Abstract
Immune checkpoint inhibitors play an important role in the treatment of malignancies. ICIs consist of monoclonal antibodies directed against inhibitory immune receptors cytotoxic T-lymphocyte antigen 4 (CTLA-4), programmed cell death 1 (PD-1), or programmed cell death-ligand 1 (PD-L1). PD-1 is a receptor expressed by T lymphocytes and has the role of inhibiting their activation. Pembrolizumab is a humanized anti-PD-1 monoclonal antibody. It can improve the immune function of T-cells, which results in significant clinical benefit in the treatment of cancer. Despite its wide use, immunotherapy is associated with a spectrum of side effects known as immune-related adverse events. We present a case of an 82-year-old patient with widespread fibroatrophic skin areas that occurred during a treatment with pembrolizumab for non-small cell lung cancer. Clinical, serological, and histopathological examinations led to the diagnosis of generalized morphea. The patient discontinued pembrolizumab and switched to chemotherapy with pemetrexed and carboplatin. A good therapeutic response was obtained with phototherapy, corticosteroids, and topical calcineurin inhibitors. A focus on the therapeutic management of this skin toxicity in oncological patients is provided.
Keywords: Scleroderma-like syndrome, Pembrolizumab, Morphea, Dermatologic management, Non-small cell lung cancer
Introduction
Immune checkpoint inhibitors (ICI) play an important role in the treatment of malignancies. They consist of monoclonal antibodies directed against inhibitory immune receptors cytotoxic T-lymphocyte antigen 4 (CTLA-4; ipilimumab), programmed cell death 1 (PD-1; nivolumab, pembrolizumab, and cemiplimab), and programmed death-ligand 1 (PD-L1; atezolizumab, avelumab, and durvalumab) [1].
Cancer cells can block immune responses through the PD-1 system by expressing the two ligands of PD-1, PD-L1 and PD-L2. Pembrolizumab is a humanized anti-PD-1 monoclonal antibody used in the treatment of several cancers such as advanced melanoma, non-small cell lung cancer (NSCLC), classical Hodgkin's lymphoma, urothelial cancer, renal cell carcinoma, squamous cell carcinoma of the head and neck, oesophageal cancer, and colorectal cancer. Despite the clinical benefit in the treatment of malignancies, immunotherapy is not devoid of side effects related to the activation of the immune system. The side effects triggered by these agents are called immune-related adverse events [2]. Skin toxicities, mainly in the form of generalized maculopapular rash and pruritus, are the most frequent, with an incidence of 30–40% in patients treated with anti-PD-1 immunotherapy. Less commonly other dermatological manifestations can occur, including vitiligo, lichenoid eruptions, psoriasis, and autoimmune skin disorders (e.g., bullous pemphigoid, dermatomyositis, alopecia areata, and scleroderma) [3]. Only a few ICI-induced scleroderma and scleroderma-like cases have been reported so far. We report a rare case of a generalized morphea/scleroderma-like syndrome that occurred during pembrolizumab therapy in a patient affected by NSCLC.
Case Report/Case Presentation
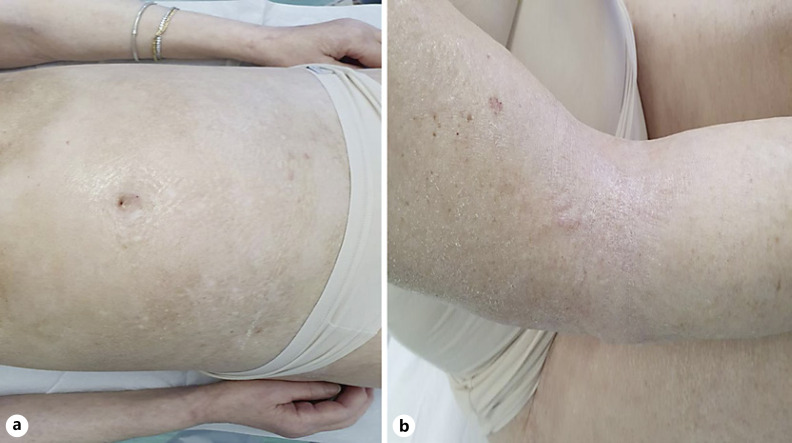
An 82-year-old Caucasian female referred to our outpatient clinic for oncological patients with a 1-month history of skin tightening. She was diagnosed with metastatic NSCLC 2 years before the admission. Since the genetic investigation and the immunohistochemistry resulted in negative for EGFR and BRAF mutations, negative for the presence of the ALK1 protein and positive for the presence of PDL-1, the patient started pembrolizumab therapy (200 mg every 3 weeks). After 7 months of therapy with pembrolizumab, she developed fibroatrophic skin areas symmetrically distributed, affecting her chest, abdomen, and proximal upper and lower limbs (shown in Fig. 1a, b). These clinical lesions rapidly appeared and were painful, caused discomfort, severe limitation in the range of motion (especially in stretching arms and thighs), and impaired daily activities and social relationships. The patient had no prior familial history of autoimmune diseases, no history of Raynaud phenomenon (a common early feature of systemic sclerosis) and the clinical evaluation for other signs and symptoms of systemic sclerosis, such as nail fold capillary changes, sclerodactyly, or acrosclerosis resulted negative.
Fig. 1.
aFibroatrophic areas of thickened skin in the abdominal region with post-inflammatory hyperpigmentation.bSkin thickening located on flexural regions of limbs.
Laboratory tests, including cell blood count, antinuclear antibodies, extractable nuclear antigen, anti-single-stranded DNA, anti-double-stranded DNA, anti-histone, anti-topoisomerase I (Scl 70), antiphospholipid, rheumatoid factor, HBV, and HCV markers were performed and the results were normal with the exception of the positivity for anti-HCV antibodies. The HCV-RNA assay detected >953,000 UI/mL copies of viral genome, so the patient underwent therapy with direct-acting antivirals. The patient was tested for Borrelia Burgdorferi, Epstein-Barr and Varicella Zoster virus infections in order to exclude localized scleroderma and scleroatrophic lichen associated disease with no significant results. To exclude paraneoplastic sclerodermia, the patient underwent positron-emission tomography/computed tomography that showed no progression of the cancer. Histopathologic examination of an incisional skin biopsy showed diffuse dermal sclerosis, atrophy of the adnexal structures, and perivascular and interstitial lymphoplasmacytic infiltrates. The absence of both clinical cutaneous features and internal organ involvement typical of sistemic sclerosis and the result of the biopsy led to a diagnosis of cutaneous generalized morphea induced by immunotherapy.
She discontinued pembrolizumab and switched to chemotherapy with pemetrexed and carboplatin. Prednisone was promptly started at a dose of 25 mg/day. In addition, she was treated with topical emollients containing vitamin E with clinical benefit. The patient stopped taking prednisone therapy after 4 weeks due to the onset of oedema. Therefore, we attempted to control the disease with topical corticosteroids and topical tacrolimus with good results, and the patient underwent phototherapy. At 3-month follow-up, the oncological disease was under control and after only 4 sessions of phototherapy the patient experienced less induration of the plaques, less tenderness and improved range of motion of the arms.
Discussion/Conclusion
The risk of developing morphea with ICIs is known, but reliable data on its prevalence and incidence are still lacking. Although the pathogenesis of cutaneous sclerosis has not been completely clarified, it is considered to be mediated by the activation of the immune system. The CD4+ T lymphocytes predominate in skin infiltrates by causing the release of interleukins (IL-2, profibrotic IL-4, IL-6, and the IL-2 receptor) and growth factors such as transforming growth factor beta (TGF-β) that initiate and perpetuate the process of fibrosis. It is believed that the primary link between immunologic alterations and fibrosis are Th2 lymphocytes that release profibrotic cytokines such as IL-4, which induce the production of TGF-β and the production of collagen by fibroblasts. Fibroblasts in patients with scleroderma have a particular phenotype characterized by abnormal collagen production and increased secretion of cytokines such as TGF-β and platelet-derived growth factor [4]. Autoantibodies are repeatedly negative.
PD-1, which is induced during T-cell activation, and its ligands, PD-L1 and PD-L2, has an important inhibitory function in the regulation of immune homoeostasis. When therapy with pembrolizumab is carried out, the regulatory function of PD-1-mediated lymphocyte activation and the maintenance of peripheral tolerance are lost [5]. Therefore, T-cell activation promoted by immunotherapeutic agents leads to immunological processes that induce skin sclerosis.
The appearance or exacerbation of sclerosis in a patient with cancer can be physically and psychologically devastating because the patient is already ravaged by the malignancy and limited therapeutic options are available. There is no unique approach to the management of immunotherapy-related cutaneous sclerosis so far. Diagnostic workup should include laboratory tests and a biopsy of the skin lesion for routine histology.
In most cases, the skin tightening can be controlled with topical treatments (emollients, skin elasticizers like vitamin E) and systemic steroids. In non-severe forms, ICI may be maintained with close monitoring. Other therapies can be considered such as topical vitamin D, topical tacrolimus, phototherapy, metotrexate, idroxyclorochine, and intravenous immunoglobulin [6]. Long-term systemic therapies with corticosteroids can lead to side effects such as immunosuppression, increased blood pressure, hyperglycaemia, and oedema that can interfere with the therapeutic management of the oncological patient. Specifically, our patient developed oedema 4 weeks after the initiation of prednisone. Therefore, we attempted to control the disease with topical corticosteroids and topical tacrolimus with good results, and the patient underwent phototherapy for its efficacy and safety profile in scleroderma-like syndrome (except for patients with melanoma history) [7].
At the same time, the lung cancer remained under control with chemotherapy. It is in line with recent data in the literature which state that patients who experience immune-related adverse events while on therapy with anti-PD-1 and anti-PD-L1 antibodies, experience improved outcomes as measured by overall response rate, progression-free survival, and overall survival [8].
In conclusion, we report a very rare case of scleroderma induced by immunotherapy. It is important to report cases of scleroderma emerging in patients treated with ICIs. Moreover, close collaboration between dermatologists and oncologists is fundamental in order to evaluate the most correct therapeutic management of the patient.
Statement of Ethics
This article was conducted ethically in accordance with the World Medical Association Declaration of Helsinki. Ethics approval is not required in accordance with local/national guidelines. The patient's written informed consent to publish the case (including publication of images) was obtained. Information revealing the subject's identity was avoided.
Conflict of Interest Statement
The authors have no conflicts of interest to declare.
Funding Sources
No funding received.
Author Contributions
Davide Fattore, Teresa Battista, and Gabriella Fabbrocini: conceptualization, validation, visualization, writing-original draft preparation, and writing − review & editing. Mario De Lucia: data curation, formal analysis, investigation, visualization, and writing-original draft preparation. Maria Carmela Annunziata: data curation, investigation, methodology, visualization, writing-original draft preparation, and writing-review & editing.
Data Availability Statement
Data are reported in the current study. Further enquiries can be directed to the corresponding author.
References
- 1.Muntyanu A, Netchiporouk E, Gerstein W, Gniadecki R, Litvinov IV. Cutaneous immune-related adverse events (irAEs) to immune checkpoint inhibitors: a dermatology perspective on management [formula: see text] J Cutan Med Surg. 2021 Jan–Feb;25((1)):59–76. doi: 10.1177/1203475420943260. [DOI] [PubMed] [Google Scholar]
- 2.Postow MA, Callahan MK, Wolchok JD. Immune checkpoint blockade in cancer therapy. J Clin Oncol. 2015;33((17)):1974–82. doi: 10.1200/JCO.2014.59.4358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sibaud V. Dermatologic reactions to immune checkpoint inhibitors: skin toxicities and immunotherapy. Am J Clin Dermatol. 2018 Jun;19((3)):345–61. doi: 10.1007/s40257-017-0336-3. [DOI] [PubMed] [Google Scholar]
- 4.Rongioletti F, Ferreli C, Atzori L, Bottoni U, Soda G. Scleroderma with an update about clinico-pathological correlation. G Ital Dermatol Venereol. 2018 Apr;153((2)):208–15. doi: 10.23736/S0392-0488.18.05922-9. [DOI] [PubMed] [Google Scholar]
- 5.Panariello L, Fattore D, Annunziata MC, Piantedosi F, Gilli M, Fabbrocini G. Bullous pemphigoid and nivolumab: dermatologic management to support and continue oncologic therapy. Eur J Cancer. 2018 Nov;103:284–6. doi: 10.1016/j.ejca.2018.08.022. [DOI] [PubMed] [Google Scholar]
- 6.Apalla Z, Nikolaou V, Fattore D, Fabbrocini G, Freites-Martinez A, Sollena P, et al. European recommendations for management of immune checkpoint inhibitors-derived dermatologic adverse events. The EADV task force “dermatology for cancer patients” position statement. J Eur Acad Dermatol Venereol. 2022;36((3)):332–50. doi: 10.1111/jdv.17855. [DOI] [PubMed] [Google Scholar]
- 7.Hassani J, Feldman SR. Phototherapy in scleroderma. Dermatol Ther. 2016 Dec;6((4)):519–53. doi: 10.1007/s13555-016-0136-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Das S, Johnson DB. Immune-related adverse events and anti-tumor efficacy of immune checkpoint inhibitors. J Immunother Cancer. 2019 Nov 15;7((1)):306. doi: 10.1186/s40425-019-0805-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data are reported in the current study. Further enquiries can be directed to the corresponding author.