ABSTRACT
To evaluate senescence mechanisms, including senescence-associated secretory phenotype (SASP), in the motor neuron disease model hSOD1-G93A, we quantified the expression of p16 and p21 and senescence-associated β-galactosidase (SA-β-gal) in nervous tissue. As SASP markers, we measured the mRNA levels of Il1a, Il6, Ifna and Ifnb. Furthermore, we explored whether an alteration of alternative splicing is associated with senescence by measuring the Adipor2 cryptic exon inclusion levels, a specific splicing variant repressed by TAR DNA-binding protein (TDP-43; encoded by Tardbp). Transgenic mice showed an atypical senescence profile with high p16 and p21 mRNA and protein in glia, without the canonical increase in SA-β-gal activity. Consistent with SASP, there was an increase in Il1a and Il6 expression, associated with increased TNF-R and M-CSF protein levels, with females being partially protected. TDP-43 splicing activity was compromised in this model, and the senolytic drug Navitoclax did not alter the disease progression. This lack of effect was reproduced in vitro, in contrast to dasatinib and quercetin, which diminished p16 and p21. Our findings show a non-canonical profile of senescence biomarkers in the model hSOD1-G93A.
KEY WORDS: Amyotrophic lateral sclerosis, Navitoclax, Senolytic, Neuroinflammation, Therapy, Cell cycle, Cryptic exon
Summary: The hSOD1-G93A mouse, a motor neuron disease model, shows an atypical profile of molecular traits of cell senescence in the lumbar spinal cord, with selective sensitivity to senolytic treatments.
INTRODUCTION
Aging is a major risk factor for developing amyotrophic lateral sclerosis (ALS) (Niccoli et al., 2017). ALS is a neurodegenerative disease characterized by losing motor neurons with an unfavorable outcome (<5% survival at 5 years after diagnosis). Cellular senescence was first described by Hayflick in the 1960s as a limitation on division of normal cells in vitro (Hayflick and Moorhead, 1961). The cellular mechanisms behind this phenomenon were later described, with an important role for cell cycle inhibitors, highlighting p16-INK4A as the major contributor (Serrano et al., 1997). Another hallmark of senescent cells is the increase in senescence-associated β-galactosidase (SA-β-gal), which is associated with an increase in lysosomal biogenesis (Kurz et al., 2000). Cellular senescence has been described as a barrier against oncogenesis, with a tradeoff where these cells can develop a pro-inflammatory status known as senescence-associated secretory phenotype (SASP). This process reflects an attempt to induce tissue repair in which senescent cells, usually accumulating DNA damage, can stimulate its clearance by the immune system. Regarding neurodegenerative diseases, several groups have independently demonstrated the presence of senescent glial cells and SASP in the central nervous system (CNS).
Another process related to aging is the change in alternative splicing (AS), a conserved mechanism that increases the complexity of the proteome. TAR DNA-binding protein (TDP-43; encoded by TARDBP) regulates many AS events in a complex way (Tollervey et al., 2011). Several pieces of evidence support the role of TDP-43 pathology in age-related neurodegenerative processes and physiological aging (McAleese et al., 2017). Most AS events regulated by TDP-43 involve the repression of a set of non-conserved (cryptic) exons that are abnormally incorporated into mRNA in ALS (Ling et al., 2015). In this line, we previously quantified the rate of inclusion of cryptic exons in nervous tissue from ALS donors and cellular models and found a positive correlation with age at death (Torres et al., 2018).
To study the potential involvement of senescence-associated phenomena in ALS, we explored the profile of senescence biomarkers, including the expression and protein levels of cell cycle modulators linked to mitosis arrest (p16 and p21). We also quantified soluble factors associated with stress-induced senescence secretory profile in motor neuron degeneration in transgenic models, linked to overexpression of mutated proteins. To further strengthen the potential translational power of our findings, we also evaluated whether known senolytic strategies can modify the phenotype of the models and compared these findings with an in vitro model of fibroblast senescence. The results demonstrate that neuroinflammation phenomena associated with SOD1-G93A overexpression are intertwined with senescence-associated secretory profiles. Not all senolytic approaches [i.e. Bcl-2/Bcl-XL (also known as BCL2L1)/Bcl-w (also known as BCL2L2)] and antioxidant ones have the same outcome, stressing adequate preclinical modeling of the disease.
RESULTS
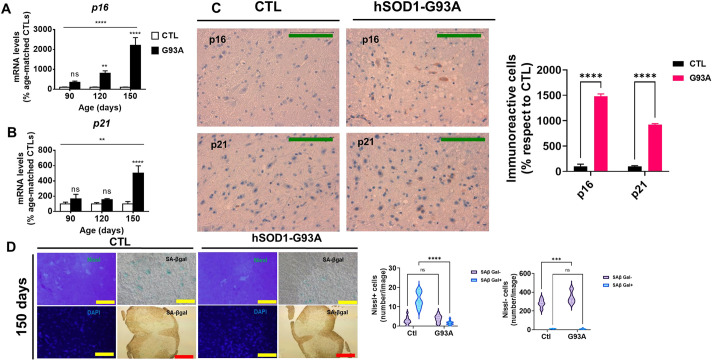
To clarify whether senescence-associated phenomena and TDP-43 dysfunction could be implicated in ALS, we measured the abovementioned variables in the familial ALS transgenic mouse model hSOD1-G93A at different disease stages. The senescence markers p16 (also known as Cdkn2a) and p21 (also known as Cdkn1a), specific biomarkers of senescent cells (Coppé et al., 2010), were analyzed in lumbar spinal cord (LSC). In control mice, we did not observe an age-related increase in senescence biomarkers such as p16, p21, Il1a, Il6 or exon retention percentage. This lack of variation is in line with the fact that changes between 90 and 150 days of age cannot be considered aging in this strain, with a medium survival ranging between 27 and 29 months (Graber et al., 2015). We proposed that G93A overexpression, most probably via secretion of neuroinflammatory mediators, might act like a pro-senescence stimulus in the CNS. Using two different techniques [immunohistochemistry (IHC) and immunofluorescence (IF)], we characterized the tissue distribution pattern of p16. The results from reverse transcription quantitative PCR (RT-qPCR) showed that the expression of p16 mRNA was progressively increased during disease evolution (Fig. 1A), whereas p21 mRNA levels were only higher at the end stage (Fig. 1B). p16 and p21 exhibited a predominantly cytoplasmic pattern (Fig. 1C; detailed in Fig. S1), in contrast to recent results from an ALS rat model that showed mainly nuclear expression (Trias et al., 2019). To define the potential senescence incidence in this model further, we also analyzed another senescence canonical biomarker, SA-β-gal activity. Although increases in SA-β-gal activity are less easily demonstrable in mouse cells than in human cells (Raffaele et al., 2020), we have shown that there are several neurons (and non-neuronal cells) exhibiting this activity, which is in line with findings of other researchers (Itahana et al., 2007). The main cellular populations expressing SA-β-gal in ventral LSC are the motor neuron cells (Nissl+ cells in the ventral horn, with a cellular size compatible with that of motor neurons). Neurons of other LSC locations and the vast majority of Nissl− cells do not show SA-β-gal activity (Fig. 1D; Fig. S2). Interestingly, SA-β-gal activity was reduced during disease progression in motor neurons and a small fraction of Nissl− cells (compatible with glia).
Fig. 1.
Senescence markers in spinal cord increase during amyotrophic lateral sclerosis (ALS) progression. (A,B) Effect of age on p16 (A) and p21 (B) mRNA expression. (C) p16 and p21 cytoplasmic staining, quantified in the right bar graph. (D) Nissl+ staining of the ventral horn of the spinal cord and senescence-associated β-galactosidase (SA-β-gal) activity in the lumbar spinal cord at 150 days, both quantified in the violin plots shown on the right. p16 and p21 expression data are expressed as mean±s.e.m. ns, P>0.05; **P<0.01; ***P<0.001; ****P<0.0001 unpaired two-tailed Student's t-test or two-way ANOVA). Green scale bars: 100 µm; yellow scale bars: 500 µm; red scale bars: 2500 µm. n=4 from each genotype and age. Ctl, control.
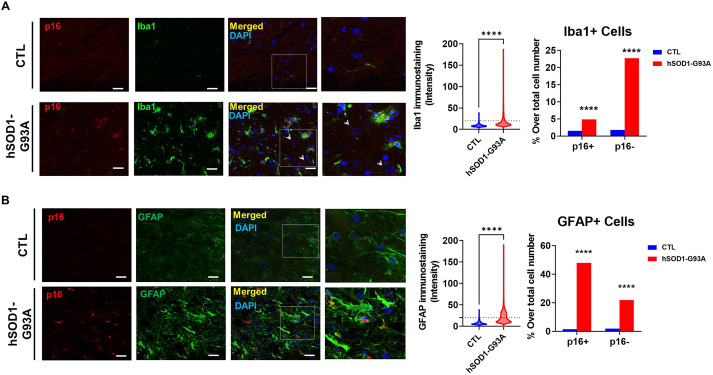
Confocal IF confirmed non-nuclear p16 staining (Fig. 2A,B, rightmost column). hSOD1-G93A overexpression led to an increase in p16 immunoreactivity, with most p16+ cells being microglia (Iba1+ cells) (Fig. 2A) or astroglia (GFAP+ cells) (Fig. 2B). Quantitative analyses of immunoreactivity distribution suggested than the percentage of p16+ cells is higher in astrocytes than in microglia (Fig. 2).
Fig. 2.
p16 immunofluorescence is present in microglia and astrocytes. (A,B) Cytoplasmic p16+ microglia (Iba1+) (A) and astrocytes (GFAP+) (B) in the lumbar spinal cord (LSC) of transgenic mice and controls. The rightmost images show amplifications of the areas within the boxes. Right panels show quantitative analyses of these images, with violin plots showing the mean cellular intensity of Iba1 (A) and GFAP (B), and horizontal dotted lines in violin plots indicating intensities used for selecting Iba1+ and GFAP+ cells. Bar graphs indicate the proportion of p16+ and p16− cells across this population. ****P<0.0001 (unpaired two-tailed Student's t-test in violin plots, or Chi-square test of proportions in bar graphs). Scale bars: 20 µm. Representative images from independent experiments (n=10 slices from at least three different animals from each genotype) are shown. Arrowheads indicate nuclei of motor neurons with p16 immunoreactivity.
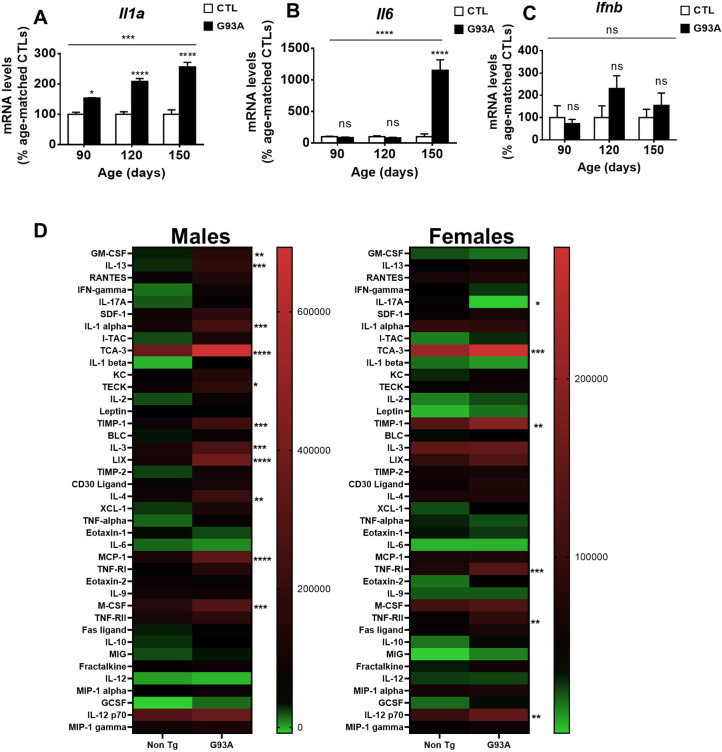
Regarding the SASP, we analyzed the expression of Ifna and Ifnb (also known as Ifnb1) (corresponding to type-I IFN response) (Nakao et al., 2020) as they are postulated as late-senescence markers and could help determine senescence progression in the LSC of this model. The expression of Ifna was not detected in any of the analyzed samples. We observed a different expression pattern between Il1a (Fig. 3A) and Il6 (Fig. 3B). Ifnb expression (Fig. 3C) was not altered over the experimental period, which could indicate that senescence in this model does not evolve in a late phase. To shed further light on the role of neuroinflammation and SASP, we evaluated an array of inflammatory proteins in the spinal cord in a small subset of samples. Sex strongly influenced the effect of G93A overexpression on the levels of these proteins, with females being partially protected against it (Fig. 3D). Thus, although in male mice, G93A overexpression explained 11.8% of the total variance (P<0.0001 in two-way ANOVA), it only explained 1.7% of the total variance in female mice (P<0.0001 in two-way ANOVA). Regarding specific proteins, TCA-3 (also known as CCL1) was the only differential protein shared by males and females (P<0.001 and P<0.05 in Bonferroni post hoc after two-way ANOVA). Using less stringent statistical analyses (Fig. S3), four different proteins were commonly changed in male and female mice [TCA-3, TIMP-I, TNF-RI (also known as TNFRSF1A) and TNF-RII (also known as TNFRSF1B)]. We also analyzed the published RNA-sequencing (RNASeq) datasets of G93A mouse-derived cells for the measured markers of senescence quantified here (Chiu et al., 2013; Liu et al., 2020; Phatnani et al., 2013). The results (Fig. S3) indicate that, in line with our findings, microglial levels of Il1a, p21 and Csf1 were increased by G93A overexpression (Chiu et al., 2013). Furthermore, increased astrocyte p21 expression was detected by independent researchers (Liu et al., 2020).
Fig. 3.
Senescence-associated secretory phenotype (SASP) markers are increased in LSC from transgenic hSOD1-G93A mice in a sex-dependent manner. (A-C) Il1a (A), Il6 (B) and Ifnb (C) mRNA expression in LSC. (D) Heat maps showing median levels (scale present right of the heatmaps) of inflammatory proteins in LSC in G93A mice at the presymptomatic stage (90 days). Il1a, Il6 and Ifnb mRNA levels are expressed as mean±s.e.m. ns, P>0.05; *P<0.05; ***P<0.001; ****P<0.0001 (Fisher’s least significant difference in post hoc test after one-way ANOVA). In A-C, n=4 from each genotype and age; in D, n=2 from each genotype and sex.
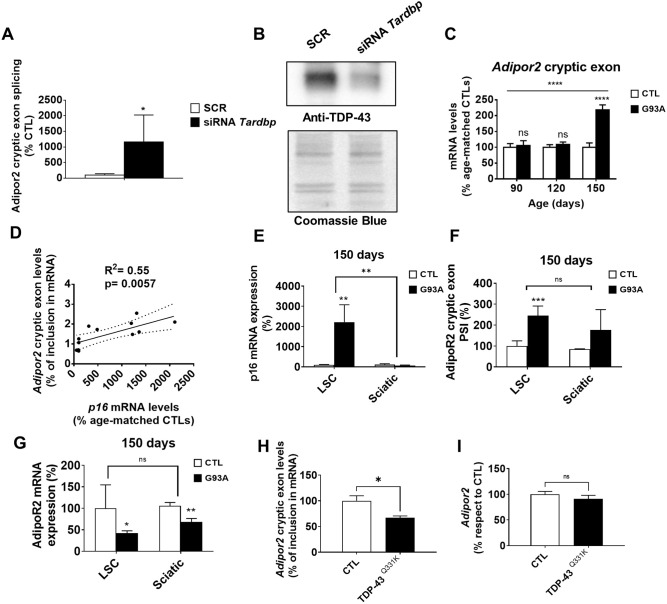
Regarding TDP-43 splicing function, in mice, it controls the inclusion of Adipor2 mRNA (Fig. 4A,B). In line with the loss of TDP-43 function in this model, cryptic exon inclusion in Adipor2 mRNA was higher in the LSC in end-stage mice (Fig. 4C) and positively correlated with p16 expression (Fig. 4D). Increased p16 seemed to be restricted to the CNS, as the sciatic nerve did not show these changes (Fig. 4E), in contrast to Adipor2 cryptic exon inclusion, which was also increased in the sciatic nerve (Fig. 4F). Noteworthily, increased Adipor2 cryptic exon was associated with loss of Adipor2 mRNA levels, suggesting increased nonsense-mediated decay in both locations (Fig. 4G). To evaluate whether enhanced TDP-43 leads to diminished Adipor2 cryptic exon inclusion in vivo, we analyzed Adipor2 splicing in the TARDBPQ331K transgenic mouse, which showed a gain of splicing function (Fratta et al., 2018). At 150 days, when these mice did not show motor neuron phenotype, Adipor2 cryptic splicing was reduced in LSC (Fig. 4H), with total Adipor2 mRNA remaining unaltered (Fig. 4I).
Fig. 4.
Cell senescence could impair TDP-43 function in G93A mice. (A,B) RT-qPCR of Adipor2 cryptic exon (A) and western blot analyses of TDP-43 in Tardbp-silenced 3T3 cells in comparison to scrambled siRNA (SCR) (B). (C) Cryptic exon in Adipor2 mRNA in LSC from 150-day-old G93A transgenic mice. (D) The inclusion ratio of Adipor2 cryptic exon positively correlated with p16 expression in LSC. (E-G) p16 expression (E), Adipor2 mRNA cryptic exons (F) and total Adipor2 mRNA (G) in sciatic nerves and LSC from 150-day-old mice. PSI, percent spliced in or cryptic exon-inclusion ratio. (H,I) Adipor2 cryptic exon (H) and total mRNA (I) in LSC from TARDBPQ331K-overexpressing 150-day-old mice. p16 and cryptic Adipor2 expression are expressed as mean±s.e.m. ns, P>0.05; *P<0.05; **P<0.01; ***P<0.001; ****P<0.0001. n=4 from each genotype and age. In A and B, data shown represent three independent experiments.
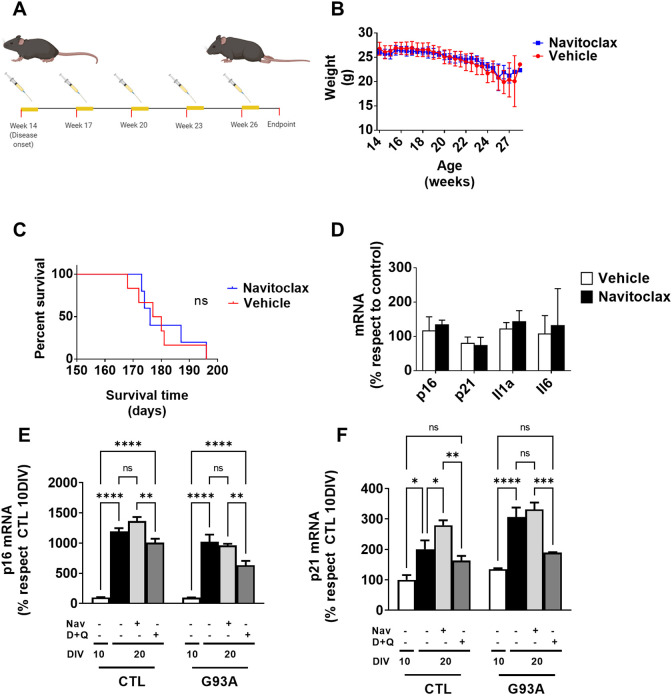
We wanted to explore the potential benefits of senolytic treatments due to the higher expression of senescence-related genes in the G93A model. We performed Navitoclax treatment following the protocol described for an Alzheimer's disease mouse model (Bussian et al., 2018). The treatment was initiated at 90 days and finished at the endpoint (Fig. 5A). We estimated the disease progression by weight loss. Navitoclax treatment did not prevent weight loss or prolong survival (Fig. 5B,C). Finally, we quantified senescence and SASP genes in the LSC. None of the analyzed genes showed statistically significant differences (Fig. 5D). Because Navitoclax treatment, which focused on Bcl-2/Bcl-XL/Bcl-w inhibition, did not significantly affect survival, we tried a different senolytic regime in a fibroblast cell culture. Previous data support using a multitarget strategy, such as dasatinib and quercetin combination, as a senolytic approach (Hickson et al., 2019). The results (Fig. 5E,F) showed that G93A expression significantly impacts both p16 (6.75% of variance explained by genotype, P<0.001, two-way ANOVA) and p21 (10.8% of variance, P<0.0001, two-way ANOVA) mRNA levels. Interestingly, although Navitoclax had a negligible effect on the concentration of these mRNAs, the dasatinib–quercetin combination significantly decreased p16 and p21 mRNA levels compared to vehicle (P<0.0005 and P<0.0001, respectively).
Fig. 5.
Navitoclax treatment does not slow disease progression in G93A mice. (A) A chronic treatment in vivo was established with five consecutive doses followed by 2 weeks of resting. (B-D) Effect of chronic Navitoclax treatment on weight loss (B), survival time (C) and expression of SASP genes (D). (E,F) Effect of alternative senolytic regime [dasatinib and quercetin (D+Q)] in comparison to Navitoclax (Nav) on the expression levels of p16 (E) and p21 (F) mRNA in vitro. DIV, days in vitro. In A-D, n=5 from each genotype. In E and F, data shown represent three different experiments. Data are expressed as mean±s.e.m. ns, P>0.05; *P<0.05; **P<0.01; ***P<0.001; ****P<0.0001 (Bonferroni post hoc analyses after two-way ANOVA).
DISCUSSION
The expression profiles of cell senescence-linked kinases (p16 and p21) suggest that senescence-associated markers and SASP are related to disease evolution in the G93A model. p16 expression is highly expressed before the symptomatology in these mice, similarly to p16+ microglia in LSC from transgenic rats (Trias et al., 2019). Both findings suggest a role for p16 in disease initiation and progression, as p16 mRNA is increased up to an early symptomatic stage (120 days), whereas p21 mRNA only increases later. p21 mRNA upregulation (related to reversible cell cycle arrest or quiescence) might derive from the late-onset activation of p53 and the DNA damage response pathway, as shown for senescent microglia (Stojiljkovic et al., 2019). Although p21 does not get upregulated in all models, it has a clear role in senescence, both in a p53- dependent and -independent fashion (Shtutman et al., 2017). Usually, senescence – specially for human cells – implies stability in cell cycle arrest. In contrast, mouse cells may not actually undergo bona fide senescence, but rather a profile of stress-induced cessation of growth and buildup of a secretory profile associated with senescence. The pattern of p16 and p21 expression reported here may reflect divergent processes sharing these biomarkers (Sharpless and Sherr, 2015). This is the case with macrophage polarization, in which p16 expression and SA-β-gal activity are physiological, reversible and not associated with cellular senescence (Hall et al., 2017).
Regarding the subcellular location of p16 and p21, cytoplasmic p16 can regulate cell migration like cyclin D1 does (Chen et al., 2013). Cytoplasmic p21 inhibits the ROCK/LIMK/Cofilin pathway through MAPK signaling, inducing cytoskeleton remodeling (Tanaka et al., 2002). Indeed, cytoskeleton regulators like RAC1 and CDC42 are implicated in ALS progression and neuroinflammation (D'Ambrosi et al., 2014). In other models of cell senescence, cytoplasmic p16 inhibits the nuclear translocation of phosphorylated regulators affecting stress responses, such as ERK1/2 (also known as MAPK3/1) (Chen et al., 2020). These results might align with previous findings of increased phosphorylated (p)ERK1/2 in ALS samples (Ayala et al., 2011) and would also agree with TDP-43 loss of function in the G93A model, discussed below. Furthermore, recent data show that nuclear TDP-43 is required for repairing DNA double-strand breaks (Mitra et al., 2019), a type of DNA damage associated with cell senescence and ALS (Wald-Altman et al., 2017; Cacabelos et al., 2014). Whether cell senescence is associated causally with disturbances in TDP-43 function will be the focus of future studies.
The potential contribution of cytoplasmic p16 and p21 to cell cycle arrest linked to senescence is unknown. It is known that cytoplasmic p21 (Lee and Helfman, 2004; Koster et al., 2010) and p16 (Evangelou et al., 2004) could interact with mediators of cell cycle. Nuclear P53/p21 and pRB/p16 are the two major pathways – in addition to telomere shortening/dysfunction – for inducing senescence (Serrano et al., 1997). Terc loss (i.e. shorter telomere length) advances the phenotype impairment in the G93A mouse model (Linkus et al., 2016). In the same report, the authors indicated that, in ALS patients, microglial cells showed near-to-significant loss of telomere length, which would be in line with our finding of increased p16 levels in Iba1+ cells. Regarding the implication of cytosolic p16 in cell cycle blockade, increased cytoplasmic p16 is observed in doxorubicin-induced senescence (Spallarossa et al., 2010), where cytosolic p16 does not change the cell cycle but induces cytoskeletal changes. Our results are also compatible with the cytoskeleton being altered in the G93A mouse model (Maximino et al., 2014) and with specific ALS forms being caused by several mutations in genes that regulate cytoskeleton (Castellanos-Montiel et al., 2020). Our findings suggest that increased p16/p21 in G93A are both cytoplasmic and nuclear. When added to the limited contribution of cytosolic p16/p21 as cell cycle inhibitors, the results reported here shall be better described as an increased expression of senescence biomarkers rather than bona fide senescence itself.
Lee et al. (2006) authors described the origin of SA-β-gal activity, another senescence marker, as being directly linked to lysosomal density. Our data on SA-β-gal activity in neurons suggest that, in contrast to in the aging mouse brain (Piechota et al., 2016), this typical marker is not associated with potential senescence. Motor neurons contain more lysosomes than other cells (Xie et al., 2015a). Of note, lysosome biogenesis (Xie et al., 2015a) and lysosomal density (Xie et al., 2015b) are compromised in this ALS mouse model, potentially explaining our results from the SA-β-gal activity assay.
Another marker commonly employed in senescence description is the cytokine increase linked to SASP. In this case, we quantified the expression of typical SASP markers Il1a and Il6, and we analyzed by an antibody array the tissue levels of several related inflammatory proteins. Il1a mRNA is increased in the presymptomatic stage and is known to be the upstream regulator of IL-6 in SASP (Orjalo et al., 2009). IL-6 is increased in cerebrospinal fluid in ALS, Alzheimer's disease and Parkinson's disease (Chen et al., 2018). Overall, our results might reflect a complex interaction between senescence, SASP, and changes in reactive glial cells and neurodegeneration. Comparison of our data with publicly available RNASeq datasets enhances the robustness of these findings. Both microglia and astrocyte RNASeq data confirm the increased amount of p21, which may be implicated, as explained above, in mitotic arrest and in cytoskeletal changes. Furthermore, these results also demonstrated increased Csf1 mRNA in microglia, in line with our finding of macrophage-colony stimulating factor (M-CSF) (its derived protein) found in G93A mice and with data on ALS patients (Akiyama et al., 1994). Interestingly, administration of GW2580, an antagonist of microglial M-CSF signaling, improves survival in G93A mice (Martínez-Muriana et al., 2016). M-CSF has also been detected in other preclinical models of ALS, including TDP-43-related ones (Hunter et al., 2021). Recent data show that M-CSF may be implicated in cellular senescence of skeletal stem cells (Ambrosi et al., 2021), and this cytokine is implicated in p53-mediated cell arrest (Azzam et al., 2013). The antibody array employed here also revealed the increased amount of TNF receptors, already involved in SASP (Schafer et al., 2020), confirming the potential role of TNF signaling in ALS (reviewed in Hensley et al., 2006) and other neurodegenerative processes (Yuan et al., 2019). Regarding TCA-3, the product of Ccl1, no previous implication in ALS has been reported. As TCA-3 is required for the clearance of senescent cells in cancer (Vilgelm et al., 2015), we speculate that its buildup in this model could signal microglial chemotaxis (Li et al., 2020) and that it is not directly related to cell senescence. In contrast, TIMP-1, another of the differential markers shown here, is a soluble factor implicated in the reprogramming of SASP, again in the context of cancer (Guccini et al., 2021), and it has been involved as a neuroprotective response in G93A mice (Izrael et al., 2018). Additionally, sex-induced differences in SASP reported here agree with the fact that there are sex differences in ALS, with men having a higher incidence and prevalence than women and unique clinical characteristics (men deteriorate more quickly) (reviewed in Blasco et al., 2012). These results can also be seen in experimental ALS models (Ajroud-Driss and Siddique, 2015). Additionally, neuroinflammatory conditions varied noticeably between mouse sexes (Sorge et al., 2015). Thus, the sex-induced differences in SASP shown here may be explained by estrogen-sensitive variations in microglia and lymphocyte function (Villa et al., 2018). In addition to its well-known role as an antineuroinflammatory factor (Vegeto et al., 2003), estrogen has protective activity that could be derived from other properties. Recent data in an unrelated preclinical model of neurodegeneration revealed that tamoxifen, a selective estrogen receptor antagonist, can increase lysosomal function and autophagy (Soldati et al., 2021), involving the signaling through the transcription factor EB. Because loss of this transcription factor has been causally linked to ALS (Cunningham et al., 2020), we could speculate that part of the protective effect of feminine sex could be related to this pathway. Other estrogen-dependent mechanisms could underlie their activity, enhancing endolysosomal acidification in neurons (Datta et al., 2021). Similarly, it is known that the activation of selected estrogen receptors, such as GP30, protects neurons by modulating autophagy in an excitotoxicity context (Yue et al., 2019).
The present data are the first to show specific alterations regarding splicing function controlled by TDP-43 in the hSOD1-G93A mouse model of ALS. Notably, loss of splicing correlates with the senescence marker p16. The present study is one of the first reports regarding cell senescence, TDP-43 dysfunction and motor neuron disease. TDP-43 pathology is present in nervous tissue during the normal aging process and age-related neurodegenerative disorders, such as Alzheimer's disease and limbic-predominant age-related TDP-43 encephalopathy (Torres et al., 2020). Because G93A seems to accelerate age-related traits, including the buildup of senescence biomarkers, as demonstrated here, we evaluated whether senescence traits were associated with TDP-43 dysfunction. Aging is linked to alterations in the splicing machinery (reviewed in Deschênes and Chabot, 2017), with age-related alteration of expression and activity of splicing regulators, such as SRSF2, promoting a cascade of splicing alterations to affect the maintenance of genomic, chromatin and DNA integrity. It remains controversial whether TDP-43 pathology is present in these mice, with previous studies showing mislocalization and biochemical modification (Shan et al., 2009) and others pointing to no pathology in G93A (Robertson et al., 2007). Our findings support the potential impairment of TDP-43 function in line with progressive neuroinflammatory changes.
Our results in two independent ALS models (G93A and Q331K mice) suggest a novel pathogenic venue, as a surrogate of TDP-43 function, ADIPOR2, is an essential regulator in intracellular lipid composition sensing (Ruiz et al., 2019). ADIPOR2 is a member of the ADIPOR family, evolutionarily conserved regulators of membrane homeostasis that work as membrane fluidity sensors and regulate phospholipid composition. Membrane rigidification triggers ADIPOR signaling, which promotes fatty acid desaturation and polyunsaturated fatty acid incorporation into membrane phospholipids until fluidity is restored (Ruiz et al., 2019). Cellular lipid homeostasis is affected in ALS, as shown by recent analyses in cellular models and tissue samples (Ramírez-Nuñez et al., 2021; Sol et al., 2021). Our data indicate that senescence-associated TDP-43 disturbances could be pathogenic through loss of Adipor2 mRNA. Whether this holds true for ALS patients will be focus of future research.
Results on Navitoclax treatment reinforce differences in molecular effectors between different neurodegenerative processes such as Alzheimer's disease and ALS. Navitoclax is an inhibitor of the antiapoptotic protein Bcl-2 (Zhu et al., 2016), essential for survival of senescent cells. Senolysis is achieved when this antiapoptotic protein is inhibited, promoting cell death (Zhu et al., 2015). Navitoclax treatment was not enough to slow the disease progression and did not extend the survival in our G93A mice. In contrast to data from Alzheimer's disease and Parkinson's disease models, we observed that Navitoclax does not prevent the increase in senescence and SASP markers. We hypothesize that the senescence-like phenotype in the G93A model is not driven by the Bcl2 expression of stressed or aged cells (Zhu et al., 2015), supporting previous data (Vukosavic et al., 1999).
Further studies are warranted to determine whether senescence-linked phenomena are mechanistically involved in ALS, clearing the pathway for therapeutic development. As a limitation of our work, we acknowledge the lack of unequivocal mechanistic insight, which may be obtained by crossbreeding G93A mice with the p16-INK4A-specific (Baker et al., 2011) or similar cellular ablation models in the future. We envisage that the translational power of senolytic treatments assayed here would be of use for further development of future therapeutic schemes. Indeed, Bcl-XL, a Bcl-2 family member, is overactive in astrocytes from G93A, providing pro-survival input, and may mediate the activation of toxic astroglia (Lee et al., 2009). Thus, specific inhibition of Bcl-XL could significantly affect disease progression. Results from in vitro modeling of cell senescence by fibroblast culture confirm the potential of the dasatinib and quercetin combination in the G93A preclinical model. Further, these results suggest that cell culture (as shown for Navitoclax) might model in vivo events, as this Bcl-2/Bcl-XL/Bcl-w inhibitory agent was unable to prevent in vitro or in vivo changes. Also, these results illustrate the complexity of senescence-like phenotypes impinged by different neurodegeneration-associated noxa.
The LSC from the hSOD1-G93A mouse, a model of familial ALS, exhibits a non-canonical profile of senescence biomarkers. This profile is characterized by an early increase in p16 and a late increase in p21, with both displaying a mainly cytoplasmic pattern in glial cells without an increase in SA-β-gal activity. In the case of SASP, it also has a dynamic profile, with increasing levels of Il1a from the presymptomatic stage onward and a sharp peak of expression in end-stage transgenic mice. Regarding AS, this tissue shows a dysfunctional splicing activity of TDP-43 in end-stage ALS mice. This is the first time that senescence markers, SASP and TDP-43-associated splicing dysfunction have been described in this ALS mouse model.
MATERIALS AND METHODS
Animal experiments
A colony of the strain B6.Cg-Tg(SOD1*G93A)1Gur/J (JAX stock #004435; referred to as hSOD1-G93A or G93A) was purchased from The Jackson Laboratory (Bar Harbor, MN, USA). Mice were maintained in C57BL/6J background. Genotyping was performed following The Jackson Laboratory's instructions. After genotyping and weaning, animals were placed in a 12:12 h dark/light cycle, at 22±2°C temperature, 50±10% relative humidity, in individual cages (at 21 days). We evaluated three different ages: 90 days (early clinical phase), 120 days and 150 days (defined as end stage, characterized by bilateral hindlimb paralyses) for age-related studies. Navitoclax (T2101, Targetmol) was diluted in 60% Phosal 50 PG (Lipoid), 30% PEG400 (91893, Sigma-Aldrich), 10% EtOH. Navitoclax was administered by oral gavage at a 50 mg/kg body dose for five consecutive days followed by 16 days of rest (n=5 per group). Treatment cycles were repeated until the clinical endpoint (righting reflex >20 s), which was achieved at the mean age of 170 days. Spinal cords and sciatic nerves were rapidly excised, frozen in liquid N2 and stored at −80°C.
A colony B6.Cg-Tg(Prnp-TARDBP*Q331K)103Dwc/J (JAX stock #017933; referred to as Q331K) was purchased from The Jackson Laboratory. Mice were placed under the same conditions as the G93A colony. Mice were maintained in C57BL/6NJ background. Ten non-transgenic mice and six transgenic littermates were euthanized at 150 days old, and LSCs were rapidly excised, snap-frozen in liquid N2 and stored at −80°C. This study was approved by the Animal Research and Ethics Committee at the University of Lleida. The care and use of all experimental animals employed here complied with relevant local animal welfare laws, guidelines and policies.
Cell culture
3T3 cells, obtained from American Type Culture Collection (#CCL-173, authenticated by provider) were maintained in Dulbecco's modified Eagle medium (DMEM; 11965, Thermo Fisher Scientific), 10% fetal bovine serum (FBS; 10270, Thermo Fisher Scientific), 100 U/ml penicillin–streptomycin (15140-122, Thermo Fisher Scientific) at 37°C and 5% CO2. For silencing, 20 nM (final concentration) Tardbp siRNA mmsiTDP-43s (5′-AGGAAUCAGCGUGCAUAUA-3′), mmsiTDP-43as (5′-UAUAUGCACGCUGAUUCCU-3′; siTdp-43) or scrambled siRNA (SCR) was mixed in 100 µl Opti-MEM (31985062, Thermo Fisher Scientific) with 2 µl RNAiMAX (13778100, Thermo Fisher Scientific) on the bottom of a well from a six-well plate and incubated for 20 min at room temperature. Then, 2 ml of DMEM (11965092, Thermo Fisher Scientific) supplemented with 10% FBS containing 100,000 3T3 cells/well were seeded onto transfection mix. After 24 h, transfection medium was removed and changed to DMEM+10% FBS. At 48 h post-transfection, cells were collected for posterior analyses.
Fibroblasts were obtained from the ears of G93A and non-transgenic littermates. Briefly, both ears were digested using 0.2 U/ml type II collagenase (LS004174, Worthington Biochemicals) for 45 min at constant shaking at 37°C. Every 15 min, supernatant was collected and centrifuged for fibroblast isolation and to prevent cell damage by excessive digestion. Digestion solution was replaced and incubated for 15 min three times. Fibroblasts were finally seeded in 60 mm plates and cultivated in DMEM+10% FBS. Non-senescent fibroblasts were collected at 10 days in vitro (DIV) and senescent fibroblasts at 20 DIV (when they stopped growing). Cells were then treated with dasatinib (250 nM)+quercetin (50 μM), Navitoclax (20 μM) or vehicle [dimethyl sulfoxide (DMSO)] for 72 h [modified from (Zhu et al., 2016)]. RNA was extracted using the TRIzol–chloroform method, as detailed below.
Western blotting
Spinal cord lysates were prepared to homogenize on ice (1:20, weight: volume) in radioimmunoprecipitation (RIPA) buffer with Protease Inhibitor Cocktail (1×) using a homogenizer device (T10 basic UltraTurraX, IKA, Staufen, Germany). After sonication, protein quantification was performed with Bradford assay (5000006, Bio-Rad). Fifteen micrograms of protein were loaded onto a 12% acrylamide SDS-PAGE gel. Membranes were blocked with I-Block (T2015, Thermo Fisher Scientific) for 1 h and incubated overnight with primary antibody anti-TDP-43 (10782-2-AP, Proteintech; 1:1000) in Tris-buffered saline containing 0.05% Tween 20 (TBS-T). After primary antibody incubation, membranes were washed three times with TBS-T and incubated with secondary antibody for 1 h. Immobilon™ Western Chemiluminiscent HRP Substrate (WBKLS0500, Merck Millipore) was used for immunodetection. For normalization, membranes were stained with Coomassie Brilliant Blue G (27815, Sigma-Aldrich). Incubation without primary antibody was employed for ensuring specificity of bands, the intensities of which were quantified with ImageLab v5.2.1 (Bio-Rad).
Antibody array
For further analyses of inflammatory profiles, we employed an antibody array (AAM-INF-1-4, RayBio) in spinal cord lysates from control (n=4, two male) and G93A (n=4, two female) 100-day-old mice according to manufacturer instructions. Briefly, spinal cord lysates, obtained and quantified as above for western blotting, were incubated with the arrays overnight at 4°C, then incubated with biotinylated secondary antibodies and horseradish peroxidase. The bioluminescence emitted by the arrays was quantified with ImageLab v5.2.1 (Bio-Rad), and dot quantification was performed by measuring the optical density of each dot using the oval tool in ImageJ. The same area was used to quantify all the arrays. The data were analyzed following the manufacturer's instructions.
RNA extraction, cDNA synthesis and RT-qPCR
First, 1 ml TRIzol reagent (AM9738, Thermo Fisher Scientific) was added to 50-100 mg tissue. The tissue was then mechanically homogenized (T10 basic UltraTurraX). Then, 200 µl chloroform was added to each sample and mixed. After 5 min of incubation at room temperature, the samples were centrifuged at 12,000 g (15 min, 4°C) to separate the phases. The aqueous phase was transferred into a new tub and mixed by vortexing with 500 µl isopropanol. After incubation for 10 min at room temperature, RNA was precipitated through centrifugation at 12,000 g (10 min, 4°C). The resulting supernatant was removed, and the pellet was washed with 75% ethanol. After vortexing, the samples were centrifuged again at 12,000 g (10 min, 4°C). The supernatant was discarded, and the RNA pellet was allowed to air dry at room temperature. The RNA was resuspended with 50 µl RNAse-free water, quantified with an ND-1000 UV/Vis spectrophotometer (Nanodrop Technologies) and stored at −80°C until further use. One microgram of RNA was used for retrotranscription employing TaqMan Reverse Transcription Reagent using random hexamers (N8080234, Thermo Fisher Scientific). RT-qPCR experiments were performed using a CFX96 instrument (Bio-Rad) with SYBR Select Master Mix (4472908, Thermo Fisher Scientific). Each 20 µl of reaction mix contained 4 µl cDNA, 10 µl SYBR Select Master Mix, 0.2 nM forwarding primer and 0.2 nM reverse primer solutions, and 4 µl PCR-grade water. The RT-qPCR run protocol was as follows: 50°C for 2 min and 95°C for 2 min, with 95°C for 15 s and 60°C for 1-min steps repeated for 40 cycles; and a melting curve test from 65°C to 95°C at a 0.1°C/s measuring rate. n=3-10 mice per group were used for RT-PCR experiments. Primers employed in these experiments are listed in Table S1. Actb expression was used as housekeeping gene to normalize other genes. Specific Adipor2 cryptic mRNA (primers annealed with cryptic exon) was normalized with Adipor2 standard transcript (the primers annealed with conserved exons).
SA-β-gal activity
Briefly, paraformaldehyde-fixed frozen sections were incubated with X-gal solution [20 mg/ml X-gal (Sigma-Aldrich), 5 mM K3Fe(CN)6, 5 mM K4Fe(CN)6 and 2 mM MgCl2] in PBS at pH 6.0 overnight at 37°C. All samples were assayed simultaneously. Then, neurons were stained with Green Fluorescent Nissl Stain (N21480, Thermo Fisher Scientific) diluted 1:150 in PBS and incubated for 20 min at room temperature. The slices were then washed three times with PBS for 10 min at room temperature and once with PBS for 2 h. Nuclei were stained with 4′,6-diamidino-2-phenylindole (DAPI; 32670, Sigma-Aldrich). Images of the stained sections were taken using an inverted microscope (IX71S8F-2, Olympus). Eight randomly selected areas of each mouse (n=2-3 per group) of the ventral horn of LSC sections were photographed at 20× magnification for visual analysis, performed in a blinded fashion. The whole section of SA-β-Gal-stained slices was photographed at 4× magnification.
IF
One control and one transgenic LSC were fixed in 4% paraformaldehyde made in PBS overnight at 4°C and cryopreserved in 30% sucrose in PBS for 48 h. The LSC was then cut at a 16 µm depth and seeded onto a gelatin-coated slide. Samples were permeabilized with 0.3% Triton X-100 PBS for 30 min and blocked with 5% bovine serum albumin in PBS for 1 h at room temperature. The primary antibodies anti-p16 (ab54210, Abcam; 1:100) and anti-GFAP (ab7260, Abcam; 1:200) or anti-Iba1 (019-19741, FUJIFILM Wako; 1:200) were diluted in 0.3% Triton X-100 PBS and incubated overnight at 4°C. The slices were washed three times with PBS for 5 min at room temperature, and then incubated with the secondary antibody (diluted 1:800 in PBS), goat anti-mouse Alexa Fluor 555 (A21422, Thermo Fisher Scientific) or goat anti-rabbit Alexa Fluor 488 (A11008, Thermo Fisher Scientific), for 1 h at room temperature in the dark. Sections were finally counterstained with 1 µg/ml DAPI in PBS for 10 min at room temperature and mounted on slides with Fluoromount-G® (0100, Southern Biotech). The same procedure was adopted for evaluating anti-p16 cellular distribution in primary fibroblasts. Samples were imaged using a laser scanning confocal microscope (Olympus FluoView FV10). Individual intensities of GFAP, p16 and Iba1 staining were quantified employing the Artificial Intelligence (AI) Software Platform Biodock 2022 (available from www.biodock.ai). We used the ‘Cell Segmentation’ AI pipeline, employing 25 pixels as the mean nucleus diameter and 25 pixels as the growth parameter. Using these conditions, at least 2500 cells were analyzed for GFAP and Iba1 immunostaining.
IHC
One control and one transgenic paraformaldehyde-fixed paraffin-embedded tissue slide were dried for 1 h at 65° before deparaffinization, rehydration and epitope retrieval in the Pre-Treatment Module (PT-LINK, Agilent Technologies-DAKO) at 95°C for 20 min in 50× Tris/EDTA buffer, pH 9. For p21 immunohistochemical staining, p21WAF1/Cip1 antibody (clone SX118, Agilent Technologies-DAKO; 1:100) was used. After incubation, the reaction was visualized with an EnVision™ FLEX Detection Kit (Agilent Technologies-DAKO) using diaminobenzidine chromogen as a substrate. For p16 immunohistochemical staining, the anti-p16-INK4A antibody was used using a CINtec® Histology Kit (clone E6H4, Roche) following the manufacturer's instructions. Sections were counterstained with Hematoxylin and quantified in a blinded fashion.
Statistical analysis
All statistical tests and graphs were performed using Prism 6 (GraphPad Software). P<0.05 was considered significant. Normalized mRNA expression was analyzed with ordinary two-way ANOVA of the variable time and genotype. Bonferroni's or Fisher’s least significant difference multiple comparisons test was used for multiple comparisons between genotypes. A linear regression was tested to evaluate the relationship between p16 and Adipor2 cryptic mRNA levels. Venn diagrams were produced using the online Venny package (v 2.1) (https://bioinfogp.cnb.csic.es/tools/venny/index.html).
Supplementary Material
Acknowledgements
This study was supported by the Scientific and Technical Service of Immunohistochemistry, Lleida Institute for Biomedical Research, Dr Pifarré Foundation, IRBLleida. We thank Prof JE Esquerda and Dr S Salvany for research support. We thank T. Yohannan for editorial assistance. We thank also the reviewers for constructive comments and suggestions.
Footnotes
Competing interests
The authors declare no competing or financial interests.
Author contributions
Conceptualization: P.T., M.E., M.P.-O.; Methodology: P.T., C.A., O.R.-N., A.F., P.A.-B., M.P., V.A.; Validation: C.A., M.P., I.F., M.P.-O.; Formal analysis: P.T., O.R.-N., P.A.-B., V.A., M.P.-O.; Investigation: P.T., C.A., A.F., V.A.; Resources: C.A., A.F., P.A.-B., M.P., I.F.; Data curation: M.E.; Writing - original draft: P.T., M.P., I.F., R.P.; Writing - review & editing: C.A., P.A.-B., R.P., M.P.-O.; Visualization: M.P.; Supervision: M.E., I.F., V.A.; Project administration: M.E., R.P., M.P.-O.; Funding acquisition: M.E., I.F., M.P.-O.
Funding
Grants supporting this work were received from the Instituto de Salud Carlos III (PI 17-00134, PI 20-0155) to M.P.-O., Generalitat de Catalunya (2017SGR696) to R.P., and Ministerio de Ciencia, Innovación y Universidades (BFU2017-83646-P, AEI, FEDER, UE) to M.E. P.T. is a ‘Margarita Salas’ fellow from the Spanish Ministry of Universities (financed by European Commission-Next Generation EU funds). Support was also received from Fundación Española Investigación Esclerosis Lateral (FUNDELA), RedELA-Plataforma Investigación and Fundació Miquel Valls (Jack Van den Hoek donation). European Regional Development Fund (FEDER) funds are acknowledged (‘A way to make Europe’). These funding bodies had no roles in the design of the study and collection, analysis and interpretation of data, or writing of the manuscript. Open Access funding provided by Universitat de Lleida. Deposited in PMC for immediate release.
References
- Ajroud-Driss, S. and Siddique, T. (2015). Sporadic and hereditary amyotrophic lateral sclerosis (ALS). Biochim. Biophys. Acta 1852, 679-684. 10.1016/j.bbadis.2014.08.010 [DOI] [PubMed] [Google Scholar]
- Akiyama, H., Nishimura, T., Kondo, H., Ikeda, K., Hayashi, Y. and Mcgeer, P. L. (1994). Expression of the receptor for macrophage colony stimulating factor by brain microglia and its upregulation in brains of patients with Alzheimer's disease and amyotrophic lateral sclerosis. Brain Res. 639, 171-174. 10.1016/0006-8993(94)91779-5 [DOI] [PubMed] [Google Scholar]
- Ambrosi, T. H., Marecic, O., Mcardle, A., Sinha, R., Gulati, G. S., Tong, X., Wang, Y., Steininger, H. M., Hoover, M. Y., Koepke, L. S.et al. (2021). Aged skeletal stem cells generate an inflammatory degenerative niche. Nature 597, 256-262. 10.1038/s41586-021-03795-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ayala, V., Granado-Serrano, A. B., Cacabelos, D., Naudí, A., Ilieva, E. V., Boada, J., Caraballo-Miralles, V., Lladó, J., Ferrer, I., Pamplona, R.et al. (2011). Cell stress induces TDP-43 pathological changes associated with ERK1/2 dysfunction: implications in ALS. Acta Neuropathol. 122, 259-270. 10.1007/s00401-011-0850-y [DOI] [PubMed] [Google Scholar]
- Azzam, G., Wang, X., Bell, D. and Murphy, M. E. (2013). CSF1 is a novel p53 target gene whose protein product functions in a feed-forward manner to suppress apoptosis and enhance p53-mediated growth arrest. PLoS One 8, e74297. 10.1371/journal.pone.0074297 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baker, D. J., Wijshake, T., Tchkonia, T., Lebrasseur, N. K., Childs, B. G., Van De Sluis, B., Kirkland, J. L. and Van Deursen, J. M. (2011). Clearance of p16Ink4a-positive senescent cells delays ageing-associated disorders. Nature 479, 232-236. 10.1038/nature10600 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blasco, H., Guennoc, A.-M., Veyrat-Durebex, C., Gordon, P. H., Andres, C. R., Camu, W. and Corcia, P. (2012). Amyotrophic lateral sclerosis: a hormonal condition? Amyotroph Lateral Scler. 13, 585-588. 10.3109/17482968.2012.706303 [DOI] [PubMed] [Google Scholar]
- Bussian, T. J., Aziz, A., Meyer, C. F., Swenson, B. L., Van Deursen, J. M. and Baker, D. J. (2018). Clearance of senescent glial cells prevents tau-dependent pathology and cognitive decline. Nature 562, 578-582. 10.1038/s41586-018-0543-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cacabelos, D., Ayala, V., Ramírez-Nunez, O., Granado-Serrano, A. B., Boada, J., Serrano, J. C. E., Cabré, R., Nadal-Rey, G., Bellmunt, M. J., Ferrer, I.et al. (2014). Dietary lipid unsaturation influences survival and oxidative modifications of an amyotrophic lateral sclerosis model in a gender-specific manner. Neuromolecular Med. 16, 669-685. 10.1007/s12017-014-8317-7 [DOI] [PubMed] [Google Scholar]
- Castellanos-Montiel, M. J., Chaineau, M. and Durcan, T. M. (2020). The neglected genes of ALS: cytoskeletal dynamics impact synaptic degeneration in ALS. Front. Cell Neurosci. 14, 594975. 10.3389/fncel.2020.594975 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen, Y.-W., Chu, H.-C., Ze-Shiang, L., Shiah, W.-J., Chou, C.-P., Klimstra, D. S. and Lewis, B. C. (2013). p16 Stimulates CDC42-dependent migration of hepatocellular carcinoma cells. PLoS One 8, e69389. 10.1371/journal.pone.0069389 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen, X., Hu, Y., Cao, Z., Liu, Q. and Cheng, Y. (2018). Cerebrospinal fluid inflammatory cytokine aberrations in Alzheimer's disease, Parkinson's disease and amyotrophic lateral sclerosis: a systematic review and meta-analysis. Front. Immunol. 9, 2122. 10.3389/fimmu.2018.02122 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen, H., Chen, H., Liang, J., Gu, X., Zhou, J., Xie, C., Lv, X., Wang, R., Li, Q., Mao, Z.et al. (2020). TGF-β1/IL-11/MEK/ERK signaling mediates senescence-associated pulmonary fibrosis in a stress-induced premature senescence model of Bmi-1 deficiency. Exp. Mol. Med. 52, 130-151. 10.1038/s12276-019-0371-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiu, I. M., Morimoto, E. T. A., Goodarzi, H., Liao, J. T., O'keeffe, S., Phatnani, H. P., Muratet, M., Carroll, M. C., Levy, S., Tavazoie, S.et al. (2013). A neurodegeneration-specific gene-expression signature of acutely isolated microglia from an amyotrophic lateral sclerosis mouse model. Cell Rep. 4, 385-401. 10.1016/j.celrep.2013.06.018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coppé, J.-P., Desprez, P.-Y., Krtolica, A. and Campisi, J. (2010). The senescence-associated secretory phenotype: the dark side of tumor suppression. Annu. Rev. Pathol. 5, 99-118. 10.1146/annurev-pathol-121808-102144 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cunningham, K. M., Maulding, K., Ruan, K., Senturk, M., Grima, J. C., Sung, H., Zuo, Z., Song, H., Gao, J., Dubey, S.et al. (2020). TFEB/Mitf links impaired nuclear import to autophagolysosomal dysfunction in C9-ALS. Elife 2020, e59419. 10.7554/eLife.59419 [DOI] [PMC free article] [PubMed] [Google Scholar]
- D'Ambrosi, N., Rossi, S., Gerbino, V. and Cozzolino, M. (2014). Rac1 at the crossroad of actin dynamics and neuroinflammation in Amyotrophic Lateral Sclerosis. Front. Cell Neurosci. 8, 279. 10.3389/fncel.2014.00279 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Datta, G., Miller, N. M., Du, W., Geiger, J. D., Chang, S. and Chen, X. (2021). Endolysosome localization of ERα is involved in the protective effect of 17α-Estradiol against HIV-1 gp120-induced neuronal injury. J. Neurosci. 41, 10365-10381. 10.1523/JNEUROSCI.1475-21.2021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deschênes, M. and Chabot, B. (2017). The emerging role of alternative splicing in senescence and aging. Aging Cell 16, 918-933. 10.1111/acel.12646 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evangelou, K., Bramis, J., Peros, I., Zacharatos, P., Dasiou-Plakida, D., Kalogeropoulos, N., Asimacopoulos, P. J., Kittas, C., Marinos, E. and Gorgoulis, V. G. (2004). Electron microscopy evidence that cytoplasmic localization of the p16INK4A “nuclear” cyclin-dependent kinase inhibitor (CKI) in tumor cells is specific and not an artifact. A study in non-small cell lung carcinomas. Biotech. Histochem. 79, 5-10. 10.1080/10520290310001659466 [DOI] [PubMed] [Google Scholar]
- Fratta, P., Sivakumar, P., Humphrey, J., Lo, K., Ricketts, T., Oliveira, H., Brito-Armas, J. M., Kalmar, B., Ule, A., Yu, Y.et al. (2018). Mice with endogenous TDP-43 mutations exhibit gain of splicing function and characteristics of amyotrophic lateral sclerosis. EMBO J. 37, e98684. 10.15252/embj.201798684 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graber, T. G., Kim, J.-H., Grange, R. W., Mcloon, L. K. and Thompson, L. V. (2015). C57BL/6 life span study: age-related declines in muscle power production and contractile velocity. Age 37, 9773. 10.1007/s11357-015-9773-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guccini, I., Revandkar, A., D'Ambrosio, M., Colucci, M., Pasquini, E., Mosole, S., Troiani, M., Brina, D., Sheibani-Tezerji, R., Elia, A. R.et al. (2021). Senescence reprogramming by TIMP1 deficiency promotes prostate cancer metastasis. Cancer Cell 39, 68-82.e9. 10.1016/j.ccell.2020.10.012 [DOI] [PubMed] [Google Scholar]
- Hall, B. M., Balan, V., Gleiberman, A. S., Strom, E., Krasnov, P., Virtuoso, L. P., Rydkina, E., Vujcic, S., Balan, K., Gitlin, I. I.et al. (2017). p16(Ink4a) and senescence-associated β-galactosidase can be induced in macrophages as part of a reversible response to physiological stimuli. Aging 9, 1867-1884. 10.18632/aging.101268 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayflick, L. and Moorhead, P. S. (1961). The serial cultivation of human diploid cell strains. Exp. Cell Res. 25, 585-621. 10.1016/0014-4827(61)90192-6 [DOI] [PubMed] [Google Scholar]
- Hensley, K., Mhatre, M., Mou, S., Pye, Q. N., Stewart, C., West, M. and Williamson, K. S. (2006). On the relation of oxidative stress to neuroinflammation: lessons learned from the G93A-SOD1 mouse model of amyotrophic lateral sclerosis. Antioxid. Redox Signal 8, 2075-2087. 10.1089/ars.2006.8.2075 [DOI] [PubMed] [Google Scholar]
- Hickson, L. J., Langhi Prata, L. G. P., Bobart, S. A., Evans, T. K., Giorgadze, N., Hashmi, S. K., Herrmann, S. M., Jensen, M. D., Jia, Q., Jordan, K. L.et al. (2019). Senolytics decrease senescent cells in humans: Preliminary report from a clinical trial of Dasatinib plus Quercetin in individuals with diabetic kidney disease. EBioMedicine 47, 446-456. 10.1016/j.ebiom.2019.08.069 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hunter, M., Spiller, K. J., Dominique, M. A., Xu, H., Hunter, F. W., Fang, T. C., Canter, R. G., Roberts, C. J., Ransohoff, R. M., Trojanowski, J. Q.et al. (2021). Microglial transcriptome analysis in the rNLS8 mouse model of TDP-43 proteinopathy reveals discrete expression profiles associated with neurodegenerative progression and recovery. Acta Neuropathol. Commun. 9, 140. 10.1186/s40478-021-01239-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Itahana, K., Campisi, J. and Dimri, G. P. (2007). Methods to detect biomarkers of cellular senescence: the senescence-associated beta-galactosidase assay. Methods Mol. Biol. 371, 21-31. 10.1007/978-1-59745-361-5_3 [DOI] [PubMed] [Google Scholar]
- Izrael, M., Slutsky, S. G., Admoni, T., Cohen, L., Granit, A., Hasson, A., Itskovitz-Eldor, J., Krush Paker, L., Kuperstein, G., Lavon, N.et al. (2018). Safety and efficacy of human embryonic stem cell-derived astrocytes following intrathecal transplantation in SOD1G93A and NSG animal models. Stem Cell Res. Ther. 9, 152. 10.1186/s13287-018-0890-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koster, , R., Di Pietro, A., Timmer-Bosscha, H., Gibcus, J. H., van den Berg, A., Suurmeijer, A. J., Bischoff, R., Gietema, J. A. and De Jong, S. (2010). Cytoplasmic p21 expression levels determine cisplatin resistance in human testicular cancer. J. Clin. Invest. 120, 3594-3605. 10.1172/JCI41939 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kurz, D. J., Decary, S., Hong, Y. and Erusalimsky, J. D. (2000). Senescence-associated (beta)-galactosidase reflects an increase in lysosomal mass during replicative ageing of human endothelial cells. J. Cell Sci. 113, 3613-3622. 10.1242/jcs.113.20.3613 [DOI] [PubMed] [Google Scholar]
- Lee, S. and Helfman, D. M. (2004). Cytoplasmic p21Cip1 is involved in Ras-induced inhibition of the ROCK/LIMK/cofilin pathway. J. Biol. Chem. 279, 1885-1891. 10.1074/jbc.M306968200 [DOI] [PubMed] [Google Scholar]
- Lee, B. Y., Han, J. A., Im, J. S., Morrone, A. and Johung, K. (2006). Senescence–associated β-galactosidase is lysosomal β-galactosidase. Aging Cell 5, 187-195. 10.1111/j.1474-9726.2006.00199.x [DOI] [PubMed] [Google Scholar]
- Lee, J., Kannagi, M., Ferrante, R. J., Kowall, N. W. and Ryu, H. (2009). Activation of Ets-2 by oxidative stress induces Bcl-xL expression and accounts for glial survival in amyotrophic lateral sclerosis. FASEB J. 23, 1739-1749. 10.1096/fj.08-121046 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li, L., Ni, L., Heary, R. F. and Elkabes, S. (2020). Astroglial TLR9 antagonism promotes chemotaxis and alternative activation of macrophages via modulation of astrocyte-derived signals: implications for spinal cord injury. J. Neuroinflammation 17, 73. 10.1186/s12974-020-01748-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ling, J. P., Pletnikova, O., Troncoso, J. C. and Wong, P. C. (2015). TDP-43 repression of nonconserved cryptic exons is compromised in ALS-FTD. Science 349, 650-655. 10.1126/science.aab0983 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Linkus, B., Wiesner, D., Meßner, M., Karabatsiakis, A., Scheffold, A., Rudolph, K. L., Thal, D. R., Weishaupt, J. H., Ludolph, A. C. and Danzer, K. M. (2016). Telomere shortening leads to earlier age of onset in ALS mice. Aging 8, 382-393. 10.18632/aging.100904 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu, W., Venugopal, S., Majid, S., Ahn, I. S., Diamante, G., Hong, J., Yang, X. and Chandler, S. H. (2020). Single-cell RNA-seq analysis of the brainstem of mutant SOD1 mice reveals perturbed cell types and pathways of amyotrophic lateral sclerosis. Neurobiol. Dis. 141, 104877. 10.1016/j.nbd.2020.104877 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martínez-Muriana, A., Mancuso, R., Francos-Quijorna, I., Olmos-Alonso, A., Osta, R., Perry, V. H., Navarro, X., Gomez-Nicola, D. and López-Vales, R. (2016). CSF1R blockade slows the progression of amyotrophic lateral sclerosis by reducing microgliosis and invasion of macrophages into peripheral nerves. Sci. Rep. 6, 25663. 10.1038/srep25663 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maximino, J. R., De Oliveira, G. P., Alves, C. J. and Chadi, G. (2014). Deregulated expression of cytoskeleton related genes in the spinal cord and sciatic nerve of presymptomatic SOD1G93A Amyotrophic Lateral Sclerosis mouse model. Front. Cell Neurosci. 8, 148. 10.3389/fncel.2014.00148 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mcaleese, K. E., Walker, L., Erskine, D., Thomas, A. J., Mckeith, I. G. and Attems, J. (2017). TDP-43 pathology in Alzheimer's disease, dementia with Lewy bodies and ageing. Brain Pathol. 27, 472-479. 10.1111/bpa.12424 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitra, J., Guerrero, E. N., Hegde, P. M., Liachko, N. F., Wang, H., Vasquez, V., Gao, J., Pandey, A., Taylor, J. P., Kraemer, B. C.et al. (2019). Motor neuron disease-associated loss of nuclear TDP-43 is linked to DNA double-strand break repair defects. Proc. Natl. Acad. Sci. USA 116, 4696-4705. 10.1073/pnas.1818415116 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakao, M., Tanaka, H. and Koga, T. (2020). Cellular senescence variation by metabolic and epigenomic remodeling. Trends Cell Biol. 30, 919-922. 10.1016/j.tcb.2020.08.009 [DOI] [PubMed] [Google Scholar]
- Niccoli, T., Partridge, L. and Isaacs, A. M. (2017). Ageing as a risk factor for ALS/FTD. Hum. Mol. Genet 26, R105-R113. 10.1093/hmg/ddx247 [DOI] [PubMed] [Google Scholar]
- Orjalo, A. V., Bhaumik, D., Gengler, B. K., Scott, G. K. and Campisi, J. (2009). Cell surface-bound IL-1α is an upstream regulator of the senescence-associated IL-6/IL-8 cytokine network. Proc. Natl. Acad. Sci. USA 106, 17031-17036. 10.1073/pnas.0905299106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phatnani, H. P., Guarnieri, P., Friedman, B. A., Carrasco, M. A., Muratet, M., O'keeffe, S., Nwakeze, C., Pauli-Behn, F., Newberry, K. M., Meadows, S. K.et al. (2013). Intricate interplay between astrocytes and motor neurons in ALS. Proc. Natl. Acad. Sci. USA 110, E756-E765. 10.1073/pnas.1222361110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piechota, M., Sunderland, P., Wysocka, A., Nalberczak, M., Sliwinska, M. A., Radwanska, K. and Sikora, E. (2016). Is senescence-associated β-galactosidase a marker of neuronal senescence? Oncotarget 7, 81099-81109. 10.18632/oncotarget.12752 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raffaele, M., Kovacovicova, K., Bonomini, F., Rezzani, R., Frohlich, J. and Vinciguerra, M. (2020). Senescence-like phenotype in post-mitotic cells of mice entering middle age. Aging 12, 13979-13990. 10.18632/aging.103637 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramírez-Nuñez, O., Jové, M., Torres, P., Sol, J., Fontdevila, L., Romero-Guevara, R., Andrés-Benito, P., Ayala, V., Rossi, C., Boada, J.et al. (2021). Nuclear lipidome is altered in amyotrophic lateral sclerosis: a pilot study. J. Neurochem. 158, 482-499. 10.1111/jnc.15373 [DOI] [PubMed] [Google Scholar]
- Robertson, J., Sanelli, T., Xiao, S., Yang, W., Horne, P., Hammond, R., Pioro, E. P. and Strong, M. J. (2007). Lack of TDP-43 abnormalities in mutant SOD1 transgenic mice shows disparity with ALS. Neurosci. Lett. 420, 128-132. 10.1016/j.neulet.2007.03.066 [DOI] [PubMed] [Google Scholar]
- Ruiz, M., Ståhlman, M., Borén, J. and Pilon, M. (2019). AdipoR1 and AdipoR2 maintain membrane fluidity in most human cell types and independently of adiponectin. J. Lipid Res. 60, 995-1004. 10.1194/jlr.M092494 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schafer, M. J., Zhang, X., Kumar, A., Atkinson, E. J., Zhu, Y., Jachim, S., Mazula, D. L., Brown, A. K., Berning, M., Aversa, Z.et al. (2020). The senescence-associated secretome as an indicator of age and medical risk. JCI Insight 5, e133668. 10.1172/jci.insight.133668 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Serrano, M., Lin, A. W., Mccurrach, M. E., Beach, D. and Lowe, S. W. (1997). Oncogenic ras provokes premature cell senescence associated with accumulation of p53 and p16INK4a. Cell 88, 593-602. 10.1016/S0092-8674(00)81902-9 [DOI] [PubMed] [Google Scholar]
- Shan, X., Vocadlo, D. and Krieger, C. (2009). Mislocalization of TDP-43 in the G93A mutant SOD1 transgenic mouse model of ALS. Neurosci. Lett. 458, 70-74. 10.1016/j.neulet.2009.04.031 [DOI] [PubMed] [Google Scholar]
- Sharpless, N. E. and Sherr, C. J. (2015). Forging a signature of in vivo senescence. Nat. Rev. Cancer 15, 397-408. 10.1038/nrc3960 [DOI] [PubMed] [Google Scholar]
- Shtutman, M., Chang, B. D. and Schools, G. P. (2017). Cellular model of p21-induced senescence. Methods Mol. Biol. 1534, 31-39. 10.1007/978-1-4939-6670-7_3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sol, J., Jové, M., Povedano, M., Sproviero, W., Domínguez, R., Piñol-Ripoll, G., Romero-Guevara, R., Hye, A., Al-Chalabi, A., Torres, P.et al. (2021). Lipidomic traits of plasma and cerebrospinal fluid in amyotrophic lateral sclerosis correlate with disease progression. Brain Commun. 3, fcab143. 10.1093/braincomms/fcab143 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soldati, C., Lopez-Fabuel, I., Wanderlingh, L. G., Garcia-Macia, M., Monfregola, J., Esposito, A., Napolitano, G., Guevara-Ferrer, M., Scotto Rosato, A., Krogsaeter, E. K.et al. (2021). Repurposing of tamoxifen ameliorates CLN3 and CLN7 disease phenotype. EMBO Mol. Med. 13, e13742. 10.15252/emmm.202013742 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sorge, R. E., Mapplebeck, J. C. S., Rosen, S., Beggs, S., Taves, S., Alexander, J. K., Martin, L. J., Austin, J.-S., Sotocinal, S. G., Chen, D.et al. (2015). Different immune cells mediate mechanical pain hypersensitivity in male and female mice. Nat. Neurosci. 18, 1081-1083. 10.1038/nn.4053 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spallarossa, P., Altieri, P., Barisione, C., Passalacqua, M., Aloi, C., Fugazza, G., Frassoni, F., Podestà, M., Canepa, M., Ghigliotti, G.et al. (2010). p38 MAPK and JNK antagonistically control senescence and cytoplasmic p16INK4A expression in doxorubicin-treated endothelial progenitor cells. PLoS One 5, e15583. 10.1371/journal.pone.0015583 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stojiljkovic, M. R., Ain, Q., Bondeva, T., Heller, R., Schmeer, C. and Witte, O. W. (2019). Phenotypic and functional differences between senescent and aged murine microglia. Neurobiol. Aging 74, 56-69. 10.1016/j.neurobiolaging.2018.10.007 [DOI] [PubMed] [Google Scholar]
- Tanaka, H., Yamashita, T., Asada, M., Mizutani, S., Yoshikawa, H. and Tohyama, M. (2002). Cytoplasmic p21(Cip1/WAF1) regulates neurite remodeling by inhibiting Rho-kinase activity. J. Cell Biol. 158, 321-329. 10.1083/jcb.200202071 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tollervey, J. R., Curk, T., Rogelj, B., Briese, M., Cereda, M., Kayikci, M., König, J., Hortobágyi, T., Nishimura, A. L., Zupunski, V.et al. (2011). Characterizing the RNA targets and position-dependent splicing regulation by TDP-43. Nat. Neurosci. 14, 452-458. 10.1038/nn.2778 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Torres, P., Ramírez-Núñez, O., Romero-Guevara, R., Barés, G., Granado-Serrano, A. B., Ayala, V., Boada, J., Fontdevila, L., Povedano, M., Sanchís, D.et al. (2018). Cryptic exon splicing function of TARDBP interacts with autophagy in nervous tissue. Autophagy 14, 1398-1403. 10.1080/15548627.2018.1474311 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Torres, P., Andrés-Benito, P., Fernàndez-Bernal, A., Ricart, M., Ayala, V., Pamplona, R., Ferrer, I. and Portero-Otin, M. (2020). Selected cryptic exons accumulate in hippocampal cell nuclei in Alzheimer's disease with and without associated TDP-43 proteinopathy. Brain 143, e20. 10.1093/brain/awaa013 [DOI] [PubMed] [Google Scholar]
- Trias, E., Beilby, P. R., Kovacs, M., Ibarburu, S., Varela, V., Barreto-Núñez, R., Bradford, S. C., Beckman, J. S. and Barbeito, L. (2019). Emergence of microglia bearing senescence markers during paralysis progression in a rat model of inherited ALS. Front. Aging Neurosci. 11, 42. 10.3389/fnagi.2019.00042 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vilgelm, A. E., Pawlikowski, J. S., Liu, Y., Hawkins, O. E., Davis, T. A., Smith, J., Weller, K. P., Horton, L. W., Mcclain, C. M., Ayers, G. D.et al. (2015). Mdm2 and aurora kinase a inhibitors synergize to block melanoma growth by driving apoptosis and immune clearance of tumor cells. Cancer Res. 75, 181-193. 10.1158/0008-5472.CAN-14-2405 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Villa, A., Gelosa, P., Castiglioni, L., Cimino, M., Rizzi, N., Pepe, G., Lolli, F., Marcello, E., Sironi, L., Vegeto, E.et al. (2018). Sex-specific features of microglia from adult mice. Cell Rep. 23, 3501-3511. 10.1016/j.celrep.2018.05.048 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vegeto, E., Belcredito, S., Etteri, S., Ghisletti, S., Brusadelli, A., Meda, C., Krust, A., Dupont, S., Ciana, P., Chambon, P.et al. (2003). Estrogen receptor-alpha mediates the brain antiinflammatory activity of estradiol. Proc. Natl. Acad. Sci. USA 100, 9614-9619. 10.1073/pnas.1531957100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vukosavic, S., Dubois-Dauphin, M., Romero, N. and Przedborski, S. (1999). Bax and Bcl-2 interaction in a transgenic mouse model of familial amyotrophic lateral sclerosis. J. Neurochem. 73, 2460-2468. 10.1046/j.1471-4159.1999.0732460.x [DOI] [PubMed] [Google Scholar]
- Wald-Altman, S., Pichinuk, E., Kakhlon, O. and Weil, M. (2017). A differential autophagy-dependent response to DNA double-strand breaks in bone marrow mesenchymal stem cells from sporadic ALS patients. Dis. Model. Mech. 10, 645-654. 10.1242/dmm.027938 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xie, Y., Zhou, B., Lin, M.-Y. and Sheng, Z.-H. (2015a). Progressive endolysosomal deficits impair autophagic clearance beginning at early asymptomatic stages in fALS mice. Autophagy 11, 1934-1936. 10.1080/15548627.2015.1084460 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xie, Y., Zhou, B., Lin, M. Y., Wang, S., Foust, K. D. and Sheng, Z. H. (2015b). Endolysosomal deficits augment mitochondria pathology in spinal motor neurons of asymptomatic fALS mice. Neuron 87, 355-370. 10.1016/j.neuron.2015.06.026 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuan, J., Amin, P. and Ofengeim, D. (2019). Necroptosis and RIPK1-mediated neuroinflammation in CNS diseases. Nat. Rev. Neurosci. 20, 19-33. 10.1038/s41583-018-0093-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yue, J., Wang, X. S., Feng, B., Hu, L. N., Yang, L. K., Lu, L., Zhang, K., Wang, Y. T. and Liu, S. B. (2019). Activation of G-protein-coupled receptor 30 protects neurons against excitotoxicity through Inhibiting excessive autophagy induced by glutamate. ACS Chem. Neurosci. 10, 4227-4236. 10.1021/acschemneuro.9b00287 [DOI] [PubMed] [Google Scholar]
- Zhu, Y., Tchkonia, T., Pirtskhalava, T., Gower, A. C., Ding, H., Giorgadze, N., Palmer, A. K., Ikeno, Y., Hubbard, G. B., Lenburg, M.et al. (2015). The Achilles’ heel of senescent cells: from transcriptome to senolytic drugs. Aging Cell 14, 644-658. 10.1111/acel.12344 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu, Y., Tchkonia, T., Fuhrmann-Stroissnigg, H., Dai, H. M., Ling, Y. Y., Stout, M. B., Pirtskhalava, T., Giorgadze, N., Johnson, K. O., Giles, C. B.et al. (2016). Identification of a novel senolytic agent, navitoclax, targeting the Bcl-2 family of anti-apoptotic factors. Aging Cell 15, 428-435. 10.1111/acel.12445 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.