Abstract
Primary neuroendocrine carcinoma of the breast (NECB) is a rare tumour with an incident rate of 0.3–0.5%. The most common metastatic sites of NECB are liver, bones, lung, pancreas, soft tissues and brain, while leptomeninges metastasis (LM) is reported rarely. This current case report describes a 50-year-old female patient with NECB and LM whose overall survival was 2 months. The report also presents the current literature regarding the knowledge of this unusual tumour and metastatic type. The current patient was diagnosed with NECB with right cerebellar metastasis, followed by LM. She underwent modified radical mastectomy of the left breast, left whole breast radiation therapy and incomplete adjuvant chemotherapy until the metastasis occurred. Whole-brain radiation therapy and a first-line salvage regimen of etoposide and cis-platinum were then undertaken. The patient died 2 months after their LM diagnosis. Primary NECB with LM is sporadic, devoid of effective treatment and associated with a poor prognosis. Consequently, it is vitally important to identify LM in order to achieve longer patient survival.
Keywords: Breast cancer, neuroendocrine carcinoma of the breast, leptomeninges metastasis, case report, review
Introduction
With an incident rate of 0.3–0.5%, 1 neuroendocrine carcinoma of the breast (NECB) is a rare and slowly developing tumour, comprising 2–5% of all breast cancers. 2 NECB was first reported in 1977, 3 when its detailed characters were described, namely small-cell tumours growing as sharply circumscribed nests and cords and bearing argyrophilic neurosecretory-type cytoplasmic granules. Despite NECB being the focus of much research since then, there is a lack of studies on advanced stage tumours. This current case report describes a 50-year-old woman with advanced NECB and leptomeninges metastasis (LM). The case report also provides a literature review describing new insights into the research into NECB.
Case report
On 1 August 2021, a 50-year-old female patient with a history of injectable breast augmentation surgery, presented to the Department of Oncology, China-Japan Friendship Hospital, Beijing, China and was diagnosed with triple-negative breast cancer with right cerebellar metastasis, followed by LM. A palpable lump of approximately 4 × 3 cm was found in her left breast at the beginning of 2020 and she had a biopsy of the left lump on 1 July 2020. The pathology revealed an invasive carcinoma and the immunohistochemical analysis was negative for oestrogen receptor (ER), progesterone receptor (PR) and human epidermal growth factor receptor 2 (HER2); and 80% of the tumour cells were Ki-67 positive. Subsequently, the patient underwent the modified radical mastectomy of the left breast and axillary lymphatic dissection. The postoperative pathology presented a high-grade neuroendocrine carcinoma (7 cm) with positive expression of CD56, chromogranin A (CgA), synaptophysin (Syn) and Ki-67 (80% of the tumour cells). At the same time, ER, PR and HER2 were all negative. Adjuvant chemotherapy consisting of 100 mg doxorubicin hydrochloride liposome on day 1 and 1000 mg cyclophosphamide on day 1 of a 21-day cycle, via intravenous drip, was administered. Only one cycle was completed and then suspended because of severe side-effects. The patient underwent left whole breast radiation therapy in 25 fractions and completed adjuvant therapy by 11 February 2021.
A regular medical check-up was conducted in May 2021 and a positron emission tomography-computed tomography scan indicated a right cerebellar metastasis. Whole-brain radiation therapy in the form of 30.6 Gy in 18 fractions was conducted to narrow the lesions, followed by 54 Gy in 18 fractions of the right cerebellum and first-line salvage chemotherapy consisting of 80 mg etoposide on days 1 and 2 and 25 mg lobaplatin on day 1 (EP) of a 21-day cycle, via intravenous drip. Only one cycle of chemotherapy was accomplished.
Unfortunately, after the first cycle of salvage chemotherapy, she experienced pain throughout her body, numbness in the legs and feet, urinary incontinence, and constipation. A brain magnetic resonance imaging (MRI) revealed the right cerebellar mass was smaller in size with less enhancement; however, LM appeared. Thus, in August 2021, the patient was transferred to the Department of Oncology, China-Japan Friendship Hospital, Beijing, China for further treatment. Physical examination showed that her muscle strength of both lower limbs was zero, dystonia and hypoesthesia presented in both legs, and abdominal reflexes were weakened with numeric rating scales scores of 7–9. Since she was admitted in a state of paraplegia with severe cancer pain, it was thought that she might have bone metastasis. Nevertheless, a bone single-photon emission computed tomography scan showed no metastasis (Figure 1). MRI scans of the brain and spine revealed no metastasis in the brain parenchyma but extensive metastases evident in the spinal cord (Figure 2). Furthermore, an enhanced MRI confirmed the existence of LM (Figure 3). The patient underwent a second cycle of EP along with supportive treatment but died on 31 August 2021, 1 month after the diagnosis of LM. A timeline of the patient’s treatment is presented in Table 1.
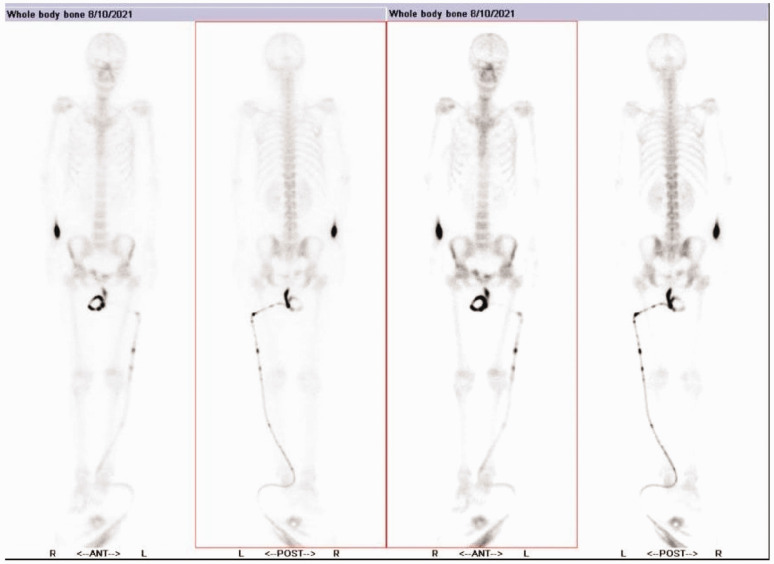
Figure 1.
A bone single-photon emission computed tomography whole-body bone scan of a 50-year-old female patient diagnosed with triple-negative breast cancer with right cerebellar metastasis and leptomeninges metastasis undertaken on 10 August 2021 showed no signs of bone metastasis. The tube on the right is a catheter attached to the patient.
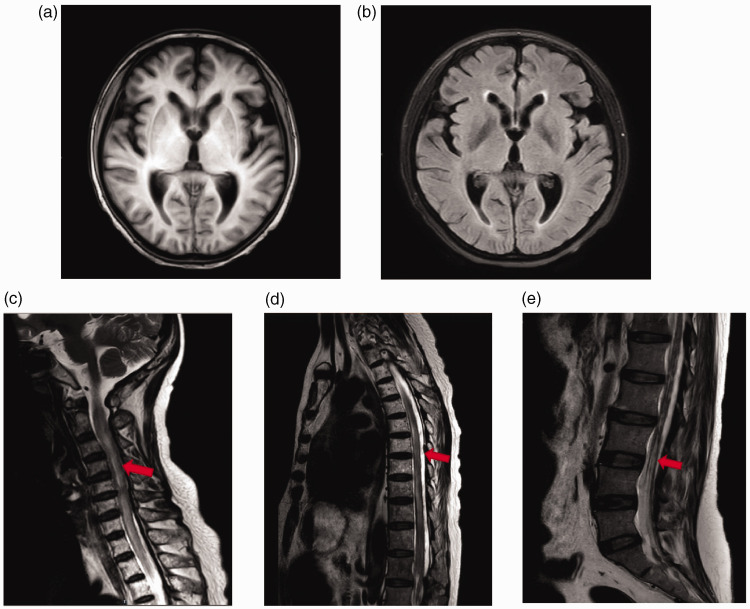
Figure 2.
Magnetic resonance imaging (MRI) scans of a 50-year-old female patient diagnosed with triple-negative breast cancer with right cerebellar metastasis and leptomeninges metastasis: (a & b) MRI scans of the brain conducted in August 2021 showed no metastatic signs and possible mild cerebral oedema; (c, d & e) MRI scans of the cervical vertebra, thoracic vertebra and lumbar vertebra demonstrated spinal cord, cauda equine and dura thickening (red arrows). An enhanced MRI was needed to verify the leptomeningeal metastasis.
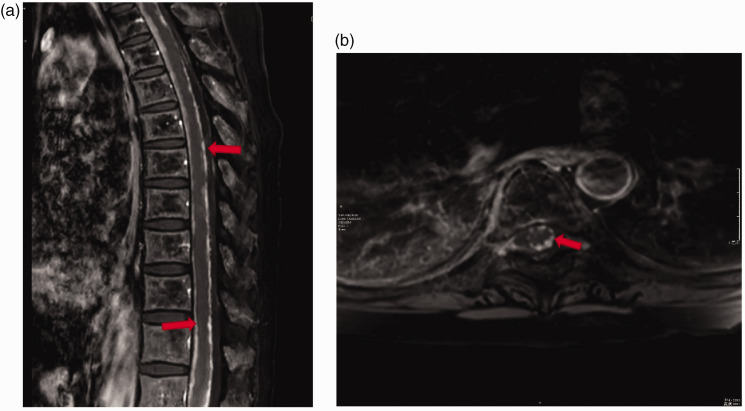
Figure 3.
Enhanced magnetic resonance imaging scans of the spinal cord of a 50-year-old female patient diagnosed with triple-negative breast cancer with right cerebellar metastasis and leptomeninges metastasis: (A) a sagittal post-gadolinium fat saturation T1-weighted image demonstrated the leptomeningeal metastasis on the thoracic segment (red arrows); (B) an axial post-gadolinium fat saturation T1-weighted image of the leptomeningeal metastasis on the 7th thoracic vertebra level (red arrow).
Table 1.
Timeline of the 50-year-old patient’s treatment.
| Beginning of 2020 | July 2020 | July 2020 to February 2021 | May 2021 to June 2021 | July 2021 | August 2021 |
|---|---|---|---|---|---|
| Found a palpable lump approximately 4 × 3 cm in the left breast and felt pain. | Left-sided modified radical mastectomy and axillary lymphatic dissection. Diagnosis neuroendocrine carcinoma of breast (diameter of tumour was 7 cm). | Adjuvant therapy. | Diagnosis of right cerebellar metastasis. Rescue treatment was undertaken. |
Patient gradually felt pain around the whole body, numbness in the legs and feet, urinary incontinence, and constipation. | Diagnosis of leptomeninges metastases on thoracic segment.
Etoposide and cis-platinum chemotherapy for a second cycle. The patient died 31 August 2021. |
The reporting of this case report conforms with the CARE guidelines. 4 The patient and her family provided consent for all treatments received. This study was approved by the Joint Institutional Review Board of China- Japan Friendship Hospital (no. 2021-31-K15).
Discussion
A search of the literature published in PubMed® from 1980 to 2021 using the terms (“neuroendocrine cancer of breast” or “small cell breast cancer” or “oat cell carcinoma of the breast”) and (“advanced” or “metastatic”) identified 12 case reports related to metastatic NEBC.5–16 The following data were extracted from each article: first author name, year of publication, patient sex, age, tumour size, family history of breast cancer, status of ER, PR and HER2, Ki-67 index, metastatic sites, disease-free survival, overall survival and treatment options (Table 2). NECB is a rare breast cancer and the median age of onset is 63 years old. 17 Of the nine articles retrieved that presented ER expression,6,7,9–14,16 seven of them6,7,9–13 were negative and one patient overexpressed HER2. 12 In terms of metastatic sites, the most common metastatic sites of NECB were the liver (seven of 12 patients; 58.3%), bones (six of 12 patients; 50.0%), lung, pancreas, soft tissues and brain (one of 12 patients; 8.3%), but few LM were reported.
Table 2.
| Authors | Year | Race | Sex | Age, years | Size, cm | Family history of breast cancer | ER | PR | HER2 | Ki-67 | Metastatic site | DFS, months | OS, months | Treatment |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Wade et al.15 | 1983 | White | Female | 52 | 10 × 8 | No | NA | NA | NA | NA | Liver, bone | NA | NA | 1. Cyclophosphamide (l000 mg/m2), doxorubicin
(40 mg/m2) and vincristine (2 mg). VP-16-213
(100 mg/m2/day) 2. Radiotherapy |
| Jundt et al.5 | 1984 | NA | Male | 52 | NA | NA | NA | NA | NA | NA | Bone | NA | 14 | Irradiation and chemotherapy |
| Papotti et al.6 | 1992 | NA | Female | 50 | 3 | NA | – | – | NA | NA | Liver, cerebellar, pulmonary | 6 | 16 | Chemotherapy with cyclophosphamide, methotrexate and fluorouracil and brain irradiation (40 Gy) |
| Francois et al.7 | 1995 | White | Female | 68 | 4.5 × 4 | NA | – | – | NA | NA | Right pleural effusion; left supraclavicular lymph node | 11 | 21 | 1. Adjuvant radiotherapy: 47.5 Gy on right inner mammary area
and 44 Gy on right supraclavicular and axillary areas 2. Rescue chemotherapy: doxorubicin (50 mg/m2, J1), cyclophosphamide (500 mg/m2, J1) and etoposide (100 mg/m2, J1–J4). |
| Adegbola et al.8 | 2005 | NA | Female | 61 | 1.7 | No | NA | NA | NA | NA | Lung | 6 | NA | VP16 and cisplatin |
| Hojo et al.10 | 2009 | Japanese | Female | 60 | 2.2 × 1.5 | NA | – | – | – | NA | Chest wall, liver | NA | 26 | 1. Carboplatin (300 mg/m2) and CPT-11
(60 mg/m2) 2. Docetaxel and 5-DFUR 3. Carboplatin (300 mg/m2) and etoposide (80 mg/m2) |
| Kinoshita et al.9 | 2008 | Japanese | Female | 31 | 3.7 × 3.1 × 2.4 | NA | – | – | – | NA | Chest wall, liver | 1 | 9 | 1. Adriamycin (50 mg/m2) and docetaxel
(60 mg/m2) 2. Cisplatin (60 mg/m2) plus irinotecan (60 mg/m2) |
| Ochoa et al.11 | 2012 | Hispanic | Female | 25 | 12 × 12 | NA | – | – | – | NA | Bone (T12, L3) | NA | 6 | Cis-platinum and etoposide chemotherapy, radiation therapy |
| Jiang et al.12 | 2014 | Chinese | Male | 79 | 2 × 1 | NA | – | + | + | NA | Cervical lymph node, pulmonary, bone, liver | 20 | 27 | Irinotecan combined with carboplatin, followed by docetaxel |
| Soe et al.16 | 2017 | NA | Female | 57 | 4.3 × 3.7 × 3.2 | No | 100% | 100% | NA | 15% | Bone | 2 | Still alive | 1. Vertebroplasty 2. Cisplatin and etoposide and octreotide injection and cisplatin and etoposide 3. Denosumab |
| Valente et al.13 | 2020 | NA | Female | 69 | 2.5 | NA | – | – | – | 90% | Liver | 96 | NA | Chemotherapy (fluorouracil/epirubicin/cyclophosphamide followed by docetaxel), radiotherapy |
| Wang et al.14 | 2021 | Chinese | Female | 62 | 1.5 × 1.5 × 1 | No | + | + | – | 50–75% | Liver, lung, bone | 52 | Still alive | 1. Adjuvant chemotherapy: pirarubicin and paclitaxel regimen.
2. Endocrine therapy 3. Etoposide and cisplatin chemotherapy as a first line treatment 4. S-1 (40 mg) combined with temozolomide (200 mg) as second-line treatment |
ER, oestrogen receptor; PR, progesterone receptor; HER2, human epidermal growth factor receptor 2; DFS, disease free survival; OS, overall survival; NA, not available; VP-16-213, VP-16-213 etoposide; VP16, VP16 etoposide; CPT-11, irinotecan (CPT-11); 5-DFUR, floxuridine.
It is widely acknowledged that LM refers to invasion of the pia mater, subarachnoid space, cerebrospinal fluid (CSF) and arachnoid mater of the brain and/or spinal cord by cancer cells. 18 Symptoms of LM depend on the location of involvement: (i) headache, seizures, nausea and vomiting would occur when the cerebrum is invaded; nuchal rigidity, Kernig sign and Brudzinski sign would appear when meningeal irritation occurs; (ii) lower extremity weakness, paraesthesia, back and neck pain, and bowel and bladder dysfunction would appear if the spine is affected; (iii) diplopia, visual acuity loss, hearing loss and facial numbness are observed when the cranial nerve is involved.18,19
As reported, almost 60% of patients with LM have associated parenchymal brain involvement. 20 Brain metastasis comprises 10–20% of advanced breast cancer and accompanying motor symptoms, including deficit and gait disturbance, seizures, headaches, cognitive dysfunction, nausea and vomiting, cranial nerve dysfunction, cerebellar symptoms and speech disturbances. 21 Consequently, the symptoms of brain metastasis are similar to LM in some aspects, such as headaches, nausea and vomiting, while LM causes more severe pain than parenchymal brain metastasis and a greater probability of lower extremity weakness. Bone metastasis comprises 65–75% of breast cancer metastatic events with a median survival of 24 months. 22 The axial skeleton is most frequently affected by bone metastasis, along with gradual neck and back pain with or without neurological complications secondary to epidural extension in vertebral metastasis.22,23 Consequently, bone metastasis is the first pathogenesis to be considered when a breast cancer patient suffers a headache, neck or back pain. As overall survival of patients with LM is shorter than 6 months after diagnosis,24,25 it is vitally important to distinguish the metastasis of parenchymal brain, bone and leptomeningeal for longer patient survival. Identifying malignant cells by CSF analysis is recognized as the diagnostic gold standard, but imaging has become the initial and often sole diagnostic tool with the development of visualization of the subarachnoid space by MRI. 20
There are two main opinions regarding NECB pathogenesis. Some researchers believe that NECB originates from the divergent differentiation of a neoplastic stem cell into epithelial and neuroendocrine cells,26,27 while others think that NECB stems from neural crest cells that migrate to the mammary glands or it originates from neuroendocrine cells present in the breast tissue. 28
Neuroendocrine tumours are composed of densely cellular, solid nests and trabeculae of cells that differ from spindle to plasmacytoid and large clear cells separated by delicate fibrovascular stroma 16 and all undifferentiated NECB cells express neuroendocrine markers.29–31 Neuron-specific enolase is recognized as the most common but not specific neuroendocrine indicator, while CgA and Syn are reliable neuroendocrine markers. 32 In addition, compared with general invasive breast cancer, ER and PR of NECB tend to be positive, and HER2 is often lowly expressed, with an older age of onset and less lymph node metastasis and mitotic figures.17,32
In 2003, the World Health Organization (WHO) formally classified NECB as an independent breast disease for the first time; and in 2012, the WHO revised the category and divided neuroendocrine carcinomas into three subtypes: (i) well-differentiated, neuroendocrine tumour; (ii) poorly differentiated, neuroendocrine carcinoma (small cell carcinoma); (iii) invasive breast carcinoma with neuroendocrine differentiation.33,34 In 2018, the WHO defined neuroendocrine tumours (NET) as an invasive tumour characterized by low/intermediate grade, neuroendocrine morphology and supported by the presence of neurosecretory granules and diffuse, uniform immunoreactivity for neuroendocrine markers. 35 Tumour stage, histological grade and mitotic counts were recognized as the main prognostic factors of NET. 35 According to WHO classifications, the current patient had a poorly differentiated NECB as she presented a triple-negative subtype with Ki-67 positive in 80% of the tumour cells, as well as CgA and Syn.
In terms of treatment, due to a lack of clinical studies, almost no formalized treatment is recommended for NECB. Like other types of breast cancer, surgery is the optimal treatment for NECB, usually followed by radiotherapy or chemotherapy depending on the size of the tumour and lymph node status, and metastasis being excluded before surgery.36,37 For early-stage NECB, the choice of adjuvant chemotherapy regimens depends on the molecular subtype based on immunohistochemistry;38–40 and the National Comprehensive Cancer Network guideline is available for reference. 41 For advanced NECB, platinum-based regimens are the most common treatment choice, such as etoposide combined with cisplatin or carboplatin (EP/EC). 16 In the literature reviewed, EP and EC were the most frequently used formulas for first-line chemotherapy.10,11,14,16 Moreover, when metastatic disease is diagnosed, the treatment strategy should be individualized depending on the general conditions and comorbidities of the patient, the extent and aggressiveness of the disease, and the biological features of the tumour. 36 The current case was diagnosed with LM, which is a type of aggressive and malignant metastasis of NECB, with a reported overall survival of 4–6 weeks. 42 Currently, the treatment options for patients with LM include intrathecal treatment (IT), radiotherapy, systemic chemotherapy, supportive therapeutic and surgery. 43
Intrathecal treatment is a promising treatment choice for patients with LM, which administers treatment directly into the subarachnoid space and avoids the blood-brain barrier. A randomized open-label phase III study of 72 LM breast cancer patients demonstrated that IT combining systemic treatment improves LM-related progression-free survival compared with systemic therapy alone. 44 It has been recommended that methotrexate, cytarabine, liposomal cytarabine and thiotepa are the most common options for IT therapy.45–52
Radiotherapy is an alternative treatment targeting bulky, symptomatic disease sites, particularly in the spine, with more efficacy and less toxicity than IT.53,54 Despite not prolonging patient survival, radiotherapy can help facilitate IT use by restoring CSF flow and relieving hydrocephalus.55,56
In addition, systemic chemotherapy has been demonstrated to improve patient survival.54,57,58 Supportive therapy could alleviate LM-related symptoms and promote the patient’s quality of life. 59 In this current case, systemic chemotherapy of EP was used as the first-line salvage treatment to delay tumour progression, with a remarkable efficacy in the first cycle but failed in the second cycle. Regarding the patient’s wishes, IT chemotherapy and radiotherapy were not administered.
This case report and the literature review have highlighted that NECB is a malignant tumour, so a comprehensive evaluation is critical to patient prognosis and treatment. Secondly, the clinical manifestations of LM are insidious, with some signs of LM such as back and neck ache, and lower extremity weakness, being easily confused with bone metastases; and other features like headache, nausea and vomiting are often confused with brain metastases. As the disease progression is rapid, timely imaging examination and keen insight are warranted. Thirdly, NECB patients with LM may not respond well to the systemic treatment of EP because different metastatic sites of NEC tumours have different sensitivity to platinum-based chemotherapy, 60 so IT may be a better treatment option.
In conclusion, primary NECB with LM is sporadic, devoid of effective treatment and is associated with a poor prognosis. Consequently, it is vitally important to identify LM in order to achieve longer patient survival. Moreover, more high-quality studies are required to explore an effective treatment for patients with advanced NECB.
Acknowledgement
We thank the patient and her family for supporting the treatment of this rare case.
Author contributions: Mengqi Yuan: responsible for the patient management, literature review, and writing; Dongmei Chen: responsible for the patient management and guidance on writing; Hongliang Sun: responsible for the interpretation of patient’s imaging results; Xiuhong Wang: responsible for the interpretation of patient’s pathological results; Donggui Wan: responsible for the treatment plan, patient management, and guidance on writing.
Declaration of conflicting interest: The authors declare that there are no conflicts of interest.
Funding: The authors disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This study was financially supported by the Wu Jieping Medical Foundation Funding (no. 320.6750.2020-07-24).
ORCID iD
Mengqi Yuan https://orcid.org/0000-0002-8972-1992
References
- 1.Lopez-Bonet E, Alonso-Ruano M, Barraza G, et al. Solid neuroendocrine breast carcinomas: incidence, clinico-pathological features and immunohistochemical profiling. Oncol Rep 2008; 20: 1369–1374. [PubMed] [Google Scholar]
- 2.Wang J, Wei B, Albarracin CT, et al. Invasive neuroendocrine carcinoma of the breast: a population-based study from the surveillance, epidemiology and end results (SEER) database. BMC Cancer 2014; 14: 147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cubilla AL, Woodruff JM. Primary carcinoid tumor of the breast: a report of eight patients. Am Surg Pathol 1977; 1: 283–292. [Google Scholar]
- 4.Gagnier JJ, Kienle G, Altman DG, et al. The CARE guidelines: consensus-based clinical case reporting guideline development. Headache 2013; 53: 1541–1547. [DOI] [PubMed] [Google Scholar]
- 5.Jundt G, Schulz A, Heitz PU, et al. Small cell neuroendocrine (oat cell) carcinoma of the male breast. Immunocytochemical and ultrastructural investigations. Virchows Arch A Pathol Anat Histopathol 1984; 404: 213–221. [DOI] [PubMed] [Google Scholar]
- 6.Papotti M, Gherardi G, Eusebi V, et al. Primary oat cell (neuroendocrine) carcinoma of the breast. Report of four cases. Virchows Arch A Pathol Anat Histopathol 1992; 420: 103–108. [DOI] [PubMed] [Google Scholar]
- 7.Francois A, Chatikhine VA, Chevallier B, et al. Neuroendocrine primary small cell carcinoma of the breast. Report of a case and review of the literature. Am J Clin Oncol 1995; 18: 133–138. [DOI] [PubMed] [Google Scholar]
- 8.Adegbola T, Connolly CE, Mortimer G. Small cell neuroendocrine carcinoma of the breast: a report of three cases and review of the literature. J Clin Pathol 2005; 58: 775–778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kinoshita S, Hirano A, Komine K, et al. Primary small-cell neuroendocrine carcinoma of the breast: report of a case. Surg Today 2008; 38: 734–738. [DOI] [PubMed] [Google Scholar]
- 10.Hojo T, Kinoshita T, Shien T, et al. Primary small cell carcinoma of the breast. Breast Cancer 2009; 16: 68–71. [DOI] [PubMed] [Google Scholar]
- 11.Ochoa R, Sudhindra A, Garcia-Buitrago M, et al. Small-cell cancer of the breast: what is the optimal treatment? A report and review of outcomes. Clin Breast Cancer 2012; 12: 287–292. [DOI] [PubMed] [Google Scholar]
- 12.Jiang J, Wang G, Lv L, et al. Primary small-cell neuroendocrine carcinoma of the male breast: a rare case report with review of the literature. Onco Targets Ther 2014; 7: 663–666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Valente I, Tringali G, Martella EM, et al. Primary neuroendocrine carcinoma of the breast: A case report of liver and lymph node metastases after eight years from diagnosis. Breast J 2020; 26: 505–507. [DOI] [PubMed] [Google Scholar]
- 14.Wang X, Shi YF, Duan JH, et al. S-1 plus temozolomide as second-line treatment for neuroendocrine carcinoma of the breast: A case report. World J Clin Cases 2021; 9: 7146–7153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wade PM, Jr., Mills SE, Read M, et al. Small cell neuroendocrine (oat cell) carcinoma of the breast. Cancer 1983; 52: 121–125. [DOI] [PubMed] [Google Scholar]
- 16.Soe AM, Joseph G, Guevara E, et al. Primary Neuroendocrine Carcinoma of the Breast Metastatic to the Bones, Which Chemotherapy? Breast J 2017; 23: 589–593. [DOI] [PubMed] [Google Scholar]
- 17.Wei B, Ding T, Xing Y, et al. Invasive neuroendocrine carcinoma of the breast: a distinctive subtype of aggressive mammary carcinoma. Cancer 2010; 116: 4463–4473. [DOI] [PubMed] [Google Scholar]
- 18.Chamberlain MC. Leptomeningeal metastasis. Curr Opin Oncol 2010; 22: 627–635. [DOI] [PubMed] [Google Scholar]
- 19.Wang N, Bertalan MS, Brastianos PK. Leptomeningeal metastasis from systemic cancer: Review and update on management. Cancer 2018; 124: 21–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Clarke JL, Perez HR, Jacks LM, et al. Leptomeningeal metastases in the MRI era. Neurology 2010; 74: 1449–1454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Chang EL, Lo S. Diagnosis and management of central nervous system metastases from breast cancer. Oncologist 2003; 8: 398–410. [DOI] [PubMed] [Google Scholar]
- 22.Coleman RE. Clinical features of metastatic bone disease and risk of skeletal morbidity. Clin Cancer Res 2006; 12: 6243s–6249s. [DOI] [PubMed] [Google Scholar]
- 23.Wang C, Xu K, Wang R, et al. Heterogeneity of BCSCs contributes to the metastatic organotropism of breast cancer. J Exp Clin Cancer Res 2021; 40: 370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Glass JP, Melamed M, Chernik NL, et al. Malignant cells in cerebrospinal fluid (CSF): the meaning of a positive CSF cytology. Neurology 1979; 29: 1369–1375. [DOI] [PubMed] [Google Scholar]
- 25.Rudnicka H, Niwinska A, Murawska M. Breast cancer leptomeningeal metastasis–the role of multimodality treatment. J Neurooncol 2007; 84: 57–62. [DOI] [PubMed] [Google Scholar]
- 26.Adams RW, Dyson P, Barthelmes L. Neuroendocrine breast tumours: breast cancer or neuroendocrine cancer presenting in the breast? Breast 2014; 23: 120–127. [DOI] [PubMed] [Google Scholar]
- 27.Abou Dalle I, Abbas J, Boulos F, et al. Primary small cell carcinoma of the breast: a case report. J Med Case Rep 2017; 11: 290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bussolati G, Gugliotta P, Sapino A, et al. Chromogranin-reactive endocrine cells in argyrophilic carcinomas (“carcinoids”) and normal tissue of the breast. Am J Pathol 1985; 120: 186–192. [PMC free article] [PubMed] [Google Scholar]
- 29.Hoang MP, Maitra A, Gazdar AF, et al. Primary mammary small-cell carcinoma: a molecular analysis of 2 cases. Hum Pathol 2001; 32: 753–757. [DOI] [PubMed] [Google Scholar]
- 30.Papotti M, Macri L, Finzi G, et al. Neuroendocrine differentiation in carcinomas of the breast: a study of 51 cases. Semin Diagn Pathol 1989; 6: 174–188. [PubMed] [Google Scholar]
- 31.Righi L, Sapino A, Marchio C, et al. Neuroendocrine differentiation in breast cancer: established facts and unresolved problems. Semin Diagn Pathol 2010; 27: 69–76. [DOI] [PubMed] [Google Scholar]
- 32.Zhang Y, Chen Z, Bao Y, et al. Invasive neuroendocrine carcinoma of the breast: a prognostic research of 107 Chinese patients. Neoplasma 2013; 60: 215–222. [DOI] [PubMed] [Google Scholar]
- 33.Lakhani SR, Ellis IO, Schnitt SJ, et al. World Health Organization Classification of Tumours of the Breast. 4th ed. Lyon: IARC Press, 2012. [Google Scholar]
- 34.Hejjane L, Oualla K, Bouchbika Z, et al. Primary neuroendocrine tumors of the breast: two case reports and review of the literature. J Med Case Rep 2020; 14: 41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Rindi G, Klimstra DS, Abedi-Ardekani B, et al. A common classification framework for neuroendocrine neoplasms: an International Agency for Research on Cancer (IARC) and World Health Organization (WHO) expert consensus proposal. Mod Pathol 2018; 31: 1770–1786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Inno A, Bogina G, Turazza M, et al. Neuroendocrine Carcinoma of the Breast: Current Evidence and Future Perspectives. Oncologist 2016; 21: 28–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Irelli A, Sirufo MM, Morelli L, et al . Neuroendocrine Cancer of the Breast: A Rare Entity. J Clin Med 2020; 9: 1452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Angarita FA, Rodriguez JL, Meek E, et al. Locally-advanced primary neuroendocrine carcinoma of the breast: case report and review of the literature. World J Surg Oncol 2013; 11: 128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Cheang MC, Chia SK, Voduc D, et al. Ki67 index, HER2 status, and prognosis of patients with luminal B breast cancer. J Natl Cancer Inst 2009; 101: 736–750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Wei X, Chen C, Xi D, et al. A case of primary neuroendocrine breast carcinoma that responded to neo-adjuvant chemotherapy. Front Med 2015; 9: 112–116. [DOI] [PubMed] [Google Scholar]
- 41.National Comprehensive Cancer Network® Clinical Practice Guidelines in Oncology-Breast Cancer (2020 Version VI) [DB/OL], http://www.nccn.org (2020, accessed 8 September 2020).
- 42.Franzoi MA, Hortobagyi GN. Leptomeningeal carcinomatosis in patients with breast cancer. Crit Rev Oncol Hematol 2019; 135: 85–94. [DOI] [PubMed] [Google Scholar]
- 43.Assi HI, Mahmoud T, Saadeh FS, et al. Management of leptomeningeal metastasis in breast cancer. Clin Neurol Neurosurg 2018; 172: 151–159. [DOI] [PubMed] [Google Scholar]
- 44.Le Rhun E, Wallet J, Mailliez A, et al. Intrathecal liposomal cytarabine plus systemic therapy versus systemic chemotherapy alone for newly diagnosed leptomeningeal metastasis from breast cancer. Neuro Oncol 2020; 22: 524–538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Chahal J, Stopeck A, Clarke K, et al. Intravenous thiotepa for treatment of breast cancer-related leptomeningeal carcinomatosis: case series. Neurol Sci 2015; 36: 1691–1693. [DOI] [PubMed] [Google Scholar]
- 46.Comte A, Jdid W, Guilhaume MN, et al. Survival of breast cancer patients with meningeal carcinomatosis treated by intrathecal thiotepa. J Neurooncol 2013; 115: 445–452. [DOI] [PubMed] [Google Scholar]
- 47.Fizazi K, Asselain B, Vincent-Salomon A, et al. Meningeal carcinomatosis in patients with breast carcinoma. Clinical features, prognostic factors, and results of a high-dose intrathecal methotrexate regimen. Cancer 1996; 77: 1315–1323. [DOI] [PubMed] [Google Scholar]
- 48.Glas M, Stuplich M, Tschampa H, et al. Liposomal cytarabine given concomitantly with radiotherapy in a patient with leptomeningeal metastasis from breast cancer. J Neurol 2008; 255: 1838–1839. [DOI] [PubMed] [Google Scholar]
- 49.Laakmann E, Witzel I, Muller V. Efficacy of Liposomal Cytarabine in the Treatment of Leptomeningeal Metastasis of Breast Cancer. Breast Care (Basel) 2017; 12: 165–167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Meissner M, Addeo A. Intrathecal Methotrexate and Craniospinal Radiotherapy Can Be an Effective Treatment of Carcinomatous Meningitis in Patients with Breast Cancer: Case Reports. Case Rep Oncol 2016; 9: 586–592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Niwinska A, Rudnicka H, Murawska M. Breast cancer leptomeningeal metastasis: the results of combined treatment and the comparison of methotrexate and liposomal cytarabine as intra-cerebrospinal fluid chemotherapy. Clin Breast Cancer 2015; 15: 66–72. [DOI] [PubMed] [Google Scholar]
- 52.Ongerboer de Visser BW, Somers R, Nooyen WH, et al. Intraventricular methotrexate therapy of leptomeningeal metastasis from breast carcinoma. Neurology 1983; 33: 1565–1572. [DOI] [PubMed] [Google Scholar]
- 53.Pan Z, Yang G, He H, et al. Concurrent radiotherapy and intrathecal methotrexate for treating leptomeningeal metastasis from solid tumors with adverse prognostic factors: A prospective and single-arm study. Int J Cancer 2016; 139: 1864–1872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Souchon R, Feyer P, Thomssen C, et al. Clinical Recommendations of DEGRO and AGO on Preferred Standard Palliative Radiotherapy of Bone and Cerebral Metastases, Metastatic Spinal Cord Compression, and Leptomeningeal Carcinomatosis in Breast Cancer. Breast Care (Basel) 2010; 5: 401–407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Chamberlain MC, Kormanik P. Carcinoma meningitis secondary to non-small cell lung cancer: combined modality therapy. Arch Neurol 1998; 55: 506–512. [DOI] [PubMed] [Google Scholar]
- 56.Gani C, Muller AC, Eckert F, et al. Outcome after whole brain radiotherapy alone in intracranial leptomeningeal carcinomatosis from solid tumors. Strahlenther Onkol 2012; 188: 148–153. [DOI] [PubMed] [Google Scholar]
- 57.Herrlinger U, Forschler H, Kuker W, et al. Leptomeningeal metastasis: survival and prognostic factors in 155 patients. J Neurol Sci 2004; 223: 167–178. [DOI] [PubMed] [Google Scholar]
- 58.Oechsle K, Lange-Brock V, Kruell A, et al. Prognostic factors and treatment options in patients with leptomeningeal metastases of different primary tumors: a retrospective analysis. J Cancer Res Clin Oncol 2010; 136: 1729–1735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Chamberlain MC. Neoplastic meningitis. Oncologist 2008; 13: 967–977. [DOI] [PubMed] [Google Scholar]
- 60.Sorbye H, Welin S, Langer SW, et al. Predictive and prognostic factors for treatment and survival in 305 patients with advanced gastrointestinal neuroendocrine carcinoma (WHO G3): the NORDIC NEC study. Ann Oncol 2013; 24: 152–160. [DOI] [PubMed] [Google Scholar]