Abstract
Background
In recent years, a large number of clinical and epidemiological studies have revealed the anti-cancer activity of propranolol in solid tumors, though the underline mechanism is yet to be clarified.
Methods
The proliferation of bladder cancer cells treated with propranolol was detected by MTS assays. In vivo tumor xenograft experiments were used to observe the effect of propranolol on bladder cancer growth in mice. The expression levels of Na+/H+ exchanger (NHE1) was measured by western blot. The frequency of CD8+ T cells and CD4+ T cells were detected via flow cytometry.
Results
In this study, propranolol inhibited the expression of NHE1 and sequentially led to a decrease of intracellular pH to 5.88 in MB49 cells and 6.85 in 5637 cells, thereafter, inhibited cell viability and induced apoptosis. Furthermore, propranolol inhibited the growth of bladder cancer in mice xenograft model. Flow cytometry found that the frequency of CD8+ T cells (34.58±2.11 vs. 32.34±0.6, P=0.35) and CD4+ T cells (57.80±2.45 vs. 51.44±0.79, P=0.06) in the spleen did not change compared with the control group, while the expression of IFN-γ, GZMB and T-bet secreted by CD8+ T cells increased respectively (IFN-γ 7.3±0.17 vs. 3.37±0.58, P=0.0017; GZMB 16.66±2.13 vs. 4.53±0.62, P=0.0034; T-bet 3.62±0.35 vs. 1.74±0.26, P=0.0027). Meantime, the expression of FoxP3 on CD4+ T cells decreased both in spleen and tumor tissue (1.53±0.11 vs. 0.91±0.1, P=0.004; 4.52±0.48 vs. 1.76±0.40, P=0.003).
Conclusions
These results suggested that propranolol exerted anti-proliferation and pro-apoptosis effects in bladder cancer cell by inhibiting Na+/H+ exchange and activated systemic anti-tumor immune response in vivo.
Keywords: Propranolol, bladder cancer, Na+/H+ exchanger (NHE1), CD8+ T cell
Introduction
Propranolol is a non-selective β-adrenergic receptor blocker which is mainly used clinically to treat hypertension (1), angina pectoris (2), myocardial infarction (3), and arrhythmia (4). Interestingly, growing evidences from pre-clinical and clinical studies support its anti-tumor activity in a variety of solid tumors (5-11). A pre-operative clinical study conducted by our team found that propranolol inhibited the proliferation of colorectal cancer by inhibiting the AKT (ATP-dependent tyrosine kinase)/MAPK (mitogen-activated protein kinase) signaling pathway and simultaneously immunomodulation activating autologous CD8+ T cells in tumor tissues (12). However, the underline mechanism is yet to be unveiled.
In tumor microenvironment (TME), the extracellular pH (pHe) is acidic at 6.2–6.9 compared with 7.3–7.4 in normal tissue, while the intracellular pH (pHi) is alkaline at 7.1–7.7 and this number is 6.9–7.2 in normal cells (13,14). Tumor cells produces large amount of lactic caused by glycolysis for a mass of energy consumption (15). Sequentially, H+ are transported outside the cell via carbonic anhydrases (CAs), the Na+-dependent Cl−/HCO3− exchangers, ATP synthase and sodium/proton exchanger isoform 1 (NHE1) (16). Among them, NHE1 is considered to be the most important factor in promoting tumor acidity (17). Activated NHE1 can promote Na+/H+ exchange and increased tumor pHi, thereafter affecting the expression of cell cycle regulators, promoting G1/S and G2/M transition, inducing phosphatidylinositol 4,5-biphosphate to accumulate in the cell membrane which increases MAPK activity then promote cell proliferation (18,19). Studies also revealed the negative influence of acidic TME on the anti-tumor immune response, including inhibiting the antigen presentation from DC cells, promoting infiltration of immune suppression cells and directly inhibiting the function of cytotoxic T cells (15,20). The activity of lymphokine-activated killer (LAK) and natural killer (NK) cells is also impaired in acidic TME (21). Tumor acidity negatively regulates CD8+ effector T cells, and the use of esomeprazole was able to restore the physiological pH of TME and promote the anti-tumor function of T cells (22). Interestingly, Hall et al. found that agonists stimulated β2 adrenergic receptors binding to NHERF (Na+/H+ exchanger regulatory factor) can regulate NHE effects which showed influence on pH in TME (23). This study explored the hypothesis that β-blockade with propranolol could suppress tumor proliferation, induce apoptosis and promote anti-tumor immune response by decreasing pHi via β-AR blocking mediated Na+/H+ exchange inhibition. We present the following article in accordance with the ARRIVE reporting checklist (available at https://tau.amegroups.com/article/view/10.21037/tau-22-113/rc).
Methods
Cell lines and reagents
MB49 cell line was purchased from Cell Bank of Typical Culture Preservation Committee, Chinese Academy of Sciences (Shanghai). And 5637 cell line was purchased from ATCC. MB49 was cultured in DMEM medium (Gibco, Life Technologies, China), 5637 was cultured in 1640 medium (Gibco, Life Technologies, China), and the medium were supplemented with 10% FBS (Gibco, Life Technologies Australia), 100 U/mL penicillin, and 100 µg/mL streptomycin at 37 °C and 5% CO2 in culture incubator.
Cell viability assays
The viability of bladder cancer cells was detected by CellTiter 96® AQueous One Solution Cell Proliferation Assay (MTS, Promega). Cells were plated in 96-well plates at a density of (2–4)×103 and treated with 20–140 µM propranolol (propranolol hydrochloride, P0884, Sigma-Aldrich, USA) for 24 and 48 h. Then MTS assays were detected absorbance at 490nm. Each test was carried out in three times.
BCECF AM fluorescence detection of intracellular pH
Standard curve detection: Use the BCECF AM fluorescent probe (Beyotime) to detect the fluorescence value of cells treated with phosphate buffer at pH 6.5, 7.0, 7.6, and 8.0. Then the fluorescence values of the cells treated with different concentrations of propranolol were detected. Construct a standard curve to calculate the intracellular pH value.
Apoptosis assays
Bladder cancer cells treated with different concentration propranolol were incubated with FITC and PI (Apoptosis detection kit, Beyotime) for 15 minutes. Then use flow cytometry to detect the rate of apoptosis.
Western blot analysis
Western blot analysis was performed on cell extracts of MB49 cell line treated with the corresponding inhibitory concentration of propranolol for 24 h. Immunoblotting was performed by adding RIPA Buffer (Cell Signaling Technology), dithiothreitol (DTT), and fresh protease and phosphatase inhibitors (Sigma) to the whole cell lysate. Cell lysates were quantified for protein content using a bicinchoninic acid (BCA) protein assay kit (Thermo). Sample were electrophoresed on 10% SDS-PAGE and electrotransferred onto nitrocellulose membrane by Bio-Rad Mini Protean 3 System (Hercules, CA) and then transferred to 0.22 µm nitrocellulose membrane (Bio-Rad) according to the standard protocol. The nitrocellulose membranes were then blocked with TBS containing 5% milk powder and 0.1% Tween-20 at 37 °C for 1 h, then the membranes were incubated with antibodies (diluted at 1:1,000) overnight at 4 °C. Antibodies specific for the following proteins were purchased from Cell Signaling Technology: MEK1/2 (L38C12) (mouse, 4694), ERK1/2 (rabbit, 9102), phosphor-ser221-MEK (rabbit, 2338), phospho-ERK1/2-Thr202/Tyr204 (rabbit, 4370), caspase3 (rabbit, 9662), cleaved-caspase3 (rabbit, 9664), Bcl-2 (mouse, 15071), Bax (rabbit, 2772). The antibody specific for β-actin (mouse, A5411) was purchased from Sigma. The antibody specific for NHE1(rabbit, ab67313) was purchased from Abcam. Quantification of the bands was done with Image Lab.
Animals and treatments
In order to analyze the anti-tumor immune response in vivo, an immunocompetent mice model, C57BL/6 male mice (Hunan Slack Jingda Experimental Animal Center), was used in this study. A total of 24 seven weeks old mice were injected subcutaneously into the right flank with 2×106 living MB49 cells in 100 µL medium without FBS. The sample size was decided according to the minimum requirement for statistical analysis. Tumor volume was measured 2 to 3 times/week and tumor volume calculated = W2×L/2 mm3. After tumor volume reached around 50 mm3, the mice were randomly divided into control and propranolol groups. The propranolol group divided into 10 mg/kg, 20 mg/kg, 30 mg/kg propranolol and gavaged every day. The dose of propranolol was determined according to our previous publication in colorectal cancer (12). The mice were raised in a SPF level experimental lab and sacrificed at the 19th day and tumors were removed for further analysis. The animal experiment protocol was approved by the Ethics Committee of Xiangya Hospital, Central South University (No. 2018sydw0211) and all experiments were completed with the approved guidelines of Xiangya Hospital, Central South University.
Immunohistochemistry assay
The paraffin-free tissue sections were treated with citrate antigen repair buffer (pH 6.0), and then the tissue sections were incubated with peroxidase blocking solution (S2023, Dako) for 15 minutes and blocked with protein (X0909, Dako) for 30 minutes. The corresponding specific primary antibodies were used: NHE1 (rabbit, ab67313, Abcam), phosphor-ser221-MEK (rabbit, 2338, CST), phospho-ERK1/2-Thr202/Tyr204 (rabbit, 4370, CST) and caspase3 (rabbit, 9662). After treatment, the slides were incubated overnight at 4 °C. Rabbit HRP conjugated secondary antibody (K4003, Dako) and hematoxylin (MHS32, Sigma) counterstain were used for treatment. The samples were observed with a digital microscope (Panoramic Viewer), and protein expression was analyzed by IOD/area (area density).
Flow cytometry
The tumor and spleen were made single cell suspension and passed directly through a 70 mm nylon cell filter (Corning). The cells were washed with flowing buffer (0.1% BSA in PBS) and incubated with Zombie NIRTM Fixable Viability Kit (Biolegend, 1:100) for 20 minutes at room temperature and then cells were stained with extracellular antibodies: anti-mouse CD3 FITC, anti-mouse CD8a APC, anti-mouse CD4 PerCP/Cy5.5, anti-mouse CD45 Brilliant Violet 510TM, anti-mouse CD 279 (PD-1) (Biolegend) and then intracellular staining: FoxP3/T-bet/IFN-γ/granzyme B (GZMB) after fixation and permeabilization according to the manufacturer’s protocol. All data were collected on a flow cytometer (BD Biosciences) and analyzed using FlowJo v10 software.
Statistical analysis
Data were presented as mean values ± SEM from three independent experiments. The two-way analysis of variance (ANOVA) test was used for tumor growth curve analyses. Differences between two groups were analyzed using unpaired Student’s t-test. Differences were considered significant at P values <0.05. All data were analyzed using SPSS software (SPSS 25.0).
Results
Propranolol inhibited cell viability and induced apoptosis in vitro
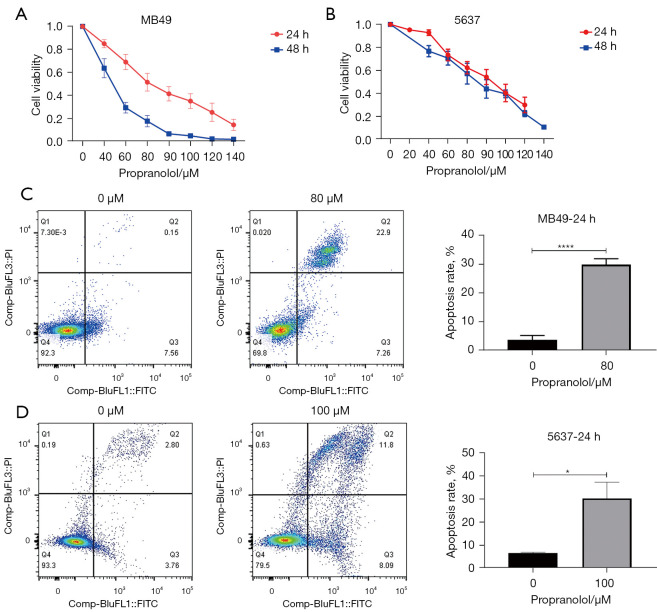
MB49 and 5637 urothelial cancer cell lines treated with propranolol for 24 and 48 h observed significant reduction in cell viability in a concentration and time dependent manner (Figure 1A,1B), with the IC50 at 24 h was 79.93 µM and 91.08 µM in MB49 and 5637 respectively. Sequentially, the cell apoptosis was assessed by flow cytometry according to the IC50. Propranolol significantly increased the apoptosis rate of MB49 (30.2% vs. 7.7%, P<0.0001) and 5637 (20% vs. 6.6%, P=0.0276) when compared with control group (Figure 1C,1D).
Figure 1.
The effect of propranolol on cell viability and apoptosis. (A,B) Cell viability of MB49 and 5637 was detected by MTS assay. (C,D) Cell apoptosis rate was detected by flow cytometry and quantification of apoptosis rate. *, P<0.05, ****, P<0.0001. All experiments were repeated three times.
Propranolol decreased the intracellular pH and the expression of NHE1
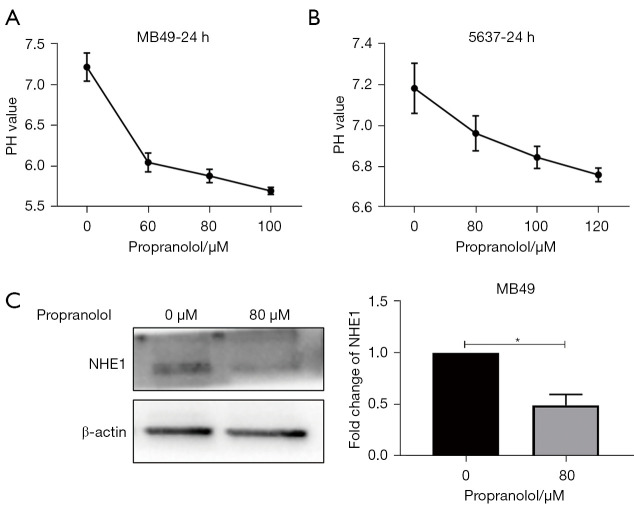
The intracellular pH was detected by BCECF fluorescent probe. When treated with increasing dosage of propranolol, the intracellular pH of MB49 and 5637 was reduced to 5.88 (80 µM) and 6.85 (100 µM) (Figure 2A,2B). Meantime, propranolol caused a nearly 50% decrease of the expression of NHE1 in MB49 cell lines (Figure 2C, P<0.05) compared with control group which explained the decrease of pHi in bladder cancer cells.
Figure 2.
The effect of propranolol on intracellular pH and NHE1 expression in vitro. (A, B) Intracellular pH of MB49 and 5637 was detected by BCECF fluorescent probe with different concentration of propranolol in 24 h. (C) The expression of NHE1 protein in MB49 treated with propranolol (80 µM) was detected by western blot and quantification of NHE1. *, P<0.05. Experiments were repeated three times. NHE1, Na+/H+ exchanger; BCECF, 2'-7'-bis(carboxyethyl)-5(6)-carboxyfluorescein.
Propranolol suppressed MAPK pathway and induced apoptosis signaling in vitro
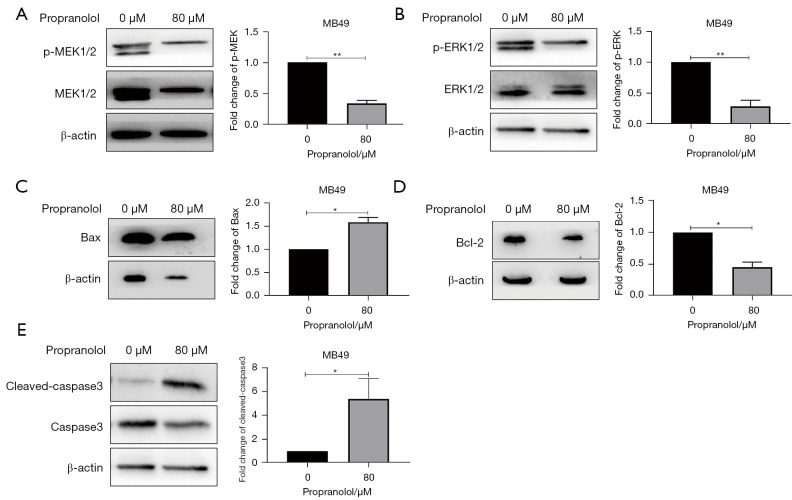
The phosphorylation level of MEK1/2 and ERK1/2 were significantly decreased 30% and 20% in propranolol group respectively when compared with control group (P<0.05, Figure 3). The expression of Bax was increased almost 50%, the expression of cleaved-caspase3 displayed a 5-fold increase (P<0.05, Figure 3C,3E), and the level of Bcl-2 was reduced 50% at the same time (P<0.05, Figure 3D). These data verified the anti-proliferation and pro-apoptosis role of propranolol in bladder cancer.
Figure 3.
The effect of propranolol on MAPK pathway and apoptosis signaling. (A-E) The expression of p-MEK1/2, p-ERK1/2, Bax, Bcl-2 and cleaved-caspase3 in MB49 treated with propranolol (80 µM) was detected by western blot. *, P<0.05, **, P<0.01. All experiments were repeated three times.
Propranolol inhibited the tumor growth in vivo
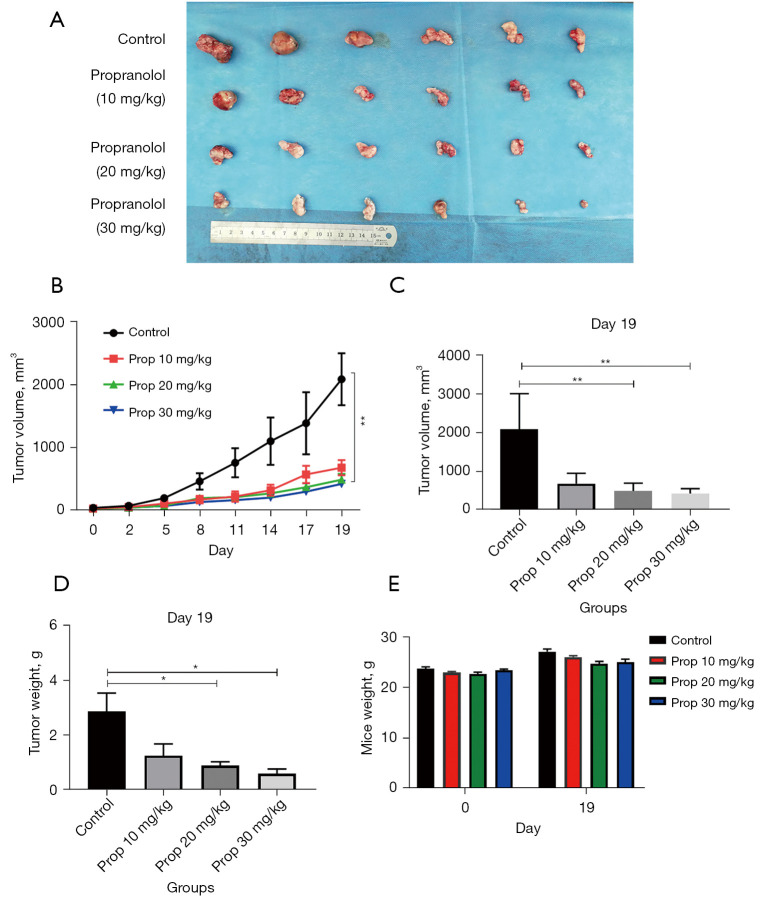
The therapeutic effect of propranolol on bladder cancer was assessed in MB49 tumor engrafted C57 mice. Once the tumor volume reached –50 mm3, mice were gavaged daily with propranolol for 10, 20, or 30 mg/kg. The mean tumor size on day 19 was significantly smaller in the three propranolol treated groups compared with treatment with vehicle alone (Control 2,090.82±412.17 vs. 688±119.86 vs. 499.14±89.59 vs. 432.32±55.81 mm3, P<0.05, Figure 4A-4C). Meantime, the tumor weight was also significantly decreased than control (2.86±0.67 vs. 1.25±0.43 vs. 0.89±1.34 vs. 0.59±1.67 g, Figure 4D). However, there was neither tumor size nor tumor weight was significantly different between propranolol 20 mg/kg and 30 mg/kg group. There was no significant change in the body weight of the mice before and after propranolol treatment (Figure 4E). Overall, these results indicated that propranolol can suppress the growth of bladder cancer without drug-limiting side effects.
Figure 4.
Propranolol inhibits the tumor growth in vivo. (A) The picture of the tumor tissue peeled off from MB49 mice on the 19th day. (B) The tumor growth curve of the MB49 mice. (C) The tumor volume of the MB49 mice on the 19th day. (D) The statistics of the mice tumor weight on the 19th day. (E) The body weight changes of propranolol-treated mice and untreated mice on day 0 and day 19. *, P<0.05; **, P<0.01, N=6 per group.
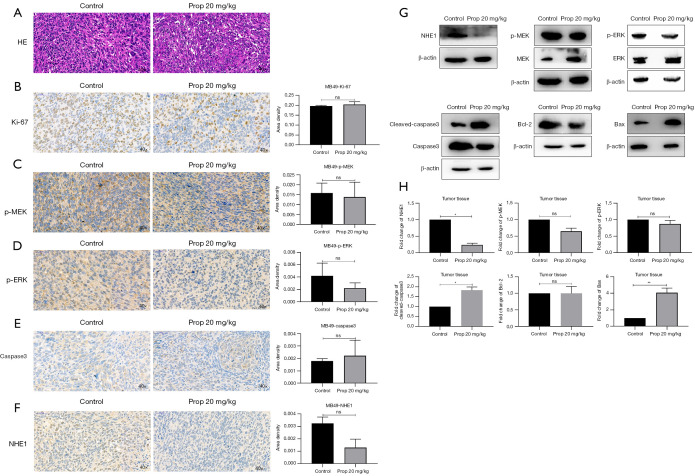
The effect of propranolol on NHE1, MAPK pathway and apoptosis signaling in vivo
The day 19 tumor tissues were stained by hematoxylin-eosin (HE) staining which showed that propranolol promoted cell necrosis in tumor, as the level of karyorrhexis and karyolysis was increased in propranolol treated group (Figure 5A). Using IHC, the expression of Ki-67, the phosphorylation level of MEK1/2, ERK1/2 and the expression of caspase3 were not significantly different with propranolol treatment (Figure 5B-5E). The expression of NHE1 displayed a trend of reduction though it was not statistically different (Figure 5F). We further assessed these markers by western blotting. Compared with the control group, the expression of NHE1 decreased by 75% (P<0.05), while the expressions of Bax and cleaved-caspase3 increased 4-fold and 1.8-fold (P<0.01 and P<0.05). Although the expressions of p-MEK1/2, p-ERK1/2, and Bcl-2 decreased slightly, this did not reach statistics significance (Figure 5G-5H). These data suggested that propranolol inhibited the expression of NHE1 in vivo at but the effect of propranolol doses that did not have dramatic effects on MAPK pathway inhibition.
Figure 5.
Propranolol affected the MAPK pathway and apoptosis signaling in vivo. (A) HE staining of control group and propranolol treatment group (hematoxylin-eosin, original magnification ×40). (B-F) Immunohistochemical detection of Ki-67, NHE1, P-MEK1/2, p-ERK1/2, caspase3 protein expression level in tumor tissue (original magnification ×40). (G-H) Western blot detection of, NHE1, p-MEK1/2, p-ERK1/2, cleaved-caspase3, Bax and Bcl-2 protein expression levels and statistics in tumor tissues. ns, no significance, *, P<0.05, **, P<0.01. Western blot was repeated three times. MAPK, mitogen-activated protein kinase; NHE1, Na+/H+ exchanger.
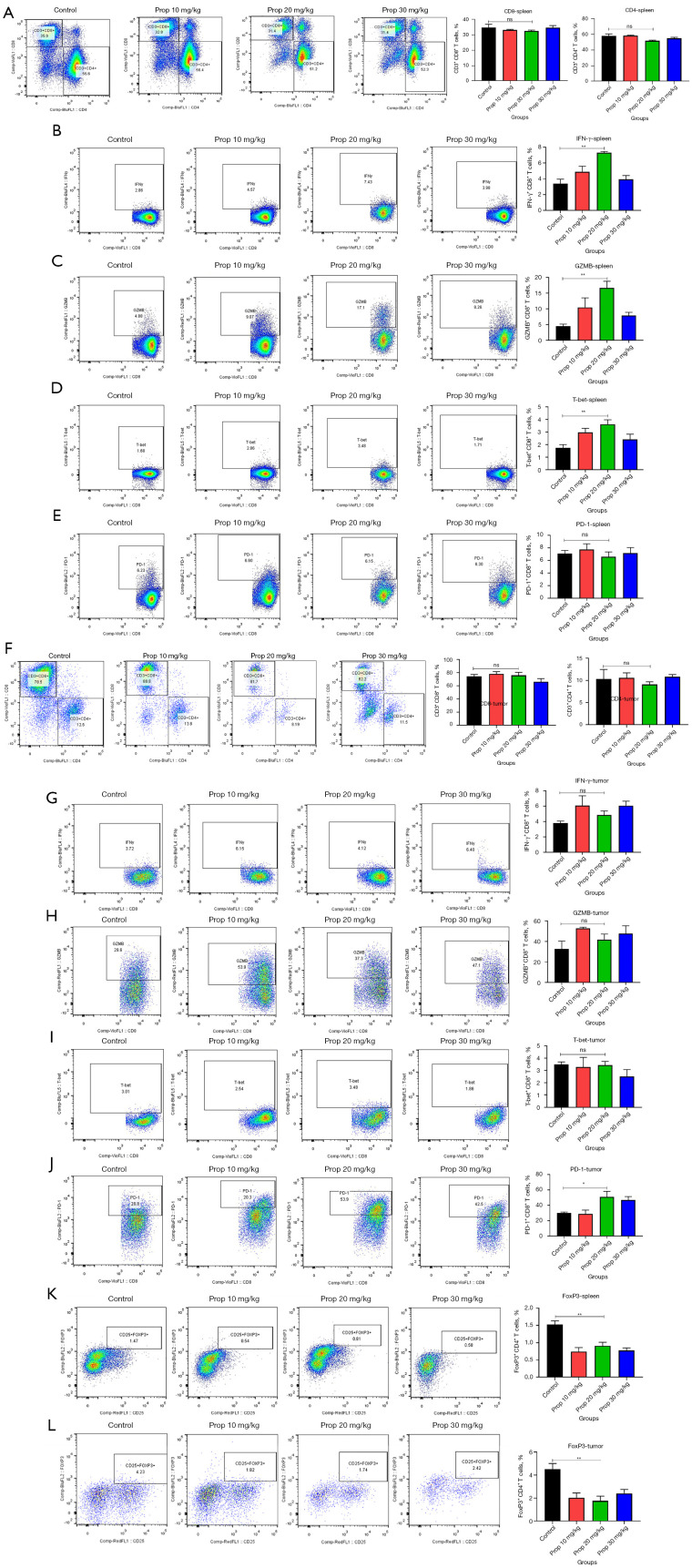
Propranolol activated anti-tumor immune response in MB49 mice
The frequency of CD4+ and CD8+ T cells in tumor tissue and spleen was assessed at day 19 via flow cytometry. Compared with the control group, propranolol did not promote the frequency of CD8+ T cells (32.34±0.6 vs. 34.58±2.11, P=0.3580, Figure 6A) and CD4+ T cells (51.44±0.79 vs. 57.80±2.45, P=0.0585, Figure 6A) in the spleen, but the frequency of IFN-γ+CD8+ T cells (3.37±0.58 vs. 7.3±0.17, P=0.0017, Figure 6B), GZMB+CD8+ T cells (4.53±0.62 vs. 16.66±2.13, P=0.0034, Figure 6C) and T-bet+CD8+ T cells (1.74±0.26 vs. 3.62±0.35, P=0.0027, Figure 6D) were significantly elevated in propranolol-treated mice compared with control. However, the expression of PD-1 on CD8+ T cells did not change significantly (7.1±0.51 vs. 6.6±0.78; P=0.6051, Figure 6E). The infiltration of CD8+ and CD4+ T cells in tumor tissues, as well as the expression of IFN-γ, GZMB, T-bet were also not significantly changed (P>0.05, Figure 6F-6I). But the expression of PD-1 was significantly reduced in tumor (30.04±1.22 vs. 51.04±7.17, P=0.0418, Figure 6J) and the frequency of FoxP3+CD4+ T cells were both significantly decreased in spleen (1.53±0.11 vs. 0.91±0.1, P=0.004, Figure 6K) and tumor tissue (4.52±0.48 vs. 1.76±0.40, P=0.0030, Figure 6L) which suggested that propranolol could potentially restore the anti-tumor immune response by counteracting immune suppressive factors.
Figure 6.
Propranolol activated anti-tumor immune response in MB49 mice. (A-E) The flow plots and quantification of CD8+, CD4+ T cells of CD3+ T cells, IFN-γ, GZMB, T-bet, PD-1 of CD8+ T cells and CD4+FoxP3+ cells in spleen of mice. (F-J) The flow plots and quantification of CD8+, CD4+ T cells of CD3+ T cells, IFN-γ, GZMB, T-bet, PD-1 of CD8+ T cells in tumor tissue. (K-L) The flow plots and quantification of CD4+FoxP3+ cells in spleen and tumor tissue of mice. *, P<0.05, **, ns, no significance, N=6 per group. IFN, interferon; GZMB, granzyme B.
Discussion
This study elucidated that propranolol treatment significantly affected in vivo bladder tumor growth. Large decreases in pHi suggested β-AR blocking mediated Na+/H+ exchange inhibition, whereas only most effects on MAPK pathway and anti-tumor immune response were observed.
NHE1 plays a major role to extrude H+ from the cytoplasm that leads to the rise of pHi (intracellular alkalinization). Reshkin et al. proved that NHE1 dependent intracellular alkalinization is a key to oncogenic proliferation and maintenance of malignant phenotype of tumor (17). NHE1 deficient cancer cells showed severely slow tumor growth in xenograft mice models (24). Therefore, pharmacological inhibition of NHE1 is rational approach to develop novel anti-cancer therapeutics (25). In addition, the activity of NHE1 can be regulated by cell surface receptors (26), such as stress hormone induced β2-AR activation could regulate NHE function via a direct binding of Na+/H+ exchanger regulatory factor (NHERF) (23). Stimulation of β2-AR by adrenaline or noradrenaline leads to growth and metastasis of many cancer types (27,28). These effects have previously been revealed to be mediated by the activation of intracellular G Protein-Coupled Receptor (GPCR) signaling pathways (29). However, certain key physiological effects of β2-AR stimulation, notably the regulation of pHi via NHE1, provided additional way to promote tumor progression. In this study, blocking β2-AR by propranolol directly decreased pHi from 7.22 to 5.88 and was associated with tumor cell death in vitro and vivo. Various cell activities require Na+/H+ exchange, including metabolism, cell cycle, angiogenesis, the maintenance of glycolysis, which may suggest that propranolol can affect tumor hallmark behaviors and exert anti-tumor activity, which requires further exploration.
Wu et al. demonstrated that low extracellular pH (pHe) does not impair the activation of T-cells, whereas it does suppress production and release of many cytokines (30). In this study, the infiltration of CD8+ and CD4+ T cells and production of IFN-γ, GZMB, T-bet in tumor tissues were not significantly changed after propranolol treatment suggesting the pHe value was not changed in line with the decrease of pHi. However, Treg cells were both significantly decreased in spleen and tumor tissue indicating that propranolol could counteract with immune suppressive factors by decreasing pHi in Treg cells.
As we know, the 5-year recurrence rate of bladder cancer is about 40%, so exploring effective treatments is imperative. This study proves that propranolol can effectively inhibit the development of bladder cancer and promote the apoptosis of tumor cells, which provides a possible mechanism for the clinical treatment of bladder cancer. This is also the first time to reveal the effectiveness of propranolol in the treatment of bladder cancer. Based on this preclinical study, our team further conducted a clinical study to explore the efficacy and safety of propranolol in the treatment of bladder cancer (NCT04493489). A large number of clinical studies of propranolol are underway, and a study on melanoma showed that propranolol treatment significantly improved the disease-free survival of patients (89% vs. 64% at 3 years; P=0.04) (31). Few adverse events were observed in the propranolol treated cancer patients from current clinical trials, which is an advantage of repurposing propranolol from a cardiac disease drug to an antitumor agent. However, in one hand, in the existing clinical trials, propranolol is generally effective in combination with other first-line antitumor drugs, which may be the limitation of propranolol as an antitumor drug in clinic. In the other hand, current clinical trials on propranolol are mostly operated in small cohort. There may be certain selection bias and statistical errors. A single-arm clinical study of gastric cancer about propranolol has completed the preliminary patient recruitment work by our team, and is undergoing efficacy evaluation and data analysis (NCT04005365). This is a good way to provides strong clinical data support for propranolol as an antitumor drug.
In summary, propranolol inhibited the growth of bladder cancer by blocking β2-AR mediated cell signaling and NHE1 inhibition caused decrease of pHi, meantime, counteracting with immune suppression in bladder cancer. Propranolol can control tumor development through a variety of mechanisms, and we found that the change of the acid-base environment of tumor cells is a new anti-cancer approach, suggesting that NHE1 may become a target for synergistic anti-tumor therapy.
Supplementary
The article’s supplementary files as
Acknowledgments
Funding: This study was supported by the National Natural Science Foundation of China (Grant Nos. 81773821 and 82173906), the Chinese National Major Project for New Drug Innovation (Grant No. 2019ZX09201-002-006), the National Key Research and Development Program (Grant No. 2016YFC0905000), the Fundamental Research Funds for the Central Universities of Central South University (Grant Nos. 198101066 and 208111176), the Key Project of the Health Commission of Hunan Province (Grant No. 202113010141), and the Natural Science Foundation of Hunan Province, China (Grant Nos. 2021JJ31063, 2021JJ31044, and 2019JJ40477).
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The animal experiment protocol was approved by the Ethics Committee of Xiangya Hospital, Central South University (No. 2018sydw0211) and all experiments were completed with the approved guidelines of Xiangya Hospital, Central South University.
Footnotes
Reporting Checklist: The authors have completed the ARRIVE reporting checklist. Available at https://tau.amegroups.com/article/view/10.21037/tau-22-113/rc
Data Sharing Statement: Available at https://tau.amegroups.com/article/view/10.21037/tau-22-113/dss
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://tau.amegroups.com/article/view/10.21037/tau-22-113/coif). HLM is on the board of directors for Vyant Bio; he is one of the founders of Clariifi LLC and a consultant to Viecure and eviCORE Health Solutions. The other authors have no conflicts of interest to declare.
References
- 1.Aronow WS. Current role of beta-blockers in the treatment of hypertension. Expert Opin Pharmacother 2010;11:2599-607. 10.1517/14656566.2010.482561 [DOI] [PubMed] [Google Scholar]
- 2.Manu P, Rogozea LM. Propranolol for Angina Pectoris. Am J Ther 2016;23:e1285-6. 10.1097/MJT.0000000000000528 [DOI] [PubMed] [Google Scholar]
- 3.Silke B, Nelson GI, Verma SP, et al. Enhanced haemodynamic effects of propranolol in acute myocardial infarction. Eur Heart J 1984;5:366-73. 10.1093/oxfordjournals.eurheartj.a061670 [DOI] [PubMed] [Google Scholar]
- 4.Kennedy HL, Brooks MM, Barker AH, et al. Beta-blocker therapy in the Cardiac Arrhythmia Suppression Trial. CAST Investigators. Am J Cardiol 1994;74:674-80. 10.1016/0002-9149(94)90308-5 [DOI] [PubMed] [Google Scholar]
- 5.Cheng Y, Gao XH, Li XJ, et al. Depression promotes prostate cancer invasion and metastasis via a sympathetic-cAMP-FAK signaling pathway. Oncogene 2018;37:2953-66. 10.1038/s41388-018-0177-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Montoya A, Varela-Ramirez A, Dickerson E, et al. The beta adrenergic receptor antagonist propranolol alters mitogenic and apoptotic signaling in late stage breast cancer. Biomed J 2019;42:155-65. 10.1016/j.bj.2019.02.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zhou C, Chen X, Zeng W, et al. Propranolol induced G0/G1/S phase arrest and apoptosis in melanoma cells via AKT/MAPK pathway. Oncotarget 2016;7:68314-27. 10.18632/oncotarget.11599 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Liao X, Che X, Zhao W, et al. The β-adrenoceptor antagonist, propranolol, induces human gastric cancer cell apoptosis and cell cycle arrest via inhibiting nuclear factor κB signaling. Oncol Rep 2010;24:1669-76. [DOI] [PubMed] [Google Scholar]
- 9.Coelho M, Moz M, Correia G, et al. Antiproliferative effects of β-blockers on human colorectal cancer cells. Oncol Rep 2015;33:2513-20. 10.3892/or.2015.3874 [DOI] [PubMed] [Google Scholar]
- 10.Lamboy-Caraballo R, Ortiz-Sanchez C, Acevedo-Santiago A, et al. Norepinephrine-Induced DNA Damage in Ovarian Cancer Cells. Int J Mol Sci 2020;21:2250. 10.3390/ijms21062250 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hu J, Lu R, Zhang Y, et al. β-adrenergic receptor inhibition enhances oncolytic herpes virus propagation through STAT3 activation in gastric cancer. Cell Biosci 2021;11:174. 10.1186/s13578-021-00687-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Liao P, Song K, Zhu Z, et al. Propranolol Suppresses the Growth of Colorectal Cancer Through Simultaneously Activating Autologous CD8+ T Cells and Inhibiting Tumor AKT/MAPK Pathway. Clin Pharmacol Ther 2020;108:606-15. 10.1002/cpt.1894 [DOI] [PubMed] [Google Scholar]
- 13.Reshkin SJ, Cardone RA, Harguindey S. Na+-H+ exchanger, pH regulation and cancer. Recent Pat Anticancer Drug Discov 2013;8:85-99. 10.2174/1574892811308010085 [DOI] [PubMed] [Google Scholar]
- 14.Cardone RA, Casavola V, Reshkin SJ. The role of disturbed pH dynamics and the Na+/H+ exchanger in metastasis. Nat Rev Cancer 2005;5:786-95. 10.1038/nrc1713 [DOI] [PubMed] [Google Scholar]
- 15.Wang JX, Choi SYC, Niu X, et al. Lactic Acid and an Acidic Tumor Microenvironment suppress Anticancer Immunity. Int J Mol Sci 2020;21:8363. 10.3390/ijms21218363 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Harguindey S, Orive G, Luis Pedraz J, et al. The role of pH dynamics and the Na+/H+ antiporter in the etiopathogenesis and treatment of cancer. Two faces of the same coin--one single nature. Biochim Biophys Acta 2005;1756:1-24. [DOI] [PubMed] [Google Scholar]
- 17.Reshkin SJ, Bellizzi A, Caldeira S, et al. Na+/H+ exchanger-dependent intracellular alkalinization is an early event in malignant transformation and plays an essential role in the development of subsequent transformation-associated phenotypes. FASEB J 2000;14:2185-97. 10.1096/fj.00-0029com [DOI] [PubMed] [Google Scholar]
- 18.Flinck M, Kramer SH, Pedersen SF. Roles of pH in control of cell proliferation. Acta Physiol (Oxf) 2018;223:e13068. 10.1111/apha.13068 [DOI] [PubMed] [Google Scholar]
- 19.Takatani-Nakase T, Matsui C, Hosotani M, et al. Hypoxia enhances motility and EMT through the Na+/H+ exchanger NHE-1 in MDA-MB-231 breast cancer cells. Exp Cell Res 2022;412:113006. 10.1016/j.yexcr.2021.113006 [DOI] [PubMed] [Google Scholar]
- 20.Rostamian H, Khakpoor-Koosheh M, Jafarzadeh L, et al. Restricting tumor lactic acid metabolism using dichloroacetate improves T cell functions. BMC Cancer 2022;22:39. 10.1186/s12885-021-09151-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Nagoshi M, Goedegebuure PS, Burger UL, et al. Successful adoptive cellular immunotherapy is dependent on induction of a host immune response triggered by cytokine (IFN-gamma and granulocyte/macrophage colony-stimulating factor) producing donor tumor-infiltrating lymphocytes. J Immunol 1998;160:334-44. [PubMed] [Google Scholar]
- 22.De Milito A, Canese R, Marino ML, et al. pH-dependent antitumor activity of proton pump inhibitors against human melanoma is mediated by inhibition of tumor acidity. Int J Cancer 2010;127:207-19. 10.1002/ijc.25009 [DOI] [PubMed] [Google Scholar]
- 23.Hall RA, Premont RT, Chow CW, et al. The beta2-adrenergic receptor interacts with the Na+/H+-exchanger regulatory factor to control Na+/H+ exchange. Nature 1998;392:626-30. 10.1038/33458 [DOI] [PubMed] [Google Scholar]
- 24.Rotin D, Steele-Norwood D, Grinstein S, et al. Requirement of the Na+/H+ exchanger for tumor growth. Cancer Res 1989;49:205-11. [PubMed] [Google Scholar]
- 25.Loo SY, Chang MK, Chua CS, et al. NHE-1: a promising target for novel anti-cancer therapeutics. Curr Pharm Des 2012;18:1372-82. 10.2174/138161212799504885 [DOI] [PubMed] [Google Scholar]
- 26.Putney LK, Denker SP, Barber DL. The changing face of the Na+/H+ exchanger, NHE1: structure, regulation, and cellular actions. Annu Rev Pharmacol Toxicol 2002;42:527-52. 10.1146/annurev.pharmtox.42.092001.143801 [DOI] [PubMed] [Google Scholar]
- 27.Blaes AH, Domingo-Musibay E, Kalinsky K. Propranolol: What is BLOCKing Its Clinical Investigation in Breast Cancer? Clin Cancer Res 2020;26:1781-3. 10.1158/1078-0432.CCR-19-3818 [DOI] [PubMed] [Google Scholar]
- 28.He RH, He YJ, Tang YJ, et al. The potential anticancer effect of beta-blockers and the genetic variations involved in the interindividual difference. Pharmacogenomics 2016;17:74-9. 10.2217/pgs.15.152 [DOI] [PubMed] [Google Scholar]
- 29.Ogasawara J, Sanpei M, Rahman N, et al. Beta-adrenergic receptor trafficking by exercise in rat adipocytes: roles of G-protein-coupled receptor kinase-2, beta-arrestin-2, and the ubiquitin-proteasome pathway. FASEB J 2006;20:350-2. 10.1096/fj.05-4688fje [DOI] [PubMed] [Google Scholar]
- 30.Calcinotto A, Filipazzi P, Grioni M, et al. Modulation of microenvironment acidity reverses anergy in human and murine tumor-infiltrating T lymphocytes. Cancer Res 2012;72:2746-56. 10.1158/0008-5472.CAN-11-1272 [DOI] [PubMed] [Google Scholar]
- 31.De Giorgi V, Grazzini M, Benemei S, et al. Propranolol for Off-label Treatment of Patients With Melanoma: Results From a Cohort Study. JAMA Oncol 2018;4:e172908. 10.1001/jamaoncol.2017.2908 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
The article’s supplementary files as