Abstract
Background:
Acute respiratory distress syndrome (ARDS) is a severe complication among patients with severe acute pancreatitis (SAP), which may be associated with increased mortality in hospitalized patients. Thus, an effective model to predict ARDS in patients with SAP is urgently required.
Methods:
We retrospectively analyzed the data from the patients with SAP who recruited in Xiangya Hospital between April 2017 and May 2021. Patients meeting the Berlin definition of ARDS were categorized into the ARDS group. Logistic regression models and a nomogram were utilized in the study. Descriptive statistics, logistic regression models, and a nomogram were used in the current study.
Results:
Comorbidity of ARDS occurred in 109 (46.58%) of 234 patients with SAP. The SAP patients with ARDS group had a higher 60-day mortality rate, an increased demand for invasive mechanical ventilation, and a longer intensive care unit (ICU) stay than those without ARDS (p < .001 for all). Partial pressure of oxygen (PaO2): fraction of inspired oxygen (FiO2) < 200, platelets <125 × 109/L, lactate dehydrogenase >250 U/L, creatinine >111 mg/dL, and procalcitonin >0.5 ng/mL were independent risk variables for development of ARDS in SAP patients. The area under the curve for the model was 0.814, and the model fit was acceptable [p = .355 (Hosmer–Lemeshow)]. Incorporating these 5 factors, a nomogram was established with sufficient discriminatory power (C-index 0.814). Calibration curve indicated the proper discrimination and good calibration in the predicting nomogram model.
Conclusion:
The prediction nomogram for ARDS in patients with SAP can be applied using clinical common variables after the diagnosis of SAP. Future studies would be warranted to verify the potential clinical benefits of this model.
Keywords: acute respiratory distress syndrome, ARDS, SAP, severe acute pancreatitis
Introduction
Acute pancreatitis (AP) is an inflammatory disease of pancreas with varying severity and progression. 1 The global incidence rate of AP is 33.74 per 100,000 person-years, and morbidity is rising gradually. 2 Around 10–20% of patients with AP have a complicated systemic inflammatory response syndrome (SIRS), and multiple organ dysfunction syndrome that can lead to the development of severe AP (SAP) with a 10–15% mortality rate. 3
Acute respiratory distress syndrome (ARDS) is the most prevalent form of organ failure in patients with SAP and remains a major cause of high in-hospital mortality. 4 Moreover, patients with SAP-associated ARDS are typically admitted to the intensive care unit (ICU) for an extended period, thereby increasing healthcare costs. Although an increasing number of studies have identified multiple possible causes of SAP-associated ARDS, including pancreatic necrosis, bacteremia, intestinal barrier failure, activation of inflammatory cascades, and diffuse alveolar damage, the pathological mechanisms are largely unknown. 5 Undoubtedly, the treatment of SAP-associated ARDS is extremely challenging. 6 It is a certainty, however, that appropriate intervention(s) in the early phase of ARDS could help improve clinical prognosis. A prediction model for SAP-associated ARDS using baseline clinical characteristics would aid in the early identification and classification of patients at high risk for SAP-associated ARDS and may provide an opportunity for early therapeutic intervention(s) prior to deteriorating development.
However, several scoring systems, such as the Systemic Inflammatory Response Syndrome (SIRS) score, Bedside Index of Severity in Acute Pancreatitis (BISAP), Modified Marshall (MMF) scoring system, and Sequential Organ Failure Assessment (SOFA) score, have good predictive capabilities for disease severity (mild, moderately severe, and severe according to the revised Atlanta classification) and mortality; however, few, if any, tools work well in predicting ARDS in SAP patients using the available data. As such, through multivariable logistic regression analysis and the development of a nomogram model, we aimed to construct an efficient ARDS prediction model using baseline clinical characteristics that could help to screen for patients who are likely to develop ARDS among those with SAP.
Methods
Study design and participants
We retrospectively collected and analyzed the data from doctor-diagnosed SAP admitted to Department of Biliary-Pancreatic Surgery and Department of Gastroenterology and ICU in Xiangya Hospital of Central South University (Changsha, China) between April 2017 and May 2021. Information of the patients and clinical data were obtained from the institutional electronic medical records by three independent investigators using a spreadsheet (Excel, Microsoft Corporation, Redmond, WA, USA), followed by data anonymization. The study protocol was approved by the Ethics Commission of Xiangya Hospital of Central South University (No. 201912477), and requirements for written informed consent were waived because patient data and analysis were anonymized.
Inclusion criteria were as follows: at least 18 years of age and diagnosed with SAP within 48 h of admission according to the Revised Atlanta classification; any organ dysfunction with ⩾grade 2 severity persisting for >48 h according to the modified Marshall grading in AP was considered SAP. 7 Individuals with a history of previous AP or chronic pancreatitis, unavailability of key data, hospital stay <48 h, malignant tumors, active tuberculosis, malignancy, and chronic obstructive pulmonary disease were excluded. Finally, 234 patients with SAP were included for analysis in the current study. In addition, if the included SAP patients met the Berlin definition for ARDS within 14 days after admission, they would be categorized into the ARDS group. 8
Measurements
Clinical information for each patient, including demographic data, preexisting condition(s), etiology, clinical signs, arterial blood gas analysis results, laboratory findings, scores (SIRS, BISAP, MMF, and SOFA), and outcome, were collected. The etiology of SAP was classified as ‘hypertriglyceridemia’, ‘biliary’, ‘alcohol’, or ‘mixed type’. The SIRS, BISAP, MMF, and SOFA scores were extracted from the admissions database. Clinical variables and outcomes including the incidence of ARDS, 14-day mortality, 30-day mortality, 60-day mortality, use of invasive mechanical ventilation, and ICU length of stay were evaluated among patients with SAP.
Statistical analysis
Data normality of the continuous variables was tested using the Shapiro–Wilk test. Normally distributed data are expressed as mean ± standard deviation, variables with non-normal distribution are reported as median [interquartile range (IQR)], and categorical variables are summarized as frequencies and percentages. Continuous data with normal distribution were compared using the Student’s t test, non-normally distributed variables were tested using the Mann–Whitney U test, and categorical variable rates were assessed using the chi-square test or Fisher’s exact test. Kaplan–Meier curves were created for each group and compared using the log-rank test. Candidate predictor variables were selected based on a priori clinical knowledge and previous literature for use in the ARDS prediction models. Factors from bivariate associations (p < .05) were included in the binary logistic regression. For calculation of odds ratio for ARDS in the logistical regression analysis, each variable was dichotomized according to cutoff values. Each cutoff values were set as the upper or lower limit of normal value or clinical cutoff based on clinical significance or previous studies.9–18 Univariate analysis was performed to assess differences in the characteristics of patients with and without ARDS. Finally, variables with an overall p < .1 in the univariate analysis were included in a multivariate stepwise logistic regression model to assess the early predictive models of ARDS in patients with SAP. A nomogram was constructed based on logistic regression and the individualized incidence of ARDS was determined by calculating the total number of points. The performance of the prediction model was measured according to a calibration curve of the nomogram and calculating the Harrell C-index. The area under the receiver operating characteristic (AUROC) curves was calculated to evaluate the discriminatory ability of the early predictive models. An AUROC > 0.7 indicates good model discrimination. The DeLong method was used to evaluate the difference in AUROC between the nomogram and the scores. All statistical analyses and graphs were generated using SPSS version 26.0 (IBM Corporation, Armonk, NY, USA), GraphPad Prism version 9.0 software (GraphPad Software Inc., San Diego, CA, USA), or R-4.1.2 software (R Foundation for Statistical Computing, Vienna, Austria), using the ‘survminer’, ‘survival’, ‘pROC’, ‘ggplot2’, ‘Hmisc’, ‘lattice’, ‘Formula’, ‘SparseM’, and ‘rms’ packages. Differences with p < .05 were considered to be statistically significant.
Results
Clinical characteristics
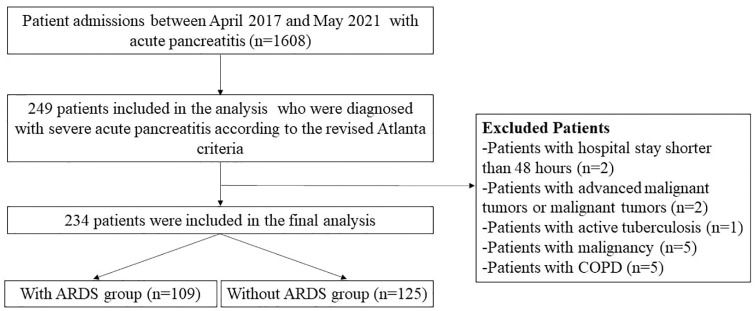
In total, 234 patients with SAP were included in the analysis: 109 (46.58%) with ARDS and 125 (53.42%) without (Figure 1). Demographic data, preexisting condition(s), clinical signs, arterial blood gas analysis results, laboratory findings, and scores on admission were compared between SAP patients with and without ARDS.
Figure 1.
Flow diagram of the study population.
SAP patients with ARDS group had a higher heart rate and respiration rate, lower pH value, and lower PaO2:FiO2 ratio than those patients without ARDS (p < .05 for all) (Table 1). Laboratory variables including the potassium, sodium, lactate dehydrogenase (LDH), blood urea nitrogen, creatinine, blood glucose, lactic acid, and procalcitonin levels were significantly elevated in SAP patients with ARDS than those without ARDS (p < .05 for all), whereas the platelet counts were lower in the SAP patients with ARDS (Table 1). There were considerably higher SIRS, MMF, and SOFA scores in the ARDS group than that in the non-ARDS group (p < .05 for all) (Table 1).
Table 1.
Baseline characteristics of SAP patients with or without ARDS.
| Characteristics | Patients without ARDS (n = 125) | Patients with ARDS (n = 109) | Total (n = 234) | p value |
|---|---|---|---|---|
| Demographic data | ||||
| Age, year | 47.00 (40.00, 55.00) | 49.00 (40.00, 56.00) | 48.00 (40.00,56.00) | .456 |
| Male | 89 (71.20) | 74 (67.89) | 163 (69.66) | .583 |
| Smoking | 49 (39.20) | 34 (31.19) | 83 (35.47) | .202 |
| Drinking | 47 (37.60) | 28 (25.69) | 75 (32.05) | .051 |
| Preexisting condition | ||||
| Chronic cardiac disease | 7 (5.60) | 4 (3.67) | 11 (4.70) | .486 |
| Hypertension | 34 (27.20) | 31 (28.44) | 65 (27.78) | .833 |
| Diabetes mellitus | 31 (24.80) | 27 (24.77) | 58 (24.79) | .996 |
| Etiology | ||||
| Hypertriglyceridemia | 64 (51.20) | 51 (46.79) | 115 (49.15) | .783 |
| Biliary | 39 (31.20) | 36 (33.03) | 75 (32.05) | |
| Alcohol | 5 (4.00) | 3 (2.75) | 8 (3.42) | |
| Mixed type | 17 (13.60) | 19 (17.43) | 36 (15.39) | |
| Clinical signs | ||||
| Heart rate, beats/min | 107.08 ± 1.83 | 114.81 ± 2.04 | 110.68 ± 1.38 | .005 |
| >110, beats/min | 56 (44.80) | 65 (59.63) | 121 (51.71) | .024 |
| Temperature, Celsius | 37.00 (36.80, 37.80) | 37.30 (36.90, 38.10) | 37.00 (36.80, 38.00) | .169 |
| Respiration rate, breaths/min | 22.00 (19.00, 26.00) | 25.00 (20.00, 31.00) | 23.50 (20.00, 29.00) | <.001 |
| >30, breaths/min | 10 (8.00) | 29 (26.61) | 39 (16.67) | <.001 |
| MAP, mmHg | 99.67 (90.00, 110.00) | 94.00 (84.67, 108.00) | 96.67 (88.00, 109.00) | .133 |
| Arterial blood gases | ||||
| PH | 7.45 (7.40, 7.50) | 7.42 (7.35, 7.49) | 7.44 (7.37, 7.50) | .030 |
| <7.350 | 13 (10.40) | 28 (25.69) | 41 (17.52) | .002 |
| PaO2:FiO2 | 209.09 (177.50, 251.35) | 172.50 (132.00, 233.75) | 193.31 (155.00, 243.90) | <.001 |
| <200 | 54 (43.20) | 72 (66.06) | 126 (53.85) | <.001 |
| PCO2, mmHg | 34.00 (30.00, 38.00) | 35.00 (30.50, 38.50) | 35.00 (30.00, 38.00) | .267 |
| Oxygen saturation, % | 96.00 (94.00, 97.00) | 96.00 (93.00, 98.00) | 96.00 (93.00, 97.00) | .983 |
| Laboratory findings | ||||
| Leucocytes counts, 109/L | 12.40 (9.55, 17.75) | 12.30 (9.20, 16.30) | 12.40 (9.20, 17.20) | .566 |
| Erythrocyte counts, 1012/L | 3.63 ± 0.08 | 3.54 ± 0.10 | 3.58 ± 0.06 | .548 |
| Hemoglobin level, g/L | 109.49 ± 2.37 | 107.59 ± 3.10 | 108.60 ± 1.92 | .535 |
| Platelet, 109/L | 206.00 (149.00, 266.00) | 155.00 (119.00, 215.00) | 179.50 (124.00, 253.00) | <.001 |
| <125, 109/L | 18 (14.40) | 44 (40.37) | 62 (26.50) | <.001 |
| Neutrophil counts,109/L | 10.20 (7.60, 15.95) | 10.80 (7.65, 13.85) | 10.50 (7.60, 15.60) | .911 |
| Lymphocyte counts, 109/L | 0.80 (0.60, 1.20) | 0.80 (0.50, 1.050 | 0.80 (0.50, 1.10) | .269 |
| Potassium, mmol/L | 3.73 (3.34, 4.20) | 3.86 (3.52, 4.31) | 3.78 (3.43, 4.23) | .019 |
| >5, mmol/L | 4 (3.2) | 6 (5.51) | 10 (4.27) | .585 |
| Sodium, mmol/L | 140.00 (136.20, 143.40) | 141.80 (139.20, 144.65) | 140.60 (137.60, 144.20) | .003 |
| >147, mmol/L | 12 (9.60) | 17 (15.60) | 29 (12.39) | .165 |
| Calcium, mmol/L | 1.98 (1.84, 2.07) | 1.93 (1.73, 2.10) | 1.97 (1.80, 2.080 | .346 |
| Albumin, g/dL | 29.25 (27.30, 33.70) | 30.20 (27.10, 33.30) | 29.70 (27.20, 33.60) | .513 |
| Total bilirubin, μmol/L | 20.15 (12.30, 36,90) | 21.1 (17.25, 41.20) | 20.90 (14.50, 38.00) | .250 |
| Aspartate aminotransferase, U/L | 25.50 (16.60, 51.10) | 23.60 (13.30, 58.70) | 24.50 (15.10, 51.90) | .382 |
| Alanine aminotransferase, U/L | 35.25 (25.30, 61.80) | 47.10 (29.35, 66.95) | 40.10 (26.80, 66.40) | .068 |
| Lactate dehydrogenase, U/L | 379.00 (249.00, 576.00) | 590.00 (412.00, 835.00) | 462.50 (296.00, 764.00) | <.001 |
| >250, U/L | 91 (72.80) | 105 (96.33) | 196 (83.76) | <.001 |
| Blood urea nitrogen, mmol/L | 6.90 (4.29, 11.42) | 10.17 (6.05, 19.73) | 8.39 (4.91, 15.44) | <.001 |
| >9.5, mmol/L | 34 (27.20) | 60 (55.05) | 94 (40.7) | <.001 |
| Creatinine, mg/dL | 74.20 (61.70, 102.10) | 119.80 (76.10, 336.20) | 90.25 (64.50, 213.10) | <.001 |
| >111, mg/dL | 28 (22.40) | 64 (58.72) | 92 (39.32) | <.001 |
| Blood glucose, mmol/L | 8.30 (6.67, 10.60) | 9.60 (7.90, 13.22) | 8.81 (6.80, 11.80) | .033 |
| >6.1, mmol/L | 97 (77.60) | 78 (71.56) | 175 (74.79) | .288 |
| Lactic acid, mmol/L | 1.30 (0.90, 1.90) | 1.50 (1.20, 2.00) | 1.40 (1.10, 1.95) | .009 |
| >1.6, mmol/L | 37 (29.60) | 45 (41.28) | 82 (35.04) | .062 |
| Triglyceride, mmol/L | 2.73 (1.69, 4.60) | 2.98 (1.80, 5.58) | 2.79 (1.72, 4.96) | .169 |
| Total serum cholesterol, mmol/L | 3.39 (2.31, 4.99) | 3.58 (2.31, 4.81) | 3.40 (2.31, 4.93) | .954 |
| High-density lipoprotein, mmol/L | 0.55 (0.39, 0.82) | 0.53 (0.37, 0.76) | 0.55 (0.38, 0.79) | .359 |
| Low-density lipoprotein, mmol/L | 2.23 (1.56, 3.34) | 2.21 (1.46, 3.15) | 2.23 (1.48, 3.19) | .719 |
| C-reactive protein, mg/dL | 165.00 (128.00, 288.50) | 236.00 (152.00, 363.00) | 205.00 (134.00, 318.00) | .111 |
| Plasma fibrinogen, g/L | 4.99 (3.76, 6.91) | 5.21 (3.90, 6.91) | 5.16 (3.85, 6.91) | .770 |
| D-dimer, mg/L | 2.90 (1.58, 6.48) | 3.85 (1.85, 9.10) | 2.99 (1.63, 6.85) | .226 |
| Procalcitonin, ng/mL | 1.17 (0.38, 3.84) | 5.48 (1.67, 15.46) | 2.32 (0.68, 8.98) | <.001 |
| >0.5, ng/mL | 85 (65.60) | 101 (92.66) | 186 (79.49) | <.001 |
| Scores | ||||
| SIRS | 6.00 (3.00, 8.00) | 7.00 (5.00, 9.000) | 6.00 (4.00, 9.000) | .001 |
| BISAP | 2.00 (2.00, 2.00) | 2.00 (2.00, 2.50) | 2.00 (2.00, 2.00) | .084 |
| MMF | 3.00 (2.00, 3.00) | 3.00 (3.00, 5.00) | 3.00 (2.00, 4.00) | <.001 |
| SOFA | 4.00 (2.00, 5.00) | 4.00 (6.00, 9.00) | 4.00 (3.00, 7.00) | <.001 |
| Outcome | ||||
| 14-day mortality | 5 (4.00) | 19 (17.43) | 24 (10.26) | .001 |
| 30-day mortality | 7 (5.60) | 32 (29.36) | 39 (16.67) | <.001 |
| 60-day mortality | 8 (6.40) | 41 (37.62) | 49 (20.94) | <.001 |
| Invasive mechanical ventilation | 12 (9.60) | 65 (59.63) | 77 (32.91) | <.001 |
| ICU length of stay, days | 2.00 (0.00, 6.00) | 10.00 (4.00, 16.00) | 5.00 (1.00, 11.00) | <.001 |
ARDS, acute respiratory distress syndrome; BISAP, bedside index of severity in acute pancreatitis; FiO2, fraction of inspired oxygen; MAP, mean artery pressure; MMF, modified Marshall scoring system; PaO2, partial pressure of oxygen; PCO2, partial pressure of carbon dioxide; PH, hydrogen ion concentration; SAP, severe acute pancreatitis; SIRS, systemic inflammatory response syndrome score; SOFA, Sequential Organ Failure Assessment score.
Data are presented as mean ± standard deviation, medians (interquartile range) and n (%).Values of p were calculated by Student’s t test, Mann–Whitney U test, chi-square test, or Fisher’s exact test, as appropriate. Values of p indicate differences between Patients with ARDS group and Patients without ARDS group.
A p value <.05 was considered statistically significant.
Clinical outcomes
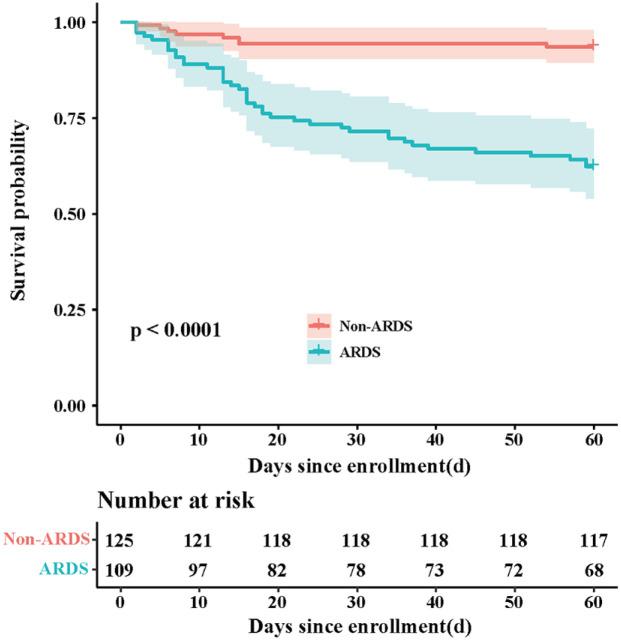
In patients with SAP, the 60-day mortality was commonly used to assess the clinical outcome, and they were 37.62% and 6.40% among SAP patients with or without ARDS, respectively (p <.001) (Table 1). Similarly, the Logistic Regression and Kaplan–Meier survival curve demonstrated a significant survival benefit among SAP patients without ARDS compared with those with ARDS (p < .001) (Table 1 and Figure 2). In addition, the SAP patients with ARDS had greater likelihood of receiving invasive mechanical ventilation and longer ICU length of stay (P < .001 for both) (Table 1).
Figure 2.
Kaplan–Meier curves of mortality for SAP patients with or without ARDS. Patients discharged home considered alive at 60 days. A log-rank test was used to evaluate differences between groups.
ARDS, acute respiratory distress syndrome; SAP, severe acute pancreatitis.
Model development
In the preliminary analysis, we revealed that higher heart rate, higher respiration rate, lower pH value, lower PaO2:FiO2 ratio, lower platelet count, and elevated LDH, elevated blood urea nitrogen, elevated creatinine, and elevated procalcitonin levels were potential predictors of ARDS development among patients with SAP using univariate analyses (p < .05 for all) (Table 2). Multiple logistic regression analysis was further performed and showed that lower PaO2:FiO2 ratio (PaO2:FiO2 < 200; odds ratio 2.045; p = .024), lower platelet count (platelet count < 125 × 109/L; odds ratio, 2.228; p = .034), elevated LDH (LDH > 250 U/L, odds ratio, 7.334; p = .001), elevated creatinine (creatinine > 111 mg/dL; odds ratio, 2.878; p = .002), and elevated procalcitonin (procalcitonin > 0.5 ng/mL; odds ratio, 3.907; p = .002) were independent risk factors for ARDS comorbidity in patients with SAP (Table 2).
Table 2.
Logistic Regression Model for predicting ARDS in SAP patients.
| Variable | Univariate | Multivariate | ||||
|---|---|---|---|---|---|---|
| Odds ratio | 95% CI | p value | Odds ratio | 95% CI | p value | |
| Heart rate > 110, beats/min | 1.820 | 1.082–3.062 | .024 | |||
| Respiratory rate > 30, breaths/min | 4.169 | 1.924–9.034 | <.001 | |||
| PH < 7.350 | 2.952 | 1.440–6.048 | .003 | |||
| PaO2:FiO2 < 200 | 2.559 | 1.504–4.353 | .001 | 2.045 | 1.099–3.806 | .024 |
| Platelet < 125, 109/L | 4.024 | 2.145–7.548 | <.001 | 2.228 | 1.062–4.671 | .034 |
| Potassium > 5, mmol/L | 1.762 | 0.484–6.415 | .390 | |||
| Sodium > 147, mmol/L | 1.740 | 0.791–3.829 | .169 | |||
| Lactate dehydrogenase > 250, U/L | 9.808 | 3.353–28.690 | <.001 | 7.334 | 2.373–22.671 | .001 |
| Blood urea nitrogen > 9.5, mmol/L | 3.277 | 1.899–5.655 | <.001 | |||
| Creatinine > 111, mg/dL | 4.927 | 2.793–8.691 | <.001 | 2.878 | 1.494–5.542 | .002 |
| Blood glucose > 6.1, mmol/L | 1.206 | 0.599–2.427 | .599 | |||
| Lactic acid > 1.6, mmol/L | 1.672 | 0.973–2.873 | .063 | |||
| Procalcitonin > 0.5, ng/mL | 5.941 | 2.638–13.383 | <.001 | 3.907 | 1.633–9.347 | .002 |
ARDS, acute respiratory distress syndrome; FiO2, fraction of inspired oxygen; PH, hydrogen ion concentration; PaO2, Partial pressure of oxygen; SAP, severe acute pancreatitis.
A p value < .05 was considered statistically significant.
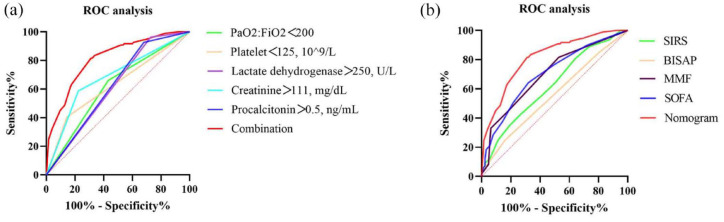
To further confirm the role of the aforementioned covariates in the predictive ability of ARDS among patients with SAP, receiver operating characteristic (ROC) curve analysis was performed (Table 3, Figure 3(a)). The area under the ROC curve (AUROC) for model combination (PaO2:FiO2 < 200; platelets < 125 × 109/L; lactate dehydrogenase > 250 U/L; creatinine > 111 mg/dL; and procalcitonin > 0.5 ng/mL) was 0.814, respectively.
Table 3.
Receiver operating characteristic curves for predicting ARDS in SAP patients.
| Variable | AUC (95% CI) | p value | Sensitivity | Specificity |
|---|---|---|---|---|
| PaO2:FiO2 < 200 | 0.614 (0.549–0.677) | .0003 | 0.661 | 0.568 |
| Platelet < 125, 109/L | 0.630 (0.564–0.692) | <.0001 | 0.404 | 0.856 |
| Lactate dehydrogenase > 250, U/L | 0.618 (0.552–0.680) | <.0001 | 0.963 | 0.272 |
| Creatinine > 111, mg/dL | 0.682 (0.618–0.741) | <.0001 | 0.587 | 0.776 |
| Procalcitonin > 0.5, ng/mL | 0.630 (0.564–0.692) | <.0001 | 0.404 | 0.856 |
| Combination | 0.814 (0.758–0.861) | <.0001 | 0.817 | 0.688 |
ARDS, acute respiratory distress syndrome; AUC, area under the curve; CI, confidence interval; FiO2, fraction of inspired oxygen; PaO2, partial pressure of oxygen; SAP, severe acute pancreatitis.
A p value < .05 was considered statistically significant.
Figure 3.
The ROC curves in prediction of ARDS in patients with SAP. (a) ROC curves of present prediction model. (b) ROC curves of SIRS, BISAP, MMF, SOFA, and nomogram.
ARDS, acute respiratory distress syndrome; BISAP, bedside index of severity in acute pancreatitis; FiO2, fraction of inspired oxygen; MMF, modified Marshall scoring system; PaO2, partial pressure of oxygen; SAP, severe acute pancreatitis; SIRS, systemic inflammatory response syndrome score; SOFA, Sequential Organ Failure Assessment score.
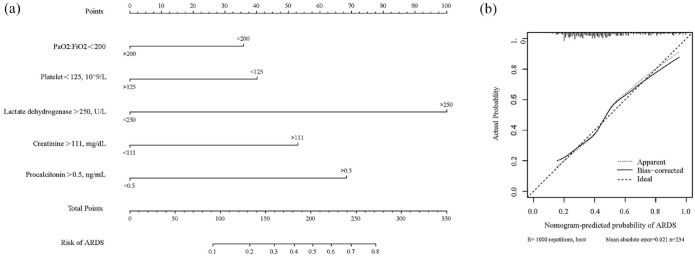
Model visualization and performance
A nomogram incorporating the ARDS prediction model incorporating the above independent predictors was constructed (Figure 4(a)), yielding a value of 0.355, indicating that the model fit was acceptable. The median C-index for the prediction nomogram was 0.814 (0.758–0.861) for the clinical data set. The calibration curve demonstrated good agreement between the estimations with the nomogram for predicting the risk for developing ARDS in patients with SAP and actual observations (Figure 4(b)). Area under the curve (AUC) comparison was based on the DeLong method to compare the AUC of the prediction model with the AUC of other clinical scoring systems. As shown in Figure 3(b) and Table 4, the AUC of the prediction model was greater than those using variables including SIRS (Z statistic, 4.521; p < .0001), BISAP (Z statistic, 6.249; p < .0001), MMF (Z statistic, 4.240; p < .0001), and SOFA (Z statistic, 3.153; p = .0016). Collectively, these results clearly indicated good prediction capability for the ARDS risk nomogram model among patients with SAP.
Figure 4.
(a) Developed ARDS prediction nomogram in patients with SAP. (b) Calibration curves of the ARDS nomogram prediction in the SAP trial.
ARDS, acute respiratory distress syndrome; FiO2, fraction of inspired oxygen; PaO2, partial pressure of oxygen; SAP, severe acute pancreatitis.
Table 4.
Predictive performance of different models.
| Variable | AUC (95% CI) | p value | Sensitivity | Specificity |
|---|---|---|---|---|
| SIRS | 0.623 (0.552–0.694) | .0012 | 0.807 | 0.360 |
| BISAP | 0.556 (0.483–0.630) | .1371 | 0.248 | 0.840 |
| MMF | 0.699 (0.632–0.767) | <.0001 | 0.817 | 0.742 |
| SOFA | 0.702 (0.639–0.760) | <.0001 | 0.642 | 0.680 |
| Nomogram | 0.814 (0.758–0.861) | <.0001 | 0.817 | 0.688 |
AUC, area under the curve; BISAP, bedside index of severity in acute pancreatitis; MMF, modified Marshall scoring system; SIRS, systemic inflammatory response syndrome score; SOFA, Sequential Organ Failure Assessment score.
A p value < .05 was considered statistically significant.
Discussion
In the current study, we observed a significantly higher mortality in SAP patients with ARDS. In addition, lower PaO2:FiO2 ratio, platelet counts, higher lactate dehydrogenase, creatinine, and procalcitonin on admission had been found to be correlated with higher risk of developing ARDS in patients with SAP. Finally, the predictive model based on above crucial variables had high sensitivity and specificity to identify high-risk population for developing ARDS in patients with SAP, which would help take early interventions to prevent ARDS progression in SAP and improve clinical outcomes.
In our study, the occurrence of ARDS in SAP was 46.58%, which was between the 15% and 60% reported in prior studies.19–22 In pneumonia-caused ARDS, increasing age and comorbidities were associated with high risk of developing ARDS. Surprisingly, there were no statistically significant differences in age and comorbidities among SAP patients with or without ARDS in the present study, which was consistent with previous studies among patients with SAP23,24 and AP,16,25 indicating that age and comorbidities is not as much of a risk factor in SAP-related ARDS as in pneumonia-initiated ARDS. Although hypertriglyceridemia-induced AP has been reported to be associated with risk of lung failure compared with other etiologies,16,26 the etiologies of SAP have no significant differences between the groups with or without ARDS. Further studies on underlying mechanism would be warranted to investigate the associations between SAP etiologies and ARDS.
In addition, our findings demonstrated the 60-day mortality rate of 37.62% and 6.40% among SAP patients with or without ARDS, respectively, confirming the critical role of ARDS in SAP prognosis. In prior studies with ARDS, the mortality rate of ARDS commonly ranged from 36% to 50%,27–29 and the mortality rate of SAP-initiated ARDS in the study fell within this range. Although ARDS has been well known as a leading cause of mortality in SAP, identifying early risk factors of ARDS in SAP is challenging. Previous studies proposed the utility of lung ultrasonography and cytokines in predicting ARDS among patients with SAP; however, individual indicator had difficulty in reflecting whole situation of the body. Besides, lung ultrasonography has not been widely used in most hospitals, particularly in primary healthcare centers. To the best of our knowledge, very few studies focused on multiple indicators using prediction score and/or a nomogram model to predict ARDS development among patients with SAP.
Herein, we developed an effective nomogram model based on several clinical indicators to predict the development of ARDS in SAP patients at the early stage of the disease. These five indicators included in our model, respiratory indexes (PaO2:FiO2), blood routine (platelet counts), blood biochemical (creatinine and LDH), and inflammatory markers (procalcitonin), were clinically meaningful and could reflect pathophysiology of SAP-initiated ARDS from several aspects. The lower PaO2:FiO2 is an indicator for hypoxemic situation, the decrease of PaO2:FiO2 may indicate a forewarning stage of ARDS development initiated by SAP.8,30 In our study, the decreased platelets were found to be an independent risk of SAP-related ARDS, suggesting that platelet activation and consumption may be involved in pathogenesis of this process.31,32 Consistently, previous studies have found lower count of platelets was not only associated with increased risk for ARDS but also with poor prognosis and hemorrhage in AP patients with ARDS.13,33–35 Increased LDH and serum creatinine levels are common indicators of multiple organ injury,14,16,36 and increased procalcitonin is a marker of systematic bacterial infection, which suggest the development of multiple organ dysfunction including ARDS in SAP. 37 Combination of procalcitonin and LDH was applied in a diagnostic model for the disease severity of AP as well. 38 Furthermore, LDH, creatinine, and procalcitonin were reported to be independent factors associated with the severity of SAP, which had more likelihood of inducing the comorbidity of ARDS.14,16,39
Collectively, our findings demonstrate that combining above clinical variables including PaO2:FiO2, platelet count, LDH, creatinine, and procalcitonin levels have a better predictive effect in predict ARDS among SAP patients than BISAP, MMF, SIRS, and SOFA scores, which have been widely used to predict mortality and organ failure in patients with SAP,40–42 thus providing a more simple, quantitative and practical predicting tool for ARDS development in SAP patients. Furthermore, a visualized nomogram would help clinician had more ability to early identify subpopulation with high ARDS risk among individual patients with SAP.
Strengths and limitations
Given the strength that we develop a model of SAP-initiated ARDS prediction that can help to take early interventions to prevent ARDS progression in SAP and improve clinical outcomes, some limitations should not be ignored. First, it was a retrospective, single-center study; as such, it may have been susceptible to the inherent limitations of retrospective analyses. Second, even though our model was proved useful for decision-making to early predict ARDS in patients with SAP, scoring tools should not replace clinical judgment. Third, the sample size of the study was relatively small, thus a larger population-based cohort study would be warranted to verify our findings in the future.
Conclusion
Lower PaO2:FiO2 ratio and platelet counts, higher LDH, creatinine, and procalcitonin levels were identified as independent risk factors for the development of ARDS in patients with SAP, and an easy-to-use nomogram was developed to predict SAP-induced ARDS. If validated, our findings could aid in establishing the appropriate level of care and guiding anticipatory management based on the prediction of ARDS.
Acknowledgments
We thank Editage (www.editage.cn) for English language editing.
Footnotes
ORCID iD: Pinhua Pan  https://orcid.org/0000-0001-5883-0531
https://orcid.org/0000-0001-5883-0531
Contributor Information
Fengyu Lin, Center of Respiratory Medicine, Xiangya Hospital, Central South University, Changsha, Hunan, China; Hunan Engineering Research Center for Intelligent Diagnosis and Treatment of Respiratory Disease, Changsha, China; National Key Clinical Specialty, Branch of National Clinical Research Center for Respiratory Disease, Xiangya Hospital, Central South University, Changsha, China; National Clinical Research Center for Geriatric Disorders, Xiangya Hospital, Changsha, China.
Rongli Lu, Center of Respiratory Medicine, Xiangya Hospital, Central South University, Changsha, Hunan, China; Hunan Engineering Research Center for Intelligent Diagnosis and Treatment of Respiratory Disease, Changsha, China; National Key Clinical Specialty, Branch of National Clinical Research Center for Respiratory Disease, Xiangya Hospital, Central South University, Changsha, China; National Clinical Research Center for Geriatric Disorders, Xiangya Hospital, Changsha, China.
Duoduo Han, Center of Respiratory Medicine, Xiangya Hospital, Central South University, Changsha, Hunan, China; Hunan Engineering Research Center for Intelligent Diagnosis and Treatment of Respiratory Disease, Changsha, China; National Key Clinical Specialty, Branch of National Clinical Research Center for Respiratory Disease, Xiangya Hospital, Central South University, Changsha, China; National Clinical Research Center for Geriatric Disorders, Xiangya Hospital, Changsha, China.
Yifei Fan, Department of Critical Care Medicine, Xijing Hospital, Air Force Military Medical University, 15th Changle West Rd, Xi’an 710032, Shaanxi, China.
Yan Zhang, Center of Respiratory Medicine, Xiangya Hospital, Central South University, Changsha 410008, Hunan, China; Hunan Engineering Research Center for Intelligent Diagnosis and Treatment of Respiratory Disease, Changsha, China; National Key Clinical Specialty, Branch of National Clinical Research Center for Respiratory Disease, Xiangya Hospital, Central South University, Changsha, China; National Clinical Research Center for Geriatric Disorders, Xiangya Hospital, Changsha, China.
Pinhua Pan, Center of Respiratory Medicine, Xiangya Hospital, Central South University, Changsha 410008, Hunan, China; Hunan Engineering Research Center for Intelligent Diagnosis and Treatment of Respiratory Disease, Changsha, China; National Key Clinical Specialty, Branch of National Clinical Research Center for Respiratory Disease, Xiangya Hospital, Central South University, Changsha, China; National Clinical Research Center for Geriatric Disorders, Xiangya Hospital, Changsha, China.
Declarations
Ethics approval and consent to participate: The study protocol was approved by the Ethics Commission of Xiangya Hospital of Central South University (No. 201912477), and requirements for written informed consent were waived because patient data and analysis were anonymized.
Consent for publication: None.
Author contributions: Fengyu Lin: Data curation; Formal analysis; Visualization; Writing – original draft.
Rongli Lu: Data curation; Visualization.
Duoduo Han: Data curation; Visualization.
Yifei Fan: Data curation; Visualization; Writing – review & editing.
Yan Zhang: Conceptualization; Methodology; Writing – review & editing.
Pinhua Pan: Conceptualization; Funding acquisition; Methodology; Resources; Supervision.
Funding: The authors disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This study was supported by National Key R&D Program of China (No. 2016YFC1304204); Key R & D Program of Hunan Province (No.2022SK2038); and the Project Program of National Clinical Research Center for Geriatric Disorders (Xiang ya Hospital, Grant No. 2020LNJJ05).
Competing interests: The authors received no financial support for the research, authorship, and/or publication of this article.
Availability of data and materials: Data are however available from the authors upon reasonable request and with permission of Xiangya Hospital of Central South University.
References
- 1. Mederos MA, Reber HA, Girgis MD. Acute pancreatitis: a review. JAMA 2021; 325: 382–390. [DOI] [PubMed] [Google Scholar]
- 2. Xiao AY, Tan ML, Wu LM, et al. Global incidence and mortality of pancreatic diseases: a systematic review, meta-analysis, and meta-regression of population-based cohort studies. Lancet Gastroenterol Hepatol 2016; 1: 45–55. [DOI] [PubMed] [Google Scholar]
- 3. Forsmark CE, Vege SS, Wilcox CM. Acute pancreatitis. N Engl J Med 2016; 375: 1972–1981. [DOI] [PubMed] [Google Scholar]
- 4. Schepers NJ, Bakker OJ, Besselink MG, et al. Impact of characteristics of organ failure and infected necrosis on mortality in necrotising pancreatitis. Gut 2019; 68: 1044–1051. [DOI] [PubMed] [Google Scholar]
- 5. Zhou J, Zhou P, Zhang Y, et al. Signal pathways and markers involved in acute lung injury induced by acute pancreatitis. Dis Markers 2021; 2021: 9947047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Schmandt M, Glowka TR, Kreyer S, et al. Secondary ARDS following acute pancreatitis: is extracorporeal membrane oxygenation feasible or futile? J Clin Med 2021; 10: 1000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Banks PA, Bollen TL, Dervenis C, et al. Classification of acute pancreatitis – 2012: revision of the Atlanta classification and definitions by international consensus. Gut 2013; 62: 102–111. [DOI] [PubMed] [Google Scholar]
- 8. Force ADT, Ranieri VM, Rubenfeld GD, et al. Acute respiratory distress syndrome: the Berlin Definition. JAMA 2012; 307: 2526–2533. [DOI] [PubMed] [Google Scholar]
- 9. Ziegler A, Schneider A, Pittman A, et al. Postoperative tachycardia in head and neck microvascular free flap patients. Otolaryngol Head Neck Surg 2019; 160: 1019–1022. [DOI] [PubMed] [Google Scholar]
- 10. Chen LD, Zhang ZY, Wei XJ, et al. Association between cytokine profiles and lung injury in COVID-19 pneumonia. Respir Res 2020; 21: 201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Cowley NJ, Owen A, Bion JF. Interpreting arterial blood gas results. BMJ 2013; 346: f16. [DOI] [PubMed] [Google Scholar]
- 12. Reed AM, Kolodecik T, Husain SZ, et al. Low pH enhances connexin32 degradation in the pancreatic acinar cell. Am J Physiol Gastrointest Liver Physiol 2014; 307: G24–G32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Wang T, Liu Z, Wang Z, et al. Thrombocytopenia is associated with acute respiratory distress syndrome mortality: an international study. PLos ONE 2014; 9: e94124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Poggiali E, Zaino D, Immovilli P, et al. Lactate dehydrogenase and C-reactive protein as predictors of respiratory failure in CoVID-19 patients. Clin Chim Acta 2020; 509: 135–138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Dai M, Fan Y, Pan P, et al. Blood urea nitrogen as a prognostic marker in severe acute pancreatitis. Dis Markers 2022; 2022: 7785497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Ding N, Guo C, Song K, et al. Nomogram for the prediction of in-hospital incidence of acute respiratory distress syndrome in patients with acute pancreatitis. Am J Med Sci 2022; 363: 322–332. [DOI] [PubMed] [Google Scholar]
- 17. Clement S, Braithwaite SS, Magee MF, et al. Management of diabetes and hyperglycemia in hospitals. Diabetes Care 2004; 27: 553–591. [DOI] [PubMed] [Google Scholar]
- 18. Chen T, Wu D, Chen H, et al. Clinical characteristics of 113 deceased patients with coronavirus disease 2019: retrospective study. BMJ 2020; 368: m1091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Zhou MT, Chen CS, Chen BC, et al. Acute lung injury and ARDS in acute pancreatitis: mechanisms and potential intervention. World J Gastroenterol 2010; 16: 2094–2099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Fei Y, Gao K, Li WQ. Prediction and evaluation of the severity of acute respiratory distress syndrome following severe acute pancreatitis using an artificial neural network algorithm model. HPB (Oxford) 2019; 21: 891–897. [DOI] [PubMed] [Google Scholar]
- 21. Samanta J, Singh S, Arora S, et al. Cytokine profile in prediction of acute lung injury in patients with acute pancreatitis. Pancreatology 2018; 18: 878–884. [DOI] [PubMed] [Google Scholar]
- 22. Lu XG, Kang X, Zhan LB, et al. Circulating miRNAs as biomarkers for severe acute pancreatitis associated with acute lung injury. World J Gastroenterol 2017; 23: 7440–7449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Zhang W, Zhang M, Kuang Z, et al. The risk factors for acute respiratory distress syndrome in patients with severe acute pancreatitis: a retrospective analysis. Medicine (Baltimore) 2021; 100: e23982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Fei Y, Gao K, Li WQ. Artificial neural network algorithm model as powerful tool to predict acute lung injury following to severe acute pancreatitis. Pancreatology 2018; 18: 892–899. [DOI] [PubMed] [Google Scholar]
- 25. Skouras C, Davis ZA, Sharkey J, et al. Lung ultrasonography as a direct measure of evolving respiratory dysfunction and disease severity in patients with acute pancreatitis. HPB (Oxford) 2016; 18: 159–169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Balint ER, Fur G, Kiss L, et al. Assessment of the course of acute pancreatitis in the light of aetiology: a systematic review and meta-analysis. Sci Rep 2020; 10: 17936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Sigurdsson MI, Sigvaldason K, Gunnarsson TS, et al. Acute respiratory distress syndrome: nationwide changes in incidence, treatment and mortality over 23 years. Acta Anaesthesiol Scand 2013; 57: 37–45. [DOI] [PubMed] [Google Scholar]
- 28. Villar J, Blanco J, Anon JM, et al. The ALIEN study: incidence and outcome of acute respiratory distress syndrome in the era of lung protective ventilation. Intensive Care Med 2011; 37: 1932–1941. [DOI] [PubMed] [Google Scholar]
- 29. Huang X, Zhang R, Fan G, et al. Incidence and outcomes of acute respiratory distress syndrome in intensive care units of mainland China: a multicentre prospective longitudinal study. Crit Care 2020; 24: 515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Brown SM, Grissom CK, Moss M, et al. Nonlinear imputation of Pao2/Fio2 from Spo2/Fio2 among patients with acute respiratory distress syndrome. Chest 2016; 150: 307–313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Thiery-Antier N, Binquet C, Vinault S, et al. Is thrombocytopenia an early prognostic marker in septic shock. Crit Care Med 2016; 44: 764–772. [DOI] [PubMed] [Google Scholar]
- 32. Menter DG, Kopetz S, Hawk E, et al. Platelet ‘first responders’ in wound response, cancer, and metastasis. Cancer Metastasis Rev 2017; 36: 199–213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Lopez-Delgado JC, Rovira A, Esteve F, et al. Thrombocytopenia as a mortality risk factor in acute respiratory failure in H1N1 influenza. Swiss Med Wkly 2013; 143: w13788. [DOI] [PubMed] [Google Scholar]
- 34. Deng H, Yu X, Gao K, et al. Dynamic nomogram for predicting thrombocytopenia in adults with acute pancreatitis. J Inflamm Res 2021; 14: 6657–6667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Lei JJ, Zhou L, Liu Q, et al. Can mean platelet volume play a role in evaluating the severity of acute pancreatitis? World J Gastroenterol 2017; 23: 2404–2413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. McFadden RG, Oliphant LD. Serum lactate dehydrogenase in interstitial lung disease. Chest 1991; 100: 1182. [DOI] [PubMed] [Google Scholar]
- 37. Mat-Nor MB, Md Ralib A, Abdulah NZ, et al. The diagnostic ability of procalcitonin and interleukin-6 to differentiate infectious from noninfectious systemic inflammatory response syndrome and to predict mortality. J Crit Care 2016; 33: 245–251. [DOI] [PubMed] [Google Scholar]
- 38. Tian F, Li H, Wang L, et al. The diagnostic value of serum C-reactive protein, procalcitonin, interleukin-6 and lactate dehydrogenase in patients with severe acute pancreatitis. Clin Chim Acta 2020; 510: 665–670. [DOI] [PubMed] [Google Scholar]
- 39. Zhang JJY, Lee KS, Ang LW, et al. Risk factors for severe disease and efficacy of treatment in patients infected with COVID-19: a systematic review, meta-analysis, and meta-regression analysis. Clin Infect Dis 2020; 71: 2199–2206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Singh VK, Wu BU, Bollen TL, et al. Early systemic inflammatory response syndrome is associated with severe acute pancreatitis. Clin Gastroenterol Hepatol 2009; 7: 1247–1251. [DOI] [PubMed] [Google Scholar]
- 41. Mofidi R, Duff MD, Wigmore SJ, et al. Association between early systemic inflammatory response, severity of multiorgan dysfunction and death in acute pancreatitis. Br J Surg 2006; 93: 738–744. [DOI] [PubMed] [Google Scholar]
- 42. Wu BU, Johannes RS, Sun X, et al. The early prediction of mortality in acute pancreatitis: a large population-based study. Gut 2008; 57: 1698–1703. [DOI] [PubMed] [Google Scholar]