Abstract
Little is known about the potential ocular adverse events following mRNA-1273 vaccine. We aimed to report a case of multiple evanescent white dot syndrome (MEWDS) developing 3 days following the administration of mRNA-1273 vaccine booster. A 71-year-old white myopic female presented with complaints of seeing “pulsating light” and scotoma with her left eye that started about 3 days following mRNA-1273 vaccine booster administration. The patient was found to have multiple scattered white-yellow outer retinal lesions on dilated fundus exam of the left eye. Visual symptoms and exam findings continued to improve without any intervention confirming a short-lived and self-limiting disease course. Clinical presentation was consistent with a clinical diagnosis of MEWDS. Ophthalmologists need to take detailed vaccination history in patients presenting with MEWDS.
Keywords: COVID-19, Multiple evanescent white dot syndrome, mRNA vaccine
Introduction
Multiple evanescent white dot syndrome (MEWDS) is a rare, usually unilateral, inflammatory chorio-retinal disease and is characterized by the appearance of multiple short-lived white dots on clinical exam. MEWDS predominantly affects young myopic women and is preceded by viral illness in about one-third of cases [1]. MEWDS has also been reported following the administration of various vaccines including inactivated influenza vaccine, HPV vaccine, hepatitis B vaccine, rabies vaccine, and following the simultaneous administration of hepatitis A and yellow fever vaccines [2, 3, 4, 5, 6]. Recently, MEWDS has also been reported following the administration of BNT162b2 mRNA vaccine (Pfizer-BioNTech, NYC, NY, and Mainz, Germany) to prevent infection from the severe acute respiratory syndrome coronavirus 2 that causes COVID-19 disease [7, 8].
The mRNA-1273 vaccine (Moderna, Cambridge, MA, USA) is an mRNA-based vaccine that is lipid nanoparticle (LNP)-encapsulated and nucleoside-modified, which encodes the severe acute respiratory syndrome coronavirus 2 spike glycoprotein and is used to prevent COVID-19 disease [9]. The mRNA-1273 vaccine is an FDA-approved 2-dose vaccine administered intramuscularly 28 days apart with a booster dose offered 6 months after completion of the two primary doses [10]. Common local and systemic side effects include pain at site of injection, headache, fatigue, myalgia, and chills [11]. Few ocular events have also been previously reported following the administration of the mRNA-1273 vaccine. These include one case of reactivation of HLA-B27-associated anterior uveitis, one case of retinal vasculitis, and 1 case of central retinal vein occlusion [7]. However, to the best of our knowledge, no other ocular morbidities − including white dot syndromes − have previously been reported following vaccination with the mRNA-1273 vaccine. We report a case of MEWDS developing 3 days following the administration of mRNA-1273 booster vaccine dose.
Case Report
A 71-year-old white female who is known to our clinic presented with a new and sudden onset of experiencing a brief episode of seeing “pulsating light” with her left eye that was immediately followed by mild blurriness of her vision in that eye as well as noticeable darkening of the left peripheral field of vision. The symptoms started 4 days prior to presentation to clinic and only 3 days following administration of mRNA-1273 booster vaccine. Patient recalled developing mild malaise and soreness at the site of injection that lasted for 1 day. Patient reported having the 1st and 2nd mRNA-1273 vaccine doses administered 8 months prior to presentation and the influenza vaccine administered 31 days prior to presentation. Patient also recalled testing positive for COVID-19 approximately 4 months prior to presentation after coming in close contact with a positive COVID-19 case. Patient admitted for only temporarily losing her senses of taste and smell for few days after testing positive for COVID-19. Patient is a myope and had a history of retinal tear in the left eye that was treated with laser barricade few years back. Patient reported having a remote history of hysterectomy more than 25 years ago for “ovarian cysts” but does not recall the exact medical condition, and medical records were not available for review. Patient is taking simvastatin 10 mg daily for hypercholesterolemia as well as vitamins B12 and D.
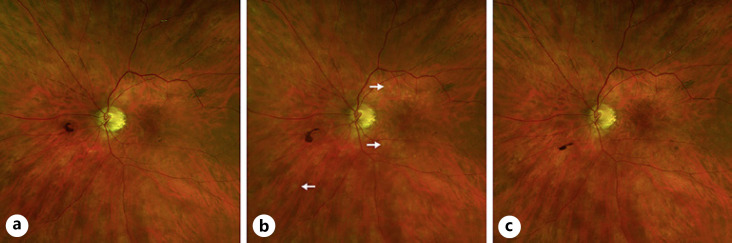
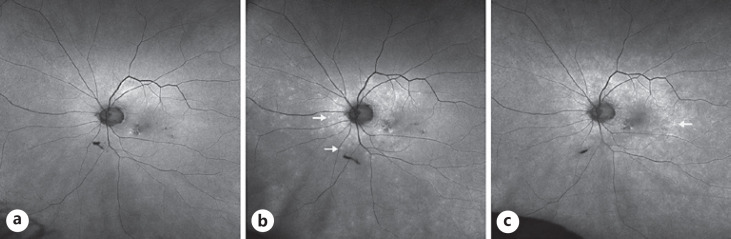
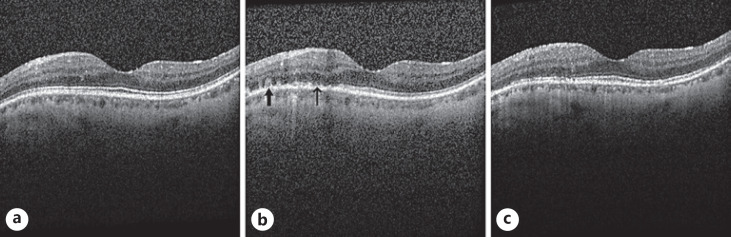
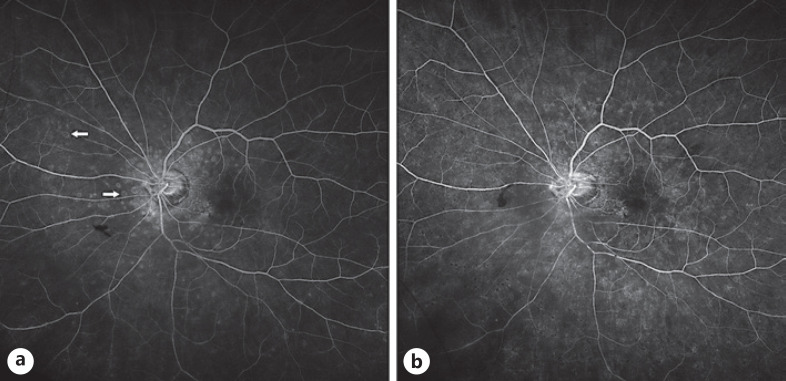
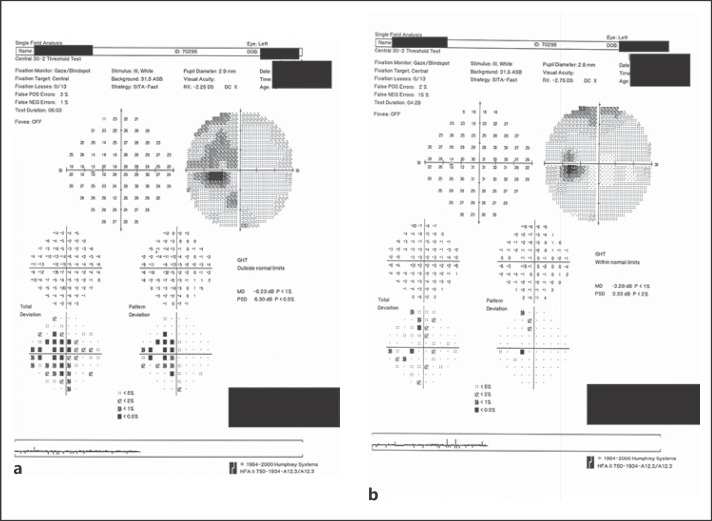
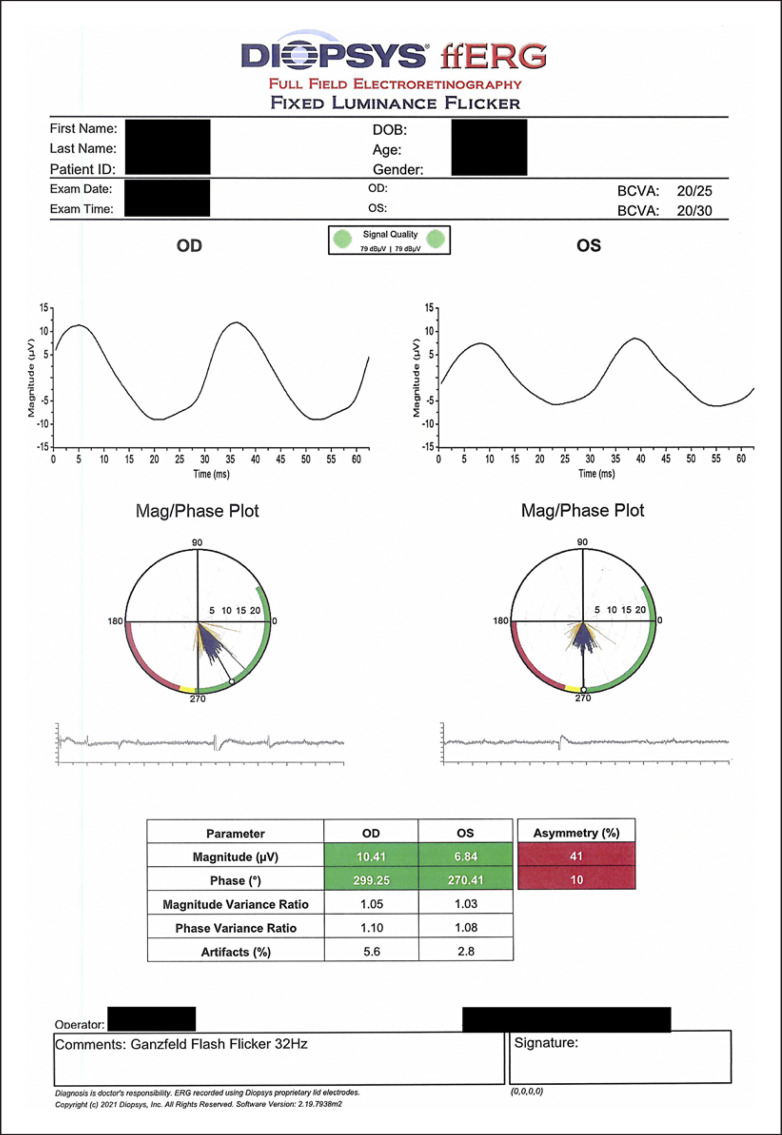
On exam, vision was 20/25 in the right eye and 20/30 in the left eye with normal pressures in both eyes. No cells or flare were seen in either the anterior chamber or vitreous in both eye. Dilated fundus exam of the left eye was significant for multiple faint outer retinal lesions in the peripapillary and peri-macular areas and extending to the periphery that were white-yellow in color (color fundus photo of left eye at baseline about 10 months prior to presentation is shown in Fig. 1a, and color fundus photo of left eye at day of presentation is shown in Fig. 1b). Dilated fundus exam was also noted for posterior vitreous detachments with evidence of myopic degeneration in both eyes and laser scars surrounding retinal hole in the left eye. The outer retinal lesions seen on exam in the left eye demonstrated hyper-autofluorescence on fundus autofluorescence (FAF) imaging (FAF image of left eye at baseline about 10 months prior to presentation is shown in Fig. 2a, and FAF image of left eye at day of presentation is shown in Fig. 2b). Spectral domain-optical coherence tomography (SD-OCT) scans showed multiple small subretinal hyper-reflective lesions in the macula that were more concentrated nasally with disruption of the ellipsoid zone (EZ) (SD-OCT scan through the macula of left eye at baseline about 8 months prior to presentation is shown in Fig. 3a, and SD-OCT scan through the macula of left eye at day of presentation is shown in Fig. 3b). Fluorescein angiography demonstrated hyper-fluorescence of the retinal lesions in a characteristic wreath pattern with no evidence of leakage (shown in Fig. 4a). Humphrey visual field (HVF) 30-2 of the left eye at baseline revealed enlarged blind spot, and full-field electroretinogram of the left eye showed mildly depressed response (shown in Fig. 5a, Fig. 6). Systemic workup to rule out autoimmune and infectious etiologies came back negative, and chest X-ray was unremarkable. A provisional diagnosis of MEWDS in the left eye was made in the setting of a recent mRNA-1273 vaccine booster shot administration. Patient continued to notice spontaneous improvement of the symptoms without any intervention. Repeat imaging about 6 weeks following presentation showed spontaneous improvement of previously documented findings (shown in Fig. 1c, 2c, 3c, 4b). Repeat HVF 30-2 of the left eye about 8 weeks following presentation also revealed resolution of enlarged blind spot (shown in Fig. 5b).
Fig. 1.
aColor fundus photo of left eye at baseline about 10 months prior to presentation.bColor fundus photo of left eye at presentation showing multiple faint white-yellow outer retinal lesions more concentrated around the nerve and the peri-macular area but also extending to the mid-periphery (arrows).cColor fundus photo of left eye 6 weeks following presentation showing the continued resolution and evolution of the outer retinal lesions.
Fig. 2.
aFundus autofluorescence (FAF) image of left eye at baseline about 10 months prior to presentation.bFAF image of left eye at presentation showing hyper-autofluorescence of the retinal lesions (arrows).cFAF image of left eye 6 weeks following presentation showing the continued resolution of previously seen lesions with the appearance of additional new lesions on the temporal macula (arrows).
Fig. 3.
aSpectral domain-optical coherence tomography (SD-OCT) scan through the macula of left eye at baseline about 8 months prior to presentation demonstrating normal retinal anatomy.bSD-OCT scan taken at the same location at presentation demonstrating multiple subretinal hyper-reflective material (thick arrow) and disruption of the ellipsoid zone (EZ, thin arrow).cRepeat SD-OCT scan 6 weeks following presentation showing continued resolution of the subretinal hyper-reflective material and the restoration of the EZ.
Fig. 4.
aFluorescein angiography (FA) image of left eye at presentation showing hyper-fluorescence of the retinal lesions with many showing a characteristic wreath pattern (arrows).bFA image of left eye 6 weeks following presentation showing nonspecific patchy fluorescence consistent with the resolution and evolution of the outer retinal lesions.
Fig. 5.
aHumphrey visual field (HVF) 30-2 of left eye at presentation showing enlarged blind spot.bHVF 30-2 of left eye 8 weeks following presentation showing resolution of the enlarged blind spot.
Fig. 6.
Full-field electroretinogram (ffERG) showing mildly depressed response of the left eye with significant asymmetry between the two eyes.
Discussion
We report here a case of MEWDS developing just 3 days following the administration of mRNA-1273 vaccine booster. The spontaneous resolution and improvement of symptoms in our case are similar to the disease course of MEWDS that has been reported following the administration of the BNT162b2 mRNA vaccine [7, 8]. However, our case presented at an older age of 71 years following a booster dose compared with an age range of presentation between 18 and 53 years following the administration of the 1st or 2nd doses of the BNT162b2 mRNA vaccine doses in the other reported cases. The benign nature of MEWDS developing following the administration of mRNA-based vaccines is consistent with the established and largely benign natural history of MEWDS in general.
While the timing of the disease development following the vaccine administration in our case suggests an association, it is difficult to establish a causation given the poorly understood pathophysiology of MEWDS as well as the poorly understood pathogenesis behind the development of ocular events following vaccinations in general. Three competing hypotheses have been suggested to explain the pathophysiology of MEWDS based on multimodal imaging analysis: first, as a primary choriocapillaris disease leading to hypoperfusion of the choriocapillaris and vaso-occlusive ischemic injury to the outer retinal layers [12]; second, as a primary disease process targeting the RPE layer and resulting in secondary injury to the outer segments of the photoreceptor layer [13]; and third, as a primary photoreceptoritis disease leading to direct injury to the inner and outer segments of the photoreceptors [14].
If a causation does exist between mRNA-based vaccines and MEWDS, it is likely an immunologic process potentially involving molecular mimicry secondary to induction of antibodies toward the S-protein that is cross-reacting with self-antigens in the eye [15]. Another possible mechanism involves immunologic reaction to the LNPs encapsulating the mRNA vaccine. LNP and liposomes, in general, are known to be related to two immunological phenomena. First, accelerated blood clearance secondary to the production of IgM antibodies toward polyethylene glycol component of the lipid layer leading to eventual rapid clearance from the blood by the spleen. This anti-polyethylene glycol antibody production has been shown following IV, SC, and IM routes of administration. Second, complement activation-related pseudo-allergy which is a non-IgE acute hypersensitivity reaction resulting from complement activation following IV administration [16, 17, 18].
In conclusion, we reported a case of MEWDS developing 3 days following the administration of an mRNA-1273 vaccine booster, which had a benign self-limiting disease course. This case underscores the importance of taking detailed history of recent vaccination in patients presenting with MEWDS, especially in the new era of COVID-19 disease, but it should not discourage vaccinations against COVID-19 disease as the benefits of vaccination significantly outweigh the risks. However, questions remain about how to best council patients who developed MEWDS shortly after vaccination against COVID-19 disease regarding any additional vaccine boosters in the future. More research is needed to better establish the exact relationship between MEWDS and mRNA-based vaccines as well as vaccinations in general.
Statement of Ethics
Ethical approval is not required for this study in accordance with local or national guidelines. Written informed consent was obtained from the patient for publication of the details of their medical case, and any accompanying images, in this manuscript.
Conflict of Interest Statement
The authors declare that there are no conflicts of interest.
Funding Sources
No funding or grant support.
Author Contributions
Rashed Alhabshan has made substantial contributions to the conception, design, and drafting of the work and revising it critically for important intellectual content and in the final approval of the version to be published. David Scales has made substantial contributions to the conception, design, and drafting of the work and revising it critically for important intellectual content and in the final approval of the version to be published.
Data Availability Statement
All data that support the findings of this study are included in this article. Further inquiries can be directed to the corresponding author.
References
- 1.dell'Omo R, Pavesio CE. Multiple evanescent white dot syndrome (MEWDS) Int Ophthalmol Clin. 2012;52((4)):221–8. doi: 10.1097/IIO.0b013e31826647ed. [DOI] [PubMed] [Google Scholar]
- 2.Ng CC, Jumper JM, Cunningham ET., Jr Multiple evanescent white dot syndrome following influenza immunization: a multimodal imaging study. Am J Ophthalmol Case Rep. 2020;19:100845. doi: 10.1016/j.ajoc.2020.100845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ogino K, Kishi S, Yoshimura N. Multiple evanescent white dot syndrome after human papillomavirus vaccination. Case Rep Ophthalmol. 2014;5((1)):38–43. doi: 10.1159/000358870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Baglivo E, Safran AB, Borruat FX. Multiple evanescent white dot syndrome after hepatitis B vaccine. Am J Ophthalmol. 1996;122((3)):431–2. doi: 10.1016/s0002-9394(14)72074-4. [DOI] [PubMed] [Google Scholar]
- 5.Yang JS, Chen CL, Hu YZ, Zeng R. Multiple evanescent white dot syndrome following rabies vaccination: a case report. BMC Ophthalmol. 2018;18((1)):312. doi: 10.1186/s12886-018-0968-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Stangos A, Zaninetti M, Petropoulos I, Baglivo E, Pournaras C. Multiple evanescent white dot syndrome following simultaneous hepatitis-A and yellow fever vaccination. Ocul Immunol Inflamm. 2006;14((5)):301–4. doi: 10.1080/09273940600932311. [DOI] [PubMed] [Google Scholar]
- 7.Bolletta E, Iannetta D, Mastrofilippo V, De Simone L, Gozzi F, Croci S, et al. Uveitis and other ocular complications following COVID-19 vaccination. J Clin Med. 2021;10((24)):5960. doi: 10.3390/jcm10245960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Rabinovitch T, Ben-Arie-Weintrob Y, Hareuveni-Blum T, Shaer B, Vishnevskia-Dai V, Shulman S, et al. UVEITIS AFTER THE BNT162b2 mRNA VACCINATION AGAINST SARS-CoV-2 INFECTION: a possible association. Retina. 2021;41((12)):2462–71. doi: 10.1097/IAE.0000000000003277. [DOI] [PubMed] [Google Scholar]
- 9.Jackson LA, Anderson EJ, Rouphael NG, Roberts PC, Makhene M, Coler RN, et al. An mRNA vaccine against SARS-CoV-2: preliminary report. N Engl J Med. 2020;383((20)):1920–31. doi: 10.1056/NEJMoa2022483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mbaeyi S, Oliver SE, Collins JP, Godfrey M, Goswami ND, Hadler SC, et al. The advisory committee on immunization practices' interim recommendations for additional primary and booster doses of COVID-19 vaccines: United States, 2021. MMWR Morb Mortal Wkly Rep. 2021;70((44)):1545–52. doi: 10.15585/mmwr.mm7044e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Baden LR, El Sahly HM, Essink B, Kotloff K, Frey S, Novak R, et al. Efficacy and safety of the mRNA-1273 SARS-CoV-2 vaccine. N Engl J Med. 2021;384((5)):403–16. doi: 10.1056/NEJMoa2035389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Papasavvas I, Mantovani A, Tugal-Tutkun I, Herbort CP., Jr Multiple evanescent white dot syndrome (MEWDS): update on practical appraisal, diagnosis and clinicopathology; a review and an alternative comprehensive perspective. J Ophthalmic Inflamm Infect. 2021;11((1)):45. doi: 10.1186/s12348-021-00279-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zicarelli F, Mantovani A, Preziosa C, Staurenghi G. Multimodal imaging of multiple evanescent white dot syndrome: a new interpretation. Ocul Immunol Inflamm. 2020;28((5)):814–20. doi: 10.1080/09273948.2019.1635169. [DOI] [PubMed] [Google Scholar]
- 14.Pichi F, Srvivastava SK, Chexal S, Lembo A, Lima LH, Neri P, et al. EN FACE OPTICAL COHERENCE TOMOGRAPHY AND OPTICAL COHERENCE TOMOGRAPHY ANGIOGRAPHY OF MULTIPLE EVANESCENT WHITE DOT SYNDROME: new insights into pathogenesis. Retina. 2016;36((Suppl 1)):S178–88. doi: 10.1097/IAE.0000000000001255. [DOI] [PubMed] [Google Scholar]
- 15.Jampol LM, Tauscher R, Schwarz HP. COVID-19, COVID-19 vaccinations, and subsequent abnormalities in the retina: causation or coincidence? JAMA Ophthalmol. 2021;139((10)):1135–6. doi: 10.1001/jamaophthalmol.2021.3483. [DOI] [PubMed] [Google Scholar]
- 16.Reichmuth AM, Oberli MA, Jaklenec A, Langer R, Blankschtein D. mRNA vaccine delivery using lipid nanoparticles. Ther Deliv. 2016;7((5)):319–34. doi: 10.4155/tde-2016-0006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Yang Q, Lai SK. Anti-PEG immunity: emergence, characteristics, and unaddressed questions. Wiley Interdiscip Rev Nanomed Nanobiotechnol. 2015;7((5)):655–77. doi: 10.1002/wnan.1339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sercombe L, Veerati T, Moheimani F, Wu SY, Sood AK, Hua S. Advances and challenges of liposome assisted drug delivery. Front Pharmacol. 2015;6:286. doi: 10.3389/fphar.2015.00286. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All data that support the findings of this study are included in this article. Further inquiries can be directed to the corresponding author.