Abstract
We report the case of a healthy man who was diagnosed with bilateral optic neuritis after COVID-19 vaccination. A 63-year-old man presented with acute painful blurred vision in his left eye and temporal pain. Eleven days before the appearance of his symptoms, he received the first dose of COVID-19 mRNA vaccination. On examination, his best corrected visual acuity was 20/20 in his right eye and counting fingers in the left eye. Funduscopic examination showed bilateral optic disc swelling, and postcontrast magnetic resonance imaging showed left optic nerve sheath enhancement, consistent with left optic perineuritis and right optic papillitis. After steroid pulse therapy, his visual function and headache showed significant improvement. A few cases of optic neuritis as a side effect of COVID-19 mRNA vaccination were reported. This report provides important information about side effects assumed to be related to the COVID-19 mRNA vaccine.
Keywords: COVID-19, mRNA vaccine, Optic neuritis
Introduction
A pandemic of coronavirus disease 2019 (COVID-19) has caused many deaths, and it continues to rage worldwide. Antivirus vaccines for COVID-19, including new types of messenger RNA (mRNA)-based vaccines, have been developed and are being widely used, and they are expected to control the outbreak.
Optic neuritis (ON) is a common clinical disease that occurs as sudden onset of visual loss that can be associated with orbital pain. Several factors are known to be involved as possible mechanisms causing ON, including infection, autoimmunity, trauma, granulomatous disease, and demyelination [1]. Past studies have reported that vaccinations, such as influenza, anthrax, polio, diphtheria, pertussis, tetanus, and others, can also cause ON [2, 3, 4].
To the best of our knowledge, there are a few reports of ON after the mRNA-1273 vaccine for COVID-19 [5]. We present a case of bilateral ON, assumed to be related to the COVID-19 vaccination.
Case Report
A 63-year-old man was seen due to awareness of blurred vision in his left eye and temporal pain. Eleven days before the appearance of the symptoms, he received the first vaccination against COVID-19 (the Pfizer-BioNTech mRNA-1273 vaccine), and the only side effect at that time was arm pain. The polymerase chain reaction test for COVID-19 was negative.
His medical history was significant for atrial fibrillation and cataract surgery in his right eye, treated only with rivaroxaban and verapamil. He had no prior neurological signs or symptoms or any family history of demyelinating disease. He drank about 150 g of alcohol daily, but he had no nutritional disorders. He also had never smoked, had never taken Sildenafil, and had never ingested paint thinner.
On examination, best corrected visual acuity was 20/20 in the right eye and counting fingers in the left eye. The relative afferent pupillary defect was positive on the left, and the indirect light reflex was normal. The anterior ocular findings showed no inflammation, an intraocular lens on the right, and a mild cataract on the left. On fundus examination, bilateral optic disc swelling was noted (Fig. 1). Goldman's perimetry (Haag-Streit, Bern, Switzerland) showed a visual field defect as a central scotoma in the left eye (Fig. 2a). The flicker value was 19.67 Hz on the right and 8.83 Hz on the left. Electroretinography was within normal range. As shown in Figure 2b, higher waveform levels were measured with visual evoked potentials (VEPs), and no latencies of the major positive peak (P100) were noted (average 101 on the right and 92 on the left).
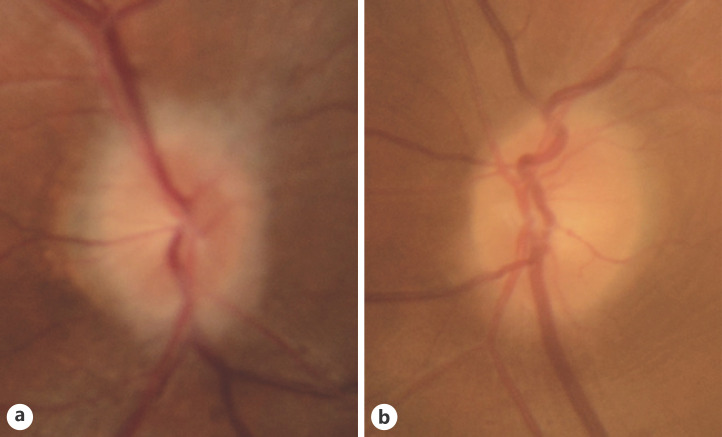
Fig. 1.
Color fundus photographs of the right eye (a) and the left eye (b). Bilateral disc swelling is seen and is especially remarkable in the right eye.
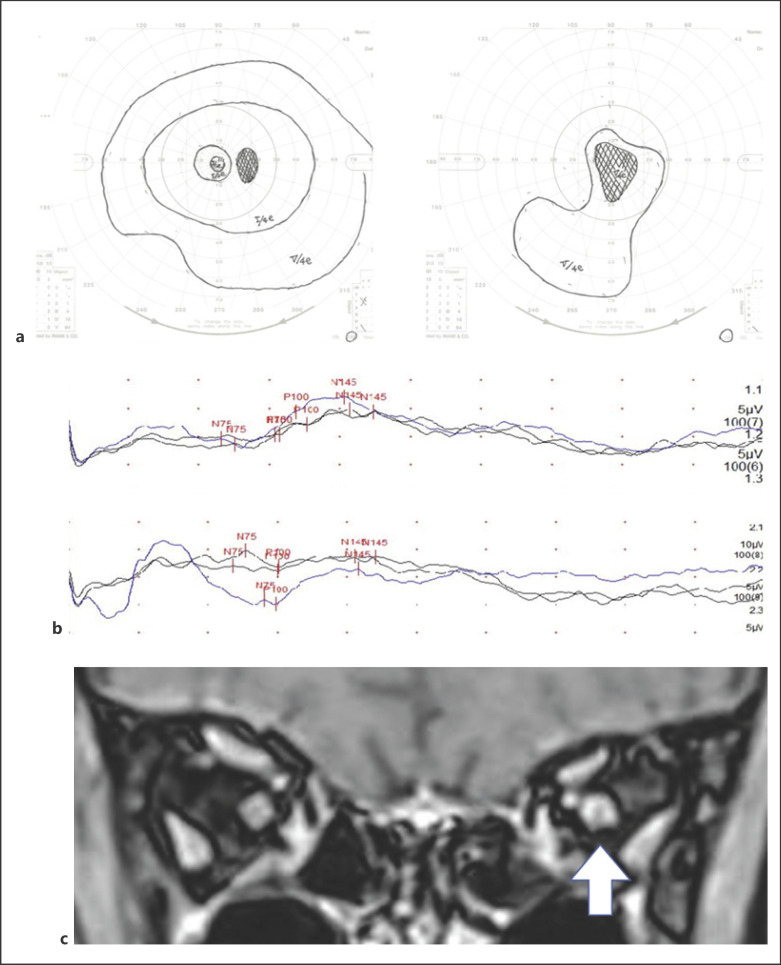
Fig. 2.
Visual field testing by Goldman perimetry (a) has been shown central scotoma on pretreatment. The pretreatment VEP (b) showed higher levels of the waveform and no latencies of P100 are shown for the right eye (upper) and the left eye (lower). Postcontrast orbital magnetic resonance imaging with fat suppression on the coronal image (c) shows the left optic nerve sheath enhancement (indicated by the white arrow).
The erythrocyte sedimentation rate was normal. Anti-aquaporin 4 antibody, IgG4, and autoimmune antibody were negative; anti-myelin oligodendrocyte glycoprotein antibody was not tested because it was not available in our hospital. The white blood cell count, C-reactive protein level, and other biochemical blood samples, including vitamin B12 levels, were within normal. Infectious diseases, including human immunodeficiency virus and syphilis, were also ruled out. Lumbar puncture was not performed as no other neurological symptoms were observed.
Postcontrast magnetic resonance imaging of the orbit showed optic nerve sheath enhancement on the left side, suggesting optic perineuritis (Fig. 2c). There were no findings suggestive of demyelinating disease and compressive lesions.
With the diagnosis of bilateral ON (optic papillitis on the right and optic perineuritis on the left), he was treated with high-dose corticosteroid pulse therapy that consisted of 3 days of methylprednisolone, 1,000 mg intravenously, and 4 days of oral prednisolone 30 mg, expected to potentially shorten the visual function recovery period.
After two courses of steroid pulse therapy, his visual function and headache showed significant improvement. Best corrected visual acuity was 18/20 for the left eye, the visual field was also improved (Fig. 3a, b), and the flicker value was 29.67 Hz on the right and 22.17 Hz on the left. Bilateral optic nerve papillae were not observed, and P100 latency on VEPs was still normal (average 91 on the right and 87 on the left), which also seemed to have improved slightly. He is now being followed carefully with tapering of the prednisone dose.
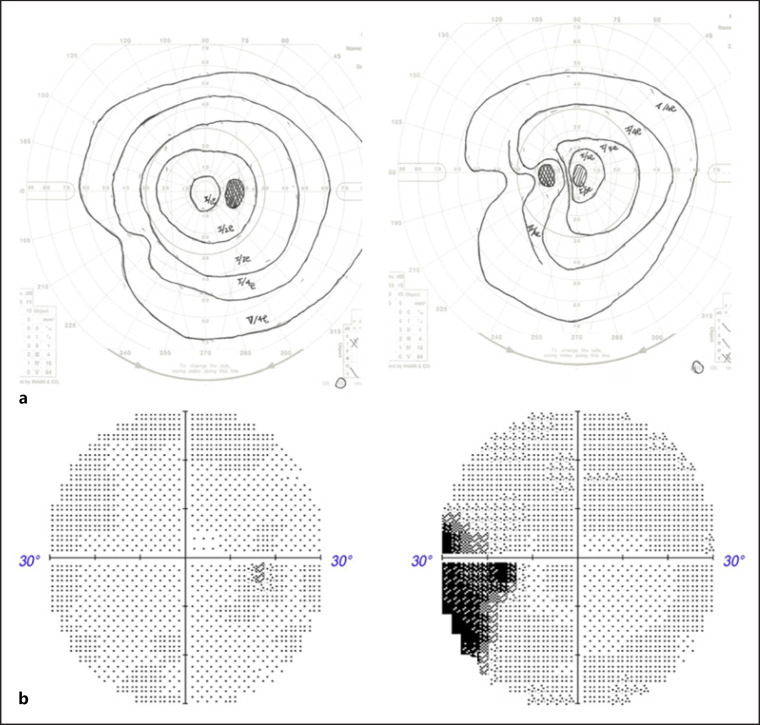
Fig. 3.
Visual field was improved after steroid therapy (Goldman perimetry (a), Humphrey visual field analyzer (b) [Carl Zeiss, Jena, Germany]).
Discussion
Many ophthalmological complications following vaccination, including vision loss, papilledema, uveitis, central serous retinopathy, acute placoid pigment epitheliopathy, central retinal vein occlusion, and others, have been reported recently [6, 7]. ON after vaccination has also been reported. Kawasaki reported two cases of anterior ON after influenza vaccination giving rise to permanent visual loss, thought to have been caused by an immune complex vasculopathy [2]. There are also reports of ON after anthrax, yellow fever, hepatitis A and hepatitis B, diphtheria, pertussis, and tetanus, and oral polio vaccinations [3, 4].
Most of these side effects might be related to immune complexes. Three mechanisms (molecular mimicry, bystander activation, and superantigen stimulation) may play roles in the development of demyelinating diseases of the central and peripheral nervous systems [8, 9]. Other papers have suggested that immune complex-mediated vascular damage may result in altered vascular permeability, perivascular inflammation, and consequent disruption of the blood-brain barrier. This may allow lymphocytes that synthesize specific viral antibodies outside the brain compartment to enter the brain, causing inflammation and demyelination [10].
Unlike previous vaccines, the mRNA vaccine is a world-first; therefore, leading to side effects may be different from that of other vaccines. The mechanism of ON following mRNA vaccination is unclear and still in the process of being elucidated. Some hypotheses for possible pathophysiological mechanisms after mRNA immunization have been reported, including increased serum cortisol, free extracellular mRNA, and polyethylene glycol [11]. The Vaccine Adverse Events Reporting System is used to evaluate adverse events potentially related to vaccinations. According to Vaccine Adverse Events Reporting System, since the vaccination was developed, there have been 58 cases of ON reported between January 1 and June 30, 2021. The mean interval from COVID-19 vaccination to ON was 9.41 days.
In the present case, the COVID-19 vaccination presumably triggered an autoimmune response and bilateral ON, while it is difficult to prove the association with the causation of vaccines. Critical visual loss occurred suddenly with retrobulbar pain, VEP latency was not found, and it recovered almost completely after steroid therapy. VEP is a noninvasive tool for studying visual function, and latency or lower levels of VEP are known to be a sensitive indicator of demyelination and subsequent remyelination in models of ON [12, 13]. Whereas the long-term visual outcome of ON is generally good, about one in three patients remains visually impaired [14]. Some studies suggested that a poor visual outcome is associated with more extensive lesions on magnetic resonance imaging and lower levels of VEP [14, 15]. Therefore, as in the present case, the prognosis of ON with higher levels of VEP and no latency after vaccination appears to be better.
With the pandemic, vaccination has been a growing need, but its clinical characteristics including ocular side effects have been inadequately identified and are still poorly understood. From the experience of this case, anti-inflammatory treatment with steroid pulse therapy may be effective for ON after COVID-19 vaccination. Additional studies are recommended, and we hope that there will be a return to normal all over the world.
Statement of Ethics
This case report was according to the tenets of the Declaration of Helsinki. The ethics approval for this report was not necessary on the basis of the ethical guidelines for medical and health research involving human subjects established by the Japanese Ministry of Education, Culture, Sports, Science, and Technology and the Ministry of Health, Labour, and Welfare. Written informed consent was obtained from the patient for publication of the details of their medical case and any accompanying images.
Conflict of Interest Statement
The authors have no conflicts of interest to declare.
Funding Sources
No funding or grant support.
Author Contributions
Hiroyasu Katayama designed the present study and data acquisition, wrote the manuscript, and approved the final version of the manuscript. Kaku Itoh drafted the work, revised it critically for important intellectual content, interpretation of the data, and substantive revision of the manuscript, and approved the final version of the manuscript. Masato Hashimoto analyzed the data, wrote the manuscript, provided conceptual advice, and approved the final version of the manuscript.
Data Availability Statement
All data are included in this paper. Further enquiries can be directed to the corresponding author.
References
- 1.Bennett JL. Optic neuritis. Continuum. 2019 Oct;25((5)):1236–64. doi: 10.1212/CON.0000000000000768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kawasaki A, Purvin VA, Tang R. Bilateral anterior ischemic optic neuropathy following influenza vaccination. J Neuroophthalmol. 1998 Mar;18((1)):56–9. [PubMed] [Google Scholar]
- 3.Kerrison JB, Lounsbury D, Thirkill CE, Lane RG, Schatz MP, Engler RM. Optic neuritis after anthrax vaccination. Ophthalmology. 2002 Jan;109((1)):99–104. doi: 10.1016/s0161-6420(01)00844-2. [DOI] [PubMed] [Google Scholar]
- 4.Arshi S, Sadeghi-Bazargani H, Ojaghi H, Savadi-Oskouei D, Hekmat S, Jastan M, et al. The first rapid onset optic neuritis after measles-rubella vaccination: case report. Vaccine. 2004 Sep 3;22((25–26)):3240–2. doi: 10.1016/j.vaccine.2004.02.044. [DOI] [PubMed] [Google Scholar]
- 5.García-Estrada C, Gómez-Figueroa E, Alban L, Arias-Cárdenas A. Optic neuritis after COVID-19 vaccine application. Clin Exp Neuroimmunol. 2022 May;13((2)):72–4. doi: 10.1111/cen3.12682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Carmeli Y, De-Medina T. Serious hepatitis B vaccine adverse reactions, are they immune-mediated? Vaccine. 1993 Oct;11((13)):1358–9. doi: 10.1016/0264-410x(93)90115-e. [DOI] [PubMed] [Google Scholar]
- 7.Stewart O, Chang B, Bradbury J. Simultaneous administration of hepatitis B and polio vaccines associated with bilateral optic neuritis. Br J Ophthalmol. 1999 Oct;83((10)):1200–1. doi: 10.1136/bjo.83.10.1194g. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Erguven M, Guven S, Akyuz U, Bilgiç O, Laloglu F. Optic neuritis following hepatitis B vaccination in a 9-year-old girl. J Chin Med Assoc. 2009 Nov;72((11)):594–7. doi: 10.1016/S1726-4901(09)70435-6. [DOI] [PubMed] [Google Scholar]
- 9.Stübgen JP. A literature review on optic neuritis following vaccination against virus infections. Autoimmun Rev. 2013 Aug;12((10)):990–7. doi: 10.1016/j.autrev.2013.03.012. [DOI] [PubMed] [Google Scholar]
- 10.Stevenson VL, Acheson JF, Ball J, Plant GT. Optic neuritis following measles/rubella vaccination in two 13-year-old children. Br J Ophthalmol. 1996 Dec;80((12)):1110–1. doi: 10.1136/bjo.80.12.1110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Fowler N, Mendez Martinez NR, Pallares BV, Maldonado RS. Acute-onset central serous retinopathy after immunization with COVID-19 mRNA vaccine. Am J Ophthalmol Case Rep. 2021 Sep;23:101136. doi: 10.1016/j.ajoc.2021.101136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Klistorner A, Graham S, Fraser C, Garrick R, Nguyen T, Paine M, et al. Electrophysiological evidence for heterogeneity of lesions in optic neuritis. Invest Ophthalmol Vis Sci. 2007 Oct;48((10)):4549–56. doi: 10.1167/iovs.07-0381. [DOI] [PubMed] [Google Scholar]
- 13.You Y, Klistorner A, Thie J, Graham SL. Latency delay of visual evoked potential is a real measurement of demyelination in a rat model of optic neuritis. Invest Ophthalmol Vis Sci. 2011 Aug 29;52((9)):6911–8. doi: 10.1167/iovs.11-7434. [DOI] [PubMed] [Google Scholar]
- 14.Berg S, Kaschka I, Utz KS, Huhn K, Lämmer A, Lämmer R, et al. Baseline magnetic resonance imaging of the optic nerve provides limited predictive information on short-term recovery after acute optic neuritis. PLoS One. 2015;10((1)):e0113961. doi: 10.1371/journal.pone.0113961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hickman SJ, Toosy AT, Miszkiel KA, Jones SJ, Altmann DR, MacManus DG, et al. Visual recovery following acute optic neuritis − a clinical, electrophysiological and magnetic resonance imaging study. J Neurol. 2004 Aug;251((8)):996–1005. doi: 10.1007/s00415-004-0477-1. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All data are included in this paper. Further enquiries can be directed to the corresponding author.