Abstract
Use of the inhalation anesthetic isoflurane may increase the risk of cognitive deficiency and neurotoxicity after birth. A growing body of evidence suggests that melatonin is an effective treatment for various types of oxidative stress damage and neurodegenerative disease. In this study, we aimed to examine the effects of melatonin on isoflurane-induced endoplasmic reticulum (ER) stress, spatial learning and memory impairment during development. The rats were grouped according to whether the rats were exposed to isoflurane or a control gas and whether they were administered melatonin or phosphate buffered saline (PBS). We administered isoflurane to 7-day-old Sprague–Dawley rat pups with intraperitoneal injections of melatonin (20 mg/kg) 15 min before and 3 h after the initiation of anesthesia. Twelve hours after isoflurane anesthesia, rats were randomly selected from each group and sacrificed. The hippocampal tissue and serum were collected to determine the levels of SIRT1, Mfn2, PERK, and other proteins or cytokines related to ER stress, apoptosis, and neuroinflammation. Subsequently, all remaining rats were assessed for spatial learning and memory deficiency 31 days after birth using the Morris water maze test. We found that melatonin attenuated isoflurane-induced ER stress and neuroapoptosis in the hippocampus and decreased the level of neuroinflammatory markers in the serum of newborn rats, resulting in improved spatial learning and memory. In addition, the neuroprotective effect of melatonin was weakened after the SIRT1/Mfn2/PERK signaling pathway was suppressed by lentivirus transfection. Therefore, our findings demonstrate that melatonin ameliorates spatial learning and memory impairment after isoflurane exposure, and these beneficial effects are associated with a reduction in ER stress, neuroapoptosis, and neuroinflammation via the SIRT1/Mfn2/PERK signaling pathway.
Keywords: Melatonin, Isoflurane, Hippocampus, SIRT1, Endoplasmic reticulum stress
Melatonin; Isoflurane; Hippocampus; SIRT1; Endoplasmic reticulum stress.
1. Introduction
In the past decade, isoflurane has been commonly used as an inhalation anesthetic during pediatric surgical procedures. Many studies have focused on isoflurane neurotoxicity and the long-term effects on cognitive dysfunction in neonates and young children [1, 2, 3]. However, the underlying mechanisms and potential targets for countering isoflurane-induced effects remain unknown.
Numerous studies have shown that the endoplasmic reticulum (ER1) is an essential organelle that performs a variety of biological functions [4, 5, 6]. When harmful stimuli or stress-related changes occur, such as the overproduction of reactive oxygen species (ROS) or elevation of intracellular calcium (Ca2+) levels, the internal ER environment is disturbed, altering the performance of normal physiological functions [7, 8, 9]. Consequently, ER stress results in protein synthesis deficiency and protein functional impairment, eventually leading to apoptosis [5, 10]. Protein synthesis is implicated in neurodevelopment and synaptogenesis after birth [11, 12, 13]. Therefore, abnormal protein synthesis and function may induce deficiency in synaptogenesis and neurodevelopmental injury [14]. Neuroapoptosis is also considered to be a main cause of cognitive dysfunction in neurodegenerative diseases [15]. However, whether ER stress is correlated with isoflurane-induced neuroapoptosis and cognitive impairment during development remains unknown.
Melatonin (N-acetyl-5-methoxytryptamine) is an amine neurohormone that is secreted by the pineal gland [16]. Melatonin possesses potent anti-oxidative, anti-inflammatory, and anti-apoptotic properties [14, 17, 18]. Furthermore, melatonin plays an important role in ameliorating cognitive dysfunction and protecting against neurodegenerative diseases such as Alzheimer's and Parkinson's disease [19, 20]. Recent research has shown that melatonin ameliorates cognitive impairment in chronic cerebral hypoperfusion, and that these beneficial effects are associated with a reduction in oxidative stress, ER stress, and apoptosis [21]. Previous studies have indicated that melatonin pre-treatment inhibits a decrease in sirtuin 1 (SIRT1) expression and circumvent the occurrence of ER stress in neonatal rats after ischemia and hypoxia [22]. Previously, we found that SIRT1, which is involved in isoflurane-induced neurotoxicity and cognitive dysfunction, was downregulated in the hippocampi of newborn rats [23]. In the present study, we aimed to investigate the potential protective effects of melatonin on ER stress and spatial learning and memory deficiency in developing rats after isoflurane-induced anesthesia. Further, we aimed to explore the underlying mechanism of action of melatonin as it relates to countering the neurotoxic effects of isoflurane.
2. Materials and method
2.1. Animals
Male and female Sprague–Dawley (SD) rats, weighing 220–250g, were obtained from the Laboratory Animal Center of Tongji Medical College, Huazhong University of Science and Technology (Wuhan, China). All the rats had ad libitum access to food and water and were housed under a 12-h light/dark cycle in standard laboratory conditions. Male and female rats were mated to induce pregnancy. Pregnant rats were reared separately until the pups were born. The procedures for animal experiments were performed in accordance with the National Research Council's Guide for the Care and Use of Laboratory Animals. The Experimental Animal Committee of Tongji Hospital, Tongji Medical College, Huazhong University of Science and Technology approved the experimental protocols.
2.2. Experimental and treatment groups
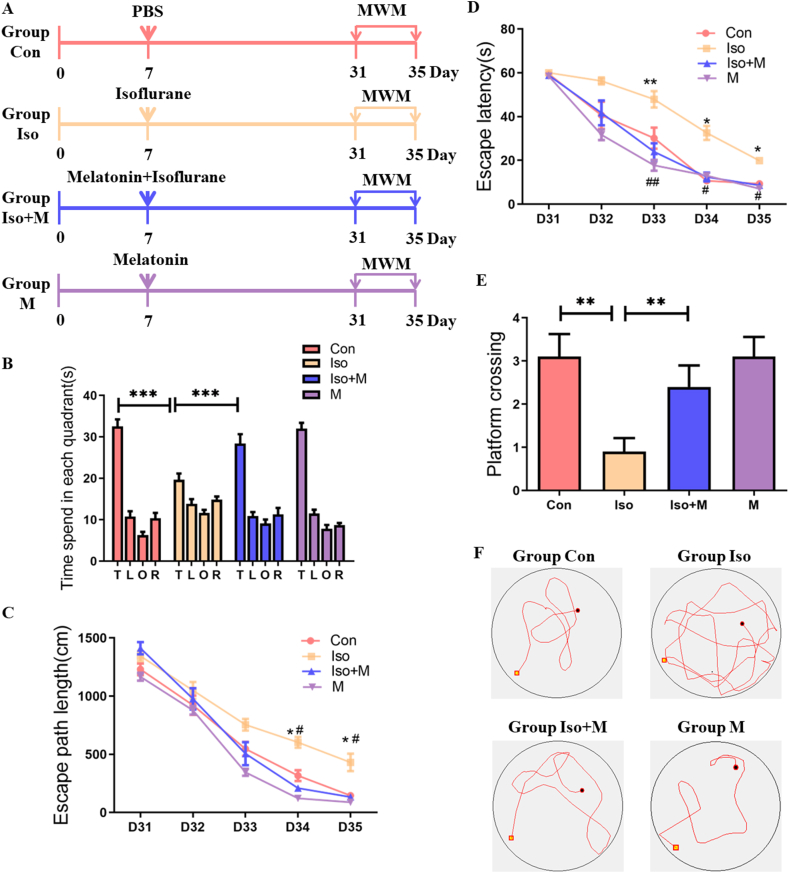
At the end of gestation, 7-day-old SD rat pups, weighing 20–25 g, were randomly selected and divided into four groups: Group Con — pups were exposed to a control gas for 6 h and an intraperitoneal (IP) injection of 100 μL PBS was administered 15 min before and 3 h after the start of control gas exposure; Group Iso — pups were exposed to 1.8% isoflurane for 6 h, and IP PBS (100 μL) was administered 15 min before and 3 h after the start of isoflurane exposure; Group Iso + M — pups were exposed to 1.8% isoflurane for 6 h, and IP melatonin (20 mg/kg) was administered 15 min before and 3 h after the start of isoflurane exposure [18]; and Group M — pups were exposed to the control gas for 6 h, and IP melatonin (20 mg/kg) was administered 15 min before and 3 h after the start of control gas exposure. Figure 1A shows the experimental design.
Figure 1.
Pretreatment with melatonin improved spatial learning and memory impairment after isoflurane exposure. (A) The schedule of four groups and behavioral tests. Isoflurane or drug treatment was performed 7 days after birth. MWM was performed on day 31–35 after birth. (B) The analysis of the time spent in each quadrant during the probe test of MWM (T: platform quadrant; L: left of platform quadrant; O: opposite to platform quadrant; R: right of platform quadrant. Quadrant: F(3,27) = 162.316, P = 0.431; Group: F(3,27) = 0.949, P < 0.001; Interaction: F(9,81) = 7.986, P < 0.001). (C) Escape path length in MWM plotted against the training days (Time: F(4,36) = 227.969, P < 0.001; Group: F(3,27) = 48.628, P < 0.001; Interaction: F(12,108) = 2.4, P = 0.009). ∗denotes p < 0.05, Con vs Iso. #denotes p < 0.05, Iso vs Iso + M. (D) Escape latency in the Morris water maze plotted against the training days (Time: F(4,36) = 152.546, P < 0.001; Group: F(3,27) = 34.765, P < 0.001; Interaction: F(12,108) = 1.358, P < 0.001). ∗denotes p < 0.05, ∗∗denotes p < 0.01, Con vs Iso. #denotes p < 0.05, ##denotes p < 0.01, Iso vs Iso + M. (E) The platform crossing times during probe trial of MWM test (F(3, 36) = 5.157, P < 0.05). (F) Representative swim paths obtained during trial 3 (session 4) from rats in the four experimental group. Data were presented as mean ± SEM (n = 10). Two-way repeated measures ANOVA followed by a by post-hoc Tukey test (n = 10). ∗denotes p < 0.05 when comparing the two groups under each end of the capped line.
2.3. Isoflurane exposure
The size of the isoflurane anesthesia box was 20 cm × 20 cm × 10 cm. The box was placed in a homoeothermic incubator, and the temperature was maintained at 37 °C. The 7-day-old rats were exposed to 1.8% isoflurane (RWD Life Science, Guangdong, China) flushed with 60% oxygen or control gas (60% oxygen, balanced with air) for 6 h in the anesthesia box. During isoflurane anesthesia, an infrared probe (OhmedaS/5 Compact, Datex-Ohmeda, Louisville, CO, USA) was used to detect the isoflurane, oxygen, and carbon dioxide concentrations.
2.4. Blood gas analysis
After the 6 h period of 1.8% isoflurane exposure, five rats from each group were randomly selected for blood gas analysis. A 100 μL blood sample was collected from the heart to determine the blood pH, arterial oxygen level, carbon dioxide tension, base excess, and blood glucose level using a blood gas analyzer (Kent Scientific Corp., Torrington, CT, USA).
2.5. Morris water maze (MWM) test
The MWM test was performed as described previously [23]. Twenty-two days after birth, the pups were separated from the mother rats. Ten rats were randomly selected from each group for the MWM test. Training for the MWM test began on 31 days after birth (D31). The pups were subjected to three training trials per day for five consecutive days. On the fifth day of training, the probe trial was performed 1 h after the training was completed. We recorded and analyzed the escape path length, time spent in each quadrant, escape latency, and platform crossings.
2.6. Western blot analysis
Western blotting was performed according to previously published protocols [23]. Twelve hours after isoflurane anesthesia, six rats were randomly selected from each group for Western blot analysis. The rats were anesthetized with an IP injection of 0.1 mL 10% pentobarbital. Quickly, the rats were placed in the supine position on a hypothermic operating table and decapitated. The skull was cut open, and the hippocampus was dissected from the exposed brain tissue. The hippocampus was homogenized in RIPA lysis and extraction buffer (150 mM sodium chloride, Triton X-100, 0.5% sodium deoxycholate, 0.1% sodium dodecyl sulfate, 50 mM Tris, pH 8.0) at 4 °C for 30 min. The samples were then centrifuged for 15 min at 4 °C, and the supernatant was collected. A bicinchoninic acid protein assay kit (Boster, Wuhan, China) was used to quantify the protein content of the supernatant. The samples were separated using 10% sodium dodecyl sulfate-polyacrylamide gel electrophoresis and transferred to polyvinylidene fluoride membranes (Millipore, Bedford, MA, USA). The bands were blocked with 5% bovine serum albumin in TBST (0.1%Tween 20 in Tris-buffered saline) for 1 h. The membrane was incubated with the primary antibodies overnight at 4 °C. The primary antibodies included the following: mouse anti-β-actin (1:2000; Qidongzi, Wuhan, China), mouse anti-SIRT1 (1:500; Cell Signaling Technology, Danvers, MA, USA), rabbit anti-mitofusin 2 (Mfn2) (1:1000; Cell Signaling Technology, Danvers, MA, USA), rabbit anti-protein kinase RNA-like ER kinase (PERK) (1:500; Affinity Biosciences, OH, USA), rabbit anti- C/EBP homologues protein (CHOP) (1:500; Affinity Biosciences, OH, USA), rabbit anti-78 kDa glucose regulated protein (GRP78) (1:500; Affinity Biosciences, OH, USA), rabbit anti-activating transcription factor 4 (ATF4) (1:500; Affinity Biosciences, OH, USA), rabbit anti-cysteine containing aspartate specific protease 12 (caspase 12) (1:500; Proteintech North America, IL, USA), rabbit anti-Bcl-2-associated X protein (Bax) (1:500; Proteintech North America, IL, USA), rabbit anti-post-synaptic density protein 95 (PSD95) (1:1000; Cell Signaling Technology, Danvers, MA, USA), rabbit anti-B-cell lymphoma-2 (Bcl-2) (1:500; Cell Signaling Technology, Danvers, MA, USA), and rabbit anti-cleaved-cysteine containing aspartate specific protease 3 (cleaved-caspase 3). The bands were then washed with TBST and incubated with horseradish peroxidase-conjugated goat anti-mouse or anti-rabbit immunoglobulin G (IgG) secondary antibodies (1:5000; Qidongzi, Wuhan, China) for 2 h at 37 °C. The bands were visualized and quantified using enhanced chemiluminescence reagents (Thermo Scientific, Waltham, MA, USA) and a ChemiDocXRS chemiluminescence imaging system (Bio-Rad, Hercules, CA, USA). The experiments were repeated at least three times.
2.7. Immunofluorescence
Brain tissue specimens were cut in the transverse plane and prepared as paraffin-embedded sections. For immunofluorescence examination, the sections were incubated overnight at 4 °C with mouse anti-cleaved-caspase 3 (1:100; Affinity Biosciences, OH, USA) and rabbit anti-caspase 12 (1:200; Proteintech North America, IL, USA) primary antibodies. The sections were washed three times and incubated with the following biotinylated secondary antibodies: FITC-conjugated AffiniPure goat anti-rabbit IgG (1:200, BOSTER, Wuhan, China) and CY3-conjugated AffiniPure goat anti-mouse IgG (1:200, BOSTER, Wuhan, China). The nuclei were stained with DAPI (BOSTER, Wuhan, China). Fluorescence images were acquired using an Olympus BX53 fluorescence microscope (Olympus Corporation, Tokyo, Japan). Finally, antigen–antibody reactions were developed using diaminobenzidine. The Image-Pro Plus software (Media Cybernetics, Silver Spring, MD, USA) was used for semiquantitative analysis.
2.8. ELISA
Rat interleukin-6 (IL-6), interleukin-1β (IL-1β), interleukin-10 (IL-10), and tumor necrosis factor-α (TNF-α) ELISA kits were purchased from MDL Biotech (Beijing, China). The protocol was followed as per the manufacturer's instructions. Six rats were randomly selected from each group for ELISA analysis. Serum was collected from whole-heart blood samples after centrifugation at 3,000 × g for 10 min. The hippocampal tissue samples were homogenized in saline, and the homogenate was centrifuged for 10 min at 2500 rpm at 4 °C to obtain the supernatant. Sample (10 μL) and dilution buffer (60 μL) was added to the wells, followed by incubation at room temperature for 60 min. The plates were washed, zymolytes were added to the wells, and the absorbance was measured on a spectrophotometer at 450 nm. The protein concentrations were calculated relative to the amount of standard protein in each sample.
2.9. Co-immunoprecipitation
Co-immunoprecipitation experiments were performed using the Pierce Co-Immunoprecipitation Kit (Thermo Scientific). The protocol was followed as per the manufacturer's instructions. Immunoprecipitation was performed using the prescribed antibodies (i.e., rabbit anti-Mfn2, 1:1000; Cell Signaling Technology, Danvers, MA, USA), followed by immunoblotting.
2.10. Primary hippocampal neuron culture
Newborn Sprague–Dawley pups (0–24 h old) were obtained from the Laboratory Animal Center of Tongji Medical College, Huazhong University of Science and Technology (Wuhan, China). Primary hippocampal neuronal cultures were produced as previously described. Briefly, hippocampi were digested with 0.125% trypsin for 15 min at 37 °C, and the cells were seeded into 6-well plates with DMEM/F12 medium (Gibco, Waltham, USA) containing 10% fetal bovine serum (Gibco, Waltham, USA). The 6-well plates were coated with Poly-L-Lysine (Sigma, MO, USA). The density of primary hippocampal neurons was 5×105 cells/well. After 4 h of incubation, the medium was replaced with a solution of Neurobasal A neuronal medium (Gibco, Waltham, USA), 2% B27 (Gibco, Waltham, USA), 1% glutamine (Gibco, Waltham, USA), and 1% penicillin/streptomycin (Gibco, Waltham, USA).
2.11. Lentiviral transfection
For the lentiviral transfection, we obtained and cultured the primary hippocampal neurons as described in section 2.10. The primary hippocampal neurons were seeded into 12-well plates at a density of 2.5×105 cells/well. The titers of SIRT1 and Mfn2 siRNA were both 2×105 TU/μL. On the third day of cell culture, the medium was discarded and 500 μL enhanced infection (ENi) solution, 5 μL polybrene, and 25 μL SIRT1 or Mfn2 siRNA housed in lentiviral vectors was added to the wells. The plate was placed in an incubator at 37 °C for 12 h. The liquid in the wells was then removed and replaced with neuronal medium containing Neurobasal A (Gibco, Waltham, USA), 2% B27 (Gibco, Waltham, USA), 1% glutamine (Gibco, Waltham, USA), and 1% penicillin/streptomycin solution (Gibco, Waltham, USA).
2.12. Statistical analysis
All data are presented as mean ± SEM and were analyzed using SPSS Statistics for Windows, version 17.0 (SPSS Inc., Chicago, Ill., USA). A two-way repeat ANOVA (treatment and time) was used to analyze the escape path length and escape latency in the MWM test. Other data were analyzed using one-way ANOVA followed by the post-hoc Tukey's test. The level of significance was set at p < 0.05.
3. Results
3.1. Physiological parameters
In blood gas test, the parameters of pH, PaCO2, PaO2, glucose, and SaO 2 were within the normal range. There were no significant between-group differences in the recorded physiological parameters (p > 0.05; Table 1).
Table 1.
Effects of isoflurane exposure on physiological parameters of arterial blood gas analysis in neonatal rats. The pH (F(3, 20) = 0.465, P = 0.710), PaCO2 (F(3, 20) = 0.247, P = 0.863), PaO2 (F(3, 20) = 0.049, P = 0.985), glucose (F(3, 20) = 0.071, P = 0.975) and SaO2 (F(3, 20) = 1.000, P = 0.413) levels did not differ significantly among the four groups. Data are presented as mean ± SEM (n = 6). PaO2, arterial oxygen tension; PaCO2, arterial carbon dioxide tension; SaO2, arterial oxygen saturation.
| Con | Iso | Iso + M | M | |
|---|---|---|---|---|
| pH | 7.28 ± 0.07 | 7.36 ± 0.04 | 7.32 ± 0.05 | 7.31 ± 0.02 |
| PaCO2 (mmHg) | 37.68 ± 3.55 | 34.57 ± 4.01 | 35.89 ± 3.89 | 39.02 ± 4.32 |
| PaO2 (mmHg) | 105.60 ± 8.12 | 103.37 ± 7.67 | 107.14 ± 6.89 | 106.63 ± 7.41 |
| Blood glucose (mmol/L) | 4.23 ± 0.82 | 4.36 ± 0.77 | 4.13 ± 0.58 | 3.94 ± 0.52 |
| SaO2 (%) | 99 ± 0.72 | 99 ± 0.97 | 99 ± 0.46 | 99 ± 0.53 |
3.2. Pretreatment with melatonin improved spatial learning and memory impairment after isoflurane exposure
The MWM was used to estimate spatial learning and memory in 31-day-old SD rats from the isoflurane exposure groups (i.e., D24 after isoflurane exposure). Isoflurane exposure impaired spatial learning and memory, indicated by less time spent in the target quarter (Figure 1B), increased escape path length (Figure 1C), and longer escape latency (Figure 1D) compared with that of the Con group. However, melatonin pretreatment ameliorated the dysfunction of spatial learning and memory induced by isoflurane exposure. Rats that were pretreated with melatonin spent more time in the target quarter (Figure 1B), had shorter travel distances (Figure 1C), and lower escape latencies (Figure 1D) compared with rats in Group Iso.
In the probe trial tests in rats from Group Iso, platform crossing decreased compared with in rats from Group Con. In support of our hypothesis, melatonin pretreatment improved isoflurane-induced spatial learning and memory impairment in juvenile rats. The results revealed that the number of platform crossings increased in the Iso + M group compared with that in the Iso group (Figure 1E). The representative swim paths obtained during trial 3 (session 4) from rats in four experimental groups (Figure 1F).
3.3. Melatonin ameliorated isoflurane-induced reduction of SIRT1 and Mfn2
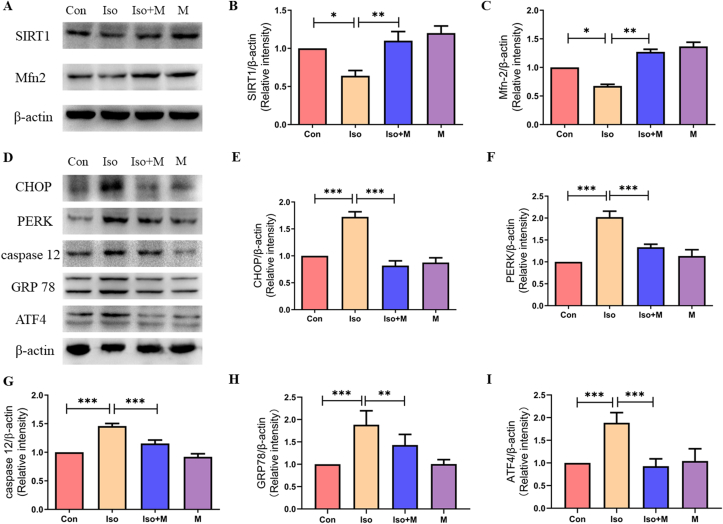
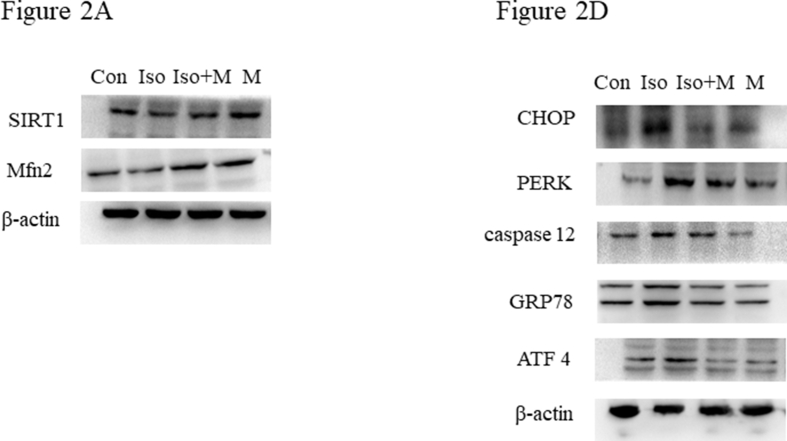
Our results demonstrated that isoflurane decreased the expression of SIRT1 and Mfn2 12 h after isoflurane exposure. Western blot results showed (Figure 2A) that pretreatment with 20 mg/kg melatonin alleviated the isoflurane-induced reduction of SIRT1 and Mfn2 in neonatal rats (Figure 2B and C).
Figure 2.
Melatonin ameliorated isoflurane-induced reduction of SIRT1 and Mfn2 and ER stress in hippocampus of new born rats. (A) Western blot bands of SIRT1 and Mfn2 in the hippocampus. (B) Hippocampal SIRT1 expression decreased after isoflurane exposure. Administration of melatonin significantly attenuated isoflurane-induced decrease in hippocampal SIRT1 expression in neonatal rats (F(3, 20) = 21.830, P < 0.001). (C) Hippocampal Mfn2 expression decreased after isoflurane exposure. Administration of melatonin significantly attenuated isoflurane-induced decrease in hippocampal Mfn2 expression in neonatal rats (F(3, 20) = 122.900, P < 0.001). (D) Western blot bands of endoplasmic reticulum stress signaling in the hippocampus. (E) Administration of melatonin significantly reduced isoflurane induced increase of endoplasmic reticulum stress marker protein CHOP expression in hippocampus of neonatal rats (F(3, 20) = 28.890, P < 0.001). (F) Administration of melatonin significantly reduced isoflurane induced increase of endoplasmic reticulum stress marker protein PERK expression in hippocampus of neonatal rats (F(3, 20) = 18.690, P < 0.001). (G) Administration of melatonin significantly reduced isoflurane induced increase of endoplasmic reticulum stress marker protein caspase 12 expression in hippocampus of neonatal rats (F(3, 20) = 26.990, P < 0.001). (H) Administration of melatonin significantly reduced isoflurane induced increase of endoplasmic reticulum stress marker protein GRP78 expression in hippocampus of neonatal rats (F(3, 20) = 26.010, P < 0.001). (I) Administration of melatonin significantly reduced isoflurane induced increase of endoplasmic reticulum stress marker protein ATF4 expression in hippocampus of neonatal rats (F(3, 20) = 32.240, P < 0.001). Data were presented as mean ± SEM (n = 6). ∗denotes p < 0.05, ∗∗denotes p < 0.01, ∗∗∗denotes p < 0.001 when comparing the two groups under each end of the capped line.
3.4. Melatonin ameliorated isoflurane-induced ER stress
Previous studies have demonstrated that Mfn2 regulates the ER stress response and cellular metabolism. ER stress leads to a series of pathological changes, such as those seen in neurodegenerative and metabolic diseases [24]. In this study, we found that isoflurane also reduced Mfn2 expression in neonatal rats. Therefore, we evaluated isoflurane-induced hippocampal ER stress and the mitigating effects of melatonin using western blotting (Figure 2D). We found that isoflurane increased the expression of CHOP, PERK, caspase 12, GRP78, and ATF4 in the hippocampi of neonatal rats, and these increases were reversed by melatonin pretreatment (Figure 2E to I).
3.5. Melatonin mitigated isoflurane-induced hippocampal neuro apoptosis and synaptogenesis obstacle in neonatal rats
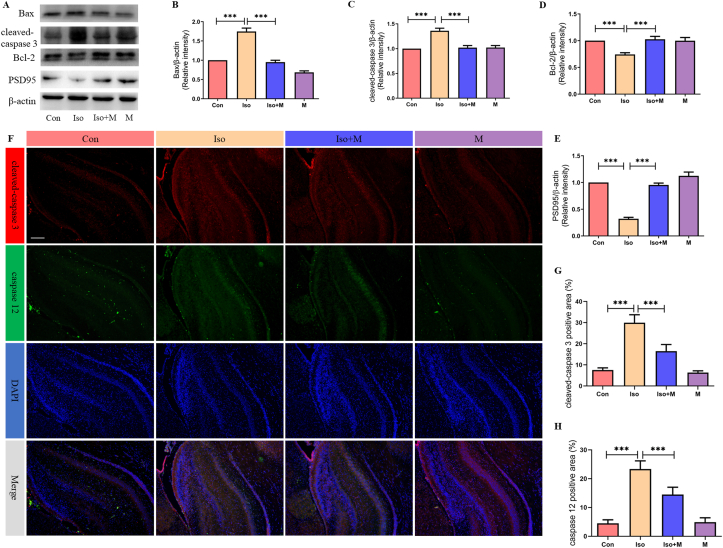
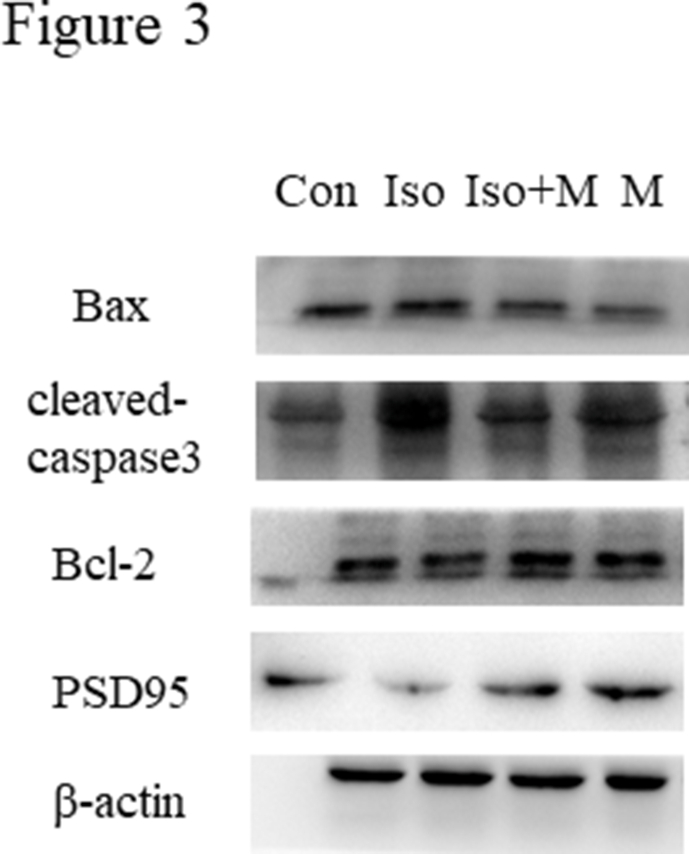
Next, we determined whether melatonin confers neuroprotective effects in the hippocampi of neonates after isoflurane exposure using western blotting. As shown in Figure 3A, isoflurane increased the expression of the pro-apoptotic factors Bax (Figure 3B) and cleaved-caspase 3 (Figure 3C), while the expression of the anti-apoptotic factor Bcl-2 was decreased (Figure 3D). However, melatonin mitigated the isoflurane-induced changes in Bax, cleaved-caspase 3, and Bcl-2 expression, thereby circumventing apoptosis in the hippocampal neurons. Isoflurane also decreased PSD95 expression in the hippocampi of neonatal rats, but this was reversed by melatonin pre-treatment (Figure 3E). We further explored the relationship between ER stress and neuronal apoptosis using immunofluorescence. Cleaved-caspase 3 and caspase 12 were simultaneously labeled for immunofluorescence analysis. Figure 3F shows that ER stress and neuronal apoptosis occurred concurrently in the hippocampal neurons, especially after isoflurane exposure.
Figure 3.
Melatonin mitigated isoflurane-induced hippocampal neuro apoptosis in neonatal rats. (A) Western blot bands of apoptosis signaling in the hippocampus. (B) The treatment of melatonin significantly reduced isoflurane induced increase of pro-apoptosis factor Bax expression in hippocampus of neonatal rats (F(3, 20) = 71.360, P < 0.001). (C) The treatment of melatonin significantly reduced isoflurane induced decrease of anti-apoptosis factor Bcl-2 expression in hippocampus of neonatal rats (F(3, 20) = 9.573, P < 0.001). (D) The treatment of melatonin significantly reduced isoflurane induced increase of pro-apoptosis factor cleaved-caspase 3 expression in hippocampus of neonatal rats (F(3, 20) = 19.910, P < 0.001). (E) The treatment of melatonin significantly increase isoflurane induced decrease of synaptic protein PSD95 expression in hippocampus of neonatal rats (F(3, 20) = 73.790, P < 0.001) (F) Immunofluorescence results of cleaved-caspase 3 and caspase 12 in hippocampus of all four groups rats. Expressions of cleaved-caspase 3 and caspase 12 in all four groups were presented through bar graphs: (G) for cleaved-caspase 3 (F(3, 20) = 109.100, P < 0.001) and (H) for caspase 12 (F(3, 20) = 105.200, P < 0.001). Data were presented as mean ± SEM (n = 6). ∗denotes p < 0.05, ∗∗denotes p < 0.01, ∗∗∗denotes p < 0.001 when comparing the two groups under each end of the capped line. Scale bar = 500 μm.
3.6. Melatonin alleviated isoflurane-induced hippocampal neuroinflammation in neonatal rats
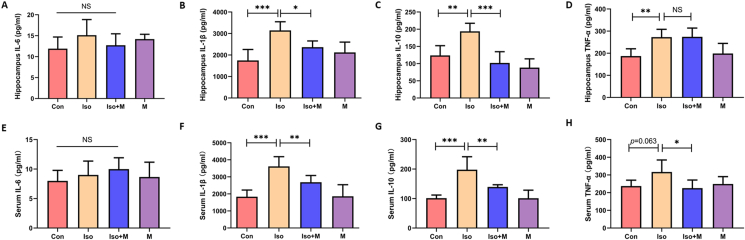
Studies have shown that neuroinflammation induced by isoflurane is associated with creating obstacles for synaptogenesis in neonatal rats, thereby causing learning and memory dysfunction as the rats become juveniles [25]. Melatonin is an anti-inflammatory and anti-oxidative stress neurohormone, and we evaluated whether melatonin could reverse isoflurane-induced neuroinflammation in neonatal rats using ELISA analyses. We found that isoflurane exposure increased the levels of IL-1β, IL-10, and TNF-α in the hippocampus, whereas melatonin effectively reversed the increase in these inflammatory factors (Figure 4B-D). Moreover, isoflurane exposure increased the levels of IL-1β and IL-10 in serum (Figure 4F and G), but not TNF-α (Figure 4H). Melatonin also effectively reversed the increase of IL-1β and IL-10 in serum (Figure 4F-G). In addition, melatonin reduced TNF-α level in serum compared with Iso group (Figure 4H). However, there were no differences in hippocampal and serum IL-6 levels among the four groups (Figure 4A and E).
Figure 4.
Melatonin alleviated isoflurane-induced neuroinflammation in hippocampus and serum of neonatal rats. Interleukin (IL)-6 level in the hippocampus (F(3, 20) = 1.639, P = 0.212) (A) and serum (F(3, 20) = 0.890, P = 0.463) (E). IL-1β level in the hippocampus (F(3, 20) = 11.000, P = 0.001) (B) and serum (F(3, 20) = 15.420, P < 0.001) (F). IL-10 level in the hippocampus (F(3, 20) = 17.110, P < 0.001) (C) and serum (F(3, 20) = 17.520, P < 0.001) (G). TNF-α level in the hippocampus (F(3, 20) = 8.695, P < 0.001) (D) and serum (F(3, 20) = 4.206, P = 0.019) (H). Data were presented as mean ± SEM (n = 6). ∗denotes p < 0.05, ∗∗denotes p < 0.01, ∗∗∗denotes p < 0.001, NS denotes not significant when comparing the two groups under each end of the capped line.
3.7. Melatonin played a neuroprotective role after isoflurane anesthesia in neonatal rats, and the effect was mediated by the SIRT1/Mfn2/PERK signaling pathway
According to our results, melatonin upregulated SIRT1 and Mfn2 expression, decreased PERK expression, and exerted neuroprotective effects on neonatal hippocampal tissue that had been exposed to isoflurane. Therefore, we further investigated the roles and relationships of SIRT1, Mfn2, and PERK in melatonin-induced neuroprotection following isoflurane exposure.
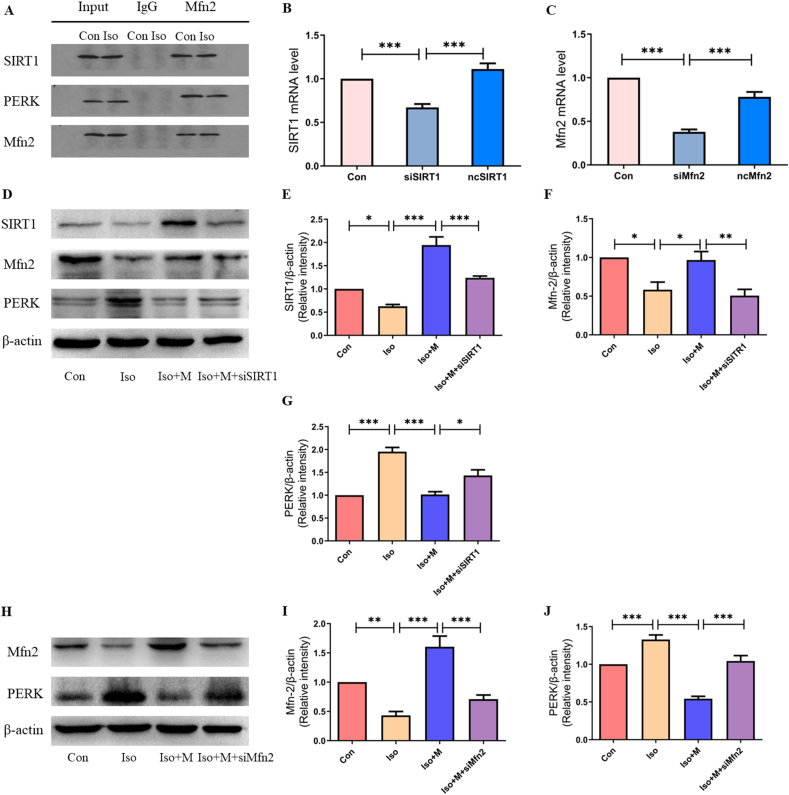
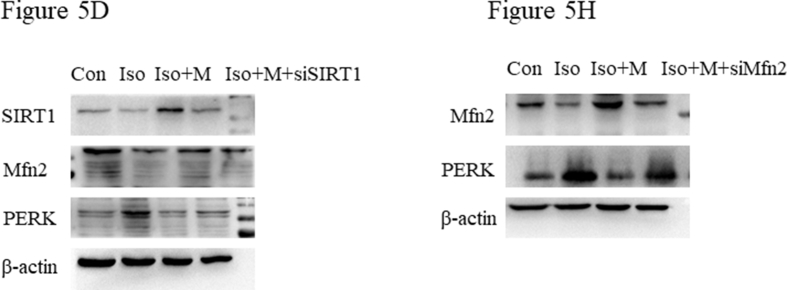
First, we examined the relationship between SIRT1, Mfn2, and PERK by co-immunoprecipitation. The co-immunoprecipitation results showed that there was an interactive relationship between SIRT1, Mfn2, and PERK in the hippocampi of neonatal rats (Figure 5A). We then downregulated SIRT1 expression using lentiviral-delivered SIRT1 siRNA in primary hippocampal neurons. The SIRT1 siRNA effectively inhibited the production of SIRT1 mRNA (Figure 5B). Further, western blotting (Figure 5D) demonstrated that isoflurane decreased SIRT1 (Figure 5E) and Mfn2 (Figure 5F) protein expression and increased PERK (Figure 5G) protein expression. However, pre-treatment with 5 mM melatonin alleviated the isoflurane-induced decrease in SIRT1 and Mfn2 expression and increase in PERK expression in primary hippocampal neurons (Figure 5D). The effect of pre-treatment with 5 mM melatonin on SIRT (Figure 5E), Mfn2 (Figure 5F), and PERK (Figure 5G) expression in primary hippocampal neurons was weakened after SIRT1 siRNA lentivirus transfection.
Figure 5.
The protective effect of melatonin after isoflurane exposure was mediated by the SIRT1/Mfn2/PERK signaling pathway. (A) The relationship of SIRT1, Mfn2 and PERK presented with Co-IP. Mfn2 was immunoprecipitated protein. SIRT1 and PERK were detected protein. (B) A graph representative of RT-PCR of SIRT1 mRNA expression (F(2,15) = 26.860, P < 0.001). (C) A graph representative of RT-PCR of Mfn2 mRNA expression (F(2,15) = 77.670, P < 0.001). (D) Western blot bands of SIRT1, Mfn2 and PERK expression after treatment with SIRT1 siRNA interference lentivirus. (E) SIRT1 expression (F(3, 20) = 35.840, P < 0.001) after treatment with SIRT1 siRNA interference lentivirus and melatonin. (F) Mfn2 expression (F(3, 20) = 9.076, P < 0.001) after treatment with SIRT1 siRNA interference lentivirus and melatonin. (G) PERK expression (F(3, 20) = 28.910, P < 0.01) after treatment with SIRT1 siRNA interference lentivirus and melatonin. (H) Western blot bands of Mfn2 and PERK expression after treatment with Mfn2 siRNA interference lentivirus. (I) Mfn2 expression (F(3, 20) = 23.500, P < 0.001) after treatment with Mfn2 siRNA interference lentivirus and melatonin. (J) PERK expression (F(3, 20) = 41.720, P < 0.001) after treatment with Mfn2 siRNA interference lentivirus and melatonin. siSIRT1: primary hippocampal neuron with SIRT1 siRNA interference lentivirus group; ncSIRT1: primary hippocampal neuron with control null siRNA interference lentivirus group; siMfn2: primary hippocampal neuron with Mfn2 siRNA interference lentivirus group; ncMfn2: primary hippocampal neuron with control null siRNA interference lentivirus group; Iso + M + siSIRT1: primary hippocampal neuron with SIRT1 siRNA interference lentivirus and pre-treatment with 5mM melatonin then received isoflurane exposure group; Iso + M + siMfn2: primary hippocampal neuron with Mfn2 siRNA interference lentivirus and pre-treatment with 5mM melatonin then received isoflurane exposure group; Data were presented as mean ± SEM (n = 6). ∗denotes p < 0.05, ∗∗denotes p < 0.01, ∗∗∗denotes p < 0.001 when comparing the two groups under each end of the capped line.
Next, we downregulated Mfn2 expression in primary hippocampal neurons using lentiviral-delivered Mfn2 siRNA. The results showed that the Mfn2 siRNA effectively inhibited Mfn2 mRNA production (Figure 5C). Western blotting (Figure 5H) demonstrated that isoflurane decreased Mfn2 (Figure 5I) and increased PERK (Figure 5J) protein expression. However, pre-treatment with 5 mM melatonin suppressed the changes in protein expression in the primary hippocampal neurons after isoflurane exposure (Figure 5H). The effect of pre-treatment with 5 mM melatonin on Mfn2 (Figure 5I) and PERK (Figure 5J) expression in primary hippocampal neurons was weakened after Mfn2 siRNA lentivirus transfection.
4. Discussion
To the best of our knowledge, this is the first study demonstrating that melatonin pre-treatment alleviates isoflurane-induced neurotoxicity and ER stress in newborn rats. We have also shown that melatonin pre-treatment improves hippocampal-dependent learning and memory dysfunction after isoflurane exposure in juvenile rats. Furthermore, we found that the protective effect of melatonin after isoflurane exposure may be mediated by the SIRT1/Mfn2/PERK signaling pathway.
Studies have shown that melatonin pre-treatment inhibits the suppression of SIRT1 expression and ameliorates ER stress in neonatal rats after ischemia and hypoxia [22]. Our previous research has shown that isoflurane reduces SIRT1 expression in new born rats, leading to developmental learning and memory impairment in juveniles [23]. We proposed that melatonin may play a protective role after isoflurane exposure in developing rats, thereby alleviating symptoms of learning and memory impairment. Previously, we showed that isoflurane reduced the expression of SIRT1 in newborn rats in a time-dependent manner, and SIRT1 expression was lowest 12 h after isoflurane exposure [23]. Based on our previous findings, we opted to measure SIRT1 expression 12 h after isoflurane exposure. The present study supports our proposition in that melatonin reversed the isoflurane-induced downregulation of SIRT1 in neonatal rats. However, in some results, the 20 mg/kg dose of melatonin provided a greater improving effect than control group because it is a high protective dose in anesthesia-induced neurodegeneration according to Yon JH et al [18]. The effects of other doses of melatonin will administrate in our future studies.
Mfn2 is a GTPase protein located in the outer membrane of mitochondria [26]. Mfn2 has an important function in the mitochondrial fusion process and participates in the functional connection between mitochondria and ER [27, 28, 29]. Studies have shown that the neuroprotective effect of SIRT1 in cerebral ischemia-reperfusion injury is related to the deacetylation of Mfn2 [27,30]. Mfn2 knockout resulted in the inhibition of SIRT1-regulated cell protection and maintenance of mitochondrial homeostasis after ischemia-reperfusion injury [31, 32]. Similarly, a lack of Mfn2 expression induces ER overexpansion or fragmentation, which activates the unfolded protein response, resulting in ER stress [33]. In contrast, the restoration of Mfn2 expression in Mfn2-deficient cells alleviates ER morphological changes and dysfunctionality induced by ER stress [17, 34, 35]. Therefore, we hypothesized that isoflurane-induced neurotoxicity in neonatal rats was related to changes in the expression of SIRT1 and Mfn2 in the hippocampi. The results of the present study showed that the expression of Mfn2 in the hippocampi of rat pups was significantly reduced after isoflurane treatment, indicating that Mfn2 may be a target of isoflurane-induced neurotoxicity in the developing brain.
Although Mfn2 is closely involved in ER functionality, whether isoflurane-induced changes to Mfn2 expression cause ER stress remains unknown. We then tested the expression of the ER stress marker proteins PERK, GRP78, ATF4, CHOP, and caspase 12 in the hippocampi of neonatal rats after isoflurane treatment. PERK, ATF6, and inositol-requiring enzyme 1 (IRE1) are the three main receptor molecules that activate CHOP during ER stress [36]. CHOP is a member of the CCAAT/enhancer binding protein family, and changes in CHOP expression directly affect the balance between proapoptotic and antiapoptotic signals [37]. The upregulation and downregulation of CHOP expression results in the activation of proapoptotic and antiapoptotic proteins, respectively [38, 39, 40]. During ER stress, caspase 12 activation is also a proapoptotic signal and is involved in neuronal apoptosis in neurodegenerative diseases [37]. Increases in caspase 12 expression in the hippocampal tissue in Alzheimer's disease effectively alleviates the production of amyloid beta and reduces neuronal apoptosis [4]. In our study, we found that isoflurane-induced ER stress may lead to dysfunctions in synaptogenesis and induce neuronal apoptosis in the developing brain. Melatonin pre-treatment alleviated isoflurane-induced ER stress and attenuated dysfunctional synaptogenesis and neuronal apoptosis in this study. Therefore, our results suggest that isoflurane-induced ER stress is related to neuronal apoptosis and learning and memory impairment in developing rats.
As risk factors for neurodegenerative diseases, neuroinflammatory responses are closely associated with cognitive dysfunction, neuronal damage, and apoptosis [15]. With disease progression, the overexpression of pro-inflammatory cytokines, such as TNF-α, IL-1β, and IL-6, results in neurodegeneration and cognitive impairment. In contrast, anti-inflammatory cytokines are significantly downregulated in neurodegenerative diseases [41]. However, our results showed that the level of the anti-inflammatory cytokine IL-10 increased after isoflurane anesthesia, and the level of the pro-inflammatory cytokine IL-6 remained unchanged. The reasons for this discrepancy may be the different time points of tissue collection and the different ELISA kits used.
Furthermore, lentiviral transfection of siRNA was performed in primary hippocampal neurons in vitro to verify the protective mechanism of melatonin. In the present study, the lentiviral delivery of SIRT1 and Mfn2 siRNA decreased the melatonin-induced upregulation of SIRT1 and Mfn2 and downregulation of PERK. This suggests that the neuroprotective function of melatonin after isoflurane exposure may be associated with the SIRT1/Mfn2/PERK signaling pathway. However whether other ER stress-related marker proteins and signaling pathways, such as ATF6 and IRE1, were involved in the protective effect requires further investigation.
Taken together, our findings demonstrate that melatonin alleviates isoflurane-induced ER stress and improves symptoms of learning and memory deficiency in juvenile rats. Therefore, melatonin provides a protective effect on hippocampal neurons after isoflurane exposure in neonatal rats following neonatal exposure to isoflurane, and the neuroprotective effect may be associated with the SIRT1/Mfn2/PERK signaling pathway. Melatonin may be a promising novel therapeutic agent for reducing ER stress and learning and memory deficiency after exposure to isoflurane during neonatal and developmental stages.
Declarations
Author contribution statement
Xi Fang: Conceived and designed the experiments; Performed the experiments; Analyzed and interpreted the data; Wrote the paper.
Qiang Han; Shiyong Li: Performed the experiments; Analyzed and interpreted the data.
Ailin Luo: Conceived and designed the experiments; Contributed reagents, materials, analysis tools or data.
Funding statement
Ailin Luo and Dr Xi Fang were supported by National Natural Science Foundation of China [81771159 & 81900140].
Data availability statement
Data included in article/supp. material/referenced in article.
Declaration of interest's statement
The authors declare no conflict of interest.
Additional information
No additional information is available for this paper.
Appendix A. Supplementary data
The following is the supplementary data related to this article:
Supplementary file-figure 3A.
Supplementary file-figure 5D and 5H.
Supplementary file-figure2A and 2D.
References
- 1.Wilder R.T., Flick R.P., Sprung J., Katusic S.K., Barbaresi W.J., Mickelson C., Gleich S.J., Schroeder D.R., Weaver A.L., Warner D.O. Early exposure to anesthesia and learning disabilities in a population-based birth cohort. Anesthesiology. 2009;110:796–804. doi: 10.1097/01.anes.0000344728.34332.5d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.DiMaggio C., Sun L.S., Li G. Early childhood exposure to anesthesia and risk of developmental and behavioral disorders in a sibling birth cohort. Anesth. Analg. 2011;113:1143–1151. doi: 10.1213/ANE.0b013e3182147f42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ing C., DiMaggio C., Whitehouse A., Hegarty M.K., Brady J., von Ungern-Sternberg B.S., Davidson A., Wood A.J., Li G., Sun L.S. Long-term differences in language and cognitive function after childhood exposure to anesthesia. Pediatrics. 2012;130:e476–485. doi: 10.1542/peds.2011-3822. [DOI] [PubMed] [Google Scholar]
- 4.Xiang C., Wang Y., Zhang H., Han F. The role of endoplasmic reticulum stress in neurodegenerative disease. Apoptosis. 2017;22:1–26. doi: 10.1007/s10495-016-1296-4. [DOI] [PubMed] [Google Scholar]
- 5.Lebeaupin C., Proics E., de Bieville C.H., Rousseau D., Bonnafous S., Patouraux S., Adam G., Lavallard V.J., Rovere C., Le Thuc O., Saint-Paul M.C., Anty R., Schneck A.S., Iannelli A., Gugenheim J., Tran A., Gual P., Bailly-Maitre B. ER stress induces NLRP3 inflammasome activation and hepatocyte death. Cell Death Dis. 2015;6 doi: 10.1038/cddis.2015.248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Fernandez A., Ordonez R., Reiter R.J., Gonzalez-Gallego J., Mauriz J.L. Melatonin and endoplasmic reticulum stress: relation to autophagy and apoptosis. J. Pineal Res. 2015;59:292–307. doi: 10.1111/jpi.12264. [DOI] [PubMed] [Google Scholar]
- 7.Feng D., Wang B., Wang L., Abraham N., Tao K., Huang L., Shi W., Dong Y., Qu Y. Pre-ischemia melatonin treatment alleviated acute neuronal injury after ischemic stroke by inhibiting endoplasmic reticulum stress-dependent autophagy via PERK and IRE1 signalings. J. Pineal Res. 2017;62 doi: 10.1111/jpi.12395. [DOI] [PubMed] [Google Scholar]
- 8.Kowalczyk M., Majsterek I., Galecki P., Talarowska M. The role of the endoplasmic reticulum stress in depression. Psychiatr. Pol. 2020;54:499–508. doi: 10.12740/PP/109130. [DOI] [PubMed] [Google Scholar]
- 9.Li W., Liu B., Wang L., Liu J., Yang X., Zheng J. Melatonin attenuates cardiac ischemia-reperfusion injury through modulation of IP3R-mediated mitochondria-ER contact. Oxid. Med. Cell. Longev. 2021;2021 doi: 10.1155/2021/1370862. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 10.Tsujii S., Ishisaka M., Hara H. Modulation of endoplasmic reticulum stress in Parkinson's disease. Eur. J. Pharmacol. 2015;765:154–156. doi: 10.1016/j.ejphar.2015.08.033. [DOI] [PubMed] [Google Scholar]
- 11.Schaeffer C., Merella S., Pasqualetto E., Lazarevic D., Rampoldi L. Mutant uromodulin expression leads to altered homeostasis of the endoplasmic reticulum and activates the unfolded protein response. PLoS One. 2017;12 doi: 10.1371/journal.pone.0175970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Godin J.D., Creppe C., Laguesse S., Nguyen L. Emerging roles for the unfolded protein response in the developing nervous system. Trends Neurosci. 2016;39:394–404. doi: 10.1016/j.tins.2016.04.002. [DOI] [PubMed] [Google Scholar]
- 13.Goswami P., Gupta S., Biswas J., Joshi N., Swarnkar S., Nath C., Singh S. Endoplasmic reticulum stress plays a key role in rotenone-induced apoptotic death of neurons. Mol. Neurobiol. 2016;53:285–298. doi: 10.1007/s12035-014-9001-5. [DOI] [PubMed] [Google Scholar]
- 14.Tajes M., Gutierrez-Cuesta J., Ortuno-Sahagun D., Camins A., Pallas M. Anti-aging properties of melatonin in an in vitro murine senescence model: involvement of the sirtuin 1 pathway. J. Pineal Res. 2009;47:228–237. doi: 10.1111/j.1600-079X.2009.00706.x. [DOI] [PubMed] [Google Scholar]
- 15.Voet S., Srinivasan S., Lamkanfi M., van Loo G. Inflammasomes in neuroinflammatory and neurodegenerative diseases. EMBO Mol. Med. 2019;11 doi: 10.15252/emmm.201810248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Xue F., Shi C., Chen Q., Hang W., Xia L., Wu Y., Tao S.Z., Zhou J., Shi A., Chen J. Melatonin mediates protective effects against kainic acid-induced neuronal death through safeguarding ER stress and mitochondrial disturbance. Front. Mol. Neurosci. 2017;10:49. doi: 10.3389/fnmol.2017.00049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Baydas G., Ozveren F., Akdemir I., Tuzcu M., Yasar A. Learning and memory deficits in rats induced by chronic thinner exposure are reversed by melatonin. J. Pineal Res. 2005;39:50–56. doi: 10.1111/j.1600-079X.2005.00212.x. [DOI] [PubMed] [Google Scholar]
- 18.Yon J.H., Carter L.B., Reiter R.J., Jevtovic-Todorovic V. Melatonin reduces the severity of anesthesia-induced apoptotic neurodegeneration in the developing rat brain. Neurobiol. Dis. 2006;21:522–530. doi: 10.1016/j.nbd.2005.08.011. [DOI] [PubMed] [Google Scholar]
- 19.Adi N., Mash D.C., Ali Y., Singer C., Shehadeh L., Papapetropoulos S. Melatonin MT1 and MT2 receptor expression in Parkinson's disease. Med. Sci. Mon. Int. Med. J. Exp. Clin. Res. 2010;16:BR61–67. [PubMed] [Google Scholar]
- 20.Shukla M., Govitrapong P., Boontem P., Reiter R.J., Satayavivad J. Mechanisms of melatonin in alleviating Alzheimer's disease. Curr. Neuropharmacol. 2017;15:1010–1031. doi: 10.2174/1570159X15666170313123454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Thangwong P., Jearjaroen P., Govitrapong P., Tocharus C., Tocharus J. Melatonin improves cognitive function by suppressing endoplasmic reticulum stress and promoting synaptic plasticity during chronic cerebral hypoperfusion in rats. Biochem. Pharmacol. 2022:198. doi: 10.1016/j.bcp.2022.114980. [DOI] [PubMed] [Google Scholar]
- 22.Carloni S., Albertini M.C., Galluzzi L., Buonocore G., Proietti F., Balduini W. Melatonin reduces endoplasmic reticulum stress and preserves sirtuin 1 expression in neuronal cells of newborn rats after hypoxia-ischemia. J. Pineal Res. 2014;57:192–199. doi: 10.1111/jpi.12156. [DOI] [PubMed] [Google Scholar]
- 23.Fang X., Han Q., Li S., Zhao Y., Luo A. Chikusetsu saponin IVa attenuates isoflurane-induced neurotoxicity and cognitive deficits via SIRT1/ERK1/2 in developmental rats. Am. J. Transl. Res. 2017;9:4288–4299. [PMC free article] [PubMed] [Google Scholar]
- 24.Zhang Y., Liu W., Zhou Y., Ma C., Li S., Cong B. Endoplasmic reticulum stress is involved in restraint stress-induced hippocampal apoptosis and cognitive impairments in rats. Physiol. Behav. 2014;131:41–48. doi: 10.1016/j.physbeh.2014.04.014. [DOI] [PubMed] [Google Scholar]
- 25.Peng L., Zhu M., Yang Y., Lu F., Liu X., Guo Q., Zhong T. Repeated neonatal isoflurane exposure is associated with higher susceptibility to chronic variable stress-induced behavioural and neuro-inflammatory alterations. Neuroscience. 2021;465:166–176. doi: 10.1016/j.neuroscience.2021.04.024. [DOI] [PubMed] [Google Scholar]
- 26.Chen H., Chan D.C. Critical dependence of neurons on mitochondrial dynamics. Curr. Opin. Cell Biol. 2006;18:453–459. doi: 10.1016/j.ceb.2006.06.004. [DOI] [PubMed] [Google Scholar]
- 27.Munoz J.P., Ivanova S., Sanchez-Wandelmer J., Martinez-Cristobal P., Noguera E., Sancho A., Diaz-Ramos A., Hernandez-Alvarez M.I., Sebastian D., Mauvezin C., Palacin M., Zorzano A. Mfn2 modulates the UPR and mitochondrial function via repression of PERK. EMBO J. 2013;32:2348–2361. doi: 10.1038/emboj.2013.168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Chen H., Detmer S.A., Ewald A.J., Griffin E.E., Fraser S.E., Chan D.C. Mitofusins Mfn1 and Mfn2 coordinately regulate mitochondrial fusion and are essential for embryonic development. J. Cell Biol. 2003;160:189–200. doi: 10.1083/jcb.200211046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Schneeberger M., Dietrich M.O., Sebastian D., Imbernon M., Castano C., Garcia A., Esteban Y., Gonzalez-Franquesa A., Rodriguez I.C., Bortolozzi A., Garcia-Roves P.M., Gomis R., Nogueiras R., Horvath T.L., Zorzano A., Claret M. Mitofusin 2 in POMC neurons connects ER stress with leptin resistance and energy imbalance. Cell. 2013;155:172–187. doi: 10.1016/j.cell.2013.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Biel T.G., Lee S., Flores-Toro J.A., Dean J.W., Go K.L., Lee M.H., Law B.K., Law M.E., Dunn W.A., Jr., Zendejas I., Behrns K.E., Kim J.S. Sirtuin 1 suppresses mitochondrial dysfunction of ischemic mouse livers in a mitofusin 2-dependent manner. Cell Death Differ. 2016;23:279–290. doi: 10.1038/cdd.2015.96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Chun S.K., Go K., Yang M.J., Zendejas I., Behrns K.E., Kim J.S. Autophagy in ischemic livers: a critical role of sirtuin 1/mitofusin 2 Axis in autophagy induction. Toxicol. Res. 2016;32:35–46. doi: 10.5487/TR.2016.32.1.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Daniele T., Hurbain I., Vago R., Casari G., Raposo G., Tacchetti C., Schiaffino M.V. Mitochondria and melanosomes establish physical contacts modulated by Mfn2 and involved in organelle biogenesis. Curr. Biol. 2014;24:393–403. doi: 10.1016/j.cub.2014.01.007. [DOI] [PubMed] [Google Scholar]
- 33.Sebastian D., Hernandez-Alvarez M.I., Segales J., Sorianello E., Munoz J.P., Sala D., Waget A., Liesa M., Paz J.C., Gopalacharyulu P., Oresic M., Pich S., Burcelin R., Palacin M., Zorzano A. Mitofusin 2 (Mfn2) links mitochondrial and endoplasmic reticulum function with insulin signaling and is essential for normal glucose homeostasis. Proc. Natl. Acad. Sci. U. S. A. 2012;109:5523–5528. doi: 10.1073/pnas.1108220109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Baydas G., Yasar A., Tuzcu M. Comparison of the impact of melatonin on chronic ethanol-induced learning and memory impairment between young and aged rats. J. Pineal Res. 2005;39:346–352. doi: 10.1111/j.1600-079X.2005.00257.x. [DOI] [PubMed] [Google Scholar]
- 35.Vadnais M.L., Lin A.M., Gerton G.L. Mitochondrial fusion protein MFN2 interacts with the mitostatin-related protein MNS1 required for mouse sperm flagellar structure and function. Cilia. 2014;3:5. doi: 10.1186/2046-2530-3-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Santos L.E., Ferreira S.T. Crosstalk between endoplasmic reticulum stress and brain inflammation in Alzheimer's disease. Neuropharmacology. 2018;136:350–360. doi: 10.1016/j.neuropharm.2017.11.016. [DOI] [PubMed] [Google Scholar]
- 37.Pinto B.A., Melo T.M., Flister K.F., Franca L.M., Kajihara D., Tanaka L.Y., Laurindo F.R., Paes A.M. Early and sustained exposure to high-sucrose diet triggers hippocampal ER stress in young rats. Metab. Brain Dis. 2016;31:917–927. doi: 10.1007/s11011-016-9830-1. [DOI] [PubMed] [Google Scholar]
- 38.Martinez G., Vidal R.L., Mardones P., Serrano F.G., Ardiles A.O., Wirth C., Valdes P., Thielen P., Schneider B.L., Kerr B., Valdes J.L., Palacios A.G., Inestrosa N.C., Glimcher L.H., Hetz C. Regulation of memory formation by the transcription factor XBP1. Cell Rep. 2016;14:1382–1394. doi: 10.1016/j.celrep.2016.01.028. [DOI] [PubMed] [Google Scholar]
- 39.Fernandez-Bautista N., Fernandez-Calvino L., Munoz A., Castellano M.M. HOP3, a member of the HOP family in Arabidopsis, interacts with BiP and plays a major role in the ER stress response. Plant Cell Environ. 2017;40:1341–1355. doi: 10.1111/pce.12927. [DOI] [PubMed] [Google Scholar]
- 40.Damiano F., Tocci R., Gnoni G.V., Siculella L. Expression of citrate carrier gene is activated by ER stress effectors XBP1 and ATF6alpha, binding to an UPRE in its promoter. Biochim. Biophys. Acta. 2015;1849:23–31. doi: 10.1016/j.bbagrm.2014.10.004. [DOI] [PubMed] [Google Scholar]
- 41.Kaur D., Sharma V., Deshmukh R. Activation of microglia and astrocytes: a roadway to neuroinflammation and Alzheimer's disease. Inflammopharmacology. 2019;27:663–677. doi: 10.1007/s10787-019-00580-x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data included in article/supp. material/referenced in article.