Abstract
Clavulanic acid is a potent inhibitor of β-lactamase enzymes and is of demonstrated value in the treatment of infections by β-lactam-resistant bacteria. Previously, it was thought that eight contiguous genes within the genome of the producing strain Streptomyces clavuligerus were sufficient for clavulanic acid biosynthesis, because they allowed production of the antibiotic in a heterologous host (K. A. Aidoo, A. S. Paradkar, D. C. Alexander, and S. E. Jensen, p. 219–236, In V. P. Gullo et al., ed., Development in industrial microbiology series, 1993). In contrast, we report the identification of three new genes, orf10 (cyp), orf11 (fd), and orf12, that are required for clavulanic acid biosynthesis as indicated by gene replacement and trans-complementation analysis in S. clavuligerus. These genes are contained within a 3.4-kb DNA fragment located directly downstream of orf9 (cad) in the clavulanic acid cluster. While the orf10 (cyp) and orf11 (fd) proteins show homologies to other known CYP-150 cytochrome P-450 and [3Fe-4S] ferredoxin enzymes and may be responsible for an oxidative reaction late in the pathway, the protein encoded by orf12 shows no significant similarity to any known protein. The results of this study extend the biosynthetic gene cluster for clavulanic acid and attest to the importance of analyzing biosynthetic genes in the context of their natural host. Potential functional roles for these proteins are proposed.
Streptomyces clavuligerus is a gram-positive, filamentous bacterium that produces clavulanic acid (see Fig. 1, compound 7), a potent inhibitor of serine β-lactamases (classes A, B, and D). The combined use of clavulanic acid and broad-spectrum β-lactam antibiotics such as amoxicillin has represented an important therapeutic strategy to combat the rapid increase in β-lactam resistance (9).
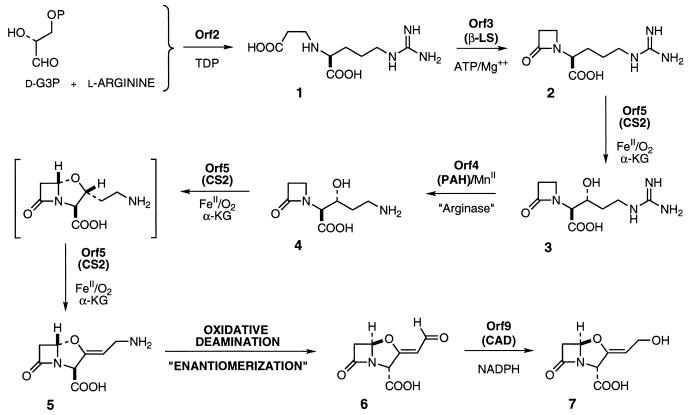
FIG. 1.
A diagram of the clavulanic acid biosynthetic pathway.
Bailey et al. first described the cloning of a genetic locus involved in clavulanic acid production by complementation of a nonproducing mutant (11). Later, Jensen et al. reported a 12-kb EcoRI fragment from the genome of S. clavuligerus which contained all the genetic information required for the production of clavulanic acid in the heterologous host, Streptomyces lividans (31). This DNA fragment is located immediately downstream of the terminal pcbC gene of the cephamycin C cluster (61). DNA sequencing revealed eight complete open reading frames (ORFs) that were assigned originally as orf2 to orf9 (25, 31, 64).
Rapid progress has been made recently to elucidate the early and middle steps of the biosynthetic pathway. The long-elusive C3 carbohydrate that is combined with l-arginine in a thiamine diphosphate-dependent step catalyzed by the protein encoded by orf2 has been identified as d-glyceraldehyde-3-phosphate (32). The product of this unusual reaction, N2-(2-carboxyethyl)-l-arginine (Fig. 1, compound 1), is cyclized by a β-lactam synthetase (B-LS) encoded by orf3 to give the monocyclic β-lactam deoxyguanidinoproclavaminic acid (Fig. 1, compound 2) (8, 38). The gene products of orf4 and orf5, proclavaminate amidino hydrolase (PAH) and clavaminate synthase isozyme 2 (CS2), respectively, function alternately to carry deoxyguanidinoproclavaminic acid by hydroxylation to guanidinoproclavaminic acid (12), to mediate an arginase-like reaction to proclavaminic acid (Fig. 1, compound 4) (1, 65), and to govern successive oxidative cyclization and desaturation to clavaminic acid (Fig. 1, compound 5) (13, 18, 23, 54).
In contrast, the final steps of the pathway from clavaminic acid (Fig. 1, compound 5) to clavulanic acid (compound 7) are poorly understood. A pathway-specific regulatory gene orf8 (claR) that controls the expression of late genes has been identified (47, 50). Downstream lies orf9 (cad) which encodes clavulanic acid dehydrogenase (CAD), an enzyme responsible for the reduction of clavulanate-9-aldehyde (compound 6) to clavulanic acid (compound 7) (42). The mechanism of the unusual “oxidative enantiomerization” between clavaminic acid (compound 5) and clavulanate-9-aldehyde (compound 6), however, remains unknown. Nonetheless, it has been observed that the allylic hydroxyl group of clavulanic acid (compound 7) is derived from molecular oxygen (34). This finding implies that the deamination of clavulanic acid does not occur by transamination, as might be expected, but by a hydroxylation process. Yet the eight genes noted above, which were believed to be sufficient to support clavulanic acid biosynthesis, encode no apparent oxygenase enzyme apart from CS2. This contradiction led us to sequence further downstream of orf9 (cad) in the hope of locating the “missing” hydroxylase gene. A further suggestion that the clavulanic acid biosynthetic cluster might be larger than previously appreciated could be found in the reported dclC locus, which mapped to ca. 4 kb downstream of orf9 (61) and complemented a clavulanic acid nonproducing mutant (11).
To test this hypothesis, we have cloned and sequenced a 3.4-kb region downstream of the 12-kb gene cluster (2). Sequence analysis revealed three complete ORFs, orf10 (cyp), orf11 (fd), and orf12. Gene disruption and trans complementation have demonstrated the involvement of these new genes in the biosynthesis of clavulanic acid. The cotranscription of orf10 (cyp) and orf11 (fd) and the high similarities of their products to cytochrome P-450 and ferredoxin proteins are proposed to accommodate the missing oxidation step required late in the biosynthesis of clavulanic acid.
MATERIALS AND METHODS
Bacterial strains and plasmids.
The bacterial strains and plasmids used in this study are listed in Table 1.
TABLE 1.
Bacterial strains and plasmids used in this study
| Strain or plasmid | Relevant characteristics | Reference or source |
|---|---|---|
| Strains | ||
| E. coli | ||
| DH5α | F−recA1 gyrA96 thi-1 hsdR17 supE44 relA1 deoR (lacZYA-argF)U196 φ80 ΔlacZΔM15 | Gibco BRL |
| JM110 | dam dcm supE44 thi leu rpsL lacY galK galT ara tonA thr Tsx Δ(lac-proAB) F′[traD36 proAB+ lacZΔM15] | Stratagene |
| NovaBlue | endA1 hsdR17 (rK12− mK12+) supE44 thi-1 recA1 gyrA96 relA1 lac [F′ proA+B+laclqZΔM15::Tn10(Tcr)] | Novagen |
| SC 12155 | β-Lactam-supersensitive indicator strain | 6 |
| K. pneumoniae subsp. pneumoniae ATCC 29665 | Indicator strain for clavulanic acid bioassay | 53 |
| S. clavuligerus | ||
| ATCC 27064 | Clavulanic acid producer, wild-type strain | ATCCa |
| RFL 10-5 | orf10 (cyp) insertional disruption mutant (orf10::tsr) | This study |
| RFL 10-5–P450ca | RFL 10-5 with plasmid pLRF38 | This study |
| RFL 5-56 | orf12 insertional disruption mutant (orf12::tsr) | This study |
| RFL 5-56–CA12 | RFL 5-56 with plasmid pLCA12 | This study |
| S. lividans TK24 | str-6; plasmidless cloning host (SLP2− SLP3−) | 27 |
| Plasmids | ||
| pBluescript II SK(−) | Phagemid; Ampr | Stratagene |
| High-copy-number Streptomyces promoter-probe vector, Thior | 60 | |
| pIJ680 | High-copy-number Streptomyces cloning vector, Thior Neor | 27 |
| pKC1139 | Streptomyces-E. coli bifunctional vector, Amr | 15 |
| pL8 | A cosmid clone isolated from a S. clavuligerus genomic library containing clavulanic acid gene cluster | 36 |
| pL1011 | pT7Blue-3 containing RT-PCR product of intergenic region of orf10 (cyp) and orf11 (fd) | This study |
| pLCA12 | pWHM1109 containing orf12 | This study |
| pLRF30 | pLRF66 containing tsr-disrupted orf10 (cyp) from pLRF450T | This study |
| pLRF37 | pUC19 containing orf12 | This study |
| pLRF38 | pKC1139 containing 1.5-kb orf10 (cyp) and upstream region | This study |
| pLRF39 | pUC19 containing tsr-disrupted orf12 | This study |
| pLRF40 | pLRF66 containing tsr-disrupted orf12 from pLRF39 | This study |
| pLRF450 | pBluescript II SK(−) containing orf10 (cyp) | This study |
| pLRF450T | pBluescript II SK(−) containing tsr-disrupted orf10 (cyp) | This study |
| pLRF66 | Derived from pIJ680 by deletion of tsr, Neor | R. Li, B. O. Bachmann, T.-K. Wu, and C. A. Townsend, unpublished data |
| pLRF90 | pBluescript II SK(−) containing a 9.0-kb EcoRI-KpnI genomic DNA fragment from S. clavuligerus covered orf10 (cyp), orf11 (fd), orf12, and downstream region | This study |
| pT7Blue-3 | E. coli cloning vector, Ampr Kanr | Novagen |
| pUC19 | E. coli cloning vector, Ampr | 55 |
| pWHM1109 | Streptomyces-E. coli biofunctional cloning and Streptomyces expression vector, Kanr Thior | 58 |
ATCC, American Type Culture Collection.
Media and culture conditions.
Escherichia coli strains were grown in either Luria broth or terrific broth (TB) as liquid medium or agar plates. S. lividans was grown on R2YE medium (27). In the case of plasmid-containing cultures, thiostrepton (20 μg/ml for S. lividans and 10 μg/ml for S. clavuligerus) or neomycin (15 μg/ml for both strains) was added. S. clavuligerus was maintained on slant-plate (SP) medium containing (per liter) 10 g of yeast extract, 10 g of glycerol, and 20 g of Bacto-agar, pH 6.8. Seed medium consisting of tryptic soy broth (Difco, Detroit, Mich.) was inoculated with spores of S. clavuligerus and was incubated at 28°C on a rotary shaker (300 rpm) for 72 h. For clavulanic acid production, mycelia from the seed cultures were inoculated into starch-aspargine (SA) medium (48) at 5%, and this culture was grown under the same conditions as the seed culture.
All restriction endonucleases, DNA ligase, and T4-DNA polymerase were purchased from New England Biolabs, Inc. (Beverly, Mass.). Pfu DNA polymerase and the Moloney murine leukemia virus (MMLV) reverse transcriptase were obtained from Stratagene (La Jolla, Calif.). All enzymes were used as recommended by the manufacturers. Apramycin was kindly provided by Eli Lilly and Company (Indianapolis, Ind.). Neomycin and ampicillin were obtained from Sigma Chemical Co. (St. Louis, Mo.). Thiostrepton and benzylpenicillin were purchased from Fluka (Ronkonkoma, N.Y.). Oligonucleotides used in this study (Table 2) were synthesized at in the Peptide/Protein Facility, Department of Biological Chemistry, The Johns Hopkins School of Medicine.
TABLE 2.
Oligonucleotide primers used in this study
| Primer | Sequencea | Function |
|---|---|---|
| ORF101 | 5′-TCTAGAATTCCATATGATGAACGAGGCAGCGC-3′ | 5′ orf10 PCR primer |
| ORF102 | 5′-GCTGCAGAAGCTTCACCAGGTCACCGGGAG-3′ | 3′ orf10 PCR primer |
| ORF12-5 | 5′-AACTGCAGCATATGGGCGACGCGGCCC-3′ | 3′ orf12 PCR primer |
| ORF12-3 | 5′-CGGGATCCGAATTCTCATCGCCGGGCG-3′ | 3′ orf12 PCR primer |
| RT 10-5 | 5′-CCGGGTGGAGCTGGAGGAGATCCT-3′ | 5′ orf10 primer for RT-PCR of CA-10-11 |
| RT 11-3 | 5′-CGGGATCCGAATTCCTAGCCCTCGGTGAC-3′ | 3′ orf11 primer for RT-PCR of CA-10-11 |
| RT 11-5 | 5′-AAGCTTGTGGAGCGGCTGACCGTC-3′ | 5′ orf11 primer for RT-PCR of CA-11-12 |
| RT 12-3 | 5′-CCGGGGCAGTCCGGACGAGGTCAG-3′ | 3′ orf12 primer for RT-PCR of CA-11-12 orf11 and orf12 |
The restriction sites introduced into primers are underlined.
Recombinant DNA procedures.
E. coli and Streptomyces plasmid DNA was isolated by standard methods (27, 55) and was purified by using the Genieprep DNA Isolation Kit (Ambion Inc., Austin, Tex.). Genomic DNA from S. clavuligerus (ATCC 27064) and disruption mutants was isolated as described by Hopwood et al. (27) and was purified with the QIAamp Tissue Kit (QIAGEN, Chatsworth, Calif.). Transformation of E. coli strains was preformed by standard procedures (55). Protoplast formation, regeneration, and DNA transformation of S. lividans TK24 was carried out as described previously (27).
For pLRF30 construction, orf10 (cyp) was amplified by PCR in a 100-μl volume containing 200 ng of pLRF90 template DNA, 100 μM concentrations each of dATP, dTTP, dCTP, and dGTP, the two primers ORF101 and ORF102 (Table 2) at 0.2 μM each, and 1× cloned Pfu reaction buffer [10 mM KCl, 10 mM (NH4)2SO4, 20 mM Tris-HCl (pH 8.8), 2 mM MgSO4, 0.1% Triton X-100, and 100 μg of bovine serum albumin per ml]. The solution was covered with mineral oil and heated to 98°C for 5 min, 1 μl (2.5 U) of Pfu DNA polymerase was added, and 30 reaction cycles were performed. The temperature and time periods used in each cycle were 95°C for 1 min, 58°C for 75 s, and 72°C for 90 s. The purified PCR product was ligated into the EcoRI-HindIII linearized pBluescript II SK(−) to give recombinant pLRF450. The 1.1-kb thiostrepton resistance gene (tsr) fragment was excised by digestion of pIJ680 with BclI and was ligated to the unique BclI internal site of orf10 (cyp) on pLRF450 to generate plasmid pLRF450T. Plasmid pLRF30, used for the disruption of orf10 (cyp) in S. clavuligerus, was constructed by insertion of the 2.3-kb tsr-disrupted orf10 (cyp) into the replicationally unstable vector pLRF66 derived from pIJ680 (R. Li, B. O. Bachmann, T.-K. Wu, and C. A. Townsend, unpublished data).
orf12 was amplified by PCR under the same conditions as orf10 (cyp) except for using primers ORF12-5 and ORF12-3 (see Table 2) and 56°C as the annealing temperature. The purified PCR product was inserted into pUC19 to give pLRF37. The tsr gene was inserted into the unique MluI site within orf12 by blunt-end ligation to generate plasmid pLRF39. The tsr-disrupted orf12 was ligated into pLRF66 to give the orf12 disruption recombinant pLRF40.
Expression vector pLRF38 was constructed by insertion of a 1.5-kb BglII-BglII orf10 (cyp) gene fragment excised from pL8 into the BamHI-linearized Streptomyces-E. coli bifunctional vector pKC1139.
To complement the orf12 mutant, pLCA12 was constructed as follows. There are two NdeI sites present in the Streptomyces expression vector pWHM1109, so it was completely digested with HindIII, followed by partial digestion with NdeI. The 9.3-kb linearized vector was purified and ligated into the NdeI-HindIII-ended orf12 fragment. Colonies were screened by colony hybridization with the orf12 probe. The recombinant plasmids with orf12 located downstream of the thiostrepton-inducible promoter (PtipA) were confirmed by endonuclease digestion.
Southern blot analysis and colony hybridization were carried out by standard methods (7) or by the shampoo method developed by May (37).
DNA sequencing and analysis.
Template DNA was purified with the QIAGEN Plasmid Mini Kit or the GeniePrep DNA Isolation Kit. Double-stranded DNA sequencing was carried out by chromosomal walking with universal and custom oligonucleotide primers. All DNA sequencing was accomplished with the PRISM Dye Terminator Cycle-sequencing Ready Reaction Kit (ABI, Foster City, Calif.) or the PE-Applied Biosystems 377 Prism DNA Sequencer in the Peptide/Protein Facility, Department of Biological Chemistry, The Johns Hopkins School of Medicine. Nucleotide sequence data were analyzed with Sequencher, version 3.0 (Gene Codes Corporation, Ann Arbor, Mich.), MacVector, version 6.5 (Oxford Molecular Ltd., Campbell, Calif.), and FramePlot, version 2.3 (found on the home page of The Society for Actinomycetes, Japan).
Transformation of S. clavuligerus.
The conditions for protoplast formation, regeneration, and DNA transformation were modified from the methods of Dominguez et al. (20), Illing et al. (28), and Malmberg et al. (35). About 109 wild-type or mutant S. clavuligerus spores were inoculated into 50 ml of YEMEG broth (20) in a 250-ml flask containing glass beads and were grown at 26°C with rotary shaking for 60 h. Mycelia were harvested by centrifugation and were washed twice with 10.3% sucrose and once with P buffer (0.31% Tris-HCl [pH 8.0], 0.368% CaCl2 · 2H2O, 0.204% MgCl2 · 6H2O, 10% sucrose, and 1% glucose). The pellet was resuspended in P buffer containing 2 mg of lysozyme per ml to a final volume of 10 ml and was incubated at 30°C for 25 min. The protoplast-mycelia mixture was filtered through a sterile cotton plug. The protoplasts were collected by centrifugation at 1,000 × g for 10 min at 4°C, were washed three times with ice-cold P buffer, and were diluted to a final concentration of approximately 109 protoplasts/ml. Before DNA transformation, about 108 protoplasts were preheated in a 45°C water bath for 10 min to inactivate the S. clavuligerus restriction system (10). The heat-treated protoplasts were transformed with 2 μg of DNA, and 500 μl of 25% (wt/vol) polyethylene glycol 1000 (NBS Biologicals, Hatfield, United Kingdom) solution was added immediately (27). After incubation at room temperature for 1 min, the transformed protoplasts were diluted with 2.5 ml of ice-cold P buffer, were collected by centrifugation, and were resuspended in 1 ml of P buffer. Each predried R2YEG regeneration plate (35) was plated with 100 μl of transformed protoplasts and was incubated at 26°C. The plates were overlaid with 1.5 ml of thiostrepton solution at a final concentration of 5 μg/ml or apramycin at a final concentration of 10 μg/ml.
Selection of double-crossover strains.
A single thiostrepton-resistant colony of S. clavuligerus (pLRF30) or S. clavuligerus (pLRF40) grown on R2YEG plates (containing 5 μg of thiostrepton per ml) was used to inoculate tryptic soy broth seed medium containing 5 μg of thiostrepton per ml. After growth for 72 h at 26°C, 1 ml of the medium was inoculated into 50 ml of YEMEG medium containing 5 μg of thiostrepton per ml. Protoplast formation was carried out as described above. Following serial dilution by 104, 105, and 106 protoplasts with P buffer, a 100-μl aliquot of diluted protoplasts was spread on predried R2YEG plates lacking antibiotics. Following 120 h of growth at 26°C, colonies were picked randomly from each strain and transferred onto SP plates containing 5 μg of thiostrepton per ml. Colonies grown on these thiostrepton-containing plates were subsequently replicated onto SP plates containing 10 μg of neomycin per ml. The resulting thiostrepton-resistant (Thior) and neomycin-sensitive (Neos) strains were analyzed by Southern hybridization and then assayed for clavulanic acid production.
Analysis of β-lactam antibiotics.
Bioassay detection of β-lactams produced by S. clavuligerus was performed by the agar plate diffusion method. Clavulanic acid was determined by the β-lactamase inhibition assay with Klebsiella pneumoniae subsp. pneumoniae and benzylpenicillin (53). Penicillin, cephalosporin, and cephamycin were detected with E. coli SC 12155 seeded in nutrition agar (6).
Clavulanic acid and its bicyclic β-lactam-containing intermediates were also detected by reaction with imidazole (16). Filtered fermentation supernatant was reacted with 0.25 equivalent volumes of 3 M imidazole reagent (pH 6.8) at 40°C for 20 min. The product of the imidazole reaction showed a maximum absorbance at 312 nm.
High-pressure liquid chromatography (HPLC) analysis was performed with a Waters 600 multisolvent delivery system consisting of a Waters 490 Programmable Multiwavelength Detector (Waters, Mississauga, Ontario, Canada) fitted with a model 7125 injector (Rheodyne, Cotati, Calif.). A 50-μl sample from the imidazole reaction mixture was analyzed on a C18 column (Partisil 5 μm octyldecyl silane column; 16) (Phenomenex, Torrance, Calif.). The mobile phase consisted of a linear gradient (25 min) from 0 to 100% methanol in 0.1% trifluoroacetic acid (flow rate 1 ml/min), and detection was set at 312 nm.
RNA isolation and reverse transcription-PCR (RT-PCR) analysis.
Cultures of S. clavuligerus grown in SA medium were harvested by centrifugation at 72 h, and the mycelia were ground in a diethyl pyrocarbonate-treated mortar and pestle under liquid nitrogen. Total RNA was isolated from about 100 mg of cell lysate with the RNeasy Mini kit (QIAGEN). The remaining DNA in the RNA samples was eliminated by treatment with RNase-free DNase I (Gibco-BRL). The reverse transcription (RT) reaction of intergenic regions of orf10 (cyp)-orf11 (fd) and orf11 (fd)-orf12 consisted of the following components: 20 μl of total RNA (15 to 20 μg), 12 μl of 4 mM deoxynucleoside triphosphate, 2 μl of 10 μM gene-specific primer (RT 11-3 or RT 12-3), 1 μl of RNase-Block (Stratagene), 5 μl of 10× MMLV buffer, and 8.5 μl of diethyl pyrocarbonate-treated ddH2O. After 10 min at room temperature, 1.5 μl of MMLV reverse transcriptase was added, and the reaction mixture was incubated at 37°C for 1 h. The PCR amplification was carried out in a 100-μl reaction mixture containing 10 μl of dimethyl sulfoxide, 10 μl of 10× Pfu buffer, 5 μl of 4 mM deoxynucleoside triphosphate, 2 μl of each sense and antisense primer (RT 10-5–RT 11-3 or RT 11-5–RT 12-3), a 10-μl sample from the RT reaction mixture, and 60 μl of ddH2O. After heating at 94°C for 3 min, 2.5 U of Pfu DNA polymerase was added and 30 reaction cycles were performed. The temperature and time period for the first five cycles were 94°C for 30 s, 48°C for 1 min, and 72°C for 1 min, and then the annealing temperature was raised to 60°C and an additional 25 cycles were carried out.
Nucleotide sequence accession number.
The GenBank accession number for orf10 (cyp), orf11 (fd), and orf12 is AF200819.
RESULTS
Identification of three ORFs located downstream of orf9 (cad).
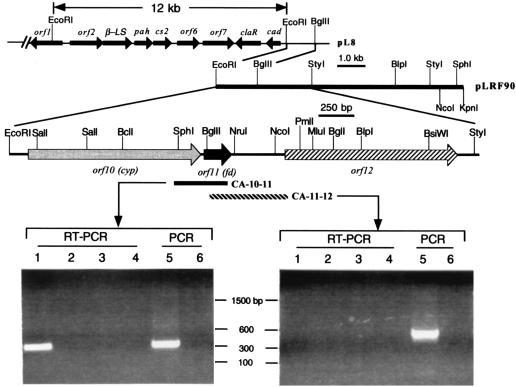
Earlier work in this laboratory has shown that the 12-kb genomic fragment encompassing orf2 to orf9 and a 1.8-kb 3′ flanking region are located at the end of the cosmid clone of S. clavuligerus, pL8 (64) (see Fig. 5). To obtain a larger DNA fragment downstream of orf9 (cad), a genomic sublibrary was constructed based on the restriction map described by Aidoo et al. (1). Genomic DNA isolated from S. clavuligerus was digested with EcoRI-KpnI, and the fragments between 8.5 and 10 kb were isolated and ligated into pBluescript II SK(−). This sublibrary was subsequently screened by colony hybridization with the 1.5-kb EcoRI-BglII fragment probe cloned from the 1.8-kb region downstream of orf9 in pL8. Of 200 colonies screened, 12 positive clones were identified. Restriction enzyme mapping revealed a 9.0-kb insert in pLRF90, and the region that hybridized to the 1.5-kb EcoRI-BglII probe was localized to its 5′ end, indicating the linkage of this fragment to orf9. A 3.4-kb EcoRI-StyI fragment from the 5′ end of pLRF90 insert was sequenced on both strands.
FIG. 5.
Restriction map of pL8 and pLRF90 and the transcriptional analysis of orf10 (cyp), orf11 (fd), and orf12 by RT-PCR. The 12-kb EcoRI fragment used for transformation of S. lividans (31) is also shown. Fragments CA-10-11 and CA-11-12 indicate the RT-PCR products with primer sets RT 10-5–RT 11-3 and RT 11-5–RT 12-3. Lane 1, RT-PCR with all components added; lane 2, no reverse transcriptase added in RT reactions; lane 3, no primer added in the RT reactions; lane 4, no RT reaction mixture added in the PCR; lane 5, PCR control with S. clavuligerus chromosomal DNA templates; lane 6, PCR control without S. clavuligerus chromosomal DNA templates.
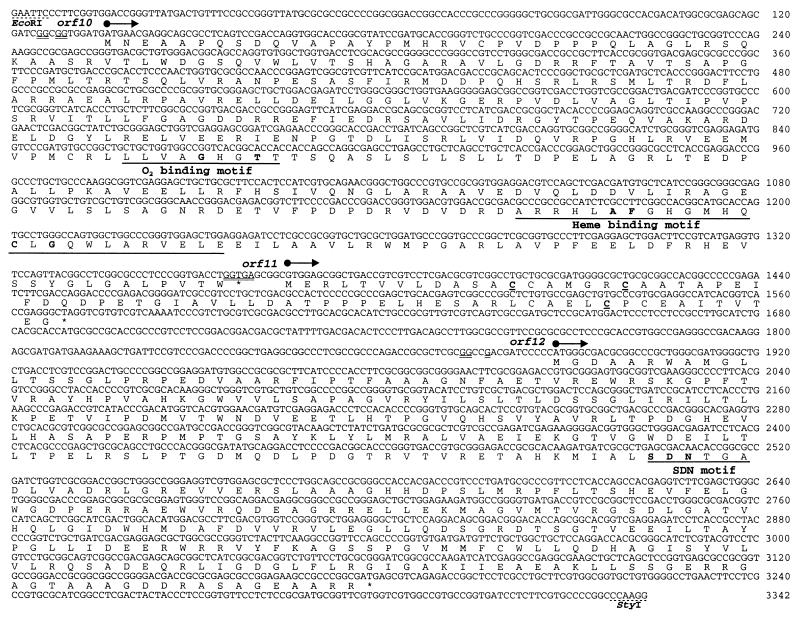
Three complete ORFs, orf10 (cyp), orf11 (fd), and orf12, were identified within the sequenced fragment by using FramePlot (30) (Fig. 2 and see Fig. 5). The three ORFs are transcribed in the same direction. The G+C content in the ORFs is 72.2% and the G+C frequencies in the codon's third position of the three ORFs are 95.1, 97, and 95.1% respectively, typical of codon usage in Streptomyces genes (14, 63). orf10 (cyp) is located 286 bp downstream of orf9 (cad) and is transcribed from the opposite strand. The start codon of orf10 (cyp) is preceded by a sequence (GGXGG) with a certain degree of complementarity to a region close to the 3′ end of the 16S rRNA that could potentially act as a ribosomal binding site (RBS) (57). orf10 (cyp) encodes a protein of 407 amino acids with a predicted mass of 44,906 Da and a calculated pI of 5.41. A BLASTP (3) search showed that the orf10 (cyp) product, P-450cla, has high sequence similarity (40 to 47% identity and 54 to 62% similarity) to cytochrome P-450 monooxygenases from several microorganisms. The greatest similarities are to the cytochrome P-450 proteins encoded by the subC gene (47% identity, 62% similarity) from Streptomyces griseolus (46) and by the putative P-450 gene from S. lividans 66 (42 and 57%) (GenBank accession no. AT-072709). Some of the homologous P-450s are involved in the biosynthesis of secondary metabolites in streptomycetes, including Streptomyces tendae (nikkomycin) (GenBank accession no. CAB46536), Streptomyces carbophilus (pravastatin) (62), Streptomyces fradiae (tylosin) (24), and Streptomyces antibioticus (oleandomycin) (52). Two highly conserved regions of cytochrome P-450 proteins exist in P-450cla (Fig. 2). The O2 binding motif is present as LLVAGHGT, including the invariant G-247 and T-250. A very strongly conserved heme-binding pocket, AFGHGMHQCLGQ, is evident and includes the invariant A-350, F-351, and G-359 residues as well as C-357 that coordinates to the heme iron.
FIG. 2.
Nucleotide and deduced amino acid sequences of the 3.4-kb EcoRI-StyI DNA fragment containing orf10 (cyp), orf11 (fd), and orf12. The deduced amino acids are shown below the nucleotides as one-letter notations. The putative RBSs are double underlined. The presumed translational start site and direction of transcription for each ORF are indicated by a labeled arrow. Restriction enzyme sites are marked by dotted lines. The highly conserved O2 and heme binding motifs in P-450cla and the SDN motif in Orf12 are single underlined. The amino acids in boldface indicate residues that are invariant in all enzymes aligned.
orf11 (fd) consists of 207 nucleotides and starts from a GTG that is located 5 bp downstream of orf10 (cyp) and is preceded by a presumed RBS (GGTGA). orf11 (fd) encodes a protein, Fdcla, of 68 amino acids with a molecular mass of 7,086 Da and a calculated pI of 4.04. A database search showed that the deduced amino acid sequence of Fdcla has a significantly high homology to the group of [3Fe-4S] ferredoxins from several Streptomyces spp. and also to [4Fe-4S] ferredoxins from Moorella thermoacetica (22) and Bacillus subtilis (56). The greatest similarity (60%) was found to ferredoxin-2 from S. griseolus containing a [3Fe-4S] cluster (44). The multiple alignment between Fdcla and homologous ferredoxins revealed the high degree of conservation of three cysteine residues essential for coordination to iron at positions 13, 19, and 58 (Fig. 2).
The third identified gene, orf12, has three possible start codons, two ATGs and one GTG, separated by 21 and 39 nucleotides, respectively. Among these, the first ATG is preferred due to the presence of a potential RBS sequence, GGXXG located 10 bp upstream. orf12 is located at 316 nucleotides downstream of orf11 (fd) and is predicted to encode a protein, Orf12, of 430 amino acids with a molecular mass of 47,081 Da and a pI of 5.52. A BLASTP search showed that this protein has significant homology (33% identity and 52% similarity) to the lpqF gene product from Mycobacterium tuberculosis (function unknown) (19). In addition, amino acid residues from 177 to 220 in Orf12 contain a conserved SDN motif, which plays a crucial role in the catalytic activity of class A β-lactamases (49).
Insertional inactivation of orf10 (cyp) and orf12 in S. clavuligerus.
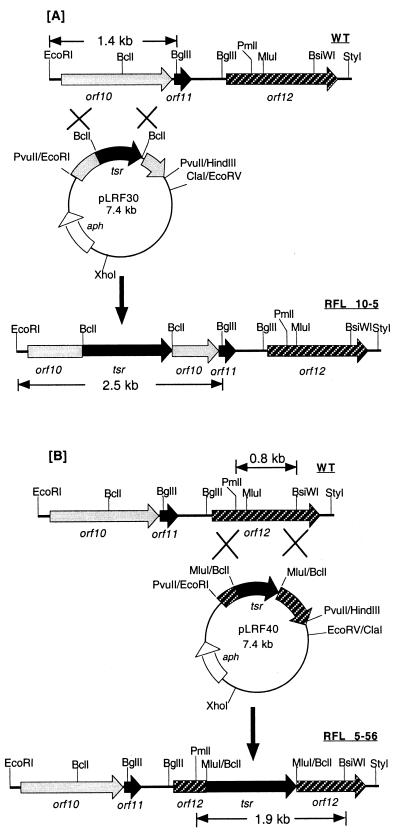
To investigate whether these three new genes were involved in clavulanic acid biosynthesis, gene disruption vectors for orf10 (cyp) and orf12 were constructed in vitro by insertion of the tsr-disrupted copy into the replicationally unstable vector pLRF66. The resulting recombinants, pLRF30 and pLRF40 (Fig. 3), isolated from S. lividans TK24 were subsequently introduced into wild-type S. clavuligerus by transformation. The primary transformants were subjected to protoplast formation and regeneration to allow vector elimination (5). Progeny screened for loss of neomycin resistance yielded strains with the Thior Neos phenotype. Five hundred colonies were screened for double crossover between the disrupted genes and their chromosomal counterparts in each case. Three Thior Neos strains were obtained from progeny of S. clavuligerus (pLRF30) and five were obtained from progeny of S. clavuligerus (pLRF40).
FIG. 3.
Construction of orf10 (cyp) and orf12 disruption mutants and restriction maps of the wild-type genes and their disrupted copies in mutants showing the expected band sizes in the Southern hybridization. The solid arrow represents the thiostrepton resistance gene (tsr), the open arrow represents neomycin resistance gene (aph), the gray arrow represents orf10 (cyp), and the cross-hatched box and arrow represent orf12. (A) The disruption of orf10 (cyp). (B) The disruption of orf12.
The replacement of wild-type orf10 (cyp) and orf12 in S. clavuligerus by tsr-disrupted copies and subsequent plasmid elimination were confirmed by Southern hybridization in the two corresponding strains, RFL 10-5 and RFL 5-56, respectively (Fig. 3A and B). Chromosomal DNA prepared from the wild type and RFL 10-5 digested with EcoRI-BglII was separately hybridized to orf10 (cyp)-specific and tsr-specific probes. As expected, the orf10 (cyp) probe showed a 1.5-kb hybridization band in the wild-type genome, and this band was replaced by a 2.6-kb band from the RFL 10-5 genome. RFL 10-5 chromosomal DNA gave exactly the same hybridization band to the tsr probe as the orf10 (cyp) probe, but no hybridization band to the tsr probe was seen in the wild-type genomic DNA (data not shown). Genomic DNA isolated from wild-type S. clavuligerus and RFL 5-56 was digested with PmlI-BsiWI and was hybridized with orf12-specific and tsr-specific probes. As expected, the 0.8-kb band that hybridized to the orf12 probe in the wild type shifted to 1.9 kb in RFL 5-56. In addition, the 1.9-kb hybridization band to the tsr probe in RFL 5-56 was missing in the wild-type chromosomal DNA (data not shown).
Characterization of RFL 10-5 and RFL 5-56 mutants.
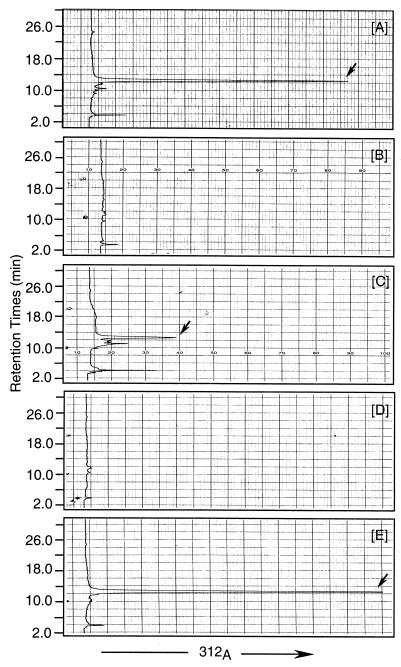
RFL 10-5 and RFL 5-56 showed identical growth characteristics and morphologies to those of wild-type S. clavuligerus when grown in liquid medium and on agar plates. To determine whether the disruption of orf10 (cyp) and orf12 affected clavulanic acid production, supernatants taken from cultures of RFL 10-5 and RFL 5-56 in SA medium were analyzed at different time points during a 144-h fermentation. No β-lactamase inhibition activities against K. pneumoniae were detected by bioassay of the two disruption mutants (data not shown). HPLC analyses of imidazole adducts showed that clavulanic acid produced in wild-type S. clavuligerus gave a peak with a retention time of 13.0 min, but this peak was absent in the two disruption strains (Fig. 4A, B, and D). These results clearly indicated that clavulanic acid biosynthesis was completely blocked in RFL 10-5 and RFL 5-56.
FIG. 4.
HPLC traces of imidazole reaction products from supernatants of the wild type (A) and disruption mutants RFL 10-5 (B) and RFL 5-56 (D) and from trans complementation strains RFL 10-5–P450ca (C) and RFL 5-56–CA12 (E). Clavulanic acid adduct peaks are marked by arrows.
To ensure that the double-crossover recombination had no effect on other aspects of secondary metabolism, supernatants were analyzed with the β-lactam-supersensitive strain E. coli SC 21255. Bioassay showed that RFL 10-5 and RFL 5-56 yielded the same levels of penicillin, cephalosporin, and cephamycin as those produced in the wild-type strain (data not shown), indicating the disruption of orf10 (cyp) and orf12 had no effect on the production of these metabolites.
trans complementation of RFL 10-5 and RFL 5-56 disruption mutants.
Two recombinant plasmids carrying a wild-type copies of orf10 (cyp) or orf12 were constructed. The 1.5-kb BglII-BglII fragment cloned in pLRF38 covered the orf10 (cyp) coding region as well as its upstream regulatory sequence so orf10 (cyp) could be expressed under the control of its native promoter. A 1.3-kb NdeI-orf12-HindIII fragment, with the NdeI site overlapping the start codon of orf12, was inserted between the NdeI and HindIII sites and downstream of the thiostrepton-inducible promoter (PtipA) of pWHM1109 to give pLCA12. RFL 10-5 and RFL 5-56 were transformed with pLRF38 and pLCA12, respectively, and apramycin- and kanamycin-resistant clones were selected. Transformants named RFL 10-5-P450cla and RFL 5-56-ORF12 were fermented along with the mutants possessing pKC1139 or pWHM1109 as controls. Bioassay and HPLC analysis of imidazole adducts showed that clavulanic acid production was observed in both RFL 10-5-P450cla and RFL 5-56-CA12 (Fig. 4C and E), whereas no clavulanic acid was produced in the mutants containing the vectors alone (data not shown). These results confirmed the targeted disruption of orf10 (cyp) and orf12 and clearly demonstrated a direct correlation between clavulanic acid biosynthesis and the presence of viable orf10 (cyp) or orf12 in S. clavuligerus.
Transcriptional analysis of orf10 (cyp), orf11 (fd), and orf12.
To examine the expression of the three new genes at the transcriptional level, an RT-PCR method was employed to detect the presence of transcripts of the intergenic regions of orf10 (cyp)-orf11 (fd) and orf11 (fd)-orf12. Two pairs of oligonucleotide primers for the amplification of the intergenic regions of orf10 (cyp)-orf11 (fd) and orf11 (fd)-orf12 were designed. cDNA was synthesized from the total RNA and antisense primer RT 11-3 or RT 12-3. PCR amplification followed by using the DNA-RNA hybrid template and either the RT10-5–RT 11-3 or RT11-5–RT 12-3 primers. A 350-bp PCR product corresponding to the size of the expected amplification product between the RT 10-5 and RT 11-3 primers was obtained (Fig. 5). This RT-PCR product was inserted into pT7Blue-3 to give plasmid pL1011 and was sequenced to show it was identical to the genomic DNA bracketed by RT 10-5 and RT 11-3. This result demonstrated that orf10 (cyp) and orf11 (fd) are expressed as a polycistronic transcript. No product was observed in the case of RT 11-5–RT 12-3 RT-PCR. In a control reaction with S. clavuligerus genomic DNA as template, the 550-bp PCR product covered by RT 11-5 and RT 12-3 was observed (Fig. 5). This indicated that orf12 is not cotranscribed with orf10 (cyp) and orf11 (fd). In addition, a promoter probing experiment was performed with pIJ486 (60), and the result showed that strong promoter activity is present in the intergenic region between orf10 (cyp) and orf11 (fd) (data not shown).
DISCUSSION
Prokaryotic genes involved in the biosynthesis of secondary metabolites are typically clustered (26). It had previously been thought that the clavulanic acid biosynthetic cluster in S. clavuligerus is composed of eight ORFs, orf2 to orf9 (2, 31). Translation of these genes does not reveal an oxygenase required in the mysterious oxidative enantiomerization of clavaminic acid (Fig. 1, compound 5) to the antipodal aldehyde (Fig. 1, compound 6) (34). This paper describes the localization of three new biosynthetic genes downstream of orf9, the determination of their nucleotide sequences, and the tentative assignment of their function. A cytochrome P-450 protein and ancillary ferrodoxin are encoded by the first two of these and are likely to mediate the heretofore missing oxidative step in the biosynthesis of clavulanic acid.
Cytochrome P-450 proteins that carry out monooxygenase reactions are widespread in nature. Of the large number of cytochrome P-450 proteins characterized in prokaryotes, some are thought to be involved in the biosynthesis of antibiotics (4, 24, 29, 39, 52). The predicted amino acid sequence of orf10 (cyp) showed close similarities to cytochrome P-450 proteins, particularly those belonging to the CYP-105 gene family (P-450SU2, P-450SU1, and P-450soy [>40%]) (41). This assignment is supported by the very strong conservation among the residues that make up the heme-binding domain and oxygen-binding site in P-450cla, including invariant residues common to all cytochrome P-450 proteins.
Ferredoxins are small, acidic, electron-transfer proteins that contain Fe-S clusters attached to the polypeptide chain by cysteine residues. orf11 (fd) was identified as a ferredoxin on the basis of its deduced amino acid sequence homology to the primary structures of Fd1 and Fd2 from S. griseolus (44) and Fdsoy from S. griseus (59). Of five cysteine residues, C-13, C-19, and C-58 align with the cysteines that are invariant in all [3Fe-4S] clusters, suggesting that Fdcla contains a [3Fe-4S] cluster as well. As observed in several other organisms, the S. clavuligerus ferredoxin gene is located downstream of and adjacent to the P-450 gene. This arrangement is highly suggestive that this is the in vivo electron transport protein functionally associated with P-450cla. The targeted disruption of orf10 (cyp) led to the complete loss of clavulanic acid production in RFL 10-5, a deficiency that could be fully restored by transformation of the mutant with a wild-type copy of orf10 (cyp). This clearly demonstrated that orf10 (cyp) and orf11 (fd) are involved in clavulanic acid biosynthesis. Therefore, the corresponding products of these genes are likely candidates to mediate the oxidative reaction between clavaminic acid (Fig. 1, compound 5) and aldehyde (compound 6).
The prototype P-450 system in prokaryotes, as characterized in Pseudomonas putida, consists of a cytochrome P-450, a ferredoxin, and a ferredoxin-NADP+ reductase, which are organized in a single operon (45). Although orf12 is located only 300 bp downstream of orf11 (fd), a BLASTP search of the amino acid sequence encoded by orf12 showed neither similarities to other ferredoxin-NADP+ reductases nor the presence of the highly conserved flavin adenine dinucleotide or flavin mononucleotide binding domains (51). Furthermore, transcriptional analysis demonstrated that orf12 is not expressed as a single operon with orf10 (cyp) and orf11 (fd). Together, these findings indicate that orf12 does not encode a ferredoxin-NADP+ reductase. Instead, this function may originate from a protein that is recruited from elsewhere in cellular metabolism (44). Apart from the uncharacterized lpqF gene product of M. tuberculosis, Orf12 does not show any revealing similarity to other proteins. Although a highly conserved SDN motif is present, other residues critical to the catalytic activity of β-lactamases are absent (43, 49). Therefore, more evidence is needed to elucidate the role of Orf12 in the clavulanic acid biosynthetic pathway.
The in vivo functional analysis of these new ORFs in the parental strain S. clavuligerus has demonstrated they are required for clavulanic acid biosynthesis. A recent study showed that the P-450 and Fd genes of S. lividans 66 are closely linked, and their products, like those encoded by orf10 (cyp) and orf11 (fd), also belong to the CYP-105 P-450 family and [3Fe-4S] ferredoxins (GenBank accession no. AT-072709). P-450cla and Fdcla share significantly high similarities to the S. lividans 66 P-450 (42.6% identity and 57% similarity) and Fd (36% identity and 46% similarity). While there is no direct experimental evidence, these findings provide a possible explanation for the earlier observation that a 12-kb S. clavuligerus genomic fragment corresponding to orf2 to orf9 was capable of producing clavulanic acid when introduced into S. lividans 66. One is misled in this instance by the expression of a presumed intact biosynthetic cluster in a heterologous host alone. The present experiments demonstrate the need for specific gene disruption and complementation in the producing strain itself to fully identify the functional genes of a biosynthetic pathway with certainty.
ACKNOWLEDGMENTS
We are grateful to T.-K. Wu for providing the S. clavuligerus genomic library clone pL8 and to J. Ravel for his comments on the transcriptional analysis. We thank C. R. Hutchinson (University of Wisconsin) for providing pIJ680, pKC1139, pWHM1109, and S. lividans TK24. We are grateful to Bristol-Myers Squibb, Inc., for providing the β-lactam indicator E. coli SC 12155.
We are pleased to acknowledge the National Institutes of Health for financial support (grant AI 14937).
REFERENCES
- 1.Aidoo K A, Wong A, Alexander D C, Rittammer R A R, Jensen S E. Cloning, sequencing and disruption of a gene from Streptomyces clavuligerus involved in clavulanic acid biosynthesis. Gene. 1994;147:41–46. doi: 10.1016/0378-1119(94)90036-1. [DOI] [PubMed] [Google Scholar]
- 2.Aidoo K A, Paradkar A S, Alexander D C, Jensen S E. Use of recombinant DNA techniques to study the production of β-lactam compounds in Streptomyces clavuligerus. In: Gullo V P, Hunter-Cevera J C, Cooper R, Johnson R K, editors. Development in industrial microbiology. Fredericksburg, Va: American Society of Industrial Microbiology; 1993. pp. 219–236. [Google Scholar]
- 3.Altschul S F, Madden T L, Schäffer A A, Zhang J, Zhang Z, Miller W, Lipman D J. Gapped BLAST and PSI-BLAST: a new generation of protein database search programs. Nucleic Acids Res. 1997;25:3389–3402. doi: 10.1093/nar/25.17.3389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Andersen J F, Tatsuta K, Gunji H, Ishijama T, Hutchinson C R. Substrate specificity of 6-deoxyerythronolide B hydroxylase, a bacterial cytochrome P450 of erythromycin A biosynthesis. Biochemistry. 1993;32:1905–1913. doi: 10.1021/bi00059a004. [DOI] [PubMed] [Google Scholar]
- 5.Anzai H, Kumada Y, Hara O, Murakami T, Itoh R, Takano E, Imai S. Replacement of Streptomyces hygroscopicus genomic segments with in vitro altered DNA sequences. J Antibiot. 1988;41:226–233. doi: 10.7164/antibiotics.41.226. [DOI] [PubMed] [Google Scholar]
- 6.Aoki H, Kubochi Y, Iguchi E, Imanaka H. Nocardicin A, a new monocyclic β-lactam antibiotic. I. Discovery, isolation and characterization. J Antibiot. 1976;29:492–500. doi: 10.7164/antibiotics.29.492. [DOI] [PubMed] [Google Scholar]
- 7.Ausubel F M, Brent R, Kingston R E, Moore D D, Seidman J G, Smith J A, Struhl K. Current protocols in molecular biology. J. New York, N.Y: Wiley and Sons; 1995. [Google Scholar]
- 8.Bachmann B O, Li R, Townsend C A. β-Lactam synthetase: a new biosynthetic enzyme. Proc Natl Acad Sci USA. 1998;95:9082–9086. doi: 10.1073/pnas.95.16.9082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Baggaley K H, Brown A G, Schofield C J. Chemistry and biosynthesis of clavulanic acid and other clavams. Nat Prod Rep. 1997;14:309–333. doi: 10.1039/np9971400309. [DOI] [PubMed] [Google Scholar]
- 10.Bailey C R, Winstanley D J. Inhibition of restriction in Streptomyces clavuligerus by heat treatment. J Gen Microbiol. 1986;132:2945–2947. doi: 10.1099/00221287-132-10-2945. [DOI] [PubMed] [Google Scholar]
- 11.Bailey C R, Butler M J, Normansell I D, Rowlands R T, Winstanley D J. Cloning a Streptomyces clavuligerus genetic locus involved in clavulanic acid biosynthesis. Bio/Technology. 1984;2:808–811. [Google Scholar]
- 12.Baldwin J E, Lloyd M D, Wha-Son B, Schofield C J, Elson S W, Baggaley K H, Nicholson N. A substrate analogue study on clavaminic acid synthase: possible clues to the biosynthetic origin of proclavamic acid. J Chem Soc Chem Commun. 1993;1993:500–502. [Google Scholar]
- 13.Baldwin J E, Adlington R M, Bryans J S, Brighen A O, Coates J B, Crouch N P, Lloyd M D, Schofield C J, Elson S W, Baggeley K H, Cassels R, Nicholson N. Isolation of dihydroclavaminic acid, an intermediate in the biosynthesis of clavulanic acid. Tetrahedron. 1991;47:4089–4100. [Google Scholar]
- 14.Bibb M J, Findlay P R, Johnson M W. The relationship between base composition and codon usage in bacterial genes and its use for the simple and reliable identification of protein coding sequences. Gene. 1984;30:157–166. doi: 10.1016/0378-1119(84)90116-1. [DOI] [PubMed] [Google Scholar]
- 15.Bierman M, Logan R, O'Brien K, Seno E T, Nagaraja Rao R, Schoner B E. Plasmid cloning vectors for the conjugal transfer of DNA from Escherichia coli to Streptomyces spp. Gene. 1992;116:43–49. doi: 10.1016/0378-1119(92)90627-2. [DOI] [PubMed] [Google Scholar]
- 16.Bird A E, Bellis J M, Gasson B C. Spectrophotometric assay of clavulanic acid by reaction with imidazole. Analyst. 1982;107:1241–1245. [Google Scholar]
- 17.Bruschi M, Guerlesquin F. Structure, function and evolution of bacterial ferredoxins. FEMS Microbiol Rev. 1988;4:155–175. doi: 10.1111/j.1574-6968.1988.tb02741.x. [DOI] [PubMed] [Google Scholar]
- 18.Busby R W, Townsend C A. A single monomeric iron center in clavaminate synthase catalyses three nonsuccessive oxidations transformation. Bioorg Med Chem. 1996;4:1059–1064. doi: 10.1016/0968-0896(96)00088-0. [DOI] [PubMed] [Google Scholar]
- 19.Cole S T, Brosch R, Parkhill J, Garnier T, Churcher C, Harris D, Gordon S V, Eiglmeier K, Gas S, Barry III C E, Tekaia F, Badcock K, Basham D, Brown D, Chillingworth T, Connor R, Davies R, Devlin K, Feltwell T, Gentles S, Hamlin N, Holroyd S, Hornsby T, Jagels K, Krogh A, McLean J, Moule S, Murphy L, Oliver S, Osborne J, Quail M A, Rajandream M A, Rogers J, Rutter S, Seeger K, Skelton S, Squares S, Squares R, Sulston J E, Taylor K, Whitehead S, Barrell B G. Deciphering the biology of Mycobacterium tuberculosis from the complete genome sequence. Nature. 1998;393:537–544. doi: 10.1038/31159. [DOI] [PubMed] [Google Scholar]
- 20.Dominguez M G, Martin J F, Mahro B, Demain A L, Liras P. Efficient plasmid transformation of the β-lactam producer Streptomyces clavuligerus. Appl Environ Microbiol. 1987;53:1376–1381. doi: 10.1128/aem.53.6.1376-1381.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Egan L A, Busby R W, Iwata-Reuyl D, Townsend C A. Probable role of clavaminic acid as the terminal intermediate in the common pathway to clavulanic acid and the antipodal clavam metabolites. J Am Chem Soc. 1997;119:2348–2355. [Google Scholar]
- 22.Elliott J I, Yang S S, Ljungdahl L G, Travis J, Reilly C F. Complete amino acid sequence of the 4Fe-4S, thermostable ferredoxin from Clostridium thermoaceticum. Biochemistry. 1982;21:3294–3298. doi: 10.1021/bi00257a007. [DOI] [PubMed] [Google Scholar]
- 23.Elson S W, Baggaley K H, Gillett J, Holland S, Nicholson N H, Sime J T, Woroniecki S R. Isolation of two novel intracellular β-lactams and a novel dioxygenase cyclising enzyme from Streptomyces clavuligerus. J Chem Soc Chem Commun. 1987;1987:1736–1738. [Google Scholar]
- 24.Fouces R, Mellado E, Diez B, Barredo J L. The tylosin biosynthetic cluster from Streptomyces fradiae: genetic organization of the left region. Microbiology. 1999;145:855–868. doi: 10.1099/13500872-145-4-855. [DOI] [PubMed] [Google Scholar]
- 25.Hodgson J E, Fosberry A P, Rawlinson N S, Ross H N M, Neal R J, Arnell J C, Earl A J, Lawlor E J. Clavulanic acid biosynthesis in Streptomyces clavuligerus: gene cloning and characterization. Gene. 1995;166:49–55. doi: 10.1016/0378-1119(95)00560-9. [DOI] [PubMed] [Google Scholar]
- 26.Hopwood D A, Sherman D H. Molecular genetics of polyketides and its comparison to fatty acid biosynthesis. Annu Rev Genet. 1990;24:37–66. doi: 10.1146/annurev.ge.24.120190.000345. [DOI] [PubMed] [Google Scholar]
- 27.Hopwood D A, Bibb M J, Chater K F, Kieser T, Bruton C J, Kieser H M, Lydiate D J, Smith C P, Ward J M, Schrempf H. Genetic manipulation of Streptomyces: a laboratory manual. Norwich, United Kingdom: The Johns Innes Foundation; 1985. [Google Scholar]
- 28.Illing G T, Normansell I D, Peberdy J F. Protoplast isolation and regeneration in Streptomyces clavuligerus. J Gen Microbiol. 1989;135:2289–2297. doi: 10.1099/00221287-135-8-2289. [DOI] [PubMed] [Google Scholar]
- 29.Inouye M, Takada Y, Muto N, Beppu T, Horinouchi S. Characterization and expression of a P-450-like mycinamicin biosynthesis gene using a novel Micronospora-Escherichia coli shuttle cosmid vector. J Biol Chem. 1994;269:27401–27408. doi: 10.1007/BF00302258. [DOI] [PubMed] [Google Scholar]
- 30.Ishikawa J, Hotta K. FramePlot: a new implementation of the Frame analysis for predicting protein-coding regions in bacterial DNA with a high G+C content. FEMS Microbiol Lett. 1999;174:251–253. doi: 10.1111/j.1574-6968.1999.tb13576.x. [DOI] [PubMed] [Google Scholar]
- 31.Jensen S E, Alexander D C, Paradkar A S, Aidoo K A. Extending the β-lactam gene cluster in Streptomyces clavuligerus. In: Baltz R H, Hegeman G D, Skatrud P L, editors. Industrial microorganisms: basic and applied molecular genetics. Washington, D.C.: American Society for Microbiology; 1993. pp. 169–176. [Google Scholar]
- 32.Khaleeli N, Li R, Townsend C A. Origin of the β-lactam carbons in clavulanic acid from a unusual thiamine pyrophosphate-mediated reaction. J Am Chem Soc. 1999;121:9223–9224. [Google Scholar]
- 33.Krol W J, Basak A, Salowe S P, Townsend C A. Oxidative cyclisation chemistry catalyzed by clavaminate synthase. J Am Chem Soc. 1989;111:7625–7626. [Google Scholar]
- 34.Krol W J, Townsend C A. The role of molecular oxygen in clavulanic acid biosynthesis: evidence for a bacterial oxidative deamination. J Chem Soc Chem Commun. 1988;1988:1234–1236. [Google Scholar]
- 35.Malmberg L H, Hu W S, Sherman D H. Precursor flux control through targeted chromosomal insertion of the lysine ɛ-aminotransferase (lat) gene in cephamycin C biosynthesis. J Bacteriol. 1993;175:6916–6924. doi: 10.1128/jb.175.21.6916-6924.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Marsh E N, Chang M D-T, Townsend C A. Two isozymes of clavaminate synthase central to clavulanic acid formation: cloning and sequencing of both genes from Streptomyces clavuligerus. Biochemistry. 1992;31:12648–12657. doi: 10.1021/bi00165a015. [DOI] [PubMed] [Google Scholar]
- 37.May B P. Southern hybridization in shampoo. BioTechniques. 1998;25:582. doi: 10.2144/98254bm06. [DOI] [PubMed] [Google Scholar]
- 38.McNaughton H J, Thirkettle J E, Zhang Z, Schofield C J, Jensen S E, Barton B, Greaves P. Beta-lactam synthetase: implications for beta-lactamase evolution. J Chem Soc Chem Commun. 1998;1998:2325–2326. [Google Scholar]
- 39.Molnar I, Aparicio J F, Haydock S F, Khaw L E, Schwecke T, Konig A, Staunton J, Leadlay P F. Organization of the biosynthetic gene cluster for rapamycin in Streptomyces hygroscopicus: analysis of genes flanking the polyketide synthase. Gene. 1996;169:1–7. doi: 10.1016/0378-1119(95)00799-7. [DOI] [PubMed] [Google Scholar]
- 40.Mosher R H, Paradkar A S, Anders C, Barton B, Jensen S E. Genes specific for the biosynthesis of clavam metabolites antipodal to clavulanic acid are clustered with the gene for clavaminate synthase 1 in Streptomyces clavuligerus. Antimicrob Agents Chemother. 1999;43:1215–1224. doi: 10.1128/aac.43.5.1215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Munro A W, Lindsay J G. Bacterial cytochromes P-450. Mol Microbiol. 1996;20:1115–1125. doi: 10.1111/j.1365-2958.1996.tb02632.x. [DOI] [PubMed] [Google Scholar]
- 42.Nicholson N H, Baggaley K H, Cassels R, Davison M, Elson S W, Fulston M, Tyler J M, Wononieck S R. Evidence that the intermediate biosynthetic precursor of clavulanic acid is its N-aldehyde analogue. J Chem Soc Chem Commun. 1994;1994:1281–1282. [Google Scholar]
- 43.Nordmann P, Naas T. Sequence analysis of PER-1 extended-spectrum β-lactamase from Pseudomonas aeruginosa and comparison with class A β-lactamases. Antimicrob Agents Chemother. 1994;38:104–114. doi: 10.1128/aac.38.1.104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.O'Keefe D P, Gibson K J, Emptage M H, Lenstra R, Romesser J A, Litle P J, Omer C A. Ferredoxins from two sulfonylurea herbicide monooxygenase systems in Streptomyces griseolus. Biochemistry. 1991;30:447–455. doi: 10.1021/bi00216a021. [DOI] [PubMed] [Google Scholar]
- 45.O'Keefe D P, Harder P A. Occurrence and biological function of cytochrome P450 monooxygenases in actinomycetes. Mol Microbiol. 1991;5:2099–2105. doi: 10.1111/j.1365-2958.1991.tb02139.x. [DOI] [PubMed] [Google Scholar]
- 46.Omer C A, Lenstra R, Litle P J, Dean C, Tepperman J M, Leto K J, Romesser J A, O'Keefe D P. Genes for two herbicide-inducible cytochromes P-450 from Streptomyces griseolus. J Bacteriol. 1990;172:3335–3345. doi: 10.1128/jb.172.6.3335-3345.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Paradkar A S, Aidoo K A, Jensen S E. A pathway-specific transcriptional activator regulates late steps of clavulanic acid biosynthesis in Streptomyces clavuligerus. Mol Microbiol. 1998;27:831–843. doi: 10.1046/j.1365-2958.1998.00731.x. [DOI] [PubMed] [Google Scholar]
- 48.Paradkar A S, Jensen S E. Functional analysis of the gene encoding the clavaminate synthase 2 isoenzyme involved in clavulanic acid biosynthesis in Streptomyces clavuligerus. J Bacteriol. 1995;177:1307–1314. doi: 10.1128/jb.177.5.1307-1314.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Peduzzi J, Farzaneh S, Reynaud A, Barthelemy M, Labia R. Characterization and amino acid sequence analysis of a new oxyimino cephalosporin-hydrolyzing class A β-lactamase from Serratia fonticola CUV. Biochim Biophys Acta. 1997;134:58–70. doi: 10.1016/s0167-4838(97)00020-4. [DOI] [PubMed] [Google Scholar]
- 50.Perez-Redondo R, Rodriguez-Garcia A, Martin J F, Liras P. The claR gene of Streptomyces clavuligerus, encoding a LysR-type regulatory protein controlling clavulanic acid biosynthesis, is linked to the clavulanate-9-aldehyde reductase (car) gene. Gene. 1998;211:311–321. doi: 10.1016/s0378-1119(98)00106-1. [DOI] [PubMed] [Google Scholar]
- 51.Ramachandra M, Seetharam R, Emptage M H. Purification and characterization of a soybean flour-inducible ferredoxin reductase of Streptomyces griseus. J Bacteriol. 1991;173:7106–7112. doi: 10.1128/jb.173.22.7106-7112.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Rodriguez A M, Olano C, Mendez C, Hutchinson C R, Salas J A. A cytochrome P450-like gene possible involved in oleandomycin biosynthesis by Streptomyces antibioticus. FEMS Microbiol Lett. 1995;127:117–120. doi: 10.1111/j.1574-6968.1995.tb07459.x. [DOI] [PubMed] [Google Scholar]
- 53.Romero J, Liras P, Martin J F. Dissociation of cephamycin and clavulanic acid biosynthesis in Streptomyces clavuligerus. Appl Microbiol Biotechnol. 1984;20:318–325. [Google Scholar]
- 54.Salowe S P, Krol W J, Iwata-Reuyl D, Townsend C A. Elucidation of the order of oxidations and identification of an intermediate in the multistep clavaminate synthase reaction. Biochemistry. 1991;30:2281–2292. doi: 10.1021/bi00222a034. [DOI] [PubMed] [Google Scholar]
- 55.Sambrook J, Fritsch E F, Maniatis T. Molecular cloning: a laboratory manual. 2nd ed. Cold Spring Harbor, N.Y: Cold Spring Harbor Laboratory Press; 1989. [Google Scholar]
- 56.Sorokin A, Azevedo V, Zumstein E, Galleron N, Ehrlichand S D, Serror P. Sequence analysis of the Bacillus subtilis chromosome region between the serA and kdg loci cloned in a yeast artificial chromosome. Microbiology. 1996;142:2005–2016. doi: 10.1099/13500872-142-8-2005. [DOI] [PubMed] [Google Scholar]
- 57.Strohl W R. Compilation and analysis of DNA sequences associated with apparent streptomycete promoters. Nucleic Acids Res. 1992;20:961–974. doi: 10.1093/nar/20.5.961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Tang L, Grimm A, Zhang Y-X, Hutchinson C R. Purification and characterization of the DNA-binding protein DnrI, a transcriptional factor of daunorubicin biosynthesis in Streptomyces peucetius. Mol Microbiol. 1996;22:801–813. doi: 10.1046/j.1365-2958.1996.01528.x. [DOI] [PubMed] [Google Scholar]
- 59.Trower M K, Lenstra R, Omer C, Buchholz S E, Sariaslani F S. Cloning, nucleotide sequence determination and expression of the genes encoding cytochrome P-450soy (soyC) and ferredoxinsoy (soyB) from Streptomyces griseus. Mol Microbiol. 1992;6:2125–2134. doi: 10.1111/j.1365-2958.1992.tb01386.x. [DOI] [PubMed] [Google Scholar]
- 60.Ward J M, Janssen G R, Kieser T, Bibb M J, Buttner M J, Bibb M J. Construction and characterisation of a series of multi-copy promoter-probe plasmid vectors for Streptomyces using the aminoglycoside phosphotransferase gene from Tn5 as indicator. Mol Gen Genet. 1986;203:468–478. doi: 10.1007/BF00422072. [DOI] [PubMed] [Google Scholar]
- 61.Ward J W, Hodgson J E. The biosynthetic genes for clavulanic acid and cephamycin production occur as a ‘super-cluster’ in three Streptomyces. FEMS Microbiol Lett. 1993;110:239–242. doi: 10.1111/j.1574-6968.1993.tb06326.x. [DOI] [PubMed] [Google Scholar]
- 62.Watanabe I, Nara F, Serizawa N. Cloning, characterization and expression of the gene encoding cytochrome P-450sca-2 from Streptomyces carbophilus involved in production of pravastatin, a specific HMG-CoA reductase inhibitor. Gene. 1995;163:81–85. doi: 10.1016/0378-1119(95)00394-l. [DOI] [PubMed] [Google Scholar]
- 63.Wright F, Bibb M J. Codon usage in the G+C rich Streptomyces genome. Gene. 1992;113:55–65. doi: 10.1016/0378-1119(92)90669-g. [DOI] [PubMed] [Google Scholar]
- 64.Wu T-K. Ph.D. thesis. Baltimore, Md: The Johns Hopkins University; 1996. [Google Scholar]
- 65.Wu T-K, Busby R W, Houston T A, McIlwaine D B, Egan L A, Townsend C A. Identification, cloning, sequencing, and overexpression of the gene encoding proclavaminate amidino hydrolase and characterization of protein function in clavulanic acid biosynthesis. J Bacteriol. 1995;177:3714–3720. doi: 10.1128/jb.177.13.3714-3720.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]