Abstract
Background
The roles of physical activity (PA) and exercise within the management of cystic fibrosis (CF) are recognised by their inclusion in numerous standards of care and treatment guidelines. However, information is brief, and both PA and exercise as multi-faceted behaviours require extensive stakeholder input when developing and promoting such guidelines.
Method
On 30th June and 1st July 2021, 39 stakeholders from 11 countries, including researchers, healthcare professionals and patients participated in a virtual conference to agree an evidence-based and informed expert consensus about PA and exercise for people with CF. This consensus presents the agreement across six themes: (i) patient and system centred outcomes, (ii) health benefits, iii) measurement, (iv) prescription, (v) clinical considerations, and (vi) future directions. The consensus was achieved by a stepwise process, involving: (i) written evidence-based synopses; (ii) peer critique of synopses; (iii) oral presentation to consensus group and peer challenge of revised synopses; and (iv) anonymous voting on final proposed synopses for adoption to the consensus statement.
Results
The final consensus document includes 24 statements which surpassed the consensus threshold (>80% agreement) out of 30 proposed statements.
Conclusion
This consensus can be used to support health promotion by relevant stakeholders for people with CF.
Keywords: respiratory disease, lifestyle, health, clinical practice
Background
Cystic fibrosis (CF) is a genetically inherited condition, predominantly characterised by an accumulation of viscous mucus in the airway and digestive tract, resulting in recurrent airway infection and inflammation, declining lung function and nutritional complications. Currently, approximately 90,000 people worldwide are diagnosed with CF, and many are now expected to live into the fifth decade of life. 1
As there is no cure for CF, it is a condition that is continually managed using pharmacological, nutritional, physiotherapy and exercise interventions. Due to the multi-faceted nature of CF treatment, many evidence-driven, expert consensus guidelines have been produced to guide clinical teams in its management.2–9 However, whilst the role of physical activity (PA) – any bodily movement resulting in energy expenditure – and exercise (as a structured sub-component of PA) has been acknowledged in guidelines and standards of care,10–13 only a solitary consensus document on the role of PA and exercise has been produced to date, focusing on recommended prescription of training. 14 Furthermore, PA and exercise have only broadly been recognised under the wider remit of physiotherapy services and treatment in guidelines, instead of as stand-alone areas of treatment. 10 Finally, reviews of PA and exercise literature have been conducted independently of one another and therefore lack a holistic appreciation of the integrated role they play in managing CF.15–17
Therefore, there is a need to provide an updated, holistic, and integrated overview of the role of PA and exercise in the management of CF, acknowledging all components of health and behaviour, as well as all sub-populations (e.g., adults, paediatric, and those with severe disease). The purpose of this consensus was to produce an evidence- and expert-driven series of statements that summarises current benefits of PA and exercise, and provide recommendations for future practices.
Methodology
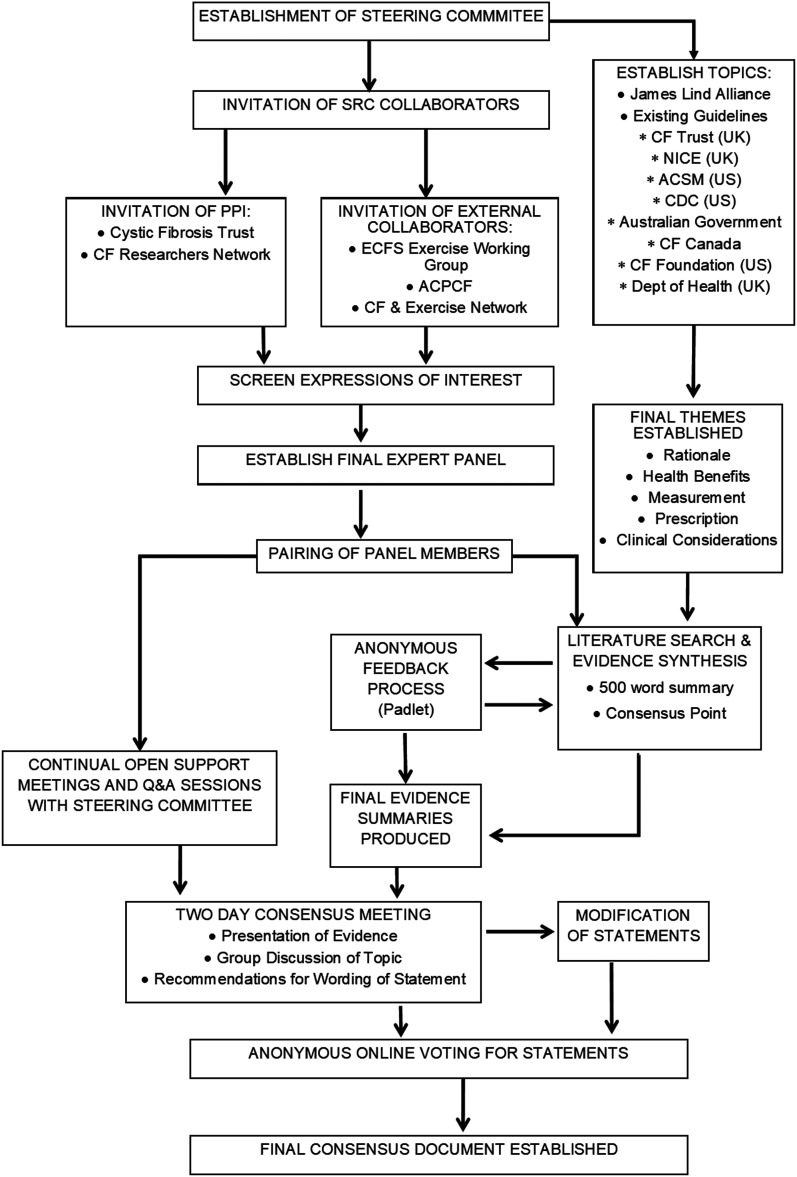
The development of this consensus document was performed in multiple stages, utilising several approaches. A full visual schematic, detailing the processes is provided in Figure 1.
Figure 1.
Flowchart detailing process involved in establishing consensus document. ACPCF, Association of Chartered Physiotherapists in Cystic Fibrosis; ACSM, American College of Sports Medicine; CDC, Centre for Disease Control; CF, cystic fibrosis; ECFS, European Cystic Fibrosis Society; NICE, National Institute for Health and Care Excellence; PPI, public and patient involvement; SRC, Strategic Research Centre.
Expert group
The expert group was made up of key stakeholders who are experts in the areas of PA and exercise, along with the provision of clinical care in CF. This group was established in two phases.
First, existing members of the ‘Youth Activity Unlimited’ group – a Strategic Research Centre (SRC) funded by the Cystic Fibrosis Trust and hosted by the University of Exeter were invited to be involved. This SRC is an international, multi-centre, research group that specialises in several areas related to PA and exercise for individuals with CF. Within this SRC-based group, a steering committee met regularly between 2020–2021 to finalise the protocol, topics for discussion, and timeline of events for the consensus. This steering committee provided the framework upon which the consensus is built, with subsequent intellectual content being provided by a wider body of researchers and applied professionals. It was decided by this initial steering committee to base the methodology for this consensus document upon the similarly structured ‘Copenhagen Consensus’ on children, youth and PA. 18
Second, a wider group of stakeholders including international and multidisciplinary academics and clinical specialists were invited to participate, to provide a wide breadth of expertise and experience. These individuals were recruited via open email advertisement, distributed via professional organisations including the Association of Chartered Physiotherapists in Cystic Fibrosis (UK), the Cystic Fibrosis and Exercise Network (UK) and the European Cystic Fibrosis Society ‘Exercise Working Group’ (EUR). The respective chairpersons of each organisation kindly disseminated an ‘Invitation Document’ via email that detailed the anticipated processes and required time commitments, as well as an ‘Expression of Interest’ form to their respective memberships (Supplementary File 1). Recipients of this ‘expression of interest’ were asked to submit a request to be involved provided: (i) they held relevant expertise in the field of PA or exercise for CF, from either a research or applied (or both) perspective in academia and/or clinical practice, and (ii) were willing and able to commit the necessary time and content, whilst adhering to specified deadlines. Expressions of interest were screened by the steering committee and following recruitment from both pathways, a total of 36 participants expressed interest, and all group members were subsequently provided with a ‘Guideline Document’ that detailed the workload and associated deadlines (Supplementary File 1).
A ‘Public and Patient Involvement’ (PPI) component was also included, thus ensuring representation of the CF community within the process. This PPI was sourced via the CF Trust (UK) and the CF Researchers Network (UK). This engagement subsequently led to a final group of 39 participants, with a composition of the group provided in Table 1.
Table 1.
Characteristics of the expert group.
| Participant characteristics | Number |
|---|---|
| Country (n) | United Kingdom (23), Australia (4), Canada (3), Ireland (3), Greece (2), Chile (1), France (1), Netherlands (1), United States of America (1) |
| SRC/External/PPI, n | 14/23/2 |
| Female/Male, n | 24/15 |
| Academic/HCP/PPI, n | 22/15/2 |
| HCP Positions, n | Physiotherapist (7), Consultant/Professor (4), kinesiologist/exercise physiologist (1), clinical researcher (1), pulmonologist (1), technical instructor (1) |
| ECR/Non-ECR †, n | 18/19 |
| Experience in CF (years, mean ± SD) | 13 ± 9 years (range = 0–36 years*) |
| Experience in CF (cumulative total, years) | 479 years |
CF: cystic fibrosis; ECR: Early Career Researcher/Professional; HCP: healthcare professional; PPI: public and patient involvement; SRC: Members of ‘Youth Activity Unlimited’ Strategic Research Centre. †Identity as an ECR was self-selected (Supplementary File 1). Academic and clinicians only included within this breakdown of ECR numbers (no PPI included). *Experience in completed number of years.
Topic selection
Topics for the consensus were selected based upon areas of research and practice that are consistently published and overlap within existing standards of care documents aligned to CF, and CF and PA/exercise literature; thus, indicating a relevance and priority to understand and evaluate these areas of CF management. The existing guidelines that are currently used in clinical practice, and informed the topic selections, were also previously identified via an international survey of healthcare professionals in CF. 19 Topics were also informed by current research priorities, as established by the CF community via a Priority Setting Partnership.20,21 To acknowledge the holistic and simultaneous prescription of both PA and exercise within clinical guidelines,10,11 these were not separated for the purposes of this consensus.
Topics were then grouped into five distinct areas to form the basis of the consensus document: (i) The Rationale for Physical Activity and Exercise in Cystic Fibrosis; (ii) Health Benefits of Physical Activity and Exercise in Cystic Fibrosis; (iii) Measurement of Physical Activity and Exercise in Cystic Fibrosis; (iv) Prescription of Physical Activity and Exercise in Cystic Fibrosis; (v) Clinical Considerations for Physical Activity and Exercise in Cystic Fibrosis.
Within the five themes, a series of specific sub-themes were identified (Table 2), which were subsequently assigned to two of the group members (excepting two sub-themes, with one author each). Where possible, experienced group members were paired with early career researchers (ECR) and practitioners to develop and train junior members, whilst maintaining rigorous standards. These pairs were subsequently responsible for literature searches, synthesis of evidence, writing of summaries, and presentation of the topic area to the wider group during the online meetings.
Table 2.
Sub-themes identified for development within the consensus.
| Main theme | Sub-theme |
|---|---|
| Rationale for physical activity and exercise | Patient oriented
outcomes Health-system oriented outcomes |
| Health benefits of physical activity and exercise | Lung health Cardiovascular health Musculoskeletal health Fitness and strength Glycaemic and inflammatory health Mental health Quality of life |
| Measurement of physical activity and exercise | Measurement of physical
activity Measurement of exercise |
| Prescription of physical activity and exercise | Optimal training modes and
styles Adherence Staffing requirements Telehealth |
| Clinical considerations for physical activity and exercise | Airway
clearance Modulators Risks Nutrition and hydration |
Evidence summaries
Initial evidence summaries were developed by each pair within the group, whereby they were requested to search relevant databases in order to collate and synthesise sufficient evidence to produce a preliminary 500-word summary on each of the aforementioned sub-themes. As per the Guideline Document in Supplementary File 1, each pair was requested to utilise the highest grade evidence available for their specific topic (e.g., meta analyses).
As there was a wide array of the topics within the consensus, each pair was allowed to select their own search criteria and databases. However, consensus members confirmed that Ovid MEDLINE, Ovid EMBASE, PsychINFO, ERIC, SPORTDiscus, ASSIA, CCTR, CINAHL, Web of Science, and Google Scholar were searched, although it is possible further undeclared databases were also searched.
In addition, evidence collated for two ongoing systematic reviews in the areas of mental health (PROSPERO: CRD42019151034) and physical health (PROSPERO: CRD42020184411 22 ) for CF were also utilised for benefit of respective authors assigned to produce summaries on those themes. Alongside these summaries, all group members were advised to provide a provisional ‘Evidence Statement’ that concisely summarised the strength, breadth, and direction of the evidence.
Once written, evidence summaries and associated statements were posted on an open, collaborative, web-platform (Padlet; San Francisco, USA), with all group members asked to provide anonymous comments and feedback on each of the summaries. The feedback process was detailed in a document sent to all group members (Supplementary File 1) and was held open for a 10-day period. Feedback included suggestions regarding the inclusion or removal of content and references, or stylistic/language modifications, as well as changes to the wording of the summaries and statements. All the feedback was collated and is provided in Supplementary File 1.
Once the feedback period had closed, all group members revised the 500-word summaries and statements. Summaries and statements were then distributed to all group members in advance of a virtual, 2-day meeting, alongside a ‘Meeting Document’ that detailed the procedures and timelines for the meeting (Supplementary File 1).
Conference meeting
A 2-day conference meeting was held on June 30th and 1 July 2021. The meeting was hosted virtually (Zoom; San Jose, USA) to facilitate maximal engagement amongst all participants, and circumvent the need for national or international travel due to global travel restrictions associated with the COVID-19 pandemic.
Over the course of the 2 days, each topic was presented by group members, whereby the existing evidence was outlined, and how the subjective strength, and depth, of the evidence informed the creation of the summary statement. Evidence was not graded, as this document was focused upon developing consensus statements and not formal recommendations for clinical practice, 23 and all topics were of differing designs and methodologies, making a singular framework with which to grade evidence impractical. Following each presentation, the evidence summaries were discussed as a group, with a view to finalising the wording on each statement, which would ultimately be voted upon by the group.
Voting of statements
Following the 2-day meeting, all of the final summary statements were collated and emailed as a survey to all attendees of the meeting. Group members were then asked to either state, via multiple-choice responses, whether they ‘agree’ or ‘do not agree’ with each statement, or whether they ‘abstain’ from voting for that particular statement. The survey was hosted on Microsoft Forms (Microsoft Corp.; Redmond, USA), and held open for 3 days to ensure all respondents had adequate time to complete the survey, with all responses being collated anonymously. The steering panel initially proposed a minimum threshold of >50% of ‘agree’ votes to be required for each statement to be included in the final document, however further consultation with the wider group led to a higher threshold of >80% being adopted, in line with recent consensus documents in CF.5,7
Results of consensus
All the evidence summaries that support the final statements are provided in Supplementary File 2. Throughout the process, the statements underwent a series of iterative changes, and the evolution of these is detailed in Supplementary File 1.
Throughout the 2-day meeting, it became apparent via discussions that a sixth category of ‘Future Directions’ was warranted, to highlight immediate priorities for clinical practice and future research. Therefore, draft statements were written by project leads (CAW and OWT), and a multiple-choice selection of future research questions was developed for inclusion as consensus statements.
Following the anonymous voting process, 21 full statements surpassed the 80% threshold, as well as a statement with three sub-sections; resulting in 24 unique components to the final document. There are all detailed in Table 3 alongside the percentage of votes for each option (agree/do not agree/abstain). Six statements failed to surpass the 80% threshold, and these are presented with their accompanying percentages of ‘agree’ votes in Supplementary File 3.
Table 3.
Final consensus statements and associated agreement from group members.
| Statement | Agree n (%) | Do not agree n (%) | Abstain n (%) | |
|---|---|---|---|---|
| The rationale for physical activity and exercise in CF – Why are these factors important for people with CF? | ||||
| 1 | Physical activity and exercise contribute to increased survival in people with cystic fibrosis and can also improve an individual’s sense of overall well-being | 36 (95) | 2 (5) | 0 (0) |
| 2 | Engagement in physical activity and exercise has the potential to positively influence the cost associated with hospitalisations, exacerbations, and antibiotic use | 34 (89) | 3 (8) | 1 (3) |
| Health benefits of physical activity and exercise in CF – How does changing these factors benefit people with cystic fibrosis? | ||||
| 3 | Physical activity and exercise can improve cardiovascular health in people with cystic fibrosis, however there is limited research on this topic | 33 (87) | 3 (8) | 2 (5) |
| 4 | Resistance training improves limb muscle strength in people with cystic fibrosis | 35 (92) | 2 (5) | 1 (3) |
| 5 | Exercise of adequate frequency and progressive intensity improves fitness in people with cystic fibrosis | 36 (95) | 2 (5) | 0 (0) |
| 6 | Health-related quality of life is an important and holistic outcome measure that should be included in physical activity and exercise intervention research, but the clinical effectiveness of physical activity and exercise interventions on the different domains of health-related quality of life remains unclear | 36 (95) | 0 (0) | 2 (5) |
| Measurement of physical activity and exercise in CF – How do we monitor these factors? | ||||
| 7 | Accelerometers provide accurate information regarding the volume and accumulation of physical activity, particularly when tailored for use in people with cystic fibrosis, but self-report data is still required to provide contextual, and complementary, information | 34 (89) | 2 (5) | 2 (5) |
| 8 | There are many safe, effective, and informative options for exercise testing in people with cystic fibrosis regardless of age or disease severity | 37 (97) | 1 (3) | 0 (0) |
| 9 | Lab-based tests provide greater insights to the determinants of exercise capacity, but field-based tests can provide useful information if lab-based tests are not available | 35 (92) | 2 (5) | 1 (3) |
| 10 | Standardised methods of exercise testing and physical activity assessment should be utilised to enable within- and between-person comparisons, facilitating the individualisation of physical activity promotion and/or exercise prescription | 36 (95) | 1 (3) | 1 (3) |
| Prescription of physical Activity and exercise in CF – How do we facilitate change and get people with cystic fibrosis moving (more)? | ||||
| 11 | An individualised and comprehensive training program, undertaken at a moderate intensity or higher, as part of the ongoing therapeutic routine is recommended in people with cystic fibrosis | 35 (92) | 2 (5) | 1 (3) |
| 12 | Lifelong adherence to habitual physical activity and exercise across the lifespan is important and challenging, due to the many competing and time-consuming activities | 34 (89) | 4 (11) | 0 (0) |
| 13 | The provision of exercise within cystic fibrosis care is becoming increasingly important and requires additional staffing and expertise at the level required to offer annual exercise testing and prescription, including the option of cardiopulmonary exercise testing. Standardisation of the education, training and roles and responsibilities of exercise specialists and other healthcare professionals responsible for the provision of exercise services is required | 36 (95) | 2 (5) | 0 (0) |
| 14 | Exercise training delivered remotely by video calling is feasible and acceptable to people with cystic fibrosis in a small number of pilot studies, and clinical teams could consider its use as a supplement to face-to-face contact | 37 (97) | 1 (3) | 0 (0) |
| Clinical considerations for physical activity and exercise in CF – What must we also consider when prescribing activity and exercise for people with cystic fibrosis | ||||
| 15 | Exercise with huff or cough improves mucus clearance, however there is no medium- or long-term evidence if exercise can adequately replace airway clearance techniques in people with cystic fibrosis | 38 (100) | 0 (0) | 0 (0) |
| 16 | The clinical efficacy of CFTR modulator therapy on the fitness and physical activity behaviours of people with cystic fibrosis warrants further study. Including exercise testing measurements as standard alongside routinely collected clinical data within cystic fibrosis registries may begin to allow a better understanding of the physiological effects of modulator therapies | 32 (84) | 3 (8) | 3 (8) |
| 17 | Physical activity and exercise are strongly recommended for people with cystic fibrosis. Although the risk is minimal, guidance should be provided to all people with cystic fibrosis around hydration, nutrition, exercise intensity and duration, based on the specific activity | 36 (95) | 1 (3) | 1 (3) |
| 18 | Both energy intake and hydration should be adjusted to meet the elevated caloric and fluid needs of an active person with cystic fibrosis. Every exercise/diet plan should be discussed with the physician and dietician to meet the individual’s body mass index targets, caloric goals, and diet preferences | 34 (89) | 2 (5) | 2 (5) |
| Future directions for physical activity and exercise in CF | ||||
| 19 | The prescription of physical activity and exercise should be individualised for people with cystic fibrosis. All aspects of disease management and personal patient preferences should be considered | 36 (95) | 1 (3) | 1 (3) |
| 20 | Clinical trials in cystic fibrosis should include outcomes associated with physical activity and exercise to understand how lifestyle parameters can be affected by pharmaceutical and non-pharmaceutical interventions | 36 (97) | 1 (3) | 0 (0) |
| 21 | Future trials of physical activity and exercise should seek to include outcomes that are currently under-reported, such as cardiovascular health, mental health, endocrine, inflammatory function, and patient reported outcomes, to help further evaluate the effects of physical activity and exercise upon overall health status | 38 (100) | 0 (0) | 0 (0) |
| 22 | Future research should examine: A. Whether
exercise can adequately replace airway clearance
techniques. B. The interactive effects of CFTR modulators, physical activity, and exercise C. How to improve adherence, including overcoming barriers, identifying enablers, and developing routines, to physical activity and structured exercise programmes |
a) 32 (84) b) 34 (89) c) 33 (87) |
— | — |
All values are presented in percentages, following voting process whereby n =38 group members cast votes. Totals may not always equal 100% due to rounding. Excluded statements provided in Supplemental File 3.
The mean agreement of all statements was 88% (±10%), ranging from 50-100%. When only considering those above the 80% acceptance threshold, mean agreement was 93% (±4%), ranging from 84-100%. Moreover, 15/24 statements surpassed a threshold of 90% agreement.
Discussion
This international, multidisciplinary, expert-driven consensus document with key stakeholders has highlighted evidence for the integration of PA and exercise in CF into standard care, whilst also highlighting gaps in current understanding and applied practice. The expert group views this as a first of its kind for CF, providing a holistic integration of topics related to PA and exercise for the management of this disease.
Encouragingly, a consensus for the majority of statements was reached by the group, and most received high agreement (>90%; Table 3). This included two statements that reached 100% agreement, being related to the role of exercise in airway clearance, and how alternative markers of health status should be considered in future trials related to physical activity and exercise in CF. The former of these points is pertinent for the CF community, having been raised as a key consideration during priority setting exercises,20,21 with consultations occurring amongst healthcare professionals and researchers. 24
Whilst most statements were agreed upon by the majority, a small number of statements had a larger proportion of abstentions, and due to the anonymous nature of the voting process, it is unclear whether this reflects lack of expertise within the group, or a lack of confidence in the existing literature to support the statement. Regardless, these statements were still agreed upon by a majority, and subsequently highlight that these could act as opportunities for future investigative research alongside the suggestions for the identified future trials. Therefore, this document can serve as a summary of the role, and benefits, of PA and exercise in managing CF and can be utilised by clinical staff and healthcare managers to direct resources and services within CF care. With the continually changing nature of CF care, particularly since the widespread introduction of modulator therapy, it is likely that the role of PA and exercise will only become more important in years to come. Therefore, this document serves to form a foundation of future care in CF in relation to the important, and multi-faceted roles of PA and exercise in changing the fitness and PA levels of people with CF on modulators.
One aspect of the process that emerged unplanned was the inclusion of future directions for research, with the identification of three specific topics:
• Whether exercise can adequately replace airway clearance techniques;
• The interactive effects of CFTR modulators, PA, and exercise;
• How to improve adherence, including overcoming barriers, identifying enablers, and developing routines, to PA and structured exercise programmes.
As previously noted, the first of these topics is currently subject to a number of studies having been previously identified by the James Lind Alliance for topics that people with CF wanted investigating. 20 The effect of modulators on exercise, fitness, and PA will be of considerable interest to people with CF, as will the need to establish guidelines for how intensive, how long, and frequent PA and exercise needs to be in order to maintain, or enhance, changing health status on modulators. Finally, as with all interventions, clinical or non-clinical, adherence is important to maximise its benefit. How changes in PA behaviour can be effectively elicited remains a challenge for both clinical groups and the general population and, subsequently, unresolved.
A lack of PA and exercise and its effect on health status is not just a concern for people of all ages in the general population, irrespective of age, but also for those with chronic medical conditions. Despite the creation of PA guidelines for healthy individuals, there are currently no guidelines that focus solely on chronic disease conditions. Physical activity guidelines for disabled adults are available in the UK,25,26 with further guidelines for children and youth planned, but these do not include conditions such as CF. Similarly, the World Health Organization have recently released PA guidelines for adults living with a disability inclusive of the following conditions; multiple sclerosis, spinal cord injury, intellectual disability, Parkinson’s disease, stroke, schizophrenia, major clinical depression, and attention deficit hyperactivity disorder. 25 Whilst our consensus statements are supportive that regular PA and exercise is beneficial in CF care, there are large gaps in the literature regarding PA/exercise proposals and recommendations in chronic disease.27-29 However, despite the lack of guidelines for people with chronic medical conditions like CF, PA should be promoted akin to healthy populations.
There are several strengths to this consensus. These include the expertise and breadth of the group, evidenced by the wealth of cumulative experience in CF (over 450 years, Table 1), and a wide range of professions including academics and several different healthcare professions; and high-quality PPI engagement to ensure the voice of the wider CF community was represented. There are also highly detailed descriptions and transparency of the methodology used (Supplementary File 1), as well as data provided on dissenting opinions with regards to each statement, 30 both of which can be often omitted in consensus documentation. Moreover, we have summarised the background as to how we formulated the statements; disclosed detailed information including funding sources, the reviewing processes, the call for group members; and provided quality stakeholder and PPI engagement in order to provide a transparent overview of the entire process. 31 Whilst the statements are not meant to be exhaustive; they do have a breadth that encompasses children, youth, adults, and differing disease states. Subsequently, the statements derived for this document will be applicable to nearly all patients with CF, as this document has been developed with a holistic approach, considering all aspects of disease management across the lifespan, as well as those with more severe disease status.
A few limitations should be acknowledged. First, the methodological approach for the formation of the consensus statements was subject to limitations with regard to finance, time, and resources available, meaning that multiple, comprehensive, systematic reviews were not feasible nor pragmatic; although this experience is not unique to our group.32–34 Nonetheless, the group sought to minimise its impact by utilising evidence from multiple sources, and adopting a multi-faceted and step-wise process (reviews, stakeholder involvement, PPI, discussions, and voting) and thus have confidence in our consensus statements. Second, this consensus document did not grade the quality of evidence, as is recommended for clinical practice guidelines or clinical recommendations. However, as noted within the methodology, the varying study designs, and thematic approaches to the holistic nature of PA and exercise made selection of a uniform grading system unfeasible. Moreover, it should be noted that grading does not typically occur in consensus documents (such as this), whereas it would be expected in a clinical practice guideline which would make explicit treatment recommendations. 23 Finally, and beyond the scope of the consensus meeting, the theoretical underpinnings for behaviour change and PA interventions were not considered. It is also acknowledged that the intervening policy implementation is important to continue the dissemination of research findings could not be included in this document.
Conclusion
This international, multi-disciplinary group has highlighted the important role of PA and exercise in the holistic management of CF and provides an evidence-informed and evidence-based summary of the literature that can be used to support important health messages for people with CF and relevant stakeholders. These consensus statements, alongside the critical future directions for the field, provide various organisations with the impetus for the initiation, implementation and promotion of PA recommendations and exercise programmes across a variety of settings, such as hospitals, outpatient clinics, the home, and schools and workplaces.
Supplemental Material
Supplemental Material for The Exeter Activity Unlimited statement on physical activity and exercise for cystic fibrosis: methodology and results of an international, multidisciplinary, evidence-driven expert consensus by Craig A Williams, Alan R Barker, Sarah Denford, Samantha B van Beurden, Mayara S Bianchim, Jessica E Caterini, Narelle S Cox, Kelly A Mackintosh, Melitta A McNarry, Sarah Rand, Jane E Schneiderman, Greg D Wells, Peter Anderson, Daniel Beever, Z Beverley, Ronan Buckley, Brenda Button, Adam J Causer, Máire Curran, Tiffany J Dwyer, Warren Gordon, Mathieu Gruet, Ryan A Harris, Elpis Hatziagorou, HJ (Erik) Hulzebos, Asterios Kampouras, Lisa Morrison, Marietta N Cámara, Clare M Reilly, Abbey Sawyer, Zoe L Saynor, James Shelley, Grace Spencer, Gemma E Stanford, Don S Urquhart, Rachel Young, and Owen W Tomlinson in Chronic Respiratory Disease
Supplemental Material for The Exeter Activity Unlimited statement on physical activity and exercise for cystic fibrosis: methodology and results of an international, multidisciplinary, evidence-driven expert consensus by Craig A Williams, Alan R Barker, Sarah Denford, Samantha B van Beurden, Mayara S Bianchim, Jessica E Caterini, Narelle S Cox, Kelly A Mackintosh, Melitta A McNarry, Sarah Rand, Jane E Schneiderman, Greg D Wells, Peter Anderson, Daniel Beever, Z Beverley, Ronan Buckley, Brenda Button, Adam J Causer, Máire Curran, Tiffany J Dwyer, Warren Gordon, Mathieu Gruet, Ryan A Harris, Elpis Hatziagorou, HJ (Erik) Hulzebos, Asterios Kampouras, Lisa Morrison, Marietta N Cámara, Clare M Reilly, Abbey Sawyer, Zoe L Saynor, James Shelley, Grace Spencer, Gemma E Stanford, Don S Urquhart, Rachel Young, and Owen W Tomlinson in Chronic Respiratory Disease
Supplemental Material for The Exeter Activity Unlimited statement on physical activity and exercise for cystic fibrosis: methodology and results of an international, multidisciplinary, evidence-driven expert consensus by Craig A Williams, Alan R Barker, Sarah Denford, Samantha B van Beurden, Mayara S Bianchim, Jessica E Caterini, Narelle S Cox, Kelly A Mackintosh, Melitta A McNarry, Sarah Rand, Jane E Schneiderman, Greg D Wells, Peter Anderson, Daniel Beever, Z Beverley, Ronan Buckley, Brenda Button, Adam J Causer, Máire Curran, Tiffany J Dwyer, Warren Gordon, Mathieu Gruet, Ryan A Harris, Elpis Hatziagorou, HJ (Erik) Hulzebos, Asterios Kampouras, Lisa Morrison, Marietta N Cámara, Clare M Reilly, Abbey Sawyer, Zoe L Saynor, James Shelley, Grace Spencer, Gemma E Stanford, Don S Urquhart, Rachel Young, and Owen W Tomlinson in Chronic Respiratory Disease
Acknowledgements
The authors would like to acknowledge Jacqueline Ali (Cystic Fibrosis Trust) and Dr Anastasiia Kovalenko (University of Exeter), whose contributions have ensured a successful delivery of this project.
Appendix
Abbreviations
- CF
cystic fibrosis
- CFRD
cystic fibrosis related diabetes
- CFTR
cystic fibrosis transmembrane conductance regulator
- ECR
early career researcher
- FEV1
forced expiratory volume in one second
- FVC
forced vital capacity
- HCP
healthcare professional
- PA
physical activity
- PPI
Public and Patient Involvement
- SRC
Strategic Research Centre
- UK
United Kingdom
Author contributions: Conceptualisation; CAW, ARB, SD, KAM, MAM, OWT. Protocol Development: CAW, ARB, SD, OWT. Literature Searching and Evidence Synthesis; Production of Initial Statements; Critical Feedback of Evidence and Statements: CAW, ARB, SD, SBvB, MSB, JEC, NSC, KAM, MAM, SR, JES, GDW, PA, ZB, RB, BB, AJC, MC, TJD, WG, MG, RAH, EH, HJEH, AK, LM, MNC, CMR, AS, ZLS, GS, JS, GES, DSU, RY, OWT. Participation in Consensus Meeting; Development of Final Statements; Endorsement of Final Statements; Critical Evaluation of Manuscript Acceptance of Final Document; Agree to be Accountable for Final Version: CAW, ARB, SD, SBvB, MSB, JEC, NSC, KAM, MAM, SR, JES, GDW, PA, DB, ZB, RB, BB, AJC, MC, TJD, WG, MG, RAH, EH, HJEH, AK, LM, MNC, CMR, AS, ZLS, GS, JS, GES, DSU, RY, OWT. Manuscript Preparation: CAW, ARB, OWT. A graphical contributions matrix is provided in Supplementary File 1.
Declaration of conflicting interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: This work is part of the ‘Youth Activity Unlimited’ Strategic Research Centre, funded by the Cystic Fibrosis Trust (SRC#008). The funder had no role in the design of the consensus, literature synthesis nor drafting of preliminary statements. Jacqueline Ali, as a representative of the Cystic Fibrosis Trust, participated in the 2-day meeting, but did not have voting rights.
Disclaimer: Despite many authors holding membership of the Association of Chartered Physiotherapists in Cystic Fibrosis, the UK Cystic Fibrosis and Exercise Technicians Network, or the European Cystic Fibrosis Society Exercise Working Group; this consensus document is not written on behalf of, nor endorsed by, any of these organisations.
Data availability statement: All results are presented within the manuscript and supplementary files.
Supplementary material: Supplemental material for this article is available online.
ORCID iDs
Jane E Schneiderman https://orcid.org/0000-0002-8271-9101
Tiffany J Dwyer https://orcid.org/0000-0001-6403-2894
Ryan A Harris https://orcid.org/0000-0002-8826-681X
Owen W Tomlinson https://orcid.org/0000-0003-4063-7682
References
- 1.Bell SC, Mall MA, Gutierrez H, et al. The future of cystic fibrosis care: A global perspective . Lancet Respir Med, 2020. 8(1): p. 65–124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Farrell PM, Rosenstein BJ, White TB, et al. Guidelines for diagnosis of cystic fibrosis in newborns through older adults: Cystic Fibrosis Foundation consensus report . J Pediatr, 2008. 153(2): p. S4–S14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Farrell PM, White TB, Ren CL, et al. Diagnosis of Cystic Fibrosis: Consensus Guidelines from the Cystic Fibrosis Foundation . J Pediatr, 2017. 181: p. S4–S15.e1. [DOI] [PubMed] [Google Scholar]
- 4.Floto RA, Olivier KN, Saiman L, et al. US Cystic Fibrosis Foundation and European Cystic Fibrosis Society consensus recommendations for the management of non-tuberculous mycobacteria in individuals with cystic fibrosis: executive summary . Thorax, 2016. 71(1): p. 88–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kapnadak SG, Dimango E, Hadjiliadis D, et al. Cystic Fibrosis Foundation consensus guidelines for the care of individuals with advanced cystic fibrosis lung disease . J Cystic Fibrosis, 2020. 19(3): p. 344–354. [DOI] [PubMed] [Google Scholar]
- 6.Moran A, Brunzell C, Cohen RC, et al. Clinical care guidelines for cystic fibrosis-related diabetes: a position statement of the American Diabetes Association and a clinical practice guideline of the Cystic Fibrosis Foundation, endorsed by the Pediatric Endocrine Society . Diabetes Care, 2010. 33(12): p. 2697–2708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ramos KJ, Smith PJ, McKone EF, et al. Lung transplant referral for individuals with cystic fibrosis: Cystic Fibrosis Foundation consensus guidelines . J Cystic Fibrosis, 2019. 18(3): p. 321–333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sinaasappel M, Stern M, Littlewood J, et al. Nutrition in patients with cystic fibrosis: a European Consensus . J Cystic Fibrosis, 2002. 1(2): p. 51–75. [DOI] [PubMed] [Google Scholar]
- 9.Yankaskas JR, Marshall BC, Sufian B, et al. Cystic Fibrosis Adult Care: Consensus Conference Report . Chest, 2004. 125(1): p. 1S–39S. [DOI] [PubMed] [Google Scholar]
- 10.Cystic Fibrosis Trust, Standards of Care and Good Clinical Practice for the Physiotherapy Management of Cystic Fibrosis. 2020: London, UK. [Google Scholar]
- 11.National Institute for Health and Care Excellence (NICE) , Cystic Fibrosis: Diagnosis and Management. 2017: London, UK. [PubMed] [Google Scholar]
- 12.Flume PA, Robinson KA, Sullivan BPO, et al. Cystic Fibrosis Pulmonary Guidelines: Airway Clearance Therapies . Respir Care, 2009. 54(4): p. 522–537. [PubMed] [Google Scholar]
- 13.Castellani C, Duff AJA, Bell SC, et al. ECFS best practice guidelines: The 2018 revision . J Cystic Fibrosis, 2018. 17(2): p. 153–178. [DOI] [PubMed] [Google Scholar]
- 14.Swisher AK, Helge H, Downs M, et al. Exercise and habitual physical activity for people with cystic fibrosis: Expert consensus, evidence-based guide for advising patients . Cardiopulmonary Phys Ther J, 2015. 26(4): p. 85–98. [Google Scholar]
- 15.Bradley J, Neill BO, Kent L, et al. Physical activity assessment in cystic fibrosis: A position statement . J Cystic Fibrosis, 2015. 14(6): p. e25-e32. [DOI] [PubMed] [Google Scholar]
- 16.Hebestreit H, Arets HGM, Aurora P, et al. Statement on exercise testing in cystic fibrosis . Respiration, 2015. 90(4): p. 332–351. [DOI] [PubMed] [Google Scholar]
- 17.Radtke T, Nevitt SJ, Hebestreit H, et al. Physical exercise training for cystic fibrosis . Cochrane Database Syst Rev, 2017. 11: p. CD002768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bangsbo J, Krustrup P, Duda J, et al. The Copenhagen Consensus Conference 2016: Children, youth, and physical activity in schools and during leisure time . Br J Sports Med, 2016. 50(19): p. 1177–1178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Denford S, Cox NS, Mackintosh KA, et al. Physical activity for cystic fibrosis: perceptions of people with cystic fibrosis, parents and healthcare professionals . ERJ Open Res, 2020. 6(3). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Rowbotham NJ, Smith S, Leighton PA, et al. The top 10 research priorities in cystic fibrosis developed by a partnership between people with CF and healthcare providers . Thorax, 2018. 73(4): p. 388–390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Rowbotham NJ, Smith SJ, Davies G, et al. Can exercise replace airway clearance techniques in cystic fibrosis? A survey of patients and healthcare professionals . J Cystic Fibrosis, 2020. 19(4): p. e19-e24. [DOI] [PubMed] [Google Scholar]
- 22.Tomlinson OW, Denford S, Barker AR, et al. The impact of physical activity and exercise interventions for physical health in people with cystic fibrosis: protocol for a systematic review . Syst Rev, 2021. 10(1): p. 64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Pacini D, Murana G, Leone A, et al. The Value and Limitations of Guidelines, Expert Consensus, and Registries on the Management of Patients with Thoracic Aortic Disease . Korean J Thorac Cardiovasc Surg, 2016. 49(6): p. 413–420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Saynor Z. P217 Exercise as airway clearance therapy (ExACT) in cystic fibrosis: a UK-based e-Delphi survey of patients, caregivers and health professionals . J Cystic Fibrosis, 2022. 21. [DOI] [PubMed] [Google Scholar]
- 25.Carty C, an der Ploeg HP, Biddle SJH, et al. The First Global Physical Activity and Sedentary Behavior Guidelines for People Living With Disability . J Phys Activity Health, 2021. 18(1): p. 86–93. [DOI] [PubMed] [Google Scholar]
- 26.Smith B. Physical activity for general health benefits in disabled adults: Summary of a rapid evidence review for the UK Chief Medical Officers’ update of the physical activity guidelines. London, UK: Public Health England, 2018. [Google Scholar]
- 27.Anderson E, Durstine JL, Physical activity, exercise, and chronic diseases: A brief review . Sports Med Health Sci, 2019. 1(1): p. 3–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.West SL, Banks L, Schneiderman JE, et al. Physical activity for children with chronic disease; a narrative review and practical applications . BMC Pediatr, 2019. 19(1): p. 12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Williams CA, Barker AR. The Role of Physical Activity, Exercise, and Fitness in Medicine. In: Williams C.A., Oades P.J. (eds). Exercise and Respiratory Disease in Paediatrics. New York: Routledge, 2022, pp. 1–20. [Google Scholar]
- 30.Shrier I., Consensus statements that fail to recognise dissent are flawed by design: a narrative review with 10 suggested improvements . Br J Sports Med, 2020. 55(10): p. 545–549. [DOI] [PubMed] [Google Scholar]
- 31.Health Research Authority . Make it Public: Transparency and Openness in Health and Social Care Research. 2020. Updated: 03/09/2020 Accessed: 22/09/2021]; Available from:https://www.hra.nhs.uk/planning-and-improving-research/policies-standards-legislation/research-transparency/make-it-public-transparency-and-openness-health-and-social-care-research/ [Google Scholar]
- 32.Tremblay MS, Chaput JP, Adamo KB, et al. Canadian 24-Hour Movement Guidelines for the Early Years (0-4 years): An Integration of Physical Activity, Sedentary Behaviour, and Sleep . BMC Public Health, 2017. 17(Suppl 5): p. 874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Rhodes RE, Guerrero MD, Vanderloo LM, et al. Development of a consensus statement on the role of the family in the physical activity, sedentary, and sleep behaviours of children and youth . Int J Behav Nutr Phys Activity, 2020. 17(1): p. 74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Tremblay MS, Warburton DER, Janssen I, et al. New Canadian physical activity guidelines . Appl Physiol Nutr Metab, 2011. 36(1): p. 36–46. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Material for The Exeter Activity Unlimited statement on physical activity and exercise for cystic fibrosis: methodology and results of an international, multidisciplinary, evidence-driven expert consensus by Craig A Williams, Alan R Barker, Sarah Denford, Samantha B van Beurden, Mayara S Bianchim, Jessica E Caterini, Narelle S Cox, Kelly A Mackintosh, Melitta A McNarry, Sarah Rand, Jane E Schneiderman, Greg D Wells, Peter Anderson, Daniel Beever, Z Beverley, Ronan Buckley, Brenda Button, Adam J Causer, Máire Curran, Tiffany J Dwyer, Warren Gordon, Mathieu Gruet, Ryan A Harris, Elpis Hatziagorou, HJ (Erik) Hulzebos, Asterios Kampouras, Lisa Morrison, Marietta N Cámara, Clare M Reilly, Abbey Sawyer, Zoe L Saynor, James Shelley, Grace Spencer, Gemma E Stanford, Don S Urquhart, Rachel Young, and Owen W Tomlinson in Chronic Respiratory Disease
Supplemental Material for The Exeter Activity Unlimited statement on physical activity and exercise for cystic fibrosis: methodology and results of an international, multidisciplinary, evidence-driven expert consensus by Craig A Williams, Alan R Barker, Sarah Denford, Samantha B van Beurden, Mayara S Bianchim, Jessica E Caterini, Narelle S Cox, Kelly A Mackintosh, Melitta A McNarry, Sarah Rand, Jane E Schneiderman, Greg D Wells, Peter Anderson, Daniel Beever, Z Beverley, Ronan Buckley, Brenda Button, Adam J Causer, Máire Curran, Tiffany J Dwyer, Warren Gordon, Mathieu Gruet, Ryan A Harris, Elpis Hatziagorou, HJ (Erik) Hulzebos, Asterios Kampouras, Lisa Morrison, Marietta N Cámara, Clare M Reilly, Abbey Sawyer, Zoe L Saynor, James Shelley, Grace Spencer, Gemma E Stanford, Don S Urquhart, Rachel Young, and Owen W Tomlinson in Chronic Respiratory Disease
Supplemental Material for The Exeter Activity Unlimited statement on physical activity and exercise for cystic fibrosis: methodology and results of an international, multidisciplinary, evidence-driven expert consensus by Craig A Williams, Alan R Barker, Sarah Denford, Samantha B van Beurden, Mayara S Bianchim, Jessica E Caterini, Narelle S Cox, Kelly A Mackintosh, Melitta A McNarry, Sarah Rand, Jane E Schneiderman, Greg D Wells, Peter Anderson, Daniel Beever, Z Beverley, Ronan Buckley, Brenda Button, Adam J Causer, Máire Curran, Tiffany J Dwyer, Warren Gordon, Mathieu Gruet, Ryan A Harris, Elpis Hatziagorou, HJ (Erik) Hulzebos, Asterios Kampouras, Lisa Morrison, Marietta N Cámara, Clare M Reilly, Abbey Sawyer, Zoe L Saynor, James Shelley, Grace Spencer, Gemma E Stanford, Don S Urquhart, Rachel Young, and Owen W Tomlinson in Chronic Respiratory Disease