Abstract
Background:
A multiple sclerosis (MS) diagnosis urges decision-making on immunotherapies, while persons with MS (PwMS) need to develop a coping concept in parallel. At this stage, PwMS ask how they themselves may contribute to controlling the disease. Evidence suggests that maintaining a healthy lifestyle (e.g. physical activity and stress management) is a key factor for healthy aging and preserving activity, while data on MS are complex.
Objectives:
Following the Medical Research Council framework, this study aimed to develop and investigate the feasibility of a new digital health application that conveys evidence-based patient information about lifestyle factors in MS and engages PwMS in relevant behaviour change techniques.
Methods:
Based on a digital health application promoting lifestyle management in breast cancer survivors, an MS-specific adaptation (‘levidex’) was developed. Feasibility was tested with 15 PwMS and eight MS experts. Subsequently, a six-week pilot study with eight PwMS was conducted. All participants provided feedback on practicability and acceptability via a questionnaire and took part in a semi-structured telephone interview. Levidex was revised after each test phase.
Results:
The final levidex tool includes 16 modules, 177 references and several other functions. Feasibility results showed that PwMS and MS experts perceived levidex as understandable (14 out of 15; 6 out of 8), trustworthy (15 out of 15; 8 out of 8), and relevant (10 out of 15; 8 out of 8). Interviews revealed potential for improvement regarding the length and complexity of some content. Piloting of the revised version confirmed good feasibility and high acceptance. Most participants felt inspired to initiate (7 out of 8) or had already implemented (5 out of 8) lifestyle changes after working with levidex.
Conclusion:
Results suggest that levidex is feasible and well-accepted by PwMS and MS experts. It might be a useful tool to support PwMS in adapting to their diagnosis and initiating health-promoting lifestyle changes.
Keywords: eHealth, evidence-based medicine, feasibility testing, piloting, digital health application, lifestyle intervention, multiple sclerosis
Introduction
The incidence of multiple sclerosis (MS) is increasing worldwide, while the inflammatory activity in the early course seems lower, possibly leading to later disability development. 1 Modified diagnostic criteria may contribute to this observation. 2 After diagnosis, persons with MS (PwMS) are shocked and often even traumatised 3 but at the same time urged to make a decision on immunotherapies. Based on their need for time to develop a coping concept and also based on complex evidence of effects and side effects of immunotherapies, not everyone embarks on treatment directly after diagnosis, 4 although immediate action is recommended. However, PwMS often ask what they themselves might contribute to optimal adaptation. Lifestyle factors or modifiable risk factors are increasingly considered relevant in MS. 5 Exercise training might have an impact on disease activity as well as on surrogates of brain integrity, 6 and the same holds true for psychological interventions.7,8 Finally, although there is a lack of controlled studies on the potential influence of dietary factors on MS, 9 adhering to guidelines for a healthy diet while taking MS-specific aspects into account 10 is highly recommended.
PwMS who identify themselves as successfully managing MS often report that maintaining a healthy lifestyle appears to be a key factor for effective self-management, 11 and they regard the provision of lifestyle-related information as very important. However, time for personal advice and individual consultation regarding alternative treatment options and lifestyle changes is limited during typical patient-neurologist encounters. PwMS frequently use Internet sources and eHealth technologies to gather information. 12 One approach to closing the gap between limited time in neurologist encounters and lifestyle-related information needs of PwMS may be the provision of evidence-based patient information (EBPI) 13 via web-based services. While many small and short-term studies have shown beneficial effects of exercise and psychological interventions, 5 Internet interventions have rarely been studied in MS. However, we were recently able to show that digital health applications can ameliorate depression 14 and fatigue 15 in PwMS. A comprehensive web-based lifestyle intervention for PwMS based on behaviour change techniques (BCTs) has not yet been investigated, to our knowledge.
The goal of this study involving PwMS and MS experts was to develop and investigate the feasibility of a new, interactive digital lifestyle management application (termed ‘levidex’) that conveys EBPI and is intended to be used as an add-on to standard care among persons with early-stage MS.
Methods
The development and testing of levidex, including the subsequent evaluation in a randomised controlled trial (RCT), 16 is part of a ῾multiphase mixed-methods study᾿ covering the first three phases of the Medical Research Council (MRC) framework for the development and evaluation of complex interventions. 17 This paper focuses on the first two phases: development and piloting of the complex intervention involving PwMS and MS experts.
Intervention development
The complex intervention levidex is an MS-specific adaptation of ‘optimune’, a digital health application developed to promote lifestyle management in breast cancer survivors with proven efficacy in an RCT.18,19 Both optimune and levidex were developed and are owned and operated by GAIA, a small-to-medium enterprise that specialises in the development and evaluation of digital health applications. Based on preliminary patient-education work14,15,20,21 and the knowledge and suggestions of the multidisciplinary study team – consisting of neurologists, psychologists, health scientists and nutritionists – MS-specific topics to add to the existing programme were identified. Like other GAIA digital health applications, optimune and levidex were developed with the proprietary software platform broca®, which uses rule-based artificial intelligence algorithms to tailor information and therapeutic exercises to individual user characteristics. Broca-based digital interventions have been examined in more than 15 RCTs.14,15,20,22,23 Using ‘simulated dialogues’ that mimic a conversational flow, broca-based digital health applications aim to engage users in therapeutic topics and exercises and continuously invite them to select one or several suitable response options. Based on individual responses, subsequent content is then tailored to match individual users’ needs and preferences. For instance, PwMS can create individual exercise plans based on their own MS disease characteristics and physical ability, or choose between a variety of recipes based on preferred diets and current cooking skills. Like all broca-based programmes, levidex incorporates a broad range of BCTs, particularly those used in cognitive behavioural therapy (CBT), motivational interviewing, and mindfulness and acceptance approaches. 24 It follows the concept of patient empowerment, 25 and several CBT techniques (e.g. behavioural activation, goal-setting, and action-planning) form the main foundation of the intervention. 16 Moreover, incorporated BCTs are linked to the domains (theoretical constructs) provided in the Theoretical Domains Framework 26 and target physical activity, dietary behaviour, psychological well-being, and stress management in MS. Based on its theory-driven approach, levidex is expected to motivate participants to change their behaviour (e.g. increase their physical activity or optimise their dietary behaviour), improve their quality of life and possibly reduce inflammatory disease activity in MS. This approach was combined with EBPI. 13 The implemented information was specifically based on an extensive health technology report examining the possible influence of modifiable risk factors on the development of MS disability 5 as well as 177 references to scientific papers. Complete citation as well as a plain language summaries with simplified information, focusing on study design and relevant findings together with the limitations, are provided in levidex. In a scientific methods section, different study designs and their inherent limitations were also explained, aiming to enable a critical appraisal of the evidence by the users.
Feasibility testing and piloting
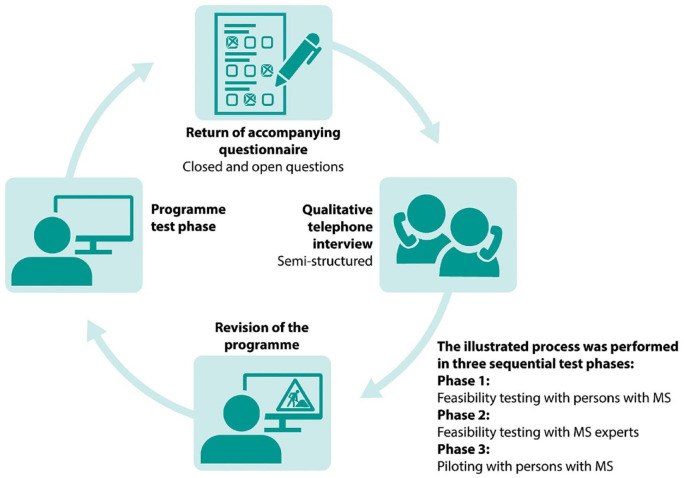
In accordance with the MRC framework, feasibility and progression criteria relating to the content and delivery of levidex as well as its practicability (e.g. login, length, and navigation), acceptability (e.g. practical applicability and motivation incentive) and perceived demand were explored in three sequential test phases that combined quantitative and qualitative methods based on guidelines for feasibility and pilot studies.27,28 The extended Consolidated Standards of Reporting Trials (CONSORT) 2010 checklist 27 for the reporting of this study is provided as Supplemental File. An overview of the methodological steps of the test phases is provided in Figure 1.
Figure 1.
Feasibility testing and piloting.
Due to the large amount of material provided in levidex, application of teach-back and think-aloud was not feasible. For this reason, questionnaires were used. Corresponding to the frameworks provided by Kowatsch et al. 29 and Allison et al., 30 the questionnaires were divided into four parts: (1) participant demographics, (2) ease of use and appearance, (3) content quality and personalisation, and (4) satisfaction and perceived benefit. Patient Determined Disease Steps (PDDS) scale 31 was used as a patient-reported outcome measure of disability in MS. Self-developed question items consisted of a mix of closed (mostly 6-point Likert-type scales) and open questions. Questionnaires were collected after each programme test phase. Responses to closed questions were analysed using descriptive statistics. Continuous variables were described using median and range, and categorical variables were expressed as counts. Responses to open questions were reviewed and categorised. Subsequently, semi-structured telephone interviews were conducted in each phase, based on the results of the filled-in questionnaires. The interviews were recorded, transcribed and analysed thematically. 32 Based on feedback discussions of the teams affiliated with UKE and GAIA, each test phase was completed with a revision of levidex based on the feedback evaluation.
Persons with relapsing-remitting MS (RRMS) were recruited for all development stages by GAIA’s collaborating partners, from the German MS Society (DMSG) and from UKE’s MS day clinic. Participants provided written informed consent and needed to be aged between 18 and 65 years and have Internet access. Feasibility participants were recruited in August 2018. In the feasibility study, participants were provided with login details for single modules (without specific allocation) of levidex for two weeks. In February 2019, a sample of eight PwMS with six weeks of access was intended for piloting, to ensure that all 16 modules were tested, two addressing each subject area. All piloting participants were asked to go through the introductory part and the booster modules. In both test phases, the sequential activation of modules was inactivated and full access was provided directly after login. The accompanying questionnaire and the qualitative telephone interview for the feasibility study focused on technical aspects of levidex.
After patient feedback in the feasibility phase, in October 2018, MS experts from different disciplines (neurologists, nutritionists, or sports scientists with an MS specialisation) from all over Germany were invited to give feedback on the revised levidex programme. After obtaining written informed consent, MS experts were provided with access to levidex for 2 weeks. They were asked to evaluate specific modules related to their expertise. Feasibility testing with MS experts focused on feedback regarding acceptability, content quality, and the motivational potential of levidex.
Results
Final levidex programme
An overview of the finalised version of levidex consisting of 16 modules is provided in Figure 2.
Figure 2.
Levidex module overview (final version).
An introductory module explains the purpose of levidex and provides users with an overview of the anticipated timeframe and content. Levidex informs PwMS that an immunotherapy treatment decision needs to be made. More precisely, it provides overview information on the effectiveness of immunotherapies, including the evaluation and interpretation of effects, as well as absolute and relative risk reduction data and possible side effects. As an alternative to very early MS therapy, levidex points to the possibility of a watch-and-wait approach over a space of one to two years, accompanied by regular neurological and magnetic resonance imaging check-ups, 33 since the natural disease course without therapy for a period up to one or two years can help to better assess MS activity and then possibly better motivate for or against therapy. It should be noted that levidex does not intend to replace or prevent immunotherapies, but is designed as an add-on to standard care. Finally, the programme encourages participants to gather more information, take time to decide, and not put too much pressure on themselves. In the following, levidex addresses three main subject areas: psychological well-being and sleep management, dietary habits and physical activity, followed by four booster sessions (see Table 1).
Table 1.
Levidex subject areas and content.
| Subject area | Content |
|---|---|
| Psychological well-being and sleep management (5 modules) | • EBPI on the potential impact of stress reduction, positive
emotion, and sleep on the immune system and
MS • Assessment of individual sleep quality and possible difficulties • Set of evidence-based CBT techniques for overcoming insomnia • Personalised suggestions for healthy sleep habits • Mindfulness practice and meditation (e.g. audio recordings and individually tailored exercises) |
| Dietary habits (3 modules) | • Assessment of individual dietary habits through screening
questions • EBPI and dietary guidelines on healthy dietary patterns • Set of behaviour change techniques [e.g. goal-setting, action-planning and mental contrasting (of pros and cons or goal obstacles and solutions)] to increase the intake of recommended food groups (e.g. vegetables, whole grain, fish) and reduce the consumption of processed foods (e.g. processed meat, snacks) which potentially increase inflammation |
| Physical activity (2 modules) | • Reflection about the participants’ current level of
physical activity • EBPI on the impact of physical activity on MS symptoms • Set of behaviour change techniques (e.g. goal-setting, action-planning) to promote the adoption of optimised physical activity behaviour |
| Booster modules (4 modules) | • Recapitulation of essential content from previous
modules • Discussion of the participants’ progress • Provision of supporting techniques for long-term maintenance of achieved behaviour change |
CBT, cognitive behavioural therapy; EBPI, evidence-based patient information; MS, multiple sclerosis.
In total, 177 references and plain language summaries are integrated in levidex. An example for each area with sources of varying quality (meta-analysis, cohort study, RCT) is given in Supplemental File 1. Levidex was designed to be accessed over one year. Each module takes about 30–45 minutes to complete, depending on reading speed, individual paths through the programme and decisions to listen to or skip optional audio exercises. The modules include tasks to be completed outside of levidex (e.g. planning exercises or shopping for certain foods) as well as exercises to engage with levidex (e.g. mindfulness meditation audio exercises). New modules are activated successively after a waiting period, allowing participants to reflect on the content and complete tasks and exercises before starting a new module. Optional e-mails and short text messages inform participants about newly available modules. In addition, brief messages are sent to provide lifestyle-related information or brief therapeutic or motivational suggestions. Handouts in PDF format (worksheets and module summaries) and all audio recordings previously encountered can be accessed directly via a menu option. Optional self-monitoring questionnaires (e.g. daily mood check-ups, weekly assessments of physical activity) with individualised feedback and scores for self-reported behaviour performance are included, to visualise achievements over time. The menu also contains an instruction manual and a glossary with additional explanations for 62 terms. Sample screenshots of levidex are given in Supplemental File 2.
Feasibility testing and piloting with PwMS
Characteristics of the participating 23 PwMS (15 in the feasibility and eight in the pilot study) are shown in Table 2. Participants were predominantly female and middle-aged. The majority had RRMS with mild impairment and had been living with MS for more than 5 years. PwMS key quotes are given in Table 3. Supplementary qualitative data are provided in Supplemental File 3.
Table 2.
Demographic and clinical characteristics of the samples of PwMS..
| Feasibility testing (n = 15) | Piloting (n = 8) | |
|---|---|---|
| Age in years, median (range) | 53 (26–60) | 47 (23–54) |
| Female, n | 11 | 4 |
| Male, n | 4 | 4 |
| Education level | ||
| Secondary school, n | 6 | 1 |
| High school/A-levels, n | 5 | 4 |
| University degree, n | 4 | 3 |
| Disease duration in years, median (range) | 7 (1–19) | 9 (2–25) |
| PDDS, median (range) | 1 (0–7) | 3 (1–4) |
| 0–normal, n | 3 | – |
| 1–mild disability, n | 5 | 3 |
| 2–moderate disability, n | 2 | – |
| 3–gait disability, n | 3 | 4 |
| 4–early cane, n | – | 1 |
| 5–late cane, n | – | – |
| 6–bilateral support, n | – | – |
| 7–wheelchair/scooter, n | 2 | – |
| 8–bedridden, n | – | – |
| MS type | ||
| RRMS, n | 10 | 5 |
| SPMS, n | 4 | 1 |
| PPMS, n | 1 | 2 |
MS, multiple sclerosis; PDDS, patient determined disease steps; PPMS, primary progressive MS; PwMS, persons with MS; RRMS, relapsing-remitting MS; SPMS, secondary progressive MS.
Table 3.
PwMS key quotes.
| Evaluation criteria | PwMS key quote |
|---|---|
| Empowerment for lifestyle adaptation | “That would have been THE perfect thing after diagnosis! To know that there are other possibilities (scientifically proven) beyond stand-alone medication. This returns a sense of trust in your own body.”[P14] |
| Barriers to digital health | “In principle, I was really pleasantly surprised, because I am not someone who actually enjoys sitting in front of a computer or playing with an app. So it really was the content I was interested in, rather than the format and yet I was surprised how well I got along with it.” [PP05] |
| Quantity and depth of information | “Yes, a lot of details that I quite simply didn’t know and that I have never seen brought together in this way before, particularly tailored to MS. Usually it’s all rather generalised and I was really surprised by the depth of information, its thoroughness. And how in actually every area there was something I hadn’t come across or heard about previously.” [PP05] |
| “I’m relatively deeply immersed in the material. For newly diagnosed patients it’s way too much input. Less is more!” [P09] | |
| Readyness | “To begin with you just want to be left in peace, or you are all-consumed with your own self and not yet. . . Caught up with your psyche and all that . . . This could very well be helpful then, but everyone is wired differently.” [PP04] |
MS, multiple sclerosis; PwMS, persons with MS.
Ease of use and appearance
The feasibility study consistently showed that the technical use of levidex was feasible. Moreover, the participants generally appreciated the layout and easy navigation (13 out of 15). However, to facilitate the usability of the programme, a print-out version of the instruction manual was compiled to be sent to future participants along with the login details. Piloting of levidex generally indicated good levels of acceptance and practicability. It was even appreciated by one participant who usually did not use digital applications. Some participants (4 out of 8) were not satisfied with the module length and considered it too long. However, participants appreciated that breaks were possible at any time. Similar to a real human conversation, the modules are designed as simulated dialogues that can only be accessed once. Here, some participants struggled with the limited repeatability of modules. One participant criticised the lack of opportunity to reread the content he had worked through in the past. Another participant even claimed a restriction of the freedom for information. Based on this feedback, short handouts available at any time including key messages were incorporated for every module. Beyond that, information provided in the modules is repeated within the last four booster modules. Information regarding the limited repeatability of the modules was additionally added to the instruction manual to avoid false expectations.
Content quality and personalisation
The majority of PwMS in the feasibility cohort agreed or strongly agreed that the content provided in levidex was understandable (14 out of 15). Whereas some participants (feasibility: 5 out of 15, piloting: 1 out of 8) perceived the optional email and short text message reminder system as excessive or annoying, this feature was appreciated by all other participants. All feasibility participants agreed or strongly agreed that levidex was trustworthy. The references, including the plain language summaries provided, were considered helpful by almost all participants who used them (12 out of 15). Some participants (feasibility: 4 out of 15, piloting: 4 out of 8) noted that the simulated dialogue offered restricted answering options and suggested including neutral answers. The additional material (e.g. audio exercises, handouts) and the glossary were helpful for all participants who used them (feasibility: 12 out of 15, piloting: 5 out of 8).
Satisfaction and perceived benefit
By using a German-style school grades format ranging from 1 (very good) to 6 (insufficient), levidex was graded as good (grade 2) in the feasibility cohort, even though some participants (4 out of 15) only perceived it as satisfactory (grade 3). In the pilot study, levidex was graded as good (grade 2) by 6 out of 8 participants. While many participants perceived levidex as relevant or highly relevant for newly diagnosed PwMS (feasibility: 10 out of 15, piloting: all participants), some already felt familiar with the content and thus perceived it as less relevant for themselves (feasibility: 5 out of 15, piloting: 5 out of 8). Nevertheless, five participants in the pilot study reported having implemented lifestyle changes through programme usage. While some participants felt that levidex was particularly suitable for newly diagnosed PwMS, others pointed out that the amount of information might be too much for this target group. Finally, the majority of all participants (20 out of 23) indicated that they would be likely or even very likely to recommend levidex to a friend or colleague who might need help regarding the handling of the disease.
Feasibility testing with MS experts
From 15 recruited MS experts, 11 signed informed consent, but only eight MS experts completed feasibility testing. The MS experts (five neurologists, two nutrition scientists, and one sports scientist) were middle-aged (median 54, range 32–54) and six were male. Expert key quotes are given in Table 4. Additional qualitative expert data are provided in Supplemental File 4.
Table 4.
MS expert key quotes.
| Evaluation criteria | MS expert key quote |
|---|---|
| Attitude towards disease modifying therapies (before revision) | “And generally I think that while the realm of drug therapy is well depicted, it probably does speak more to the sceptical viewpoint here, and what doesn’t come through strongly enough, I think, is that there are patients with highly active MS who really do benefit from immunotherapy, and less so in other cases.” [E03] |
| Quantity, quality and depth of information | “I think the glossary is really good, and these are absolutely the themes that are often raised in seminars and in consultations and they are the ones I must be ready to offer an opinion on. [. . .]So it is very serious, and very readable, and I believe that an impacted person who is in search of an answer to a particular point, and many people affected are on such quests in my experience, will truly find an answer. [. . .] And I think for those who want to know more, this [the plain language abstracts] is wonderful. And it remains an individual choice, what to click on or not. And above all the scope of the description of the study is just right. Short and to the point and all the essentials are in there.”[E05] |
| MS and diet | “In my opinion it’s a confidence-building measure to say, okay, we know there are these other things circulating on the internet at the moment, and this is our position on those.” [E04] |
| “How should I change my nutrition, how should I optimise my sleep? It would be best to work on physical exercise, too, not forgetting whatever else, stress management, say. But at some point, it could all get a little much, right? [. . .] I have to think of too many things at once. And just when I’m familiarising myself with one subject, the next one is already tapping at the door.” [E06] |
MS, multiple sclerosis.
Ease of use and appearance
Most participants (7 out of 8) regarded the arrangement of modules within levidex as plausible. The majority (7 out of 8) perceived it as visually appealing. Only one expert (neurologist) perceived the visualisation as rather monotonous and very text-heavy, which is why he recommended including videos and more images for more graphic variety.
Content quality and personalisation
Findings confirmed understandability, as most experts (6 out of 8) agreed that it was understandable for PwMS. One expert (sports scientist) perceived the content tailoring of the modules on physical activity as appropriate, but overloaded. The participating experts also generally perceived the introductory module as too long and complex. Some of the participating neurologists particularly expressed their concern about a too critical review of immunotherapies in the introductory module. As immunotherapy decision-making is not at the core of the intervention, this part was excluded from levidex. After the revision, levidex now only encourages immunotherapy decision-making and refers users to the treating neurologist for further immunotherapy-related matters (see ‘Final levidex programme’). In addition, the first module was split into two parts. More break options and a progress bar were incorporated throughout the modules, to visualise the remaining module duration. Six of eight experts rated levidex as highly trustworthy and relevant for PwMS. The implemented plain language summaries and the glossary were highly appreciated. Moreover, two experts (a nutrition scientist and a neurologist) appreciated the evidence communication regarding MS and nutrition, as well as on lifestyle in general, being aware of the complex evidence situation in this field. Some of the participating neurologists pointed out that the answer options in the simulated dialogues were too limited, which is why they perceived them as predefined and out of context in places.
Satisfaction and perceived benefit
Overall, levidex was graded as good (grade 2), even though some participants (3 out of 8) only perceived it as satisfactory (grade 3). The experts were also asked to assess the motivational potential for long-term use of levidex. The feedback in this regard was mixed. Although almost all experts (7 out of 8) ranked the application as rather motivating to highly motivating, they stressed that motivation is highly individual and that not all PwMS might be amenable to behaviour change based only on a digital health application. Some participants were afraid that levidex might be too demanding with regard to parallel behaviour changes in many different areas. To make the pursued behaviour change more attainable, three experts recommended including more targeted and specific goal-setting, based on positive formulations and practical examples. For this reason, more frequent and more targeted nutritional impulses were added to the optional emails and short text message reminders.
Discussion
This article reports on the development, feasibility testing, piloting, and revision of a new digital health application (levidex) for PwMS based on the MRC framework. Throughout the development phase, relevant findings for modifications were gathered, and the initial version of levidex was adapted accordingly. Levidex was rated as comprehensive and complex, but our results consistently show that it is feasible and well accepted by both MS experts and PwMS. Most relevantly, trust in levidex and perceived relevance for newly diagnosed PwMS was high. While some MS experts raised concerns regarding the complexity of some content, it was rated as understandable and easy to navigate by PwMS. Nevertheless, concerns expressed by PwMS and MS experts will be addressed in a mixed-methods process evaluation conducted in parallel to an ongoing RCT, to inform refinement of the intervention. 16 Participating PwMS particularly appreciated the glossary, summarising handouts and the audio exercises that were provided.
Levidex is an individually tailored digital health application that uses simulated dialogues to mimic a conversational flow, which is a special approach of the intervention. Moreover, a unique feature of levidex is that it conveys EBPI. Participating MS experts particularly appreciated the scientific citations and plain language summaries, which increase perceived credibility of the intervention. Participating PwMS expressed a special interest in diet. This corresponds with survey data 21 showing a high unmet need of PwMS for evidence and education on the potential influence of dietary behaviour on MS disease course. However, the interpretation of the available evidence on diet and MS is very complex, as it is mostly based on observational studies. Conclusive RCTs investigating the influence of complex dietary patterns are lacking and supplementation studies with Vitamin D, fatty acids and other single nutrients have not yet demonstrated clear evidence.9,10,34 Considering this, the question arises as to how complex evidence can be presented to PwMS. EBPI should be committed to both the comprehensive presentation of scientific evidence and patient-centeredness, and thus to delivering a comprehensible presentation of scientific information with practical relevance.33,35 To integrate both demands, different complexity levels of EBPI should be made accessible, based on individual health literacy and needs. 36 Responsive digital technologies such as broca, which was used for levidex, offer an ideal tool for this approach. However, possibilities for personalisation within the context of digital interventions designed as a simulated conversational flow are limited. Changing lifestyle habits is a difficult endeavour, and a wide range of domains such as knowledge acquisition, skill development, motivation, goal-setting, self-monitoring, and social support need to be addressed to successfully initiate and maintain behaviour change.37,38 Although our data support the idea that levidex can motivate PwMS to initiate lifestyle changes, the question arises as to whether a digital health application can be enough to enable long-term behaviour change.
Very few studies have rigorously assessed the benefits of web-based interventions for MS. 39 However, recent meta-analytic evidence shows that the additional benefit of guided compared to unguided interventions might be small. 40 Compared to cost-intensive and time-consuming face-to-face interventions with high implementation barriers, levidex could become a cost-effective and supportive add-on to standard care that can easily be implemented even in remote regions. In light of the COVID-19 pandemic, the year 2020 clearly showed the feasibility and usefulness of web-based health care. 41 Levidex adds to this.
While levidex was initially developed for newly diagnosed PwMS, feasibility testing and piloting was performed with participants in later stages of MS and with advanced MS disease knowledge. This cohort was selected because newly diagnosed PwMS can be overwhelmed or even traumatised after receiving an MS diagnosis 3 and therefore might not yet be able to judge which stage of MS a lifestyle intervention might be most suitable for. Results indicated that it could be a helpful guide for all stages of MS. As participants were mostly at the age of about 50, levidex also appeared to be suitable for older adults, who are associated with lower eHealth literacy. 42 This offers potential for future adaptations of levidex. Building on our findings, preliminary data from a cohort of 40 PwMS in more advanced disease stages of MS indicate high acceptance of an adapted version specifically tailored to the needs of later MS stages. Nevertheless, levidex might be especially beneficial for PwMS in the early stage, as they are young and not substantially impaired or threatened in the short term, and thus qualified to induce behaviour change more easily. A currently ongoing RCT will therefore assess whether levidex can effectively change patient behaviour and impact on inflammatory disease activity among newly diagnosed PwMS. 16
Limitations
The study findings are limited by the small sample size in feasibility testing and piloting. The sample consisted of PwMS who were diagnosed more than five years ago and mostly recruited via UKE’s MS day clinic in Hamburg. As the programme covers at least 153 days in the intended participant timeline, at this stage, we were not able to test the whole sequence of modules. These limitations will be addressed in the ongoing RCT (NCT03968172) throughout Germany, targeting persons with a recent (<1 year) MS diagnosis and providing a follow-up of 1–2 years. It will assess whether levidex can effectively change patient behaviour, improve quality of life, and impact on inflammatory disease activity among newly diagnosed PwMS. 16 Based on the experience in the development of several individually tailored digital health applications,14,15,20,43 levidex aimed to provide a broad range of pre-programmed response options. We are not aware of any other intervention delivering more individualisation while being completely software-based. However, possibilities for individialisation within the context of digital interventions designed as a simulated conversational flow are limited.
Conclusion
Levidex has the potential to close the gap between limited time in neurologist encounters and lifestyle-related information needs of PwMS. Beyond that, it can possibly enable PwMS to adjust to MS at an early stage, especially by optimising lifestyle habits.
Supplemental Material
Supplemental material, sj-docx-1-tan-10.1177_17562864221118729 for ‘That would have been the perfect thing after diagnosis’: development of a digital lifestyle management application in multiple sclerosis by Nicole Krause, Karin Riemann-Lorenz, Anne Christin Rahn, Jana Pöttgen, Sascha Köpke, Björn Meyer, Frithjof Thale, Herbert Temmes, Markus van de Loo, Stefan M. Gold and Christoph Heesen in Therapeutic Advances in Neurological Disorders
Supplemental material, sj-docx-2-tan-10.1177_17562864221118729 for ‘That would have been the perfect thing after diagnosis’: development of a digital lifestyle management application in multiple sclerosis by Nicole Krause, Karin Riemann-Lorenz, Anne Christin Rahn, Jana Pöttgen, Sascha Köpke, Björn Meyer, Frithjof Thale, Herbert Temmes, Markus van de Loo, Stefan M. Gold and Christoph Heesen in Therapeutic Advances in Neurological Disorders
Supplemental material, sj-docx-3-tan-10.1177_17562864221118729 for ‘That would have been the perfect thing after diagnosis’: development of a digital lifestyle management application in multiple sclerosis by Nicole Krause, Karin Riemann-Lorenz, Anne Christin Rahn, Jana Pöttgen, Sascha Köpke, Björn Meyer, Frithjof Thale, Herbert Temmes, Markus van de Loo, Stefan M. Gold and Christoph Heesen in Therapeutic Advances in Neurological Disorders
Supplemental material, sj-docx-4-tan-10.1177_17562864221118729 for ‘That would have been the perfect thing after diagnosis’: development of a digital lifestyle management application in multiple sclerosis by Nicole Krause, Karin Riemann-Lorenz, Anne Christin Rahn, Jana Pöttgen, Sascha Köpke, Björn Meyer, Frithjof Thale, Herbert Temmes, Markus van de Loo, Stefan M. Gold and Christoph Heesen in Therapeutic Advances in Neurological Disorders
Acknowledgments
The authors want to thank all participating persons with MS and MS experts for their involvement and support in the further development process of the levidex programme.
Footnotes
ORCID iD: Nicole Krause  https://orcid.org/0000-0001-6681-7054
https://orcid.org/0000-0001-6681-7054
Supplemental material: Supplemental material for this article is available online.
Contributor Information
Nicole Krause, Institute of Neuroimmunology and Multiple Sclerosis (INIMS), University Medical Center Hamburg-Eppendorf, Martinistraße 52, 20246 Hamburg, Germany.
Karin Riemann-Lorenz, Institute of Neuroimmunology and Multiple Sclerosis (INIMS), University Medical Center Hamburg-Eppendorf, Hamburg, Germany.
Anne Christin Rahn, Institute of Neuroimmunology and Multiple Sclerosis (INIMS), University Medical Center Hamburg-Eppendorf, Hamburg, Germany; Nursing Research Unit, Institute for Social Medicine and Epidemiology, University of Lübeck, Lübeck, Germany.
Jana Pöttgen, Institute of Neuroimmunology and Multiple Sclerosis (INIMS), University Medical Center Hamburg-Eppendorf, Hamburg, Germany; Department of Neurology, University Medical Center Hamburg-Eppendorf, Hamburg, Germany.
Sascha Köpke, Institute of Nursing Science, Faculty of Medicine, University of Cologne and University Hospital Cologne, Cologne, Germany.
Björn Meyer, Research and Development Department, GAIA Group, Hamburg, Germany.
Frithjof Thale, Research and Development Department, GAIA Group, Hamburg, Germany.
Herbert Temmes, German Multiple Sclerosis Society, Federal Association, Hannover, Germany.
Markus van de Loo, German Multiple Sclerosis Society, Federal Association, Hannover, Germany.
Stefan M. Gold, Institute of Neuroimmunology and Multiple Sclerosis (INIMS), University Medical Center Hamburg-Eppendorf, Hamburg, Germany Charité–Universitätsmedizin Berlin, Klinik für Psychiatrie und Psychotherapie und Med. Klinik m.S. Psychosomatik, Berlin, Germany.
Christoph Heesen, Institute of Neuroimmunology and Multiple Sclerosis (INIMS), University Medical Center Hamburg-Eppendorf, Hamburg, Germany; Department of Neurology, University Medical Center Hamburg-Eppendorf, Hamburg, Germany.
Declarations
Ethics approval and consent to participate: The study has been approved by the Ethics Committee of the Hamburg Chamber of Physicians (PV6015). Informed consent was obtained from all study participants. The subsequent RCT has been prospectively registered at ClinicalTrials.gov (NCT03968172).
Consent for publication: All study participants consented to a psyeudonymised publication of the data collected in this study.
Author contributions: Nicole Krause: Conceptualisation; Data curation; Formal analysis; Investigation; Methodology; Project administration; Visualisation; Writing – original draft.
Karin Riemann-Lorenz: Conceptualisation; Funding acquisition; Methodology; Resources; Supervision; Writing – review & editing.
Anne Christin Rahn: Conceptualisation; Funding acquisition; Investigation; Methodology; Project administration; Resources; Writing – review & editing.
Jana Pöttgen: Conceptualisation; Funding acquisition; Methodology; Resources; Writing – review & editing.
Sascha Köpke: Conceptualisation; Funding acquisition; Methodology; Resources; Writing – review & editing.
Björn Meyer: Conceptualisation; Funding acquisition; Resources; Software; Writing – review & editing.
Frithjof Thale: Conceptualisation; Resources; Software; Writing – review & editing.
Herbert Temmes: Conceptualisation; Funding acquisition; Writing – review & editing.
Markus van de Loo: Resources; Writing – review & editing.
Stefan M. Gold: Conceptualisation; Funding acquisition; Methodology; Resources; Writing – review & editing.
Christoph Heesen: Conceptualisation; Funding acquisition; Investigation; Methodology; Project administration; Resources; Supervision; Writing – original draft; Writing – review & editing.
Funding: The authors disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This study was publicly funded by the Innovationsfonds, Innovationsausschuss beim Gemeinsamen Bundesausschuss, Wegelystraße 8, 10623 Berlin, Germany (grant no. 01VSF17015). The funding body was not involved in any study-related aspect.
Competing interests: The authors declared the following potential conflicts of interest with respect to the research, authorship, and/or publication of this article: CH has received research grants, speaker honoraria, and travel grants from Biogen, Celgene, Genzyme, Merck, and Roche. JP has received research grants, speaker honoraria, and travel grants from Bayer, Celgene, and Merck. BM and FT are employed by GAIA, the company that developed, owns, and operates levidex. NK, KR-L, ACR, SK, HT, and MvdL have nothing to declare.
Availability of data and material: All relevant data are within the manuscript and its supplemental material. The transcripts from the interviews contain sensitive data that could possibly give conclusions about the study participants.
References
- 1. Ellenberger D, Flachenecker P, Haas J, et al. Is benign MS really benign? What a meaningful classification beyond the EDSS must take into consideration. Mult Scler Relat Disord 2020; 46: 102485. [DOI] [PubMed] [Google Scholar]
- 2. Thompson AJ, Banwell BL, Barkhof F, et al. Diagnosis of multiple sclerosis: 2017 revisions of the McDonald criteria. Lancet Neurol 2018; 17: 162–173. [DOI] [PubMed] [Google Scholar]
- 3. Chalfant AM, Bryant RA, Fulcher G. Posttraumatic stress disorder following diagnosis of multiple sclerosis. J Trauma Stress 2004; 17: 423–428. [DOI] [PubMed] [Google Scholar]
- 4. Chalmer TA, Baggesen LM, Nørgaard M, et al. Early versus later treatment start in multiple sclerosis: a register-based cohort study. Eur J Neurol 2018; 25: 1262–e110. [DOI] [PubMed] [Google Scholar]
- 5. Hempel S, Graham GD, Fu N, et al. A systematic review of modifiable risk factors in the progression of multiple sclerosis. Mult Scler 2017; 23: 525–533. [DOI] [PubMed] [Google Scholar]
- 6. Dalgas U, Hvid LG, Kwakkel G, et al. Moving exercise research in multiple sclerosis forward (the MoXFo initiative): developing consensus statements for research. Mult Scler 2020; 26: 1303–1308. [DOI] [PubMed] [Google Scholar]
- 7. Mohr DC, Lovera J, Brown T, et al. A randomized trial of stress management for the prevention of new brain lesions in MS. Neurology 2012; 79: 412–419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Mohr DC, Goodkin DE, Islar J, et al. Treatment of depression is associated with suppression of nonspecific and antigen-specific T(H)1 responses in multiple sclerosis. Arch Neurol 2001; 58: 1081–1086. [DOI] [PubMed] [Google Scholar]
- 9. Parks NE, Jackson-Tarlton CS, Vacchi L, et al. Dietary interventions for multiple sclerosis-related outcomes. Cochrane Database Syst Rev 2020; 5: CD004192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Holton KF, Kirkland AE. Moving past antioxidant supplementation for the dietary treatment of multiple sclerosis. Mult Scler 2020; 26: 1012–1023. [DOI] [PubMed] [Google Scholar]
- 11. Ghahari S, Forwell SJ, Suto MJ, et al. Multiple sclerosis self-management model: personal and contextual requirements for successful self-management. Patient Educ Couns 2019; 102: 1013–1020. [DOI] [PubMed] [Google Scholar]
- 12. Marrie RA, Leung S, Tyry T, et al. Use of eHealth and mHealth technology by persons with multiple sclerosis. Mult Scler Relat Disord 2018; 27: 13–19. [DOI] [PubMed] [Google Scholar]
- 13. Bunge M, Muhlhauser I, Steckelberg A. What constitutes evidence-based patient information? Overview of discussed criteria. Patient Educ Couns 2010; 78: 316–328. [DOI] [PubMed] [Google Scholar]
- 14. Twomey C, O’Reilly G, Meyer B. Effectiveness of an individually-tailored computerised CBT programme (Deprexis) for depression: a meta-analysis. Psychiatry Res 2017; 256: 371–377. [DOI] [PubMed] [Google Scholar]
- 15. Pöttgen J, Moss-Morris R, Wendebourg JM, et al. Online fatigue management program for patients with multiple sclerosis – a randomized controlled trial. Mult Scler 2015; 21(Suppl. 11): 41–42.25145691 [Google Scholar]
- 16. Krause N, Riemann-Lorenz K, Steffen T, et al. Study protocol for a randomised controlled trial of a web-based behavioural lifestyle programme for emPOWERment in early Multiple Sclerosis (POWER@MS1). BMJ Open 2021; 11: e041720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Craig P, Dieppe P, Macintyre S, et al. Developing and evaluating complex interventions: the new Medical Research Council guidance. Int J Nurs Stud 2013; 50: 587–592. [DOI] [PubMed] [Google Scholar]
- 18. Holtdirk F, Mehnert A, Weiss M, et al. Protocol for the Optimune trial: a randomized controlled trial evaluating a novel Internet intervention for breast cancer survivors. Trials 2020; 21: 117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Holtdirk F, Mehnert A, Weiss M, et al. Results of the Optimune trial: a randomized controlled trial evaluating a novel Internet intervention for breast cancer survivors. PLoS ONE 2021; 16: e0251276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Fischer A, Schröder J, Vettorazzi E, et al. An online programme to reduce depression in patients with multiple sclerosis: a randomised controlled trial. Lancet Psychiatry 2015; 2: 217–223. [DOI] [PubMed] [Google Scholar]
- 21. Riemann-Lorenz K, Eilers M, von Geldern G, et al. Dietary interventions in multiple sclerosis: development and pilot-testing of an evidence based patient education program. PLoS ONE 2016; 11: e0165246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Zill JM, Christalle E, Meyer B, et al. The effectiveness of an internet intervention aimed at reducing alcohol consumption in adults. Dtsch Arztebl Int 2019; 116: 127–133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Meyer B, Weiss M, Holtkamp M, et al. Effects of an epilepsy-specific Internet intervention (Emyna) on depression: results of the ENCODE randomized controlled trial. Epilepsia 2019; 60: 656–668. [DOI] [PubMed] [Google Scholar]
- 24. Abraham C, Michie S. A taxonomy of behavior change techniques used in interventions. Health Psychol 2008; 27: 379–387. [DOI] [PubMed] [Google Scholar]
- 25. Werbrouck A, Swinnen E, Kerckhofs E, et al. How to empower patients? A systematic review and meta-analysis. Transl Behav Med 2018; 8: 660–674. [DOI] [PubMed] [Google Scholar]
- 26. Cane J, O’Connor D, Michie S. Validation of the theoretical domains framework for use in behaviour change and implementation research. Implement Sci 2012; 7: 37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Eldridge SM, Chan CL, Campbell MJ, et al. CONSORT 2010 statement: extension to randomised pilot and feasibility trials. Br Med J 2016; 355: i5239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. O’Cathain A, Hoddinott P, Lewin S, et al. Maximising the impact of qualitative research in feasibility studies for randomised controlled trials: guidance for researchers. Pilot Feasibility Stud 2015; 1: 32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Kowatsch T, Otto L, Harperink S, et al. A design and evaluation framework for digital health interventions. Inf Technol 2019; 61: 253–263. [Google Scholar]
- 30. Allison R, Hayes C, McNulty CAM, et al. A comprehensive framework to evaluate websites: literature review and development of GoodWeb. JMIR Form Res 2019; 243: e14372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Learmonth YC, Motl RW, Sandroff BM, et al. Validation of patient determined disease steps (PDDS) scale scores in persons with multiple sclerosis. BMC Neurol 2013; 13: 37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Kuckartz U. Qualitative text analysis: a guide to methods, practice & using software. London: SAGE, 2014. [Google Scholar]
- 33. Hemmer B, Bayas A, Berthele A, et al. Diagnose und Therapie der Multiplen Sklerose, Neuromyelitis-optica-Spektrum-Erkrankungen und MOG-IgG-assoziierten Erkrankungen. S2k-Leitlinie: Leitlinien für Diagnostik und Therapie in der Neurologie, 2021, www.dgn.org/leitlinien
- 34. Jagannath VA, Filippini G, Di Pietrantonj C, et al. Vitamin D for the management of multiple sclerosis. Cochrane Database Syst Rev 2018; 9: CD008422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Steckelberg A, Berger B, Köpke S, et al. Kriterien für evidenzbasierte Patienteninformationen. Z Ärztl Fortbild Qualitätssich 2005; 99: 353–357. [PubMed] [Google Scholar]
- 36. Kasper J, Heesen C, Muhlhauser I. [Evidence-based patient information: the example of immunotherapy for patients with multiple sclerosis]. Bundesgesundheitsblatt Gesundheitsforschung Gesundheitsschutz 2009; 52: 77–85. [DOI] [PubMed] [Google Scholar]
- 37. Kwasnicka D, Dombrowski SU, White M, et al. Theoretical explanations for maintenance of behaviour change: a systematic review of behaviour theories. Health Psychol Rev 2016; 10: 277–296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Michie S, West R, Sheals K, et al. Evaluating the effectiveness of behavior change techniques in health-related behavior: a scoping review of methods used. Transl Behav Med 2018; 8: 212–224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Lavorgna L, Brigo F, Moccia M, et al. E-Health and multiple sclerosis: an update. Mult Scler 2018; 24: 1657–1664. [DOI] [PubMed] [Google Scholar]
- 40. Baumeister H, Reichler L, Munzinger M, et al. The impact of guidance on Internet-based mental health interventions – a systematic review. Internet Interv 2014; 1: 205–215. [Google Scholar]
- 41. Fagherazzi G, Goetzinger C, Rashid MA, et al. Digital health strategies to fight COVID-19 worldwide: challenges, recommendations, and a call for papers. J Med Internet Res 2020; 1622: e19284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Kickbusch P, Jürgen M, Apfel F, et al. Health literacy: the solid facts. World Health Organization, Regional Office for Europe, 2013, https://apps.who.int/iris/handle/10665/326432 [Google Scholar]
- 43. Meyer B, Berger T, Caspar F, et al. Effectiveness of a novel integrative online treatment for depression (Deprexis): randomized controlled trial. J Med Internet Res 2009; 11: e15. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material, sj-docx-1-tan-10.1177_17562864221118729 for ‘That would have been the perfect thing after diagnosis’: development of a digital lifestyle management application in multiple sclerosis by Nicole Krause, Karin Riemann-Lorenz, Anne Christin Rahn, Jana Pöttgen, Sascha Köpke, Björn Meyer, Frithjof Thale, Herbert Temmes, Markus van de Loo, Stefan M. Gold and Christoph Heesen in Therapeutic Advances in Neurological Disorders
Supplemental material, sj-docx-2-tan-10.1177_17562864221118729 for ‘That would have been the perfect thing after diagnosis’: development of a digital lifestyle management application in multiple sclerosis by Nicole Krause, Karin Riemann-Lorenz, Anne Christin Rahn, Jana Pöttgen, Sascha Köpke, Björn Meyer, Frithjof Thale, Herbert Temmes, Markus van de Loo, Stefan M. Gold and Christoph Heesen in Therapeutic Advances in Neurological Disorders
Supplemental material, sj-docx-3-tan-10.1177_17562864221118729 for ‘That would have been the perfect thing after diagnosis’: development of a digital lifestyle management application in multiple sclerosis by Nicole Krause, Karin Riemann-Lorenz, Anne Christin Rahn, Jana Pöttgen, Sascha Köpke, Björn Meyer, Frithjof Thale, Herbert Temmes, Markus van de Loo, Stefan M. Gold and Christoph Heesen in Therapeutic Advances in Neurological Disorders
Supplemental material, sj-docx-4-tan-10.1177_17562864221118729 for ‘That would have been the perfect thing after diagnosis’: development of a digital lifestyle management application in multiple sclerosis by Nicole Krause, Karin Riemann-Lorenz, Anne Christin Rahn, Jana Pöttgen, Sascha Köpke, Björn Meyer, Frithjof Thale, Herbert Temmes, Markus van de Loo, Stefan M. Gold and Christoph Heesen in Therapeutic Advances in Neurological Disorders