Abstract
Background
Glioblastoma (GBM) is the first most frequent type of primary malignant brain tumors in adults. It is basically confined to the brain, and extracranial metastases (ECM) are rare. The genomic features of GBM with ECM are not fully elucidated.
Case Description
Here, we present a case of a male patient with headache and left eye vision loss for 2 months who had a left occipital lobe tumor. GBM of grade IV [isocitrate dehydrogenase 1 (IDH-1) wild type] was diagnosed based on the histological profiles of intracranial tumor according to the World Health Organization standard. ECM of GBM located in the mediastinal lymph node occurred 6 months after resection of the intracranial tumor. High throughput gene sequencing was performed using ECM lesions. Mutated genes included tumor protein 53 (TP53), CUB and Sushi multiple domains 3 (CSMD3), poly(ADP-ribose) polymerase family member 4 (PARP4), and PTEN. The patient underwent surgery, radiotherapy, chemotherapy, and anti-angiogenic drug treatment. Unfortunately, the patient died 8 months after surgery.
Conclusions
ECM of GBM is rare, and its prognosis is very poor. Mutated genes in ECM included TP53, CSMD3, PARP4, and PTEN in our case. Genomic analysis provides important insights into GBM and its ECM.
Keywords: Glioblastoma (GBM), gene sequencing, extracranial metastases (ECM), case report
Introduction
Glioblastoma (GBM) is the most common primary malignant brain tumors in adults. It accounts for more than 60% of primary brain tumors (1). Its median overall survival (OS) is generally 13 months (2). Surgery is the main treatment, followed by radiation therapy and chemotherapy with temozolomide (TMZ). However, patients are still prone to recurrence of intracranial lesions (3,4).
Extracranial metastasis (ECM) is a rare phenomenon of GBM with an estimated incidence of less than 2% (5). The prognosis of patients with ECM is poor. The median time from ECM to death is 1.5 months (6). Great progress has recently been made in the genomic analysis of GBM tumorigenesis and recurrence. However, after a systematic search on PubMed and Google Scholar, genomic features of GBM with ECM was found in 5 reports (Table 1) (7-11).
Table 1. Reported cases of gene variants of glioblastoma with extracranial metastasis.
| First author and year of publication | Number of cases | Location of the primary tumor | Location of extracranial metastasis | Sample type | Genes |
|---|---|---|---|---|---|
| Tamai, 2019 | 1 | Right temporal lobe | Cervical spine, left lung | 1. Primary glioblastoma | 1. TERT |
| 2. Cervical spinal metastases | 2. TERT | ||||
| 3. Lung metastases | 3. TERT | ||||
| Anderson, 2020 | 1 | Left frontal lobe | Pelvic bones | 1. Intracranial tumors | 1./2. TP53; NF1; RB1; CDKN2A |
| 2. Right iliac bone | |||||
| Mohme, 2020 | 1 | Right temporal lobe | Vertebral bodies | 1. Primary glioblastoma | 1./2./3. Ch7; Ch5; Ch9; TPDF52L3; HIVEP2 |
| 2. Recurrence glioblastoma | 3. PIK3CA; NF1; TP53; CDKN2A/B | ||||
| 3. Vertebral metastasis | |||||
| Noch, 2021 | 10 | Frontal lobe (3 cases), temporal lobe (3 cases), parietal lobe (1 case), frontoparietal lobe (2 cases), occipital lobe (1 case) | Bone (6 cases), lymph nodes (2 cases), lung (2 cases), leptomeninges/dura (3 cases), liver (1 case) | 1. Intracranial tumor | 1. TP53; RB1; PTEN; TERT; ATRX; NF1 |
| 2. Extracranial metastases | 2. TP53; RB1; PTEN; TERT; EGFR; MET; CDKN2A | ||||
| Rong, 2021 | 1 | Left temporal lobe | Vertebral and thoracolumbar spine, pelvic, and left femur | 1. Cervical spine metastases | 1. TP53; MPL |
| 2. Thoracolumbar metastases | 2. ANTXR1; TLR8 |
There are no reports on how to treat GBM patients with ECM. In recent years, immunotherapy and targeted therapy have developed rapidly in the treatment of solid tumors (12). For example, targeted medicine aiming at tumor protein 53 (TP53) mutation, AZD1775, received much attention in the treatment of recurrent GBM (13). However, better treatments are expected for GBM.
We present a GBM patient with ECM that was diagnosed by collecting a mediastinal lymph node sample via mediastinoscopy. High-throughput gene sequencing was performed using the mediastinal lymph node. The result provides useful information about gene mutations in GBM genesis and metastasis. We present the following case in accordance with the CARE reporting checklist (available at https://tcr.amegroups.com/article/view/10.21037/tcr-22-955/rc).
Case presentation
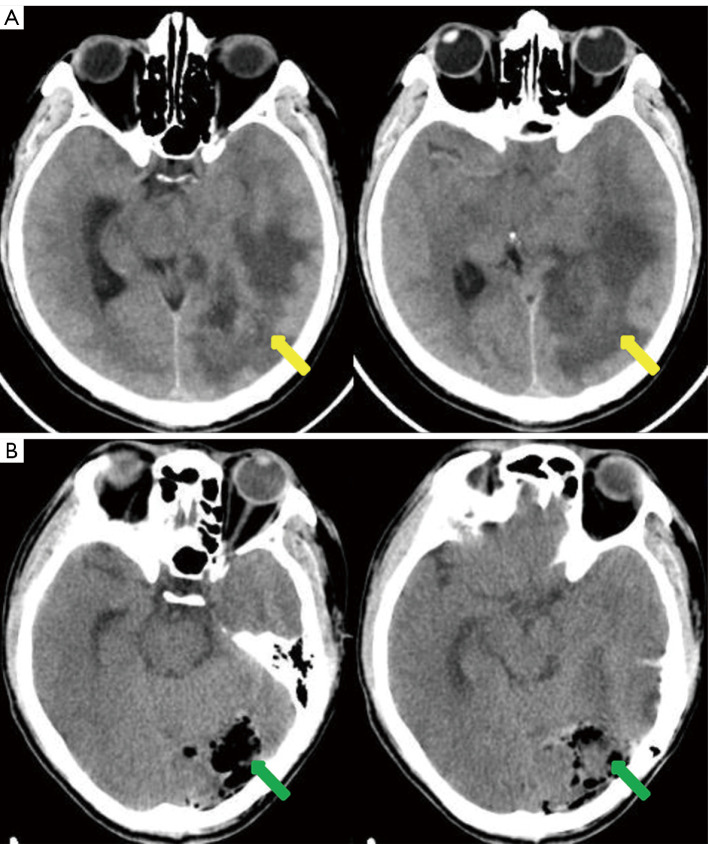
A previously healthy 43-year-old male patient was admitted to our hospital because of headache and left eye vision loss for 2 months. Two days after admission, cranial computed tomography (CT) revealed a left occipital lobe tumor (Figure 1A). Four days after admission, the patient underwent intracranial tumor resection (Figure 1B). The chest CT did not show obvious lesions (Figure 2). The abovementioned symptoms disappeared after the operation. The Karnofsky performance score increased to 90 points after surgery. Histological analysis revealed GBM of grade IV according to the World Health Organization (WHO) standard [isocitrate dehydrogenase 1 (IDH-1) wild type] (Figure 3).
Figure 1.
Cranial CT images before and after surgery. (A) Preoperative cranial CT showing a large tumor in left occipital lobe (yellow arrow). Peritumoral edema was obvious. (B) Cranial CT on the first day after surgery showing successful tumor resection (green arrow). CT, computed tomography.
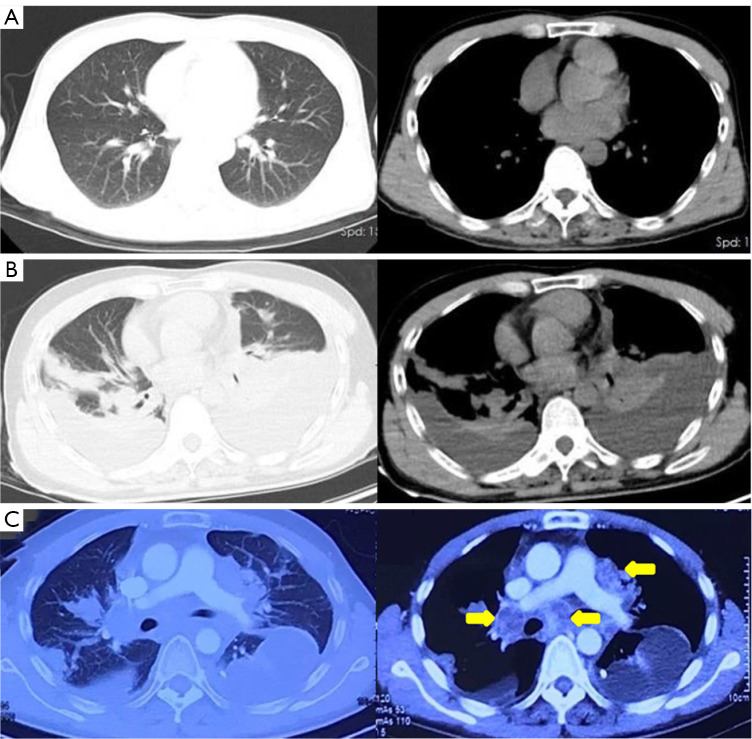
Figure 2.
Serial chest CT images during the treatment. (A) No obvious abnormality on chest CT images before intracranial surgery. (B) Extracranial metastasis with bilateral pleural effusion and mediastinal lymph node enlargement 6 months after intracranial surgery. (C) Enhanced chest CT images 7 months after intracranial surgery showing mediastinal mass significantly larger than before (yellow arrow). CT, computed tomography.
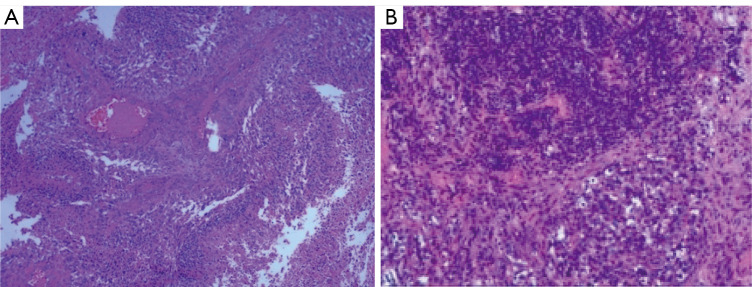
Figure 3.
Pathology of glioblastoma. (A) Brain tissue: GFAP (+), olig-2 (+), P53 (+), NeuN (−), IDH-1 (−), CD34 (−), ATRX (+), and Ki-67 (40%). Tumor cells grew intensively and diffusely, blood vessels proliferated obviously in the stromal. A large area of necrosis was found focally, and the tumor cells arranged in palisade shape around the necrosis (B) mediastinal lymph nodes: GFAP (+), S-10 (+), P53 (+), Syn (+), Ki-67 (30%), CK (−), CD34 (−), P63 (−). Nest-like heterotypic cells distributed in the proliferative lymphoid tissue. Hematoxylin-eosin staining; A, ×10, B, ×200.
One week after operation, the patient began to receive adjuvant chemotherapy with TMZ (150 mg daily for 5 days per cycle of 28 days for 5 cycles). After the first cycle of chemotherapy, whole brain radiotherapy (54 Gy in 6 weeks using linear accelerator) was conducted with concomitant TMZ chemotherapy. The treatment went well. The patient had only mild gastrointestinal reactions.
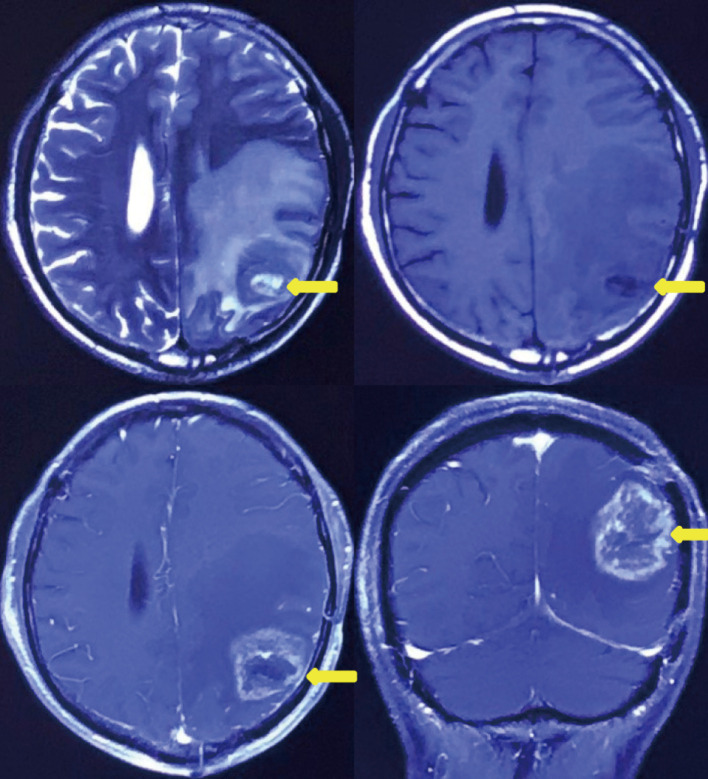
Six months after surgery, cranial magnetic resonance imaging revealed local recurrence in the surgical field and the left thalamus for the first time (Figure 4). However, the patient had no obvious symptoms. Then, the patient received a sixth cycle of chemotherapy with TMZ. One week after chemotherapy, the patient gradually developed cough and dyspnea. Seven months after surgery, chest CT revealed bilateral pleural effusions, pericardial effusion, and a mediastinal mass (Figure 2B). Thoracentesis was performed for bilateral pleural effusions. The analysis of the pleural effusions confirmed that they were transudates. The cytological examination of the pleural effusion did not reveal tumor cells. Half a month after drainage, the patient underwent chest enhancement CT scan, which showed that the mediastinal mass was significantly larger than before (Figure 2C).
Figure 4.
Cranial magnetic resonance imaging showing recurrence of glioblastoma. Recurrence of tumor in left occipitotemporal lobe 6 months after intracranial surgery (yellow arrow).
Mediastinoscopy was carried out to obtain lymph nodes for histological examination. Histological analysis revealed metastatic GBM. The patient did not take any corticosteroid or immunosuppressor at the time when the ECM was diagnosed. We performed high-throughput gene sequencing using the mediastinal lymph nodes metastases that examined 528 genes closely related to tumors [lllumina Solexa Genome Analyzer platform, GBM related genes included IDH-1/2, epidermal growth factor receptor (EGFR), platelet-derived growth factor receptor, TP53, cyclin-dependent kinase inhibitor 2 A/B, phosphatase and tensin homolog (PTEN), telomerase reverse transcriptase, phosphatidylinositol-4,5-bisphosphate 3-kinase A, murine double minute 2, neurofibromatosis type 1, glioma-associated oncogene homolog 1. Tested genes did not include O6-methylguanine-DNA methyltransferase (MGMT), chr: 1P, chr: 19Q, chr: 7, chr: 10]. Mutated genes included TP53, CUB and Sushi multiple domains 3 (CSMD3), poly(ADP-ribose) polymerase family member 4 (PARP4), and PTEN (Table 2). The TP53 gene had the highest mutation peak. The tumor mutation burden (TMB) of ECM lesions was calculated to be 8.667 Muts/Mb, and no microsatellite instability was detected. The patient received a course of treatment with bevacizumab (200 mg, d 1) plus TMZ (100 mg, d 1–14). Although the pleural effusion decreased, the patient’s impaired respiratory status continued to worsen, and he ultimately died of respiratory failure 8 months after surgery. The timeline of the patient’s treatment was shown in Figure 5.
Table 2. The likely pathogenic variants identified in mediastinal lymph nodes metastases.
| Genes | Variants | Abundance | Clinical significance |
|---|---|---|---|
| TP53 | Exon 5 c.451C>T (p.P151S) | 61.32% | Missense mutation (putative driver) |
| CSMD3 | Exon 69 c.10574T>C (p.V3525A) | 3.95% | Missense mutation (unknown significance) |
| PARP4 | Exon 29 c.3509C>T (p.T1170I) | 8.04% | Missense mutation (unknown significance) |
| PTEN | Exon 6 c.611C>T (p.P204L) | 50.83% | Missense mutation (putative driver) |
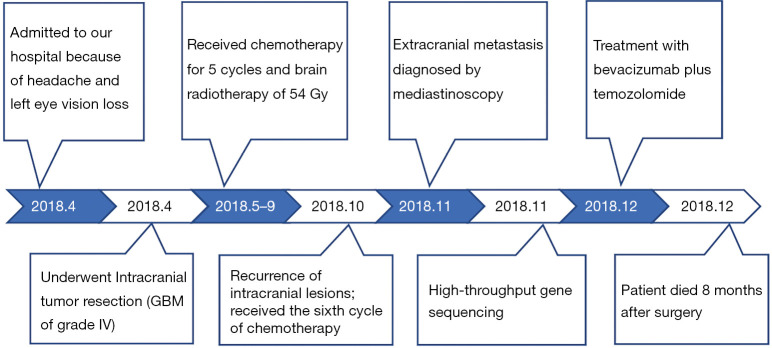
Figure 5.
Timeline of the patient’s treatment. GBM, glioblastoma.
All procedures performed in this study were in accordance with the ethical standards of the institutional and/or national research committee(s) and with the Helsinki Declaration (as revised in 2013). Written informed consent was obtained from the patient’s wife for publication of this case report and accompanying images. A copy of the written consent is available for review by the editorial office of this journal.
Discussion
Relatively little is known about the occurrence of ECM of GBM and the underlying pathogenesis due to its rarity. Epidemiologically, young patients are prone to ECM (1,14,15), possibly because they have a longer OS than elderly patients (16). A meta-analysis of GBM patients with ECM including 150 cases showed that the median time from initial diagnosis to the diagnosis of ECM was 9.0 months. The most common metastatic organs are bones, lymph nodes and lungs (1).
In this case, mediastinal lymph node metastasis was confirmed by pathology 6 months after surgery. Improving the understanding of the ECM of GBM is helpful for preventing misdiagnosis and mistreatment. This patient’s prognosis was poor. Progression-free survival (PFS) and OS were only 6 and 9 months, respectively. This may be related to the IDH-1 wild type status. Patients without IDH mutations had a shorter median PFS time (1.4 vs. 4.7 years) and a lower 5-year survival rate (14% vs. 42%) than those with IDH-1 mutations. IDH mutation status may be a strong independent predictor of PFS (17) .
The discoveries of various gene mutations greatly promote the understanding of glioma genesis and the practice of neuro-oncology. Except IDH-1/2 mutation, MGMT promoter methylation, 1p19q codeletion, TP53 mutation, PTEN mutation, etc. have offered new insights on prognosis and management of GBM, including predicted response to chemotherapy and radiation treatment (18,19). Compared with GBM researches, gene data of GBM with ECM are limit. Similar to previous studies (8-11), TP53 and PTEN mutations in ECM lesion were identified in the patient. Despite the role of genetic mutations in GBM with ECM is still not fully clear, ongoing research on this topic will ultimately implement a personalized or precision medicine for this deadly disease.
The pathways of ECM from GBM, although not fully understood, involved surgical intervention, cerebrospinal fluid pathway, blood vessels, lymphatic vessels. Scalp tumor masses/cutaneous tumor tissue, spinal cord metastasis was possibly associated with surgical intervention (stereotactic biopsy or craniotomy), cerebrospinal fluid pathway, respectively (20,21). In addition, it was demonstrated that approximately 20% of GBM patients have circulating tumor cells in their peripheral blood (22). Hematogenous dissemination through neovascularization of tumors is considered to be involved in ECM. Recent rediscoveries of lymphatic vessels within the dura mater surrounding the brain make ECM possible via lymphatic vessels (23,24). In our case, without evidence of other ECM sites, the mediastinal lymph nodes metastases and history of craniotomy implied the pathway of surgical intervention and lymphatic vessels involved in the ECM.
In the past decade, the treatment of GBM has progressed very little. The standard of treatment for GBM patients remains surgery for gross total resection followed by the Stupp protocol, namely radiotherapy and concomitant chemotherapy with TMZ, an alkylating agent (25). However, once GBM relapses, there are limited options for treatment (26). Lomustine, an alkylating agent like TMZ, is commonly given with procarbazine and vincristine as part of the PCV regimen (procarbazine, lomustine, and vincristine) and reserved for treatment of patients with recurrent GBM, especially for patients with primary MGMT methylated GBM (27). Another treatment option for recurrent GBM is the angiogenesis inhibitor bevacizumab (28). This patient used bevacizumab after ECM. However, it had no significant effect on survival. Other chemotherapeutic agents, such as irinotecan (29), carboplatin/cisplatin (30), when given as monotherapy or in combination with other agents, particularly TMZ and bevacizumab, have yielded promising results in preliminary studies of recurrent GBM.
The other treatment for GBM is the alternating electric field (AEF) generator NovoTTF/Optune (31). Recently, the combination of AEFs and maintenance TMZ chemotherapy demonstrated improved progression-free and OS in randomized prospective phase III trials (32,33). However, it is mainly for the treatment of intracranial lesions.
The preferred treatment of patients with ECM is undocumented. According to the treatment principles of other malignant tumors, systemic drug therapy may be more important. In recent years, molecular targeted therapy and immunotherapy have been successful in other solid cancers. Some new treatments have been explored in GBM.
Targeted therapy is generally targeted at specific gene mutations in the body. Previous study reported biologically relevant alterations in three common core pathways in GBM, namely, TP53, Rb and receptor tyrosine kinase (RTK)/Ras/phosphoinositide 3-kinase (PI3K) signaling (34). This patient had a TP53 mutation with a high mutation peak. TP53 mutation-related drugs include AZD1775, MLN8237, ALT-801 and P53-SLP vaccines, which have antitumor effects in clinical trials (35,36). Among them, researches on AZD1775 are relatively more common. AZD1775 can produce a wide range of cytotoxic effects in vitro and can eliminate brain tumors in mice. In addition, AZD1775 has been shown to have good penetrability in recurrent GBM (13). Therefore, whether AZD1775 combined with chemotherapy or radiotherapy is a new method for the treatment of TP53 mutant GBM deserves further research.
In addition, the patient had three coexisting gene mutations of CSMD3, PARP4 and PTEN, but their clinical significance was not clear. Other potential therapies include EGFR-tyrosine kinase inhibitor (TKI), vaccines, chimeric antigen receptor (CAR) T-cell therapies, virotherapy and immune checkpoint inhibitors (CPIs) (37). However, they are still at the research stage.
Immune CPIs, including anti-T-lymphocyte-associated protein 4 (CTLA-4), anti-programmed cell death-1 (PD-1), and anti-programmed cell death-ligand 1 (PD-L1) antibodies, are promising immunotherapy approaches for GBM. However, the superior efficacy of CPIs over the standard of care was not shown in recent clinical trials (CheckMate 143, NCT02017717; CheckMate 498, NCT02617589). Possible mechanisms of resistance to CPIs include intertumoral/intratumoral heterogeneity, low TMB, systemic and local immunosuppression (38). Cases of response to anti PD-1 therapy in GBM patients with a high TMB were reported (39,40). The patient in our case had as high TMB as 8.667 Muts/Mb, who might benefit from CPIs. Unfortunately, CPIs were not available in our hospital during his treatments.
ECM of GBM is rare, and its prognosis is very poor. Mutated genes in ECM included TP53, CSMD3, PARP4, and PTEN in our case. Genomic analysis provides important insights into GBM and its ECM. We look forward to new treatments to improve the survival of GBM patients with ECM.
Supplementary
The article’s supplementary files as
Acknowledgments
The authors acknowledge Dr. Rui Tang for the help during preparation of the manuscript.
Funding: None.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. All procedures performed in this study were in accordance with the ethical standards of the institutional and/or national research committee(s) and with the Helsinki Declaration (as revised in 2013). Written informed consent was obtained from the patient’s wife for publication of this case report and accompanying images. A copy of the written consent is available for review by the editorial office of this journal.
Footnotes
Reporting Checklist: The authors have completed the CARE reporting checklist. Available at https://tcr.amegroups.com/article/view/10.21037/tcr-22-955/rc
Conflicts of Interest: Both authors have completed the ICMJE uniform disclosure form (available at https://tcr.amegroups.com/article/view/10.21037/tcr-22-955/coif). The authors have no conflicts of interest to declare.
References
- 1.Hanif F, Muzaffar K, Perveen K, et al. Glioblastoma Multiforme: A Review of its Epidemiology and Pathogenesis through Clinical Presentation and Treatment Asian Pac J Cancer Prev 2017;18:3-9. [DOI] [PMC free article] [PubMed]
- 2.Pietschmann S, von Bueren AO, Kerber MJ, et al. An individual patient data meta-analysis on characteristics, treatments and outcomes of glioblastoma/ gliosarcoma patients with metastases outside of the central nervous system. PLoS One 2015;10:e0121592. 10.1371/journal.pone.0121592 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Tykocki T, Eltayeb M. Ten-year survival in glioblastoma. A systematic review. J Clin Neurosci 2018;54:7-13. 10.1016/j.jocn.2018.05.002 [DOI] [PubMed] [Google Scholar]
- 4.Burri SH, Gondi V, Brown PD, et al. The Evolving Role of Tumor Treating Fields in Managing Glioblastoma: Guide for Oncologists. Am J Clin Oncol 2018;41:191-6. 10.1097/COC.0000000000000395 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Amitendu S, Mak SK, Ling JM, et al. A single institution experience of the incidence of extracranial metastasis in glioma. J Clin Neurosci 2012;19:1511-5. 10.1016/j.jocn.2011.08.040 [DOI] [PubMed] [Google Scholar]
- 6.Lun M, Lok E, Gautam S, et al. The natural history of extracranial metastasis from glioblastoma multiforme. J Neurooncol 2011;105:261-73. 10.1007/s11060-011-0575-8 [DOI] [PubMed] [Google Scholar]
- 7.Tamai S, Kinoshita M, Sabit H, et al. Case of metastatic glioblastoma with primitive neuronal component to the lung. Neuropathology 2019;39:218-23. 10.1111/neup.12553 [DOI] [PubMed] [Google Scholar]
- 8.Anderson KJ, Tan AC, Parkinson J, et al. Molecular and clonal evolution in recurrent metastatic gliosarcoma. Cold Spring Harb Mol Case Stud 2020;6:a004671. 10.1101/mcs.a004671 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Noch EK, Sait SF, Farooq S, et al. A case series of extraneural metastatic glioblastoma at Memorial Sloan Kettering Cancer Center. Neurooncol Pract 2021;8:325-36. 10.1093/nop/npaa083 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Rong T, Zou W, Qiu X, et al. A Rare Manifestation of a Presumed Non-Osteophilic Brain Neoplasm: Extensive Axial Skeletal Metastases From Glioblastoma With Primitive Neuronal Components. Front Oncol 2021;11:760697. 10.3389/fonc.2021.760697 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mohme M, Maire CL, Schliffke S, et al. Molecular profiling of an osseous metastasis in glioblastoma during checkpoint inhibition: potential mechanisms of immune escape. Acta Neuropathol Commun 2020;8:28. 10.1186/s40478-020-00906-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Atkins MB, Larkin J. Immunotherapy Combined or Sequenced With Targeted Therapy in the Treatment of Solid Tumors: Current Perspectives. J Natl Cancer Inst 2016;108:djv414. 10.1093/jnci/djv414 [DOI] [PubMed] [Google Scholar]
- 13.Sanai N, Li J, Boerner J, et al. Phase 0 Trial of AZD1775 in First-Recurrence Glioblastoma Patients. Clin Cancer Res 2018;24:3820-8. 10.1158/1078-0432.CCR-17-3348 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Blume C, von Lehe M, van Landeghem F, et al. Extracranial glioblastoma with synchronous metastases in the lung, pulmonary lymph nodes, vertebrae, cervical muscles and epidural space in a young patient - case report and review of literature. BMC Res Notes 2013;6:290. 10.1186/1756-0500-6-290 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Piccirilli M, Brunetto GM, Rocchi G, et al. Extra central nervous system metastases from cerebral glioblastoma multiforme in elderly patients. Clinico-pathological remarks on our series of seven cases and critical review of the literature. Tumori 2008;94:40-51. 10.1177/030089160809400109 [DOI] [PubMed] [Google Scholar]
- 16.Ohgaki H, Kleihues P. Epidemiology and etiology of gliomas. Acta Neuropathol 2005;109:93-108. 10.1007/s00401-005-0991-y [DOI] [PubMed] [Google Scholar]
- 17.Metellus P, Coulibaly B, Colin C, et al. Absence of IDH mutation identifies a novel radiologic and molecular subtype of WHO grade II gliomas with dismal prognosis. Acta Neuropathol 2010;120:719-29. 10.1007/s00401-010-0777-8 [DOI] [PubMed] [Google Scholar]
- 18.Montemurro N. Glioblastoma Multiforme and Genetic Mutations: The Issue Is Not Over Yet. An Overview of the Current Literature. J Neurol Surg A Cent Eur Neurosurg 2020;81:64-70. 10.1055/s-0039-1688911 [DOI] [PubMed] [Google Scholar]
- 19.Chen R, Ravindra VM, Cohen AL, et al. Molecular features assisting in diagnosis, surgery, and treatment decision making in low-grade gliomas. Neurosurg Focus 2015;38:E2. 10.3171/2015.1.FOCUS14745 [DOI] [PubMed] [Google Scholar]
- 20.Mentrikoski M, Johnson MD, Korones DN, et al. Glioblastoma multiforme in skin: a report of 2 cases and review of the literature. Am J Dermatopathol 2008;30:381-4. 10.1097/DAD.0b013e31817532c4 [DOI] [PubMed] [Google Scholar]
- 21.Rivero-Garvía M, Boto GR, Pérez-Zamarrón A, et al. Spinal cord and brain glioblastoma multiforme without previous craniotomy. J Neurosurg Spine 2008;8:601. 10.3171/SPI/2008/8/6/601 [DOI] [PubMed] [Google Scholar]
- 22.Müller C, Holtschmidt J, Auer M, et al. Hematogenous dissemination of glioblastoma multiforme. Sci Transl Med 2014;6:247ra101. 10.1126/scitranslmed.3009095 [DOI] [PubMed] [Google Scholar]
- 23.Sun BL, Wang LH, Yang T, et al. Lymphatic drainage system of the brain: A novel target for intervention of neurological diseases. Prog Neurobiol 2018;163-164:118-43. 10.1016/j.pneurobio.2017.08.007 [DOI] [PubMed] [Google Scholar]
- 24.Frank S, Kuhn SA, Brodhun M, et al. Metastatic glioblastoma cells use common pathways via blood and lymphatic vessels. Neurol Neurochir Pol 2009;43:183-90. [PubMed] [Google Scholar]
- 25.Stupp R, Hegi ME, Mason WP, et al. Effects of radiotherapy with concomitant and adjuvant temozolomide versus radiotherapy alone on survival in glioblastoma in a randomised phase III study: 5-year analysis of the EORTC-NCIC trial. Lancet Oncol 2009;10:459-66. 10.1016/S1470-2045(09)70025-7 [DOI] [PubMed] [Google Scholar]
- 26.Ma R, Taphoorn MJB, Plaha P. Advances in the management of glioblastoma. J Neurol Neurosurg Psychiatry 2021;92:1103-11. 10.1136/jnnp-2020-325334 [DOI] [PubMed] [Google Scholar]
- 27.Herrlinger U, Tzaridis T, Mack F, et al. Lomustine-temozolomide combination therapy versus standard temozolomide therapy in patients with newly diagnosed glioblastoma with methylated MGMT promoter (CeTeG/NOA-09): a randomised, open-label, phase 3 trial. Lancet 2019;393:678-88. 10.1016/S0140-6736(18)31791-4 [DOI] [PubMed] [Google Scholar]
- 28.Diaz RJ, Ali S, Qadir MG, et al. The role of bevacizumab in the treatment of glioblastoma. J Neurooncol 2017;133:455-67. 10.1007/s11060-017-2477-x [DOI] [PubMed] [Google Scholar]
- 29.Vredenburgh JJ, Desjardins A, Reardon DA, et al. Experience with irinotecan for the treatment of malignant glioma. Neuro Oncol 2009;11:80-91. 10.1215/15228517-2008-075 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Roci E, Cakani B, Brace G, et al. Platinum-based chemotherapy in recurrent high-grade glioma patients: retrospective study. Med Arch 2014;68:140-3. 10.5455/medarh.2014.68.140-143 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Swanson KD, Lok E, Wong ET. An Overview of Alternating Electric Fields Therapy (NovoTTF Therapy) for the Treatment of Malignant Glioma. Curr Neurol Neurosci Rep 2016;16:8. 10.1007/s11910-015-0606-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Stupp R, Taillibert S, Kanner AA, et al. Maintenance Therapy With Tumor-Treating Fields Plus Temozolomide vs Temozolomide Alone for Glioblastoma: A Randomized Clinical Trial. JAMA 2015;314:2535-43. 10.1001/jama.2015.16669 [DOI] [PubMed] [Google Scholar]
- 33.Stupp R, Taillibert S, Kanner A, et al. Effect of Tumor-Treating Fields Plus Maintenance Temozolomide vs Maintenance Temozolomide Alone on Survival in Patients With Glioblastoma: A Randomized Clinical Trial. JAMA 2017;318:2306-16. 10.1001/jama.2017.18718 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Brennan CW, Verhaak RG, McKenna A, et al. The somatic genomic landscape of glioblastoma. Cell 2013;155:462-77. 10.1016/j.cell.2013.09.034 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Alimbetov D, Askarova S, Umbayev B, et al. Pharmacological Targeting of Cell Cycle, Apoptotic and Cell Adhesion Signaling Pathways Implicated in Chemoresistance of Cancer Cells. Int J Mol Sci 2018;19:1690. 10.3390/ijms19061690 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Tay BQ, Wright Q, Ladwa R, et al. Evolution of Cancer Vaccines-Challenges, Achievements, and Future Directions. Vaccines (Basel) 2021;9:535. 10.3390/vaccines9050535 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Muir M, Gopakumar S, Traylor J, et al. Glioblastoma multiforme: novel therapeutic targets. Expert Opin Ther Targets 2020;24:605-14. 10.1080/14728222.2020.1762568 [DOI] [PubMed] [Google Scholar]
- 38.Majd N, Kamiya-Matsuoka C, de Groot J. The path forward for anti-programmed cell death-1 therapy in gliomas. Curr Opin Neurol 2019;32:864-71. 10.1097/WCO.0000000000000746 [DOI] [PubMed] [Google Scholar]
- 39.Bouffet E, Larouche V, Campbell BB, et al. Immune Checkpoint Inhibition for Hypermutant Glioblastoma Multiforme Resulting From Germline Biallelic Mismatch Repair Deficiency. J Clin Oncol 2016;34:2206-11. 10.1200/JCO.2016.66.6552 [DOI] [PubMed] [Google Scholar]
- 40.Johanns TM, Miller CA, Dorward IG, et al. Immunogenomics of Hypermutated Glioblastoma: A Patient with Germline POLE Deficiency Treated with Checkpoint Blockade Immunotherapy. Cancer Discov 2016;6:1230-6. 10.1158/2159-8290.CD-16-0575 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
The article’s supplementary files as