Abstract
Background
Compared to the general population, adults with chronic obstructive pulmonary disease (COPD) have an increased prevalence of osteoporosis. Despite the known risk factors and potential complications of compromised bone health in COPD, little is known about whether poor bone health is routinely suspected. We measured, in people with COPD, the prevalence of those who had one or more indicators suggestive of suspected poor bone health, and compared the characteristics of those with versus without these indicators.
Methods
Data were collected from adults with COPD presenting to three tertiary hospitals. Indicators of suspected poor bone health were defined as any of the following criteria: (i) self-reported problems with bone health, (ii) previous imaging for bone health, (iii) history of fragility fracture or, (iv) advised to use medication/supplements to optimise bone health. Characteristics compared between those with versus without indicators of suspected poor bone health comprised age, sex, body mass index (BMI), FEV1% predicted and recruitment setting.
Results
361 participants were included (age 70 ± 10, BMI 27.9 ± 7.8 kg/m2, FEV1% predicted 49 ± 20; 161 [45%] female). Indicators suggestive of suspected poor bone health were present in 53% (95% confidence interval [CI] 47–58) of the participants. The odds of this outcome increased with advancing age (odds ratio; OR [95% CI] 1.05 [1.03 to 1.08]) and being female (OR [95% CI] 3.4 [2.2 to 5.7]) .
Conclusion
In people with COPD, the odds of having indicators suggestive of suspected poor bone health increase with advancing age and in females. Further work is required to promote the importance of bone health in this population.
Keywords: bone health, osteoporosis, treatable traits, COPD
Introduction
Adults with chronic obstructive pulmonary disease (COPD) have many extra pulmonary manifestations or ‘treatable traits’, including poor bone health. 1 Optimal management of treatable traits is now widely recognised as a priority for adults with COPD. In this population, poor bone health which is characterised by loss of bone mineral density (BMD), likely results from systemic inflammation, smoking, sedentary lifestyle and prolonged use of inhaled corticosteroids. 2 A loss of BMD on its own is asymptomatic and is often undetected until a fracture occurs. Although it is widely accepted that treatable traits should be optimised, no guidelines currently exist outlining how bone health should be managed in adults with COPD. Specifically, there are no recommendations regarding how and when bone health should be screened and what routine interventions be put in place to optimise BMD.
Low BMD is clinically important in adults with COPD as they have known balance deficits that increase falls risk. A fall coupled with a low BMD increases the likelihood of fracture. Consequences of a fracture in this population include loss of independence, impaired quality of life and higher rates of mortality.
Despite these consequences, it is unclear what proportion of adults with COPD have evidence that a low BMD has been suspected. The aims of this study in adults with COPD were to: determine the prevalence of those who had one or more indicators suggestive of suspected poor bone health; and compare the characteristics of those with versus without indicators suggestive of suspected poor bone health.
Methods
This is a cross-sectional exploratory study that is nested within a larger mixed-methods study. That is, data used in this study were collected prospectively as part of the OPTIMAL study 3 and oral and written consent was obtained from each participant. Briefly, between August 2020 and January 2021, adults with COPD were sequentially recruited if they were hospitalized for an exacerbation or attended an outpatient respiratory clinic at one of three participating hospitals in Australia. People were approached if they were independently ambulant, did not reside in a residential care facility and were able to understand English. Data were sought through medical records and participant interview, and included: age, sex, body mass index (BMI), forced expiratory volume in one second (FEV1 % predicted), past medical history, previous dual-energy X-ray absorptiometry scan (DXA), medication use, and recruitment setting (in-patient vs out-patient).
Participant data were examined to classify the sample into two groups: (i) those with indicators, and (ii) those without indicators suggestive of suspected poor bone health. These indicators were one or more of the following: (i) diagnosis of osteopenia/osteoporosis (either self-reported or stated in their medical record), (ii) previous DXA, (iii) history of a fragility fracture, or (iv) were advised to take medications/supplements known to enhance bone health.
Statistical analysis
Predictors of group membership were explored using logistic regression. Predictors included: age, sex, BMI, FEV1 % predicted and recruitment setting. As these analyses were hypothesis-generating, no a priori sample size calculation was undertaken.
Results
Of the 682 people approached, data were available for 361 (Table 1). The most common reasons data were not available were a failure to meet the inclusion criteria (n = 78) and/or unwillingness to participate (n = 240).
Table 1.
Characteristics of the participants (n = 361).
| Participant characteristics | |
|---|---|
| Age, yr | 70 ± 10 |
| Sex, F | 161 (45%) |
| BMI, kg/m2 | 27.9 ± 7.8 |
| FEV1, % predicted | 49 ± 20 |
| Recruited as an outpatient, n | 206 (57%) |
Data are presented as mean ± standard deviation or number (%).
Abbreviations: BMI (body mass index), FEV1 % predicted (forced expiratory volume in 1 second, expressed as a percentage of the predicted value).
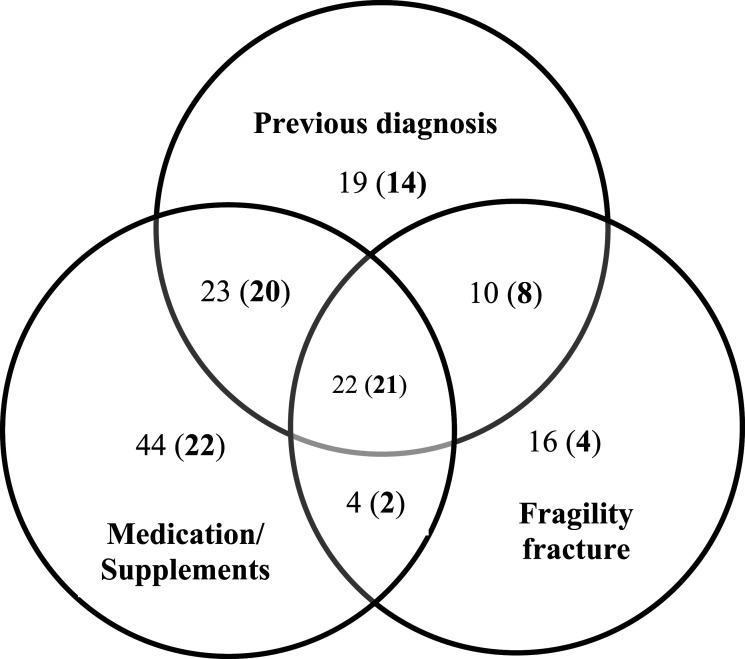
The prevalence of participants who had indicators of suspected poor bone health was 53% (95% confidence interval [CI] 47–58). Previous DXA scan was reported by 143 participants (40%). The overlap between indicators of poor bone health is shown in Figure 1. The characteristics of those with (n = 190) versus without (n = 171) indicators of suspected poor bone health are shown in Table 2. The odds of having indicators of suspected poor bone health increased with age (odds ratio; OR [95% CI] 1.05 [1.03 to 1.08]) and being female (OR [95% CI] 3.4 [2.2 to 5.7]) (Table 2).
Figure 1.
Interaction (overlap) between the indicators of poor bone health. Numbers outside brackets are: Total number of participants in each respective category. Numbers inside brackets are: Participants who reported having a dual-energy X-ray absorptiometry scan (DXA) within each respective category.
Table 2.
Characteristics of participants classified as having versus not having indicators of poor bone health.
| Indicators of suspected poor bone health (n = 190) | No indicators of suspected poor bone health (n = 171) | p value | OR | 95% CI | |
|---|---|---|---|---|---|
| Age, yr | 72 ± 9 | 67 ± 10 | <.01 | 1.05 | 1.03 to 1.08 |
| Sex, female | 111 (58%) | 50 (29%) | <.01 | 3.40 | 2.20 to 5.30 |
| BMI, kg/m2 | 28 ± 8 | 28 ± 8 | .472 | 0.99 | 0.96 to 1.02 |
| FEV1, % predicted | 49 ± 21 | 48 ± 19 | .570 | 1.00 | 0.99 to 1.02 |
| Outpatient, n | 105 (55%) | 101 (59%) | .466 | 0.86 | 0.56 to 1.30 |
Data are presented as mean ± standard deviation or number (%) unless otherwise stated.
Abbreviations: BMI (body mass index), FEV1 % predicted (forced expiratory volume in 1 second, expressed as a percentage of the predicted value), odds ratio (OR).
Discussion
This study found that in a sample of adults with COPD who presented to tertiary hospitals, just over half had indicators suggestive of suspected poor bone health. The odds of this outcome increased with age and being female. Of note, disease severity (FEV1 % predicted) did not increase the odds of having indicators of poor bone health.
In a recent meta-analysis that included adults with COPD across a range of settings, the pooled prevalence of osteoporosis was 38% (95% CI 34 – 43), 2 which is similar to the proportion of our sample reporting a previous DXA scan. However, in our sample, an additional 13% of participants had an indicator of suspected poor bone health, but had not undergone a previous DXA. This suggests that the use of a DXA to screen bone health may be underutilised in this population.
Compared to non-COPD populations, a diagnosis of COPD increases the odds of having osteoporosis (OR [95% CI] 2.83 [2.00 to 4.03]). 2 Interestingly, only 26% of our participants had been advised to take medications/supplements known to enhance bone health. The benefits of calcium and vitamin D for bone health have been extensively reported and often prescribed as a preventive measure in ‘at risk’ populations. In this sample, only 60% of participants with a diagnosis of osteoporosis and 50% of participants who reported a history of a fragility fracture stated they had been advised to take these medication/supplements. Given the low cost and negligible risk profile of vitamin D and calcium, our data suggest there is scope to increase their use by people with COPD.
In other ‘at risk’ groups, such as postmenopausal women and men with prostate cancer undergoing androgen suppression, 4 progressive resistance training and impact loading exercises have been used to preserve or enhance BMD. 4 Although people with COPD who participate in pulmonary rehabilitation programs (PRP) engage in exercise, the effect of PRP on BMD is unknown. It is likely commonly prescribed exercises in PRP (i.e. aerobic and functional resistance) elicit insufficient ground reaction forces to stimulate bone remodeling, thus influence bone health. As international societies emphasise the importance of tailoring PRP to address an individual’s ‘treatable traits’, further investigation is needed to determine the feasibility, tolerance and effects on BMD of impact loading exercises in adults with COPD.
This study is novel in that it highlighted there appears to be no consistent approach to how bone health is screened and managed in a large sample of adults with COPD interfacing with a tertiary hospital. It does not appear DXA scans are routinely ordered to assess bone health once an adult has been diagnosed with COPD, or preventative measures put in place such as the use of over the counter supplements including Vitamin D and Calcium. Limitations of this study are that much of the data collected were self-reported by the patient, and results of the DXA scan, if done, were not recorded. Future studies looking at current practices for managing bone health in adults with COPD should also gather information from the clinicians treating these patients.
In summary, our study demonstrated that approximately half of adults with COPD who presented to tertiary hospitals had indicators suggestive of suspected poor bone health. Consistent with the general population, the odds were higher with advancing age and in females. An increased use of standardised screening tools, such as the Fracture Risk Assessment Tool (FRAX) could be useful to ensure bone health is considered in the overall management of adults with COPD. 5 Despite the known risk factors for loss of bone density, and outcomes linked to fractures in this population, to our knowledge there are no consistent tools used to monitor fracture risk or bone health. Combined with clinical guidelines recommending treatable traits of COPD to be optimized, 1 further work is required to promote early detection and prevention of BMD loss in this population.
Acknowledgements
The authors would like to thank the physiotherapists involved in the OPTIMAL study who collected the data used in this study.
Footnotes
Declaration of conflicting interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The OPTIMAL study is funded by a Department of Health Research Translation Project grant (Round-13). EC PhD program is supported by the Amgen Clinical Grants Program, administered by the OA-ANZBMS Research Fund. A-MH is supported by a National Health and Medical Research Council (Australia) Career Development Award.
Ethics: Data for this study was collected as part of the OPTIMAL study, with ethics approved by the South Metropolitan Health Service Human Research Ethics Committee (RGS0000003704) and reciprocal approval from Royal Perth Hospital, Sir Charles Gairdner Hospital and Curtin University (HRE2020–0095).
ORCID iDs
Erin Cecins https://orcid.org/0000-0002-2807-7822
Vinicius Cavalheri https://orcid.org/0000-0001-8620-7499
References
- 1.Agusti A, Bel E, Thomas M, et al. Treatable traits: toward precision medicine of chronic airway diseases. Eur Respir J. 2016; 47(2):410–419. [DOI] [PubMed] [Google Scholar]
- 2.Chen Y-W, Ramsook AH, Coxson HO, et al. Prevalence and risk factors for osteoporosis in individuals with COPD: a systematic review and meta-analysis. Chest. 2019; 156(6):1092–1110. [DOI] [PubMed] [Google Scholar]
- 3.Hug S, Cavalheri V, Gucciardi DF, et al. OPTImising the implementation of pulMonary rehAbiLitation in people with chronic obstructive pulmonary disease (the OPTIMAL study): mixed methods study protocol. BMC Pulm Med. 2020; 20(1):286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Newton RU, Galvão DA, Spry N, et al. Exercise mode specificity for preserving spine and hip bone mineral density in prostate cancer patients. Med Sci Sports Exerc. 2019; 51(4):607–614. [DOI] [PubMed] [Google Scholar]
- 5.Akyea RK, McKeever TM, Gibson J, et al. Predicting fracture risk in patients with chronic obstructive pulmonary disease: a UK-based population-based cohort study. BMJ Open. 2019;9(4), e024951. [DOI] [PMC free article] [PubMed] [Google Scholar]