. S.M. receives lecture fees, honoraria, or other fees from AstraZeneca K.K., Bristol‐Myers Squibb K.K., Chugai Pharmaceutical Co., Ltd., Eli Lilly and Company, MSD K.K., Pfizer Japan Inc., and Taiho Pharmaceutical Co., Ltd. M.T. (Toi) receives lecture fees, honoraria, or other fees from AstraZeneca K.K., Daiichi‐Sankyo Co., Ltd., and Eli Lilly and Company, research funds from AFI Corporation, Astellas Pharma Inc., AstraZeneca K.K., GL Sciences Inc., Kyoto Breast Cancer Research Network, Kyowa‐Kirin Co., Ltd., Shimadzu Corporation, Taiho Pharmaceutical Co., Ltd., and Yakult Honsha Co., Ltd., scholarship endowments from Chugai Pharmaceutical Co., Ltd., Eisai Co., Ltd., and Nippon Kayaku Co., Ltd., and is a member of the editorial board of Cancer Science. S.O. receives lecture fees, honoraria, or other fees from AstraZeneca K.K., Chugai Pharmaceutical Co., Ltd., Eli Lilly and Company, and Pfizer Japan Inc., research funds from Eisai Co., Ltd., and is a member of the editorial board of Cancer Science. The other authors have no conflict of interest to disclose.
ETHICAL APPROVAL
This study was conducted in accordance with the Declaration of Helsinki and the Ethical Guidelines for Clinical Research of the Japanese Ministry of Health, Labor and Welfare. An independent ethics committee for each participating site approved the protocol and any modifications. This trial was registered with ClinicalTrials.gov (NCT02514681), Japan Registry of Clinical Trials (jRCTs041180153), and the University Hospital Medical Information Network (UMIN000018202).
CONSENT TO PARTICIPATE
Each participant provided written informed consent before enrolment.
CONSENT FOR PUBLICATION
No identifying patient data were presented for publication.
Supporting information
Figure S1
Figure S2
Figure S3
Figure S4
Table S1
Table S2
Table S3
Table S4
Table S5
Table S6
Table S7
Table S8
Table S9
Table S10
Table S11
Table S12
ACKNOWLEDGMENTS
This study was supported by the Japan Breast Cancer Research Group. Research funding was provided to the Japan Breast Cancer Research Group by Chugai Pharmaceutical Corporation under a research agreement. We would like to thank patients, their families, researchers from 93 institutions, and Clinical Research Coordinators for participating in the PRECIOUS trial. Patient registration, randomization, and data management were performed by Chikako Hibi and Shina Kurai of the JBCRG Data Center. The data were analyzed by Masao Shionoya of Mebics. Medical writing and editorial assistance were provided by Mark Snape, Yoshiko Okamoto, and Yasutomo Yutoku of inScience Communications, Springer Healthcare. Finally, I would like to express my gratitude to all the Japan Breast Cancer Research Group staff, including Michiro Soma, Nobuko Aoki, Kiyomi Nishizawa, and Mieko Abe, for their great contribution to the completion of this study.
Yamamoto Y, Iwata H, Taira N, et al. Pertuzumab retreatment for HER2‐positive advanced breast cancer: A randomized, open‐label phase III study (PRECIOUS). Cancer Sci. 2022;113:3169‐3179. doi: 10.1111/cas.15474
Trial Registration: ClinicalTrials.gov (NCT02514681), Japan Registry of Clinical Trials (jRCTs041180153), University Hospital Medical Information Network (UMIN000018202).
DATA AVAILABILITY STATEMENT
Deidentified patient data will be made available upon reasonable request. Requests for data access should be made in writing, including details of how the data will be used, and addressed to the corresponding author. Approval for data sharing will be considered based on the scientific merit, feasibility, and timeliness of the request.
REFERENCES
- 1. Aihara T, Toyama T, Takahashi M, et al. The Japanese Breast Cancer Society Clinical Practice Guideline for systemic treatment of breast cancer, 2015 edition. Breast Cancer. 2016;23(3):329‐342. [DOI] [PubMed] [Google Scholar]
- 2. Giordano SH, Temin S, Davidson NE. Systemic therapy for patients with advanced human epidermal growth factor receptor 2‐positive breast cancer: ASCO clinical practice guideline update summary. J Oncol Pract. 2018;14(8):501‐504. [DOI] [PubMed] [Google Scholar]
- 3. Krop IE, Kim SB, González‐Martín A, et al. Trastuzumab emtansine versus treatment of physician's choice for pretreated HER2‐positive advanced breast cancer (TH3RESA): a randomised, open‐label, phase 3 trial. Lancet Oncol. 2014;15(7):689‐699. [DOI] [PubMed] [Google Scholar]
- 4. Verma S, Miles D, Gianni L, et al. Trastuzumab emtansine for HER2‐positive advanced breast cancer. N Engl J Med. 2012;367(19):1783‐1791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Murthy RK, Loi S, Okines A, et al. Tucatinib, trastuzumab, and capecitabine for HER2‐positive metastatic breast cancer. N Engl J Med. 2020;382(7):597‐609. [DOI] [PubMed] [Google Scholar]
- 6. Modi S, Saura C, Yamashita T, et al. Trastuzumab deruxtecan in previously treated HER2‐positive breast cancer. N Engl J Med. 2019;382(7):610‐621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Rugo HS, Im SA, Cardoso F, et al. Efficacy of margetuximab vs trastuzumab in patients with pretreated ERBB2‐positive advanced breast cancer: a phase 3 randomized clinical trial. JAMA Oncol. 2021;7(4):573‐584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. von Minckwitz G, du Bois A, Schmidt M, et al. Trastuzumab beyond progression in human epidermal growth factor receptor 2‐positive advanced breast cancer: a german breast group 26/breast international group 03‐05 study. J Clin Oncol. 2009;27(12):1999‐2006. [DOI] [PubMed] [Google Scholar]
- 9. von Minckwitz G, Schwedler K, Schmidt M, et al. Trastuzumab beyond progression: overall survival analysis of the GBG 26/BIG 3‐05 phase III study in HER2‐positive breast cancer. Eur J Cancer. 2011;47(15):2273‐2281. [DOI] [PubMed] [Google Scholar]
- 10. Baselga J, Cortés J, Kim S‐B, et al. Pertuzumab plus trastuzumab plus docetaxel for metastatic breast cancer. N Engl J Med. 2011;366(2):109‐119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Dang C, Iyengar N, Datko F, et al. Phase II study of paclitaxel given once per week along with trastuzumab and pertuzumab in patients with human epidermal growth factor receptor 2‐positive metastatic breast cancer. J Clin Oncol. 2015;33(5):442‐447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Bachelot T, Ciruelos E, Schneeweiss A, et al. Preliminary safety and efficacy of first‐line pertuzumab combined with trastuzumab and taxane therapy for HER2‐positive locally recurrent or metastatic breast cancer (PERUSE). Ann Oncol. 2019;30(5):766‐773. [DOI] [PubMed] [Google Scholar]
- 13. Perez EA, López‐Vega JM, Petit T, et al. Safety and efficacy of vinorelbine in combination with pertuzumab and trastuzumab for first‐line treatment of patients with HER2‐positive locally advanced or metastatic breast cancer: VELVET Cohort 1 final results. Breast Cancer Res. 2016;18(1):126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Araki K, Fukada I, Yanagi H, et al. First report of eribulin in combination with pertuzumab and trastuzumab for advanced HER2‐positive breast cancer. Breast. 2017;35:78‐84. [DOI] [PubMed] [Google Scholar]
- 15. Urruticoechea A, Rizwanullah M, Im SA, et al. Randomized phase III trial of trastuzumab plus capecitabine with or without pertuzumab in patients with human epidermal growth factor receptor 2‐positive metastatic breast cancer who experienced disease progression during or after trastuzumab‐based therapy. J Clin Oncol. 2017;35(26):3030‐3038. [DOI] [PubMed] [Google Scholar]
- 16. Iyengar NM, Smyth LM, Lake D, et al. Efficacy and safety of gemcitabine with trastuzumab and pertuzumab after prior pertuzumab‐based therapy among patients with human epidermal growth factor receptor 2‐positive metastatic breast cancer: a phase 2 clinical trial. JAMA Netw Open. 2019;2(11):e1916211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Japan Clinical Oncology Group . Common Terminology Criteria for Adverse Events (CTCAE) version 4.0. 2021. Available at: http://www.jcog.jp/doctor/tool/ctcaev4.html. Accessed 12 April 2021.
- 18. Schwartz LH, Litière S, de Vries E, et al. RECIST 1.1—update and clarification: from the RECIST committee. Eur J Cancer. 2016;62:132‐137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Brady MJ, Cella DF, Mo F, et al. Reliability and validity of the Functional Assessment of Cancer Therapy – breast quality‐of‐life instrument. J Clin Oncol. 1997;15(3):974‐986. [DOI] [PubMed] [Google Scholar]
- 20. Cortés J, Baselga J, Im YH, et al. Health‐related quality‐of‐life assessment in CLEOPATRA, a phase III study combining pertuzumab with trastuzumab and docetaxel in metastatic breast cancer. Ann Oncol. 2013;24(10):2630‐2635. [DOI] [PubMed] [Google Scholar]
- 21. Eton DT, Cella D, Yost KJ, et al. A combination of distribution‐ and anchor‐based approaches determined minimally important differences (MIDs) for four endpoints in a breast cancer scale. J Clin Epidemiol. 2004;57(9):898‐910. [DOI] [PubMed] [Google Scholar]
- 22. Lee‐Hoeflich ST, Crocker L, Yao E, et al. A central role for HER3 in HER2‐amplified breast cancer: implications for targeted therapy. Cancer Res. 2008;68(14):5878‐5887. [DOI] [PubMed] [Google Scholar]
- 23. Citri A, Skaria KB, Yarden Y. The deaf and the dumb: the biology of ErbB‐2 and ErbB‐3. Exp Cell Res. 2003;284(1):54‐65. [DOI] [PubMed] [Google Scholar]
- 24. Tzahar E, Waterman H, Chen X, et al. A hierarchical network of interreceptor interactions determines signal transduction by Neu differentiation factor/neuregulin and epidermal growth factor. Mol Cell Biol. 1996;16(10):5276‐5287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Junttila TT, Akita RW, Parsons K, et al. Ligand‐independent HER2/HER3/PI3K complex is disrupted by trastuzumab and is effectively inhibited by the PI3K inhibitor GDC‐0941. Cancer Cell. 2009;15(5):429‐440. [DOI] [PubMed] [Google Scholar]
- 26. Franklin MC, Carey KD, Vajdos FF, Leahy DJ, de Vos AM, Sliwkowski MX. Insights into ErbB signaling from the structure of the ErbB2‐pertuzumab complex. Cancer Cell. 2004;5(4):317‐328. [DOI] [PubMed] [Google Scholar]
- 27. Nahta R, Hung MC, Esteva FJ. The HER‐2‐targeting antibodies trastuzumab and pertuzumab synergistically inhibit the survival of breast cancer cells. Cancer Res. 2004;64(7):2343‐2346. [DOI] [PubMed] [Google Scholar]
- 28. Scheuer W, Friess T, Burtscher H, Bossenmaier B, Endl J, Hasmann M. Strongly enhanced antitumor activity of trastuzumab and pertuzumab combination treatment on HER2‐positive human xenograft tumor models. Cancer Res. 2009;69(24):9330‐9336. [DOI] [PubMed] [Google Scholar]
- 29. Diéras V, Miles D, Verma S, et al. Trastuzumab emtansine versus capecitabine plus lapatinib in patients with previously treated HER2‐positive advanced breast cancer (EMILIA): a descriptive analysis of final overall survival results from a randomised, open‐label, phase 3 trial. Lancet Oncol. 2017;18(6):732‐742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Perez EA, Barrios C, Eiermann W, et al. Trastuzumab emtansine with or without pertuzumab versus trastuzumab plus taxane for human epidermal growth factor receptor 2‐positive, advanced breast cancer: primary results from the phase III MARIANNE study. J Clin Oncol. 2017;35(2):141‐148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Yamashita‐Kashima Y, Shu S, Osada M, et al. Combination efficacy of pertuzumab and trastuzumab for trastuzumab emtansine‐resistant cells exhibiting attenuated lysosomal trafficking or efflux pumps upregulation. Cancer Chemother Pharmacol. 2020;86(5):641‐654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Gianni L, Pienkowski T, Im YH, et al. Efficacy and safety of neoadjuvant pertuzumab and trastuzumab in women with locally advanced, inflammatory, or early HER2‐positive breast cancer (NeoSphere): a randomised multicentre, open‐label, phase 2 trial. Lancet Oncol. 2012;13(1):25‐32. [DOI] [PubMed] [Google Scholar]
- 33. Schneeweiss A, Chia S, Hickish T, et al. Pertuzumab plus trastuzumab in combination with standard neoadjuvant anthracycline‐containing and anthracycline‐free chemotherapy regimens in patients with HER2‐positive early breast cancer: a randomized phase II cardiac safety study (TRYPHAENA). Ann Oncol. 2013;24(9):2278‐2284. [DOI] [PubMed] [Google Scholar]
- 34. von Minckwitz G, Procter M, de Azambuja E, et al. Adjuvant pertuzumab and trastuzumab in early HER2‐positive breast cancer. N Engl J Med. 2017;377(2):122‐131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. von Minckwitz G, Huang C‐S, Mano MS, et al. Trastuzumab emtansine for residual invasive HER2‐positive breast cancer. N Engl J Med. 2018;380(7):617‐628. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1
Figure S2
Figure S3
Figure S4
Table S1
Table S2
Table S3
Table S4
Table S5
Table S6
Table S7
Table S8
Table S9
Table S10
Table S11
Table S12
Data Availability Statement
Deidentified patient data will be made available upon reasonable request. Requests for data access should be made in writing, including details of how the data will be used, and addressed to the corresponding author. Approval for data sharing will be considered based on the scientific merit, feasibility, and timeliness of the request.


