Abstract
Biobanks are biorepositories that collect, process, store, catalog, and distribute human biological samples, and record the associated data. The role and action field of these strategic infrastructures for implementing precision medicine in translational research is continuously evolving. To ensure the optimal quality at all stages of biobanking, specific protocols are required and should be elaborated according to updated guidelines, recommendations, laws, and rules. This article illustrates the standard operating procedures, including protocols, troubleshooting, and quality controls, of a fully certified biobank in a referral Cancer Center. This model involves all clinical departments and research groups to support the dual mission of academic cancer centers, i.e. to provide high-quality care and high-quality research. All biobanking activities based on the type of biological specimens are detailed and the most tricky methodological aspects are discussed, from patients’ informed consent to specimen management.
Keywords: biobank, translational research, biomarkers, cancer research, tissue samples, liquid biopsy, standard operating procedures, quality control
Introduction
Modern oncologic research requires that high-quality biological samples and the associated data are collected, tracked, processed, stored, cataloged, and distributed to research groups and collaborating partners (Kinkorová, 2015). This integrated biobanking approach has led to breakthroughs in both biomarker discovery and drug development (Pagni et al., 2019). Biobanks thus represent essential resources for basic, translational, and clinical research but they also act as key players linking academic research and the pharmaceutical biotechnology industry (Vaught et al., 2011; Hewitt and Watson, 2013; Jose et al., 2018; Coppola et al., 2019). Moreover, the ability to integrate not only clinical information but also biospecimens into big data repositories has intensified the centrality of biobanks (Margolis et al., 2014; Kinkorová, 2015; Drosou et al., 2017; Kinkorová and Topolčan, 2020). This is especially important as biorepositories have begun to incorporate patient information with comprehensive clinicopathologic, epidemiologic, and demographic data, together with multi-omics molecular information (Braun et al., 2014; Luo et al., 2014; Leff and Yang, 2015; Saifuddin et al., 2017; Bycroft et al., 2018; Hulsen et al., 2019; Bonnechère et al., 2021). The collection of this increasing amount of data requires strict quality controls and standard operating procedures (SOPs). The genomic and post-genomic field area has generated a high demand for high-quality biospecimen and data. Biorepositories in cancer research support scientists and clinicians to obtain disease-specific insights. For these reasons, biobanks should be established and updated following international guidelines, such as those from the International Agency for Research on Cancer (IARC), U.S. National Cancer Institute, United Kingdom. Confederation of Cancer Biobanks, and International Society for Biological and Environmental Repositories (ISBER), recommendations, laws, and rules (Vaught et al., 2009; Sanchini et al., 2016; Mendy et al., 2017). In this respect, networking is essential for sharing materials and data among institutions and research groups, particularly for the study of rare diseases (Montserrat and Taruscio, 2019).
Here, we present the organization of a fully certified (UNI EN ISO 9001:2015 - Certiquality) biobank in a referral Cancer Center, which is an integral part of the Italian node of the European Research Infrastructure on Biobanking (BBMRI-ERIC) (Salvaterra and Corfield, 2017). This facility works in compliance with the new standard ISO 20387:2018 “Biotechnology - Biobanking - General requirements for Biobanks”. All SOPs herein reported fulfilled the BBMRI-ERIC quality control and audits (https://www.bbmri-eric.eu/services/quality-management/). Protocols and best practices for the collection of surgical tissue samples, as well as biofluids (e.g., plasma, serum, blood, urine), cell cultures, and peripheral blood mononuclear cells (PBMC), are described in detail (Kanof et al., 2001; Elliott and Peakman, 2008; Guerin et al., 2010; Mallone et al., 2011; Fisher et al., 2018; Hojat et al., 2019; Rolfo et al., 2021). This model enables collaboration among research groups and industry, allowing patients to be an integral part of translational research.
Scientific/ethical approval and patients’ recruitment
All procedures involving biobanks must be approved by the local Scientific and Technical Committee, the Ethics Committee, and the directors of the involved clinical Units and surgical programs, according to the 1964 Declaration of Helsinki, the 2018 General Data Protection Regulation (GDPR), and subsequent amendments (Sanchini et al., 2016). In this prototype, the GDPR is represented by the Scientific Research Participation Agreement (SRPA), which is the standard informed consent that patients sign to donate biological samples, sensitive data, or genetic data at the European Institute of Oncology in Milan, Italy. The SRPA should be obtained from all patients for the storage, processing, and use of the data obtained for scientific purposes. Only the signed SRPA allows for biospecimen collection. Through this agreement, each patient can express his or her will and modalities of scientific research participation. Given the complexity of the concepts described in the SRPA, it is advisable to share educational material with the patients. For example, as a reminder of their first visit, patients can receive a text message whereby the SRPA information is provided. In addition, short engaging videos broadcasted in the waiting rooms can be employed to inform patients about the importance and implications of the SRPA. An example of a cartoon on biobank used at the European Institute of Oncology, Milan, Italy is freely available online (https://vimeo.com/679070846). During each phase of the hospitalization, SRPA can be administered to the patient by qualified professionals, including biobank staff, nurses, physicians, and biomedical personnel. If patients agree to participate in any study, they should receive all the specific study information approved by the Ethics Committee. Informed consent in the form of SRPA is obtained from all patients for their material to be stored in the biobank and used for further studies. Hence, SOPs, guidelines, and recommendations do not permit the collection and storage of biospecimens in the absence of patients’ consent. Therefore, SRPA should be continuously updated to inform patients properly and comprehensively. To solve any patient’s withdrawal from the previous SRPA, a patient sample take-out methodology should be implemented (Schmanski et al., 2021). To obtain the collected samples, researchers, or external collaborators (for-profit or non-profit) should apply to the Biobank Scientific-Technical Committee and/or the Institutional Ethics Committee. A specific form has to be filled by the applicant for evaluation and linked to an approved evaluation of the project.
Management of biological samples
After previous verification of the patients’ SRPA, each biological sample can be collected and treated. Samples may include: fresh and frozen tissue samples related to the patients who underwent surgery; fresh and frozen tissue biopsies; cytological samples (e.g., needle aspirations, excreted, ascites, pleural fluids) from needle aspiration or brushing/scraping, and from affixing to surgically removed tissues for small lesions; biofluids (blood, serum, plasma, PBMC, oral swab, urine, feces, ascites) of patients in pre-hospitalization, patients enrolled in clinical trials, and any other subjects involved in screening projects. Each phase of samples collection should be compliant with the latest ISO standards (e.g. ISO 20387:2018).
Check-in, aliquoting, and distribution
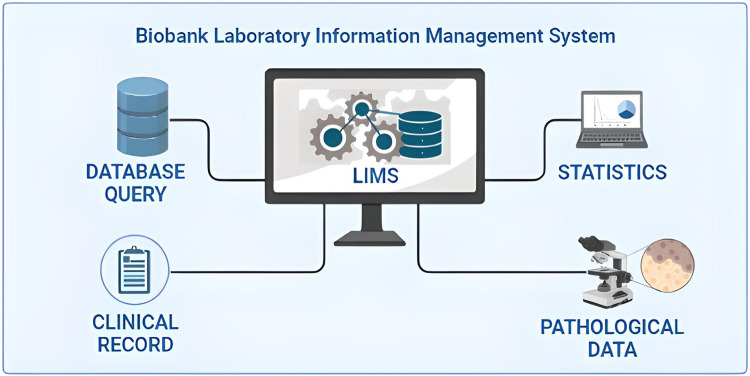
When surgical intervention is scheduled, the Biobank data manager should check in the Surgery Plan if the patient is eligible for samples collection by the signed SRPA presence. If eligible, a surgery plan for biobank must be prepared to check the correspondence of the patient’s inclusion/exclusion criteria, and the patient’s consent for clinical trial or research project. To store and track the significant amount of data generated from the processing and analysis of patients’ biological samples, the use a Laboratory Information Management System (LIMS) software named is highly recommended. This software is considered the biobank neural network because it should ideally interact bidirectionally with all the other softwares and applications used in the Institution, as portrayed in Figure 1. All processing and analysis are tracked by the LIMS, allowing the biobank operators to minimize possible errors, as each type of aliquot is electronically recorded using barcodes (Figure 2). Further, all samples should be divided into subcategories according to the processing, such as the fresh sample, and the frozen sample. The aliquots’ ID is a sequential series of numbers and letters generated by LIMS, according to SOPs, as exemplified for biofluids in Table 1 and tissue samples in Table 2. An example of an ID code is: “12-B1-00100-01” where “12” indicates the year (2012), “B1” indicates the anatomical origin (left breast), and “00100-01” identifies the sample. Once an aliquot is requested, a database query allows the retrieval of the requested samples. This process needs a tracked form with information, a short description of the approved research project, and information on the principal investigator (PI). Consequently, the LIMS generates the requested ID for each aliquot sorted into a picklist. Later, the biobank technicians can check the ID list to retrieve the requested aliquots. Last, barcoded tubes (e.g. Nunc™ Coded Cryobank Vial Systems, Thermo Scientific, Waltham, Massachusetts, US) and relative data can be delivered.
FIGURE 1.
Integration of the laboratory information management system (LIMS) of biobanks in the critical junction of data from different sources.
FIGURE 2.
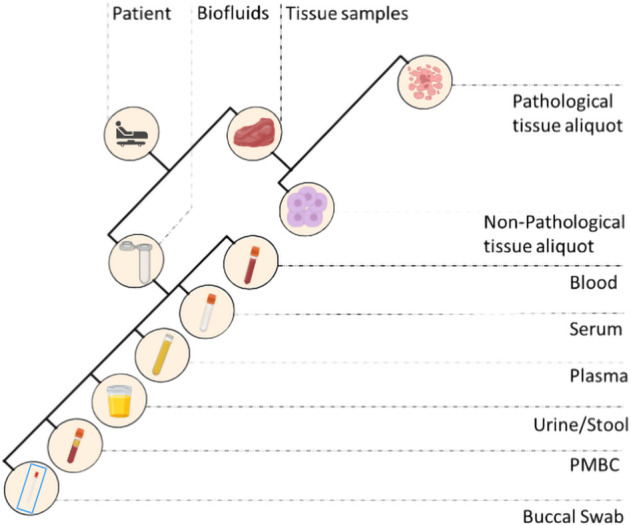
Different types of biospecimens collected in a standard biobank. PMBC, peripheral blood mononuclear cells.
TABLE 1.
Examples of non-tissue sample types and corresponding biobank codes.
| Matrix | Code |
|---|---|
| ASCITES | AS |
| WHOLE BLOOD | BL |
| BRONCHOSCOPY | BS |
| BUCCAL SWAB | BU |
| BUFFY COAT | BC |
| CYTOLOGICAL SAMPLE, NOS | CY |
| FECES | F |
| PERIPHERAL BLOOD MONONUCLEAR CELLS (PBMCs) | PB |
| BLOOD PLASMA | PL |
| BLOOD SERUM | SE |
| TUBE BRUSHING | TB |
| URINE | U |
TABLE 2.
Examples of tissue sample types and corresponding biobank codes.
| Atrix | Code |
|---|---|
| ABDOMEN, NOS | A |
| ADRENAL GLAND | AG |
| BONE TISSUE | BO |
| BONE MARROW | BM |
| BRAIN | BR |
| BREAST, NOS | B |
| BREAST - LEFT | B1 |
| BREAST - RIGHT | B2 |
| MESENTERY | M |
| CERVIX | CE |
| COLON | C |
| ESOPHAGUS | E |
| FIMBRIA, NOS | FI |
| FIMBRIA - LEFT | FI1 |
| FIMBRIA - RIGHT | FI2 |
| KIDNEY | K |
| ILEUM | I |
| LARYNX | LA |
| LIVER | LI |
| LUNG, NOS | L |
| LUNG - LEFT | L1 |
| LUNG - RIGHT | L2 |
| LYMPH NODE | LN |
| LYMPH NODE-ABDOMINAL | LNA |
| MESENTERY | M |
| NASOPHARYNX | NA |
| OMENTUM | OM |
| ORAL CAVITY | OR |
| OROPHARYNX | OP |
| OVARY, NOS | O |
| OVARY - LEFT | O1 |
| OVARY - RIGHT | O2 |
| PANCREAS | PA |
| PHARYNX | PH |
| PERITONEUM | PE |
| PROSTATE | p |
| RECTUM | R |
| SKIN | SK |
| SOFT TISSUES | ST |
| STOMACH | S |
| TESTIS | TE |
| THYMUS | TH |
| THYROID | T |
| TONGUE | TO |
| FALLOPIAN TUBE | TU |
| URINARY BLADDER | UB |
| UTERUS CORPUS | UC |
Sample types
Tissue
During the gross examination of the surgical sample, the pathologist determines whether there is sufficient material (i.e. exceeding diagnosis) for research purposes. When available/possible, the non-pathological counterpart is also collected. Normal and tumor samples are labelled and placed in sterile Petri dishes on ice and divided into fresh and/or optimal cutting temperature (OCT) compound aliquots. Samples are then frozen for 3 min at −120°C in isopentane before transfer to cryopreservation rooms and stored at −80°C. It is important to perform a quality check for each frozen aliquot before distribution and use it to obtain a histological assessment of the cellularity on hematoxylin and eosin (H&E) cryosections (Table 3).
TABLE 3.
Representative quality Control Form of tissue sections included in OCT and frozen.
| Label with biobank code | — |
|---|---|
| Tumor Tissue (%) | — |
| Tumor Tissue Description | — |
| Normal Tissue Counterpart (%) | — |
| Normal Tissue Counterpart Description | — |
| Necrotic Tissue (%) | — |
| Adipose Tissue (%) | — |
| Stromal Tissue (%) | — |
| Inflammation | ☐ Absent |
| ☐ Sparse | |
| ☐ Intermediate | |
| ☐ Extensive | |
| Diameter of Section (mm) | |
| Conclusion | ☐ Insufficient |
| ☐ Sufficient | |
| Notes | — |
| Technical Operator | — |
| Pathologist Operator | — |
Whole blood
For whole blood samples collection, 6 ml labeled vacutainer tubes, containing anti-coagulant Na2 ethylenediaminetetraacetic acid (EDTA), are used by the nurses at the assessment centers. Following the collection, the biobank technicians process the sample using its medical record number and the episode code and register each aliquot in the biobank software. For clinical studies, or project-specific requirements, the blood is prepared for shipment, otherwise it is firstly collected using a blood amount of 900 μL in 1 ml barcoded cryotubes for one or more aliquots, and stored at −80°C.
Blood serum
For blood serum samples collection, 6 ml labeled vacutainer tubes containing a thixotropic barrier gel at the bottom of the tube, are initially used. Tubes are left for clotting for 3 h at room temperature (RT), and then centrifugated on a refrigerated centrifuge at 828 x g for 10 min. Depending on the initial amount of whole blood taken, the biobank technicians’ rate 450 μL of serum in the 0.5 ml barcoded cryotubes for the maximum amount of aliquots, and stored at −80°C. When the last aliquot serum volume is < 450 μL, the sample is registered as “leftover”.
Blood plasma and cf-DNA/RNA
To separate the plasma from the whole blood, blood-filled vacutainers need to be centrifuged at 2,000 x g for 10 min at RT. After centrifugation, the upper plasma layer should be removed and transferred to a sterile 15 ml conical tube for a second centrifugation at 16,000 x g for 10 min at RT to remove contaminating blood cells. Then, the obtained plasma can be transferred to a barcoded cryotube for the maximum amount of aliquots, depending on the volume. The remaining blood is collected for subsequent cf-DNA and/or cf-RNA purification. Aliquots should be stored at −80°C.
Peripheral blood mononuclear cells
For peripheral blood mononuclear cells (PBMC) isolation, the blood is drawn in 7.5 ml labeled vacutainer tubes, containing Na2 EDTA as an anticoagulant reagent. The blood is subsequently transferred into an empty 50 ml conical tube and diluted in a 1:1 ratio using phosphate buffered saline (PBS) 1X (e.g., 15 ml of blood +15 ml of PBS 1X). Again, the diluted blood is layered on the top of a clean 50 ml conical tube containing 15 ml of Ficoll, without mixing the two solutions. After centrifugation at 400 x g for 30 min at RT, the white layer containing PBMC is collected and placed in a new sterile 50 ml conical tube. PBMCs are washed by adding 45 ml of PBS, mixed, and centrifuged at 400 x g for 10 min at 4°C. After discarding the supernatant, the pellet containing PBMCs is resuspended in PBS and counted using Tuerks solution and single-use slide for counting cells (e.g. Biosigma S.P.A. Cat. no. 347143/001). Cells are washed once more with PBS and resuspended at 2-3x10ˆ6 cells/mL of FSB +10% dimethyl sulfoxide (DMSO) to be frozen. Finally, 1 ml of resuspended cells are transferred in cryotubes for storage at −80°C.
Stool and buccal swab
Feces and buccal swabs are collected in 15 ml tubes (e.g. Stool Sample Collection and Stabilization Kit Canvax Cat. no 0013), containing 50 mM Tris-HCl, 10 mM NaCl, and 10 mM EDTA pH 7.5 and stored at −80°C.
Disaster recovery plan
Biobanks are dedicated to managing valuable and possibly irreplaceable biological specimens. Therefore, biomaterials and associated data must be managed and protected carefully as their loss can destroy years of research efforts and costs, and potentially result in damage to the Institution (Eng and Tan, 2019). For this reason, risk management and practical crisis management plans must be established for any biobank (Parry-Jones et al., 2017). It is essential to define a data protection program that must satisfy various needs that may range from remote data only (backup) to disaster recovery (as a set of technological measures and organizational processes aimed at restoring systems, data, and infrastructures necessary to provide biobank services during emergencies) and to ensure continuity of service and recovery of materials and data during emergencies (Cicek and Olson, 2020).
Staff training programs
The quality and quantity of samples and data stored in a biobank directly depend on the biobank personnel, including data managers and technicians (Hartung et al., 2021). Modern biobanking must rely on high-level training programs for biobank employees not only to allow harmonization of correct sample handling but also to ensure safety and quality (Kinkorová and Topolčan, 2020). Not surprisingly, training certificates of biobank employees are needed for the accreditation process (Williams et al., 2019). Types of training programs include master’s programs, certificate courses, and workshops. Due to the paucity of available formal training programs, biobanks often train most of their new staff on site (Castellanos-Uribe et al., 2020). Learning about teamwork, personnel safety, patient privacy, biospecimen quality, and SOPs is crucial not only for efficiency and productivity but also for the personnel’s career success. A well-designed training program should include helpful tips, tricks, and troubleshooting. International collaboration and exchange programs might facilitate the process of creating next-generation biobanking staff.
Representative results
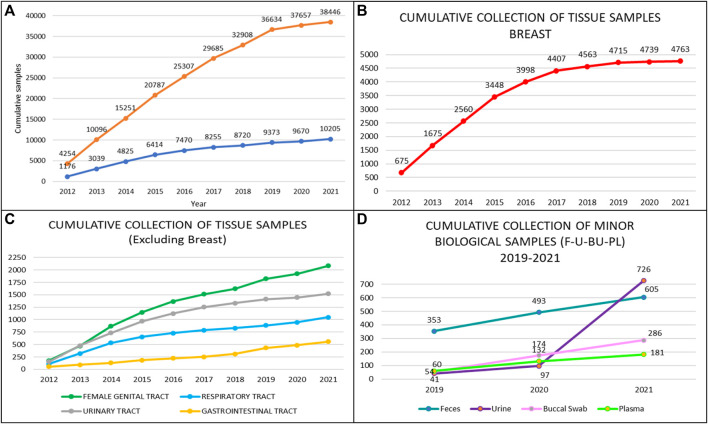
A total of 38,446 annotated biofluids and a total of 10,205 tissue samples were collected by the Biobank for Translational and Digital Medicine Unit at the IEO, European Institute of Oncology, Milan, Italy from April 2012 to December 2021 (Figure 3A). The highest number of samples were related to breast cancer, urological malignancies, tumors of the female genital tract, head and neck carcinomas, and lung cancer (Figures 3B,C). The cumulative analysis of plasma, buccal swab, urine, and stool samples revealed a significant increase in the number of collected samples, particularly for the urine in patients with urological malignancies, reaching a total number of 726 urine samples (Figure 3D). These samples have been divided into multiple aliquots related to specific projects or clinical trials and to the institutional universal collection of samples. Taken together, 28 different projects were responsible for the vast majority of aliquot distribution, for a total number of 28,852 aliquots, as detailed in Table 4. The total number of tissue samples whose aliquots were employed for research purposes were 8,383/10,205 (82%). These data confirm the fundamental role of certified biobanks not only for samples collection but also for samples distribution and use by research groups.
FIGURE 3.
Types and number of samples collected by the biobank of the European Institute of Oncology by year. (A) Cumulative collection of tissue and blood/serum samples; (B) Cumulative collection of breast tissue samples; (C) Cumulative collection of tumor samples from the ovary, prostate, lung, and colon; (D) Cumulative collection of non-tissue samples, i.e. feces, saliva/swab, plasma, and urine.
TABLE 4.
Distribution of samples aliquots from the biobank of the European Institute of Oncology.
| Project ID | 2012 | 2013 | 2014 | 2015 | 2016 | 2017 | 2018 | 2019 | 2020 | 2021 | Total |
|---|---|---|---|---|---|---|---|---|---|---|---|
| T-CELL | — | — | — | — | — | — | 46 | 182 | 144 | 137 | 509 |
| BLADDER | — | — | — | — | 5 | 16 | — | — | — | — | 21 |
| BREAST-1 | 1,168 | 1,611 | 1,340 | 756 | 258 | 65 | 217 | 160 | 12 | 40 | 5,627 |
| BREAST-2 | — | — | — | — | — | — | 26 | 79 | 100 | 65 | 270 |
| BREAST-3 | — | — | — | — | — | — | — | 122 | 122 | 90 | 334 |
| COLON-1 | — | — | — | — | — | — | 22 | — | 185 | — | 207 |
| COLON-2 | — | — | — | 46 | 85 | 97 | 105 | 37 | — | — | 370 |
| COLON-3 | — | — | — | — | — | — | 20 | 14 | 13 | — | 47 |
| COLON-4 | — | — | — | — | — | — | — | 563 | — | — | 563 |
| COSMOS | 5 | 14 | 4 | 3,000 | 363 | — | — | — | — | — | 3,386 |
| FAM | — | — | — | — | 164 | — | — | — | — | — | 164 |
| H&N-1 | — | — | — | 6 | 46 | 22 | 56 | 51 | 20 | 134 | 335 |
| H&N-2 | — | — | — | — | — | — | — | — | — | 35 | 35 |
| H&N-3 | — | — | — | — | — | — | — | — | — | 65 | 65 |
| LUNG-1 | 246 | 311 | 338 | 103 | 26 | 20 | — | — | — | — | 1,044 |
| LUNG-2 | — | — | — | — | — | — | — | 80 | 498 | 342 | 920 |
| LUNG-3 | — | — | — | — | — | — | 186 | — | — | — | 186 |
| MEL-1 | — | — | — | — | — | — | — | — | — | 2 | 2 |
| MEL-2 | — | — | — | — | — | — | — | 628 | 752 | 417 | 1,797 |
| miRNA | 120 | 117 | 206 | 214 | 50 | — | — | — | — | — | 707 |
| OVARY-1 | 320 | 569 | 696 | 561 | 402 | 312 | 204 | 664 | 146 | 166 | 4,040 |
| OVARY-2 | 26 | 113 | 102 | 77 | 318 | ||||||
| PROSTATE | 275 | 545 | 299 | 200 | 127 | 128 | 181 | 66 | 23 | 1,844 | |
| SARCOMA | — | — | — | — | — | — | — | — | — | 9 | 9 |
| SKIN | — | — | — | — | — | — | 23 | 10 | — | 47 | 80 |
| STOMACH | — | — | — | — | — | — | — | — | — | 193 | 193 |
| TEST COVID | — | — | — | — | — | — | — | 692 | 692 | ||
| THYMUS | — | — | — | — | 2 | 19 | 24 | 18 | 11 | 13 | 87 |
| TOTAL | 2,134 | 3,167 | 2,883 | 4,886 | 1,528 | 679 | 1,136 | 2,787 | 2,820 | 1,832 | 23,852 |
Discussion
The transversal role of biobanks in scientific research, particularly in oncologic pathology, and basic and clinical sciences, has put these important infrastructures on the front line of personalized medicine evolution (Kinkorová, 2015; Coppola et al., 2019). Indeed, cancer is still a leading cause of morbidity and mortality worldwide (Sung et al., 2021). For these reasons, the understanding of cancer pathogenesis, mechanisms of disease, and biomarkers discovery at the multi-omics level is becoming an urgent clinical need, akin the support in drug discovery (Kinkorová, 2015; Yan et al., 2018; Szustakowski et al., 2021). When a biobank is established several challenges, from methodological to operative and ethical issues need to be assessed. The first important point is to manage the existing institution data. This can be done by using a laboratory information management system software able to receive and integrate different types of information, from clinical to pathological and digital data. In literature, several valuable softwares have been used to implement biobank databases (Tukacs et al., 2012; Paul et al., 2017; Fthenou et al., 2019; Im et al., 2019), and some freeware can be obtained for biobank management (Voegele et al., 2013; Willers et al., 2021). A biobank consent form is another critical step in data acquisition and management (Beskow et al., 2017; Kinkorová et al., 2019; Kasperbauer et al., 2021; Schmanski et al., 2021). The application of an adequate SRPA is a necessary agreement on legal and ethical aspects of the patient’s data storage and usage (D'Abramo et al., 2015; Sanchini et al., 2016). All SOPs described in this work are continuously under evaluation and improvement and they are currently compliant with the ISO 20387:2018 standards. It should be noted, however, that standardization and improvement of pre-analytical procedures for in-vitro diagnostics is a continuous process. The most updated high-priority pre-analytical CEN and ISO standard documents as well as corresponding External Quality Assessment (EQA) schemes and implementation tools are detailed in Table 5. Not only following adequate SOPs is essential to secure research achievements, but also have qualified personnel, aware of the biobank’s role and potential (Caixeiro et al., 2016; Kintossou et al., 2020). Another important aspect related to the multidisciplinary collaboration in biobanking is represented by the pathologist (Angerilli et al., 2021). Pathologists are the only professionals able to ensure both the tissue sampling for diagnosis and the biobank. Finally, it should be mentioned that the efforts and resources invested to set up and sustain a biobank are significant and such work should be traced and, most importantly, recognized in scientific publications (Howard et al., 2018). In this respect, the Bioresource Research Impact Factor/Framework (BRIF) initiative was proposed for transparency and to promote the responsible and effective use of biomaterials (Cambon-Thomsen, 2003). Another point that is worth mentioning is related to the integration of artificial intelligence (AI) and machine learning into modern biobanks (Kinkorová and Topolčan, 2020; Eccher et al., 2021; Narita et al., 2021; Rizzo et al., 2022). This would allow for the integration of a digitalized database with digital pathology and high throughput molecular data, potentially representing a quantum leap in biobanking.
TABLE 5.
European Committee for Standardization Technical Committee (CEN/TC) 140 in vitro diagnostic medical devices published standards. All projects are sorted by date and available at https://www.spidia.eu/projects/standard-documents (Accessed 28 July 2022).
| References | Date | Title |
|---|---|---|
| CEN/TS 17811:2022 | 22 June 2022 | Molecular in vitro diagnostic examinations - Specifications for pre-examination processes for urine and other body fluids - Isolated cell free DNA |
| CEN/TS 17747:2022 | 20 April 2022 | Molecular in vitro diagnostic examinations - Specifications for pre-examination processes for exosomes and other extracellular vesicles in venous whole blood - DNA, RNA and proteins |
| CEN/TS 17742:2022 | 30 March 2022 | Molecular in vitro diagnostic examinations - Specifications for pre-examination processes for venous whole blood - Isolated circulating cell free RNA from plasma |
| EN ISO 20776-2:2022 | 19 January 2022 | Clinical laboratory testing and in vitro diagnostic test systems - Susceptibility testing of infectious agents and evaluation of performance of antimicrobial susceptibility test devices - Part 2: Evaluation of performance of antimicrobial susceptibility test devices against References broth micro-dilution (ISO 20776-2:2021) |
| CEN/TS 17688-2:2021 | 22 December 2021 | Molecular in vitro diagnostic examinations - Specifications for pre-examination processes for Fine Needle Aspirates (FNAs) - Part 2: Isolated proteins |
| CEN/TS 17688-1:2021 | 22 December 2021 | Molecular in vitro diagnostic examinations - Specifications for pre-examination processes for Fine Needle Aspirates (FNAs) - Part 1: Isolated cellular RNA |
| CEN/TS 17688-3:2021 | 22 December 2021 | Molecular in vitro diagnostic examinations - Specifications for pre-examination processes for Fine Needle Aspirates (FNAs) - Part 3: Isolated genomic DNA |
| EN ISO 4307:2021 | 3 November 2021 | Molecular in vitro diagnostic examinations - Specifications for pre-examination processes for saliva - Isolated human DNA (ISO 4307:2021) |
| EN ISO 16256:2021 | 27 October 2021 | Clinical laboratory testing and in vitro diagnostic test systems - Broth micro-dilution References method for testing the in vitro activity of antimicrobial agents against yeast fungi involved in infectious diseases (ISO 16256:2021) |
| EN ISO 6717:2021 | 8 September 2021 | In vitro diagnostic medical devices - Single-use containers for the collection of specimens from humans other than blood (ISO 6717:2021) |
| EN ISO 20166-4:2021 | 28 July 2021 | Molecular in vitro diagnostic examinations - Specifications for preexamination processes for formalin-fixed and paraffin-embedded (FFPE) tissue - Part 4: In situ detection techniques (ISO 20166-4:2021) |
| EN ISO 23162:2021 | 14 July 2021 | Basic semen examination - Specification and test methods (ISO 23162:2021) |
| EN ISO 17511:2021 | 2 June 2021 | In vitro diagnostic medical devices - Requirements for establishing metrological traceability of values assigned to calibrators, trueness control materials and human samples (ISO 17511:2020) |
| EN ISO 23118:2021 | 2 June 2021 | Molecular in vitro diagnostic examinations - Specifications for pre-examination processes in metabolomics in urine, venous blood serum and plasma (ISO 23118:2021) |
| EN ISO 20184-3:2021 | 26 May 2021 | Molecular in vitro diagnostic examinations - Specifications for pre-examination processes for frozen tissue - Part 3: Isolated DNA (ISO 20184-3:2021) |
| CEN/TS 17626:2021 | 5 May 2021 | Molecular in vitro diagnostic examinations - Specifications for pre-examination processes for human specimen - Isolated microbiome DNA |
| EN ISO 20776-1:2020 | 1 July 2020 | Susceptibility testing of infectious agents and evaluation of performance of antimicrobial susceptibility test devices - Part 1: Broth micro-dilution References method for testing the in vitro activity of antimicrobial agents against rapidly growing aerobic bacteria involved in infectious diseases (ISO 20776-1:2019, including Corrected version 2019-12) |
| EN ISO 22367:2020 | 11 March 2020 | Medical laboratories - Application of risk management to medical laboratories (ISO 22367:2020) |
| CEN/TS 17390-1:2020 | 22 January 2020 | Molecular in vitro diagnostic examinations - Specifications for pre-examination processes for circulating tumor cells (CTCs) in venous whole blood - Part 1: Isolated RNA |
| CEN/TS 17390-2:2020 | 22 January 2020 | Molecular in vitro diagnostic examinations - Specifications for pre-examination processes for circulating tumor cells (CTCs) in venous whole blood - Part 2: Isolated DNA |
| CEN/TS 17390-3:2020 | 22 January 2020 | Molecular in vitro diagnostic examinations - Specifications for pre-examination processes for circulating tumor cells (CTCs) in venous whole blood - Part 3: Preparations for analytical CTC staining |
| EN ISO 20186-3:2019 | 23 October 2019 | Molecular in-vitro diagnostic examinations - Specifications for pre-examination processes for venous whole blood - Part 3: Isolated circulating cell free DNA from plasma (ISO 20186-3:2019) |
| EN ISO 20186-1:2019 | 27 March 2019 | Molecular in vitro diagnostic examinations - Specifications for pre-examination processes for venous whole blood - Part 1: Isolated cellular RNA (ISO 20186-1:2019) |
| EN ISO 20186-2:2019 | 27 March 2019 | Molecular in vitro diagnostic examinations - Specifications for pre-examination processes for venous whole blood - Part 2: Isolated genomic DNA (ISO 20186-2:2019) |
| EN ISO 15195:2019 | 6 February 2019 | Laboratory medicine - Requirements for the competence of calibration laboratories using References measurement procedures (ISO 15195:2018) |
| EN ISO 20166-3:2019 | 23 January 2019 | Molecular in vitro diagnostic examinations - Specifications for pre-examination processes for formalin-fixed and paraffin-embedded (FFPE) tissue - Part 3: Isolated DNA (ISO 20166-3:2018) |
| EN ISO 20166-2:2018 | 19 December 2018 | Molecular in vitro diagnostic examinations - Specifications for pre-examinations processes for formalin-fixed and paraffin-embedded (FFPE) tissue - Part 2: Isolated proteins (ISO 20166-2:2018) |
| EN ISO 20166-1:2018 | 19 December 2018 | Molecular in vitro diagnostic examinations - Specifications for pre-examination processes for formalin-fixed and paraffin-embedded (FFPE) tissue - Part 1: Isolated RNA (ISO 20166-1:2018) |
| EN ISO 20184-1:2018 | 19 December 2018 | Molecular in vitro diagnostic examinations - Specifications for pre-examination processes for frozen tissue - Part 1: Isolated RNA (ISO 20184-1:2018) |
| EN ISO 20184-2:2018 | 12 December 2018 | Molecular in vitro diagnostic examinations - Specifications for pre-examination processes for frozen tissue - Part 2: Isolated proteins (ISO 20184-2:2018) |
| EN ISO 6710:2017 | 6 September 2017 | Single-use containers for human venous blood specimen collection (ISO 6710:2017) |
| EN ISO 22870:2016 | 30 November 2016 | Point-of-care testing (POCT) - Requirements for quality and competence (ISO 22870:2016) |
| EN ISO 15197:2015 | 10 June 2015 | In vitro diagnostic test systems - Requirements for blood-glucose monitoring systems for self-testing in managing diabetes mellitus (ISO 15197:2013) |
| EN ISO 23640:2015 | 10 June 2015 | In vitro diagnostic medical devices - Evaluation of stability of in vitro diagnostic reagents (ISO 23640:2011) |
| EN ISO 19001:2013 | 20 March 2013 | In vitro diagnostic medical devices - Information supplied by the manufacturer with in vitro diagnostic reagents for staining in biology (ISO 19001:2013) |
| EN ISO 15189:2012 | 1 November 2012 | Medical laboratories - Requirements for quality and competence (ISO 15189:2012, Corrected version 2014-08-15) |
| EN ISO 18113-5:2011 | 19 October 2011 | In vitro diagnostic medical devices - Information supplied by the manufacturer (labelling) - Part 5: In vitro diagnostic instruments for self-testing (ISO 18113-5:2009) |
| EN ISO 18113-2:2011 | 19 October 2011 | In vitro diagnostic medical devices - Information supplied by the manufacturer (labelling) - Part 2: In vitro diagnostic reagents for professional use (ISO 18113-2:2009) |
| EN ISO 18113-3:2011 | 19 October 2011 | In vitro diagnostic medical devices - Information supplied by the manufacturer (labelling) - Part 3: In vitro diagnostic instruments for professional use (ISO 18113-3:2009) |
| EN ISO 18113-4:2011 | 19 October 2011 | In vitro diagnostic medical devices - Information supplied by the manufacturer (labelling) - Part 4: In vitro diagnostic reagents for self-testing (ISO 18113-4:2009) |
| EN ISO 18113-1:2011 | 19 October 2011 | In vitro diagnostic medical devices - Information supplied by the manufacturer (labelling) - Part 1: Terms, definitions and general requirements (ISO 18113-1:2009) |
| EN ISO 15193:2009 | 1 May 2009 | In vitro diagnostic medical devices - Measurement of quantities in samples of biological origin - Requirements for content and presentation of References measurement procedures (ISO 15193:2009) |
| EN ISO 15194:2009 | 1 May 2009 | In vitro diagnostic medical devices - Measurement of quantities in samples of biological origin - Requirements for certified References materials and the content of supporting documentation (ISO 15194:2009) |
| EN 14136:2004 | 19 May 2004 | Use of external quality assessment schemes in the assessment of the performance of in vitro diagnostic examination procedures |
| EN ISO 18153:2003 | 15 August 2003 | In vitro diagnostic medical devices - Measurement of quantities in biological samples - Metrological traceability of values for catalytic concentration of enzymes assigned to calibrators and control materials (ISO 18153:2003) |
| EN 13975:2003 | 19 March 2003 | Sampling procedures used for acceptance testing of in vitro diagnostic medical devices - Statistical aspects |
| EN 13612:2002/AC:2002 | 18 December 2002 | Performance evaluation of in vitro diagnostic medical devices |
| EN 13641:2002 | 8 May 2002 | Elimination or reduction of risk of infection related to in vitro diagnostic reagents |
| EN 13532:2002 | 17 April 2002 | General requirements for in vitro diagnostic medical devices for self-testing |
| EN 13612:2002 | 20 March 2002 | Performance evaluation of in vitro diagnostic medical devices |
| EN 12322:1999/A1:2001 | 24 October 2001 | In vitro diagnostic medical devices - Culture media for microbiology - Performance criteria for culture media |
| EN 12322:1999 | 21 April 1999 | In vitro diagnostic medical devices - Culture media for microbiology - Performance criteria for culture media |
| EN 1659:1996 | 20 November 1996 | In vitro diagnostic systems - Culture media for microbiology - Terms and definitions |
Acknowledgments
The authors would like to thank all the patients that during the last decade actively participated in our research programs through the donation of their biospecimens. Without them, this research would not be possible. We are also grateful to all the personnel working at IEO, nurses, technicians, biologists, doctors, and the Directors of all the clinical and research units. The authors are grateful to Pier Paolo Di Fiore and Giancarlo Pruneri for their guidance since the very beginning of our Biobank Unit. Finally, we dedicate this work to Umberto Veronesi, the founder of IEO, and his pioneering approach to integrating cancer research and patient care.
Data availability statement
Requests to access the datasets should be directed to B4M= ED@ieo.it.
Ethics statement
The studies involving human participants were reviewed and approved by IEO IRB. The patients/participants provided their written informed consent to participate in this study.
Author contributions
Methodology, GB, MC, GT, CC, EG-R, MM, NF, Investigation, NF, GB, MC Writing—original draft, GB, LZ, Writing—Review and Editing, GB, MI, MF, AA, RO, GV, and NF.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
- Angerilli V., Galuppini F., Pagni F., Fusco N., Malapelle U., Fassan M. (2021). The role of the pathologist in the next-generation era of tumor molecular characterization. Diagnostics 11 (2), 339. 10.3390/diagnostics11020339 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beskow L. M., Lin L., Dombeck C. B., Gao E., Weinfurt K. P. (2017). Improving biobank consent comprehension: A national randomized survey to assess the effect of a simplified form and review/retest intervention. Genet. Med. 19 (5), 505–512. 10.1038/gim.2016.157 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonnechère B., Liu J., Thomson A., Amin N., Van Duijn C. M. (2021). Ethnicity influences risk of dementia in the UK Biobank. Alzheimer's. Dementia 17 (10), e056077. 10.1002/alz.056077 [DOI] [Google Scholar]
- Braun K. L., Tsark J. U., Powers A., Croom K., Kim R., Gachupin F. C., et al. (2014). Cancer patient perceptions about biobanking and preferred timing of consent. Biopreserv. Biobank. 12 (2), 106–112. 10.1089/bio.2013.0083 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bycroft C., Freeman C., Petkova D., Band G., Elliott L. T., Sharp K., et al. (2018). The UK Biobank resource with deep phenotyping and genomic data. Nature 562 (7726), 203–209. 10.1038/s41586-018-0579-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caixeiro N. J., Byun H. L., Descallar J., Levesque J. V., de Souza P., Soon Lee C. (2016). Health professionals' opinions on supporting a cancer biobank: Identification of barriers to combat biobanking pitfalls. Eur. J. Hum. Genet. 24 (5), 626–632. 10.1038/ejhg.2015.191 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cambon-Thomsen A. (2003). Assessing the impact of biobanks. Nat. Genet. 34 (1), 25–26. 10.1038/ng0503-25b [DOI] [PubMed] [Google Scholar]
- Castellanos-Uribe M., Gormally E., Zhou H., Matzke E., Watson P. H. (2020). Biobanking education. Biopreserv. Biobank. 18 (1), 1–3. 10.1089/bio.2019.29062.mjc [DOI] [PubMed] [Google Scholar]
- Cicek M. S., Olson J. E. (2020). Mini-Review of laboratory operations in biobanking: Building biobanking resources for translational research. Front. Public Health 8, 362. 10.3389/fpubh.2020.00362 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coppola L., Cianflone A., Grimaldi A. M., Incoronato M., Bevilacqua P., Messina F., et al. (2019). Biobanking in health care: Evolution and future directions. J. Transl. Med. 17 (1), 172. 10.1186/s12967-019-1922-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- D'Abramo F., Schildmann J., Vollmann J. (2015). Research participants' perceptions and views on consent for biobank research: A review of empirical data and ethical analysis. BMC Med. Ethics 16, 60. 10.1186/s12910-015-0053-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drosou M., Jagadish H. V., Pitoura E., Stoyanovich J. (2017). Diversity in big data: A review. Big Data 5 (2), 73–84. 10.1089/big.2016.0054 [DOI] [PubMed] [Google Scholar]
- Eccher A., Fontanini G., Fusco N., Girolami I., Graziano P., Rocco E. G., et al. (2021). Digital slides as an effective tool for programmed death ligand 1 combined positive score assessment and training: Lessons learned from the "programmed death ligand 1 key learning program in head-and-neck squamous cell carcinoma. J. Pathol. Inf. 12, 1. 10.4103/jpi.jpi_63_20 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elliott P., Peakman T. C. (2008). The UK Biobank sample handling and storage protocol for the collection, processing and archiving of human blood and urine. Int. J. Epidemiol. 37 (2), 234–244. 10.1093/ije/dym276 [DOI] [PubMed] [Google Scholar]
- Eng C. B., Tan W. L. (2019). Disaster prevention and recovery. Methods Mol. Biol. 1897, 31–41. 10.1007/978-1-4939-8935-5_4 [DOI] [PubMed] [Google Scholar]
- Fisher W. E., Cruz-Monserrate Z., McElhany A. L., Lesinski G. B., Hart P. A., Ghosh R., et al. (2018). Standard operating procedures for biospecimen collection, processing, and storage: From the consortium for the study of chronic pancreatitis, diabetes, and pancreatic cancer. Pancreas 47 (10), 1213–1221. 10.1097/mpa.0000000000001171 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fthenou E., Al Emadi A., Mahal F. F., Chettupuzhakaran L. T., Al Thani A., Afifi N. (2019). Conception, implementation, and integration of heterogenous information technology infrastructures in the Qatar biobank. Biopreserv. Biobank. 17 (6), 494–505. 10.1089/bio.2019.0067 [DOI] [PubMed] [Google Scholar]
- Guerin J. S., Murray D. W., McGrath M. M., Yuille M. A., McPartlin J. M., Doran P. P. (2010). Molecular medicine Ireland guidelines for standardized biobanking. Biopreserv. Biobank. 8 (1), 3–63. 10.1089/bio.2010.8101 [DOI] [PubMed] [Google Scholar]
- Hartung M. L., Baber R., Herpel E., Specht C., Brucker D. P., Schoneberg A., et al. (2021). Harmonization of biobank education for biobank technicians: Identification of learning objectives. BioTech. 10 (2), 7. 10.3390/biotech10020007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hewitt R., Watson P. (2013). Defining biobank. Biopreserv. Biobank. 11 (5), 309–315. 10.1089/bio.2013.0042 [DOI] [PubMed] [Google Scholar]
- Hojat A., Wei B., Olson M. G., Mao Q., Yong W. H. (2019). Procurement and storage of surgical biospecimens. Methods Mol. Biol. 1897, 65–76. 10.1007/978-1-4939-8935-5_7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howard H. C., Mascalzoni D., Mabile L., Houeland G., Rial-Sebbag E., Cambon-Thomsen A. (2018). How to responsibly acknowledge research work in the era of big data and biobanks: Ethical aspects of the Bioresource research impact factor (BRIF). J. Community Genet. 9 (2), 169–176. 10.1007/s12687-017-0332-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hulsen T., Jamuar S. S., Moody A. R., Karnes J. H., Varga O., Hedensted S., et al. (2019). From big data to precision medicine. Front. Med. 6, 34. 10.3389/fmed.2019.00034 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Im K., Gui D., Yong W. H. (2019). An introduction to hardware, software, and other information technology needs of biomedical biobanks. Methods Mol. Biol. 1897, 17–29. 10.1007/978-1-4939-8935-5_3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jose R., Rooney R., Nagisetty N., Davis R., Hains D. (2018). Biorepository and integrative genomics initiative: Designing and implementing a preliminary platform for predictive, preventive and personalized medicine at a pediatric hospital in a historically disadvantaged community in the USA. Epma J. 9 (3), 225–234. 10.1007/s13167-018-0141-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanof M. E., Smith P. D., Zola H. (2001). Isolation of whole mononuclear cells from peripheral blood and cord blood. Curr. Protoc. Immunol. 7, 1. 10.1002/0471142735.im0701s19 [DOI] [PubMed] [Google Scholar]
- Kasperbauer T. J., Schmidt K. K., Thomas A., Perkins S. M., Schwartz P. H. (2021). Incorporating biobank consent into a healthcare setting: Challenges for patient understanding. AJOB Empir. Bioeth. 12 (2), 113–122. 10.1080/23294515.2020.1851313 [DOI] [PubMed] [Google Scholar]
- Kinkorová J., Topolčan O. (2020). Biobanks in the era of big data: Objectives, challenges, perspectives, and innovations for predictive, preventive, and personalised medicine. EPMA J. 11 (3), 333–341. 10.1007/s13167-020-00213-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kinkorová J., Topolčan O., Kučera R. (2019). Informed consent in the newly established biobank. Int. J. Environ. Res. Public Health 16 (20), 3943. 10.3390/ijerph16203943 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kinkorová J. (2015). Biobanks in the era of personalized medicine: Objectives, challenges, and innovation: Overview. Epma J. 7 (1), 4. 10.1186/s13167-016-0053-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kintossou A. K., N’dri M. K., Money M., Cissé S., Doumbia S., Soumahoro M.-K., et al. (2020). Study of laboratory staff’ knowledge of biobanking in Côte d’Ivoire. BMC Med. Ethics 21 (1), 88. 10.1186/s12910-020-00533-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leff D. R., Yang G.-Z. (2015). Big data for precision medicine. Engineering 1 (3), 277–279. 10.15302/J-ENG-2015075 [DOI] [Google Scholar]
- Luo J., Guo X. R., Tang X. J., Sun X. Y., Yang Z. S., Zhang Y., et al. (2014). Intravital biobank and personalized cancer therapy: The correlation with omics. Int. J. Cancer 135 (7), 1511–1516. 10.1002/ijc.28632 [DOI] [PubMed] [Google Scholar]
- Mallone R., Mannering S. I., Brooks-Worrell B. M., Durinovic-Belló I., Cilio C. M., Wong F. S., et al. (2011). Isolation and preservation of peripheral blood mononuclear cells for analysis of islet antigen-reactive T cell responses: Position statement of the T-cell workshop committee of the immunology of diabetes society. Clin. Exp. Immunol. 163 (1), 33–49. 10.1111/j.1365-2249.2010.04272.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Margolis R., Derr L., Dunn M., Huerta M., Larkin J., Sheehan J., et al. (2014). The national institutes of health's big data to knowledge (BD2K) initiative: Capitalizing on biomedical big data. J. Am. Med. Inf. Assoc. 21 (6), 957–958. 10.1136/amiajnl-2014-002974 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mendy M., Caboux E., Lawlor R. T., Wright J., Wild C. P. (2017). Common minimum technical standards and protocols for biobanks dedicated to cancer research. Lyon FR: IARC Technical Publications International Agency for Research on Cancer. [PubMed] [Google Scholar]
- Montserrat A., Taruscio D. (2019). Policies and actions to tackle rare diseases at European level. Ann. Ist. Super. Sanita 55 (3), 296–304. 10.4415/ann_19_03_17 [DOI] [PubMed] [Google Scholar]
- Narita A., Ueki M., Tamiya G. (2021). Artificial intelligence powered statistical genetics in biobanks. J. Hum. Genet. 66 (1), 61–65. 10.1038/s10038-020-0822-y [DOI] [PubMed] [Google Scholar]
- Pagni F., Guerini-Rocco E., Schultheis A. M., Grazia G., Rijavec E., Ghidini M., et al. (2019). Targeting immune-related biological processes in solid tumors: We do need biomarkers. Int. J. Mol. Sci. 20 (21), E5452. 10.3390/ijms20215452 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parry-Jones A., Hansen J., Simeon-Dubach D., Bjugn R. (2017). Crisis management for biobanks. Biopreserv. Biobank. 15 (3), 253–263. 10.1089/bio.2016.0048 [DOI] [PubMed] [Google Scholar]
- Paul S., Gade A., Mallipeddi S. (2017). The state of cloud-based biospecimen and biobank data management tools. Biopreserv. Biobank. 15 (2), 169–172. 10.1089/bio.2017.0019 [DOI] [PubMed] [Google Scholar]
- Rizzo P. C., Girolami I., Marletta S., Pantanowitz L., Antonini P., Brunelli M., et al. (2022). Technical and diagnostic issues in whole slide imaging published validation studies. Front. Oncol. 12, 918580. 10.3389/fonc.2022.918580 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rolfo C., Mack P., Scagliotti G. V., Aggarwal C., Arcila M. E., Barlesi F., et al. (2021). Liquid biopsy for advanced nsclc: A consensus statement from the international association for the study of lung cancer. J. Thorac. Oncol. 16 (10), 1647–1662. 10.1016/j.jtho.2021.06.017 [DOI] [PubMed] [Google Scholar]
- Saifuddin S. R., Devlies W., Santaolalla A., Cahill F., George G., Enting D., et al. (2017). King's health partners' prostate cancer biobank (KHP PCaBB). BMC Cancer 17 (1), 784. 10.1186/s12885-017-3773-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salvaterra E., Corfield J. (2017). Advances in biobanking practice through public and private collaborations. Sharjah United Arab Emirates: Bentham Science Publishers. [Google Scholar]
- Sanchini V., Bonizzi G., Disalvatore D., Monturano M., Pece S., Viale G., et al. (2016). A trust-based pact in research biobanks. From theory to practice. Bioethics 30 (4), 260–271. 10.1111/bioe.12184 [DOI] [PubMed] [Google Scholar]
- Schmanski A., Roberts E., Coors M., Wicks S. J., Arbet J., Weber R., et al. (2021). Research participant understanding and engagement in an institutional, self-consent biobank model. J. Genet. Couns. 30 (1), 257–267. 10.1002/jgc4.1316 [DOI] [PubMed] [Google Scholar]
- Sung H., Ferlay J., Siegel R. L., Laversanne M., Soerjomataram I., Jemal A., et al. (2021). Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. Ca. Cancer J. Clin. 71 (3), 209–249. 10.3322/caac.21660 [DOI] [PubMed] [Google Scholar]
- Szustakowski J. D., Balasubramanian S., Kvikstad E., Khalid S., Bronson P. G., Sasson A., et al. (2021). Advancing human genetics research and drug discovery through exome sequencing of the UK Biobank. Nat. Genet. 53 (7), 942–948. 10.1038/s41588-021-00885-0 [DOI] [PubMed] [Google Scholar]
- Tukacs E., Korotij A., Maros-Szabo Z., Molnar A. M., Hajdu A., Torok Z. (2012). Model requirements for biobank software systems. Bioinformation 8 (6), 290–292. 10.6026/97320630008290 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vaught J., Kelly A., Hewitt R. (2009). A review of international biobanks and networks: Success factors and key benchmarks. Biopreserv. Biobank. 7 (3), 143–150. 10.1089/bio.2010.0003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vaught J., Rogers J., Myers K., Lim M. D., Lockhart N., Moore H., et al. (2011). An NCI perspective on creating sustainable biospecimen resources. J. Natl. Cancer Inst. Monogr. 2011 (42), 1–7. 10.1093/jncimonographs/lgr006 [DOI] [PubMed] [Google Scholar]
- Voegele C., Bouchereau B., Robinot N., McKay J., Damiecki P., Alteyrac L. (2013). A universal open-source electronic laboratory notebook. Bioinformatics 29 (13), 1710–1712. 10.1093/bioinformatics/btt253 [DOI] [PubMed] [Google Scholar]
- Willers C., Lynch T., Chand V., Islam M., Lassere M., March L. (2021). A versatile, secure, and sustainable all-in-one biobank-registry data solution: The A3BC REDCap model. Biopreserv. Biobank. 20 (3), 244–259. 10.1089/bio.2021.0098 [DOI] [PubMed] [Google Scholar]
- Williams R. R., Gupta D., Yong W. H. (2019). Orientation and training of new biobank personnel. Methods Mol. Biol. 1897, 51–63. 10.1007/978-1-4939-8935-5_6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yan H. H. N., Siu H. C., Law S., Ho S. L., Yue S. S. K., Tsui W. Y., et al. (2018). A comprehensive human gastric cancer organoid biobank captures tumor subtype heterogeneity and enables therapeutic screening. Cell Stem Cell 23 (6), 882–897. 10.1016/j.stem.2018.09.016 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Requests to access the datasets should be directed to B4M= ED@ieo.it.