Abstract
Heart metastases from urothelial carcinoma of the bladder have rarely been reported in the literature. We present a case complicated by symptomatic disseminated intravascular coagulation in a 67-year-old woman. A rapid and sustained recovery from hemostatic troubles was obtained following fibrinogen supplementation combined with second-line paclitaxel chemotherapy.
Keywords: Bladder cancer, Urothelial carcinoma, Heart metastasis, Disseminated intravascular coagulation
Introduction
Bladder cancer is the tenth most common malignancy worldwide, and urothelial carcinoma (UC) accounts for 90% of all bladder cancers [1]. The most frequent metastatic sites are lymph nodes, lungs, liver, and bones. Heart metastases from UC of the bladder are uncommon, with less than 20 cases reported in the literature [2, 3, 4, 5, 6]. According to a recent review, only 3.9% of patients with bladder cancer are likely to develop heart metastases, and diagnosis is mainly performed during autopsy analyses [7].
Disseminated intravascular coagulation (DIC) occurs in up to 7% of solid tumors, mainly pancreas, prostate, and breast tumors, upon tumor progression in most cases [8]. DIC is characterized by systemic activation of blood coagulation resulting simultaneously in microvascular thrombi formation, organ dysfunction, and consumption of clotting factors and platelets, with a high risk of thrombosis and uncontrolled hemorrhage. Here, we report the successful management of a symptomatic DIC with enhanced fibrinolysis due to a right ventricular metastasis of UC of the bladder.
Case Presentation
A 67-year-old woman was referred to the emergency unit of Saint-Louis Hospital in November 2020. She complained of a fever for 3 days with a persistent macroscopic hematuria. She had been diagnosed in October 2019 with a locally advanced UC of the bladder with metastatic left external iliac lymph nodes. She received 4 cycles of preoperative chemotherapy with methotrexate, vinblastine, doxorubicin, and cisplatin followed by the surgical removal of the bladder, uterus, cervix, vagina, and ovaries with urinary diversion using Bricker's ileal conduit technique. Histological results retrieved a ypT3bN2 tumor. Metastatic relapse in pelvic and retroperitoneal lymph nodes occurred in September 2020, while a 9-mm thickening was described in the right ventricle. She received 4 cycles of the anti-PD1 immune checkpoint inhibitor pembrolizumab followed by antalgic irradiation because of progressive, disabling pelvic pain in October 2020.
On admission to the emergency unit in November 2020, a major hyperleukocytosis linked to a urinary tract infection as well as severe hemostasis disorders were observed: grade 2 thrombocytopenia (platelet count: 66 × 109/L, normal range 150–400 × 109/L), low prothrombin time (PT) (36%, normal range >70–130%), low fibrinogen level (0.43 g/L; normal range 2–4 g/L), and elevated D-Dimers >20,000 ng/mL (normal <500 ng/mL). Before this episode, anterior hemostasis blood tests showed no abnormalities. Antibiotic therapy for methicillin-sensitive Staphylococcus aureus in urine test had no efficacy on hemostasis troubles, leading to a transfer into the oncology unit for further investigations.
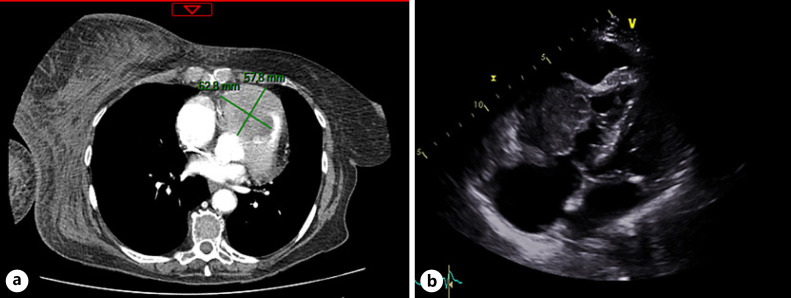
A physical exam showed a major ecchymosis over the left calf and a large hematoma over the right arm which appeared within 2 days before admission. There was no evidence of exterior bleeding. There were no other significant findings on cardiovascular, respiratory, abdominal, and neurologic examination. Laboratory analysis confirmed persistent grade 2 thrombopenia (50 × 109/L), low PT (36%), and low fibrinogen level (0.36 g/L). There was no significant trouble with conduction or repolarization in the electrocardiogram. Computed tomography showed an active hemorrhage of the right axillary artery in three different sites, precluding any embolization. Concomitantly, a growing mass of 63 mm in the right ventricle that extended into the right pulmonary outflow tract, with a small pericardial effusion, was seen (Fig. 1a). Transthoracic echocardiography evidenced a 46 × 37 mm mass in the pulmonary infundibulum responsible for a severe obstruction of the pulmonary valve and a non-compressive pericardial effusion of 20 mm (Fig. 1b).
Fig. 1.
Heart metastases.aComputed tomography imaging of the right ventricular mass.bApical view of the transthoracic echocardiogram showing a large echogenic mass filling the right ventricle.
Seven days upon admission, we diagnosed thrombotic microangiopathy associated with DIC, with clinically high blood pressure and positive schizocytes on a peripheral blood smear. ADAMST13 activity was in the normal range (57 IU/dL). Systemic autoimmune investigations did not report any autoimmune disorder nor antiplatelet antibody detectable. In addition, there was no liver failure. Coagulation disorders related to intrinsic coagulation pathway deficiency (factors VIII, IX, XI) or acquired von Willebrand factor syndrome were excluded. No deficit of complement factors was observed, and serum protein electrophoresis showed no abnormalities.
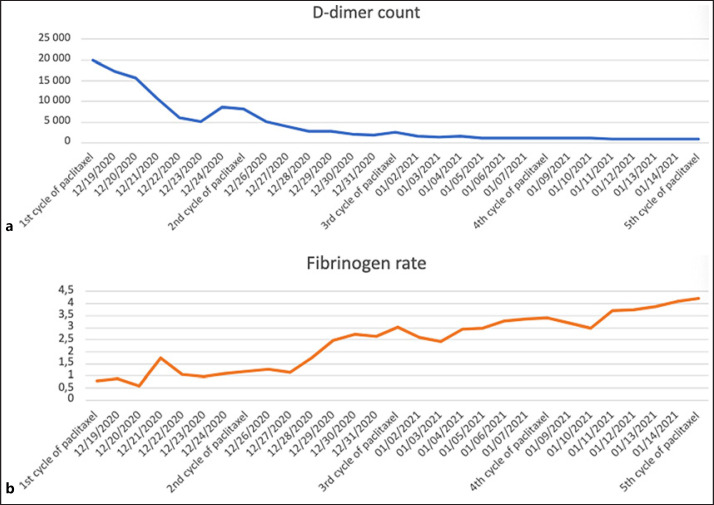
Fibrinogen supplementation based on plasma-derived fibrinogen concentrate was used to reach a fibrinogen level of over 1 g/L. After 7 days of intensive monitoring including blood and platelet transfusions, the bleeding had stopped, and the patient was stable with a fibrinogen level of around 1 g/L. Hence, we initiated second-line chemotherapy based on weekly paclitaxel at the dose of 60 mg/m2. Three days after the first cycle of systemic chemotherapy, D-dimer levels started to decrease, and then platelets, fibrinogen, and PT recovered normal range after 3 cycles of systemic chemotherapy (Fig. 2). After 30 days, the patient's clinical status was consistent with a hospital discharge. The pursuit of weekly paclitaxel chemotherapy as well as the initiation of a daily curative anticoagulation treatment with heparin to prevent a thrombus of the heart metastasis from embolizing were decided. After 10 cycles of weekly paclitaxel chemotherapy, hemostasis troubles were completely resolved, and the mass volume on computed tomography was stable. The patient ultimately died of progressive disease 4 months later without recurrence of DIC.
Fig. 2.
D-dimer (ng/mL) (a) and fibrinogen (g/L) (b) levels during paclitaxel chemotherapy.
Discussion/Conclusion
DIC with enhanced fibrinolysis is associated with severe coagulation activation and marked fibrinolytic activation responsible for more easily dissolved hemostatic plugs leading to severe bleeding symptoms [9]. It was mainly described in hematological malignancies such as acute promyelocytic leukemia [10] or prostate cancer [11]. Rare cases of bladder cancer-related DIC have been reported in the literature [12, 13] but, to our knowledge, this is the first case of enhanced-fibrinolytic type DIC occurring during the course of UC. As no obvious triggering event was found, our hypothesis was a chronic balanced-fibrinolytic type DIC evolving silently, which finally progressed to DIC with enhanced fibrinolysis upon the progression of UC through the development of ventricular metastases.
A coagulation/fibrinolysis imbalance may occur in various diseases such as sepsis, trauma, or cancer. In addition, interactions between the tumoral process and the hemostatic system are complex: malignant cells have both prothrombotic and fibrinolytic properties at once with a poorly understood prothrombotic tendency described in tumor pathologies. Therefore, thromboembolic events or severe bleeding symptoms may occur because of disturbances in the balance of coagulation. One of the mechanisms of cancer-related hypercoagulability includes the expression of tissue factor (TF) by circulating tumor cells. In normal situations, TF is expressed by cells surrounding blood vessels, initiating rapid coagulation activation in case of vessel injury [14]. A high level of tumor TF expression is found in many cancers but is rather uncommon in UC. As hemostasis disorders resolved quickly after initiation of chemotherapy, it could be hypothesized that the tumor progression through the right ventricular metastasis resulted in a massive exposure of tumor antigens and TF to circulating plasma, triggering the coagulation cascade, and therefore leading to enhanced-fibrinolytic DIC.
Therapy of DIC is complex, consisting in reversing the underlying triggering disorder while providing patients the appropriate supportive treatment. The difficulty is whether the trigger cause can be treated and amended without worsening the consumption of platelets and the risk of bleeding. Consequently, management of DIC with enhanced fibrinolysis in patients with solid cancer is a challenge, as antifibrinolytic therapy may cause life-threatening thrombosis and organ failure.
We conclude that (i) cardiac metastases should be considered in cancer patients with otherwise unexplained DIC, (ii) DIC with enhanced fibrinolysis is not restricted to prostate cancer among solid tumors, and (iii) a close collaboration between medical oncologists and biological hematologists is required in order to adequately diagnose and rapidly manage this severe but reversible complication.
Statement of Ethics
Written informed consent was obtained from the patient's son for publication of this case report and any accompanying images. The Ethical Review Board of Saint-Louis Hospital waived the need for ethical approval of this case report.
Conflict of Interest Statement
The authors have no conflict to declare.
Funding Sources
This study did not receive any funding.
Author Contributions
Investigation: Clara Helal, Tiphaine Lambert, Bérangère S. Joly, and Stéphane Culine; data curation: Clara Helal and Tiphaine Lambert; writing − original draft preparation: Clara Helal and Tiphaine Lambert, writing − review and editing: Clara Helal, Tiphaine Lambert, Bérangère S. Joly, Clément Dumont, Hélène Gauther, and Stéphane Culine.
Data Availability Statement
All data generated or analyzed during this study are included in this article. Further inquiries can be directed to the corresponding author.
References
- 1.Richters A, Aben KKH, Kiemeney LALM. The global burden of urinary bladder cancer: an update. World J Urol. 2020 Nov;38((8)):1895–904. doi: 10.1007/s00345-019-02984-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bussani R, De-Giorgio F, Abbate A, Silvestri F. Cardiac metastases. J Clin Pathol. 2007 Jan;60((1)):27–34. doi: 10.1136/jcp.2005.035105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Doshi TV, Doshi JV, Makaryus JN, Makaryus AN. A rare case of successfully treated cardiac metastasis from transitional cell bladder cancer. Am J Ther. 2013 May–Jun;20((3)):307–10. doi: 10.1097/MJT.0b013e3181f6c131. [DOI] [PubMed] [Google Scholar]
- 4.Nakashima Y, Tanioka K, Kubo T, Yamasaki N, Yamasaki I, Syuin T, et al. Metastatic cardiac tumor from urothelial carcinoma detected by transthoracic echocardiography: a case report. J Med Case Rep. 2015 Nov;9:257. doi: 10.1186/s13256-015-0740-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Yamac AH, Insanic D, Bockmeyer C. Cardiac metastasis from a urothelial cell carcinoma: a commented case report. Cardiovasc Pathol. 2014 May–Jun;23((3)):178–80. doi: 10.1016/j.carpath.2014.01.005. [DOI] [PubMed] [Google Scholar]
- 6.Schreiner M, Schneider B, Pauls S. Cardiac metastasis of a recurrent urothelial carcinoma of the bladder. Rofo. 2015 Dec;187((12)):1124–6. doi: 10.1055/s-0041-104181. [DOI] [PubMed] [Google Scholar]
- 7.Taguchi S. Comprehensive review of the epidemiology and treatments for malignant adult cardiac tumors. Gen Thorac Cardiovasc Surg. 2018 May;66((5)):257–62. doi: 10.1007/s11748-018-0912-3. [DOI] [PubMed] [Google Scholar]
- 8.Levi M, Sivapalaratnam S. Disseminated intravascular coagulation: an update on pathogenesis and diagnosis. Expert Rev Hematol. 2018 Aug;11((8)):663–72. doi: 10.1080/17474086.2018.1500173. [DOI] [PubMed] [Google Scholar]
- 9.Asakura H. Classifying types of disseminated intravascular coagulation: clinical and animal models. J Intensive Care. 2014 Mar;2((1)):20. doi: 10.1186/2052-0492-2-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ikezoe T. Advances in the diagnosis and treatment of disseminated intravascular coagulation in haematological malignancies. Int J Hematol. 2021 Jan;113((1)):34–44. doi: 10.1007/s12185-020-02992-w. [DOI] [PubMed] [Google Scholar]
- 11.Hyman DM, Soff GA, Kampel LJ. Disseminated intravascular coagulation with excessive fibrinolysis in prostate cancer: a case series and review of the literature. Oncology. 2011;81((2)):119–25. doi: 10.1159/000331705. [DOI] [PubMed] [Google Scholar]
- 12.Chadachan VM, Lee SK. Disseminated intravascular coagulation complicating urothelial malignancy. Singapore Med J. 2012 Aug;53((8)):e161–2. [PubMed] [Google Scholar]
- 13.John T, Davis ID. Ventricular metastasis resulting in disseminated intravascular coagulation. World J Surg Oncol. 2005 May;3((1)):29. doi: 10.1186/1477-7819-3-29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hisada Y, Mackman N. Tissue factor and cancer: regulation, tumor growth, and metastasis. Semin Thromb Hemost. 2019 Jun;45((04)):385–95. doi: 10.1055/s-0039-1687894. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All data generated or analyzed during this study are included in this article. Further inquiries can be directed to the corresponding author.